
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 November 2024
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1437688
This article is part of the Research Topic Autoimmunity: novel insights and future perspectives View all 37 articles
Background: Autoimmune diseases (ADs) are a category of conditions characterized by misrecognition of autologous tissues and organs by the immune system, leading to severe impairment of patients’ health and quality of life. Increasing evidence suggests a connection between fluctuations in plasma metabolites and ADs. However, the existence of a causal relationship behind these associations remains uncertain.
Methods: Applying the two-sample mendelian randomization (MR) method, the reciprocal causality between plasma metabolites and ADs was analyzed. We took the intersection of two metabolite genome-wide association study (GWAS) datasets for GWAS-meta and obtained 1,009 metabolites’ GWAS data using METAL software. We accessed GWAS summary statistics for 5 common ADs, inflammatory bowel disease (IBD), multiple sclerosis (MS), type 1 diabetes (T1D), systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) from published GWAS data. MR analyses were performed in discovery and replication stage simultaneously. Meanwhile, the reverse MR analysis was conducted to investigate the possibility of reverse causal association. Furthermore, a series of sensitivity analyses were conducted to validate the robustness of the results. These statistical analyses were conducted using R software. Finally, the web version of MetaboAnalyst 5.0. was applied to analyze metabolic pathways. Ultimately, we conducted ELISA assays on plasma samples from patients to validate the results.
Results: 4 metabolites were identified to have causal relationships with IBD, 2 metabolites with MS, 13 metabolites with RA, and 4 metabolites with T1D. In the reverse MR analysis, we recognized causality between SLE and 22 metabolites, IBD and 4 metabolites, RA and 22 metabolites, and T1D and 37 metabolites. Additionally, 4 significant metabolic pathways were identified in RA by metabolic pathway analysis in the forward MR analysis. Correspondingly, in the reverse, 11 significant metabolic pathways in RA, 8 in SLE, and 4 in T1D were obtained using identical approaches. Furthermore, the protective role of glutamate was confirmed through ELISA assays.
Conclusions: Our research established a reciprocal causality between plasma metabolites and ADs. Furthermore, diverse metabolic pathways correlated with ADs were uncovered. Novel insights into the prediction and diagnosis were provided, as well as new targets for precise treatment of these conditions were discovered.
Autoimmune diseases (ADs) are a group of disorders in which the immune system mistakenly targets and destroys healthy tissues and organs within the body. This can result in inflammation, tissue damage, and dysfunction of multiple organ systems (1). The number of people affected by autoimmune diseases is approximately 10% of the population worldwide and is expected to continue to increase globally (2). Autoimmune diseases frequently impact multiple organs within the body, posing significant challenges and financial burdens in terms of treatment. This is undoubtedly a heavy pressure on both individuals and society (3). The etiology of ADs is believed to involve an intricate combination of hereditary, external, hormonal, mental stress and immune factors (4). However, the exact causes of autoimmune diseases are currently not understood completely.
Plasma encompasses a spectrum of small molecule metabolites that frequently engage in diverse biological processes and contribute to the pathogenesis of various diseases. The application of these metabolites in the diagnosis of human diseases is increasingly recognized and is a focal point of contemporary medical research (5, 6). Plasma metabolites play a significant role in their native state. Valine, as an example, is an essential amino acid in its natural state, playing a crucial role in protein synthesis (7). Homocitrulline, as another example, is a derivative of an amino acid that facilitates in the cellular removal of excess ammonia, thereby maintaining ammonia homeostasis (8). Although the pathogenesis of ADs is still unclear, in recent years, more and more evidence has shown that metabolic abnormalities are closely related to the development of ADs. For example, a metabolomics study on systemic lupus erythematosus (SLE) found a significant decrease in many serum metabolites including valine (9). Similarly, in another study on amino acid analysis in patients with inflammatory bowel disease (IBD), significant changes in valine levels were also observed (10). At the same time, previous studies have demonstrated significant variations of plasma homocitrulline level in Type 1 diabetes (T1D) patients with various symptoms (11). These previous studies have suggested that there is some correlation between metabolites and ADs, and even that metabolite levels may be related to the severity of disease symptoms. Nevertheless, due to the presence of numerous confounding factors, it is hard to determine whether the potential causal relationship exist between plasma metabolites and ADs through traditional cross-sectional studies.
With the development of high-throughput technology, it has become feasible to simultaneously evaluate a vast number of plasma metabolites. Metabolomics is progressively assuming a crucial role in the investigation of disease occurrence and progression in ADs (12). Genome-wide association study (GWAS) greatly facilitates the comprehensive investigation of the underlying genetic factors contributing to complex diseases. Mendelian randomization (MR), a statistical method, provides confounder-free estimates effectively. It employs genetic variation as an unbiased instrumental variable (IV) to study the casual relationship between exposure and outcome (13). MR has been proved to be a powerful statistical approach, as the outcomes are less susceptible to be affected by unknown confounding variables or reverse causality (14). MR analysis has been extensively utilized in diverse situations, encompassing some studies related to plasma metabolites or ADs (15, 16).
In this study, we conducted a comprehensive two-sample bidirectional MR analysis to (1) assess the causality of human plasma metabolites on 5 ADs, including IBD, multiple sclerosis (MS), T1D, SLE and rheumatoid arthritis (RA). (2) reversely determine the causal relationship of 5 ADs on plasma metabolites. (3) explore potential metabolic pathways that may contribute to elucidate the mechanisms of ADs.
We conducted a comprehensive evaluation of the potential causal relationship between human plasma metabolites and the likelihood of ADs using a bidirectional two-sample MR design. A well-executed MR study should adhere to three fundamental assumptions: (1) instrumental variable assumption—the genetic variation should exhibit a direct association with exposure; (2) independence assumption—the genetic variant is randomly assigned and is independent of any confounding factors that may affect the outcome; (3) exclusion restriction assumption—the genetic variation have no direct effect on the outcome, and affect the outcome variable only through the effect of the exposure variable. To ensure data integrity, we acquired individual GWAS datasets for plasma metabolites and ADs, thereby avoiding overlap of samples. The summary of this study was depicted in Figure 1. The overview of the research workflow.
The genetic statistics for plasma metabolites come from two different GWAS data: One is from a German Chronic Kidney Disease (referred to as GCKD below) involving a cohort of 5023 German individuals, and summary statistics for study GCST90264176-GCST90266872 (17) were obtained from the NHGRI-EBI GWAS Catalog (18) on 05/06/2023. We only selected plasma metabolites for further study and urine metabolites were excluded. The other is from a Canadian Longitudinal Study of Aging (referred to as CLSA below) including 8,299 Canadian individuals, and summary statistics for study GCST90199621-GCST90200711 (19) were also acquired from the NHGRI-EBI GWAS Catalog (18) on 05/06/2023. These are the two large-scale GWAS data published to date for plasma metabolites which were published in 2023 and they shared the same platform called Metabolon HD4 thus perfectly increasing the data consistency. We meta-analyzed summary statistics from GCKD and CLSA cohorts using METAL software (20).
During the discovery phase, summary statistics for each of the 5 ADs were obtained from the NHGRI-EBI GWAS Catalog: GCST004131 (25,042 IBD cases and 34,915 controls) (21), GCST90132223 (22,350 RA cases and 74,823 controls) (22), GCST003156 (5,201 SLE cases and 9,066 controls) (23) and GCST010681 (9,266 T1D cases and 15,574 controls) (24). In order to facilitate correspondence with diseases, these GWAS datasets were named as followed: IBD_GCST004131, RA_GCST90132223, SLE_GCST003156 and T1D_GCST010681. Data for MS was obtained from the IEU Open GWAS Project, so we called MS_IEU (47,429 MS cases and 68,374 controls) below (25).
For the replication phase, the FinnGen study provided the replication outcome samples for IBD, RA, T1D, and MS. To distinguish these GWAS datasets, we have all prefixed them with the name of the disease: IBD_Finngen (8,704 IBD cases and 300,450 controls), RA_Finngen (12,555 RA cases and 240,862 controls), T1D_Finngen (8,671 T1D cases and 255,466 controls) and MS_Finngen (2,182 MS cases and 373,987 controls) (26). The samples for SLE were obtained from the NHGRI-EBI GWAS Catalog: GCST90014462 (624 SLE cases and 324,074 controls), then we called it SLE _GCST90014462 below (27).
Initially, we identified single nucleotide polymorphisms (SNPs) for each plasma metabolite, which met the association threshold of P < 5 × 10–6 (28–30). The commonly employed threshold for genome-wide significance, P < 5 × 10–8 is applied to select genetic instruments. However, imposing such conditions will result in a significant reduction in the quantity of metabolites that can be analyzed. To obtain a more comprehensive evaluation, we relaxed the threshold to P < 5 × 10–6. In reverse, as a standard practice, SNPs were selected with association thresholds at P < 5 × 10–8 for each AD analyzed. Next, link-age disequilibrium between the SNPs was calculated, and we only retained those SNPs with a r2 < 0.001 (clumping window size = 10,000 kb). In addition, SNPs with minor allele frequency (MAF) ≤ 0.01, SNPs with alleles that form a palindrome and have intermediate allele frequencies and SNPs with F-statistics ≤ 10 were all excluded. Then, we removed SNPs associated with disease and potential confounding factors, which were checked in the Phenoscanner of R software (31, 32). Finally, SNPs that exhibited a stronger correlation with the outcome rather than with the exposure were removed based on the Steiger Test (33).
In our MR analyses, the standard inverse variance weighted (IVW) method, Simple mode test, MR Egger test, Weighted mode test and Weighted-median method were employed to evaluate the causal relationship between plasma metabolites and ADs, and IVW method is the primary and most significant approach among them. Subsequently, we carried out diverse sensitivity analyses simultaneously to ensure the reliability and robustness of the results: (1) Cochran’s Q was calculated to assess heterogeneity among the individual causal correlation, where pleiotropy is considered present when P < 0.05. (2) MR-Egger and MR-PRESSO regression tests were applied to examine the potential impact of horizontal pleiotropy. In both methods, we delete the data with a P < 0.05.
