Abstract
Purpose:
To explore whether tumor-associated lymphatic vessel density (LVD) could be a biomarker for the prognosis of patients with esophageal cancer after radical resection.
Methods:
A systematic literature search was performed through PubMed, EMBASE, Wanfang Data, and Cochrane Library from the inception of databases until March 19, 2024. The selected studies investigated overall survival (OS) and/or recurrence-free survival (RFS) of patients with esophageal cancer with different levels of LVD after radical resection. The OS and RFS data were pooled as hazard ratios (HR) and 95% confidential interval (CI). Furthermore, the standardized mean differences (SMDs) and 95% CI were aggregated to evaluate the correlation between LVD and clinicopathological features.
Results:
A total of 10 retrospective studies of 1,201 patients were finally included for the meta-analysis. Patients with esophageal cancer with a high level of LVD exhibited worse OS (HR 1.65, 95% CI 1.18 to 2.31) and RFS (HR 1.57, 95% CI 1.09 to 2.26) than those with a low level of LVD. Subgroup analysis of different pathological subtypes revealed that patients with esophageal adenocarcinoma with a high level of LVD had significantly worse RFS (HR 2.84, 95% CI 1.61 to 5.02) than those with a low level of LVD; while patients with esophageal squamous cell carcinoma with a high level of LVD had similar OS (HR 1.52, 95% CI 0.93 to 2.47) and RFS (HR 1.03, 95% CI 0.72 to 1.48) to those with a low level of LVD. Furthermore, tumors with lymph node metastasis had significantly higher levels of LVD than those without lymph node metastasis (SMD = 1.11, 95% CI 0.54 to 1.67). Tumors at the stages III-IV had significantly higher levels of LVD than those at the stages I-II (SMD = 1.62, 95% CI 0.90 to 2.34).
Conclusion:
A high level of LVD in tumor was associated with worse survival of patients with esophageal cancer after radical resection, especially in patients with esophageal adenocarcinoma. Tumor-associated LVD is a new parameter that should be measured in postoperative pathology for predicting the prognosis of patients with esophageal cancer.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/ PROSPERO, identifier CRD42024553766.
Introduction
Esophageal cancer (EC) is one of the frequently diagnosed cancers and leading causes of cancer-related deaths worldwide, with an estimated 511,000 new cases and 445,000 deaths in the world, according to global cancer statistics in 2022 (1). Pathologically, EC is classified into esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) as well as other rare types (2). EAC typically occurs in the lower third of the esophagus and is more common in developed countries, while ESCC typically occurs in the upper two-thirds of the esophagus and is more common in developing countries (3–5). Although radical resection is the most optimal therapeutic approach for early-stage EC, the prognosis remains dismal with a 5-year overall survival (OS) rate of approximately 13%-18% (6–8). It is crucial to identify novel prognostic markers to manage EC patients (9).
Tumor-associated lymphangiogenesis is the formation of new lymphatic vessels in tumor, which is driven by growth factors secreted from tumor cells and cells in the tumor microenvironment (10, 11). The lymphangiogenesis can be quantified by measuring lymphatic vessel density (LVD) (12, 13). High levels of LVD in tumor are associated with the worse survival of patients with hepatocellular carcinoma (13), intrahepatic cholangiocarcinoma (14), and hilar cholangiocarcinoma (15) after radical resection. Previous studies have reported that the levels of LVD are associated with worse survival of patients with EC after radical resection (16–23), while others have shown that the levels of LVD are not significantly associated with the prognosis of patients with EC after radical resection (24, 25). The difference may stem from a relatively smaller sample size (16–25). Hence, the prognostic value of LVD for EC is not clarified.
In this study, we performed a systemic review and meta-analysis to clarify the role of lymphangiogenesis in the prognosis of EC, and whether LVD could be a prognostic indicator for patients with EC after radical resection.
Materials and methods
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (26), and the study protocol was registered in an official repository (PROSPERO registration number: CRD42024553766).
Search strategy
A systematic literature search of PubMed, EMBASE, Wanfang Data, and Cochrane Library was performed, from the inception of databases to March 19, 2024, to identify all relevant studies on the association between LVD and survival outcomes in patients with EC after radical resection. The search strategy utilized the terms: (“lymphangiogenesis” OR “lymphangiogeneses” OR “lymph vessel” OR “lymphatic vessel” OR “lymph microvessel” OR “lymphatic microvessel” OR “podoplanin” OR “D2-40”) AND (“esophageal” OR “oesophageal”). Filters for study types, geographical location, or language limits were not applied. The reference lists of the retrieved articles were also consulted.
Article selection
Two investigators (J.L. and Q.B.W.) independently reviewed the titles, abstracts, and full texts of the articles, based on the pre-specified inclusion criteria: (1) studies focusing on EC patients, who underwent the radical resection; (2) determining LVD in tumor samples using immunohistochemistry; (3) categorizing patients into high and low LVD groups, based on a specified cut-off value; (4) reporting survival outcomes of OS and/or recurrence-free survival (RFS). Studies were excluded based on the following criteria: (1) duplication, only the study with the largest sample size was included if the same population was used in multiple studies; (2) non-clinical study articles: e.g., case report, conference abstract, review, meta-analysis.
Data extraction
The relevant information was extracted from every eligible article by two independent reviewers (J.L. and Q.B.W.) in predefined formats, including the following items: the first author’s name, year of publication, country/region, the sample size of groups with different levels of LVD, cut-off value of LVD, sex, mean/median age, the pathological subtypes of the tumor, differentiation grade, location of tumor, the extent of the tumor invasion, lymph node invasive status, TNM stage, median follow up, OS, RFS, hazard ratio (HR), and corresponding 95% confidence interval (CI). The mean and standard deviation (SD) of LVD in groups with or without lymph node metastasis and groups with different TNM stages were also extracted. Unavailable raw data from articles were tried to obtain from the corresponding authors. If HR and 95% CI were not available, they were calculated from Kaplan-Meier curves using Tierney’s method (27).
Quality assessment
The quality of the included studies was assessed by two independent reviewers (J.L. and Q.B.W.) using the Newcastle-Ottawa scale (NOS). Studies with scores between 7 and 9 were considered good quality, studies with scores between 4 and 6 were considered fair quality, while studies with a score of ≤ 3 were considered low quality (28, 29). In case of discrepancies between the investigators (J.L. and Q.B.W.) during the processes of article selection, data extraction, and quality assessment, a senior reviewer (X.C. or Q.Y.L. or Y.K.) participated in a discussion to make a final decision.
Statistical analyses
The HR and 95% CI were aggregated to evaluate the effect of LVD on OS and RFS in patients with EC after radical resection. A pooled HR greater than 1 and a 95% CI that did not cross 1 indicated a worse survival outcome in the group with a high level of LVD compared to that with a low level of LVD. The standardized mean difference (SMD) and 95% CI were aggregated to evaluate the correlation between the levels of LVD and lymph node metastasis or higher TNM stages. A pooled SMD greater than 0 and a 95% CI that did not cross 0 indicated a higher level of LVD in groups with lymph node metastasis or higher TNM stages. The I-square (I2) test was utilized to assess the heterogeneity of the meta-analysis results, with a threshold of 50% considered significant heterogeneity (30). In cases where I2 exceeded 50%, the random effects model was chosen to calculate the combined estimates, otherwise, the fixed effects model was applied (31, 32). Subgroup analysis was performed based on the pathological subtypes of EC. Egger’s and Begg’s regression analysis of funnel plot was conducted to evaluate any possible publication bias (33). All the statistical analyses were conducted by the Stata 18.0 software (Stata, Texas, USA).
