- Department of Hematology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, China
Our case demonstrated unique cytomegalovirus (CMV) encephalitis post-haploidentical donor hematopoietic stem cell transplantation (HID-HSCT), with early findings on diffusion-weighted brain magnetic resonance imaging (MRI) in the absence of any neurologic symptoms. A 54-year-old Chinese man with acute lymphoblastic leukemia (Philadelphia chromosome-negative) underwent HID-HSCT. After HSCT, the patient developed CMV viremia and severe acute graft-versus-host disease. Recurrence of CMV viremia was observed. On day 129, brain MRI was performed to determine the cause for the intermittent fever. Diffusion-weighted imaging (DWI) revealed several bright spots in the cortex of the frontal lobes and anterior angle of the left lateral ventricle. Subsequently, he developed transplant-associated thrombotic microangiopathy, posterior reversible encephalopathy syndrome, and enlargement of lesions alongside the ventricular wall on a brain MRI series. Metagenomic next-generation sequencing (NGS) of the cerebrospinal fluid (CSF) led to the final diagnosis of CMV encephalitis. Although ganciclovir combined with foscarnet was administered, the patient’s consciousness deteriorated, followed by respiratory failure. The patient died on day 198. Additionally, we performed a systematic review to comprehensively analyze this disease. Regarding treatment, immunological therapies, including virus-specific T cells from a third donor and CMV-cytotoxic T lymphocytes, may be more effective. This case report and systematic review underscores the complexities of managing CMV ventriculoencephalitis in HSCT recipients and emphasizes the importance of early diagnosis by brain MRI and CSF polymerase chain reaction or NGS and ongoing research in improving outcomes.
Introduction
Cytomegalovirus (CMV) infection is a serious complication in hematopoietic stem cell transplantation (HSCT) recipients. CMV encephalitis is a rare but often fatal occurrence following allogeneic-HSCT (allo-HSCT). Early diagnosis and effective therapy are paramount, especially with the limited efficacy of conventional anti-CMV drugs because of drug resistance (1–7). Screening for this complication using brain MRI may be helpful. Recently, adoptive treatment using CMV-specific cytotoxic T lymphocytes (CTLs) has emerged as a promising therapeutic approach for post-HSCT CMV infection (8, 9). Here, we report the dynamic brain magnetic resonance imaging (MRI) findings in a patient who developed CMV ventriculoencephalitis from an asymptomatic onset to a fatal outcome. Additionally, we provide a systematic review of similar cases to highlight the key diagnostic and prognostic features of this rare disease.
Case presentation
A 54-year-old Chinese man with acute lymphoblastic leukemia (Philadelphia chromosome-negative) underwent haploidentical donor HSCT (HID-HSCT) on December 1, 2019, from his 29-year-old son. The conditioning regimen included busulfan (3.2 mg/kg/day for three days), cyclophosphamide (1.8 g/m2/day for two days), etoposide (20 mg/kg/day for two days), and rabbit anti-thymocyte globulin (2.5 mg/kg/day for four days). Acute graft-versus-host disease (aGVHD) prophylaxis comprised cyclosporine A (CsA), short-course methotrexate (MTX), and mycophenolate mofetil (MMF).
Nine days after HSCT, the patient developed engraftment syndrome, for which methylprednisolone (MP) was initiated at 1 mg/kg/day. On day 22, he developed EBV viremia with DNA titers of 6.41×103 copies/mL in nuclear cells. Low dose of rituximab (100 mg qw for two doses) was administered for preemptive therapy and tapering of steroids. After two weeks, blood test was negative for EBV. On day 26, he experienced diarrhea, and after excluding other reasons especially infective causes from the stool culture, he was clinically diagnosed with aGVHD (grade II). Despite an increase in the MP dose to 2 mg/kg/day, the patient’s symptoms persisted. On day 30, foscarnet (PFA) was initiated to address CMV reactivation with a load < 1000 copies/mL.
On day 33, diarrhea deteriorated to 1500 mL daily with bloody stools and abdominal pain, and steroid-refractory aGVHD was suspected. After that, the anti-CD25 antibody basiliximab at 20 mg was given on days 33,36,40, and 47 as along with ruxolitinib 5 mg bid and tapering of steroids. The symptoms improved after four doses, but recurred on day 53, and one dose of mesenchymal stem cells (5×107) was administered. On day 70, the patient’s diarrhea was completely controlled. Minimal residual disease and CMV were negative, and his condition was deemed sufficiently stable for discharge on day 83.
On day 98, the patient was readmitted owing to “diarrhea and anorexia”. Blood test revealed a recurrence of CMV infection below 1000 copies/mL with severe thrombocytopenia. After eliminating aGVHD, relapse, transplant-associated thrombotic microangiopathy (TA-TMA), and drug-induced thrombocytopenia, the low platelet count was attributed to the viral infection. Imaging was performed to identify the cause for the intermittent fever, which revealed several bright spots on diffusion-weighted image (DWI) located in the cortex of the frontal lobes and the anterior angle of the left lateral ventricle in the brain MRI (black arrows in Figures 1A, E, I) compared to nothing specific in the T1 (Figures 1B, F, J), T2 (Figures 1C, G, K), and T2-fluid attenuated inversion recovery (FLAIR) images (Figures 1D, H, L) on day 129, despite the absence of neurological symptoms (Figure 1). Lumbar puncture was performed with RBC and WBC counts and glucose and protein levels within normal ranges in the cerebrospinal fluid (CSF). However, tests for microorganisms, including CMV, were not performed. After treated with PFA and eltrombopag, whole blood CMV DNA became negative twice, and blood counts returned to normal levels. The patient continued to be followed in the clinic.