Statistical analyses were conducted using R software (version 4.3.1). Forest plot was performed using package “forestploter” (version 1.1.0) (https://CRAN.R-project.org/package=forestploter). We conducted MR analyses using the “TwoSampleMR” package (version 0.5.6) (34). We used “ieugwasr” package (version 0.1.5) (https://github.com/MRCIEU/ieugwasr) to analyze linkage disequilibrium.
Metabolic pathways were investigated using the web version of MetaboAnalyst 5.0. (https://www.metaboanalyst.ca/) (35). To identify potential metabolite groups or pathways that may be associated with ADs’ biological process, pathway analyses modules and functional enrichment analyses were employed. In this study, the Kyoto Encyclopedia of Genes and Genomes (KEGG) database was utilized, and a significance level of 0.05 was applied for pathway analyses.
This study has been approved by the Ethics Committee of The First Affiliated Hospital of Xiamen University (Xiamen, China; approval number: XMYY-2022KY121). Informed consent was obtained from all patients. Plasma samples were collected from a control group of 12 patients without ADs who had internal fixation removed (6 males, 6 females), as well as two groups of patients who underwent joint replacement surgery: one group with 12 patients with RA (4 males, 8 females), and another group with 12 patients with SLE (3 males, 9 females).
Glutamate concentrations were quantified in human plasma according to manufacturer’s instructions (Glutamate ELISA kit KA1909, Novus Biologicals).
Experimental data analysis was conducted using SPSS v29.0 (SPSS Inc.) or GraphPad Prism v10.0.2 (GraphPad Inc.). Data were tested for normality and equal variances, followed by 2-sample, 2-tailed t tests or 3-way ANOVA with Dunnett’s post hoc tests. P < 0.05 was considered significant. Mean ± SD or box plots representing interquartile range, median, and all data points are presented in the figures.
Our workflow is summarized in Figure 1. The genetic statistics for plasma metabolites come from two different GWAS data, each with 1,296 and 1,091 metabolites, respectively. 1,009 metabolites were obtained after intersection using METAL software. To investigate the impact of metabolites on diseases, we selected SNPs as IVs after a set of quality assurance procedures. To be specific, in the discovery stage, for IBD, 683 SNPs (P < 5.0 × 10−6, r2 < 0.001) involved with 64 metabolites were extracted from IBD_GCST004131. In the case of RA, 1,489 SNPs associated with 97 metabolites were selected from RA_GCST90132223. For SLE, 540 SNPS were selected from SLE_GCST003156, covering 55 metabolites. Regarding T1D, a total of 933 SNPs were chosen from T1D_GCST010681, encompassing 69 metabolites. Lastly, in the case of MS, 357 SNPs related to 47 metabolites were chosen from the MS_IEU study. Then, in the replication stage, the same screening criteria for selecting IVs are applied (Supplementary Tables S1–S10).
At the same time, we also wanted to explore the effects of autoimmune diseases on plasma metabolites, so a reverse MR analysis was performed. Among the SNPs associated with each genus, those that reached the locus-wide significance threshold (P < 5.0 × 10−8) were selected as potential IVs (Supplementary Tables S11–S20). Statistically, all F-statistics for the validity test were all above the standard threshold of 10, indicating a strong genetic instrument.
Next, we employed five methodologies, respectively, IVW, MR Egger, Weighted median, Weighted mode, and Simple mode, to assess the causal associations of plasma metabolites on ADs as shown in Figure 2 and Supplementary Figure S1. During the discovery phase, 23 diverse metabolites were preliminarily identified by IVW method to have significant causal relationship with these autoimmune diseases, including 3 unknown components and the remaining 20 known metabolites involved in 4 metabolic pathways. Specifically, they were as follows:
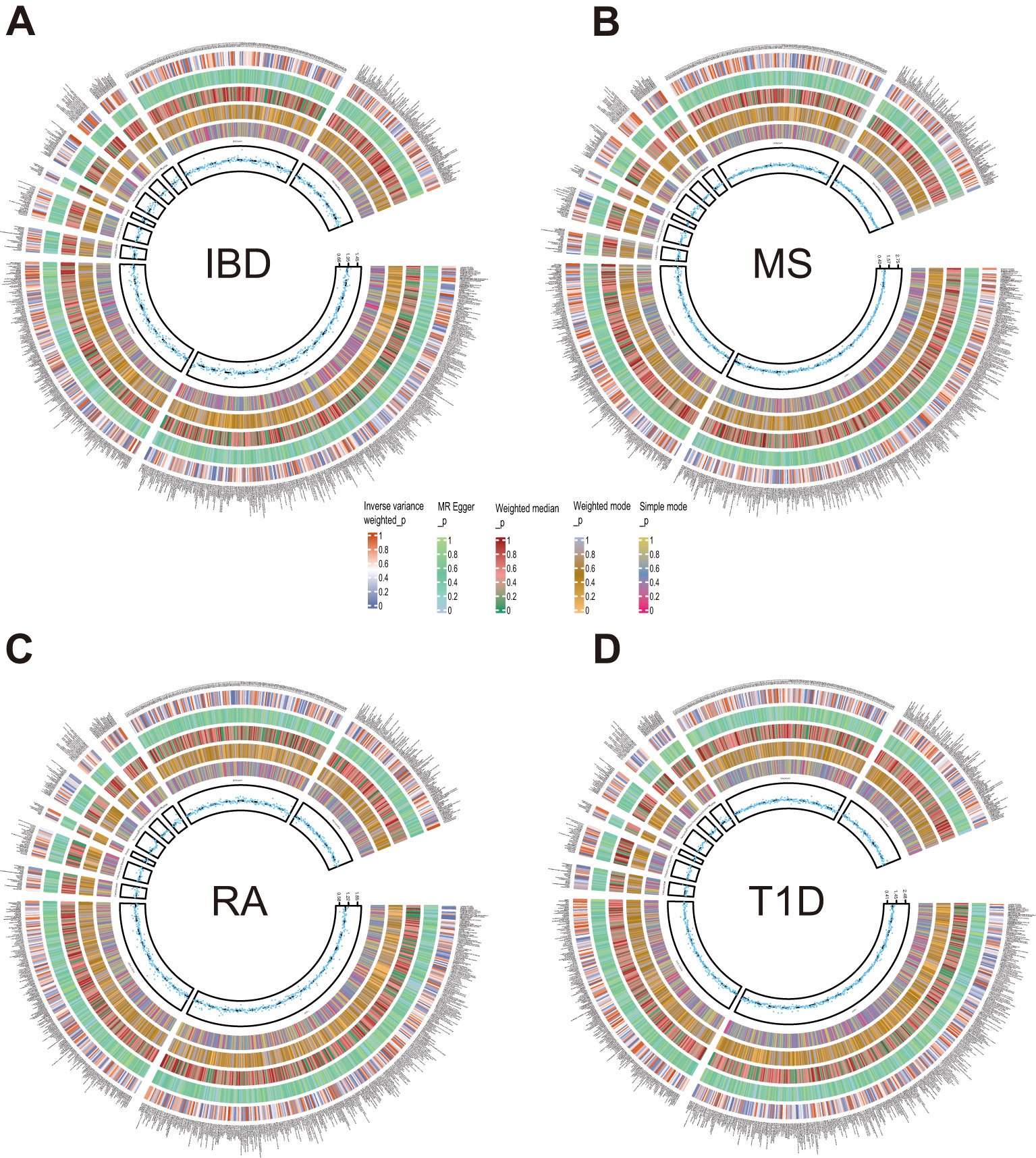
Figure 2. Circle diagrams of the discovery sample in the forward MR analysis. The complete results of the forward MR analysis, showing the causal effects of plasma metabolites on ADs. (A) IBD, (B) MS, (C) RA, (D) T1D. Five statistical methods, respectively, IVW, MR Egger, Weighted median, Weighted mode, and Simple mode, are represented by five circles from inner to outer.
4 metabolites from 2 pathways for IBD: 2-hydroxy-4-(methylthio)butanoic acid (odds ratio (OR) = 1.095; 95% confidence interval (95% CI) 1.025-1.171; P = 0.008), sphingomyelin (d18:2/16:0, d18:1/16:1)* (OR = 0.704; 95% CI 0.557-0.890; P = 0.003), sphingosine (OR = 0.886; 95% CI 0.801-0.979; P = 0.018), X-12839 (OR = 1.161; 95% CI 1.049-1.285; P = 0.004).
2 metabolites from 2 pathways for MS: N-acetylcitrulline (OR = 1.526; 95% CI 1.183-1.968; P = 0.001), threonate (OR = 0.757; 95% CI 0.589-0.972; P = 0.029).
13 metabolites from 2 pathways for RA: 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPC (P-16:0/20:4)* (OR = 1.160; 95% CI 1.066-1.262; P = 0.001), 1-arachidonoyl-GPC (20:4n6)* (OR = 1.184; 95% CI 1.050-1.335; P = 0.006), 1-oleoyl-2-linoleoyl-GPE (18:1/18:2)* (OR = 0.900; 95% CI 0.845-0.958; P = 0.001), 1-palmitoleoyl-2-linolenoyl-GPC (16:1/18:3)* (OR = 0.885; 95% CI 0.797-0.982; P = 0.021), 1-palmitoyl-2-linoleoyl-GPC (16:0/18:2) (OR = 0.615; 95% CI 0.423-0.895, P = 0.011), 1-palmitoyl-2-linoleoyl-GPE (16:0/18:2) (OR = 0.898; 95% CI 0.838-0.961; P = 0.002), 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4) (OR = 1.317; 95% CI 1.172-1.480; P = 0.000), 1-stearoyl-2-linoleoyl-GPE (18:0/18:2)* (OR = 0.897; 95% CI 0.816-0.986; P = 0.024), 1-stearoyl-2-oleoyl-GPE (18:0/18:1) (OR = 0.893; 95% CI 0.798-1.000; P = 0.050), 5alpha-androstan-3beta,17beta-diol monosulfate (2) (OR = 0.890; 95% CI 0.813-0.974; P = 0.011), androsterone sulfate (OR = 0.937; 95% CI 0.891-0.985; P = 0.011), metabolonic lactone sulfate (OR = 0.946; 95% CI 0.905-0.989; P = 0.014), X-26109 (OR = 0.923; 95% CI 0.881-0.967; P = 0.001).
