
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 25 September 2024
Sec. Alloimmunity and Transplantation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1437176
 Yuanfeng Zhang1,2†
Yuanfeng Zhang1,2† Yan Wang1,2†
Yan Wang1,2† Runzhi Ma1
Runzhi Ma1 Li Liu1
Li Liu1 Jiali Sun1*
Jiali Sun1* Xin Chen1
Xin Chen1 Donglin Yang1
Donglin Yang1 Aiming Pang1
Aiming Pang1 Rongli Zhang1
Rongli Zhang1 Qiaoling Ma1
Qiaoling Ma1 Weihua Zhai1
Weihua Zhai1 Yi He1
Yi He1 Jialin Wei1
Jialin Wei1 Tingting Zhang1
Tingting Zhang1 Erlie Jiang1
Erlie Jiang1 MingZhe Han1
MingZhe Han1 Sizhou Feng1*
Sizhou Feng1*Introduction: Currently, no study has determined whether platelet transfusion refractoriness (PTR) post-hematopoietic stem cell transplantation (HSCT) before engraftment in patients with myelodysplastic syndrome (MDS) would impacts clinical outcomes.
Methods: We performed a MDS-specific retrospective analysis to determine whether PTR in one-month post-HSCT in patients with MDS could influence outcomes.
Results and discussion: Among the 315 patients enrolled, 110 (34.9 %) had PTR from stem cell infusion to one-month post-HSCT. Baseline characteristics of the PTR and non-PTR groups were similar. We found that patients with PTR had a slower and lower rate of platelet engraftment by day 28, as well as a slower recovery of neutrophils. The median days of neutrophil and platelet engraftment were 14 days (9-23) and 17 days (8-28) in the PTR groups versus 13 days (9-23) and 15 days (7-28) in the non-PTR group (P<0.001). By day 28, 84 of 110 patients (76.4%) with PTR achieved platelet engraftment compared with 181 of 205 patients (88.3%) without PTR achieving platelet engraftment (P=0.007). In addition, patients in the PTR group received significantly more red blood cell (median, 17 units vs. 10 units, P<0.001) and platelet transfusions (median, 13 units vs. 7 units, P<0.001). However, the overall survival was similar between the two groups. PTR in one-month post-HSCT, haploidentical donor, and ferritin level>1041ng/ml (median level) were independent adverse factors of platelet engraftment.
Myelodysplastic syndrome (MDS) is a malignant hematologic disease in adults characterized by cytopenia and a high risk of transformation into acute leukemia in certain subgroups (1). Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative therapy available for these patients. Platelet transfusion refractoriness (PTR) is defined as an inadequate increase in platelet count after transfusion that may impair the survival of patients undergoing allo-HSCT (2–6). MDS patients usually experience heavy transfusion dependence before transplantation, which may contribute to a high PTR rate (7). However, different mechanisms may lead to PTR before and after allo-HSCT (8). For instance, non-immune-mediated factors, rather than immune-mediated factors, mainly play a role in post-HSCT PTR (5). The reason for this phenomenon may partially be that hematologic recovery after HSCT is from the donor. Currently, no study has determined whether PTR post-HSCT before engraftment in patients would impacts clinical outcomes. Therefore, we performed this retrospective study to compare the outcomes of MDS patients with or without PTR post-HSCT in one month over the same period of investigation.
Between 2010 and July 2023, 340 patients with MDS or MSD/MPN, who consecutively underwent allo-HSCT, were screened. The enrollment criteria included patients diagnosed with MDS or MDS/MPN according to the fourth edition of WHO who underwent allo-HSCT during this period (1). The exclusion criteria included patients with incomplete data (N=5), those who died (N=6), or those not evaluated for PTR (N=14) at one-month post-HSCT. Finally, 315 patients were enrolled in this study. This study was approved by the Ethics Committee of our hospital (IIT2021011-EC-1).