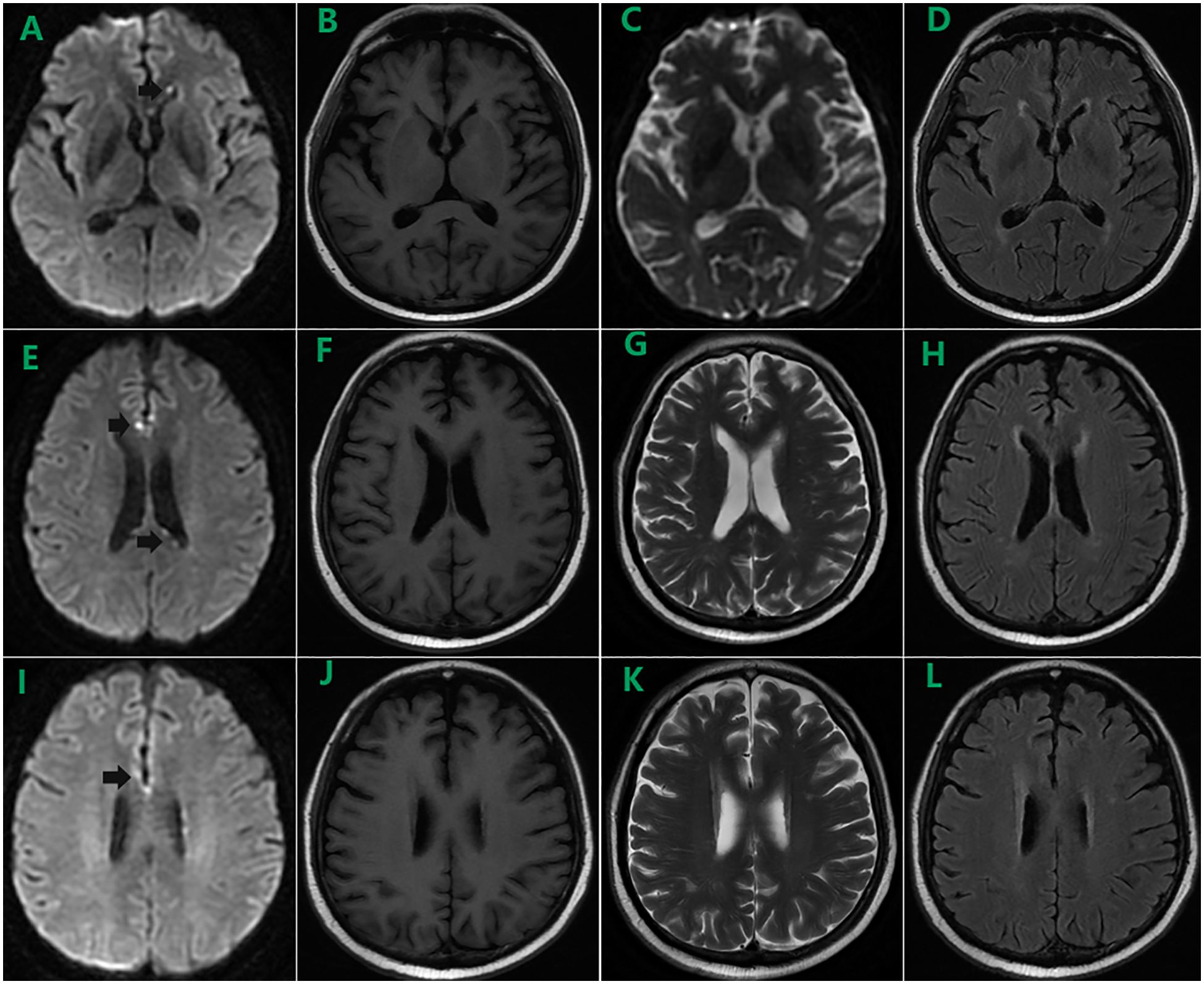
Figure 1. Brain MRI on day 129 showed several bright spots on diffusion-weighted image (DWI) located in the cortex of the frontal lobes and the anterior angle of the left lateral ventricle (black arrows in A, E, I) compared to nothing specific in the T1 (B, F, J), T2 (C, G, K), and T2-fluid attenuated inversion recovery (FLAIR) images (D, H, L).
During follow-up, the patient experienced persistent anorexia. On day 157, he reported blurred vision on the left eye, and fundus image revealed several exudates in the upper area of the retina and a hemorrhage below the macular area. Moreover, the whole blood samples were qualitatively positive for CMV DNA. On day 159, enhanced brain MRI revealed multiple abnormal punctate-signals in the anterior medulla oblongata, bifrontal cortex, and bilateral lateral ventricles. Despite ongoing antiviral therapy with foscarnet, blood CMV nucleic acid test results remained positive. On day 166, the patient experienced severely decreased hemoglobin and platelet counts, increased free hemoglobin, and repeated seizures from day 174. On day 176, brain MRI T2-FLAIR image revealed posterior reversible encephalopathy syndrome (PRES) (Figures 2E–L) and enlargement of lesions among the lateral ventricles (Figures 2A–H), suspecting TA-TMA. Treatments include plasmapheresis, discontinuation of CsA, control of CMV infection, and alternatives, including corticosteroids and MMF to prevent aGVHD. On day 185, brain MRI revealed the disappearance of lesions related to PRES and new lesions surrounding the fourth ventricle wall (Figure 3).
