
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 22 October 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1433774
This article is part of the Research Topic Understanding Complications and Outcomes of Anti-BCMA Treatments in Relapsed Multiple Myeloma View all articles
Chimeric antigen receptor T-cell (CAR-T) therapy has ushered in a new era for the treatment of multiple myeloma (MM). Numerous clinical studies, especially those involving B-cell maturation antigen (BCMA)-directed CAR-T, have shown remarkable efficacy in patients with relapsed or refractory multiple myeloma (R/R MM). However, a considerable number of patients still experience disease recurrence or progression after BCMA CAR-T treatment, which is attributed to various factors, including antigen escape, CAR-T manufacturing factors, T cell exhaustion, inhibitory effects of tumor microenvironment and impact of prior treatments. The scarcity of effective treatment options following post-CAR-T disease recurrence, coupled with the lack of well-established salvage regimens, leaves patients who do relapse facing a bleak prognosis. In recent years, some academic institutions have achieved certain results in salvage treatments of patients with relapse after BCMA CAR-T treatment through secondary infusion of BCMA CAR-T, changing to non-BCMA-directed CAR-T, double-target CAR-T, bispecific antibodies or other novel therapies. This review summarizes the mechanisms of resistance or relapse after BCMA CAR-T administration and the available data on current salvage treatments, hoping to provide ideas for optimizing clinical salvage therapies.
Multiple myeloma (MM), characterized by presence of abnormal clonal plasma cells, is the second most common hematological malignancy (1, 2). Over the years, the development of hematopoietic stem cell transplantation, immunomodulatory drugs, proteasome inhibitors and monoclonal antibodies has significantly improved the prognosis of multiple myeloma patients (3–7). Despite these advances, MM remains incurable and disease relapse is inevitable in most patients. Recent years, chimeric antigen receptor T-cell (CAR-T) therapies, especially those targeting B-cell maturation antigen (BCMA), a cell surface glycoprotein primarily expressed by plasma cells and some mature B cells, have shown remarkable efficacy in patients with relapsed or refractory MM (R/R MM) (8–11). To date, the US Food & Drug Administration (FDA) has approved two anti-BCMA CAR-T cell products, idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) for the treatment of R/R MM. Some patients exhibit durable responses–for instance, in the LEGEND-2 trial, 16.2% of participants experienced lasting responses and remained relapse-free, with the longest remission being as long as 6.4 years (12). However, a considerable proportion of patients eventually experience disease relapse or progression. This underscores the necessity to explore salvage therapeutic strategies following BCMA CAR-T therapy recurrence. In this review, we summarize the mechanisms of relapse after BCMA CAR-T treatment and the current data on salvage treatment options, providing recommendations for optimal treatment strategies for patients following BCMA CAR-T relapse.
Despite the encouraging achievements of anti-BCMA CAR-T therapy in R/R MM (11, 13), the issue of recurrence in MM patients is equally deserving serious attention. The resistance mechanisms are closely related to antigen escape, factors of CAR-T production, T cell exhaustion, the interaction between tumor cells and complex tumor microenvironment and the influence of previous treatment (Figure 1). A precise grasp of these mechanisms can help guide subsequent medical decisions and overcome the resistance.
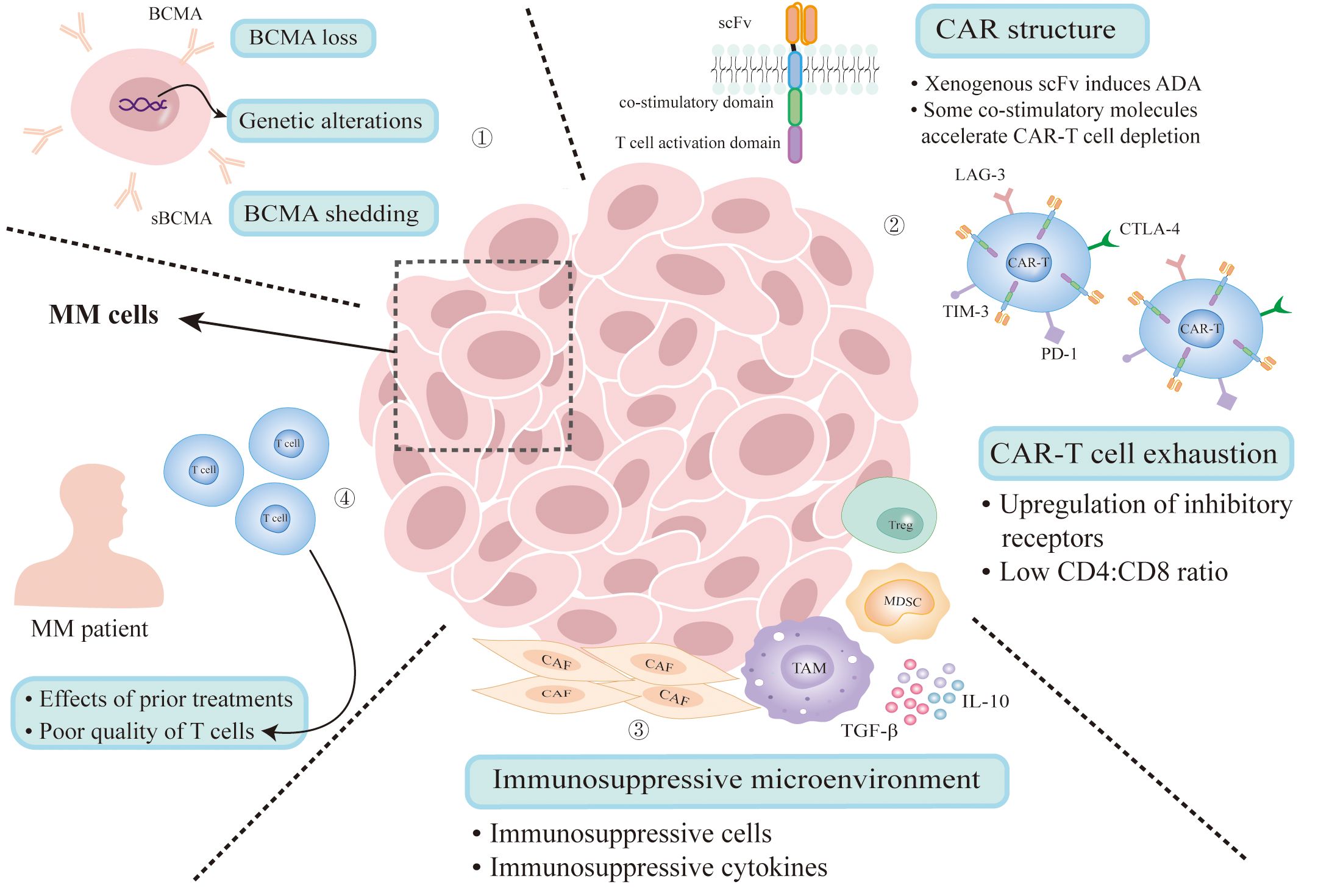
Figure 1. Mechanisms of resistance to BCMA CAR-T therapy. (1) Antigen escape due to genetic alterations and clonal selection under therapeutic pressure. Gamma secretase cleaves BCMA from the surface of MM cells that releases sBCMA. (2) The presence of anti-scFv antibodies results in reduced CAR-T cell count and loss of activity. Some co-stimulatory molecules can accelerate CAR-T cell depletion. CAR-T cell exhaustion results from persisting antigenic stimulation and immunosuppressive microenvironment can also lead to disease relapse. (3) Immunosuppressive microenvironment impairs CAR-T cell activation. (4) Multiple treatment regimens will impact the environment of CAR-T therapy and the quality of T cells collected from MM patients is poor. MM, multiple myeloma; BCMA, B-cell maturation antigen; sBCMA, soluble BCMA; ADA, anti-CAR antibodies; CAF, cancer-associated fibroblast; TAM, tumor associated macrophage; MDSC, myeloid-derived suppressor cell; Treg, regulatory T-cell.
Compared to normal cells, BCMA is highly expressed in myeloma cells. It has been confirmed that overexpression and activation of BCMA are closely related to the progression of MM, which makes it the most attractive therapeutic target in MM (9, 14). However, MM is a highly heterogeneous malignancy with a large number of subclones in the same patient, suggesting MM may be particularly susceptible to evading single antigen-targeted therapy. Brudno et al. found that 1 in 16 patients had a loss of BCMA in myeloma cells after BCMA CAR-T cell infusion (15). Similarly, Ali et al. found that one of the eight patients who were continuously followed in their study had a BCMA-negative recurrence (16). The mechanism of these phenomena might be clonal selection under therapeutic pressure: myeloma cells with high BCMA expression are eliminated by BCMA CAR-T cells, while low BCMA expression cells are selected to survive. Over time, these antigen-negative subclones could expand and dominate the tumor population, leading to antigen loss.
