- Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, China
Background: Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can cause diverse clinical manifestations in multiple organ systems. Child-onset SLE (cSLE) is associated with significantly higher morbidity and mortality than adult-onset SLE. The traditional treatments for SLE (glucocorticoids, antimalarials, conventional and biological disease-modifying antirheumatic drugs) often have significant adverse effects and may not fully control disease activity. Tofacitinib is an oral Janus kinase (JAK) inhibitor that inhibits the JAK-STAT pathway and has the potential to reduce SLE severity.
Methods: cSLE patients who received tofacitinib and had at least one follow-up visit were retrospectively examined. Case histories, laboratory test results, and treatment regimens were analyzed at disease onset, initiation of tofacitinib treatment, and 1, 3, 6, 9, 12, 18, and 24 months after starting tofacitinib.
Results: We examined 9 patients with refractory cSLE. All patients were female and the average age at diagnosis was 10.67 years. At initiation of tofacitinib, the average age was 13.28 years and the average disease duration was 2.62 years. Four patients experienced alleviation of symptoms and reduced their daily prednisone dosages; one of them also discontinued cyclosporine A and two of them also discontinued belimumab. The other 5 patients experienced no apparent benefit.
Conclusion: Tofacitinib may provide clinical benefits for some patients with refractory cSLE, and can also allow reduction in the glucocorticoid dosage. Tofacitinib has the potential as an adjunctive treatment for some patients with cSLE.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with clinical manifestations of varying severity in multiple organs and organ systems. Although adult-onset SLE is more common, child-onset SLE (cSLE) is typically more severe, and is associated with a higher mortality rate, more frequent organ involvement, higher disease activity, and the potential need for long-term high-dose immunosuppressive therapy (1, 2). The management of cSLE is particularly challenging because children generally have poor tolerance of the medications typically used to control adult SLE (1, 3). The current treatment options for cSLE include glucocorticoids, antimalarials, and conventional and biological disease modifying drugs (DMARDs), but these can have significant adverse effects and may not fully control disease activity (4, 5).
Tofacitinib is an oral Janus kinase (JAK) inhibitor that has high selectivity for JAK1 and JAK3, and recent studies reported it had potential as a treatment for cSLE (4, 6–10). Cytokines such as type I interferon (IFN), interleukin 12 (IL-12), and IL-23 (which connect the innate and adaptive immune systems), and IL-21 (which activates interactions of T-cells and B-cells) contribute to the characteristic inflammation of SLE. Activation of these cytokines requires increased JAK-mediated signaling: type I IFN activates JAK1/tyrosine kinase 2 (TYK2); IL-12 and IL-23 activate JAK2/TYK2; and IL- 21 activates JAK1/JAK3. Because JAK inhibitors block the signaling of multiple cytokines, they are a promising class of drugs for the treatment of SLE, which has heterogeneous immune manifestations (4, 8, 11–13). The tofacitinib-mediated inhibition of the JAK-STAT pathway therefore appears to be theoretically well-suited for treatment of SLE. In fact, tofacitinib has demonstrated good efficacy as a treatment for several other autoimmune diseases, including adult rheumatoid arthritis (14–16), and recent studies have examined its possible efficacy and safety as a treatment for SLE (8, 9, 17, 18).
This study retrospectively collected clinical data of patients with refractory cSLE who received tofacitinib at our hospital, and analyzed multiple clinical observations to assess its potential efficacy and safety as a treatment for cSLE. Given the difficulties that cSLE patients often face, and their failure to achieve long-term disease control without adverse effects, it is crucial to identify new treatments. This study aimed to assess the potential for expanding the use of JAK inhibitors by examining the effect of tofacitinib on refractory cSLE.
Methods
Patients
All included cSLE patients were treated at the Pediatric Nephrology and Rheumatology Department of Shengjing Hospital, China Medical University, between January 2013 and December 2023, and received tofacitinib treatment with at least one follow-up visit. Concurrent use of other medications (glucocorticoids, antimalarials, conventional and biological DMARDs, etc.) was allowed. The diagnosis of SLE was based on the 1997 American College of Rheumatology (ACR) criteria (19), the 2012 Systemic Lupus International Collaborating Clinics (SLICC) revised criteria (20), or the 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) criteria (21).
Data collection
Patient data were collected retrospectively, and included general information (gender, age, and disease duration), clinical symptoms (fever, rash, arthritis, oral ulcers, photosensitivity, hair loss, serositis, etc.), and laboratory test results (white blood cell count, platelet count, serum complement levels, anti-dsDNA antibody status, urine protein quantification, urine sediment, red blood cell count, SLE Disease Activity Index-2000 score [SLEDAI-2K, range: 0–105], and SLE Damage Index [SDI, range: 0–47]). These data were collected at disease onset, at the start of tofacitinib treatment, and at 1, 3, 6, 9, 12, 18, and 24 months after initiation of tofacitinib. Records of previous treatments, including use of glucocorticoids, antimalarials, and conventional and biological DMARDs, were also analyzed.
