
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Immunol. , 20 June 2024
Sec. B Cell Biology
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1441999
This article is a correction to:
Translatability of findings from cynomolgus monkey to human suggests a mechanistic role for IL-21 in promoting immunogenicity to an anti-PD-1/IL-21 mutein fusion protein
 Mark A. Kroenke1*
Mark A. Kroenke1* Marta Starcevic Manning2
Marta Starcevic Manning2 Christina L. Zuch de Zafra3†
Christina L. Zuch de Zafra3† Xinwen Zhang4
Xinwen Zhang4 Kevin D. Cook5
Kevin D. Cook5 Michael Archer6†
Michael Archer6† Martijn P. Lolkema7
Martijn P. Lolkema7 Jin Wang2
Jin Wang2 Sarah Hoofring2
Sarah Hoofring2 Gurleen Saini2†
Gurleen Saini2† Famke Aeffner3
Famke Aeffner3 Elizabeth Ahern8
Elizabeth Ahern8 Elena Garralda Cabanas9
Elena Garralda Cabanas9 Ramaswamy Govindan10
Ramaswamy Govindan10 Mun Hui11
Mun Hui11 Shalini Gupta2
Shalini Gupta2 Daniel T. Mytych1
Daniel T. Mytych1By Kroenke MA, Starcevic Manning M, Zuch de Zafra CL, Zhang X, Cook KD, Archer M, Lolkema MP, Wang J, Hoofring S, Saini G, Aeffner F, Ahern E, Cabanas EG, Govindan R, Hui M, Gupta S and Mytych DT (2024). Front. Immunol. 15:1345473. doi: 10.3389/fimmu.2024.1345473
Error in Figure/Table
In the published article, there was an error in Figure 2A as published. The Figure 2A y-axis should read “Serum concentration (ng/mL)”. The corrected Figure 2 is attached.
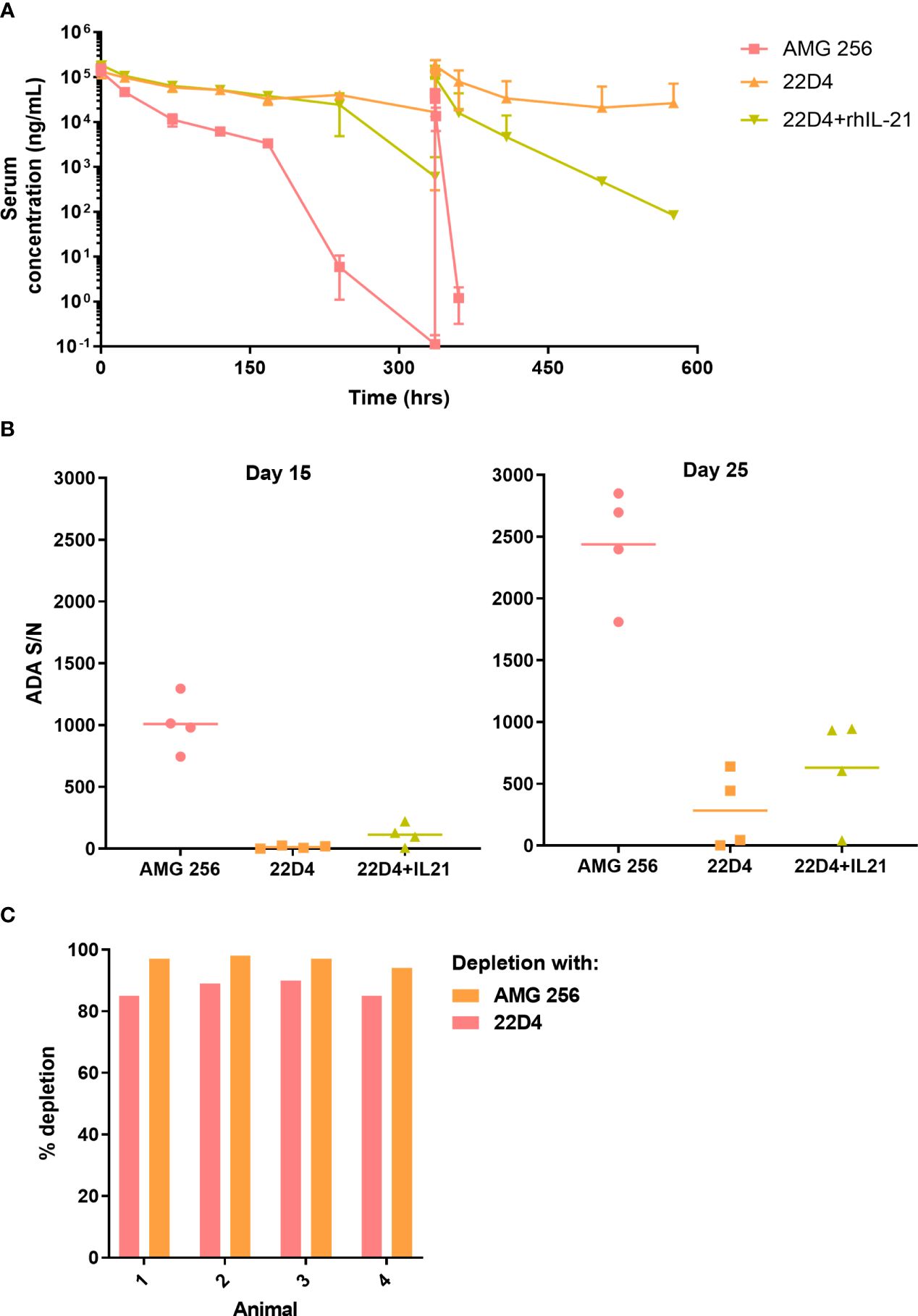
Figure 2 IL-21 mutein domain enhanced the antibody response to 22D4 in cynomolgus monkeys. Cynomolgus monkeys were dosed with 5 mg/kg AMG 256, 5 mg/kg 22D4, or 5 mg/kg 22D4 plus 0.1 mg/kg recombinant human IL-21. (A) AMG 256 or 22D4 serum levels were measured over time in each of the 3 treatment