- 1Department of Neurology, SUNY Upstate Medical University, Syracuse, NY, United States
- 2Department of Neuroscience, Johns Hopkins School of Medicine, Baltimore, MD, United States
Post-pump chorea (PPC) is characterized by the development of choreiform movements following cardiopulmonary bypass (CPB) surgery. PPC occurs almost exclusively in children, and its pathophysiology remains unclear. Here we present an adult case of PPC after bovine aortic valve replacement (AVR) which exhibited dramatic and reproducible response to steroid, suggesting the presence of occult neuroinflammation. This observation suggests a novel underlying mechanism in certain subgroups of PPC, which is likely a heterogeneous condition to start with. Further research into the pathomechanisms of PPC could offer insights into managing this otherwise symptomatic control-only condition.
Introduction
Choreiform movement, when seen after surgeries on cardiopulmonary bypass (CPB) pump, is known as post-pump chorea (PPC) (1, 2). Besides generalized chorea, comorbid encephalopathy and behavioral changes have been reported (1, 2). The first case series describing PPC was published in the 1960s, about children experiencing extrapyramidal symptoms and ultimately death after congenital heart surgeries with deep hypothermia and circulatory arrest (3). PPC is now well-characterized in the pediatric population, with an estimated incidence of 1.1–1.2% (2, 4). Conversely, PPC was not recognized in adults until the 2000s, as a rare complication with an incidence of 0.046% (2 out of 4,345 cases) in a single-center study (1, 5).
The clinical features and disease course of PPC are highly variable, with few consistent characteristics identified (1, 2, 4, 6). Abnormal movements, in a majority of cases generalized chorea, usually appear within 14 days post-surgery (3–12 days in children, 1–14 days in adults) after a latency period of 1–7 days (1, 2, 6). In children, additional symptoms such as orofacial dyskinesia, postural instability, dysphagia, and dysarthria can occur (2, 6). Common risk factors for PPC include deep hypothermia (below 25°C), rapid rewarming (above 0.18°C/minutes), long bypass time (over one hour), and extended total arrest (more than 45 min) (1, 2, 6). PPC lacks definite biomarkers and hence is a diagnosis by history and exclusion, except that at times (estimated around 38.8% in one study) magnetic resonance imaging (MRI) may reveal symmetrical caudate-putamen T2 hyperintensity (1, 2, 6). Regarding the disease course, approximately half of the cases resolve completely, while the remainder become persistent and irreversible (1, 2, 6). MRI changes may be associated with a higher likelihood of persistent diseases; poor outcome with neurocognitive developmental disorder can be seen in children, but age by itself is not an outcome predictor (1, 2, 6–12). Treatment primarily focuses on symptom control, traditionally with antipsychotics, anti-seizure medications, and benzodiazepines and more recently with vesicular monoamine transporter 2 (VMAT2) inhibitors (1, 2, 6). Out of the available VMAT2 inhibitors, tetrabenazine have demonstrated some efficacy, providing partial relief in two adult PPC cases (7, 11). For medically refractory individuals, deep brain stimulation targeting the globus pallidus internus may be considered (13, 14).
The underlying pathogenesis for PPC remains elusive (7). Hypoxia, microthromboembolism, metabolic insults, and acquired acanthocytosis have been proposed as potential mechanisms (15). However, these hypotheses do not fully explain the characteristics of PPC, such as the differences in children vs. in adult, not to mention that most cases lack etiology-specific pathognomonic findings (1, 7–12). Here, we present an adult male who developed PPC after an on-pump bovine aortic valve replacement (AVR). His clinical course suggests an inflammatory mechanism which may provide insight into some of the unknown aspects of PPC.
Case description
DD, a 66-year-old otherwise healthy gentleman, received a bovine AVR for aortic stenosis. Surgery was performed under mild hypothermia (30–32°C), with 72 minutes on-pump and 62 of total arrest. Out of the surgery he appeared encephalopathic, initially presumed to be perioperative stress and sedatives/analgesia. However, his mentation did not improve with time and instead progressed to the extent that, by postoperative day (POD) 7, his family was alarmed by the out-of-character hypervigilance and aggression (Figure 1C). By week three, he exhibited abnormal movement of frontal, bucco-lingual, axial, and appendicular chorea-ballismus, manifesting as flow of forehead wrinkling, eye closure, chewing/smacking, and jerking of extremities (Supplementary Video 1). These movements were involuntary, unpredictable, non-suppressible, non-distractible, and absent in sleep. Motor, sensory, and cerebellar functions were intact, specifically no oculomotor disturbance, loss of tone, dysarthria or dysphagia. Examination of other systems is unremarkable, including absence of constitutional symptoms or rheumatological stigmata. There was no movement disorder running in the family.