In addition to clonal selection, genetic alterations could also lead to antigen loss. Samur et al. found that biallelic loss of BCMA is one of the resistance mechanisms to anti-BCMA CAR-T cell therapy by performing single-cell transcriptome profiling of bone marrow samples serially collected from a patient treated with ide-cel (17). Similarly, Lee et al. found that biallelic loss of TNFRSF17 gene encoding BCMA can result in the loss of the BCMA antigen, while extracellular domain mutations can lead to loss of functional BCMA epitopes, significantly attenuating the binding between CAR-T cells and their cognate epitopes, thereby causing disease recurrence (18). Based on the above, targeting other antigens, or constructing dual-target CAR-T, can overcome the loss of BCMA.
Furthermore, BCMA can be cleaved from the surface of MM cells by gamma secretase, releasing soluble BCMA (sBCMA), which presents in most R/R MM patients. It can not only decrease functional BCMA on the surface of MM cells, but interfere with BCMA-directed CAR-T cells (19, 20). In this case, gamma secretase inhibitors have shown promise for improving the efficacy of BCMA CAR-T therapy (21).
CAR-T is constructed by modifying T cells isolated from patients, thus the condition of T cells has an important impact on the durability and efficacy of CAR-T. One study found that apheresis samples from patients with earlier stages of MM exhibited a higher percentage of memory T cells and CD4/CD8 ratio than those from R/R MM patients, suggesting that T cells from patients with early treatment stage are more suitable for CAR-T manufacturing and CAR-T cells may be more effective if they are manufactured from patients before onset of recurrent/refractory (22).
The structural design of CAR molecules determines the property of CAR-T cells. The co-stimulatory domain, an important component of CAR, presenting in the second-generation and later CAR-T products, significantly affects the function of CAR-T cells, such as depletion rate, anti-tumor activity and persistence (23, 24). CD28, 4-1BB, ICOS (CD278), OX40 (CD134) are the most commonly used co-stimulatory molecules. CD28 triggers powerful T cell activation but accelerates CAR-T cell depletion. In the contrary, 4-1BB can improve CAR-T cell depletion and promote the expansion of stem cell memory T cells. Besides, the combination of ICOS and 4-1BB can significantly improve the persistence of CAR-T cells. OX40 shows the ability to promote T cell proliferation and memory formation (23, 25–28). In the view of the importance of co-stimulatory factors and their existing shortcomings, new co-stimulatory molecules or combination structures are still being explored to improve CAR-T function.
In addition, the emergence of anti-CAR antibodies may be another important factor leading to CAR-T resistance. Most BCMA-targeting CAR-T cells have derived their scFv from mouse or other non-human species. Xenogenous scFv can induce the appearance of anti-CAR antibodies (ADA), resulting in reduced CAR-T cell count and loss of activity (29–31). By analyzing 17 R/R MM patients treated with LCAR-B38M, Xu et al. found that 6 out of 7 patients with relapse or disease progression showed high levels of ADA and their residual CAR-T cells in peripheral blood were deeply reduced. This suggests that ADA may pose a high risk of relapse after treatment (32). A study of ADA testing of 55 R/R MM patients participating in LCAR-B38M treatment found that 34 (62%) were ADA positive, and the frequency of ADA in cyclophosphamide plus fludarabine group was lower than that in cyclophosphamide group, suggesting the choice of lymphodepleting regimen has an important influence on ADA generation. However, the presence of ADA had no effect on overall survival (OS), progression-free survival (PFS), or duration of response (DOR) (33).The impact of ADA presence on BCMA CAR-T efficacy remains controversial. Additional data is necessary to confirm these observations, and strategies to develop whole humanized scFv or antigen-recognition domains composed of fully humanized pure heavy chain antibody variable domains may overcome the potential adverse impact generated by ADA (29, 34–36), advancing the efficacy of CAR-T cell therapy.
Persisting antigenic stimulation and immunosuppressive tumor microenvironment can cause T cell exhaustion, defined as the inhibition of T cell proliferation and effector function (37–39). After BCMA CAR-T cell infusion, the senescence phenotype in CD8-positive T cells increased more than that in CD4-positive T cells, and an increase in the CD4:CD8 ratio in the infusion products was associated with better amplification and clinical outcomes (15, 40). In addition, Ledergor et al. found that patients exhibiting durable responses to CAR-T cell therapy possessed a significantly higher proportion of CD8+ T-effector memory cells. Conversely, in patients with transient responses to treatment, there was an increase in cytotoxic CD4+ CAR-T cells, which expanded early in vivo after infusion but expressed exhaustion markers while maintaining polyclonality. Furthermore, they discovered that myeloid cells in the myeloma niche may contribute to CD4+ CAR-T cell dysfunction via transforming growth factor beta (TGF-β) (41).
Increased expression of multiple inhibitory receptors is another feature of T cell depletion, including programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte antigen 4 (CTLA-4), T-cell immunoglobulin domain and mucin domain-3 (TIM-3), lymphocyte activation gene 3 (LAG-3), etc. (42, 43). It has been found in a variety of cancers that the expression of these inhibitory receptors is up-regulated in CAR-T cells of patients who have failed CAR-T therapy (42, 43). Previous studies have demonstrated that the deletion of the inhibitory co-receptor CTLA-4 and the blocking of PD-1 can enhance CAR-T function (44, 45). In MM, researchers added an anti-PD-1 short hairpin RNA to BCMA CAR-T cell, and it has showed reduced T-cell exhaustion and increased percentage of memory T cells in vitro (46), improving the efficacy of CAR-T cell therapy.
MM microenvironment contains many tumor-supporting and immunosuppressive components, such as myeloid derived suppressor cells (MDSCs), regulatory T-cells (Tregs), tumor-associated macrophages and a variety of immunosuppressive cytokines (such as IL-10, TGF-β, etc.) (47, 48). A study has revealed that MM patients treated with BCMA CAR-T cells developing macrophage-activated syndrome-like manifestations, leading to lower 1-year survival rates and progression-free survival times (49). Li et al. conducted a single-cell sequencing on a patient who relapsed for the first time after BCMA CAR-T treatment 22 months later. They found that immune cells including T cells, natural killer (NK) cells, dendritic cells (DC), neutrophils, and monocytes/macrophages showed tumor-promoting phenotypes when disease relapsed (50), revealing tumor microenvironment is one of the underlying mechanisms of R/R MM recurrence after BCMA CAR-T cell therapy. Sakemura et al. revealed that a high presence of cancer-associated fibroblasts (CAFs) can suppress the anti-tumor activity of CAR-T cells. These CAFs concurrently produced elevated levels of inhibitory cytokines, such as TGF-β, and upregulated the surface expression of inhibitory ligands such as programmed cell death-ligand 1 (PD-L1). Additionally, they observed a significant increase expression of PD-1 on CAR-T cells, which contributes to their functional impairment. Dual-specific CAR-T cells targeted against both MM cells and CAFs were capable of overcoming the suppressive effects imposed by CAFs, thereby enhancing the anti-tumor efficacy of the CAR-T cell therapy (51).
Most R/R MM patients have received multiple treatment regimens prior to BCMA CAR-T therapy (52, 53), and these regimens may impact the efficacy and duration of BCMA CAR-T cell. Studies have shown that the use of alkylating agents such as cyclophosphamide and bendamustine prior to leukapheresis could lead to the depletion of T cells and was associated with poor CAR-T manufacturing, particularly detrimental for autologous CAR-T therapies (54–56). Additionally, Ramos et al. found that patients receiving a fludarabine-based lymphodepletion had more durable responses to CAR-T therapy compared to those receiving bendamustine alone (57), which may explain why bendamustine is used less frequently as a lymphodepleting chemotherapy compared to fludarabine/cyclophosphamide regimen (58). Another study revealed that patients treated with daratumumab as bridging therapy had better response rates after CAR-T cell transfusions compared to patients who did not receive daratumumab. At the same time, daratumumab has immunomodulatory effects on the bone marrow tumor microenvironment, thereby reducing environmental resistance (8, 59). Moreover, there are now two BCMA CAR-T products approved by the FDA for the treatment of early-stage multiple myeloma. Therefore, it is advisable to select appropriate preconditioning regimens and effective less toxic bridging therapies prior to CAR-T treatment based on the patients’ situation, with the goal of optimizing the outcomes of CAR-T therapy.