The conditioning regimen and graft-versus-host (GvHD) prophylaxis were determined by physicians. The backbone protocol was a myeloablative conditioning regimen composed of busulfan 3.2 mg/kg/day for three days, cyclophosphamide 40 mg/kg/day for two days, fludarabine 30 mg/m2/day for three days, and cytarabine 2-4g/m2/day at divided dose for three days (9). Fludarabine and cytarabine were replaced with cladribine at 5mg/m2/day and idarubicin at 12 mg/m2/day for three days, respectively. In some cases, we also added 5-day decitabine to the conditioning (10). Patients with a matched unrelated donor (MUD) or haploidentical donor (HID) received rabbit anti-thymocyte globulin (ATG) at 2.5 mg/kg/day of 4 days. Low-dose methotrexate (MTX, 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11) and tacrolimus or cyclosporine were administered for the prevention of acute graft versus host disease (aGvHD). A regimen of tacrolimus or cyclosporine, short-term MTX, and mycophenolate mofetil (MMF) was administered to patients who underwent transplantation from a MUD or HID (Supplementary Table S1). Antibacterial, antifungal, and antiviral prophylactic agents were administered according to our protocol (11).
Neutrophil and platelet engraftment (12), aGvHD (13), chronic GvHD (cGvHD) (14), graft failure (GF) (15), and primary cause of death (COD) (16) were defined based on previously reported criteria. In our study, the platelet transfusion protocol is single-donor (apheresis) products, and a 12-hour corrected count increment (CCI) was calculated 12h after platelet transfusion with the following formula: (12h post-transfusion platelet count/μL−pre-transfusion platelet count/μL) ×body surface area/2.5[×1011] (mean number of platelets transfused in our center). PTR was defined as a CCI<5×109/L on two sequential occasions (5, 17). Therapy-related mortality (TRM) was defined as death without relapse. Treatment failure after HSCT was defined as death, relapse, or GF, whichever occurred first. Disease-free survival (DFS) was defined as the time from the second month post-HSCT to treatment failure or last follow-up. Overall survival (OS) was defined as the time from the second month post-HSCT to death or last follow-up.
The primary objective of this study was to compare the major outcomes of patients with MDS who underwent allo-HSCT with or without PTR in one-month post-HSCT.
All patients attended an outpatient department or telephone follow-up. The final follow-up date was in July 2023. Continuous and categorical variables were compared using the Mann–Whitney U test, chi-square test, and Fisher’s exact test, respectively. The median follow-up of patients was calculated using the reverse Kaplan–Meier method. The cumulative incidences (CI) of GvHD and TRM were estimated using the competing risk model and compared using Gray’s test. Death was considered as a competing event for GvHD. The probabilities of OS and DFS were estimated using the Kaplan–Meier method, and differences were compared using the log–rank test. Variables with P values ≤0.05 in the univariate analysis were entered in multivariate analyses which were used to identify factors impacting engraftment. We used R software packages (R 4.0.5), GraphPad Prism 5, and SPSS 25.0, to perform the statistical analyses. Figures were generated using GraphPad Prism 5. All P values were two-sided, and the results were considered statistically significant at P <0.05.
The basic characteristics of the enrolled patients are summarized in Table 1. In short, among the 315 patients analyzed, 110 (34.9%) were diagnosed with PTR in one-month post-HSCT. The median age of the patients in the two groups was comparable (45 vs. 45 years, P=0.521). Remarkably, 64 patients and 102 patients had received platelet transfusion twice or more before transplantation in the PTR and non-PTR group, respectively. Among those patients, 41 (64.06%) and 27 (26.47%) patients experienced PTR pre-HSCT with significant difference between the two groups (P<0.001). There were no differences in terms of patient sex, diagnosis, pre-HSCT ferritin level, donor age, donor source, donor-recipient relationship, donor-patient sex match, blood types of donors to recipients, or conditioning regimen between the two groups. In addition, the numbers of infused mononuclear and CD34+ cells were not significantly different between the two groups. In addition, anti-HLA antibodies result before-HSCT were available in 38, 92, and 6 patients of MSD, HID, and MUD donors, respectively. Among those patients, 12 (31.58%), 26 (28.26%), and 2 (33.33%) patients were positive for anti-HLA I antibodies, respectively. Of the 25 patients tested for HPA and other antibodies before HSCT, 18 were negative, while 4 were positive for IIb/IIIa antibodies, 2 were positive forIa/IIa, and one was positive for both IIb/IIIa andIa/IIa. The median duration of refractoriness was 13 days (range: 3 to 232), and only 7 patients suffered PTR after engraftment.