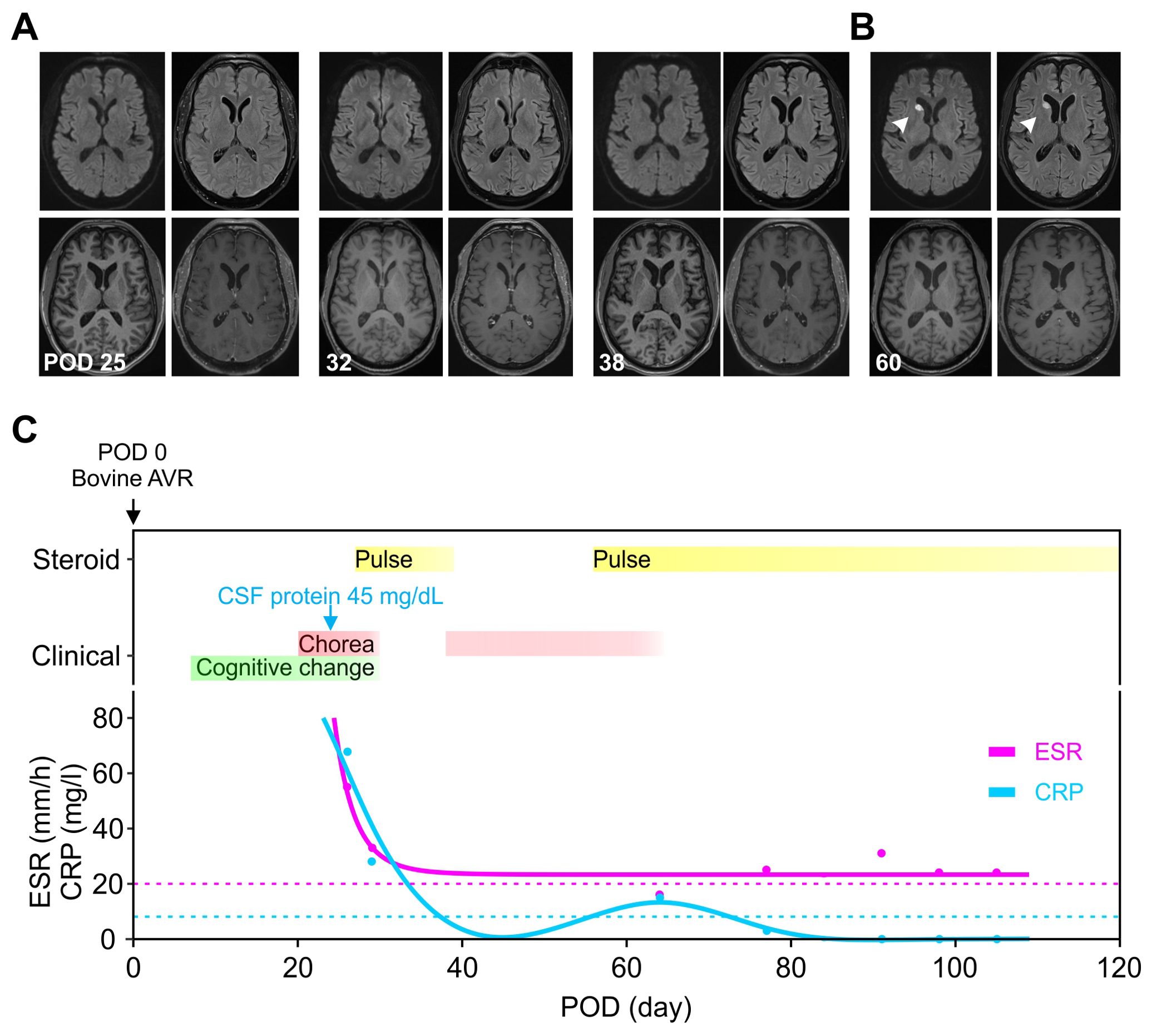
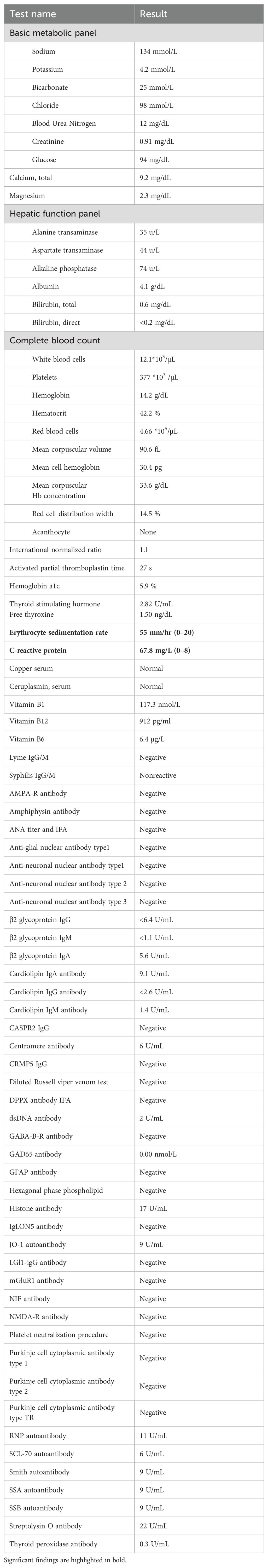
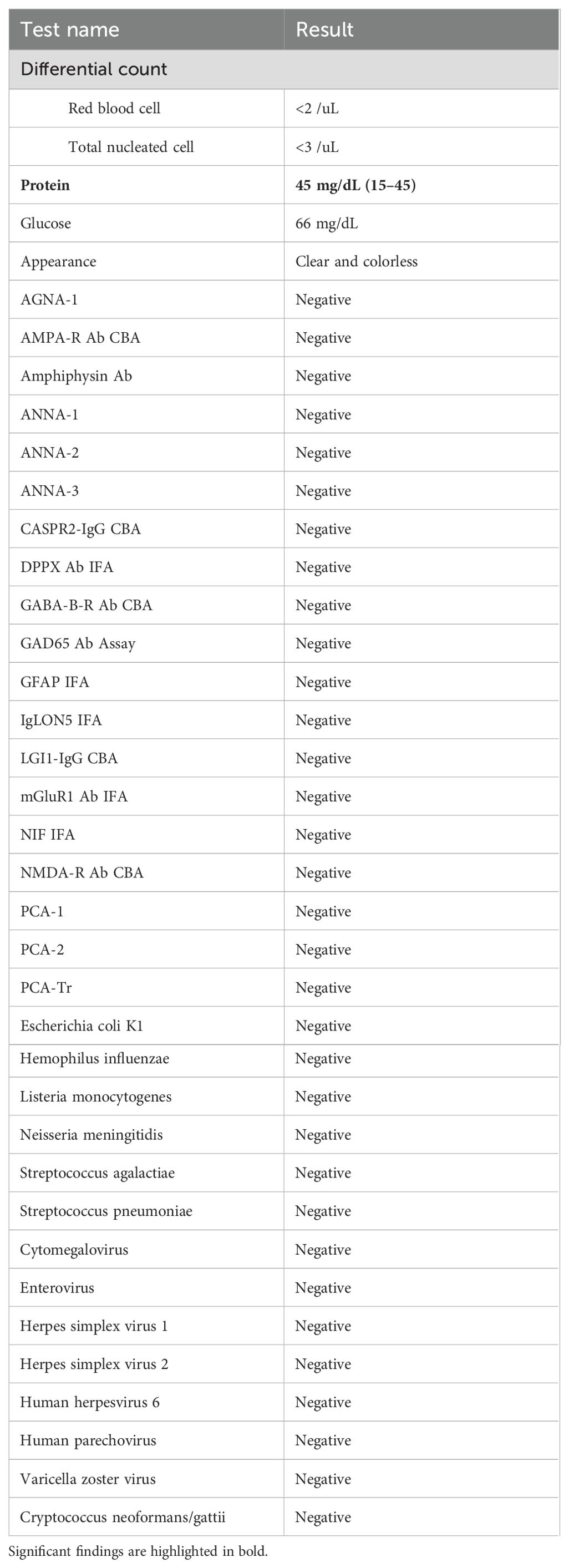
Figure 1. Evolution of clinical presentation, inflammatory biomarkers and imaging findings. (A) The MRIs were unremarkable on three occasions, including when the symptoms were most severe (POD 25) and during the recurrence (POD 38). (B) An acute ischemic stroke at the head of the right caudate (white arrowhead) was seen incidentally on POD 60 when the chorea was resolving. (C) The timing of immunomodulation (yellow) and clinical symptoms (red and green) was plotted against the days after valve replacement. The initial presentation began with cognitive changes (green, POD 7) and chorea (red, POD 14), then with symptom resolution on POD 30 during the pulse steroid (POD 27–31). Oral steroid was stopped on POD 39 with symptoms recurrence around POD 46 and then at maximum around POD 54–55. The second course of pulse steroid lasted from POD 56–60 with significant clinical improvement by POD 58. The inflammatory markers ESR (magenta) and CRP (cyan) were trended (dotted lines denote upper limit of normal range). AVR, aortic valve replacement; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; POD, postoperative day.