Results
Study selection
The comprehensive literature search process is illustrated in the flow diagram (Figure 1). A total of 3,291 records were retrieved from PubMed (784), EMBASE (2,000), Wanfang Data (490), Cochrane Library (16), and reference lists (1). After removing duplications, 2,762 records remained, and of them, 16 studies underwent full-text evaluation after the exclusion of 2,741 records through evaluating their titles and abstracts, according to the inclusion and exclusion criteria. Subsequently, 6 studies were excluded for reasons, such as the absence of subgroups into high and low LVD groups and no available data. Ultimately, 10 retrospective studies were included in the meta-analysis (16–25), and most studies had high quality, except one with fair quality (Supplementary Table 1).
Figure 1
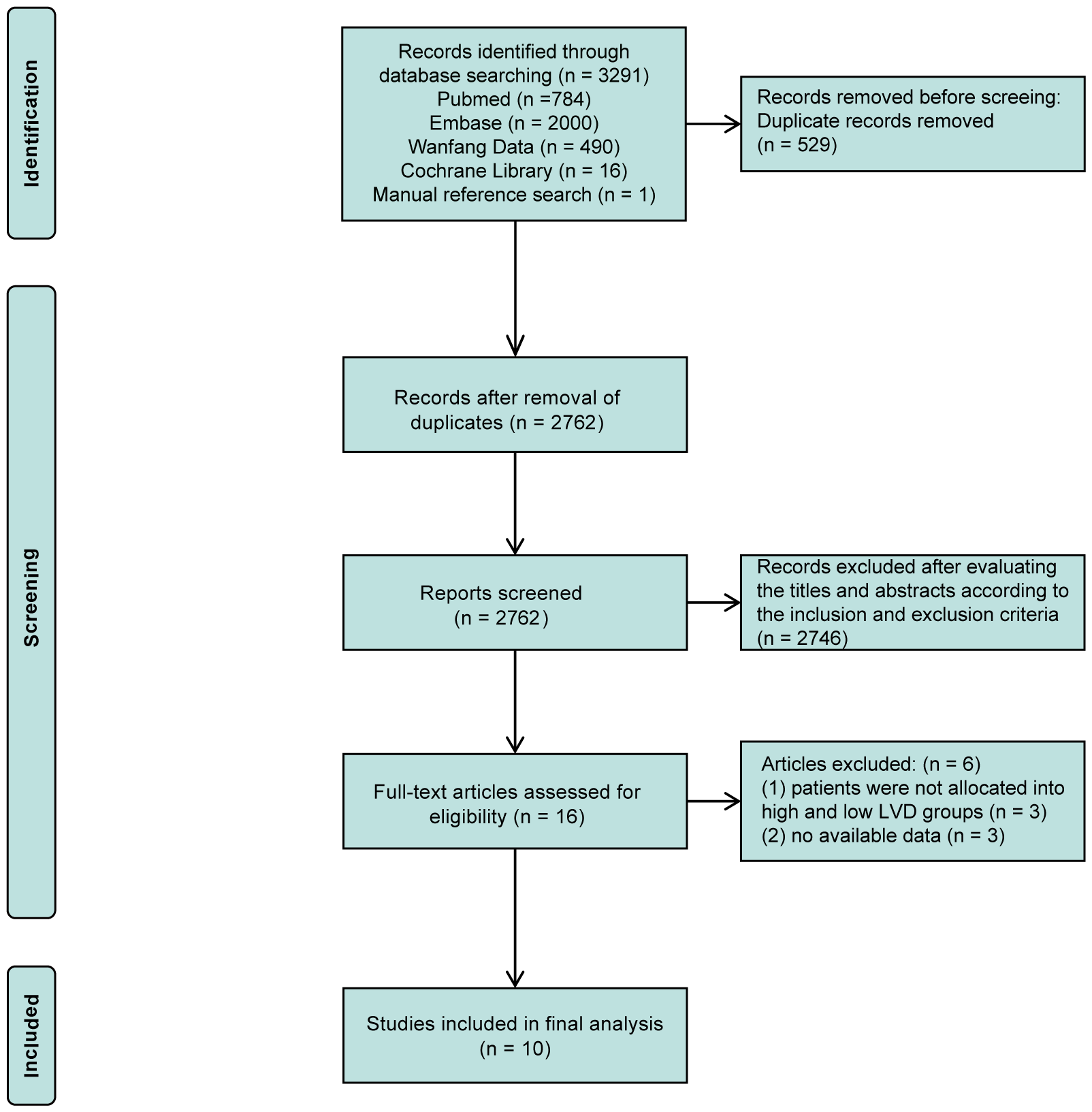
PRISMA flow diagram of the literature search and selection.
Study and patient characteristics
The baseline characteristics of the 10 eligible studies are presented in Table 1. The studies covered 14 years (2007-2021). Seven studies were conducted in China (16–19, 21, 23, 24), one in Japan (25), one in Austria (22), and one in Poland (20). A total of 1,201 patients with EC after radical resection were included in these studies, with 927 male (77%) and 274 female (23%), and an age ranged from 35 to 81 years old. The degrees of LVD were evaluated by immunohistochemistry with the lymphatic endothelial cell marker D2-40 (a.k.a. podoplanin) in all 10 studies. Patients were stratified into the high and low LVD groups, based on median LVD in five studies (18, 20–22, 24), mean LVD in one study (25), and a specific cut-off value in the remaining four studies (16, 17, 19, 23). Among the 10 studies, 6 studies reported the number of patients with different levels of LVD in tumor (17–19, 22–24), including 454 (48.8%) patients with a high level of LVD in tumor and 477 (51.2%) patients with a low level of LVD in tumor, while other 4 studies did not reported the number of patients with different levels of LVD in tumor, but reported HR and 95% CI about the role of different levels of LVD in tumor on prognosis (16, 20, 21, 25). Baseline comparisons between the group with a high level of LVD and the group with a low level of LVD were performed in 9 studies (17–25). There was no significant difference in sex, age, pathologic subtypes, differentiation grade, and tumor location between these two groups. The median follow-up time periods ranged from 28.5 to 54.4 months.
Table 1
| Ref. | Year of publication | Country | No. of patient (high vs. low) | Cut-off value | No. of Male (%) | Mean or median age (yr) | Pathologic subtypes EAC (n)/ ESCC (n) |
Location of tumor (upper/midthoracic/lower) | Differentiation grade (good/moderate/poor) | Extent of tumor invasion (T1/T2/T3/T4) | Lymph node invasion (Y/N) | TNM (I/II/III/IV) | Median follow-up (M) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakayama Y et al. (25) | 2007 | Japan | 29¶ | 7.2/HPF | 23 (79.3) | 61.3 | EAC (21)/ ESCC (8) |
NA | 3/20/5 | 4/5/16/4 | 9/20 | 3/6/12/8※ | 54.4 |
| Li CH et al. (24) | 2008 | China | 54 vs. 24 | 7.4/HPF | 56 (71.8) | 53.8 | ESCC (78) | NA | 36/30/11 | 3/12/60/3 | 55/23 | 2/49/24/3* | 33.0 |
| Bu XH et al. (16) | 2012 | China | 60¶ | 25/HPF | 40 (66.7) | 58.0 | ESCC (60) | NA | good/moderate + poor: 20/40 | NA | 34/26 | NA※ | 49.5 |
| Schoppmann SF et al. (22) | 2013 | Austria | 164 vs. 181 | 12/HPF | 269 (77.9) | 63.0 | EAC (194)/ ESCC (151) |
NA | 17/181/147 | 58/94/177/16 | 122/203 | 58/94/177/16⁑ | 52.0 |
| Kozlowski M et al. (20) | 2013 | Poland | 74¶ | 38/HPF | 60 (81.1) | 66.0 | EAC (42)/ ESCC (32) |
4/26/44 | 7/32/35 | T1+T2/T3+T4: 21/53 | 25/49 | NA⁑ | 28.5 |
| Xie LX et al. (23) | 2013 | China | 61 vs. 67 | 13/HPF | 106 (82.8) | 60.0 | EAC (128) | NA | good + moderate/poor: 51/77 | T1/T2+T3/T4: 0/85/43 | 42/86 | NA* | 45.0 |
| Ma W et al. (21) | 2014 | China | 107¶ | 4.4/HPF | 85 (79.4) | 60.0 | ESCC (107) | 11/66/36 | 25/53/29 | 14/23/62/8 | 69/38 | 17/53/37/0⁑ | 40.0 |
| Chen B et al. (18) | 2014 | China | 54 vs. 70 | median (?) | 98 (79.0) | 59.0 | ESCC (124) | NA | 22/93/9 | T1+T2/T3+T4: 17/107 | 58/66 | I+II/III+IV: 66/58⁑ | NA |
| Chen GQ et al. (19) | 2014 | China | 83 vs. 123 | 17/HPF | 152 (73.7) | 58.6 | ESCC (206) | 25/113/68 | 40/118/48 | 33/110/63/0 | NA | NA⁑ | 55.9 |
| Tang SJ et al. (17) | 2021 | China | 38 vs. 12 | 3/HPF | 38 (76.0) | 62.5 | ESCC (50) | upper + midthoracic/lower: 30/20 | 12/18/20 | T1+T2/T3+T4: 15/35 | 26/24 | NA⁂ | NA |
Characteristics of patients with esophageal cancer with high and low levels of lymphatic vessel density after radical resection.