The major clinical outcomes of the patients are shown in Table 2. More patients in the PTR group experienced febrile neutropenia (40.37% vs. 23%, P=0.002), but the rates of bloodstream infection before engraftment (16.36% vs. 9.27%, P=0.093) and CMV reactivation (40% vs. 48.3%, P=0.157) were similar between the two groups. Only one patient experienced primary graft rejection and PTR. Eventually, 84 out of 110 patients (76.4%) with PTR achieved platelet engraftment at day 28, while 181 out of 205 patients (88.3%) without PTR achieved platelet engraftment at day 28 (P=0.007). The median days of neutrophil and platelet engraftment were one and two days slower among patients in the PTR group, respectively, than among patients without PTR (P<0.001). No differences were observed in the CI of 100-day grades I to IV and III to IV aGvHD between the two groups (Figure 1). Until the last follow-up, patients in the PTR group received significantly more red blood cell (RBC) and platelet (PLT) transfusions. The median units of RBC and PLT transfusions were 17 (range: 2-143.5) and 13 (range: 3-85) in the PTR group versus 10 (range: 0-77.5) and 7 (range: 1-75) in the non-PTR group (P<0.001). Among the 68 patients with PTR pre-HSCT, only 28 of 52 (53.8%) patients who received rabbit anti-thymocyte globulin (rATG) or porcine anti-lymphocyte globulin (pALG) during conditioning experienced PTR post-HSCT compared to 13 of 16 (81.3%) patients who did not receive rATG or pALG (P=0.05) suffered PTR post-HSCT.
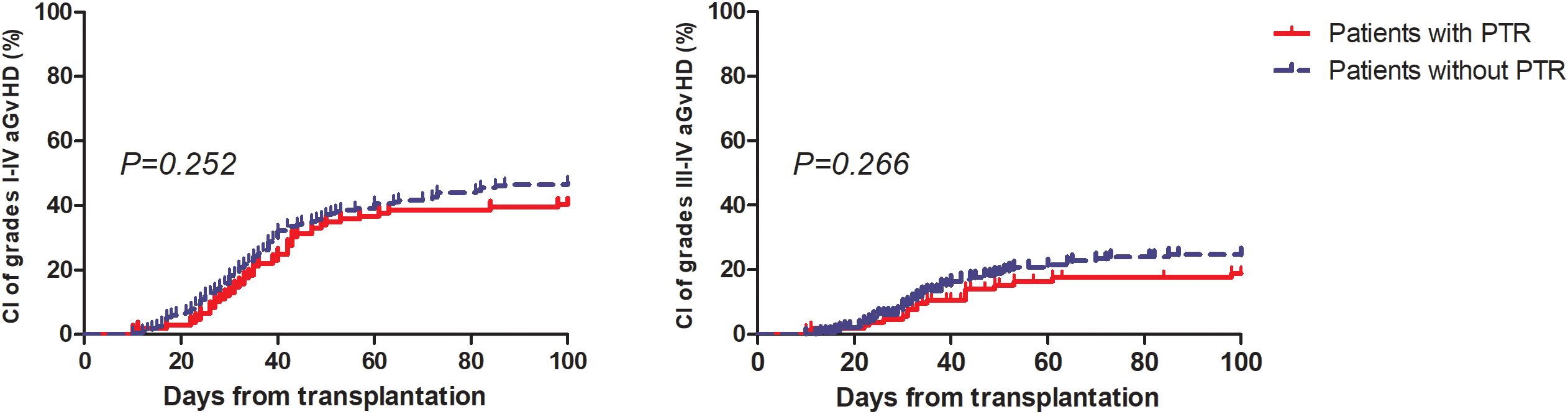
Figure 1. Cumulative incidence of grade I to IV aGvHD and III to IV aGvHD among patients with and without PTR.