Evaluation of treatment efficacy and adverse reactions
The efficacy of tofacitinib in each patient was classified into one of the following four groups:
Complete Remission: No disease activity without the use of glucocorticoids, but with the use of antimalarials allowed.
Clinical Remission: Clinically quiescent but serologically active disease, with high levels of serological markers (anti-dsDNA positivity and/or hypocomplementemia), with prednisone dosage below 5 mg/day.
Alleviated: Some resolution of clinical symptoms and improvement of laboratory test results.
Unalleviated: No resolution or further deterioration of clinical symptoms and laboratory test results.
At each follow-up visit after the initiation of tofacitinib treatment, all adverse events were recorded in detail.
Ethics
Patients and their guardians were provided with thorough information about tofacitinib, and were told it is a novel oral protein tyrosine kinase inhibitor that was approved by the U.S. FDA for treatment of polyarticular juvenile idiopathic arthritis in children aged 2 years and older, but that it is not yet approved for treatment of cSLE in China. Written informed consent was obtained from all patients and their guardians before treatment, and these records were kept in the medical files. Because this clinical study is descriptive and retrospective, it did not require approval from the Ethics Committee, according to Chinese regulations. After providing informed consent, all patients and their guardians consented to the use of the data for research purposes.
Results
Clinical characteristics of patients
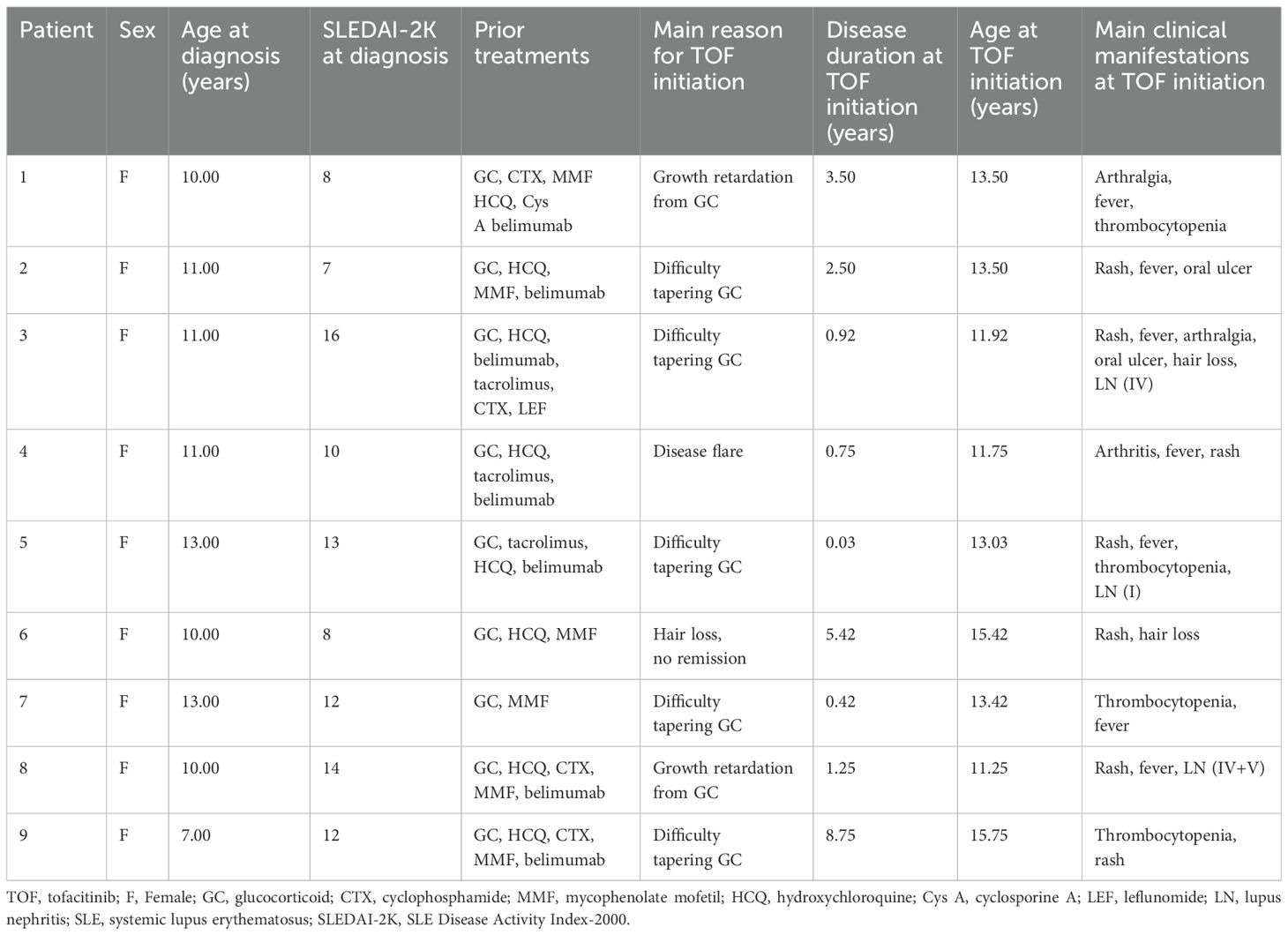
We included 9 children with refractory cSLE in this study (Table 1). All the children were females and the average age at diagnosis of cSLE was 10.67 years (range: 7–13 years). The average age at the start of tofacitinib treatment was 13.28 years (range: 11.25–15.75 years) and the average disease duration at the start of tofacitinib treatment was 2.62 years (range: 0.03–8.75 years). The average SLEDAI-2K score at disease onset was 11.11 (range: 7–16); 6 patients had moderate disease activity (SLEDAI-2K score: 7–12) and 3 patients had severe disease activity (SLEDAI-2K: >12). Cases 3, 7, and 8 had SDI scores of 1. Three patients also had concurrent lupus nephritis (LN), 1 each with class IV, class I, and class IV+V. All patients used several medications before starting tofacitinib (range: 2–6), including glucocorticoids, hydroxychloroquine (HCQ), conventional DMARDs (mycophenolate mofetil [MMF], cyclophosphamide [CTX], tacrolimus, cyclosporine A [Cys A], leflunomide [LEF]), and a biological DMARD (belimumab). All patients were given tofacitinib because of difficulty in reducing the glucocorticoid dose or glucocorticoid-induced growth retardation (7 patients), disease relapse (1 patient), or persistent hair loss (1 patient).