Serum hematological, metabolic, and infectious studies were normal, except for the elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (Table 1, Figure 1C). Survey for relevant toxins or environmental exposure also returned negative. Computed tomographic angiogram and MRI showed no vascular or structural lesions (Figure 1A, POD 25). Spot video-electroencephalography did not reveal epileptogenicity. A comprehensive panel checking other inflammatory and autoimmune markers was unremarkable (Table 1). Cell counts and glucose in the cerebrospinal fluid (CSF) were normal with protein at the upper limit (Table 2, Figure 1C). Advanced CSF tests for infectious, demyelinating, inflammatory, autoimmune, paraneoplastic, and degenerative markers did not yield any etiology (Table 2).
The behavior and movement continued to worsen despite aggressive symptomatic control, and course was further complicated by total insomnia. His continuous video-electroencephalography demonstrated mild, generalized slowing without epileptogenicity, consistent with non-specific encephalopathy. We eventually initiated dexmedetomidine infusion for sedation and movement control. Forty-eight hours after, DD was able to resume prior sleep routine and gradually behavioral improvement, with nevertheless persistent and later nearly continuous choreiform movement unresponsive to clonazepam (0.5 mg twice daily [BID]) and valproic acid (1000–2250 mg) hitherto plus trials of quetiapine (25–100 mg for 13 days) and risperidone (0.25–2 mg for 12 days). It is worth noting that his behavioral changes rendered the use of VMAT2 inhibitors unfavorable.
