- Department of Dermatology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Biological drugs are extensively used to treat various inflammatory diseases, including psoriasis, atopic dermatitis (AD), and rheumatoid arthritis. While generally effective and safe, these therapies have been increasingly associated with secondary development of vitiligo, especially with anti-TNF α and anti-IL17 drugs. Dupilumab, an IL-4 receptor alpha antagonist used in moderate to severe AD, rarely induces vitiligo. This study reports two cases of new-onset vitiligo following dupilumab treatment for AD. The first case involves an 80-year-old male who developed vitiligo patches appeared on the chest, back, and lower limbs after 2 months of dupilumab therapy. Despite discontinuation of dupilumab, the vitiligo did not regress. The second case describes a 14-year-old female who experienced depigmentation on her forehead one month into dupilumab treatment, with partial improvement of vitiligo lesions over time despite continued therapy. This phenomenon may be due to dupilumab blocking type 2 inflammation, disrupting normal skin homeostasis, and exacerbating type 1 inflammation. These cases, supplemented with a literature review, highlight the potential for biologic drug-induced vitiligo and underscore the need for awareness of such adverse events in clinical practice. The mechanisms underlying this phenomenon likely involve disruption of the Th1/Th2/Th17 cytokine balance, suggesting that targeted therapies may inadvertently exacerbate type 1 inflammation, leading to vitiligo. With the rising use of biologics, clinicians should carefully consider the risk of vitiligo when prescribing these treatments.
1 Introduction
Biological drugs have become a common treatment for many inflammatory conditions due to their effectiveness and high safety profile (1–3). Atopic dermatitis and vitiligo are chronic, inflammatory skin diseases that can significantly impact the quality of life of those affected (4, 5). Dupilumab, a human interleukin-4 receptor alpha antagonist, inhibits