4 metabolites from 2 pathways for T1D: beta-hydroxyisovaleroylcarnitine (OR = 0.709; 95% CI 0.531-0.946; P = 0.020), 1-palmitoyl-2-dihomo-linolenoyl-GPC (16:0/20:3n3 or 6)* (OR = 0.622; 95% CI 0.427-0.905; P = 0.013), glycohyocholate (OR = 1.236; 95% CI 1.045-1.463; P = 0.014), X-21310 (OR = 0.653; 95% CI 0.516-0.825; P = 0.000).
These causal relationships were further supported by the replication samples, as depicted in Figure 3. Two samples were used to enhance the confidence of causal association. We only established a causal relationship between a metabolite and an AD when the metabolite was identified as a protective factor or a risk factor simultaneously by discovery and replication sets, and both sets of results have statistical significance (P < 0.05). The graphs showing the intersection of discovery and replication samples in the forward analysis are listed in Supplementary Figure S3.
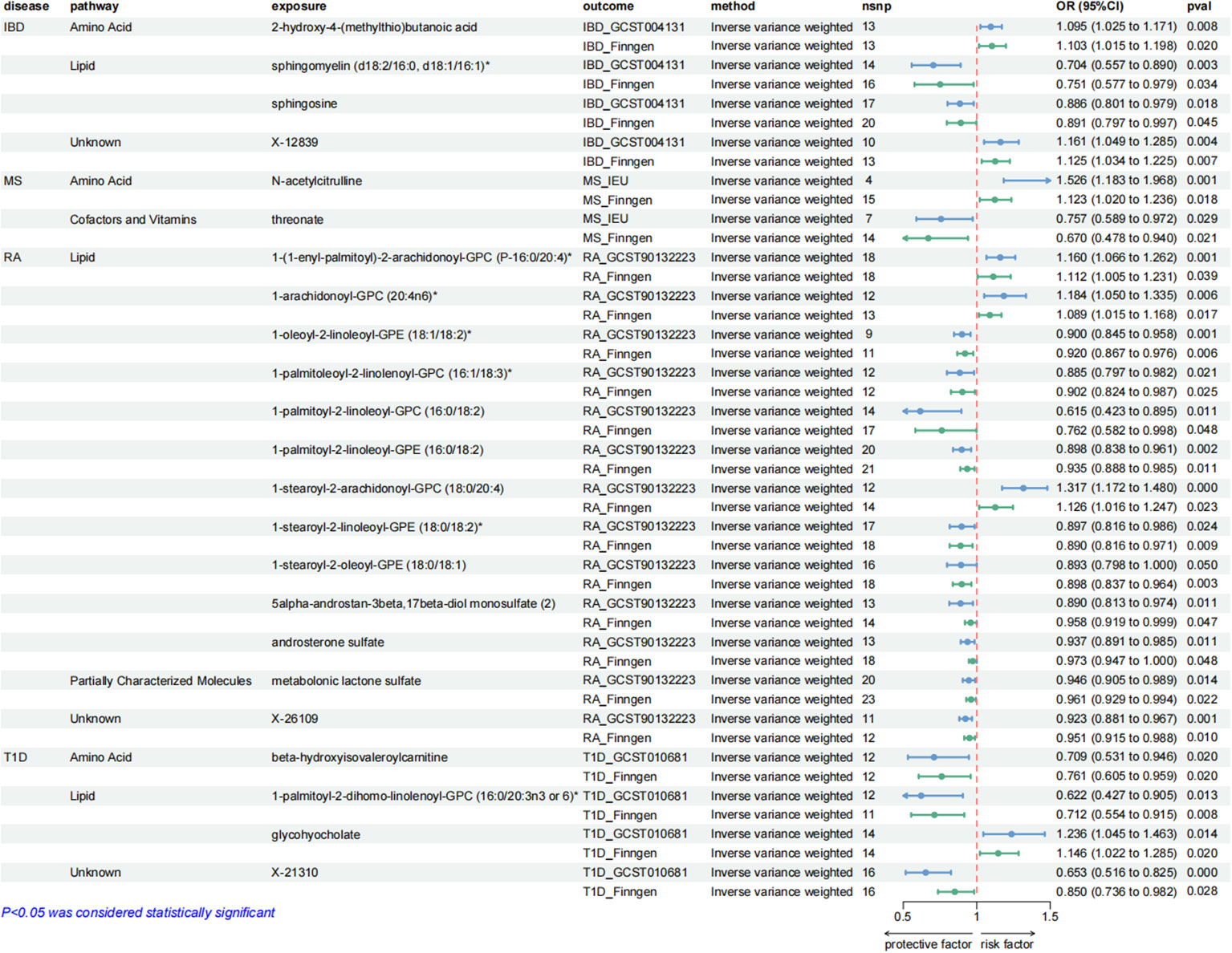
Figure 3. Forest plots showing the causal relationships between plasma metabolites and ADs outcomes. All causal relationships were derived from the fixed-effect IVW analysis and can be supported by both discovery and replication samples.
After exploring the causal effects of autoimmune diseases on plasma metabolites, we further conducted a reverse MR analysis, as depicted in Figure 4 and Supplementary Figure S2. According to the preliminary identification by IVW, IBD had a causal relationship with 4 metabolites, 2 of which were from the amino acid metabolic pathway and 1 was from the lipid metabolic pathway.
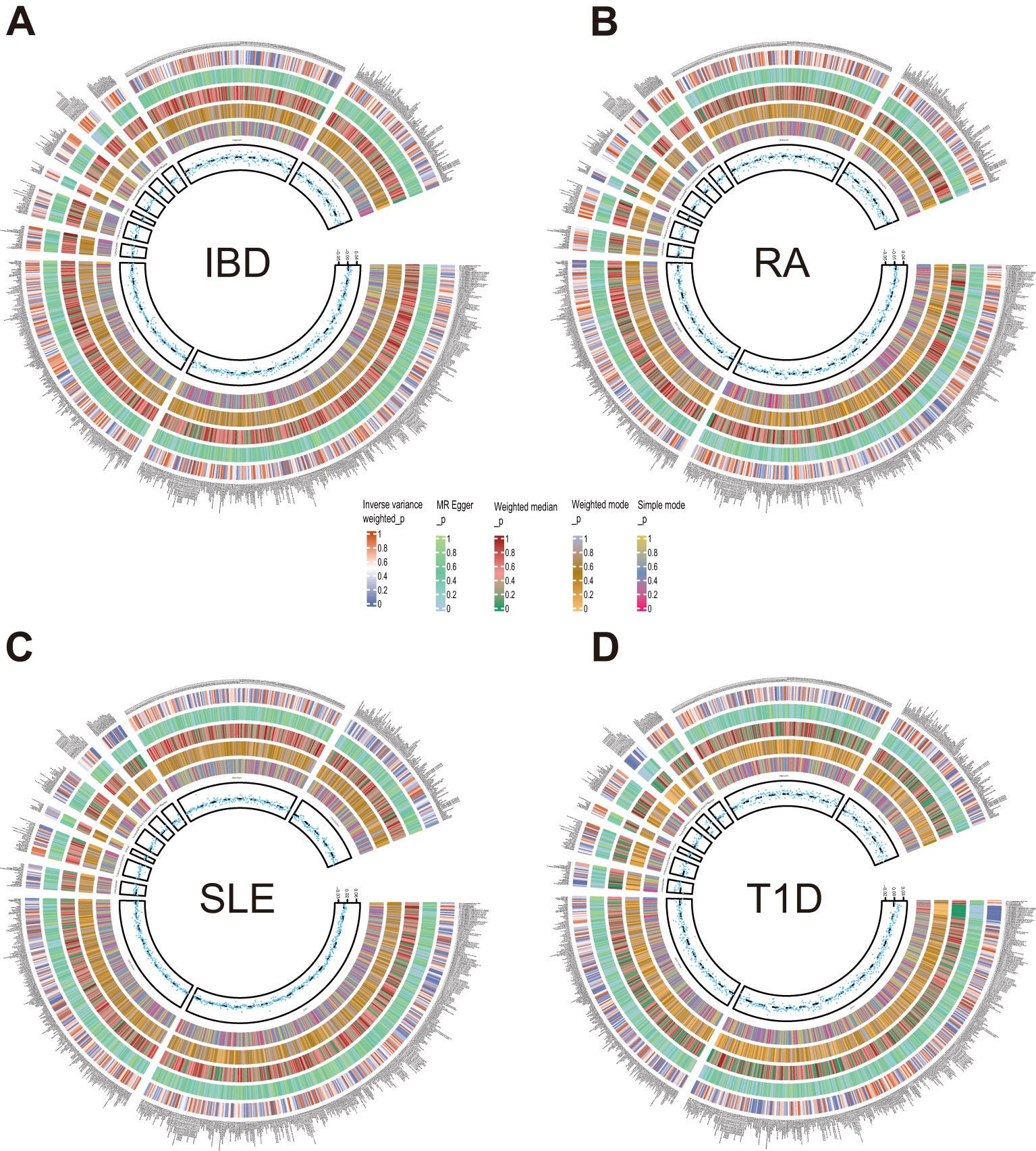
Figure 4. Circle diagrams of the discovery sample in the reverse MR analysis. The complete results of the reverse MR analysis, showing the causal effects of ADs on plasma metabolites. (A) IBD, (B) RA, (C) SLE, (D) T1D. Five statistical methods, respectively, IVW, MR Egger, Weighted median, Weighted mode, and Simple mode, are represented by five circles from inner to outer.