In multivariate analysis (Table 3), PTR post-HSCT in one-month, male sex, and bloodstream infection were independent adverse risk factors of neutrophil engraftment, while PTR post-HSCT at one month, haploidentical donor, and ferritin>1041ng/ml pre-HSCT were independently associated with poor platelet engraftment.
At 5 years, the estimated OS and DFS rates in patients with PTR were 71.6% (95%CI: 61-79.9) and 71.7% (95%CI: 61.6-79.7), respectively, in contrast to 74.7% (95%CI: 67.1-80.8) (P=0.584) and 74.1% (95%CI: 66.9-79.9) (P=0.679) in patients without PTR (Figure 2).
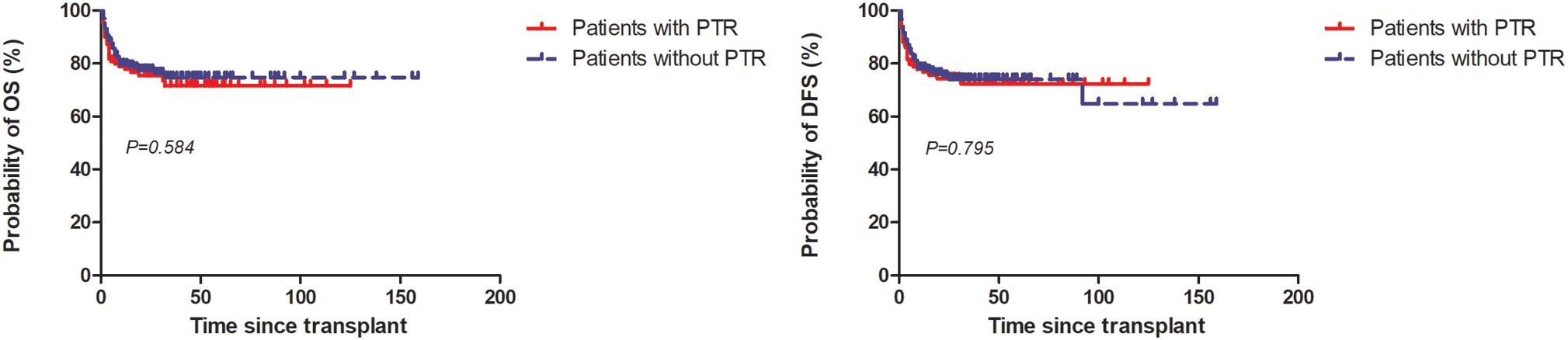
Figure 2. Overall survival (OS) and disease-free survival (DFS) among patients with and without PTR.
A total of 74 deaths had occurred at the last follow-up. The primary COD values are listed in Table 4. Strikingly, fatal hemorrhages and all hemorrhagic events in one month post-HSCT between the two groups were similar (Supplementary Table S2).
To the best of our knowledge, this is the first retrospective study with a large sample size to evaluate the effects of post-HSCT PTR before engraftment in patients with MDS or MDS/MPN. Our study showed PTR in one-month post-HSCT did not influence the survival of these patients, nor did the incidence of aGvHD. However, it delayed the engraftment of neutrophils and platelets and significantly decreased the rate of platelet engraftment on day 28.
In accordance with previous studies, we found PTR in one-month post-HSCT did not influence the survival of patients with MDS. Tan et al. evaluated the impact of PTR on survival before and after cord blood cell transplantation (CBT). In contrast to patients without PTR, they identified that only PTR 31–45 days after CBT, instead of PTR before or after HSCT in one month, was significantly associated with inferior survival (6). This finding was also consistent with another study from Spain that demonstrated PTR in one-month post-HSCT could significantly decrease the survival rate of patients undergoing peripheral blood stem cell (PBSC) transplantation but marginally influenced the survival of patients receiving UCB (5). In the MDS-specific setting of our study, we applied PBSC as the main stem cell source and focused on PTR from stem cell infusion to engraftment to minimize the effect of virus reactivation and aGvHD, most of which occurred one month later. However, other studies have found that patients with PTR have significantly inferior survival compared to those without PTR (2, 4, 18). These differences may be partially explained by the different hematological diseases analyzed, treatment procedures, and study periods.