¶, the total number of high and low groups; *, adopted to the 6th edition of AJCC/UICC TNM staging for esophageal cancer; ⁑, adopted to the 7th edition of AJCC/UICC TNM staging for esophageal cancer; ⁂, adopted to the 8th edition of AJCC/UICC TNM staging for esophageal cancer; ※, not available of version information of TNM staging; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; HPF, high-power field; NA, not available; TNM, tumor node metastasis classification.
Overall survival
The effects of LVD on OS were assessed in 9 studies (16–22, 24, 25) (Table 2). Seven studies reported that patients with a high level of LVD in tumor had significantly worse OS than those with a low level of LVD in tumor (16–22), and two studies reported that patients with a high level of LVD in tumor had a similar OS to those with a low level of LVD in tumor (24, 25). Data pooled from those 9 studies exhibited that patients with a high level of LVD in tumor had significantly worse OS than those with a low level of LVD in tumor (HR = 1.65, 95% CI 1.18 to 2.31, Figure 2).
Table 2
| Ref. | Year of publication | Group | Overall survival | |||
|---|---|---|---|---|---|---|
| One-year | Three-year | Five-year | P (log-rank) | |||
| Nakayama Y et al. (25) | 2007 | High | 73.44% | 34.36% | 29.40% | 0.083 |
| Low | 89.72% | 64.77% | 62.50% | |||
| Li CH et al. (24) | 2008 | High | 93.99% | 42.30% | NA | 0.283 |
| Low | 96.63% | 52.20% | NA | |||
| Bu XH et al. (16) | 2012 | High | 77.00% | 46.50% | 38.90% | < 0.050 |
| Low | 98.70% | 84.51% | 62.50% | |||
| Schoppmann SF et al. (22) | 2013 | High | 80.00% | 36.00% | 30.00% | < 0.001 |
| Low | 85.00% | 61.00% | 54.00% | |||
| Kozlowski M et al. (20) | 2013 | High | 74.98% | 26.17% | 19.03% | 0.003 |
| Low | 97.56% | 62.16% | 49.00% | |||
| Ma W et al. (21) | 2014 | High | 81.38% | 43.90% | NA | 0.042 |
| Low | 92.78% | 59.10% | NA | |||
| Chen B et al. (18) | 2014 | High | 83.05% | 24.10% | 12.40% | < 0.001 |
| Low | 88.76% | 65.79% | 56.57% | |||
| Chen GQ et al. (19) | 2014 | High | 93.14% | 80.96% | 54.20% | 0.017 |
| Low | 94.19% | 84.61% | 71.50% | |||
| Tang SJ et al. (17) | 2021 | High | 71.10% | 34.20% | 21.10% | < 0.050 |
| Low | 83.30% | 66.70% | 58.30% | |||
Overall survival of patients with esophageal cancer with high and low levels of lymphatic vessel density after radical resection.
Figure 2
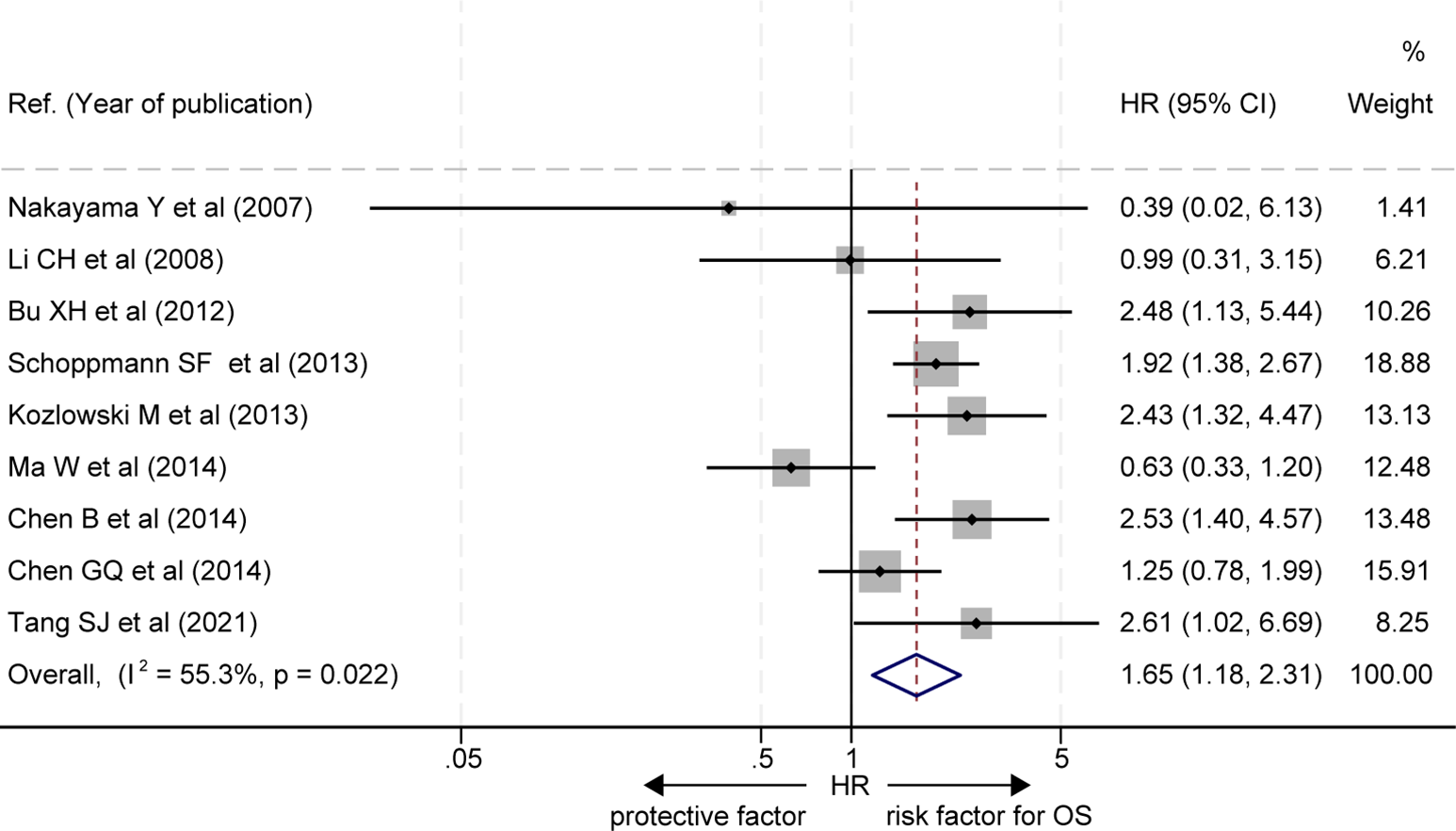
Comparison of overall survival between patients with esophageal cancer with a high level of LVD and those with a low level of LVD after radical resection. CI, confidence interval; HR, hazard ratio; LVD, lymphatic vessel density.