Efficacy and adverse reactions to tofacitinib
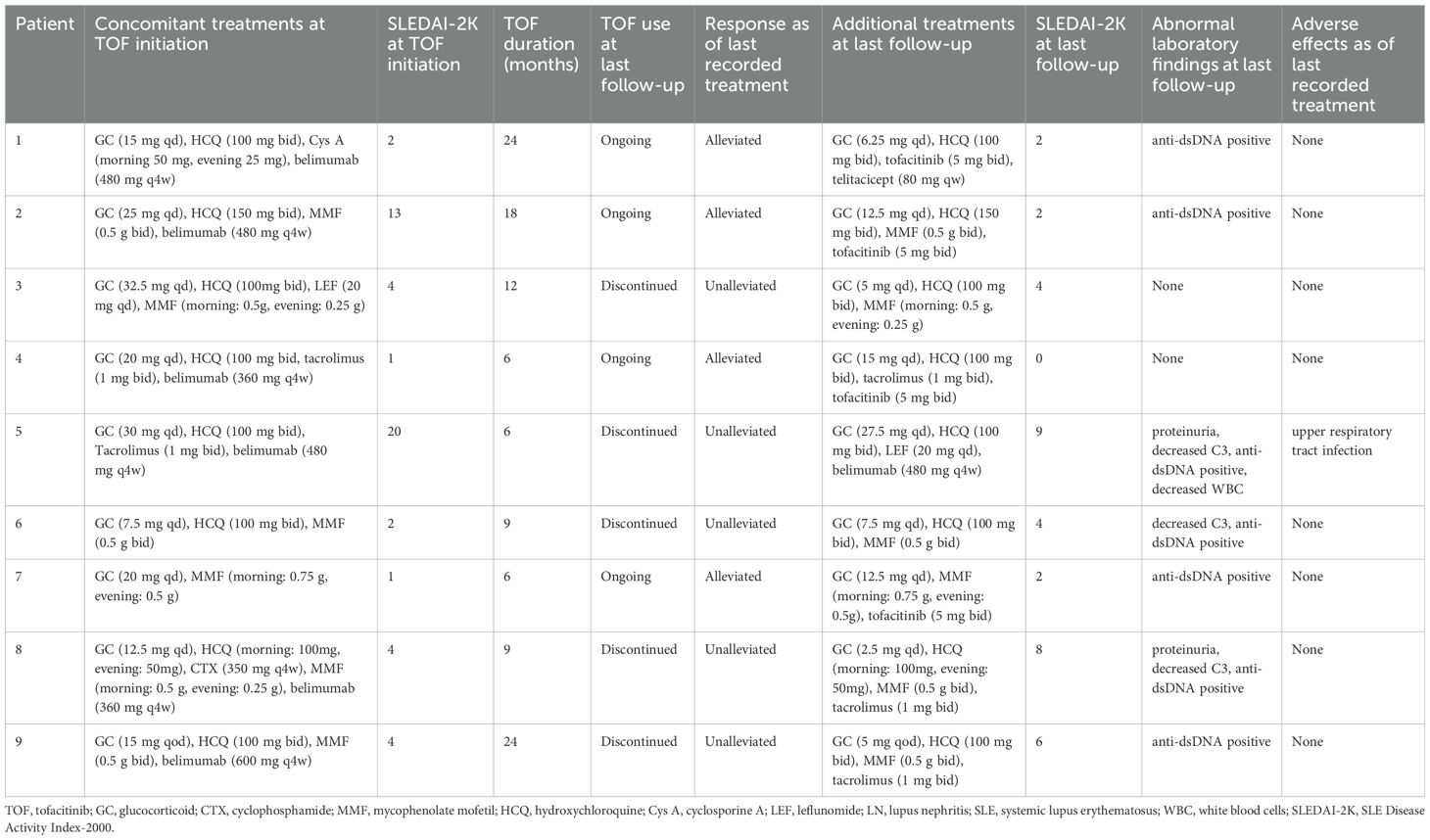
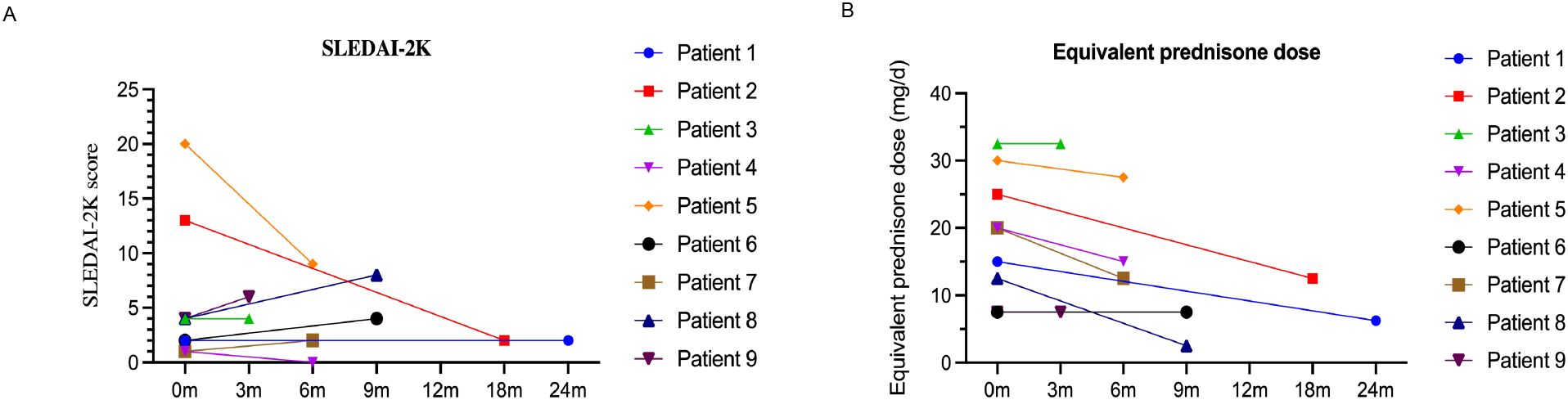
We analyzed the dosing, efficacy, and safety of tofacitinib in all 9 patients, each of whom had at least one follow-up visit between January 2013 and December 2023 (Table 2). Specific information for each of the nine cases is given below. The median SLEDAI-2K score of the 9 patients at tofacitinib initiation was 4.00 (interquartile range: [IQR]: 1.50, 8.50). At the last follow-up, the median SLEDAI-2K score was 2.00 (IQR: 1.00, 6.00) (Figure 1A), with no significant difference. The SDI score was 0 in all cases at tofacitinib initiation; case 5 had an SDI score of 1 at the last follow-up. Among all 9 patients, 4 cases (1, 2, 4, and 7) experienced disease alleviation and continued this therapy, and the other 5 cases experienced no disease alleviation and discontinued this therapy. The 4 patients who achieved alleviation all reduced their dosages of prednisone (case 1: 15 mg/day to 6.25 mg/day, case 2: 25 mg/day to 12.5 mg/day, case 4: 20 mg/day to 15 mg/day, case 7: 20 mg/day to 12.5 mg/day; Figure 1B). In addition, case 1 discontinued CysA, and cases 2 and 4 discontinued belimumab. The 5 cases who discontinued tofacitinib received other immunosuppressants (cyclophosphamide, tacrolimus, or others) to control disease activity and reduce corticosteroid usage (Table 2), as described below.