Furthermore, our analysis also demonstrated a significant causal correlation between RA and 22 metabolites. These metabolites encompassed 8 from the amino acid pathways, 8 from the lipid metabolism pathways, 1 from the energy pathways, and 1 from the xenobiotic pathways.
Similarly, SLE was found to have a causal link with 22 metabolites. These metabolites compromised 7 from the lipid metabolism pathways, 4 metabolites from the amino acid pathways, 3 from the nucleotide pathways, 3 from the xenobiotic pathways, 2 from the carbohydrate pathways and 1 from the peptide pathways.
Additionally, a causal relationship between 37 plasma metabolites and T1D was identified. Among them, 22 were from the lipid metabolism pathways, 10 were from the amino acid pathways, 2 were from the partially characterized molecules pathways, 2 were from the xenobiotic pathways and 1 was from the carbohydrate pathways. Figures 5–7.
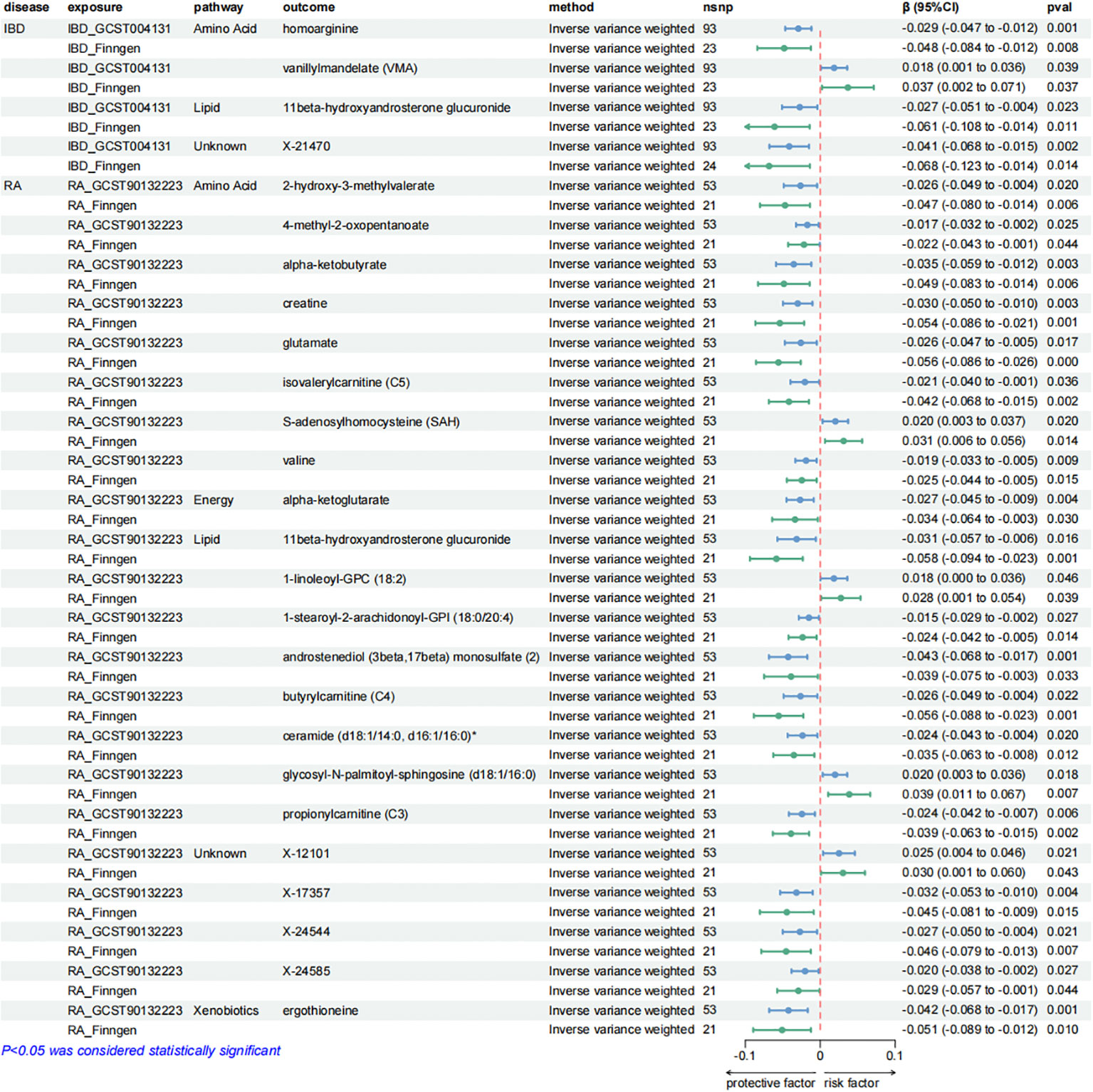
Figure 5. Forest plots presenting the results of reverse MR analysis (1). Causal effects of IBD and RA on plasma metabolites using IVW method, which were supported by both discovery and replication samples.
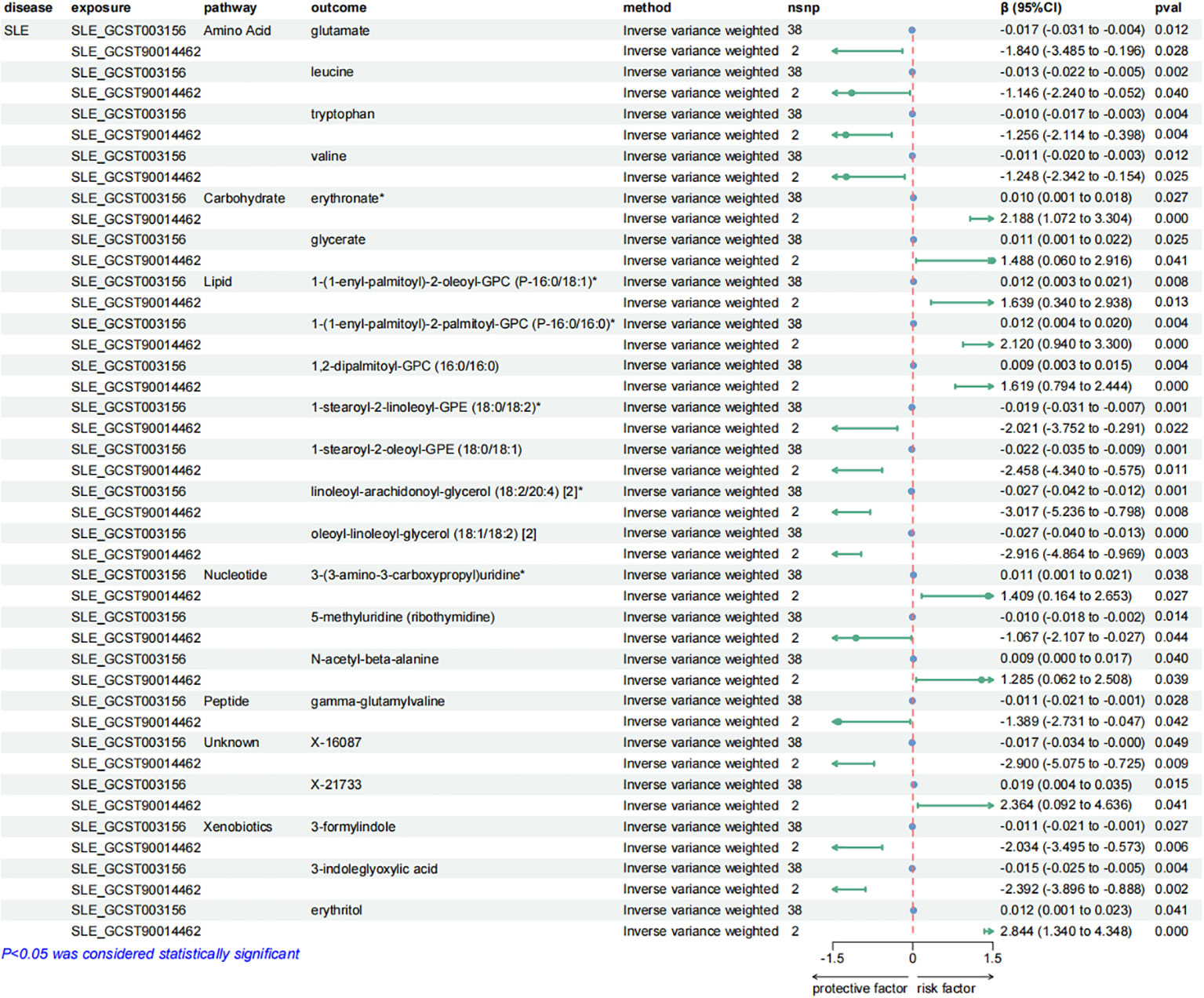
Figure 6. Forest plots presenting the results of reverse MR analysis (2). Causal effects of SLE on plasma metabolites using IVW method, which were supported by both discovery and replication samples.