In light of elevated inflammatory markers and high-normal CSF protein, we empirically started him on intravenous pulse methylprednisone (1000 mg) for five days from POD 27–31. This led to remarkable improvement with minimal symptoms at POD 30, associated with normalization of CRP and ESR (Figure 1C). Follow-up MRIs from POD 32 and 38 did not show any significant change (Figure 1A). Due to acute gastrointestinal bleed, he was unable to complete a 10-day short prednisone taper. Despite concomitant use of clonazepam 0.5 mg BID and valproic acid 500 mg BID, upon discontinuation of steroid, chorea recurred immediately within the same week (POD 46) along with the elevation of inflammatory markers (Figure 1C). A second round of pulse methylprednisone (POD 56–60) was administered with his abnormal movement visibly decreased in magnitude and frequency (POD 58) along with the course of steroid. This allowed symptom resolution and discharge to an acute rehabilitation facility upon completion of the pulse steroid. We had him on an extended prednisone taper with 10 mg decrements every 7 days, for a total of 6 weeks, along with the clonazepam 0.5 BID maintained for symptomatic control. The fourth MRI from POD 60 revealed incidentally an acute infarct at the right caudate (Figure 1B). He otherwise remained in full remission at his 3, 6, & 9-month outpatient follow-up (Figures 1C).