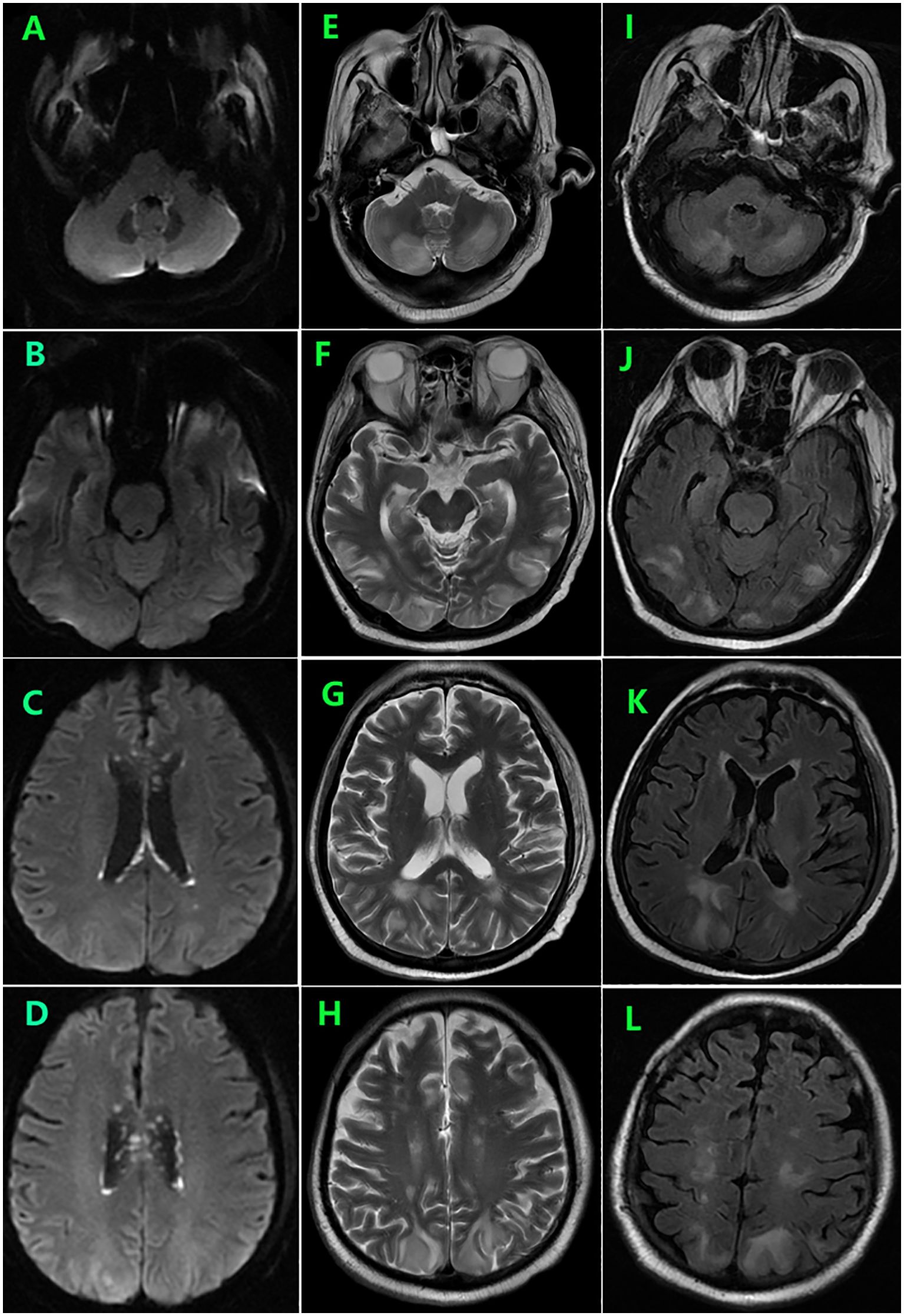
Figure 2. Brain MRI on day 176 showed symmetrical areas of hyperintensity in both occipital, frontal, and lobes and cerebellar involvement consistent with posterior reversible encephalopathy syndrome (PRES) (E–L) as well as enlargement of lesions among the lateral ventricles (A–D).
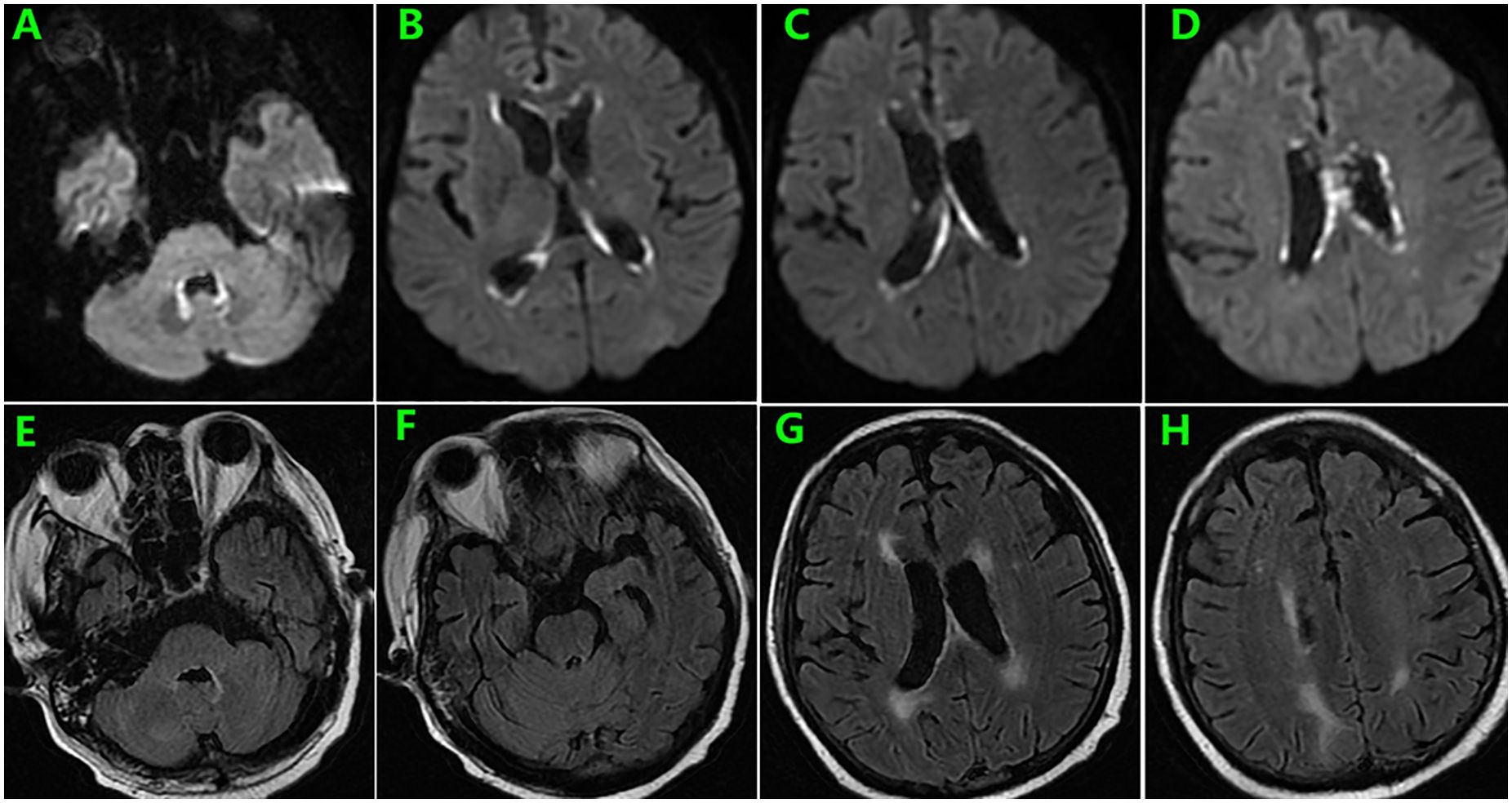
Figure 3. Brain MRI on day 185 showed resolution of lesions related to PRES (E–H) and new lesions surrounding the fourth ventricle wall as well as enlargement of former presentations along the lateral ventricles (A–D).
Although transient clinical improvement was achieved, the patient’s cognitive function deteriorated rapidly, manifesting as lethargy and orientation disturbance. On day 188, lumbar puncture was performed, and CSF was tested using metagenomic next-generation sequencing technology (mNGS), which detected CMV infection with 790,633 sequencing reads. CSF polymerase chain reaction (PCR) was also qualitatively positive. The patient was diagnosed with CMV ventriculoencephalitis. PFA (60 mg/kg q8h) combined with ganciclovir (GCV) (5 mg/kg q12h), and immunosuppressive drugs was discontinued. However, the patient’s condition did not improve, resulting in respiratory failure, and died on day 198. The timeline (treatment and clinical findings) of patient outcomes after transplantation is shown in Supplementary Figure 1.
Systematic review
A systematic search of PubMed, Scopus, Web of Science, and Embase databases for studies published between January 1, 2020 and December 31, 2022, using the search terms “hematopoietic stem cell transplantation” or “HSCT” with “cytomegalovirus encephalitis” or “CMV encephalitis” was performed. Cases or case series meeting the following criteria were enrolled (1): patients undergoing allo-HSCT and (2) patients suffering from CMV encephalitis with details of diagnostic method, treatment, and outcomes. Patients who did not write in English were excluded.