During the period of treatment with tofacitinib, 8 patients demonstrated good tolerance, based on follow-up periods of 6 to 24 months. However, case 5 experienced an upper respiratory tract infection (nasal congestion, mild cough).
Case 1
Disease duration: 3.5 years.
Prior treatment: prednisone (15 mg qd), HCQ (100 mg bid), Cys A (morning: 50 mg, evening: 25 mg), belimumab (480 mg q4w IV).
Reason for tofacitinib addition: Growth retardation due to prolonged glucocorticoid use.
Tofacitinib dosage: 5 mg bid.
Follow-up: 24 months, Cys A was discontinued, prednisone dosage was reduced (6.25 mg qd), belimumab changed to telitacicept (80 mg qw SQ), height increased by 5 cm.
SLEDAI-2K score: 2 (anti-dsDNA positivity).
Clinical outcome: Alleviated, tofacitinib continued, no adverse reactions.
Case 2
Disease duration: 2.5 years.
Prior treatment: prednisone (25 mg qd), HCQ (150 mg bid), MMF (0.5 g bid), belimumab (480 mg q4w IV).
Reason for tofacitinib addition: Difficulty reducing prednisone dose.
Tofacitinib dosage: 5 mg bid.
Follow-up: 18 months, prednisone dosage reduced (12.5 mg qd), belimumab discontinued.
SLEDAI-2K score: 2 (anti-dsDNA positivity).
Clinical outcome: Alleviated, tofacitinib continued, no adverse reactions.
Case 3
Disease duration: 0.92 years.
Complication: lupus nephritis class IV.
Prior treatment: prednisone (32.5 mg qd), HCQ (100 mg bid), LEF (20 mg qd), MMF (morning: 0.5 g, evening: 0.25 g).
Reason for tofacitinib addition: Difficulty reducing prednisone dose.
Tofacitinib dosage: 5 mg bid.
Follow-up: 3 months, prednisone could not be reduced, standard CTX pulse therapy (350 mg q4w) initiated.
SLEDAI-2K score: 4.
Follow-up at 12 months: prednisone dosage reduced (5 mg qd), LEF discontinued.
SLEDAI-2K score: 0.
Clinical outcome: Unalleviated, tofacitinib discontinued (6 months), no adverse reactions.
Case 4
Disease duration: 0.75 years.
Prior treatment: prednisone (20 mg qd), HCQ (100 mg bid), tacrolimus (1 mg bid), belimumab (360 mg q4w IV).
Reason for tofacitinib addition: Recurring fever and rash.
Tofacitinib dosage: 5 mg bid.
Follow-up: 6 months, prednisone dosage reduced (15 mg qd), belimumab discontinued.
SLEDAI-2K score: 0.
Clinical outcome: Alleviated, tofacitinib continued, no adverse reactions.
Case 5
Disease duration: 0.03 years.
Complication: lupus nephritis class I.
Prior treatment: prednisone (30 mg qd), HCQ (100 mg bid), tacrolimus (1 mg bid), belimumab (480 mg q4w IV).
Reason for tofacitinib addition: Difficulty reducing prednisone dose.
Tofacitinib dosage: 5 mg bid.
Follow-up: 6 months, prednisone dosage reduced (27.5 mg qd), added LEF (20 mg qd).
SLEDAI-2K score: 9 (moderate disease activity).
Clinical outcome: Unalleviated, tofacitinib discontinued, an upper respiratory tract infection occurred, and resolved with symptomatic treatment.
Case 6
Disease duration: 5.42 years.
Prior treatment: prednisone (7.5 mg qd), HCQ (100 mg bid), MMF (0.5 g bid).
Reason for tofacitinib addition: persistent hair loss, no remission.
Tofacitinib dosage: 5 mg bid.
Follow-up: 9 months, no change in hair loss.
SLEDAI-2K score: 4 (anti-dsDNA positivity, low serum complement C3).
Clinical outcome: Unalleviated, tofacitinib discontinued (9 months), no adverse reactions.
Case 7
Disease duration: 0.42 years.
Prior treatment: prednisone (20 mg qd), MMF (morning: 0.75 g, evening: 0.5 g), no HCQ due to visual field loss.
Reason for tofacitinib addition: difficulty reducing prednisone dose.
Tofacitinib dosage: 5 mg bid.
Follow-up: 6 months, prednisone dosage reduced (12.5 mg qd).
SLEDAI-2K score: 2 (anti-dsDNA positivity).
Clinical outcome: Alleviated, tofacitinib continued, no adverse reactions.
Case 8
Disease duration: 1.25 years.
Complication: lupus nephritis class IV+V.
Prior treatment: prednisone (12.5 mg qd), HCQ (morning: 100 mg, evening: 50 mg), CTX (350 mg q4w), MMF (morning: 0.5 g, evening: 0.25 g), belimumab (360 mg q4w IV).