Relapse after anti-BCMA CAR-T cell therapy has been common, with more than 50% of patients having relapsed after CAR-T cells infusion (12, 60, 61). Our recently updated 5-year follow-up results of LEGEND-2 trial demonstrated that 71.6% of patients appeared disease progression (PD) after partial response (PR) or better (12). Most patients before treated with BCMA CAR-T have undergone rigorous preconditioning, thus subsequent therapy options remain limited. There is currently a lack of recommended salvage therapy for patients who relapse after CAR-T cell therapy. Here, we introduce and summarize the existing follow-up treatments after recurrence of CAR-T treatment, with selected trials highlighted in Table 1, so as to provide reference for improving the prognosis of patients who progress after CAR-T treatment.
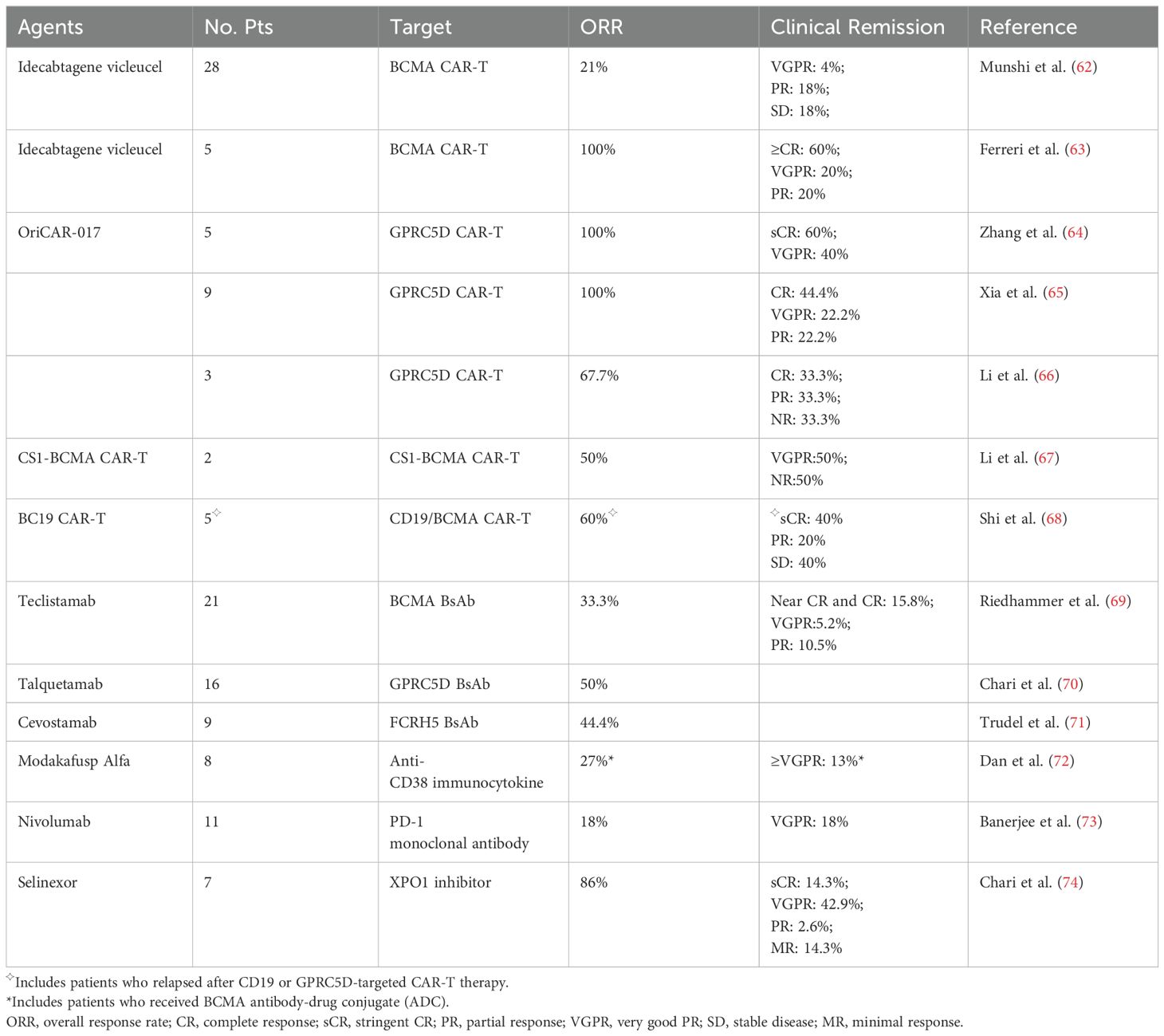
Table 1. Treatment efficacy in multiple myeloma patients with disease relapse after BCMA CAR-T cell therapy.
Disease progression after BCMA CAR-T treatment is partly due to the short duration of CAR-T cells in vivo result from CAR-T product factors. Therefore, secondary infusion of the same BCMA CAR-T product or full human-derived BCMA CAR-T can be performed to improve the persistence of CAR-T in vivo and thus improve the survival prognosis of patients.
Munshi et al. conducted a single-group phase II study of ide-cel which a total of 128 patients received ide-cel infusions. After PD, 28 patients received ide-cel reinfusion and 6 patients (21%) had a second response, with durations of response ranging from 1.9 to 6.8 months. It is noting that all the patients who had a response were retreated with a higher dose than their initial dose (62). This suggests that the same CAR-T product has limited efficacy as a salvage treatment for patients who relapse after BCMA CAR-T therapy.
In a retrospective study of R/R MM patients treated with commercial ide-cel, five of the patients had previously undergone BCMA CAR-T therapy. Two of them had received ide-cel as their prior CAR construct in early phase trials, two had received an allogeneic CAR, and one had received an autologous CAR with a non-viral transduction. This subgroup had the best response rate to ide-cel (overall response rate [ORR]: 100%; ≥complete response [CR]: 60%) (63). A clinical trial of BCMA CAR-T with a fully human scFv (CT103A) enrolled 18 consecutive adult subjects with BCMA-positive R/R MM, including 4 with prior murine BCMA CAR exposures. The ORR was 100%, and 72.2% of patients achieved CR or stringent CR (sCR). For the 4 murine BCMA CAR–exposed patients, 3 achieved sCR, and 1 achieved a very good partial response (VGPR) (36). Similarly, a study reported that two patients with PD after infusion of murine CAR-T, including 1 patient with extramedullary disease (EMD) at PD, achieved VGPR and PR after rescue therapy with humanized BCMA CAR-T cells (75). In another clinical trial of fully human anti-BCMA CAR-T (HRC0202), 7 R/R MM patients previously exposed to anti-BCMA CAR-T therapy were evaluated. The ORR was 71.4% (5/7), and 3 patients achieved sCR/CR. The median PFS (mPFS) was 269 days (76). These studies indicate that fully human anti-BCMA CAR-T is a promising treatment for R/R MM patients who relapsed or refractory from prior non-human-derived anti-BCMA CAR-T infusion.
Patients with antigen loss or low, variable BCMA expression may benefit from new target CAR-T therapies, particularly CAR-T directed at G protein–coupled receptor class C group 5 member D (GPRC5D), which presents as an appealing alternative. Previous studies have shown that GPRC5D was highly expressed on myeloma cells. Except bone marrow, GPRC5D expression is observed in keratin synthesis, lung tissue, and the inferior olivary nucleus (77, 78). In addition, GPRC5D is a seven-pass transmembrane receptor protein, so it is unlikely to be shed in the serum, greatly reducing the risk of disease recurrence due to antigen shedding (79, 80). Besides, expression of GPRC5D is independent of BCMA (79), making GPRC5D CAR-T capable of killing MM cells in spite of BCMA expression. In a phase 1 dose-escalation study, 17 R/R MM patients received anti-GPRC5D CAR-T cell infusion. Ten had previously received BCMA-targeted therapy, of which eight had received BCMA CAR-T therapy. Responses were observed in 7 of 10 patients who had received previous BCMA therapies (81). In a single center, phase 1 trial, 10 R/R MM patients were treated with GPRC5D CAR-T cells (OriCAR-017), and five of them had been treated with BCMA CAR-T. 100% of ORR was observed, with 60% (6/10) of them achieving sCR (64). Recently, at 2023 American Society of Hematology (ASH) annual meeting, a clinical trial of BMS-986393, a GPRC5D targeted CAR-T product, 75% (21/28) of ORR was observed in patients treated with prior BCMA therapies, including BCMA CAR-T cells (NCT04674813) (82). From the above results, we can see that GPRC5D is an active target in multiple myeloma and has also responded well in patients who had failed in prior anti-BCMA therapy.