Thirty-one studies involving 38 patients were included (Table 1). The patients’ median age was 29.5 years (range; 1.5–65 years). Among these patients, HID, mismatched or matched unrelated donors (MMUD or MUD), and umbilical donors (UD) accounted for 94.7% (36/38), whereas almost all patients (37, 97.4%) had previously suffered from CMV viremia. Regarding CMV serostatus, only one patient and donor were negative, whereas 24 were positive for either the patient or donor (D+/R- 3, D-/R+ 9, D+/R+ 12). Regarding anti-CMV treatment, 28 of the 38 patients received both GCV or valganciclovir (VGC) and PFA for CMV viremia. Excluding one patient with CD4+ cell >200/ul, low CD4+ cell counts during onset (range; 0–132/uL) were observed. Notably, the median number of days to CMV encephalitis onset was 180 days post-transplantation. Of the 20 patients with mutation tests in the CSF, 11 (55%) had mutations in UL97 or 54, whereas 10 of 14 patients had mutations in UL97 or 54 in the peripheral blood (PB). Notably, compartmentalization of viruses present in the PB and CSF was observed in five patients (3, 4, 10–12). Only 10 patients were alive during follow-up, with a survival rate of 26.3%; the leading cause of death was uncontrolled CMV infection. Particularly, three were recovered by GCV, PFA, and cidofovir (CDV) or VGC (13–15) while the remaining seven patients were successfully treated by virus-specific T cells (VST) from a third-party donor (n=1) (16), CMV-CTLs (n=1) (5), donor lymphocyte infusion (DLI) or immune globulin (IG) with or without drugs (n=4) (17–20), or CMV-specific IG alone (n=1) (21).