IL-4 and IL-3 signaling (6). It is widely used in patients with moderate to severe atopic dermatitis (AD) who do not respond adequately to conventional therapies. Adverse events such as injection-site reactions, paradoxical erythema, ophthalmic complications, and alopecia areata have drawn the attention of dermatologists (7). However, cases of dupilumab-induced vitiligo in patients with atopic dermatitis are rare in existing literature, and there is a lack of cases in Chinese. Here, we present two instances of new-onset vitiligo in Chinese patients with atopic dermatitis who received dupilumab injections for their condition.
2 Case reports
2.1 Case 1
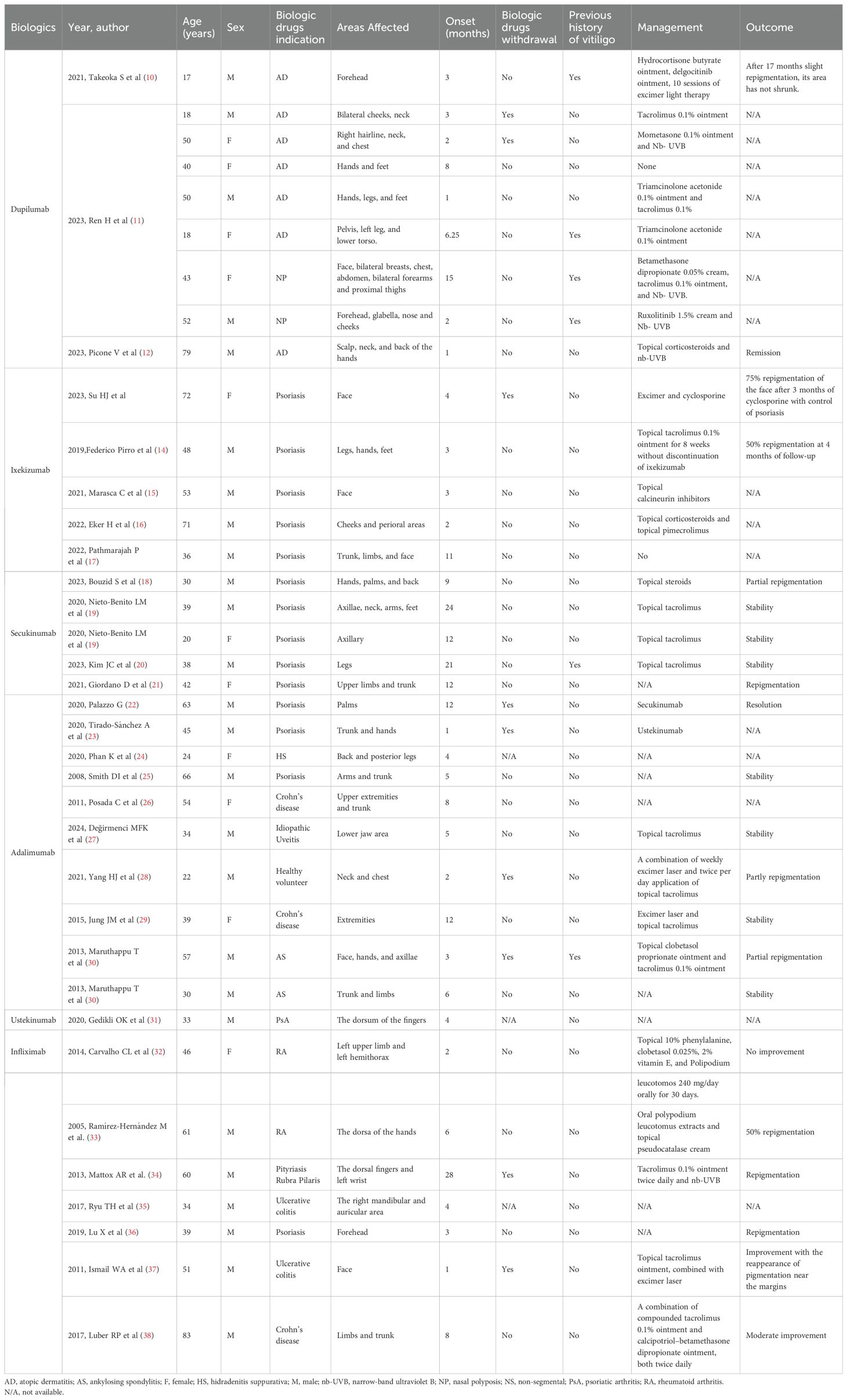
In October 2023, an 80-year-old male patient with AD visited our department due to a poor response to repeated antihistamine treatment and a fungal infection following the use of topical steroids. The patient had a history of AD for more than 30 years; however, he reported no personal or family history of vitiligo. A physical examination revealed erythema, papules, and thickened plaques on the trunk and lower limbs, with a Scoring of Atopic Dermatitis (SCORAD] score: 50.5, an Investigator Global Assessment (IGA] score: 5, and a Numeric Pain Rating Scale (NRS of 10). Laboratory tests revealed an increased serum immunoglobulin E (IgE) level (2990 IU/mL; normal: 5-136 IU/mL). Fungal microscopy confirmed a fungal infection in both lower limbs. According to the typical clinical manifestations of the patient and subsequent auxiliary examinations, AD was identified. Given the poor efficacy of conventional therapies and the presence of a fungal infection, the patient was administered dupilumab (600 mg induction dose and then 300 mg every 2 weeks). After 2 months, there was a slight improvement in erythema and papules (SCORAD: 43.6; IGA: 3; NRS: 4) (Figure 1A). However, he developed irregular light white patches on the chest, back, and lower limbs with a Vitiligo Area Score Index (VASI) score of 1.175. The patient requested to continue treatment with dupilumab.