Compared to single-target CAR-T cells, double-target CAR-T cells increase the coverage of antigen, decrease the possibility of myeloma relapse by BCMA-negative clones, thus have better anti-myeloma effect (83–85).
CS1, also known as CD319 and SLAMF7, is expressed at high levels on multiple myeloma cells (86, 87). Li et al. found that the MM cells of relapsed patients after anti-BCMA CAR-T cell therapy with BCMA loss still maintained CS1 expression. Therefore, they constructed a bispecific CS1-BCMA CAR-T cell to augment BCMA targeting with CS1. Fourteen patients who had not received BCMA CAR-T and two who had relapsed after BCMA CAR-T cell therapy, received CS1-BCMA CAR-T cell transfusions. The ORR was 81%. Of the two patients who received prior BCMA CAR-T therapy, one obtained VGPR but the other one with solitary EMD did not respond (67).
Researchers found that although CD19 is only expressed on a subset of MM cells, these cells are typically less differentiated and possess clonogenic capacity, considered to be poorly differentiated MM cells or myeloma-like stem cells (88, 89). Even with low CD19 expression, these cells can still be eliminated by CD19 CAR-T cells (89, 90). Therefore, therapies targeting this subpopulation may have synergistic effects with other treatments, helping to eliminate less differentiated myeloma cells. Based on the above, Shi et al. designed bispecific BC19 CAR-T cells targeting BCMA/CD19 and evaluate antimyeloma activity in 50 patients. Five patients in this study had received prior BCMA, CD19, or GPRC5D-targeted CAR-T cell treatment. Two of them achieved sCR, one achieved PR, and the other two had stable disease (SD) (68). These studies suggest that dual-target CAR-T has promising clinical activity in R/R MM patients even after BCMA CAR-T cell treatment.
Bispecific antibodies (BsAb) are designed to bind to targets on tumor cells and cytotoxic immune effector cells (T cells/NK cells) to create immune synapses that cause T/NK cells to activate and direct them to attack and destroy tumor cells (91, 92).
Teclistamab is a BCMA×CD3 directed bispecific antibody. In the C cohort of MajesTEC-1 trial, 11 of the 25 evaluable patients had previously received BCMA CAR-T. They achieved an ORR of 45% after treated with teclistamab, and their responses deepened over time (93). A retrospective study analyzing 123 patients treated with teclistamab found that among the 21 patients previously treated with BCMA CAR-T, the ORR was only 33.3%, with a significantly shorter median PFS of 1.8 months. Furthermore, the duration of response to teclistamab in patients achieving an overall response did not differ from those who had not received prior BCMA CAR-T therapy (69). These suggest a lower response rate to teclistamab after BCMA-directed CAR-T cell therapy. However, another retrospective analysis of teclistamab included 106 R/R MM patients, of whom 56 had previously received BCMA-directed therapy, including 42 who had previously undergone BCMA CAR-T treatment. These patients achieved response rates comparable to those seen in the overall patient population (ORR: 59% vs 66%) (94), while the long-term efficiency outcome was not known.
Talquetamab, a bispecific antibody that against GPRC5D and CD3, recruits and activates T cells to kill GPRC5D-expressing myeloma cells (70, 95). In a study presented at 2023 ASH meeting, 50 of 70 patients with CAR-T exposure (48/50 anti-BCMA CAR-T) were treated with talquetamab. Among them whose efficacy could be assessed, their ORR was 72.9%, similar to the overall population (96). This supports that talquetamab can serve as a multifunctional treatment option to provide effective responses in BCMA CAR-T exposed R/R MM patients.
Fc receptor-homolog 5 (FcRH5), also known as CD307, is a membrane protein. It is only expressed on B cell lineage and at a higher level on myeloma cells than normal B cells. Cevostamab is a FcRH5×CD3 BsAb that facilitates T cell-directed killing of myeloma cells. Of the patients participating in the cevostamab monotherapy study, 9 patients who had received BCMA were efficacy evaluable and they achieved an ORR at 44.4% (71).
In addition to BsAb, the combination of BsAb and monoclonal antibody (mAb) is also another choice of rescue measures. Daratumumab, a CD38 mAb, has shown a good safety and efficacy profile when used in combination with other anti-myeloma drugs (97, 98). In the phase 2 TRIMM-2 trial (NCT04108195), 65 R/R MM patients were enrolled to receive talquetamab plus daratumumab. Prior treatments included anti-CD38 (88%), anti-BCMA (54%), BsAb (25%), and anti-BCMA CAR-T (17%) therapy. In patients exposed to prior anti-BCMA therapy, ORR was 74%. At 12 months, 86% of the responders (89% of patients ≥CR) were still responding (99). These results suggest that combining BsAb with anti-CD38 mAb can yield robust responses in patients who have relapsed after BCMA CAR-T.
Modakafusp alfa is an immunocytokine fusion protein combining attenuated interferon alpha (IFNɑ) to be molecules attached to an anti-CD38 IgG4 mAb, delivering IFNɑ to CD38-expressing cells such as myeloma cells. Among 30 patients treated with modakafusp 1.5 mg/kg Q4W, 16 patients (50%) had previously received anti-BCMA therapy, of which 8 (27%) had received CAR-T therapy. The ORR was 43% and the median PFS was 5.7 months. In patients with prior anti-BCMA therapy (half received BCMA CAR-T cell therapy), the ORR was 27%, of which 13%≥VGPR (72).
Checkpoint inhibitors can theoretically improve CAR-T cell activity. To characterize the efficacy of nivolumab (a monoclonal antibody targeting PD-1 on T-cells) in MM after CAR-T failure, Banerjee et al. enrolled 11 patients. After nivolumab monotherapy, their ORR was 18% (2/11) (73). However, this is only a prospective study with a small number of patients included in the study. More trials are needed to verify the efficacy of PD-1 monoclonal antibody in BCMA CAR-T relapsed populations.
In addition to salvage with T-cell–engaging therapies after CAR-T, other saving methods or multiple follow-up treatments can be used based on patient characteristics. Selinexor is an oral small molecule nuclear export protein export protein 1 (XPO1) inhibitor that promotes apoptosis in malignant tumor cells such as myeloma cells (100, 101). The activity of selinexor was preserved regardless of prior therapy. Therefore, Chari et al. identified seven patients who had progressed after BCMA CAR-T to receive a selinexor combination immediately after CAR-T progression and the responses included 1 sCR, 3 VGPR, 2 PR and 1 minimal response (74). While the findings reported here are derived from a small cohort of patients, the trial demonstrated the anti-myeloma activity of selinexor irrespective of prior treatment history and without evidence of cross-resistance. Adenosine has a highly immunosuppressive effect, and CD73 can catalyze adenosine monophosphate (AMP)-producing adenosine. ORIC-533 is a small molecule inhibitor of CD73 that inhibits CD73 to block adenosine production to restore and enhance immune function. A recent study demonstrated a preliminary evidence of immune activation after ORIC-533 in heavily pretreated R/R MM patients. Seventeen patients were treated with four dose levels of ORIC-533, 59% of whom had previously received BCMA/CD3 bispecific therapy or anti-BCMA CAR-T therapy. After one treatment cycle, an increase in the abundance of circulating NK and CD8+ T cells was observed (102).
Radiotherapy (RT) is a local control method. A study involved 13 patients with locally progressive bone or soft tissue plasmacytomas. Five of them only received bridging RT before CAR-T, four received salvage RT after CAR-T failure, and another four received both bridging and salvage RT. The median overall survival of the cohort was 16.2 months, the local control rate was 100%, and no radiation-related toxicity was observed (103). Iopofosine I-131 is a phospholipid radioactive conjugate that causes apoptosis by causing double-stranded DNA breaks. The six patients receiving a total dose >60 mCi had an ORR of 50% and the clinical benefit (stable or better disease) of 100% (104). In addition, two case reports introduced the clinical effects of myeloablative monophasic hematopoietic stem cell transplantation and triple MAPK inhibition strategies for saving BCMA CAR-T recurrence, indicating their feasibility (105, 106).