groups. (B) The ADA response in each dosing group was assessed on day 15 and day 25 by UNISA. (C) Domain characterization was performed on AMG 256 dosed animals at the day 25 time point. Serum samples were pre-treated with either AMG 256 or 22D4 and re-tested in the antibody assay. Percent depletion indicates the signal change from the pre-treated sample relative to the untreated sample.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: PD-1, IL-21, immunogenicity, anti-drug antibodies, mutein, IgE
Citation: Kroenke MA, Manning MS, Zuch de Zafra CL, Zhang X, Cook KD, Archer M, Lolkema MP, Wang J, Hoofring S, Saini G, Aeffner F, Ahern E, Cabanas EG, Govindan R, Hui M, Gupta S and Mytych DT (2024) Corrigendum: Translatability of findings from cynomolgus monkey to human suggests a mechanistic role for IL-21 in promoting immunogenicity to an anti-PD-1/IL-21 mutein fusion protein. Front. Immunol. 15:1441999. doi: 10.3389/fimmu.2024.1441999
Received: 01 June 2024; Accepted: 13 June 2024;
Published: 20 June 2024.
Edited and Reviewed by:
Michael Dougan, Massachusetts General Hospital and Harvard Medical School, United StatesCopyright © 2024 Kroenke, Manning, Zuch de Zafra, Zhang, Cook, Archer, Lolkema, Wang, Hoofring, Saini, Aeffner, Ahern, Cabanas, Govindan, Hui, Gupta and Mytych. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark A. Kroenke, bWtyb2Vua2VAYW1nZW4uY29t
†Present address: Christina L. Zuch de Zafra, Nonclinical Sciences, Seagen, South San Francisco, CA, United States Michael Archer, Drug Safety and Pharmacovigilance, Atara Biotherapeutics, Thousand Oaks, CA, United States Gurleen Saini, Immunogenicity Characterization, Boehringer Ingelheim, Ridgefield, CT, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.