Reason for tofacitinib addition: growth retardation due to prolonged glucocorticoid use.
Tofacitinib dosage: 5 mg bid.
Follow-up: 9 months, prednisone dosage reduced (2.5 mg qd), CTX and belimumab discontinued, MMF dosage adjusted (0.5 g bid), added tacrolimus (1 mg bid), height increased by 3 cm.
SLEDAI-2K score: 8 (proteinuria, decreased C3, anti-dsDNA positive).
Clinical outcome: Unalleviated, tofacitinib discontinued, no adverse reactions.
Case 9
Disease duration: 8.75 years.
Prior treatment: prednisone (15 mg qod), HCQ (100 mg bid), MMF (0.5 g bid), belimumab (600 mg q4w IV).
Reason for tofacitinib addition: difficulty reducing prednisone dose.
Tofacitinib dosage: 5 mg bid.
Follow-up: 3 months, no reduction in prednisone dosage, disease activity observed, SLEDAI-2K score: 6 (decreased C3, anti-dsDNA positive, fever, decreased WBC), added tacrolimus (1 mg bid).
Follow-up at 24 months: prednisone dosage reduced (5 mg qod).
SLEDAI-2K score: 2 (anti-dsDNA positivity).
Clinical outcome: Unalleviated, tofacitinib discontinued (3 months), no adverse reactions during follow-up.
Discussion
SLE is a systemic autoimmune inflammatory disease of unknown etiology that has heterogeneous clinical manifestations, an uncertain clinical course, and highly variable patient prognosis. The goal of treatment is to achieve disease remission or lower disease activity and to improve patient quality-of-life (8, 13). Unlike other autoimmune diseases, there are no well-established biological DMARD therapies for SLE, although the current treatments include belimumab (22, 23), telitacicept (24, 25), and anti-CD20 monoclonal antibodies (26). However, the clinical outcomes from these molecular targeted therapies are still unsatisfactory, possibly because the highly heterogeneous immunological abnormalities in SLE patients mean that multiple cells and molecules must be targeted. Thus, a drug that has a single molecular target may not provide consistent efficacy in a population of these patients (8). This indicates the need to identify additional treatments for SLE or a treatment that can target multiple molecules or cells.
cSLE can affect organs and organ systems simultaneously or sequentially, and these patients may experience unpredictable flares, high morbidity, and high mortality (27). Antimalarials and glucocorticoids are still the most commonly used drugs for treating cSLE. MMF or intravenous CTX is often recommended as induction therapy for class III and class IV LN, and calcineurin inhibitors (Cys A, tacrolimus, and voclosporin) are also good options for treatment of cSLE patients with LN. Rituximab (a biological DMARD that targets CD20 on B cells) can be combined with another DMARD for refractory LN, and the U.S. FDA recently approved belimumab (a biological DMARD that targets B-cell activating factor) for the treatment of cSLE in children who are more than 5-years-old (27). Phase II randomized controlled trials in adult SLE patients showed that anti-IFN therapy (sifalimumab and anifrolumab) (27) and some JAK inhibitors (8, 9, 17, 18) provided beneficial effects. These recent findings led us to examine the JAK inhibitor tofacitinib as a treatment option for patients with refractory cSLE.
To our best knowledge, the present retrospective clinical cohort study of 9 female patients with refractory cSLE is the largest study to examine tofacitinib as a treatment for cSLE. Our results showed that adding oral tofacitinib to a treatment regimen led to disease alleviation in 4 patients, all of whom also reduced their daily prednisone dosages; 1 of these patients also discontinued cyclosporine A and 2 other patients also discontinued belimumab. As of the last follow-up, all four of these patients had low disease activity and continued using tofacitinib. However, the addition of tofacitinib provided no significant benefit for the other 5 patients. Nonetheless, because some cSLE patients appear to benefit from tofacitinib treatment, we suggest that this drug should be considered when other multi-drug treatments are ineffective.
Similar to our findings, other recent studies also reported therapeutic benefits of JAK inhibitors when given to patients with SLE. For example, Pin et al. (10) studied the effect of different JAK inhibitors in 7 patients with early-onset pediatric inflammatory disorders, including a 9-year-old girl who experienced resolution of most symptoms after standard combined therapy, but who had unresolved headache, fatigue, and irritability. After adding baricitinib and gradually reducing the glucocorticoid dose, this girl’s mood improved significantly and her erythrocyte sedimentation rate returned to normal. Another study of adults with SLE found that tofacitinib led to decreased arthritis and rash, and allowed use of a lower steroid dose (28). A recent study used the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score to assess skin manifestations in adults with mild-to-moderate SLE who received tofacitinib, and reported that 3 patients with refractory cutaneous lesions experienced improved CLASI scores (29). A case report confirmed the beneficial effect of tofacitinib for refractory alopecia in a patient with SLE (30). A recent study described a patient with periorbital discoid lupus erythematosus who experienced significant improvement after treatment with a 2% tofacitinib ointment (18).