Chen et al. first reported the outcomes of 20 patients who received subsequent anti-myeloma therapies (sAMT) following progression on HDS269B, a BCMA CAR-T. Subsequent treatments included anti-CD38 mAb-based therapy, pomalidomide-based therapy, daratumumab-based therapy, and further rounds of CAR-T. Nine patients (45%) went on to receive more than three sAMTs. Apart from three patients who could not be evaluated, the ORR to the first sAMT was 35%, and the clinical benefit rate was 65% (60).
Van et al. analyzed the salvage treatments and outcomes of 79 R/R MM patients from two academic institutions who had progression of disease after treatment with BCMA CAR-T. The ORR for first-line salvage therapy was 43.4% and the median PFS was 3.5 months. Median overall survival was 17.9 months after multiline salvage therapy and researchers did not find ORR to decrease significantly up to beyond the fifth line of salvage therapy. The first-line treatment options include CAR-T Trials , non-BCMA-directed bispecific trial , venetoclax-based therapy , chemotherapy , BCMA-directed bispecific trial , selinexor-based therapy , autologous hematopoietic stem cell transplantation (Auto-HSCT) , doublet/triplet/quadruplet combination of approved agents , other in combinations (including MAPKi, checkpoint inhibitor or other trial) and BCMA antibody-drug conjugate (ADC) , the ORRs of these schemes were 100%, 75%, 66.7%, 57.9%, 50%, 40%, 33.3%, 31.8%, 9.1% and 0%, respectively (61). This study indicates that though the response rate and/or duration of each salvage treatment option after BCMA CAR-T recurrence is modest, there are a wide range of management strategies can be used as salvage anti-myeloma treatment options.
Reyes et al. analyzed 78 R/R MM patients who received BCMA CAR-T therapy. Of 42 patients with post-CAR-T PD, 37 (88%) received ≥1 follow-up salvage treatment with a median of 3 (range: 1-8) lines. ORR in patients who subsequently received BCMA CAR-T, BCMA-targeted BsAb, anti-CD38 antibodies, alkylators, proteosome inhibitors (PIs), or immunomodulatory drugs (IMiDs) as salvage therapy was 75%, 60%, 52.6%, 46.3%, 40.6% and 37.5%, respectively. In addition, they found that patients who had previously failed to respond to a specific drug class prior to CAR-T were able to show a renewed response after CAR-T relapsed (107). In the updated follow-up from last year, 58 patients received salvage therapy, and first-line salvage strategies included alkylating agent therapy, CD38-based combination therapy, and BCMA-directed alternative therapy. The ORR for first-line salvage therapy was 41%. The ORR of the other BCMA-directed CAR-T was 89% and the ORR of the BCMA-directed BsAb was 60% of all salvage treatment lines. In CD38-oriented combinations, ORR was 80% when combined with BsAb and the ORR was 44% in combination with IMiD and/or PI. The alkylator-based therapies were also effective with an ORR of 50% (108). Indeed, in their cohort, T-cell engaging rescue therapies appeared to be more effective. A recent retrospective study explored the prognosis and optimal treatment sequence for patients who relapsed after BCMA CAR-T or double antibody therapy. Among the 38 patients studied, 17 were in the CAR-T group and 21 in the BiAbs group, all of whom received salvage therapy after relapse. To identify the optimal treatment sequence, these patients were divided into four groups: CAR-T followed by BiAbs, CAR-T followed by other schemes, BiAbs followed by other BiAbs and BiAbs followed by other schemes. Results showed that nearly 90% of patients in the CAR-T followed by BiAbs group achieved at least a partial response (≥PR), with 38% achieving at least a complete response (≥CR). In contrast, the other three groups had lower ORR ranging from 30% to 50% and fewer instances of deep remission. Besides, the treatment sequence of CAR-T followed by BiAbs also produced a longer mPFS than CAR-T followed with other schemes, suggesting BiAbs could be an optimal therapeutic option after BCMA CAR-T (109).
In our recent retrospective analysis of R/R MM patients treated with LCAR-B38M enrolled in the LEGEND-2 trial showed that 34 (76%) patients had evidence of PD and 32 patients received salvage therapy, including PI-based combination therapy , second BCMA CAR-T , and combination chemotherapy . The ORR for this cohort was 50.0% with a median PFS was 16.3 months. In addition, the analysis found that despite 67% of the patients who received PI-based combination salvage therapy having been previously exposed to PI, this salvage therapy had a significantly higher ORR (80%) compared to second CAR-T infusion (30%) and chemotherapy (0%) (110).
In recent years, anti-BCMA CAR-T cell therapy has achieved impressive results in R/R MM patients with generally manageable side effects. However, certain challenges persist, such as the occurrence of recurrences even after anti-BCMA CAR-T cell therapy. Indeed, standards or recommended guidelines for post-CAR-T salvage therapy have not yet been established, highlighting a significant area of unmet need in multiple myeloma treatment. In this review, after summarizing the data of various salvage therapies, it appears that the efficacy of BCMA CAR-T therapy as a salvage therapy after prior BCMA CAR-T relapse seems to be limited, and other T-cell–engaging therapies, such as CAR-T targeting other targets, dual-target CAR-T, bispecific antibodies, etc., demonstrate notable clinical activity, with high response rate and long-lasting response. Currently, the data on dual-target CAR-T therapy for salvage treatment after BCMA CAR-T relapse primarily focus on combinations of BCMA with CS1 or CD19 (67, 68). Ongoing research is exploring the effectiveness of combining BCMA with other targets such as TACI, GPRC5D, CD38, CD24, and more. Clinical trials involving these combinations have shown promising anti-myeloma activity (111–115), indicating their potential to rescue patients experiencing recurrence in the future.
In addition, there are other molecules expressed on the MM cell surface that can be used as CAR-T targets, such as GPRC5D. Most CD138+ cells express both BCMA and GPRC5D, but the expression of the two is independent of each other. And the antigen escape risk of GPRC5D is very low, which makes it a hot research direction in recent years (77). CAR-T targeting CD38, Natural Killer Group 2D (NKG2D) ligand, FcRH5, and kappa light chains are also under continuous development and are expected to be a promising outcome for rescue therapy (116–120). Besides, patients are disassociated from previous therapeutic agents such as PIs and IMiDs during BCMA CAR-T therapy. MM resistant cells could be targeted and eliminated by CAR-T cells, potentially allowing patients to resume their original regimen following CAR-T relapse. Moreover, CAR-T therapy may have a positive effect on bone marrow microenvironment (110). New anti-myeloma drugs such as selinexor and carfilzomib also provide a new salvage option.
On the other hand, consolidate/maintain the efficacy of CAR-T after CAR-T cell infusion to achieve a longer duration of remission may provide potential clinical benefits for high-risk MM patients. One study reported a patient whose disease continued to progress after multiple chemotherapy regimens, mouse anti-BCMA CAR-T, and human anti-BCMA CAR-T. But he achieved VGPR lasting for more than 8 months after human-derived anti-BCMA CAR-T combined with lenalidomide regimen, indicating that lenalidomide as maintenance therapy increases the expansion and efficacy of anti-BCMA CAR-T (121). Besides, Zhao et al. found that pomalidomide maintained T cell viability, promoted cell proliferation and significantly enhanced TNF-ɑ and IFN-γ secretion in vitro studies. Three R/R MM patients with EMD achieved 100% ORR after receiving the combination therapy (122). Similarly, a prospective study presented at the ASH meeting in 2023 reported that long-term oral administration of pomalidomide (4 mg/day) after BCMA CAR-T cell infusion can enhance the efficacy of BCMA CAR-T cell therapy, prolong PFS, and reduce the recurrence rate in patients with R/R MM (123). The above findings indicate that combination with immunomodulator can effectively enhance the efficacy of CAR-T. Certainly, the application of the combination therapy still requires strict clinical trial verification.
In conclusion, conducting long-term follow-up and larger studies is imperative to elucidate the role of these new therapies following BCMA CAR-T therapy. This will help determine the optimal sequence and combination regimen for treating relapsed/refractory multiple myeloma patients after CAR-T therapy relapse (124). Furthermore, with BCMA CAR-T now approved for earlier lines of treatment, the goal of post-CAR-T therapy should extend beyond merely rescuing patients from imminent death. Instead, the focus should be on strategically planning treatments to further prolong patient survival. Continued exploration of more effective options for managing relapse is equally essential. Ultimately, CAR-T therapy will become an even more powerful weapon against multiple myeloma.