Discussion
PPC, the emergence of chorea following CPB, was initially described in children and recently identified as a rarity in adults; to date, 18 cases of adult-onset PPC have been reported (1, 5, 7, 9–11, 15–19). Existing literature helps identify several consistent features (1, 5, 7, 9–11, 15–19). In concordance with these reports, our case developed early cognitive change and shortly after, generalized choreiform movement around POD 14 (1, 20). He experienced prolonged bypass duration and circulatory arrest, both known risk factors for PPC (1, 20). An extensive workup ruled out other etiologies (1, 2, 4). Therefore, we conclude that this presentation is consistent with an adult-onset PPC after bovine AVR, and that the distinct and robust response to one particular type of treatment, in contrast to the commonly-observed variable outcomes, may implicate an underlying singular pathomechanism.
The prevailing theory of PPC pathogenesis involves cerebral vasoconstriction and increased blood viscosity due to hypoxia, alkalosis, hypothermia, and/or rewarming, despite the lack of supportive evidence (1, 2, 4, 7). Our case demonstrated a reproducible immunosuppression response in symptoms and biomarkers (Figure 1C), suggesting neuroinflammation, either primary neural or secondary to systemic, could be a potential pathophysiology. As a matter of fact, neuroinflammation has been reported in various other post-CPB surgery neurological complications, such as chronic neurocognitive impairment or progressive supranuclear palsy-like Mokri syndrome (21–24).
It is well-recognized that cardiac surgery with bypass induces systemic and cerebral inflammation, leading to disrupted blood-brain-barrier and cellular injury (25). The inflammation may result from “contact activation” of maladaptive cascades in response to the internal surfaces of CPBs (26). Additionally, nonhuman extracellular matrix molecules such as α-1,3-galactose or N-glycolylneuraminic acid have been implicated in immune-related graft failure, potentially making the bovine bioprosthesis an immunogenic source (27, 28). Lastly, metabolic-immune signaling such as the hypoxia-inducible factor-1α pathway have also been shown to shift the immune balance toward a pro-inflammatory state (29, 30). Perioperative ischemia and reperfusion could induce such pathways, eventually contributing to the neural dysfunction observed in PPC.
PPC likely represents a heterogeneous condition, and the proposed inflammatory mechanism, along with other previously noted pathogenic processes, provides a more comprehensive understanding (1, 2, 4, 26–30). For example, deep hypothermia and circulatory arrest, both known risk factors for PPC, have been linked to heightened neuroinflammation, manifested by increased pro-inflammatory cytokines in the blood and CSF (31–33). The neuroinflammatory mechanism may also elucidate some of the distinctions between PPC in children and adults (1, 2, 4, 5). Children, having limited antigen exposure a priori, are believed to have less mature immune systems and more prone to autoimmunity and inflammation (34, 35). The proposed mechanism may also provide justification for the highly-variable course, particularly the late-onset and/or persistent cases, considering that the bovine tissue can continue being an antigen-presenting source long after surgery (1, 2, 4, 6, 27, 28). Furthermore, cerebral inflammation can lead to vascular injury or plasma hyperviscosity, both recognized risks for small vessel occlusion, potentially serving as a reciprocal feed-forward mechanism in the persistent PPC case (36, 37). The delayed right caudate infarct observed on POD 60 (Figure 1A), though incongruent in time course and unilaterality with the clinical presentation, may reflect the indolent nature of this process.
In summary, PPC is a heterogeneous collection of diseases with various mechanisms (38). The remarkable response to immunosuppression demonstrated in this case may thus suggest a subgroup of PPC with immune/inflammation-related chorea (1, 2, 6). Given the constraint of a single case and the lack of a control group, our preliminary observation requires cautious interpretation without presuming the causality. Further large-scale investigations into the pathophysiology and management of PPC are needed (39–41).