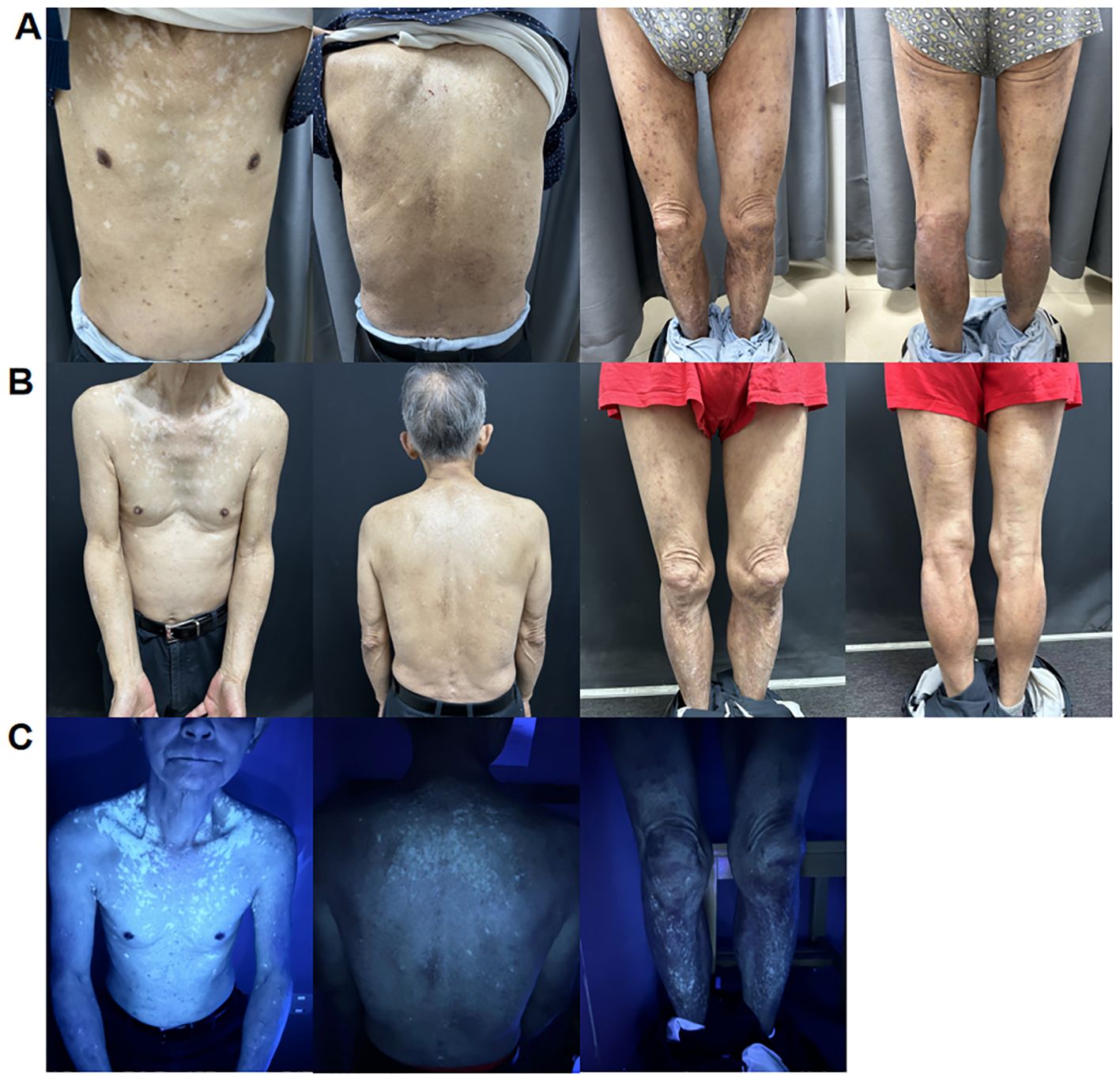
Figure 1. (A) Irregular light white patches sparing the chest, back, and lower limb area after 2 months of treatment with dupilumab. (B) Further enlargement of white patches on the trunk and lower limbs after 5 months of treatment with dupilumab. (C) Woolamp examination of depigmented patches on the chest, back, and lower limbs showing white fluorescence, confirming the diagnosis of vitiligo.
After 5 months of treatment, his erythema and papules essentially vanished (SCORAD: 27.7; IGA: 2; NRS: 2). However, the white patches on his trunk enlarged, and Wood lamp examination revealed bright white patches, indicating that the patient was in the progression stage of vitiligo (VASI: 1.51) (Figures 1B, C). It was decided to discontinue dupilumab treatment and use glycyrrhizin. Eight weeks after the cessation of dupilumab, the patches had not reduced in size.
2.2 Case 2
A 14-year-old female patient presented with moderate to severe atopic dermatitis, which she had experienced since the age of 4. She had no personal or familial history of vitiligo. Despite treatments with antihistamines and topical corticosteroids, the results were unsatisfactory. Physical examination revealed erythema, papules, and thickened plaques in the hands and both upper limbs, with (SCORAD: 48.4; IGA: 5; NRS: 10). Laboratory assay results revealed increased IgE levels. The patient received dupilumab treatment, receiving an initial loading dose of 600 mg, followed by 300 mg every 2 weeks.