BF: Visualization, Writing – original draft, Writing – review & editing. RL: Writing – original draft, Writing – review & editing. GG: Visualization, Writing – review & editing. ZL: Visualization, Writing – review & editing. AH: Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Joshua DE, Bryant C, Dix C, Gibson J, Ho J. Biology and therapy of multiple myeloma. Med J Aust. (2019) 210:375–80. doi: 10.5694/mja2.50129
2. Dimopoulos MA, Jakubowiak AJ, McCarthy PL, Orlowski RZ, Attal M, Bladé J, et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. (2020) 10:17. doi: 10.1038/s41408-020-0273-x
3. Rafae A, van Rhee F, Al Hadidi S. Perspectives on the treatment of multiple myeloma. Oncologist. (2024) 29:200–12. doi: 10.1093/oncolo/oyad306
4. Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. (2020) 370:m3176. doi: 10.1136/bmj.m3176
5. Uckun FM, Qazi S, Demirer T, Champlin RE. Contemporary patient-tailored treatment strategies against high risk and relapsed or refractory multiple myeloma. EBioMedicine. (2018) 39:612–20. doi: 10.1016/j.ebiom.2018.12.004
6. Garfall AL. New biological therapies for multiple myeloma. Annu Rev Med. (2024) 75:13–29. doi: 10.1146/annurev-med-050522-033815
7. Sperling AS, Anderson KC. Facts and hopes in multiple myeloma immunotherapy. Clin Cancer Res. (2021) 27:4468. doi: 10.1158/1078-0432.CCR-20-3600
8. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. (2019) 380:1726–37. doi: 10.1056/NEJMoa1817226
9. Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. (2020) 34:985–1005. doi: 10.1038/s41375-020-0734-z
10. San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. (2023) 389:335–47. doi: 10.1056/NEJMoa2303379
11. Martino M, Canale FA, Alati C, Vincelli ID, Moscato T, Porto G, et al. CART-cell therapy: Recent advances and new evidence in multiple myeloma. Cancers. (2021) 13:2639. doi: 10.3390/cancers13112639
12. Xu J, Wang B-Y, Yu S-H, Chen S-J, Yang S-S, Liu R, et al. Long-term remission and survival in patients with relapsed or refractory multiple myeloma after treatment with LCAR-B38M CAR T cells: 5-year follow-up of the LEGEND-2 trial. J Hematol Oncol. (2024) 17:23. doi: 10.1186/s13045-024-01530-z
13. Sellner L, Fan F, Giesen N, Schubert M-L, Goldschmidt H, Müller-Tidow C, et al. B-cell maturation antigen-specific chimeric antigen receptor T cells for multiple myeloma: Clinical experience and future perspectives. Int J Cancer. (2020) 147:2029–41. doi: 10.1002/ijc.33002
14. Tai Y-T, Acharya C, An G, Moschetta M, Zhong MY, Feng X, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. (2016) 127:3225–36. doi: 10.1182/blood-2016-01-691162
15. Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T cells genetically modified to express an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. (2018) 36:2267–80. doi: 10.1200/JCO.2018.77.8084
16. Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. (2016) 128:1688–700. doi: 10.1182/blood-2016-04-711903
17. Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai Y-T, Prabhala R, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun. (2021) 12:868. doi: 10.1038/s41467-021-21177-5
18. Lee H, Ahn S, Maity R, Leblay N, Ziccheddu B, Truger M, et al. Mechanisms of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in multiple myeloma. Nat Med. (2023) 29:2295–306. doi: 10.1038/s41591-023-02491-5
19. Meinl E, Krumbholz M. Endogenous soluble receptors sBCMA and sTACI: Biomarker, immunoregulator and hurdle for therapy in multiple myeloma. Curr Opin Immunol. (2021) 71:117–23. doi: 10.1016/j.coi.2021.06.015
20. Zhou X, Rasche L, Kortüm KM, Mersi J, Einsele H. BCMA loss in the epoch of novel immunotherapy for multiple myeloma: from biology to clinical practice. Haematologica. (2022) 108:958–68. doi: 10.3324/haematol.2020.266841
21. Cowan AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby EN, et al. [amp]]gamma;-Secretase inhibitor in combination with BCMA chimeric antigen receptor T-cell immunotherapy for individuals with relapsed or refractory multiple myeloma: a phase 1, first-in-human trial. Lancet Oncol. (2023) 24:811–22. doi: 10.1016/S1470-2045(23)00246-2
22. Garfall AL, Dancy EK, Cohen AD, Hwang W-T, Fraietta JA, Davis MM, et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. (2019) 3:2812–5. doi: 10.1182/bloodadvances.2019000600
23. Honikel MM, Olejniczak SH. Co-stimulatory receptor signaling in CAR-T cells. Biomolecules. (2022) 12:1303. doi: 10.3390/biom12091303
24. Peng J-J, Wang L, Li Z, Ku C-L, Ho P-C. Metabolic challenges and interventions in CAR T cell therapy. Sci Immunol. (2023) 8:eabq3016. doi: 10.1126/sciimmunol.abq3016
25. Berger TR, Maus MV. Mechanisms of response and resistance to CAR T cell therapies. Curr Opin Immunol. (2021) 69:56–64. doi: 10.1016/j.coi.2021.02.010
26. Dai Q, Han P, Qi X, Li F, Li M, Fan L, et al. 4-1BB signaling boosts the anti-tumor activity of CD28-incorporated 2nd generation chimeric antigen receptor-modified T Cells. Front Immunol. (2020) 11:539654. doi: 10.3389/fimmu.2020.539654
27. Labanieh L, Mackall CL. CAR immune cells: design principles, resistance and the next generation. Nature. (2023) 614:635–48. doi: 10.1038/s41586-023-05707-3
28. Pietrobon V, Todd LA, Goswami A, Stefanson O, Yang Z, Marincola F. Improving CAR T-cell persistence. Int J Mol Sci. (2021) 22:10828. doi: 10.3390/ijms221910828
29. Khan AN, Chowdhury A, Karulkar A, Jaiswal AK, Banik A, Asija S, et al. Immunogenicity of CAR-T Cell therapeutics: Evidence, mechanism and mitigation. Front Immunol. (2022) 13:886546. doi: 10.3389/fimmu.2022.886546
30. Manier S, Ingegnere T, Escure G, Prodhomme C, Nudel M, Mitra S, et al. Current state and next-generation CAR-T cells in multiple myeloma. Blood Rev. (2022) 54:100929. doi: 10.1016/j.blre.2022.100929
31. Zeng W, Zhang P. Resistance and recurrence of Malignancies after CAR-T cell therapy. Exp Cell Res. (2022) 410:112971. doi: 10.1016/j.yexcr.2021.112971
32. Xu J, Chen L-J, Yang S-S, Sun Y, Wu W, Liu Y-F, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U.S.A. (2019) 116:9543–51. doi: 10.1073/pnas.1819745116
33. Zhao W-H, Wang B-Y, Chen L-J, Fu W-J, Xu J, Liu J, et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: a phase 1, single-arm, open-label, multicenter study in China (LEGEND-2). J Hematol Oncol. (2022) 15:86. doi: 10.1186/s13045-022-01301-8
34. Mikkilineni L, Natrakul DA, Lam N, Manasanch EE, Mann J, Weissler KA, et al. Rapid anti-myeloma activity by T cells expressing an anti-BCMA CAR with a human heavy-chain-only antigen-binding domain. Mol Ther. (2024) 32:503–26. doi: 10.1016/j.ymthe.2023.12.018
35. Kumar SK, Baz RC, Orlowski RZ, Anderson LD Jr, Ma H, Shrewsbury A, et al. Results from lummicar-2: A phase 1b/2 study of fully human B-cell maturation antigen-specific CAR T cells (CT053) in patients with relapsed and/or refractory multiple myeloma. Blood. (2020) 136:28–9. doi: 10.1182/blood-2020-139802
36. Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai H, et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood. (2021) 137:2890–901. doi: 10.1182/blood.2020008936
37. Gumber D, Wang LD. Improving CAR-T immunotherapy: Overcoming the challenges of T cell exhaustion. EBioMedicine. (2022) 77:103941. doi: 10.1016/j.ebiom.2022.103941
38. Tang L, Zhang Y, Hu Y, Mei H. T Cell Exhaustion and CAR-T immunotherapy in hematological Malignancies. BioMed Res Int. (2021) 2021:6616391. doi: 10.1155/2021/6616391