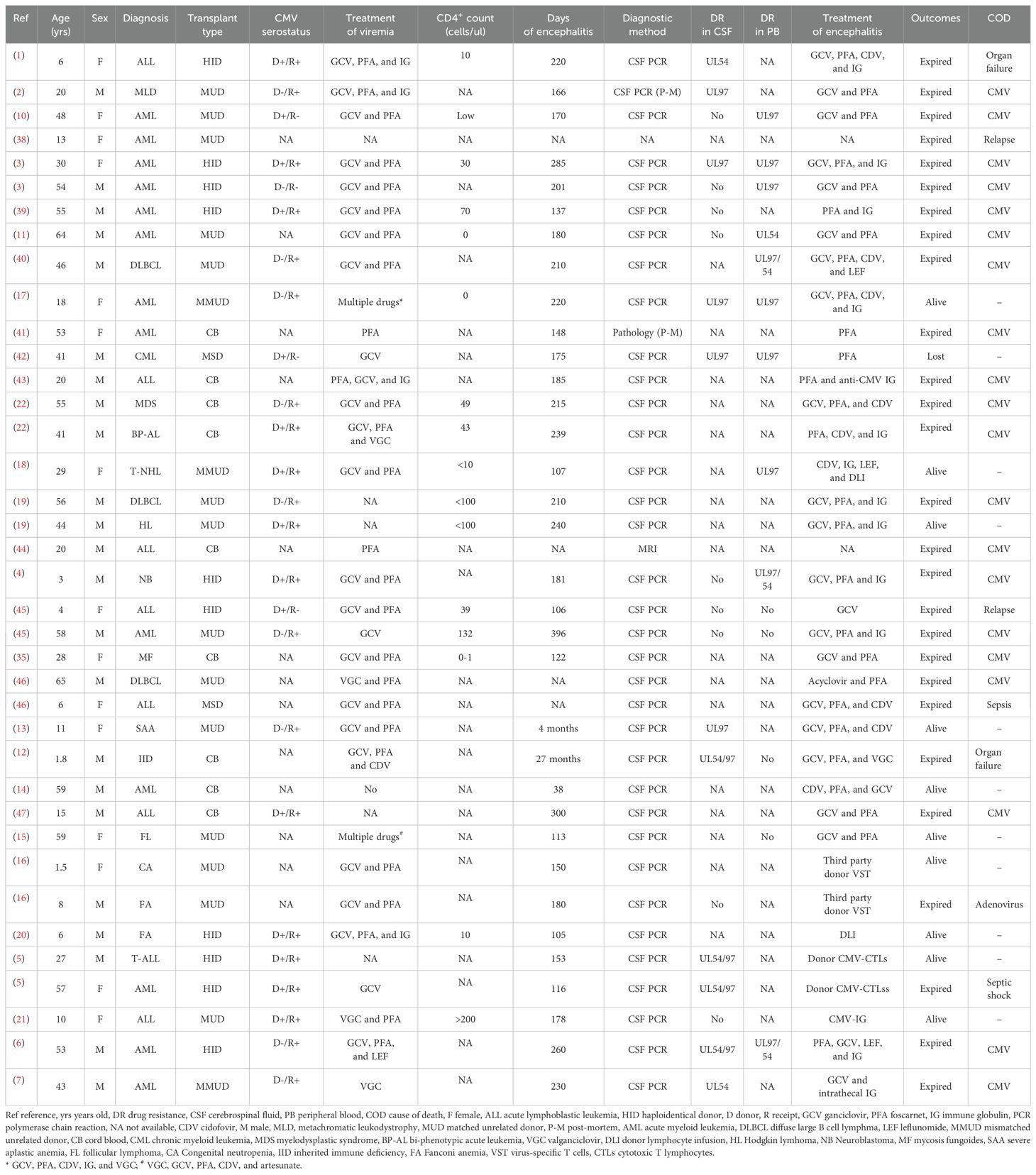
Table 1. Details CMV encephalitis among patients undergoing allogeneic transplantation in literature.
Discussion and conclusion
CMV encephalitis is an extremely rare post-HSCT complication. In retrospective studies, the incidence ranges from 0.1%–2.3% (22, 23). However, the prognosis is poor and should not be neglected.
Several factors contribute to the development of the condition. First, impaired T cell immune function caused by intensive GVHD prophylaxis conditioning regimen including ATG or anti-CD52 antibody, severe aGVHD, and delayed immune reconstitution following UC transplantation, are crucial in its emergence. Second, CMV viremia is another risk factor. Moreover, prolonged exposure to anti-CMV drugs, such as GCV and PFA may lead to drug resistance mutations in UL97 or 54. Therefore, CMV reactivation prophylaxis with new drugs such as letermovir may reduce this fatal complication. Primary prophylaxis with letermovir has been found to be a significant beneficial factor in preventing refractory or resistant CMV infections, reducing non-relapse mortality at week 48 and clinically significant CMV infections and diseases after allo-HSCT (24). Third, compartmentalization of anti-viral sensitive CMV in the CSF may indicate an insufficient concentration of GCV, PFA, or CDV across the blood-brain barrier (11). Our case was an HID-HSCT recipient with ATG added to the conditioning regimen, severe aGVHD post-HSCT, and poor immune reconstitution marked as a low CD4+ cell count (only 37/uL). Previously, CMV reactivation was approximately 85% according to the ATG-based regimen in HID-HSCT (25–28) and CMV drug resistance was approximately 14.5% (29).
Clinically, the diagnosis of CMV encephalitis depends on CSF PCR while the typical finding of an “owl eye sign” by brain pathology is difficult to perform (30). In our case, based on a series of brain MRIs and reported literature (31), we found extremely early findings of CMV encephalitis presenting as bright spots on DWI and dynamic changes in these lesions. Our case revealed that routine MRI screens, especially DWI images, may be important in early diagnosis (31). Specifically, brain DWI and mutation detection should be performed in HSCT patients with relapsed or refractory CMV viremia in the absence of neurological symptoms. For patients with suspected encephalitis, a CSF test panel that includes CMV, human herpesvirus 6, EBV, and herpes simplex virus tests should be performed. Moreover, if CMV is a probable pathogen, CMV mutation detection both in the PB and CSF should be implemented (32–34).