In subgroup analysis based on pathological subtypes of EC, three out of the 9 studies did not stratify for EAC or ESCC subgroups (20, 22, 25), and the OS of patients with a high level of LVD in tumor was significantly worse than those with a low level of LVD in these studies (HR = 1.99, 95% CI 1.49 to 2.65, Figure 3, the upper panel). Six studies focused on the ESCC subgroup (16–19, 21, 24), and patients with a high level of LVD in tumor had OS, similar to those with a low level of LVD in these studies (HR = 1.52, 95% CI 0.93 to 2.47, Figure 3, the lower panel).
Figure 3
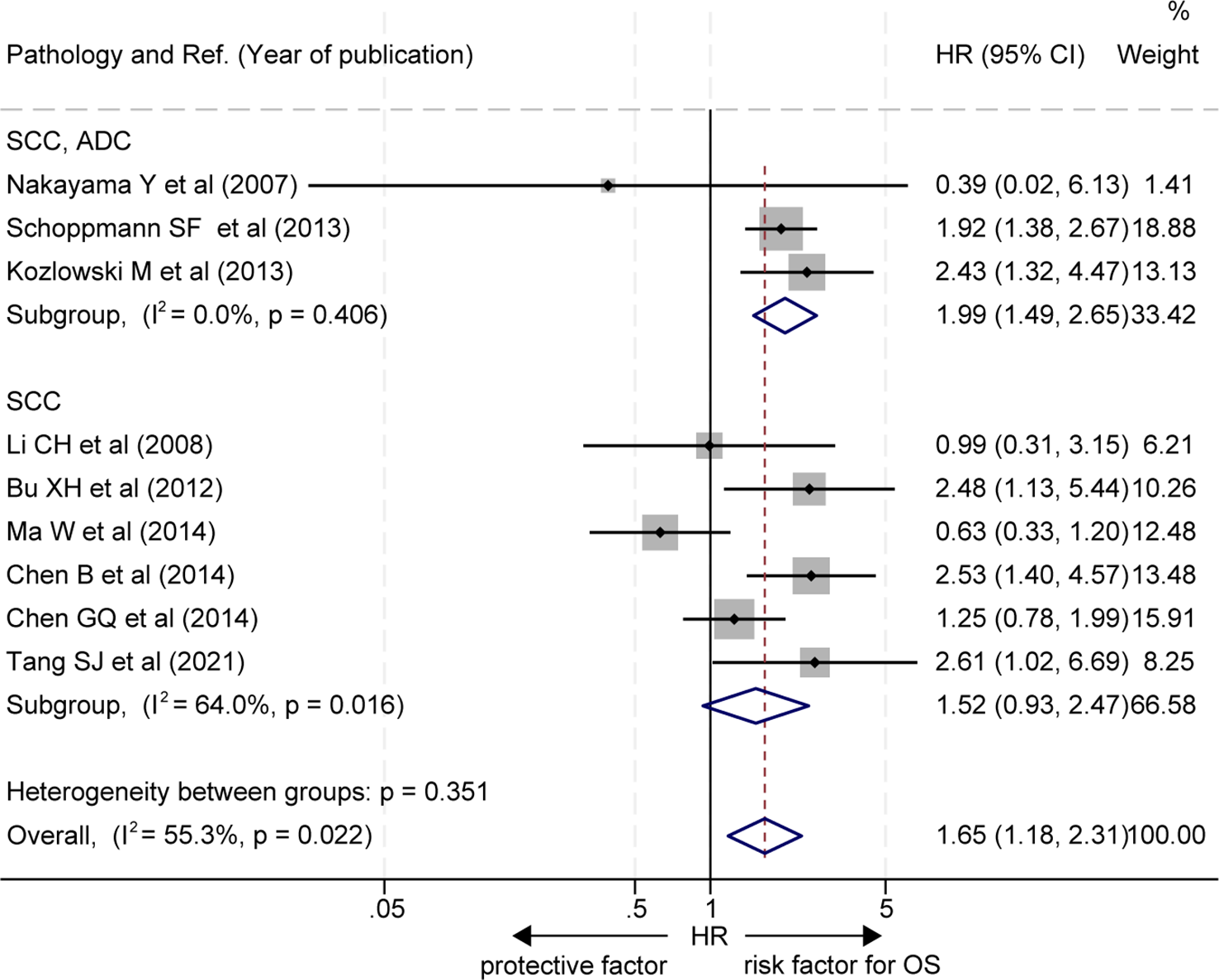
Subgroup comparison of overall survival between patients with esophageal cancer with a high level of LVD and those with a low level of LVD based on pathologic subtypes. CI, confidence interval; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; LVD, lymphatic vessel density.
Recurrence-free survival
The effects of LVD on RFS were assessed in 5 studies (19–23) (Table 3). Five studies consistently reported that patients with a high level of LVD in tumor had significantly worse RFS than those with a low level of LVD in tumor (19–23). Data pooled from those 5 studies indicated that patients with a high level of LVD in tumor had significantly worse RFS than those with a low level of LVD in tumor (HR = 1.57, 95% CI 1.09 to 2.26, Figure 4).
Table 3
| Ref. | Year of publication | Group | Recurrence-free survival | |||
|---|---|---|---|---|---|---|
| One-year | Three-year | Five-year | P (log-rank) | |||
| Schoppmann SF et al. (22) | 2013 | High | 62.00% | 32.00% | 28.00% | < 0.001 |
| Low | 75.00% | 49.00% | 48.00% | |||
| Kozlowski M et al. (20) | 2013 | High | 62.20% | 23.76% | 15.87% | 0.010 |
| Low | 86.44% | 52.03% | 31.74% | |||
| Xie LX et al. (23) | 2013 | High | 72.31% | 21.32% | 12.48% | < 0.001 |
| Low | 88.15% | 60.56% | 53.22% | |||
| Ma W et al. (21) | 2014 | High | 64.44% | 43.90% | NA | 0.035 |
| Low | 90.35% | 60.60% | NA | |||
| Chen GQ et al. (19) | 2014 | High | 88.74% | 74.10% | 56.48% | 0.030 |
| Low | 91.55% | 80.10% | 71.27% | |||
Recurrence-free survival of patients with esophageal cancer with high and low levels of lymphatic vessel density after radical resection.