Conclusions
We present a case of adult-onset PPC with remarkable and reproducible responsiveness to steroid treatment. This phenomenon suggests an underlying neuroinflammatory or neuroimmunological mechanism. We propose possible causes of inflammation and immunogenicity and illustrate how this hypothesis can address the unknown aspects of PPC. Further studies based on this observation may advance the understanding of its pathophysiology and targeted management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MI: Data curation, Writing – review & editing, Methodology. MZ: Data curation, Writing – original draft, Writing – review & editing, Conceptualization, Methodology. NO: Writing – original draft. YG: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing, Methodology. EY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank our colleagues at SUNY for discussions and comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1458022/full#supplementary-material
Glossary
AVR: Aortic valve replacement
BID: twice daily
CPB: Cardiopulmonary bypass
CSF: Cerebrospinal fluid
MRI: Magnetic resonance imaging
POD: Postoperative day
PPC: Post-pump chorea
References
1. Ahn JH, Song J, Choi I, Youn J, Cho JW. Risk factors and prognosis of adult-onset post-pump chorea. J Neurol Sci. (2021) 422. doi: 10.1016/j.jns.2021.117328
2. Medlock MD, Cruse RS, Winek SJ, Geiss DM, Horndasch RL, Schultz DL, et al. A 10-year experience with postpump chorea. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc. (1993) 34:820–6. doi: 10.1002/ana.410340611
3. Björk VO, Hultquist G. Brain damage in children after deep hypothermia for open-heart surgery. Thorax. (1960) 15. doi: 10.1136/thx.15.4.284
4. DeLeon S, Ilbawi M, Arcilla R, Cutilletta A, Egel R, Wong A, et al. Choreoathetosis after deep hypothermia without circulatory arrest. Ann Thorac Surg. (1990) 50:714–9. doi: 10.1016/0003-4975(90)90668-V
5. de Bie RM, Surie S, Kloek JJ, Biervliet JD, de Beaumont EM, Rutten PM, et al. Chorea in adults after pulmonary endarterectomy with deep hypothermia and circulatory arrest. Ann Intern Med. (2008) 149:842. doi: 10.7326/0003-4819-149-11-200812020-00025
6. Wong P, Barlow C, Hickey P, Jonas R, Castaneda A, Farrell D, et al. Factors associated with choreoathetosis after cardiopulmonary bypass in children with congenital heart disease. Circulation. (1992) 86.
7. Di Luca DG, Swinkin E, Lang A. Teaching video neuroImage: reversible caudate changes in a patient with post-pump chorea. Neurology. (2022) 98:731–2. doi: 10.1212/WNL.0000000000200339
8. Gavish R, Straussberg R. A rare presentation of postpump hemichorea. Isr Med Assoc J IMAJ. (2019) 21:286–7.
9. Miró JM, Matellano FV. Chorea following extracorporeal circulation in adults: A case report and brief literature review. Neurol Barc. (2020) 35:519–21.
10. Park KW, Choi N, Ryu HS, Kim HJ, Lee CS, Chung SJ. Post-pump chorea and progressive supranuclear palsy-like syndrome following major cardiac surgery. Mov Disord Clin Pract. (2020) 7:78–82. doi: 10.1002/mdc3.12867
11. Savadi-Oskouei S, Savadi-Oskouei D, Baghbani-Oskouei A. Post-pump chorea: choreoathetosis following pulmonary thromboendarterectomy. J Exp Clin Neurosci. (2020) 7.