At the one-month follow-up visit, improvements in the AD skin lesions were observed (SCORAD: 14.6; IGA: 1; NRS: 3). However, a patch of depigmentation appeared on her forehead, accompanied by gray hair (VASI: 0.24). Dermatoscopy and wood lamp examination confirmed the diagnosis of vitiligo (Figure 2). Treatment with topical hydrocortisone butyrate ointment was initiated, and dupilumab therapy was continued at the request of the patient. After five months, partial improvement of the vitiligo lesions was observed.
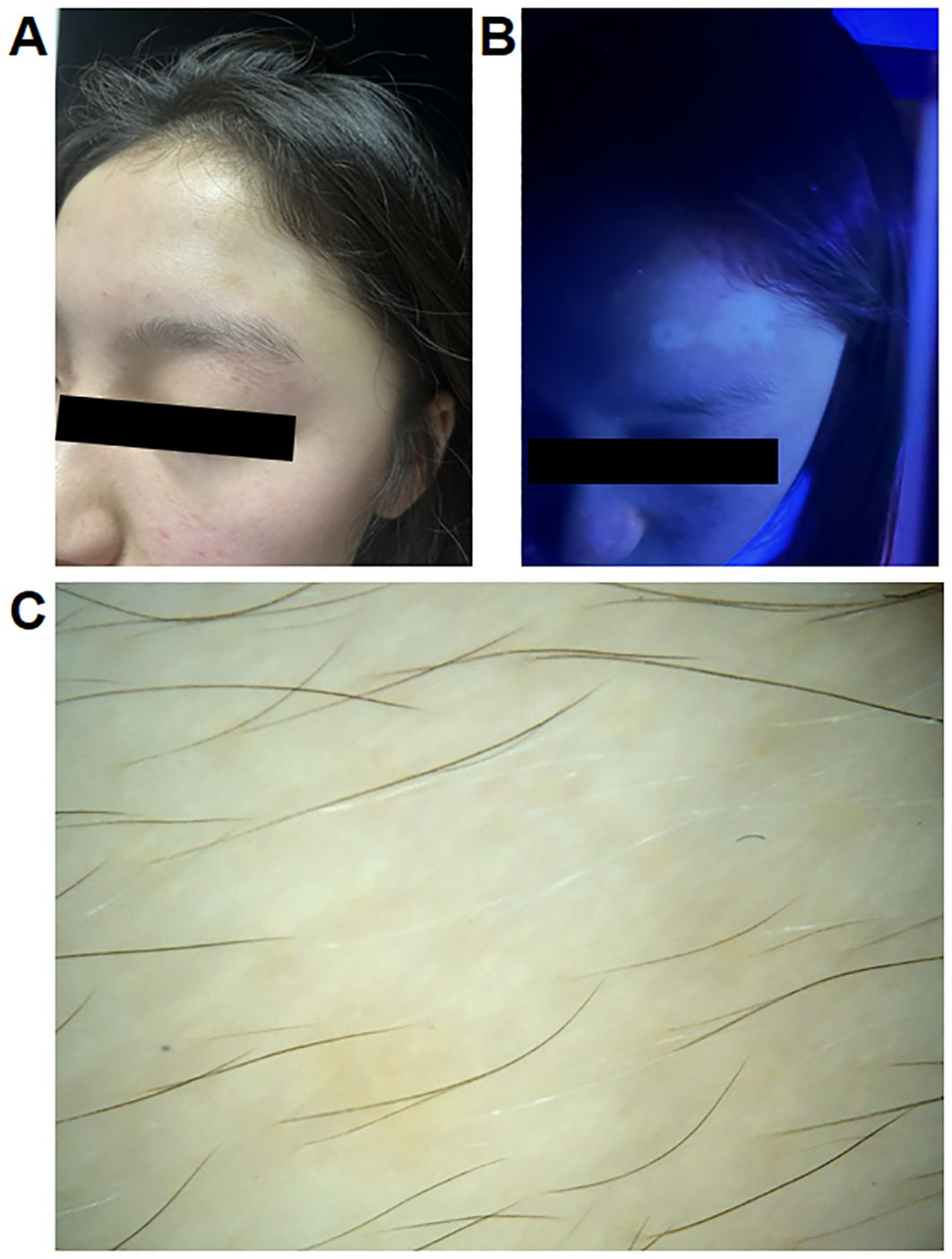
Figure 2. (A) Clinical photographs of a 14-year-old female patient with atopic dermatitis who developed vitiligo after treatment with dupilumab. (B, C) Wood lamp examination and dermatoscopy of the patient.