Figure 4
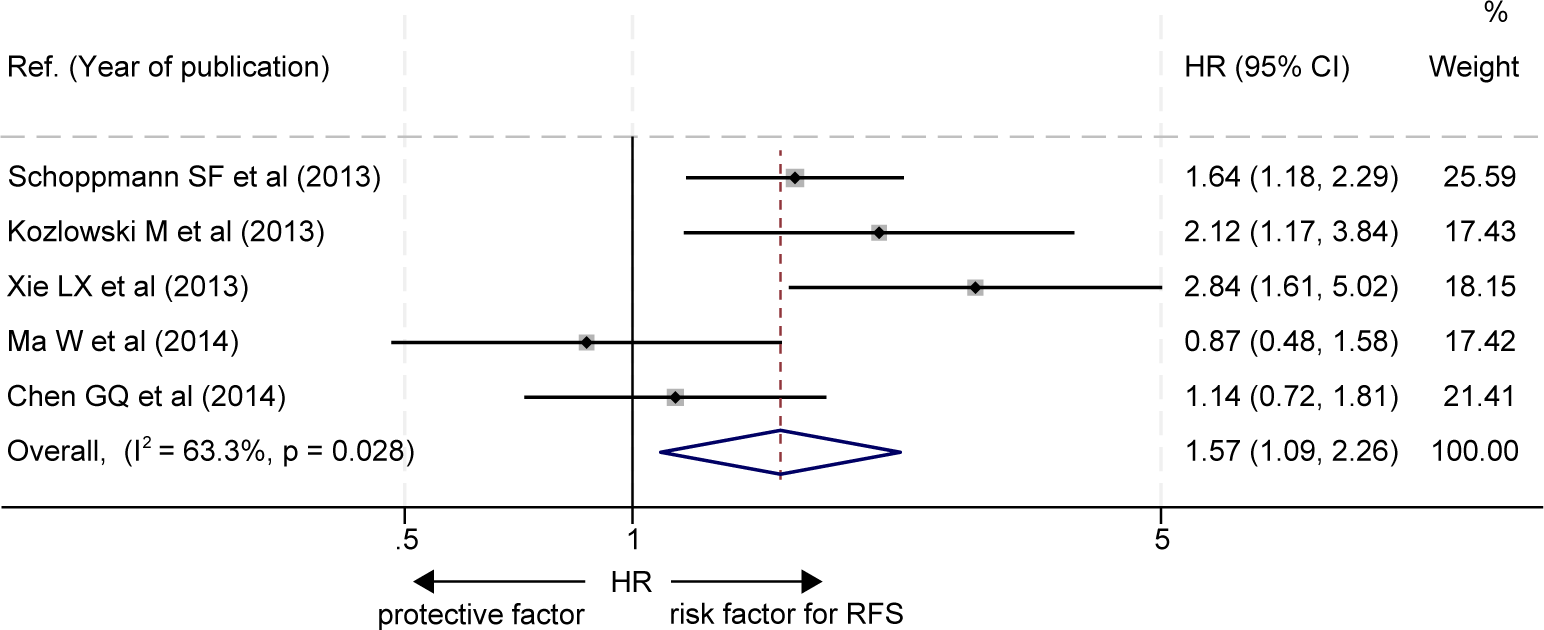
Comparison of recurrence-free survival between patients with esophageal cancer with a high level of LVD and those with a low level of LVD after radical resection. CI, confidence interval; HR, hazard ratio; LVD, lymphatic vessel density.
Furthermore, two out of the five studies did not stratify EC pathological subgroups (20, 22), and the RFS of patients with a high level of LVD in tumor was significantly worse than those with a low level of LVD in these studies (HR = 1.74, 95% CI 1.31 to 2.33, Figure 5, the upper panel). One study focused on the EAC subgroup (23), and the RFS of patients with a high level of LVD in tumor was significantly worse than those with a low level of LVD in the study (HR = 2.84, 95% CI 1.61 to 5.02, Figure 5, the middle panel). Two studies focused on the ESCC subgroup (19, 21), and patients with a high level of LVD in tumor had RFS, similar to those with a low level of LVD in these studies (HR = 1.03, 95% CI 0.72 to 1.48, Figure 5, the lower panel).
Figure 5
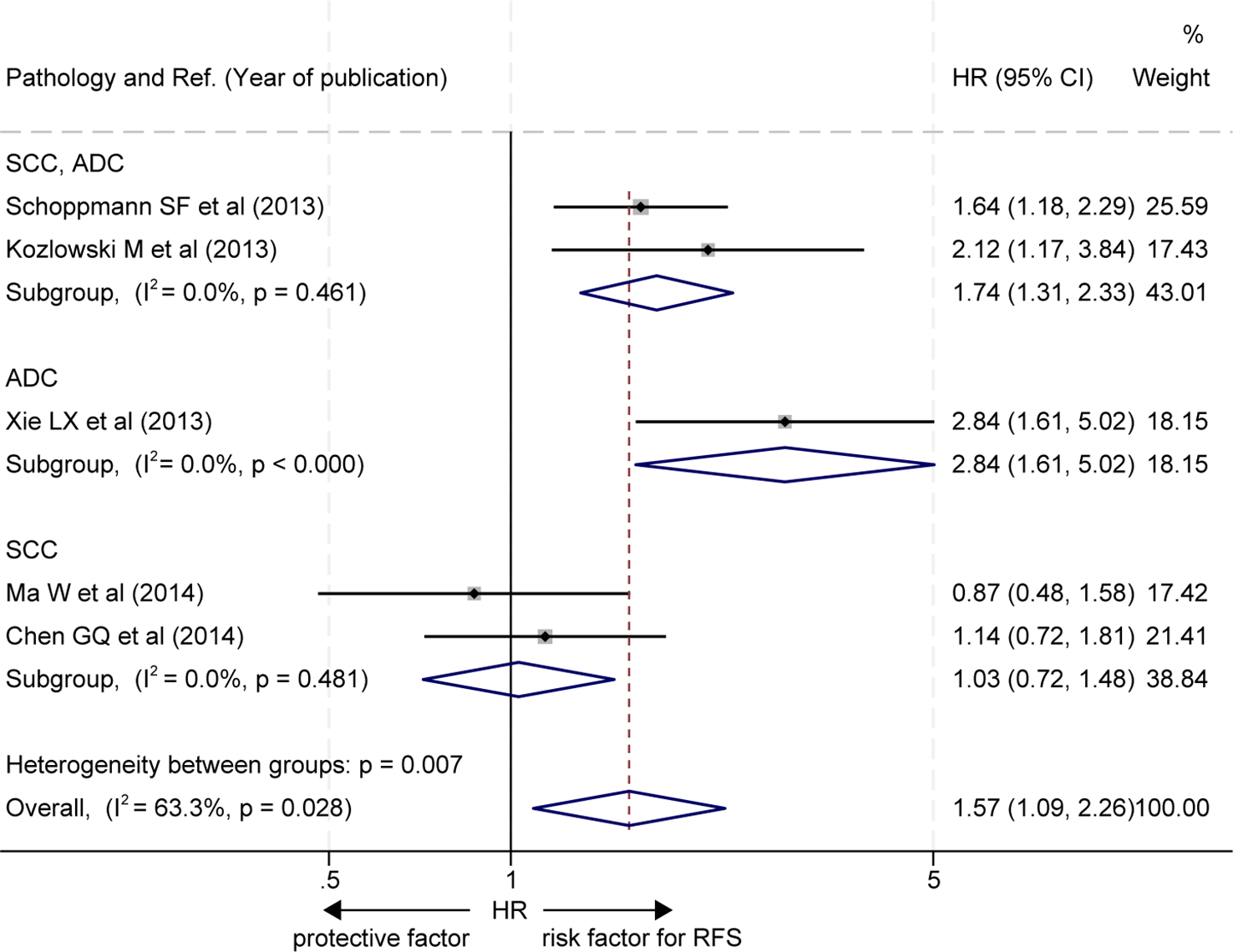
Subgroup comparison of recurrence-free survival between patients with esophageal cancer with a high level of LVD and those with a low level of LVD based on pathologic subtypes. CI, confidence interval; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; LVD, lymphatic vessel density.