Three clinical trials have examined the effect of tofacitinib on SLE. A phase Ib trial (NCT02535689) that began in 2015 evaluated the safety of tofacitinib in SLE patients (8). This study stratified patients with mild-to-moderate disease activity based on STAT4 risk allele status, and compared the safety and tolerability of tofacitinib (5 mg bid) plus standard treatment with standard treatment plus placebo. Most adverse events in the tofacitinib group were mild-to-moderate upper respiratory tract infections that resolved spontaneously or after oral antibiotic treatment, and there were no reports of herpes zoster reactivation or any venous thromboembolism events (9). Two other ongoing phase I/II trials are examining the effects of oral tofacitinib on discoid lupus, cutaneous lupus, and SLE with moderate-to-severe skin symptoms (NCT03159936, NCT03288324) (8).
There is evidence that oral tofacitinib is well-tolerated. In fact, only one of our patients experienced a mild upper respiratory tract infection, and there were no other adverse reactions, consistent with previous reports (8). The present study was a single-center retrospective study with a small sample size that focused on refractory cSLE and showed that tofacitinib appeared to benefit some patients. However, larger-scale clinical trials are needed to validate these findings and to examine the benefit of introducing tofacitinib at different stages of cSLE treatment. Randomized controlled trials are also required to more comprehensively assess its efficacy and safety.
In conclusion, our study of the treatment of patients with refractory cSLE using a combination of oral tofacitinib and other drugs showed that some patients experienced improvements and were able to reduce their glucocorticoid dosages, and that this drug was well-tolerated with a low incidence of adverse reactions. However, due to our small sample size and the retrospective design of this study, larger and more thorough prospective studies are needed to verify these preliminary results and also to evaluate the long-term effects and safety of tofacitinib when used as a treatment for cSLE.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements because [reason ethics approval was not required]. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LH: Conceptualization, Methodology, Writing – original draft. PZ: Methodology, Writing – review & editing. CZ: Conceptualization, Methodology, Writing – review & editing. XW: Project administration, Writing – review & editing. YD: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. (2012) 59:345–64. doi: 10.1016/j.pcl.2012.03.007
2. Pineles D, Valente A, Warren B, Peterson MG, Lehman TJ, Moorthy LN. Worldwide incidence and prevalence of pediatric onset systemic lupus erythematosus. Lupus. (2011) 20:1187–92. doi: 10.1177/0961203311412096
3. Rubinstein TB, Knight AM. Disparities in childhood-onset lupus. Rheum Dis Clin North Am. (2020) 46:661–72. doi: 10.1016/j.rdc.2020.07.007
4. Pan L, Lu MP, Wang JH, Xu M, Yang SR. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr. (2020) 16:19–30. doi: 10.1007/s12519-019-00229-3
5. Groot N, de Graeff N, Avcin T, Bader-Meunier B, Brogan P, Dolezalova P, et al. European evidence-based recommendations for diagnosis and treatment of childhood-onset systemic lupus erythematosus: the SHARE initiative. Ann Rheum Dis. (2017) 76:1788–96. doi: 10.1136/annrheumdis-2016-210960
6. Chuprin J, McCormack L, Richmond JM, Rashighi M. Evaluating the use of JAK inhibitors in inflammatory connective tissue diseases in pediatric patients: an update. Expert Rev Clin Immunol. (2022) 18:263–72. doi: 10.1080/1744666x.2022.2047022
7. Welzel T, Winskill C, Zhang N, Woerner A, Pfister M. Biologic disease modifying antirheumatic drugs and Janus kinase inhibitors in paediatric rheumatology - what we know and what we do not know from randomized controlled trials. Pediatr Rheumatol Online J. (2021) 19:46. doi: 10.1186/s12969-021-00514-4
8. Nakayamada S, Tanaka Y. Novel JAK inhibitors under investigation for systemic lupus erythematosus: - where are we now? Expert Opin Investig Drugs. (2023) 32:901–08. doi: 10.1080/13543784.2023.2264172
9. Hasni SA, Gupta S, Davis M, Poncio E, Temesgen-Oyelakin Y, Carlucci PM, et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat Commun. (2021) 12:3391. doi: 10.1038/s41467-021-23361-z