In addition, we did not observe that patients in the PTR group experienced higher rates of aGvHD, BSI, or fatal hemorrhagic complications. A major concern with PTR is the potentially increased risk of bleeding. However, our findings contradicted this hypothesis. This may be due to recent improvements in supportive care, such as high-efficacy anti-infection prophylaxis, matched transfusion techniques, and the application of thrombopoietin receptor agonists (19–22). Moreover, delayed successful platelet engraftment may counteract the negative effects of early PTR. In our study, although only 76.4% of patients with PTR achieved platelet engraftment on day 28, most of the remaining patients became platelet independent at approximately 40 days post-HSCT.
Indeed, PTR post-HSCT at one month was independently associated with slower platelet engraftment in our study. This finding is consistent with that of another study (6). They identified patients suffering from PTR 0–15 days after CBT as an independent adverse factor in achieving neutrophil engraftment, and PTR 0–30 days after CBT as an independent poor factor in achieving platelet recovery. In addition, we found a higher rate of platelet non-engraftment on day 28 among patients in the PTR group, which is consistent with the findings of another study (23). However, the time to platelet engraftment was similar between the two groups.
We also found that haploidentical transplantation and ferritin>1041ng/ml (median level in our study) were independent risk factors for platelet engraftment. Raj et al. reported that compared to patients with MSD, patients with HID had lower and slower recovery of platelets. The median number of days to platelet engraftment was 14 (95%CI: 14-15) and 28 (95%CI: 27-31), respectively (24). A Chinese study also demonstrated a lower rate of platelet engraftment on day 100 after haploidentical transplantation, in contrast to that in MDS patients (25). High pre-HSCT ferritin levels could increase the rate of infection and impair the function of progenitors by reactive oxygen species. Malki et al. reported that iron overload characterized by a high ferritin level of >2000ng/ml in UCB could lead to delayed platelet engraftment. At 3 months, the CI of platelet engraftment in patients with ferritin levels of >2000 ng/ml was 52% (95%CI: 0.29-0.71) compared to 81% (95%CI:0.69-0.88) in patients with ferritin ≤2000 ng/ml (P=0.044) (26).
Our study has some limitations. First, as a retrospective study, selection bias is unavoidable; for instance, we may prefer HLA-matched or cross-matched platelets in patients with PTR, which increases the transfusion effect and improves survival. Second, for those with PTR, we did not uniformly perform tests to further identify the reasons. We descried the clinical conditions such as febrile neutropenia (FN), BSI, and bleeding related with PTR in details in Table 1, Supplementary Table S2, respectively. We noticed that FN and BSI were significantly and marginally different between the two groups while bleeding events was similar between them. Therefore, massive platelet consumption caused by infection and drug interaction may also cause PTR post-HSCT (6). Based on our research, our recommendation is to perform anti-class I human leukocyte Antigens (HLA), anti-human platelet antigens (HPA), anti-membrane glycoproteins, and anti-36 antibodies among patients with PTR to differentiate immunologic from non-immunologic factor and guider treatment further (27).