Correlation between the levels of LVD and lymph node metastasis
The data about the correlation between the levels of LVD and lymph node metastasis was extracted from five studies (16, 21, 23–25) (Table 4). Four studies reported that tumors with lymph node metastasis had significantly higher levels of LVD than those without lymph node metastasis (16, 21, 23, 24), and one study reported that tumors with lymph node metastasis had a similar level of LVD to those without lymph node metastasis (25). Data pooled from those five studies indicated that tumors with lymph node metastasis had significantly higher levels of LVD than those without lymph node metastasis (SMD = 1.11, 95% CI 0.54 to 1.67, Figure 6A).
Table 4
| Ref. | Year of publication | Lymph node metastasis | No. | Lymphatic vessel density | P | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| Nakayama Y et al. (25) | 2007 | Positive | 20 | 7.97 | 3.26 | 0.0745 |
| Negative | 9 | 5.60 | 3.21 | |||
| Li CH et al. (24) | 2008 | Positive | 23 | 7.73 | 1.86 | 0.030 |
| Negative | 55 | 5.38 | 1.43 | |||
| Bu XH et al. (16) | 2012 | Positive | 26 | 37.79 | 4.99 | 0.001 |
| Negative | 34 | 25.82 | 5.76 | |||
| Xie LX et al. (23) | 2013 | Positive | 86 | 16.651 | 6.073 | 0.001 |
| Negative | 42 | 12.319 | 4.096 | |||
| Ma W et al. (21) | 2014 | Positive | 38 | 6.09 | 4.06 | 0.010 |
| Negative | 69 | 4.15 | 3.92 | |||
Correlation between the levels of lymphatic vessel density and lymph node metastasis in patients with esophageal cancer after radical resection.
SD, standard deviation.
Figure 6
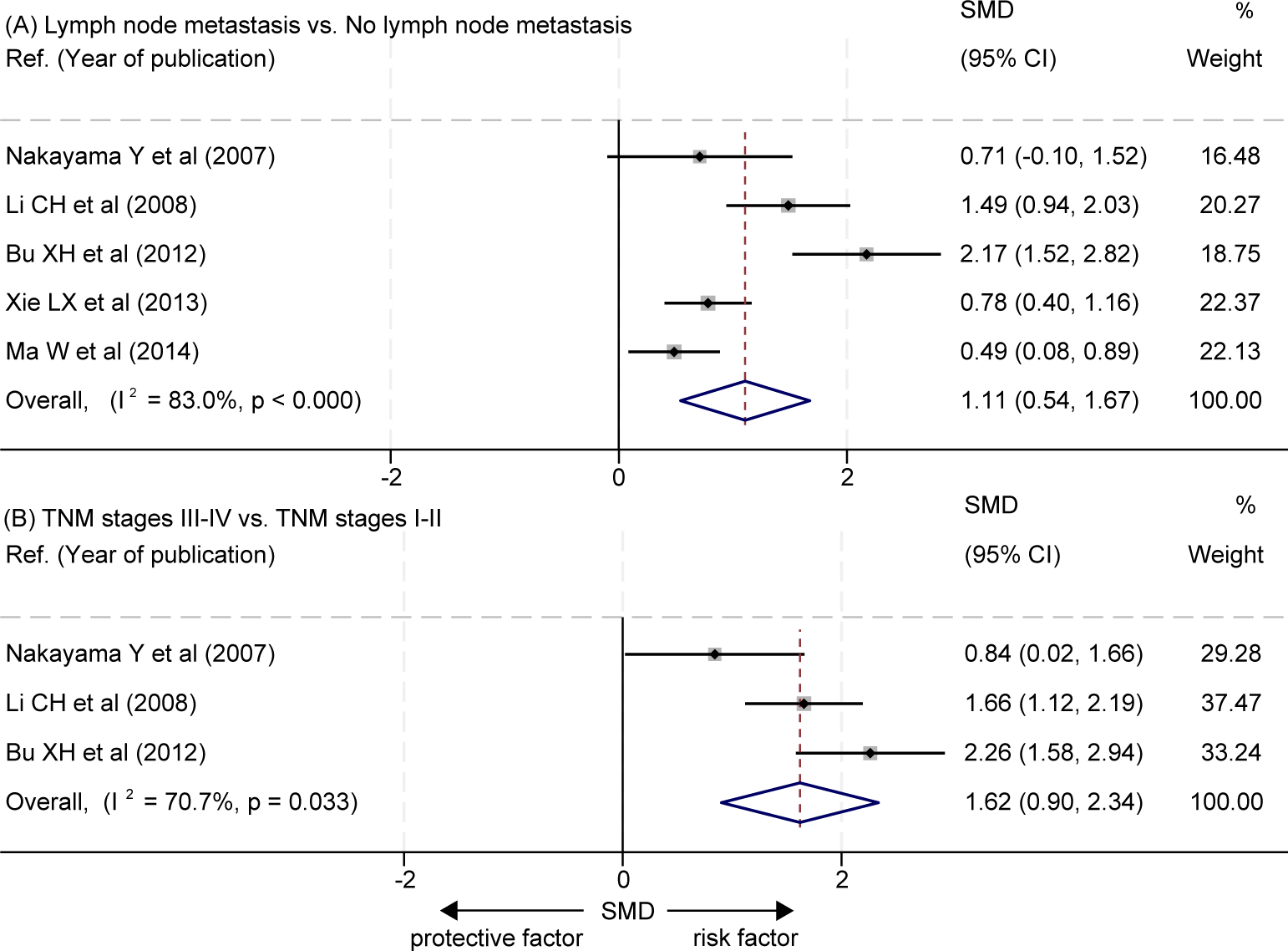
Correlation between the levels of LVD and clinicopathological features in patients with esophageal cancer after radical resection. (A) Correlation between the levels of LVD and lymph node metastasis. (B) Correlation between the levels of LVD and TNM stages. CI, confidence interval; LVD, lymphatic vessel density; SMD, standardized mean difference; TNM, tumor node metastasis classification.
Correlation between the levels of LVD and higher TNM stages
The data about the correlation between the levels of LVD and TNM stages was extracted from three studies (16, 24, 25) (Table 5). The three studies consistently reported that tumors at the stages III-IV had significantly higher levels of LVD than those at the stages I-II (16, 24, 25). The pooled data also indicated that tumors at the stages III-IV had significantly higher levels of LVD than those at the stages I-II (SMD = 1.62, 95% CI 0.90 to 2.34, Figure 6B).
Table 5
| Ref. | Year of publication | TNM stages | No. | Lymphatic vessel density | P | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| Nakayama Y et al. (25) | 2007 | III-IV | 20 | 8.16 | 3.15 | 0.0351 |
| I-II | 9 | 5.47 | 3.00 | |||
| Li CH et al. (24) | 2008 | III-IV | 27 | 7.18 | 1.35 | 0.013 |
| I-II | 51 | 4.27 | 1.91 | |||
| Bu XH et al. (16) | 2012 | III-IV | 20 | 39.37 | 3.52 | 0.001 |
| I-II | 40 | 26.83 | 6.20 | |||
Correlation between the levels of lymphatic vessel density and TNM stages in patients with esophageal cancer after radical resection.
SD, standard deviation; TNM, tumor node metastasis classification.
Publication bias
There was no significant publication bias in funnel plots for OS (Egger’s P = 0.428 and Begg’s P = 0.602, Supplementary Figure 1A) or RFS (Egger’s P = 0.724 and Begg’s P = 0.807, Supplementary Figure 1B).