3 Discussion
Here, we present two cases of de novo vitiligo, a Th1 cell-mediated inflammatory skin disease, following dupilumab therapy for AD. Vitiligo is a common mucocutaneous depigmentation disorder caused by cytotoxic CD8+ T cell-mediated destruction of melanocytes (5). Recent studies highlighted significant changes in the expression of inflammatory cytokines in vitiligo lesions (8, 9). Consistent with this, previous reports have documented cases of vitiligo induced by biologic drugs such as anti-IL17 agents and anti-TNF α drugs. Despite only two cases of biological agent-induced vitiligo reported in a nationwide cohort study, this adverse event warrants attention. Published cases of biologic drug-induced vitiligo are summarized in Table 1 (10–38). Thirty-seven cases (26 male patients and 11 female patients) of vitiligo associated with biologic drug use have been reported. Of these cases, 10 were related to adalimumab, nine were related to dupilumab, seven were related to infliximab, five were related to ixekizumab, five were related to secukinumab, and one was related to ustekinumab. Most patients received conventional therapy after developing vitiligo, such as topical steroids, topical tacrolimus and phototherapy. Fourteen patients showed varying degrees of discoloration after treatment, and seven patients showed stable condition of vitiligo after treatment. Notably, six patients chose to continue using biologics, their white patch also improved after treatment.
The mechanisms underlying the development of vitiligo during biological therapy remain unknown. Previous studies suggested that blocking the TNF α pathway may have therapeutic potential in treating vitiligo skin lesions. However, some clinical cases have also reported that TNF α inhibitors may be associated with the emergence or progression of vitiligo (23–38). The underlying mechanism of anti-TNF α agents-induced vitiligo may involve local changes in cytokine balance and the activation of alternative pathways such as type I interferon (39). Additionally, TNF α may decrease regulatory T cell (Treg) production and activation and impair Treg skin homing that allows T cells to self-react to melanocytes (40). IL-17 antagonist-induced vitiligo highlights the delicate balance between Th1 and Th17 regulation. The absence of one of these effector cytokines can promote a response dominated by the other. A previous animal study suggested that IL-17 deficiency contributes to increased IFN-γ+Th1 cells and an elevated Th1 response (41). Additionally, because of the association between the onset of vitiligo and genetic variations, some studies suggested that biologics may cause vitiligo by intervening in the innate immunity of specific genetically susceptible patients (42, 43). Vitiligo is related to other immune diseases; therefore, vitiligo development may be coincidental and related to underlying diseases (40).
Vitiligo and AD are common chronic autoimmune inflammatory skin diseases. Previous studies indicated that patients with AD are at a higher risk of developing autoimmune diseases such as vitiligo. Conversely, individuals with vitiligo have an elevated incidence of AD compared to those without vitiligo (44, 45). The treatment of vitiligo is more difficult than that of AD; however, the use of ruxolitinib may allow repigmentation after vitiligo (46).
Dupilumab is a fully human monoclonal antibody targeting the IL-4 receptor α, inhibiting both IL-4 and IL-13 signaling. Only a few isolated cases of dupilumab-induced vitiligo have been reported. This study contributes to the existing literature by documenting additional cases, specifically highlighting instances of vitiligo induced by dupilumab in Chinese patients. We speculated that this phenomenon occurs due to dupilumab disrupting the balance between helper T cell (Th) 2 and Th1/Th17 pathways. Sushama S et al. suggested that dupilumab-induced IL-4 inhibition leads to Th1/Th17 polarization, resulting in increased expression of IL-17, IL-2, TNF-α, and IFN-γ, which are implicated in the pathogenesis of vitiligo (47). Dupilumab blocks type 2 inflammation, disrupting normal skin homeostasis and potentially exacerbating type 1 inflammation, thereby inducing vitiligo.