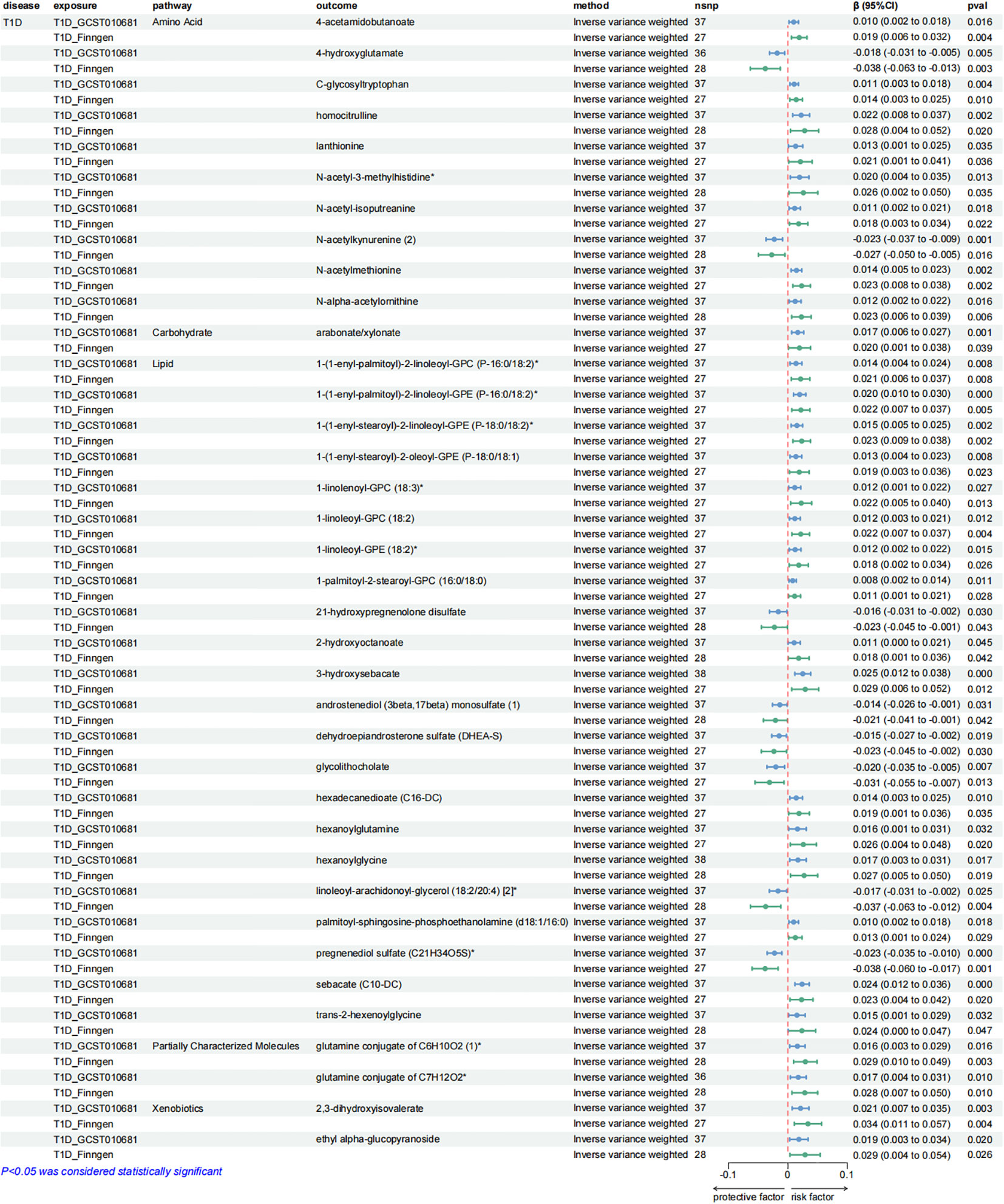
Figure 7. Forest plots presenting the results of reverse MR analysis (3). Causal effects of T1D on plasma metabolites using IVW method, which were supported by both discovery and replication samples.
Consistent with the preceding, as was the case with forward MR analysis, these findings were supported by replication samples. The graphs displaying the intersection of discovery and replication samples in the reverse analysis are listed in Supplementary Figure S4.
A series of sensitivity analyses were conducted to minimize the impact of horizontal pleiotropy on the MR estimate as possible, aiming to obtain more robust results. We utilized MR-Egger and MR-PRESSO regression tests to examine the potential horizontal pleiotropy impact. Through screening, samples with P < 0.05 in both methods were deleted to provide more reliable results. Horizontal pleiotropy was not found in all relevant metabolites applied in Figures 3, 5–7.
At the same time, in order to quantify the heterogeneity among the individual causation effects Cochran’s Q was calculated. Through this test, in the forward MR analysis, Cochran’ Q-derived P values indicated the absence of detected heterogeneity in the causal relationships 11 metabolites with RA, 3 metabolites with T1D and all metabolites with IBD and MS (Supplementary Table S21-S28). At the same time, in the reverse MR analysis, no heterogeneity was detected in the causal association in 21 metabolites with SLE, 36 metabolites with T1D and all metabolites with IBD and RA (Supplementary Tables S29–36).
In the forward MR analysis, metabolic pathway analysis revealed that, only in RA, 4 significant metabolic pathways were found, as shown in Figure 8A. Our results show that the “Glycosylphosphatidylinositol (GPI)-anchor biosynthesis”, “alpha-Linolenic acid metabolism”, “Linoleic acid metabolism”, and “Glycerophospholipid metabolism” pathways were found to be involved in the pathogenetic process of RA (Supplementary Table S37).
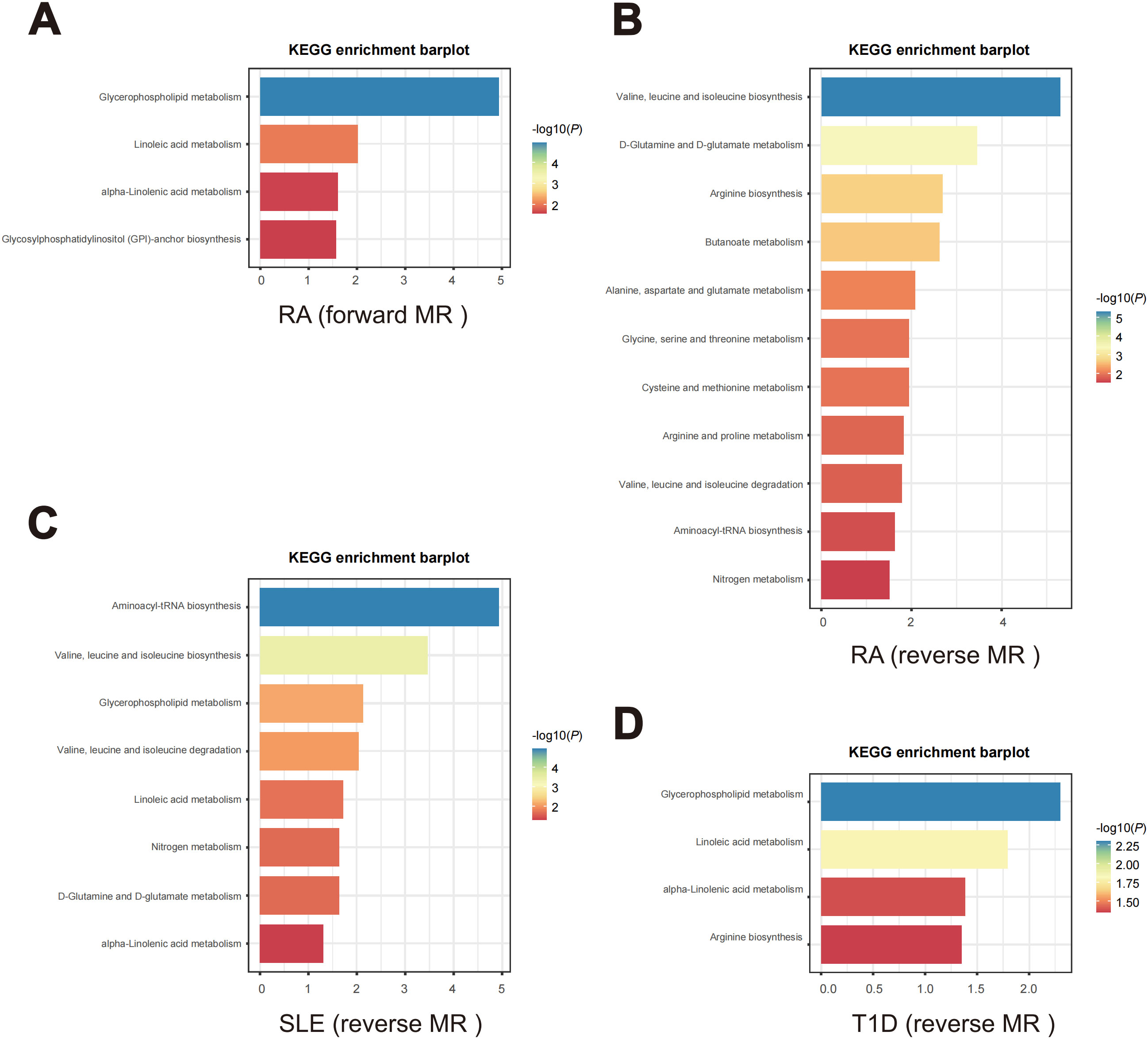
Figure 8. Enriched key metabolic pathways associated with ADs. (A) Metabolic pathways in RA identified through the forward MR analysis. (B-D) Metabolic pathways in the reverse MR analysis for different ADs. (B) RA, (C) SLE, (D) T1D.
In the reverse MR analysis, as shown in Figures 8B–D, 14 significant metabolic pathways involved 3 ADs were identified (Supplementary Tables S38–S40). Specifically, “Arginine biosynthesis”, “Aminoacyl-tRNA biosynthesis”, “Arginine and proline metabolism”, “Alanine, aspartate and glutamate metabolism”, “Butanoate metabolism”, “Cysteine and methionine metabolism”, “D-Glutamine and D-glutamate metabolism”, “Glycine, serine and threonine metabolism”, “Nitrogen metabolism pathways”, “Valine, leucine and isoleucine biosynthesis” and “Valine, leucine and isoleucine degradation” were noticed to be involved in the pathogenetic process of RA. Meanwhile, SLE is observed to be associated with the “Aminoacyl-tRNA biosynthesis”, “alpha-Linolenic acid metabolism”, “D-Glutamine and D-glutamate metabolism”, “Glycerophospholipid metabolism”, “Linoleic acid metabolism”, “Nitrogen metabolism”, “Valine, leucine and isoleucine degradation” and “Valine, leucine and isoleucine biosynthesis” pathways. And T1D is associated with the “alpha-Linolenic acid metabolism”, “Arginine biosynthesis”, “Glycerophospholipid metabolism” and “Linoleic acid metabolism” pathways.