Discussion
This study investigated the potential role of tumor-associated LVD in the prognosis of patients with EC after radical resection. While earlier studies have explored the impact of different levels of tumor-associated LVD on the prognosis of patients with EC after radical resection (16–25), the findings from these studies remain controversial due to the relatively smaller sample size. Therefore, we conducted this meta-analysis of 1,201 cases from 10 studies and our findings revealed that a high level of LVD in tumor was associated with worse OS and RFS of patients with EC after radical resection. Hence, tumor-associated lymphangiogenesis may have an unfavorable impact on the prognosis of patients with EC. This is a key inference in our study, and some evidence supports the inference: firstly, lymph node metastasis is a well-established prognostic factor for recurrence and poor outcomes in patients with EC after radical resection (34). With the increase in the number of positive lymph nodes, the OS rate of patients with EC decreases significantly (35). Secondly, lymphatic vessels function as a channel for malignant tumor cells to metastasize into lymph nodes and distant organs (36). The step of tumor cell entry into lymphatic vessels was termed lymphatic vascular invasion (37). Patients with EC with positive lymphatic vascular invasion have a worse prognosis compared to those without lymphatic vascular invasion (38–40). Thirdly, a high level of tumor-associated LVD probably increases the probability of lymphatic vascular invasion and lymph node metastasis (41, 42), based on the fact that an increase in LVD correlates with a higher probability of lymph node metastasis and higher TNM stages (17, 18, 20, 23, 24). Given that tumor-associated lymphangiogenesis may be an early and essential event of lymphatic vessel invasion and lymph node metastasis, future studies may explore the value of LVD as a biomarker of recurrence or prognosis of patients with EC after radical resection when no lymphatic vessel invasion and lymph node metastasis is found.
Subgroup analysis based on pathological subtypes of EC exhibited that ESCC patients had similar OS and RFS periods after radical resection regardless of the levels of LVD in the tumors. However, as regards EAC, the predominant pathological subtype in America and the Western world, patients with a high level of LVD in their tumors had worse RFS than those with a low level of LVD after radical resection. Apparently, tumor-associated LVD had different prognosis roles in ESCC and EAC. The significant role of tumor-associated LVD in EAC may reflect a metastatic tendency of adenocarcinoma, which more relies on the lymphatic system than squamous cell carcinoma (43, 44). Future prospective studies with larger sample sizes may verify our observation. We tried to perform subgroup analysis based on other clinicopathological features, like TNM stages, lymph node metastasis, and tumor cell differentiation. However, no study evaluated the role of LVD in the prognosis of these different subgroups of EC.
We recognized our study had limitations. Firstly, all data included in this study were obtained from retrospective cohort studies. Secondly, most patients in the study came from China, which may reflect the most prevalent region of the most prevalent type, ESCC, worldwide and may reduce the generalizability of our findings to other regions. Thirdly, although the cut-off value of high and low levels of LVD was generally determined by the median or mean value of LVD, no uniform cut-off value was used across different studies, which may increase the heterogeneity in the meta-analysis.
In conclusion, this study systematically reviewed the prognostic outcome of patients with EC with different levels of LVD after radical resection. Patients with EC with a high level of LVD had worse OS and RFS than those with a low level of LVD especially in patients with EAC.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JL: Data curation, Writing – original draft, Formal analysis, Validation, Visualization. QBW: Data curation, Formal analysis, Validation, Writing – original draft, Visualization. YBL: Data curation, Formal analysis, Validation, Writing – original draft, Visualization. XMC: Formal analysis, Data curation, Validation, Writing – original draft, Visualization. WLL: Formal analysis, Data curation, Validation, Writing – original draft, Visualization. YKL: Formal analysis, Data curation, Validation, Writing – original draft, Visualization. XC: Supervision, Writing – review & editing, Conceptualization, Data curation, Formal analysis. QYL: Supervision, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Validation. YK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from the National Natural Science Foundation of China (82103173 and 82460461), the Yunnan Province Young and Middle Scholarly and Technical Leader-B Program (202205AC160063), the Yunnan Fundamental Research Project for Excellent Young Scholars (202401AW070003), the Provincial First-class Discipline Construction Project of Clinical Medicine of the Second Affiliated Hospital of Kunming Medical University (YK), the Yunnan Province Sci-Tech Department-KMU Applied Basic Research Co-Fund (202301AY070001-235), and the Collaborative Research Program of the Second Affiliated Hospital of Kunming Medical University (2022dwhz13). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1453482/full#supplementary-material
Abbreviations
CI, confident interval; EAC, esophageal adenocarcinoma; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; LVD, lymphatic vessel density; NOS, Newcastle-Ottawa scale; OS, overall survival; RFS, recurrence-free survival; TNM, tumor node metastasis classification
References
1
Bray F Laversanne M Sung H Ferlay J Siegel RL Soerjomataram I et al . Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2
Chen R Zheng R Zhang S Wang S Sun K Zeng H et al . Patterns and trends in esophageal cancer incidence and mortality in China: An analysis based on cancer registry data. J Natl Cancer Cent. (2023) 3:21–7. doi: 10.1016/j.jncc.2023.01.002
3
Arnold M Ferlay J Van Berge Henegouwen MI Soerjomataram I . Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. (2020) 69:1564–71. doi: 10.1136/gutjnl-2020-321600
4
Zheng R Zhang S Zeng H Wang S Sun K Chen R et al . Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. (2022) 2:1–9. doi: 10.1016/j.jncc.2022.02.002
5
Sheng C Zhang X Liu B Lynn HS Chen K Dai H . Interplay between oral health and lifestyle factors for cancer risk in rural and urban China: a population-based cohort study. J Natl Cancer Cent. (2023) 3:279–85. doi: 10.1016/j.jncc.2023.10.004
6
Shi B Li C Xia W Chen Y Chen H Xu L et al . Construction a new nomogram prognostic model for predicting overall survival after radical resection of esophageal squamous cancer. Front Oncol. (2023) 13:1007859. doi: 10.3389/fonc.2023.1007859
7
Zhang R Wang Z Kang X Wang X Zhang B Ng H-l et al . Quality control indices for standardized diagnosis and treatment of esophageal cancer in China (2022 edition). J Natl Cancer Cent. (2023) 3:167–74. doi: 10.1016/j.jncc.2023.07.005
8
Mao Y Gao S Li Y Chen C Hao A Wang Q et al . Minimally invasive versus open esophagectomy for resectable thoracic esophageal cancer (NST 1502): a multicenter prospective cohort study. J Natl Cancer Cent. (2023) 3:106–14. doi: 10.1016/j.jncc.2023.02.002
9
Wagner PD Srivastava S . National Cancer Institute’s early detection research network: a model organization for biomarker research. J Natl Cancer Cent. (2023) 3:93–9. doi: 10.1016/j.jncc.2023.05.002
10
Liu X Li X Wei H Liu Y Li N . Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front Immunol. (2023) 14:1209056. doi: 10.3389/fimmu.2023.1209056
11
Viúdez-Pareja C Kreft E García-Caballero M . Immunomodulatory properties of the lymphatic endothelium in the tumor microenvironment. Front Immunol. (2023) 14:1235812. doi: 10.3389/fimmu.2023.1235812
12
Martin SG Rakha E Storr SJ . Quantifying lymphatic vessel density in human tissue samples. Methods Mol Biol. (2022) 2441:183–9. doi: 10.1007/978-1-0716-2059-5_14
13
Thelen A Jonas S Benckert C Weichert W Schott E Bötcher C et al . Tumor-associated lymphangiogenesis correlates with prognosis after resection of human hepatocellular carcinoma. Ann Surg Oncol. (2009) 16:1222–30. doi: 10.1245/s10434-009-0380-1
14
Thelen A Scholz A Weichert W Wiedenmann B Neuhaus P Gessner R et al . Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. (2010) 105:1123–32. doi: 10.1038/ajg.2009.674
15
Thelen A Scholz A Benckert C Weichert W Dietz E Wiedenmann B et al . Tumor-associated lymphangiogenesis correlates with lymph node metastases and prognosis in hilar cholangiocarcinoma. Ann Surg Oncol. (2008) 15:791–9. doi: 10.1245/s10434-007-9774-0
16
Bu XH Gong XJ Chen X . Expression of FGF3 and Podoplanin in esophageal squamous cell carcinoma and its relationship to prognosis. Chin J Oncol Prev Treat. (2012) 19:663–6. doi: 10.16073/j.cnki.cjcpt.2012.09.011
17
Tang SJ . The expression of VEGFR-3, MCM2 and the correlation analysis of D2-40-labeled MLVD with the prognosis of esophageal squamous cell carcinoma. [dissertation/master’s thesis]. Zunyi Medical University, Guizhou (2021).