Concerning treatment, despite aggressive antiviral therapy, many cases have poor prognosis (22, 35), highlighting the urgent need for novel treatment strategies. The high mortality of this disease may be ascribed to the low concentration of anti-viral drugs in the CSF, drug resistance, and poor recovery of the immune system. Patients successfully treated by anti-viral drug combinations experienced a long-term course and tapering of immunosuppressants (13–15).Successful cases of immunological therapies, including VST from a third donor (16), CMV-CTLs (5), DLI (18, 20), and CMV-specific IG (21), have been reported. In a retrospective study, six out of 10 patients with CMV encephalitis who may benefit from CMV-CTLs were salvaged (30). However, the time-consuming production of cells and the urgency of this disease may limit the application of CTLs in practice, although we may fear the potential GVHD effects of DLI. In clinical trials, the application of off-the-shelf CMV-specific CTLs has shown great benefits in treating viral reactivation (36); however, further studies are required to determine the benefits of CMV-specific T cells in the treatment of CMV-encephalitis, provided the poor understanding of the penetration of CMV-specific CTLs in the CSF.
There are several limitations to its diagnosis and treatment. First, we did not perform CSF microbiological analysis at the beginning because of the limited knowledge on this disease. Second, the compartmentalization of viruses following allo-HSCT should be focused. Third, we may consider tapering CsA rapidly to help reconstitute the immune system and administering DLI in the absence of aGVHD to treat this disease. Fourth, in our patient, as CMV was detected using a qualitative method, the quantitative level of CMV DNA in the peripheral blood was monitored regularly, not less than once weekly, and mutation detection was initiated as refractory CMV viremia was suspected after the second recurrence of CMV viremia on day 157 (37).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Writing – review & editing. NL: Writing – original draft. JZ: Writing – original draft. YL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1450576/full#supplementary-material
References
1. Seo SK, Regan A, Cihlar T, Lin DC, Boulad F, George D, et al. Cytomegalovirus ventriculoencephalitis in a bone marrow transplant recipient receiving antiviral maintenance: clinical and molecular evidence of drug resistance. Clin Infect Dis. (2001) 33:e105–8. doi: 10.1086/cid.2001.33.issue-9
2. Julin JE, Van Burik JH, Krivit W, Webb C, Holman CJ, Clark HB, et al. Ganciclovir-resistant cytomegalovirus encephalitis in a bone marrow transplant recipient. Transplant Infect Dis. (2002) 4:201–6. doi: 10.1034/j.1399-3062.2002.02005.x
3. Wolf DG, Lurain NS, Zuckerman T, Hoffman R, Satinger J, Honigman A, et al. Emergence of late cytomegalovirus central nervous system disease in hematopoietic stem cell transplant recipients. Blood. (2003) 101:463–5. doi: 10.1182/blood-2002-07-1982
4. Jeong T-D, Sung H, Choi S-H, Lee S-O, Yoon H-K, Kim M-N, et al. Cytomegalovirus ventriculoencephalitis with compartmentalization of antiviral-resistant cytomegalovirus in a T cell-depleted haploidentical peripheral blood stem cell transplant recipient. Diagn Microbiol Infect Dis. (2012) 74:307–10. doi: 10.1016/j.diagmicrobio.2012.07.006
5. Ke P, Bao X, Zhou J, Li X, Zhuang J, He X, et al. Donor CMV-specific cytotoxic T lymphocytes successfully treated drug-resistant cytomegalovirus encephalitis after allogeneic hematopoietic stem cell transplantation. Hematology. (2020) 25:43–7. doi: 10.1080/16078454.2019.1710945
6. Jung J-Y, Nho D, Cho S-Y, Lee D-G, Choi S-M, Kim H-J, et al. Intra-host diversity of drug-resistant cytomegalovirus: A case report of cytomegalovirus infection after allogeneic hematopoietic cell transplantation. J infection chemotherapy. (2022) 28:1415–8. doi: 10.1016/j.jiac.2022.05.020
7. Pang I, Singhabahu S, Novitzky-Basso I, Mazzulli T, Husain S, Mattsson J. Intrathecal cytomegalovirus immunoglobulin for CMV encephalitis post allogeneic stem cell transplantation. IDCases. (2022) 29. doi: 10.1016/j.idcr.2022.e01608
8. Wang X, Yu U, Yang C, Wang C, Zhang X, Li Y, et al. Cytomegalovirus (CMV)-specific cytotoxic T lymphocyte therapy resolve CMV diseases and refractory CMV infections in paediatric recipients of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. (2022) 57:271–5. doi: 10.1038/s41409-021-01499-0
9. Pei XY, Zhao XY, Liu XF, Mo XD, Lv M, Xu LP, et al. Adoptive therapy with cytomegalovirus-specific T cells for cytomegalovirus infection after haploidentical stem cell transplantation and factors affecting efficacy. Am J Hematol. (2022) 97:762–9. doi: 10.1002/ajh.26535
10. Hamprecht K, Eckle T, Prix L, Faul C, Einsele H, Jahn G. Ganciclovir-resistant cytomegalovirus disease after allogeneic stem cell transplantation: pitfalls of phenotypic diagnosis by in vitro selection of an UL97 mutant strain. J Infect Dis. (2003) 187:139–43. doi: 10.1086/jid.2003.187.issue-1