39. Zhang X, Zhang H, Lan H, Wu J, Xiao Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. (2023) 14:1101495. doi: 10.3389/fimmu.2023.1101495
40. Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. (2019) 129:2210–21. doi: 10.1172/JCI126397
41. Ledergor G, Fan Z, Wu K, McCarthy E, Hyrenius-Wittsten A, Starzinski A, et al. CD4+ CAR-T cell exhaustion associated with early relapse of multiple myeloma after BCMA CAR-T cell therapy. Blood Adv. (2024) 8:3562–75. doi: 10.1182/bloodadvances.2023012416
42. Xia A, Zhang Y, Xu J, Yin T, Lu X-J. T cell dysfunction in cancer immunity and immunotherapy. Front Immunol. (2019) 10:1719. doi: 10.3389/fimmu.2019.01719
43. Ruella M, Korell F, Porazzi P, Maus MV. Mechanisms of resistance to chimeric antigen receptor-T cells in haematological Malignancies. Nat Rev Drug Discovery. (2023) 22:976–95. doi: 10.1038/s41573-023-00807-1
44. Agarwal S, Aznar MA, Rech AJ, Good CR, Kuramitsu S, Da T, et al. Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Immunity. (2023) 56:2388–2407.e9. doi: 10.1016/j.immuni.2023.09.001
45. Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)–modified T cells: refueling the CAR. Blood. (2017) 129:1039–41. doi: 10.1182/blood-2016-09-738245
46. Ouyang W, Jin S-W, Xu N, Liu W-Y, Zhao H, Zhang L, et al. PD-1 downregulation enhances CAR-T cell antitumor efficiency by preserving a cell memory phenotype and reducing exhaustion. J Immunother Cancer. (2024) 12:e008429. doi: 10.1136/jitc-2023-008429
47. Holthof LC, Mutis T. Challenges for immunotherapy in multiple myeloma: Bone marrow microenvironment-mediated immune suppression and immune resistance. Cancers (Basel). (2020) 12:988. doi: 10.3390/cancers12040988
48. Kankeu Fonkoua LA, Sirpilla O, Sakemura R, Siegler EL, Kenderian SS. CAR T cell therapy and the tumor microenvironment: Current challenges and opportunities. Mol Ther Oncolytics. (2022) 25:69–77. doi: 10.1016/j.omto.2022.03.009
49. Kennedy VE, Wong C, Huang C-Y, Kambhampati S, Wolf J, Martin TG, et al. Macrophage activation syndrome-like (MAS-L) manifestations following BCMA-directed CAR T cells in multiple myeloma. Blood Adv. (2021) 5:5344–8. doi: 10.1182/bloodadvances.2021005020
50. Li W, Zhang B, Cao W, Zhang W, Li T, Liu L, et al. Identification of potential resistance mechanisms and therapeutic targets for the relapse of BCMA CAR-T therapy in relapsed/refractory multiple myeloma through single-cell sequencing. Exp Hematol Oncol. (2023) 12:44. doi: 10.1186/s40164-023-00402-5
51. Sakemura R, Hefazi M, Siegler EL, Cox MJ, Larson DP, Hansen MJ, et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to CART-cell therapy in multiple myeloma. Blood. (2022) 139:3708–21. doi: 10.1182/blood.2021012811
52. Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. (2023) 388:1002–14. doi: 10.1056/NEJMoa2213614
53. Parikh RH, Lonial S. Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice. CA Cancer J Clin. (2023) 73:275–85. doi: 10.3322/caac.21771
54. Rytlewski J, Madduri D, Fuller J, Campbell TB, Mashadi-Hossein A, Thompson EG, et al. Effects of prior alkylating therapies on preinfusion patient characteristics and starting material for CAR T cell product manufacturing in late-line multiple myeloma. Blood. (2020) 136:7–8. doi: 10.1182/blood-2020-134369
55. Hansen DK, Sidana S, Peres LC, Colin Leitzinger C, Shune L, Shrewsbury A, et al. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: real-world experience from the myeloma CAR T consortium. J Clin Oncol. (2023) 41:2087–97. doi: 10.1200/JCO.22.01365
56. Jo T, Yoshihara S, Okuyama Y, Fujii K, Henzan T, Kahata K, et al. Risk factors for CAR-T cell manufacturing failure among DLBCL patients: A nationwide survey in Japan. Br J Haematology. (2023) 202:256–66. doi: 10.1111/bjh.18831
57. Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu M-F, Ivanova A, et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory hodgkin lymphoma. J Clin Oncol. (2020) 38:3794–804. doi: 10.1200/JCO.20.01342
58. Sidana S, Ahmed N, Akhtar OS, Heim M, Brazauskas R, Hansen DK, et al. Real world outcomes with idecabtagene vicleucel (Ide-cel) CAR-T cell therapy for relapsed/refractory multiple myeloma. Blood. (2023) 142:1027–7. doi: 10.1182/blood-2023-181762
59. Bisht K, Fukao T, Chiron M, Richardson P, Atanackovic D, Chini E, et al. Immunomodulatory properties of CD38 antibodies and their effect on anticancer efficacy in multiple myeloma. Cancer Med. (2023) 12:20332–52. doi: 10.1002/cam4.6619
60. Chen D, Wang X, Chen Z, Jiang S, Jiang H, Fu W, et al. Subsequent anti-myeloma therapy after maturation antigen (BCMA) chimeric antigen receptor (CAR)-T cell (HDS269B) treatment in patients with relapsed/refractory multiple myeloma. Am J Hematol. (2022) 97:E478–81. doi: 10.1002/ajh.26745
61. Van Oekelen O, Nath K, Mouhieddine TH, Farzana T, Aleman A, Melnekoff DT, et al. Interventions and outcomes of patients with multiple myeloma receiving salvage therapy after BCMA-directed CAR T therapy. Blood. (2023) 141:756–65. doi: 10.1182/blood.2022017848
62. Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. (2021) 384:705–16. doi: 10.1056/NEJMoa2024850
63. Ferreri CJ, Hildebrandt MAT, Hashmi H, Shune LO, McGuirk JP, Sborov DW, et al. Real-world experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted therapy. Blood Cancer J. (2023) 13:1–9. doi: 10.1038/s41408-023-00886-8
64. Zhang M, Wei G, Zhou L, Zhou J, Chen S, Zhang W, et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematology. (2023) 10:e107–16. doi: 10.1016/S2352-3026(22)00372-6
65. Xia J, Li H, Yan Z, Zhou D, Wang Y, Qi Y, et al. Anti–G Protein–Coupled Receptor, Class C Group 5 Member D chimeric antigen receptor T cells in patients with relapsed or refractory multiple myeloma: A single-arm, Phase II trial. J Clin Oncol. (2023) 41:2583–93. doi: 10.1200/JCO.22.01824
66. Li S, Yuan Z, Liu L, Li Y, Luo L, Chen Y, et al. Safety and efficacy of GPRC5D CAR T cell therapy in relapsed/refractory multiple myeloma patients. Blood. (2023) 142:3472. doi: 10.1182/blood-2023-179147
67. Li C, Xu J, Luo W, Liao D, Xie W, Wei Q, et al. Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma. Leukemia. (2024) 38:149–59. doi: 10.1038/s41375-023-02065-x
68. Shi M, Wang J, Huang H, Liu D, Cheng H, Wang X, et al. Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun. (2024) 15:3371. doi: 10.1038/s41467-024-47801-8
69. Riedhammer C, Bassermann F, Besemer B, Bewarder M, Brunner F, Carpinteiro A, et al. Real-world analysis of teclistamab in 123 RRMM patients from Germany. Leukemia. (2024) 38:365–71. doi: 10.1038/s41375-024-02154-5
70. Chari A, Minnema MC, Berdeja JG, Oriol A, Van De Donk NWCJ, Rodríguez-Otero P, et al. Talquetamab, a T-cell–redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. (2022) 387:2232–44. doi: 10.1056/NEJMoa2204591
71. Trudel S, Cohen AD, Krishnan AY, Fonseca R, Spencer A, Berdeja JG, et al. Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): Updated results from an ongoing Phase I study. Blood. (2021) 138:157. doi: 10.1182/blood-2021-147983
72. Vogl DT, Atrash S, Holstein SA, Nadeem O, Benson DM, Suryanarayan K, et al. Final results from the first-in-human Phase 1/2 study of Modakafusp Alfa, an immune-targeting attenuated cytokine, in patients (Pts) with relapsed/refractory multiple myeloma (RRMM). Blood. (2022) 140:1357–9. doi: 10.1182/blood-2022-162253