Taken together, our study indicates that among patients with MDS or MDS/MPN, PTR post-HSCT in one-month could impair hematopoietic recovery but not survival. Future studies should explore the underlying mechanisms and interventions for this phenomenon.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Institute of Hematology and Blood Diseases Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
YZ: Writing – original draft, Methodology. YW: Data curation, Investigation, Writing – original draft. RM: Data curation, Investigation, Writing – review & editing. LL: Data curation, Investigation, Writing – review & editing. JS: Conceptualization, Writing – review & editing. XC: Data curation, Investigation, Writing – review & editing. DY: Data curation, Investigation, Writing – review & editing. AP: Data curation, Investigation, Writing – review & editing. RZ: Data curation, Investigation, Writing – review & editing. QM: Data curation, Investigation, Writing – review & editing. WZ: Data curation, Investigation, Writing – review & editing. YH: Data curation, Investigation, Writing – review & editing. JW: Data curation, Investigation, Writing – review & editing. TZ: Data curation, Investigation, Writing – review & editing. EJ: Data curation, Investigation, Writing – review & editing. MH: Writing – review & editing. SF: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.Tianjin Municipal Science and Technology Commission Grant (21JCZDJC01170), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-060, 2021-I2M-1-017, 2023-I2M-2-007), National Nature Science Foundation of China (82170217), and Clinical research project of Tianjin Society of Hematology and Regenerative Medicine (No. 2022 TSHRM08003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1437176/full#supplementary-material
1. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544%JBlood
2. Toor AA, Choo SY, Little JA. Bleeding risk and platelet transfusion refractoriness in patients with acute myelogenous leukemia who undergo autologous stem cell transplantation. Bone Marrow Transplant. (2000) 26:315–20. doi: 10.1038/sj.bmt.1702490
3. Christou G, Kekre N, Petrcich W, Tokessy M, Neurath D, Giulivi A, et al. Impact of platelet transfusion on toxicity and mortality after hematopoietic progenitor cell transplantation. Transfusion. (2015) 55:253–8. doi: 10.1111/trf.12817
4. Fu Q, Xu L, Zhang X, Wang Y, Chang Y, Liu K, et al. Platelet transfusion refractoriness after T-cell-replete haploidentical transplantation is associated with inferior clinical outcomes. Sci China Life Sci. (2018) 61:569–77. doi: 10.1007/s11427-017-9110-0
5. Solves P, Sanz J, Freiria C, Santiago M, Villalba A, Gomez I, et al. Factors influencing platelet transfusion refractoriness in patients undergoing allogeneic hematopoietic stem cell transplantation. Ann Hematol. (2018) 97:161–7. doi: 10.1007/s00277-017-3168-6
6. Tanoue S, Konuma T, Kato S, Oiwa-Monna M, Isobe M, Jimbo K, et al. Platelet transfusion refractoriness in single-unit cord blood transplantation for adults: risk factors and clinical outcomes. Biol Blood Marrow Transplant. (2018) 24:1873–80. doi: 10.1016/j.bbmt.2018.05.006
7. Saris A, Pavenski K. Human leukocyte antigen alloimmunization and alloimmune platelet refractoriness. Transfus Med Rev. (2020) 34:250–7. doi: 10.1016/j.tmrv.2020.09.010
8. Juskewitch JE, Norgan AP, De Goey SR, Duellman PM, Wakefield LL, Gandhi MJ, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion. (2017) 57:2828–35. doi: 10.1111/trf.14316
9. Wang J, Wang Q, Zhang H, He Y, Huang Y, Zhang R, et al. Moderate to severe marrow fibrosis as a more advanced risk factor for MDS and MDS-AML patients with excess of blasts receiving allogeneic hematopoietic stem cell transplantation. Transplant Cell Ther. (2021) 27:666.e661–666.e669. doi: 10.1016/j.jtct.2021.05.006
10. Cao Y-G, He Y, Zhang S-D, Liu Z-X, Zhai W-H, Ma Q-L, et al. Conditioning regimen of 5-day decitabine administration for allogeneic stem cell transplantation in patients with myelodysplastic syndrome and myeloproliferative neoplasms. Biol Blood Marrow Transplant. (2020) 26:285–91. doi: 10.1016/j.bbmt.2019.09.001
11. Zhang Y, Huo J, Liu L, Shen Y, Chen J, Zhang T, et al. Comparison of hematopoietic stem cell transplantation outcomes using matched sibling donors, haploidentical donors, and immunosuppressive therapy for patients with acquired aplastic anemia. Front Immunol. (2022) 13:837335. doi: 10.3389/fimmu.2022.837335
12. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Jin S, et al. Comparison of efficacy and health-related quality of life of first-line haploidentical hematopoietic stem cell transplantation with unrelated cord blood infusion and first-line immunosuppressive therapy for acquired severe aplastic anemia. Leukemia. (2020) 34:3359–69. doi: 10.1038/s41375-020-0933-7
13. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. (1995) 15:825–8.
14. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401.e381. doi: 10.1016/j.bbmt.2014.12.001
15. Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. (1989) 73:606–13. doi: 10.1182/blood.V73.2.606.bloodjournal732606
16. Copelan E, Casper JT, Carter SL, van Burik J-AH, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. (2007) 13:1469–76. doi: 10.1016/j.bbmt.2007.08.047
17. Gao M, Huang J, Shao Y, Ge M, Li X, Zhang J, et al. Efficacy of anti-thymocyte globulin for platelet transfusion refractoriness in serious aplastic anemia patients. Transfus Apher Sci. (2022) 61:103376. doi: 10.1016/j.transci.2022.103376
18. Kerkhoffs J-LH, Eikenboom JCJ, van de Watering LMG, van Wordragen-Vlaswinkel RJ, Wijermans PW, Brand A. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion. (2008) 48:1959–65. doi: 10.1111/j.1537-2995.2008.01799.x
19. Reid R, Bennett JM, Becker M, Chen Y, Milner L, Phillips GL, et al. Use of eltrombopag, a thrombopoietin receptor agonist, in post-transplantation thrombocytopenia. Am J Hematol. (2012) 87:743–5. doi: 10.1002/ajh.23225
20. Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood marrow tr. (2016) 22:919–24. doi: 10.1016/j.bbmt.2016.01.018
21. Zhou M, Qi J, Gu C, Wang H, Zhang Z, Wu D, et al. Avatrombopag for the treatment of thrombocytopenia post hematopoietic stem-cell transplantation. Ther Adv Hematol. (2022) 13:20406207221127532. doi: 10.1177/20406207221127532
22. Zheng X, Zhang H, Guo W, Cao Y, Liu X, Zhai W, et al. Herombopag promotes platelet engraftment and decreases platelet transfusion after allogeneic hematopoietic stem cell transplantation. Eur J haematol. (2023) 110:527–33. doi: 10.1111/ejh.13925
23. Scott A, Baidya S, Butler J, Morris K, Thornton A, Kennedy GA. Pretransplant platelet transfusion refractoriness is not associated with platelet nonengraftment in T-replete hematopoietic progenitor cell transplantation for hematologic Malignancies. Transfusion. (2016) 56:164–9. doi: 10.1111/trf.13263
24. Raj K, Eikema D-J, Sheth V, Koster L, de Wreede LC, Blaise D, et al. Comparison of outcomes for HLA-matched sibling and haplo-identical donors in Myelodysplastic syndromes: report from the chronic Malignancies working party of EBMT. Blood Cancer J. (2022) 12. doi: 10.1038/s41408-022-00729-y
25. Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. (2016) 30:2055–63. doi: 10.1038/leu.2016.110
26. Malki MMA, Song JY, Yang D, Cao T, Aldoss I, Mokhtari S, et al. Iron overload is associated with delayed engraftment and increased nonrelapse mortality in recipients of umbilical cord blood hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2020) 26:1697–703. doi: 10.1016/j.bbmt.2020.06.002
Keywords: myelodysplastic syndrome, transplantation, platelet transfusion refractoriness, stem, cell
Citation: Zhang Y, Wang Y, Ma R, Liu L, Sun J, Chen X, Yang D, Pang A, Zhang R, Ma Q, Zhai W, He Y, Wei J, Zhang T, Jiang E, Han M and Feng S (2024) Impact of platelet transfusion refractoriness in the first 30 days post-hematopoietic stem cell transplantation on outcomes of patients with myelodysplastic syndrome. Front. Immunol. 15:1437176. doi: 10.3389/fimmu.2024.1437176
Received: 23 May 2024; Accepted: 05 September 2024;
Published: 25 September 2024.
Edited by:
Ying-Jun Chang, Peking University People’s Hospital, ChinaReviewed by:
Roberto Crocchiolo, Niguarda Ca’ Granda Hospital, ItalyCopyright © 2024 Zhang, Wang, Ma, Liu, Sun, Chen, Yang, Pang, Zhang, Ma, Zhai, He, Wei, Zhang, Jiang, Han and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiali Sun, c3VuamlhbGlAaWhjYW1zLmFjLmNu; Sizhou Feng, c3pmZW5nQGloY2Ftcy5hYy5jbg==; ZG9jdG9yX3N6aGZlbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.