With the increasing use of biological agents in patients with skin diseases, clinicians should consider the possibility of adverse reactions like vitiligo when selecting targeted biological therapies. However, our study also has some limitations, including a small sample size and the possibility of potential confounding factors. Future clinical studies with larger sample sizes can better clarify the possible mechanisms of biologics induced vitiligo, and future studies could examine if JAK inhibition may be superior in patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by the First Affiliated Hospital of Chongqing Medical University, Chongqing for the studies involving humans because this study is a case report. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Conceptualization, Data curation, Investigation, Writing – original draft. TC: Data curation, Investigation, Writing – original draft. XP: Writing – original draft. SC: Conceptualization, Data curation, Supervision, Writing – review & editing. JC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. YC: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (n82073462) and the Natural Science Foundation of Chongqing (2023NSCQ-MSX0321).
Acknowledgments
We are grateful to the patients for permitting the publication of their data for this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bellinato F, Gisondi P, Girolomoni G. Latest advances for the treatment of chronic plaque psoriasis with biologics and Oral small molecules. Biologics. (2021) 15:247–53. doi: 10.2147/BTT.S290309
2. Ratchataswan T, Banzon TM, Thyssen JP, Weidinger S, Guttman-Yassky E, Phipatanakul W. Biologics for treatment of atopic dermatitis: Current status and future prospect. J Allergy Clin Immunol Pract. (2021) 9:1053–65. doi: 10.1016/j.jaip.2020.11.034
3. Snyder CL, Gibson RS, Porter ML, Kimball AB. Secukinumab in the treatment of hidradenitis suppurativa. Immunotherapy. (2023) 15:1449–57. doi: 10.2217/imt-2023-0103
4. Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. (2019) 40:84–92. doi: 10.2500/aap.2019.40.4202
5. Frisoli ML, Essien K, Harris JE. Vitiligo: Mechanisms of pathogenesis and treatment. Annu Rev Immunol. (2020) 38:621–48. doi: 10.1146/annurev-immunol-100919-023531
6. Napolitano M, Fabbrocini G, Potestio L, Fontanella G, Picone V, Bennardo L, et al. A 24-weeks real-world experience of dupilumab in adolescents with moderate-to-severe atopic dermatitis. Dermatol Ther. (2022) 35:e15588. doi: 10.1111/dth.15588
7. Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J Am Acad Dermatol. (2021) 84:139–47. doi: 10.1016/j.jaad.2020.08.051
8. Yu HS, Chang KL, Yu CL, Li HF, Wu MT, Wu CS, et al. Alterations in IL-6, IL-8, GM-CSF, TNF-alpha, and IFN-gamma release by peripheral mononuclear cells in patients with active vitiligo. J Invest Dermatol. (1997) 108:527–9. doi: 10.1111/1523-1747.ep12289743
9. Jin R, Zhou M, Lin F, Xu W, Xu A. Pathogenic Th2 cytokine profile skewing by IFN-γ-responding vitiligo fibroblasts via CCL2/CCL8. Cells. (2023) 12:217. doi: 10.3390/cells12020217
10. Takeoka S, Kamata M, Yokoi I, Takehara A, Tada Y. Rapid enlargement of vitiligo vulgaris after initiation of dupilumab for atopic dermatitis: A case report. Acta Derm Venereol. (2021) 101:adv00581. doi: 10.2340/actadv.v101.545
11. Ren H, Akabane AL, Kelleher K, Halverstam C, Hicks M, Schachter JR, et al. Vitiligo induced by dupilumab treatment: A case series. J Eur Acad Dermatol Venereol. (2023) 37:2259–61. doi: 10.1111/jdv.19132
12. Picone V, Napolitano M, Torta G, Fabbrocini G, Patruno C. Vitiligo during dupilumab therapy. JAAD Case Rep. (2023) 36:51–3. doi: 10.1016/j.jdcr.2023.03.025