18
Chen B Fang WK Wu ZY Xu XE Wu JY Fu JH et al . The prognostic implications of microvascular density and lymphatic vessel density in esophageal squamous cell carcinoma: Comparative analysis between the traditional whole sections and the tissue microarray. Acta Histochem. (2014) 116:646–53. doi: 10.1016/j.acthis.2013.12.003
19
Chen GQ Tian H Yue WM Li L Li SH Qi L et al . SIRT1 expression is associated with lymphangiogenesis, lymphovascular invasion and prognosis in pN0 esophageal squamous cell carcinoma. Cell Biosci. (2014) 4:48. doi: 10.1186/2045-3701-4-48
20
Kozlowski M Niklinska W Naumnik W Niklinski J . THORACIC SURGERY Intratumoral lymphatic vessel density, and intratumoral and peritumoral lymphatic vessel invasion as predictive factors of lymph node metastasis and prognostic factors in esophageal cancer. Kardiochirurgia i Torakochirurgia Polsk. (2013) 10(2):120–9. doi: 10.5114/kitp.2013.36131
21
Ma W Wang K Yang S Wang J Tan B Bai B et al . Clinicopathology significance of podoplanin immunoreactivity in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. (2014) 7:2361–71.
22
Schoppmann SF Jesch B Zacherl J Riegler MF Friedrich J Birner P . Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery. (2013) 153(4):526–34. doi: 10.1016/j.surg.2012.10.007
23
Xie LX Zhai TT Yang LP Yang E Zhang XH Chen JY et al . Lymphangiogenesis and prognostic significance of vascular endothelial growth factor C in gastro-oesophageal junction adenocarcinoma. Int J Exp Pathol. (2013) 94:39–46. doi: 10.1111/iep.12005
24
Li CH . The investigation of the relation between the expression and lymphatic metastasis in the esophageal carcinoma. [dissertation/master’s thesis]. Shandong Academy of Medical Sciences, Shangdong (2008).
25
Nakayama Y Matsumoto K Nagato M Inoue Y Katsuki T Minagawa N et al . Significance of lymphangiogenesis as assessed by immunohistochemistry for podoplanin in patients with esophageal carcinoma. Anticancer Res. (2007) 27:619–25.
26
Moher D Liberati A Tetzlaff J Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–269, w64. doi: 10.7326/0003-4819-151-4-200908180-00135
27
Tierney JF Stewart LA Ghersi D Burdett S Sydes MR . Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
28
Cao H Gong Y Wang Y . The prognostic impact of myosteatosis on overall survival in gynecological cancer patients: A meta-analysis and trial sequential analysis. Int J Cancer. (2022) 151:1997–2003. doi: 10.1002/ijc.34179
29
Steinberg J Hughes S Hui H Allsop MJ Egger S David M et al . Risk of COVID-19 death for people with a pre-existing cancer diagnosis prior to COVID-19-vaccination: A systematic review and meta-analysis. Int J Cancer. (2024) 154:1394–412. doi: 10.1002/ijc.34798
30
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
31
Leonard T Duffy JC . A Bayesian fixed effects analysis of the Mantel-Haenszel model applied to meta-analysis. Stat Med. (2002) 21:2295–312. doi: 10.1002/sim.1048
32
Zhou J Ke Y Lei X Wu T Li Y Bao T et al . Meta-analysis: The efficacy of metformin and other anti-hyperglycemic agents in prolonging the survival of hepatocellular carcinoma patients with type 2 diabetes. Ann Hepatol. (2020) 19:320–8. doi: 10.1016/j.aohep.2019.11.008
33
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
34
Zhang Y Zhang Y Peng L Zhang L . Research progress on the predicting factors and coping strategies for postoperative recurrence of esophageal cancer. Cells. (2022) 12:114. doi: 10.3390/cells12010114
35
Xiao ZF Yang ZY Miao YJ Wang LH Yin WB Gu XZ et al . Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys. (2005) 62:82–90. doi: 10.1016/j.ijrobp.2004.08.046
36
Petrova TV Koh GY . Biological functions of lymphatic vessels. Science. (2020) 369:eaax4063. doi: 10.1126/science.aax4063
37
Fujimoto N Dieterich LC . Mechanisms and clinical significance of tumor lymphatic invasion. Cells. (2021) 10:2585. doi: 10.3390/cells10102585
38
Wang A Tan Y Geng X Chen X Wang S . Lymphovascular invasion as a poor prognostic indicator in thoracic esophageal carcinoma: a systematic review and meta-analysis. Dis Esophagus. (2019) 32:1–8. doi: 10.1093/dote/doy083
39
Yang J Lu Z Li L Li Y Tan Y Zhang D et al . Relationship of lymphovascular invasion with lymph node metastasis and prognosis in superficial esophageal carcinoma: systematic review and meta-analysis. BMC Cancer. (2020) 20:176. doi: 10.1186/s12885-020-6656-3
40
Wang A Tan Y Wang S Chen X . The prognostic value of separate lymphatic invasion and vascular invasion in oesophageal squamous cell carcinoma: a meta-analysis and systematic review. BMC Cancer. (2022) 22:1329. doi: 10.1186/s12885-022-10441-6
41
Hu Z Zhao X Wu Z Qu B Yuan M Xing Y et al . Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets. Signal Transduct Target Ther. (2024) 9:9. doi: 10.1038/s41392-023-01723-x
42
Wu Z Qu B Yuan M Liu J Zhou C Sun M et al . CRIP1 reshapes the gastric cancer microenvironment to facilitate development of lymphatic metastasis. Adv Sci (Weinh). (2023) 10:e2303246. doi: 10.1002/advs.202303246
43
Deng HY Wang ZQ Wang YC Li G Luo J Chen LQ et al . Oesophageal adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-matched study. Eur J Cardiothorac Surg. (2017) 52:958–62. doi: 10.1093/ejcts/ezx222
44
Alexiou C Khan OA Black E Field ML Onyeaka P Beggs L et al . Survival after esophageal resection for carcinoma: the importance of the histologic cell type. Ann Thorac Surg. (2006) 82:1073–7. doi: 10.1016/j.athoracsur.2006.03.012
Summary
Keywords
esophageal cancer, lymphangiogenesis, lymphatic vessel density, meta-analysis, overall survival, prognosis, radical resection, recurrence-free survival
Citation
Li J, Wang Q-B, Liang Y-B, Chen X-M, Luo W-L, Li Y-K, Chen X, Lu Q-Y and Ke Y (2024) Tumor-associated lymphatic vessel density is a reliable biomarker for prognosis of esophageal cancer after radical resection: a systemic review and meta-analysis. Front. Immunol. 15:1453482. doi: 10.3389/fimmu.2024.1453482
Received
23 June 2024
Accepted
02 September 2024
Published
20 September 2024
Volume
15 - 2024
Edited by
Zhijie Wang, National Cancer Center of China, China
Reviewed by
Zhonghua Wu, The First Affiliated Hospital of China Medical University, China
Rui Wan, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Updates
Copyright
© 2024 Li, Wang, Liang, Chen, Luo, Li, Chen, Lu and Ke.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiong Chen, cxiongzpc@fjmu.edu.cn; Qi-Yu Lu, luqiyu0714@126.com; Yang Ke, keyang1218@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.