12. Stimming EF, Bega D. Chorea. Contin Lifelong Learn Neurol. (2022) 28:1379–408. doi: 10.1212/CON.0000000000001169
13. Telford R, Vattoth S. MR anatomy of deep brain nuclei with special reference to specific diseases and deep brain stimulation localization. Neuroradiol J. (2014) 27:29–43. doi: 10.15274/NRJ-2014-10004
14. Aoyagi K, Higuchi Y, Okahara Y, Yakufujiang M, Matsuda T, Yamanaka Y, et al. Effects of bilateral pallidal deep brain stimulation on chorea after pulmonary thromboendarterectomy with deep hypothermia and circulatory arrest: a case report. Acta Neurochir (Wien). (2018) 160:393–5. doi: 10.1007/s00701-017-3433-4
15. Das P, Shinozaki G, McAlpine D. Post-pump chorea—Choreiform movements developing after pulmonary thromboendarterectomy for chronic pulmonary hypertension presenting as “Functional. Mov Disord Psychosom. (2011) 52:459–62. doi: 10.1016/j.psym.2011.01.022
16. Bisciglia M, London F, Hulin J, Peeters A, Ivanoiu A, Jeanjean A. Choreoathetotic syndrome following cardiac surgery. J Clin Anesth. (2017) 36:59–61. doi: 10.1016/j.jclinane.2016.08.043
17. Passarin MG, Romito S, Avesani M, Alessandrini F, Petrilli G, Santini F, et al. Late-onset choreoathetotic syndrome following heart surgery. Neurol Sci. (2010) 31:95–7. doi: 10.1007/s10072-009-0171-2
18. Saft C, Reber D, Streuer M, Andrich J. Post pump chorea in a 77-year-old male. Neurol Sci. (2011) 32:699–701. doi: 10.1007/s10072-011-0583-7
19. Hamzi MA, Hassani K, El Kabbaj D. Late-onset choreoathetotic syndrome following heart surgery in adults with end-stage renal disease. Saudi J Kidney Dis Transplant. (2018) 29. doi: 10.4103/1319-2442.225180
20. Surie S, Tijssen MA, Biervliet JD, Beaumont EM, Kloek JJ, Rutten PM, et al. Chorea in adults following pulmonary endarterectomy. Mov Disord. (2010) 25:1101–4. doi: 10.1002/mds.23044
21. Zhuang YM, Xu JY, Zheng K, Zhang H. Research progress of postoperative cognitive dysfunction in cardiac surgery under cardiopulmonary bypass. Ibrain. (2023). doi: 10.1002/ibra.12123
22. Van Harten A, Scheeren T, Absalom A. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. (2012) 67:280–93. doi: 10.1111/j.1365-2044.2011.07008.x
23. Tisel SM, Ahlskog JE, Duffy JR, Matsumoto JY, Josephs KA. PSP-like syndrome after aortic surgery in adults (Mokri syndrome. Neurol Clin Pract. (2020) 10:245–54. doi: 10.1212/CPJ.0000000000000708
24. van Ton AMP, Duindam HB, van Tuijl J, Li WW, Dieker H-J, Riksen NP, et al. Neuroinflammation in cognitive decline post-cardiac surgery (the FOCUS study): an observational study protocol. BMJ Open. (2021) 11:e044062.
25. Reinsfelt B, Ricksten S-E, Zetterberg H, Blennow K, Fredén-Lindqvist J, Westerlind A. Cerebrospinal fluid markers of brain injury, inflammation, and blood-brain barrier dysfunction in cardiac surgery. Ann Thorac Surg. (2012) 94:549–55. doi: 10.1016/j.athoracsur.2012.04.044
26. Rodríguez-López JM, Iglesias-González JL, Lozano-Sánchez FS, Palomero-Rodríguez MÁ, Sánchez-Conde P. Inflammatory response, immunosuppression and arginase activity after cardiac surgery using cardiopulmonary bypass. J Clin Med. (2022) 11. doi: 10.3390/jcm11144187
27. Salama A, Couvrat-Desvergnes G, Lorent M, Evanno G, Le Berre L, Hruba P, et al. Increased late graft loss in kidney recipients with serum sickness disease following anti-thymocyte globulin induction: relation with an anti-NEU5GC response. Am Transpl Congr. (2015) 15:1.