13. Su HJ, Chan YP, Shen PC, Ku CL, Ng CY. Anti-IL-17A antibody-associated de novo vitiligo: Case report and review of literature. Front Immunol. (2022) 13:1077681. doi: 10.3389/fimmu.2022.1077681
14. Pirro F, Caldarola G, De Simone C, Moretta G, Giovanardi G, Peris K. Multiple paradoxical reactions during ixekizumab therapy. Dermatol Ther. (2019) 32:e12852. doi: 10.1111/dth.12852
15. Marasca C, Fornaro L, Martora F, Picone V, Fabbrocini G, Megna M. Onset of vitiligo in a psoriasis patient on ixekizumab. Dermatol Ther. (2021) 34:e15102. doi: 10.1111/dth.15102
16. Eker H, Kaya İslamoğlu ZG, Demirbaş A. Vitiligo development in a patient with psoriasis vulgaris treated with ixekizumab. Dermatol Ther. (2022) 35:e15314. doi: 10.1111/dth.15314
17. Pathmarajah P, Benjamin-Laing Z, Abdurrahman M, Grunova A, Sinclair C. Generalized vitiligo in a psoriatic patient treated with ixekizumab. Dermatol Ther. (2022) 35:e15872. doi: 10.1111/dth.15872
18. Bouzid S, Hammami-Ghorbel H, Chamli A, Aounti I, Daly W, Kochbati S, et al. Secukinumab-induced vitiligo: A new case report and review of the literature. Thérapie. (2023) 78:754–6. doi: 10.1016/j.therap.2022.12.004
19. Nieto-Benito LM, Baniandrés-Rodríguez O. New-onset vitiligo during treatment with secukinumab: Report of two cases and review of the literature. Clin Drug Investig. (2020) 40:1089–91. doi: 10.1007/s40261-020-00964-w
20. Kim JC, Lee ES. Progression of pre-existing vitiligo during secukinumab treatment for psoriasis. Ann Dermatol. (2023) 35:S117–21. doi: 10.5021/ad.21.078
21. Giordano D, Magri F, Persechino F, Lepore A, Verde R, Capalbo A, et al. Vitiligo with progressive repigmentation during secukinumab treatment in a patient with psoriatic arthritis: A case report. Case Rep Dermatol. (2021) 13:209–15. doi: 10.1159/000510831
22. Palazzo G. Resolution of post-adalimumab vitiligo with secukinumab in a patient with psoriasis vulgaris. Oxf Med Case Rep. (2020) 2020:omz134. doi: 10.1093/omcr/omz134
23. Tirado-Sánchez A, Bonifaz A. Simultaneous bullous pemphigoid and vitiligo associated with adalimumab therapy in a patient with psoriasis vulgaris. Indian Dermatol Online J. (2020) 11:229–31. doi: 10.4103/idoj.IDOJ_53_19
24. Phan K, Charlton O, Smith SD. New onset vitiligo in a patient with hidradenitis suppurativa treated with adalimumab. Dermatol Ther. (2020) 33:e13347. doi: 10.1111/dth.13347
25. Smith DI, Heffernan MP. Vitiligo after the resolution of psoriatic plaques during treatment with adalimumab. J Am Acad Dermatol. (2008) 58:S50–2. doi: 10.1016/j.jaad.2006.05.035
26. Posada C, Flórez A, Batalla A, Alcázar JJ, Carpio D. Vitiligo during treatment of Crohn’s disease with adalimumab: Adverse effect or co-occurrence? Case Rep Dermatol. (2011) 3:28–31. doi: 10.1159/000324619
27. Değirmenci MFK, Yalçındağ FN. Vitiligo in a patient receiving adalimumab for idiopathic uveitis. Turk J Ophthalmol. (2024) 54:112–5. doi: 10.4274/tjo.galenos.2024.04575
28. Yang HJ, Lee WJ, Lee MW, Choi JH, Chang SE. A case of new-onset vitiligo in a healthy volunteer after administration of adalimumab. Ann Dermatol. (2021) 33:474–6. doi: 10.5021/ad.2021.33.5.474
29. Jung JM, Lee YJ, Won CH, Chang SE, Lee MW, Choi JH, et al. Development of vitiligo during treatment with adalimumab: A plausible or paradoxical response? Ann Dermatol. (2015) 27:620–1. doi: 10.5021/ad.2015.27.5.620
30. Maruthappu T, Leandro M, Morris SD. Deterioration of vitiligo and new onset of halo naevi observed in two patients receiving adalimumab. Dermatol Ther. (2013) 26:370–2. doi: 10.1111/dth.12002
31. Gedikli OK, Kilic G. New-onset vitiligo as an unusual cutaneous reaction under ustekinumab therapy in patients with psoriatic arthritis. Acta Reumatol Port. (2020) 45:301–3.