To validate our findings, we experimentally confirmed the role of glutamate, identified as a protective factor in both SLE and RA from our previous results (Figures 5, 6). In previous studies, glutamate has been identified as a pivotal excitatory neurotransmitter in the central nervous system, essential for cognitive function, memory, mood regulation, stress response, and immune modulation via its anti-inflammatory properties and involvement in glutathione synthesis (36–39). Using an ELISA kit, we found that the glutamate concentration in the control group (healthy individuals) was the highest at 57.18 ± 13.54 µM, significantly higher than those in the RA group (39.70 ± 10.95 µM, P < 0.01, n=12) and the SLE group (43.52 ± 9.321 µM, P < 0.05, n=12) (Figure 9A). Additionally, no significant differences in glutamate concentrations were observed between male and female plasma (Figure 9B). The consistency of these experimental results with our MR data corroborates the reliability and robustness of our findings.
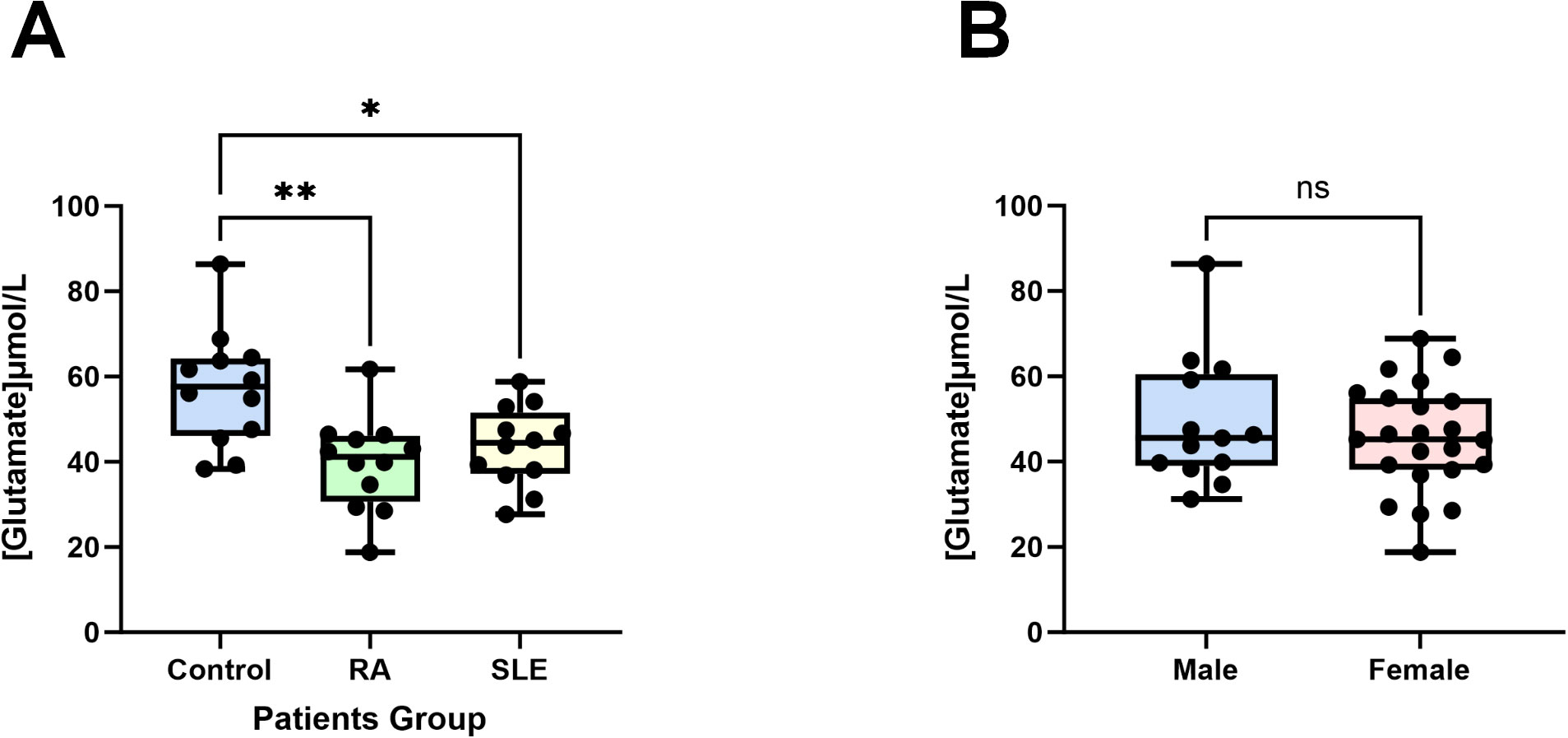
Figure 9. Glutamate concentrations in patients’ plasma. (A) Compared to the control group (healthy individuals, n = 12), the plasma glutamate concentrations in patients with RA (n = 12) and SLE (n = 12) were significantly reduced. (B) There are no differences in male (n = 13) and female (n = 23) plasma glutamate concentrations. Data are presented as the mean ± SD; ns: not significant, *P < 0.05, **P < 0.01.
During this study, a bidirectional two-sample MR analysis was performed to assess the reciprocal causal correlation between plasma metabolites and 5 prevalent ADs (IBD, MS, T1D, SLE and RA). Furthermore, the results of pleiotropy at the gene level were excluded by sensitivity analysis, rendering the results of the study more robust and reliable. Through the Combination of evidence from both discovery and replication samples, we identified 4 metabolites that have causal relationships with IBD, 2 with MS, 13 with RA, and 4 with T1D. In the reverse MR study, we recognized causal relationships between SLE and 22 metabolites, IBD and 4 metabolites, RA and 22 metabolites, and T1D and 37 metabolites.
For the last few years, ADs have been increasingly acknowledged as a metabolism-related disease and changes in metabolites related to various ADs have been continuously discovered through metabolomics (12, 40–43). However, the existing studies have not been able to clarify the specific relationship between metabolites and ADs. Our research not only confirmed the presence of specific plasma metabolites causally related to ADs but also identified key metabolic pathways of ADs. The finding offered new perspectives into the prediction and diagnosis of ADs, along with providing potential targets for precision treatment.
In the results of the study, we first focused on the causality between lipid metabolites and ADs. Our research results have shown that there is a causal relationship between lipid-related metabolites and every type of ADs. In our study, sphingosine was found to be a protective factor for IBD. Sphingosine can generate S1P through a phosphorylation reaction, which is a bioactive lipid molecule (44). Several studies have shown that sphingosine-1-phosphate (S1P) has a regulatory effect in IBD, and selective S1P agonists have anti-inflammatory properties, which also indirectly validated our study (45, 46). These findings suggested novel targets for IBD therapy, and our study provided further support for this notion, highlighting the crucial role of sphingosine as a protective factor in the management of IBD progression.
In addition, our study indicated that 11 plasma metabolites of the lipid metabolism pathway participated in the pathogenesis of RA. In another study of inflammatory bowel disease serum metabolome, three metabolites overlapped with ours. Contrary to their study, we believed that 1-oleoyl-2-linoleoyl-GPE (18:1/18:2)* and 1-stearoyl-2-linoleoyl-GPE (18:0/18:2)* are protective factors for RA, but they confirmed that they are risk factors for Crohn’s disease. Interestingly, as revealed in our study, 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4) was considered to be a risk factor for RA while they indicated it would be a protective factor for Crohn’s disease (47). Although the causality between these metabolites and different diseases is not completely consistent, it is jointly confirmed that these metabolites do have a causal connection with the occurrence and development of autoimmune diseases.
Our results also found a causal relationship between alterations in other metabolites involved in lipid metabolism and ADs, for example, for T1D, glycohyocholate is a risk factor. In multiple metabolomic studies related to asthma, it has also been observed that there are significant differences in levels of glycohyocholate between diseased children and the control group (48, 49). In a study on nonalcoholic fatty liver disease, after all confounding factors are excluded, the proportion of glycohyocholate remains significantly correlated with the presence of nonalcoholic fatty liver disease (50). All these pieces of evidence collectively indicated the causal link between abnormal levels of glycohyocholate and the occurrence of autoimmune-related diseases.
Undoubtedly, lipid metabolism imbalance plays a crucial role in the self-immune response, although the specific mechanisms by which lipid metabolism imbalance leads to inflammatory reactions and aberrant activation of immune system remain unclear (51–53). Based on these known evidences and our findings, these plasma metabolites may hold therapeutic targets for the treatment of ADs and promising research direction.
At the same time, not only in lipid metabolism, changes in other metabolites belonging to other pathways such as metabolites from cofactors and vitamin metabolism pathway are also causally related to ADs. According to our research, threonate is a protective factor for MS. As a chronic autoimmune disease, MS mainly impacts the central nervous system, which includes brain and spinal cord (54). In previous studies, threonate was often used as an important component of L-Threonic acid Magnesium salt, which was crucial in the treatment of orthopedic diseases and Alzheimer’s disease (55–57). Research findings point that threonine is a unique molecule that effectively regulates the structural and synaptic architecture and function of the central nervous system (58). According to the research, supplemental intake of threonate may help improve neurodegenerative conditions and cognitive function in MS patients. Correspondingly, threonate also has antioxidative and anti-inflammatory properties, which may potentially benefit the prevention and treatment of MS.