10. Pin A, Tesser A, Pastore S, Moressa V, Valencic E, Arbo A, et al. Biological and clinical changes in a pediatric series treated with off-label JAK inhibitors. Int J Mol Sci. (2020) 21:7767. doi: 10.3390/ijms21207767
11. Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U.S.A. (2003) 100:2610–5. doi: 10.1073/pnas.0337679100
12. Tanaka Y. State-of-the-art treatment of systemic lupus erythematosus. Int J Rheum Dis. (2020) 23:465–71. doi: 10.1111/1756-185x.13817
13. González-García A, Cusácovich I, Ruiz-Irastorza G. Treatment of systemic lupus erythematosus: new therapeutic options. Rev Clin Esp (Barc). (2023) 223:629–39. doi: 10.1016/j.rceng.2023.11.001
14. Rakieh C, Conaghan PG. Tofacitinib for treatment of rheumatoid arthritis. Adv Ther. (2013) 30:713–26. doi: 10.1007/s12325-013-0047-y
15. Tanaka Y, Yamaoka K. JAK inhibitor tofacitinib for treating rheumatoid arthritis: from basic to clinical. Mod Rheumatol. (2013) 23:415–24. doi: 10.1007/s10165-012-0799-2
16. Vyas D, O'Dell KM, Bandy JL, Boyce EG. Tofacitinib: The First Janus Kinase (JAK) inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother. (2013) 47:1524–31. doi: 10.1177/1060028013512790
17. Richter P, Cardoneanu A, Burlui AM, Macovei LA, Bratoiu I, Buliga-Finis ON, et al. Why do we need JAK inhibitors in systemic lupus erythematosus? Int J Mol Sci. (2022) 23:11788. doi: 10.3390/ijms231911788
18. Mazori DR, Min MS, Kassamali B, Brichta L, Merola JF, Vleugels RA, et al. Use of tofacitinib, 2%, ointment for periorbital discoid lupus erythematosus. JAMA Dermatol. (2021) 157:880–82. doi: 10.1001/jamadermatol.2021.1198
19. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40:1725. doi: 10.1002/art.1780400928
20. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. (2012) 64:2677–86. doi: 10.1002/art.34473
21. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:1400–12. doi: 10.1002/art.40930
22. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. doi: 10.1002/art.30613
23. Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. (2011) 377:721–31. doi: 10.1016/s0140-6736(10)61354-2
24. Chen R, Fu R, Lin Z, Huang C, Huang W. The efficacy and safety of telitacicept for the treatment of systemic lupus erythematosus: a real life observational study. Lupus. (2023) 32:94–100. doi: 10.1177/09612033221141253
25. Fan Y, Gao D, Zhang Z. Telitacicept, a novel humanized, recombinant TACI-Fc fusion protein, for the treatment of systemic lupus erythematosus. Drugs Today (Barc). (2022) 58:23–32. doi: 10.1358/dot.2022.58.1.3352743
26. Rodziewicz M, Mendoza-Pinto C, Dyball S, Munguía-Realpozo P, Parker B, Bruce IN. Predictors and prognostic factors influencing outcomes of anti-CD20 monoclonal antibodies in systemic lupus erythematosus: A systematic review update. Semin Arthritis Rheum. (2024) 65:152346. doi: 10.1016/j.semarthrit.2023.152346
27. Trindade VC, Carneiro-Sampaio M, Bonfa E, Silva CA. An update on the management of childhood-onset systemic lupus erythematosus. Paediatr Drugs. (2021) 23:331–47. doi: 10.1007/s40272-021-00457-z
28. You H, Zhang G, Wang Q, Zhang S, Zhao J, Tian X, et al. Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: the experience from a single centre. Ann Rheum Dis. (2019) 78:1441–43. doi: 10.1136/annrheumdis-2019-215455
29. Benucci M, Bernardini P, Coccia C, De Luca R, Levani J, Economou A, et al. JAK inhibitors and autoimmune rheumatic diseases. Autoimmun Rev. (2023) 22:103276. doi: 10.1016/j.autrev.2023.103276
Keywords: systemic lupus erythematosus (SLE), child, tofacitinib, jak-stat, DMARD
Citation: Hou L, Zhou P, Zhao C, Wang X and Du Y (2024) Tofacitinib for child-onset systemic lupus erythematosus. Front. Immunol. 15:1457821. doi: 10.3389/fimmu.2024.1457821
Received: 01 July 2024; Accepted: 23 September 2024;
Published: 08 October 2024.
Edited by:
Andras Perl, Upstate Medical University, United StatesReviewed by:
Ryu Watanabe, Osaka Metropolitan University, JapanMikhail Kostik, Saint Petersburg State Pediatric Medical University, Russia
Copyright © 2024 Hou, Zhou, Zhao, Wang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Du, MTgzODkwNzg4NUBxcS5jb20=
 Ling Hou
Ling Hou Peng Zhou
Peng Zhou Chengguang Zhao
Chengguang Zhao Yue Du
Yue Du