28. Senage T, Paul A, Le Tourneau T, Fellah-Hebia I, Vadori M, Bashir S, et al. The role of antibody responses against glycans in bioprosthetic heart valve calcification and deterioration. Nat Med. (2022) 28:283–94. doi: 10.1038/s41591-022-01682-w
29. Fritzenwanger M, Jung C, Goebel B, Lauten A, Figulla HR. Impact of short-term systemic hypoxia on phagocytosis, cytokine production, and transcription factor activation in peripheral blood cells. Mediators Inflammation. (2011) 2011). doi: 10.1155/2011/429501
30. Wang T, Jiao Y, Zhang X. Immunometabolic pathways and its therapeutic implication in autoimmune diseases. Clin Rev Allergy Immunol. (2021) 60:55–67. doi: 10.1007/s12016-020-08821-6
31. Puwei S, Siyu M, ZhuoGa D, Kede W, Zhaocong Y, Patel N, et al. The potential value of cuprotosis in myocardial immune infiltration that occurs in pediatric congenital heart disease in response to surgery with cardiopulmonary bypass. Immun Inflammation Dis. (2023) 11. doi: 10.1002/iid3.795
32. Chen Q, Lei Y-Q, Liu J-F, Wang Z-C, Cao H. Beneficial effects of chlorogenic acid treatment on neuroinflammation after deep hypothermic circulatory arrest may be mediated through CYLD/NF-κB signaling. Brain Res. (2021) 1767. doi: 10.1016/j.brainres.2021.147572
33. Stern M, Kok WF, Doorduin J, Jongman RM, Jainandunsing J, Nieuwenhuijs-Moeke GJ, et al. Mild and deep hypothermia differentially affect cerebral neuroinflammatory and cold shock response following cardiopulmonary bypass in rat. Brain Behav Immun. (2024) 119:96–104. doi: 10.1016/j.bbi.2024.03.046
34. Murdaca G, Greco M, Borro M, Gangemi S. Hygiene hypothesis and autoimmune diseases: A narrative review of clinical evidences and mechanisms. Autoimmun Rev. (2021) 20. doi: 10.1016/j.autrev.2021.102845
35. Wasko NJ, Nichols F, Clark RB. Multiple sclerosis, the microbiome, TLR2, and the hygiene hypothesis. Autoimmun Rev. (2020) 19. doi: 10.1016/j.autrev.2019.102430
36. Mun KT, Hinman JD. Inflammation and the link to vascular brain health: timing is brain. Stroke. (2022) 53:427–36. doi: 10.1161/STROKEAHA.121.032613
37. Bonaventura A, Potere N. Blood hyperviscosity: A novel link between hyperinflammation and hypercoagulability in COVID-19. J Am Coll Cardiol. (2022) 80:329–31. doi: 10.1016/j.jacc.2022.04.061
38. Chen Y, Dai J, Tang L, Mikhailova T, Liang Q, Li M, et al. Neuroimmune transcriptome changes in patient brains of psychiatric and neurological disorders. Mol Psychiatry. (2023) 28:710–21. doi: 10.1038/s41380-022-01854-7
39. van Ton AMP, Leijte GP, Franssen GM, Bruse N, Booij J, Doorduin J, et al. Human in vivo neuroimaging to detect reprogramming of the cerebral immune response following repeated systemic inflammation. Brain Behav Immun. (2021) 95:321–9. doi: 10.1016/j.bbi.2021.04.004
40. Rosca EC, Bilavu R, Cornea A, Simu M. Chorea following SARS-CoV-2 infection and vaccination: a systematic review of reported cases. Int J Infect Dis. (2023). doi: 10.1016/j.ijid.2023.07.001
41. Vreeland A, Calaprice D, Or-Geva N, Frye RE, Agalliu D, Lachman HM, et al. Postinfectious inflammation, autoimmunity, and obsessive-compulsive disorder: sydenham chorea, pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection, and pediatric acute-onset neuropsychiatric disorder. Dev Neurosci. (2023) 45:361–74. doi: 10.1159/000534261
Keywords: chorea (non-Huntington’s), post-pump chorea, cardiopulmonary bypass (CPB), neuroinflammation, bioprosthetic aortic valve, hypoxia inducible factor
Citation: Iqbal M, Zaman M, Ojha N, Gau YTA and Young EI (2024) The known and unknown of post-pump chorea: a case report on robust steroid responsiveness implicating occult neuroinflammation. Front. Immunol. 15:1458022. doi: 10.3389/fimmu.2024.1458022
Received: 01 July 2024; Accepted: 26 August 2024;
Published: 10 September 2024.
Edited by:
Paolo Immovilli, Guglielmo da Saliceto Hospital, ItalyReviewed by:
Prashant A. Natteru, Mayo Clinic, United StatesMarcelo Mendonça, Champalimaud Foundation, Portugal
Copyright © 2024 Iqbal, Zaman, Ojha, Gau and Young. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yung-Tian A. Gau, YW5qaWUuZ2F1QG5paC5nb3Y=; Eufrosina I. Young, eW91bmdldUB1cHN0YXRlLmVkdQ==
†These authors share first authorship
‡ These authors share last authorship
 Muhammad Iqbal1†
Muhammad Iqbal1† Yung-Tian A. Gau
Yung-Tian A. Gau