Furthermore, pathway analyses revealed diverse metabolic pathways related to autoimmune responses. Our results have shown that metabolic pathways involved in autoimmune diseases are often not singular, proving that an AD is often associated with multiple metabolisms. Furthermore, we have observed an overlap of metabolic pathways among various autoimmune diseases, indicating a tendency for the metabolic pathways involved in ADs to intersect. According to our research, “Glycerophospholipid metabolism”, “alpha-Linolenic acid metabolism” and “Linoleic acid metabolism” pathways were all involved in the pathogenetic process of RA, SLE and T1D. Interestingly, all of these metabolic pathways are interconnected with lipid metabolism. Lipid metabolites, as elucidated above, exert a pivotal influence in the etiology and progression of autoimmunity disorders. In a study based on multi-omics analysis, glycerophospholipid metabolism was revealed to play a key role in the occurrence and development of RA (59). Similarly, in another UPLC-MS/MS-based plasma lipidomics study, glycerophospholipid metabolism of lipid metabolites was demonstrated to be upregulated in SLE patients, further supporting our findings (60). Meanwhile, the previous study has reported the amelioration of ω-3 polyunsaturated fatty acids on T1D (61). Alpha-Linolenic acid, as a type of ω-3 polyunsaturated fatty acids, aligns with our findings, providing further evidence that this metabolic pathway plays a significant role in ADs. Equally, through another MR Study, the researchers demonstrated that linoleic acid exerts a protective function in the development of RA and SLE (62). Human body can consume these lipids from common food, so these metabolism researches provided dietary guidelines for the prevention of ADs. The opportunity for individuals to prevent ADs to some extent through the moderate intake of lipids in the daily dietary makes this discovery highly significant. Combined with previous perceptions, we speculated that it may be due to the occurrence of metabolic pathway disorders, especially lipid related metabolism disorders, leading to metabolic changes and immune dysfunction (63–66). Whereas, it also suggests that we can get new directions for prevention and treatment of ADs through the intervention of these metabolic pathways and crucial metabolites in the pathways.
At the level of genetic variation, identifying the causal effects of metabolites and metabolic pathways on ADs reveals potential targets for treatment or intervention. Subsequent validation in cellular and animal models, such as gene overexpression or knockdown experiments, along with metabolite intervention studies, can further confirm these causal relationships. Based on these findings, the development of drugs targeting specific metabolites or pathways can be pursued, potentially involving small molecule compounds or biologics to modulate the activity of these metabolites. On the other hand, understanding the causal influence of disease on metabolites enables the proactive prediction of individuals at risk of developing autoimmune conditions. Further development of risk scoring systems or the assessment of representative biomarkers can be employed to achieve this. According to the results of these risk predictions, dietary advice or pharmacological interventions can be provided to high-risk individuals to delay or prevent the onset of ADs. Of course, the efficacy and safety of these interventions need to be tested in human subjects through randomized controlled trials and other clinical study designs. After clinical application, long-term monitoring of high-risk individuals can be conducted to assess the onset and the long-term effects of intervention measures in real-time.
There were several strengths to this MR study. First, the most up-to-date and extensive databases of metabolites were selected. Meta-analysis of the two groups of data was conducted employing METAL software, resulting in 1,009 metabolites obtained through intersection (20). Meanwhile, the whole-genome data of 5 common ADs were acquired from 10 GWAS datasets, with each AD consisting of discovery and validation sets. This enabled us to conduct a comprehensive and systematic analysis of the metabolic profiles associated with the development of ADs. Second, our MR design investigated the causal relationship between plasma metabolites and ADs from the perspective of forward and reverse respectively, analyzing both the influence of metabolites on ADs and the impact of ADs on metabolites, which are mutually causal. This design facilitates the study more comprehensive, while the extensive sensitivity analysis ensures the reliability and robustness of our inferences. Ultimately, we conducted ELISA assays using plasma samples from the patients. The findings from these assays were in concordance with the results of the MR analysis, further substantiating the reliability of our analytical outcomes.
Our study still possessed certain limitations. Firstly, our study reported the causal relationship between ADs and metabolites, covering a relatively comprehensive metabolite spectrum, but the mechanism and function of many metabolites in diseases have not been fully elaborated, and require further investigation. Secondly, the data primarily originated from a European population, which limits the extrapolation of our results across ethnic groups. Thirdly, beyond the ELISA results, we did not explore gender disparities within our study. To address this gap, future research should incorporate a gender-based analysis and consider additional factors associated with gender, thereby enhancing our understanding of the mechanisms underlying ADs.
From the perspective of genetics, our systematic investigation revealed the causal relationship between plasma metabolites and diverse ADs, offering valuable insights into their etiology and underlying mechanisms. Furthermore, our findings provided potential inspiration for further accurate diagnosis and precision treatment strategies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XY: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. PY: Funding acquisition, Writing – review & editing. JH: Data curation, Formal Analysis, Software, Writing – original draft, Writing – review & editing. DC: Funding acquisition, Supervision, Writing – review & editing. BH: Funding acquisition, Project administration, Supervision, Writing – review & editing. GR: Funding acquisition, Supervision, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was sponsored by grants from National Natural Science Foundation of China (grant numbers: 82202749), Postdoctoral Science Foundation of China (grant numbers: 2022M722663), Natural Science Foundation of Fujian Province (grant numbers: 2022J05303) and Medical and Health Guidance Project of Xiamen City (grant number: 3502Z20224ZD1020 and 3502Z20224ZD1003).
We express our gratitude to the NHGRI-EBI for their release of the most recent GWAS summary statistics, as well as to the IEU Open GWAS Project for their contribution in making these data available. Additionally, we would like to extend our acknowledgement to the participants and investigators involved in the FinnGen study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1437688/full#supplementary-material
1. Rose NR. Prediction and prevention of autoimmune disease in the 21st century: A review and preview. Am J Epidemiol. (2016) 183:403–6. doi: 10.1093/aje/kwv292
2. Cao F, Liu YC, Ni QY, Chen Y, Wan CH, Liu SY, et al. Temporal trends in the prevalence of autoimmune diseases from 1990 to 2019. Autoimmun Rev. (2023) 22:103359. doi: 10.1016/j.autrev.2023.103359
3. Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol. (2023) 80:102266. doi: 10.1016/j.coi.2022.102266
4. Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. (2008) 7:209–13. doi: 10.1016/j.autrev.2007.11.007
5. Beckwith-Hall BM, Thompson NA, Nicholson JK, Lindon JC, Holmes E. A metabonomic investigation of hepatotoxicity using diffusion-edited 1H NMR spectroscopy of blood serum. Analyst. (2003) 128:814–8. doi: 10.1039/b302360p
6. Zhang A, Sun H, Wang X. Serum metabolomics as a novel diagnostic approach for disease: a systematic review. Anal Bioanal Chem. (2012) 404:1239–45. doi: 10.1007/s00216-012-6117-1
7. Savizi ISP, Maghsoudi N, Motamedian E, Lewis NE, Shojaosadati SA. Valine feeding reduces ammonia production through rearrangement of metabolic fluxes in central carbon metabolism of CHO cells. Appl Microbiol Biotechnol. (2022) 106:1113–26. doi: 10.1007/s00253-021-11755-4
8. Mao Y, Shi D, Li G, Jiang P. Citrulline depletion by ASS1 is required for proinflammatory macrophage activation and immune responses. Mol Cell. (2022) 82:527–41 e7. doi: 10.1016/j.molcel.2021.12.006
9. Ouyang X, Dai Y, Wen JL, Wang LX. (1)H NMR-based metabolomic study of metabolic profiling for systemic lupus erythematosus. Lupus. (2011) 20:1411–20. doi: 10.1177/0961203311418707
10. Jagt JZ, Struys EA, Ayada I, Bakkali A, Jansen EEW, Claesen J, et al. Fecal amino acid analysis in newly diagnosed pediatric inflammatory bowel disease: A multicenter case-control study. Inflammation Bowel Dis. (2022) 28:755–63. doi: 10.1093/ibd/izab256
11. Winther SA, Henriksen P, Vogt JK, Hansen TH, Ahonen L, Suvitaival T, et al. Gut microbiota profile and selected plasma metabolites in type 1 diabetes without and with stratification by albuminuria. Diabetologia. (2020) 63:2713–24. doi: 10.1007/s00125-020-05260-y
12. Kang J, Zhu L, Lu J, Zhang X. Application of metabolomics in autoimmune diseases: insight into biomarkers and pathology. J Neuroimmunol. (2015) 279:25–32. doi: 10.1016/j.jneuroim.2015.01.001
13. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomization studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
14. Elliott J, Bodinier B, Bond TA, Chadeau-Hyam M, Evangelou E, Moons KGM, et al. Predictive accuracy of a polygenic risk score-enhanced prediction model vs a clinical risk score for coronary artery disease. JAMA. (2020) 323:636–45. doi: 10.1001/jama.2019.22241