11. Miller GG, Boivin G, Dummer JS, McConnell T, Becher MW, Kassim A, et al. Cytomegalovirus ventriculoencephalitis in a peripheral blood stem cell transplant recipient. Clin Infect Dis. (2006) 42:e26–9. doi: 10.1086/499366
12. Frange P, Boutolleau D, Leruez-Ville M, Touzot F, Cros G, Heritier S, et al. Temporal and spatial compartmentalization of drug-resistant cytomegalovirus (CMV) in a child with CMV meningoencephalitis: Implications for sampling in molecular diagnosis. J Clin Microbiol. (2013) 51:4266–9. doi: 10.1128/JCM.02411-13
13. Tam DY, Cheng FW, Chan PK, Leung WK, Lee V, Shing MK, et al. Intact survival of refractory CMV limbic encephalitis in a patient with severe aplastic anemia after unrelated bone marrow transplantation. J Pediatr Hematol Oncol. (2012) 34:472–4. doi: 10.1097/MPH.0b013e318243501b
14. Ikegame K, Kato R, Fujioka T, Okada M, Kaida K, Ishii S, et al. Detection of donor-derived CMV-specific T cells in cerebrospinal fluid in a case of CMV meningoencephalitis after cord blood stem cell transplantation. Int J Hematol. (2013) 97:287–90. doi: 10.1007/s12185-012-1231-6
15. Baghban A, Malinis M. Ganciclovir and foscarnet dual-therapy for cytomegalovirus encephalitis: A case report and review of the literature. J Neurological Sci. (2018) 388:28–36. doi: 10.1016/j.jns.2018.02.029
16. Alonso L, Rudilla F, Gimeno R, Codinach M, Blanco M, Querol S, et al. Successful treatment of post-transplant CMV meningoencephalitis with third-party CMV virus-specific T cells: Lessons learned. Pediatr Transplant. (2019) 23:e13584. doi: 10.1111/petr.13584
17. Hubacek P, Keslova P, Formankova R, Pochop P, Cinek O, Zajac M, et al. Cytomegalovirus encephalitis/retinitis in allogeneic haematopoietic stem cell transplant recipient treated successfully with combination of cidofovir and foscarnet. Pediatr Transplant. (2009) 13:919–22. doi: 10.1111/j.1399-3046.2008.01070.x
18. Akpek G, Mikulski M, Kleinberg M, Badros A, Yanovich S, Rapoport AP. Cellular therapy with sequential unmanipulated donor lymphocyte infusions in drug-resistant cytomegalovirus (CMV) encephalitis. Blood. (2011) 117:5772–4. doi: 10.1182/blood-2011-02-334060
19. Candoni A, Simeone E, Buttignol S, Volpetti S, Lazzarotto T, Pipan C, et al. Late onset cytomegalovirus encephalitis after reduced-intensity conditioning allogeneic SCT: An emerging neurological complication. Bone Marrow Transplant. (2011) 46:455–6. doi: 10.1038/bmt.2010.123
20. Rastogi N, Yadav SP. Successful treatment of cytomegalovirus encephalitis post TCR-alpha-beta/CD19 depleted haploidentical stem cell transplant by unmanipulated donor lymphocyte infusions. Pediatr Hematol Oncol. (2019) 36:520–2. doi: 10.1080/08880018.2019.1663326
21. Maximova N, Marcuzzi A, Del Rizzo I, Zanon D, Maestro A, Barbi E, et al. Standard treatment–refractory cytomegalovirus encephalitis unmasked by immune reconstitution inflammatory syndrome and successfully treated with virus-specific hyperimmune globulin. Clin Trans Immunol. (2020) 9. doi: 10.1002/cti2.v9.11
22. Reddy SM, Winston DJ, Territo MC, Schiller GJ. CMV central nervous system disease in stem-cell transplant recipients: An increasing complication of drug-resistant CMV infection and protracted immunodeficiency. Bone Marrow Transplant. (2010) 45:979–84. doi: 10.1038/bmt.2010.35
23. Crisinel PA, Duval M, Crisinel DT, Mallette B, Bellier N, Vachon MF, et al. Risk of cytomegalovirus infection and disease after umbilical cord blood transplantation in children. Can J Infect Dis Med Microbiol. (2013) 24:e11–e5. doi: 10.1155/2013/159691
24. Sassine J, Khawaja F, Shigle TL, Handy V, Foolad F, Aitken SL, et al. Refractory and resistant cytomegalovirus after hematopoietic cell transplant in the letermovir primary prophylaxis era. Clin Infect Dis. (2021) 73:1346–54. doi: 10.1093/cid/ciab298
25. Chang YJ, Wu DP, Lai YR, Liu QF, Sun YQ, Hu J, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic Maligna ncies: A multicenter, open-label, randomized controlled study. J Clin Oncol. (2020) 38:3367–76. doi: 10.1200/JCO.20.00150
26. Chang YJ, Wang Y, Xu LP, Zhang XH, Chen H, Chen YH, et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. (2020) 13:27. doi: 10.1186/s13045-020-00860-y
27. Gao XN, Lin J, Wang LJ, Li F, Li HH, Wang SH, et al. Risk factors and associations with clinical outcomes of cytomegalovirus reactivation after haploidentical versus matched-sibling unmanipulated PBSCT in patients with hematologic Malignancies. Ann Hematol. (2020) 99:1883–93. doi: 10.1007/s00277-020-04156-6
28. Zhang YY, Mo WJ, Zuo YY, Zhou M, Zhang XH, Wang Y, et al. Comparable survival outcome between transplantation from haploidentical donor and matched related don or or unrelated donor for severe aplastic anemia patients aged 40 years and older: A retrospective m ulticenter cohort study. Clin Transplant. (2020) 34:e13810. doi: 10.1111/ctr.13810
29. Shmueli E, Or R, Shapira MY, Resnick IB, Caplan O, Bdolah-Abram T, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis. (2014) 209:557–61. doi: 10.1093/infdis/jit475