32. Carvalho CLDB, Ortigosa LCM. Segmental vitiligo after infliximab use for rheumatoid arthritis–A case report. Bras Dermatol. (2014) 89:154–6. doi: 10.1590/abd1806-4841.20142887
33. Ramírez-Hernández M, Marras C, Martínez-Escribano JA. Infliximab-induced vitiligo. Dermatology. (2005) 210:79–80. doi: 10.1159/000081494
34. Mattox AR, Chappell JA, Hurley MY. New-onset vitiligo during long-term, stable infliximab treatment of pityriasis rubra pilaris. J Drugs Dermatol. (2013) 12:217–9.
35. Ryu TH, Lee DW, Choi JE, Ahn HH, Kye YC, Seo SH. A Type II segmental vitiligo developed under infliximab treatment for ulcerative colitis. Ann Dermatol. (2017) 29:826–7. doi: 10.5021/ad.2017.29.6.826
36. Lu X, Gao Y, Ding Y. Vitiligo in a patient receiving infliximab for chronic plaque psoriasis. Dermatol Ther. (2019) 32:e12917. doi: 10.1111/dth.12917
37. Ismail WA, Al-Enzy SA, Alsurayei SA, Ismail AE. Vitiligo in a patient receiving infliximab for refractory ulcerative colitis. Arab J Gastroenterol. (2011) 12:109–11. doi: 10.1016/j.ajg.2011.03.001
38. Luber RP, Chamberlain AJ, Sparrow MP. New onset vitiligo following commencement of infliximab in Crohn disease. Intern Med J. (2017) 47:972–3. doi: 10.1111/imj.13498
39. Massara A, Cavazzini L, La Corte R, Trotta F. Sarcoidosis appearing during Anti-tumor Necrosis Factor alpha therapy: A new “class effect” paradoxical phenomenon. Two case reports and literature review. Semin Arthritis Rheum. (2010) 39:313–9. doi: 10.1016/j.semarthrit.2008.11.003
40. Méry-Bossard L, Bagny K, Chaby G, Khemis A, Maccari F, Marotte H, et al. New-onset vitiligo and progression of pre-existing vitiligo during treatment with biological agents in chronic inflammatory diseases. J Eur Acad Dermatol Venereol. (2017) 31:181–6. doi: 10.1111/jdv.13759
41. Das G, Sheridan S, Janeway CA Jr. The source of early IFN-gamma that plays a role in Th1 priming. J Immunol. (2001) 167:2004–10. doi: 10.4049/jimmunol.167.4.2004
42. Birol A, Kisa U, Kurtipek GS, Kara F, Kocak M, Erkek E, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol. (2006) 45:992–3. doi: 10.1111/j.1365-4632.2006.02744.x
43. Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. (2007) 356:1216–25. doi: 10.1056/NEJMoa061592
44. Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: A systematic review and meta-analysis. JAMA Dermatol. (2015) 151:522–8. doi: 10.1001/jamadermatol.2014.3324
45. Acharya P, Mathur M. Association of atopic dermatitis with vitiligo: A systematic review and meta-analysis. J Cosmet Dermatol. (2020) 19:2016–20. doi: 10.1111/jocd.13263
46. Tavoletti G, Avallone G, Conforti C, Roccuzzo G, Maronese CA, Mattioli MA, et al. Topical ruxolitinib: A new treatment for vitiligo. J Eur Acad Dermatol Venereol. (2023) 37:2222–30. doi: 10.1111/jdv.19162
Keywords: dupilumab-induced vitiligo, atopic dermatitis, biological therapy, inflammatory skin diseases, cytokine imbalance
Citation: Shao X, Chen T, Pan X, Chen S, Chen Y and Chen J (2024) Biologic drugs induced vitiligo: case reports and review of literature. Front. Immunol. 15:1455050. doi: 10.3389/fimmu.2024.1455050
Received: 27 June 2024; Accepted: 03 December 2024;
Published: 17 December 2024.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Devis Benfaremo, Marche Polytechnic University, ItalyElizabeth Spencer, Icahn School of Medicine at Mount Sinai, United States
Carlo Alberto Maronese, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italy
Adeeb Bulkhi, Umm Al Qura University, Saudi Arabia
Copyright © 2024 Shao, Chen, Pan, Chen, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Chen, c2h1YW5nY2hlbjA3QGhvdG1haWwuY29t; Yangmei Chen, Y2hlbnlhbmdtZWkxMDIzQDE2My5jb20=; Jin Chen, Y2hlbmppbjc3OTFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xinyi Shao†
Xinyi Shao† Shuang Chen
Shuang Chen Jin Chen
Jin Chen