15. Gu Y, Jin Q, Hu J, Wang X, Yu W, Wang Z, et al. Causality of genetically determined metabolites and metabolic pathways on osteoarthritis: a two-sample mendelian randomization study. J Transl Med. (2023) 21:357. doi: 10.1186/s12967-023-04165-9
16. Yang H, Song J, Li A, Lv L, Sun X, Mao Y, et al. Genetically predicted levels of folate, vitamin B(12), and risk of autoimmune diseases: A Mendelian randomization study. Front Immunol. (2023) 14:1139799. doi: 10.3389/fimmu.2023.1139799
17. Schlosser P, Scherer N, Grundner-Culemann F, Monteiro-Martins S, Haug S, Steinbrenner I, et al. Genetic studies of paired metabolomes reveal enzymatic and transport processes at the interface of plasma and urine. Nat Genet. (2023) 55:995–1008. doi: 10.1038/s41588-023-01409-8
18. Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, et al. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. (2023) 51:D977–D85. doi: 10.1093/nar/gkac1010
19. Chen Y, Lu T, Pettersson-Kymmer U, Stewart ID, Butler-Laporte G, Nakanishi T, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet. (2023) 55:44–53. doi: 10.1038/s41588-022-01270-1
20. Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. (2010) 26:2190–1. doi: 10.1093/bioinformatics/btq340
21. de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. (2017) 49:256–61. doi: 10.1038/ng.3760
22. Ishigaki K, Sakaue S, Terao C, Luo Y, Sonehara K, Yamaguchi K, et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat Genet. (2022) 54:1640–51. doi: 10.1038/s41588-022-01213-w
23. Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. (2015) 47:1457–64. doi: 10.1038/ng.3434
24. Forgetta V, Manousaki D, Istomine R, Ross S, Tessier MC, Marchand L, et al. Rare genetic variants of large effect influence risk of type 1 diabetes. Diabetes. (2020) 69:784–95. doi: 10.2337/db19-0831
25. International Multiple Sclerosis Genetics C. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. (2019) 365(6460):eaav7188. doi: 10.1126/science.aav7188
26. Kurki MI, Karjalainen J, Palta P, Sipila TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
27. Glanville KP, Coleman JRI, O’Reilly PF, Galloway J, Lewis CM. Investigating pleiotropy between depression and autoimmune diseases using the UK biobank. Biol Psychiatry Glob Open Sci. (2021) 1:48–58. doi: 10.1016/j.bpsgos.2021.03.002
28. Zhao Y, Quan E, Zeng T, Huang Z, Luo Y, Peng L, et al. Type 1 diabetes, its complications, and non-ischemic cardiomyopathy: a mendelian randomization study of European ancestry. Cardiovasc Diabetol. (2024) 23:31. doi: 10.1186/s12933-023-02117-7
29. Cai Y, Liu K, Wu P, Yuan R, He F, Zou J. Association of mTORC1−dependent circulating protein levels with cataract formation: a mendelian randomization study. BMC Genomics. (2022) 23:719. doi: 10.1186/s12864-022-08925-7
30. Luo J, le Cessie S, Blauw GJ, Franceschi C, Noordam R, van Heemst D. Systemic inflammatory markers in relation to cognitive function and measures of brain atrophy: a Mendelian randomization study. Geroscience. (2022) 44:2259–70. doi: 10.1007/s11357-022-00602-7
31. Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. (2016) 32:3207–9. doi: 10.1093/bioinformatics/btw373
32. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
33. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PloS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
34. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
35. Chong J, Xia J. Using metaboAnalyst 4.0 for metabolomics data analysis, interpretation, and integration with other omics data. Methods Mol Biol. (2020) 2104:337–60. doi: 10.1007/978-1-0716-0239-3_17
36. Pal MM. Glutamate: the master neurotransmitter and its implications in chronic stress and mood disorders. Front Hum Neurosci. (2021) 15:722323. doi: 10.3389/fnhum.2021.722323
37. Newsholme P, Diniz VLS, Dodd GT, Cruzat V. Glutamine metabolism and optimal immune and CNS function. Proc Nutr Soc. (2023) 82:22–31. doi: 10.1017/S0029665122002749
38. Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. (2014) 345:1494–8. doi: 10.1126/science.1250469
39. Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. (2018) 10(11):1564. doi: 10.3390/nu10111564
40. Blanco LP, Kaplan MJ. Metabolic alterations of the immune system in the pathogenesis of autoimmune diseases. PloS Biol. (2023) 21:e3002084. doi: 10.1371/journal.pbio.3002084
41. Checa A, Xu N, Sar DG, Haeggstrom JZ, Stahle M, Wheelock CE. Circulating levels of sphingosine-1-phosphate are elevated in severe, but not mild psoriasis and are unresponsive to anti-TNF-alpha treatment. Sci Rep. (2015) 5:12017. doi: 10.1038/srep12017
42. Stathopoulou C, Nikoleri D, Bertsias G. Immunometabolism: an overview and therapeutic prospects in autoimmune diseases. Immunotherapy. (2019) 11:813–29. doi: 10.2217/imt-2019-0002
43. Takeshima Y, Iwasaki Y, Fujio K, Yamamoto K. Metabolism as a key regulator in the pathogenesis of systemic lupus erythematosus. Semin Arthritis Rheumatol. (2019) 48:1142–5. doi: 10.1016/j.semarthrit.2019.04.006
44. Pyne S, Adams DR, Pyne NJ. Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Prog Lipid Res. (2016) 62:93–106. doi: 10.1016/j.plipres.2016.03.001
45. Verstockt B, Vetrano S, Salas A, Nayeri S, Duijvestein M, Vande Casteele N, et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2022) 19:351–66. doi: 10.1038/s41575-021-00574-7
46. Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev. (2017) 16:495–503. doi: 10.1016/j.autrev.2017.03.007
47. Di’Narzo AF, Houten SM, Kosoy R, Huang R, Vaz FM, Hou R, et al. Integrative analysis of the inflammatory bowel disease serum metabolome improves our understanding of genetic etiology and points to novel putative therapeutic targets. Gastroenterology. (2022) 162:828–43 e11. doi: 10.1053/j.gastro.2021.11.015
48. Schjodt MS, Gurdeniz G, Chawes B. The metabolomics of childhood atopic diseases: A comprehensive pathway-specific review. Metabolites. (2020) 10(12):511. doi: 10.3390/metabo10120511
49. Crestani E, Harb H, Charbonnier LM, Leirer J, Motsinger-Reif A, Rachid R, et al. Untargeted metabolomic profiling identifies disease-specific signatures in food allergy and asthma. J Allergy Clin Immunol. (2020) 145:897–906. doi: 10.1016/j.jaci.2019.10.014
50. Caussy C, Hsu C, Singh S, Bassirian S, Kolar J, Faulkner C, et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment Pharmacol Ther. (2019) 49:183–93. doi: 10.1111/apt.2019.49.issue-2
51. Robinson G, Pineda-Torra I, Ciurtin C, Jury EC. Lipid metabolism in autoimmune rheumatic disease: implications for modern and conventional therapies. J Clin Invest. (2022) 132(2):e148552. doi: 10.1172/JCI148552
52. Ryu H, Kim J, Kim D, Lee JE, Chung Y. Cellular and molecular links between autoimmunity and lipid metabolism. Mol Cells. (2019) 42:747–54. doi: 10.14348/molcells.2019.0196
53. Lei Q, Yang J, Li L, Zhao N, Lu C, Lu A, et al. Lipid metabolism and rheumatoid arthritis. Front Immunol. (2023) 14:1190607. doi: 10.3389/fimmu.2023.1190607
54. Naghashpour M, Jafarirad S, Amani R, Sarkaki A, Saedisomeolia A. Update on riboflavin and multiple sclerosis: a systematic review. Iran J Basic Med Sci. (2017) 20:958–66. doi: 10.22038/IJBMS.2017.9257
55. He JH, Tong NW, Li HQ, Wu J. Effects of L-threonate on bone resorption by osteoclasts in vitro. Sichuan Da Xue Xue Bao Yi Xue Ban. (2005) 36:225–8.
56. Zhang C, Hu Q, Li S, Dai F, Qian W, Hewlings S, et al. A magtein((R)), magnesium L-threonate, -based formula improves brain cognitive functions in healthy chinese adults. Nutrients. (2022) 14(24):5235. doi: 10.3390/nu14245235
57. Li W, Yu J, Liu Y, Huang X, Abumaria N, Zhu Y, et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol Brain. (2014) 7:65. doi: 10.1186/s13041-014-0065-y
58. Sun Q, Weinger JG, Mao F, Liu G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology. (2016) 108:426–39. doi: 10.1016/j.neuropharm.2016.05.006
59. Jian C, Wei L, Wu T, Li S, Wang T, Chen J, et al. Comprehensive multi-omics analysis reveals the core role of glycerophospholipid metabolism in rheumatoid arthritis development. Arthritis Res Ther. (2023) 25:246. doi: 10.1186/s13075-023-03208-2
60. Chen J, Liu C, Ye S, Lu R, Zhu H, Xu J. UPLC-MS/MS-based plasma lipidomics reveal a distinctive signature in systemic lupus erythematosus patients. MedComm (2020). (2021) 2:269–78. doi: 10.1002/mco2.v2.2
61. Bi X, Li F, Liu S, Jin Y, Zhang X, Yang T, et al. omega-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J Clin Invest. (2017) 127:1757–71. doi: 10.1172/JCI87388
62. Zhao JV, Schooling CM. Role of linoleic acid in autoimmune disorders: a Mendelian randomization study. Ann Rheum Dis. (2019) 78:711–3. doi: 10.1136/annrheumdis-2018-214519
63. Dong K, Gao ZW, Zhang HZ. The role of adenosinergic pathway in human autoimmune diseases. Immunol Res. (2016) 64:1133–41. doi: 10.1007/s12026-016-8870-2
64. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. (2015) 278:369–95. doi: 10.1111/joim.2015.278.issue-4
65. Ramaswamy M, Tummala R, Streicher K, Nogueira da Costa A, Brohawn PZ. The pathogenesis, molecular mechanisms, and therapeutic potential of the interferon pathway in systemic lupus erythematosus and other autoimmune diseases. Int J Mol Sci. (2021) 22(20):11286. doi: 10.3390/ijms222011286
Keywords: Mendelian randomization, plasma metabolites, autoimmune diseases, inflammatory bowel disease, multiple sclerosis, type 1 diabetes, systemic lupus erythematosus, rheumatoid arthritis
Citation: Yuan X, Yang P, Hu J, Cai D, Hu B, Rui G and Lin Z (2024) Causal association between plasma metabolites and diverse autoimmune diseases: a two-sample bidirectional mendelian randomization study. Front. Immunol. 15:1437688. doi: 10.3389/fimmu.2024.1437688
Received: 24 May 2024; Accepted: 21 October 2024;
Published: 07 November 2024.
Edited by:
Mihaela Adriana Ilie, Länssjukhuset i Kalmar, SwedenReviewed by:
Robert Cody Sharp, University of Florida, United StatesCopyright © 2024 Yuan, Yang, Hu, Cai, Hu, Rui and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoshan Hu, eG1oYnNAMjFjbi5jb20=; Gang Rui, cmVpZ2FuZ0AxNjMuY29t; Zhiming Lin, em1saW5AeG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.