30. Meng XY, Fu HX, Zhu XL, Wang JZ, Liu X, Yan CH, et al. Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation. Ann Hematol. (2020) 99:2659–70. doi: 10.1007/s00277-020-04201-4
31. Renard T, Daumas-Duport B, Auffray-Calvier E, Bourcier R, Desal H. Cytomegalovirus encephalitis: Undescribed diffusion-weighted imaging characteristics. Original aspects of cases extracted from a retrospective study, and from literature review. J Neuroradiol. (2016) 43:371–7. doi: 10.1016/j.neurad.2016.03.004
32. Schmidt-Hieber M, Schwender J, Heinz WJ, Zabelina T, Kühl JS, Mousset S, et al. Viral encephalitis after allogeneic stem cell transplantation: a rare complication with distinct characteristics of different causative agents. Haematologica. (2011) 96:142–9. doi: 10.3324/haematol.2010.029876
33. Zhang XH, Zhang JM, Han W, Chen H, Chen YH, Wang FR, et al. Viral encephalitis after haplo-identical hematopoietic stem cell transplantation: Causative viral spectrum, characteristics, and risk factors. Eur J Haematology. (2017) 98:450–8. doi: 10.1111/ejh.2017.98.issue-5
34. Wu M, Huang F, Jiang X, Fan Z, Zhou H, Liu C, et al. Herpesvirus-associated central nervous system diseases after allogeneic hematopoietic stem cell transplantation. PloS One. (2013) 8:e77805. doi: 10.1371/journal.pone.0077805
35. Matsukawa T, Goto H, Takahashi K, Asanuma S, Yasumoto A, Takahata M, et al. A fatal case of cytomegalovirus ventriculoencephalitis in a mycosis fungoides patient who received multiple umbilical cord blood cell transplantations. Int J Hematol. (2012) 95:217–22. doi: 10.1007/s12185-012-1003-3
36. Withers B, Blyth E, Clancy LE, Yong A, Fraser C, Burgess J, et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv. (2017) 1:2193–205. doi: 10.1182/bloodadvances.2017010223
37. Chemaly RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. (2019) 68:1420–6. doi: 10.1093/cid/ciy696
38. Hazard E, Bastard C, Callat MP, Schneider P, Vannier JP. De novo acute myeloblastic leukemia with trilineage abnormalities and a t(3;12) in a child. Med Pediatr Oncol. (2003) 40:397–400. doi: 10.1002/mpo.10226
39. Zeiser R, Grüllich C, Bertz H, Pantazis G, Hufert FT, Bley TA, et al. Late cytomegalovirus polyradiculopathy following haploidentical CD34+-selected hematopoietic stem cell transplantation. Bone Marrow Transplant. (2004) 33:243–5. doi: 10.1038/sj.bmt.1704311
40. Battiwalla M, Paplham P, Almyroudis NG, McCarthy A, Abdelhalim A, Elefante A, et al. Leflunomide failure to control recurrent cytomegalovirus infection in the setting of renal failure after allogeneic stem cell transplantation. Transplant Infect disease: an Off J Transplant Soc. (2007) 9:28–32. doi: 10.1111/j.1399-3062.2006.00170.x
41. Ando T, Mitani N, Yamashita K, Takahashi T, Ohama E, Miyata H, et al. Cytomegalovirus ventriculoencephalitis in a reduced- intensity conditioning cord blood transplant recipient. Transplant Infect disease: an Off J Transplant Soc. (2010) 12:441–5. doi: 10.1111/j.1399-3062.2010.00503.x
42. Arslan F, Tabak F, Avşar E, Midilli K, Mert A, Ozaras R, et al. Ganciclovir-resistant cytomegalovirus encephalitis in a hematopoietic stem cell transplant recipient. J neurovirology. (2010) 16:174–8. doi: 10.3109/13550281003682539
43. Lee S, Kim S-H, Choi S-M, Lee D-G, Kim S-Y, Lee J-W, et al. Cytomegalovirus ventriculoencephalitis after unrelated double cord blood stem cell transplantation with an alemtuzumab-containing preparative regimen for philadelphia-positive acute lymphoblastic leukemia. J Korean Med Sci. (2010) 25:630–3. doi: 10.3346/jkms.2010.25.4.630
44. Seok JH, Ahn KJ, Park HJ. Diffusion MRI findings of cytomegalovirus-associated ventriculitis: A case report. Br J Radiol. (2011) 84:e179–e81. doi: 10.1259/bjr/31561378
45. Colombo AA, Giorgiani G, Rognoni V, Villani P, Furione M, Bonora MR, et al. Differential outcome of neurological HCMV infection in two hematopoietic stem cell transplant recipients. BMC Infect Dis. (2012) 12. doi: 10.1186/1471-2334-12-238
46. Sarva H, Graber J, Remanan R, Rosenblum M, Omuro A. CMV encephalitis in BMT recipients. Bone Marrow Transplant. (2012) 47:318–20. doi: 10.1038/bmt.2011.80
Keywords: cytomegalovirus, encephalitis, transplantation, diffusion-weighted image, case report
Citation: Li N, Zhao J, Liu Y and Zhang Y (2024) Dynamic findings of brain magnetic resonance imaging in a haploidentical hematopoietic stem cell transplantation recipient with cytomegalovirus ventriculoencephalitis: a case report and systematic review. Front. Immunol. 15:1450576. doi: 10.3389/fimmu.2024.1450576
Received: 17 June 2024; Accepted: 05 September 2024;
Published: 20 September 2024.
Edited by:
Rita Maccario, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Antonio Piralla, San Matteo Hospital Foundation (IRCCS), ItalyGaurav Sutrave, The University of Sydney, Australia
Copyright © 2024 Li, Zhao, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghui Liu, bGl1eWluZ2h1aXlhbnRhaUAxMjYuY29t; Yuanfeng Zhang, eXVhbmZlbmd6aGFuZzEyNkAxMjYuY29t
†These authors have contributed equally to this work
 Nannan Li
Nannan Li Jing Zhao†
Jing Zhao† Yuanfeng Zhang
Yuanfeng Zhang