73. Banerjee R, Lynch RC, Wu Q, Simon S, Ujjani CS, Till BG, et al. A phase 2 study of nivolumab for relapsed/refractory multiple myeloma or Non-Hodgkin Lymphoma following CAR-T therapy. Blood. (2023) 142:3509. doi: 10.1182/blood-2023-173202
74. Chari A, Vogl DT, Jagannath S, Jasielec J, Unger TJ, DeCastro A, et al. Selinexor-based regimens for the treatment of myeloma refractory to chimeric antigen receptor T cell therapy. Br J Haematol. (2020) 189:e126–30. doi: 10.1111/bjh.16550
75. Cui R, Li P, Li Q, Mu J, Jiang Y, Jiang Y, et al. Humanized BCMA CAR- T cell salvage therapy in two refractory multiple myeloma patients who progressed after their murine BCMA CAR-T cell therapy. Zhonghua Xue Ye Xue Za Zhi. (2021) 42:502–7. doi: 10.3760/cma.j.issn.0253-2727.2021.06.010
76. Wang Q, Wei R, Guo S, Min C, Zhong X, Huang H, et al. An alternative fully human anti-BCMA CAR-T shows response for relapsed or refractory multiple myeloma with anti-BCMA CAR-T exposures previously. Cancer Gene Ther. (2024) 31:420–6. doi: 10.1038/s41417-023-00712-0
77. Smith EL, Harrington K, Staehr M, Masakayan R, Jones J, Long TJ, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. (2019) 11:eaau7746. doi: 10.1126/scitranslmed.aau7746
78. Elemian S, Al Hadidi S. Targeting GPRC5D in multiple myeloma. Expert Rev Anticancer Ther. (2024) 0:1–10. doi: 10.1080/14737140.2024.2343114
79. Pillarisetti K, Edavettal S, Mendonça M, Li Y, Tornetta M, Babich A, et al. A T-cell–redirecting bispecific G-protein–coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. (2020) 135:1232–43. doi: 10.1182/blood.2019003342
80. Rodriguez-Otero P, van de Donk NWCJ, Pillarisetti K, Cornax I, Vishwamitra D, Gray K, et al. GPRC5D as a novel target for the treatment of multiple myeloma: a narrative review. Blood Cancer J. (2024) 14:24. doi: 10.1038/s41408-023-00966-9
81. Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al. GPRC5D-targeted CAR T cells for myeloma. N Engl J Med. (2022) 387:1196–206. doi: 10.1056/NEJMoa2209900
82. Bal S, Htut M, Nadeem O, Anderson LD, Koçoğlu H, Gregory T, et al. BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-targeted chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory multiple myeloma (RRMM): Updated results from a Phase 1 study. Blood. (2023) 142:219–9. doi: 10.1182/blood-2023-181857
83. Choi T, Kang Y. Chimeric Antigen Receptor (CAR) T-cell therapy for multiple myeloma. Pharmacol Ther. (2022) 232:108007. doi: 10.1016/j.pharmthera.2021.108007
84. Soekojo CY, Chng WJ. Treatment horizon in multiple myeloma. Eur J Haematol. (2022) 109:425–40. doi: 10.1111/ejh.13840
85. Furqan F, Shah NN. Multispecific CAR T Cells deprive lymphomas of escape via antigen loss. Annu Rev Med. (2023) 74:279–91. doi: 10.1146/annurev-med-042921-024719
86. Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. (2008) 14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246
87. Chu E, Wu J, Kang SS, Kang Y. SLAMF7 as a promising immunotherapeutic target in multiple myeloma treatments. Curr Oncol. (2023) 30:7891–903. doi: 10.3390/curroncol30090573
88. Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. (2004) 103:2332–6. doi: 10.1182/blood-2003-09-3064
89. Paiva B, Puig N, Cedena M, de Jong B, Ruiz Y, Rapado I, et al. Differentiation stage of myeloma plasma cells: biological and clinical significance. Leukemia. (2017) 31:382–92. doi: 10.1038/leu.2016.211
90. Nerreter T, Letschert S, Götz R, Doose S, Danhof S, Einsele H, et al. Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat Commun. (2019) 10:3137. doi: 10.1038/s41467-019-10948-w
91. Cho S-F, Yeh T-J, Anderson KC, Tai Y-T. Bispecific antibodies in multiple myeloma treatment: A journey in progress. Front Oncol. (2022) 12:1032775. doi: 10.3389/fonc.2022.1032775
92. Lancman G, Sastow DL, Cho HJ, Jagannath S, Madduri D, Parekh SS, et al. Bispecific antibodies in multiple myeloma: present and future. Blood Cancer Discovery. (2021) 2:423–33. doi: 10.1158/2643-3230.BCD-21-0028
93. Touzeau C, Krishnan AY, Moreau P, Perrot A, Usmani SZ, Manier S, et al. Efficacy and safety of teclistamab (tec), a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in patients (pts) with relapsed/refractory multiple myeloma (RRMM) after exposure to other BCMA-targeted agents. JCO. (2022) 40:8013–3. doi: 10.1200/JCO.2022.40.16_suppl.8013
94. Dima D, Davis JA, Ahmed N, Jia X, Sannareddy A, Shaikh H, et al. Safety and efficacy of teclistamab in patients with relapsed/refractory multiple myeloma: A real-world experience. Transplant Cell Ther. (2024) 30:308.e1–308.e13. doi: 10.1016/j.jtct.2023.12.016
95. Liu L, Krishnan A. Talquetamab in multiple myeloma. Haematologica. (2023) 109:718–24. doi: 10.3324/haematol.2023.283931
96. Jakubowiak AJ, Anguille S, Karlin L, Chari A, Schinke C, Rasche L, et al. Updated results of talquetamab, a GPRC5D×CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma with prior exposure to T-cell redirecting therapies: Results of the Phase 1/2 MonumenTAL-1 study. Blood. (2023) 142:3377. doi: 10.1182/blood-2023-187242
97. Nooka AK, Kaufman JL, Hofmeister CC, Joseph NS, Heffner TL, Gupta VA, et al. Daratumumab in multiple myeloma. Cancer. (2019) 125:2364–82. doi: 10.1002/cncr.32065
98. Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:1582–96. doi: 10.1016/S1470-2045(21)00466-6
99. Bahlis NJ, Weisel K, Mateos M-V, Goldschmidt H, Martin T, Morillo D, et al. S192: Talquetamab (Tal) + daratumumab (Dara) in patients (Pts) with relapsed/refractory multiple myeloma (RRMM): Update TRIMM-2 results. Hemasphere. (2023) 7:e90062ca. doi: 10.1097/01.HS9.0000967680.90062.ca
100. Richter J, Madduri D, Richard S, Chari A. Selinexor in relapsed/refractory multiple myeloma. Ther Adv Hematol. (2020) 11:2040620720930629. doi: 10.1177/2040620720930629
101. Goldsmith SR, Liu L, Shiah K. Selinexor therapy for multiple myeloma and non-Hodgkin lymphomas. Curr Opin Oncol. (2022) 34:524–30. doi: 10.1097/CCO.0000000000000866
102. Rodriguez C, Solomon SR, Paul B, Nadeem O, Patel M, Wang J, et al. Preliminary results of the Oral CD73 inhibitor, Oric-533, in relapsed/refractory multiple myeloma (RRMM). Blood. (2023) 142:4761. doi: 10.1182/blood-2023-173730
103. Ababneh HS, Yee AJ, Raje NS, Martin S, Frigault MJ, Ng AK, et al. Radiation therapy as a bridging and salvage strategy in patients with relapsed or refractory multiple myeloma undergoing BCMA-targeted CAR T-cell therapy. Radiother Oncol. (2023) 189:109933. doi: 10.1016/j.radonc.2023.109933
104. Longcor J, Callander N, Oliver K, Chanan-Khan A, Ailawadhi S. Iopofosine I-131 treatment in late-line patients with relapsed/refractory multiple myeloma post anti-BCMA immunotherapy. Blood Cancer J. (2022) 12:130. doi: 10.1038/s41408-022-00725-2
105. Qian Y, Qian Z, Zhao X, Pan W, Wei X, Meng H, et al. Successful treatment of relapsed/refractory extramedullary multiple myeloma with anti-BCMA CAR-T cell therapy followed by haploidentical hematopoietic stem cell transplantation: A case report and a review of the contemporary literature. Front Med (Lausanne). (2021) 8:649824. doi: 10.3389/fmed.2021.649824
106. Elnaggar M, Agte S, Restrepo P, Ram M, Melnekoff D, Adamopoulos C, et al. Triple MAPK inhibition salvaged a relapsed post-BCMA CAR-T cell therapy multiple myeloma patient with a BRAF V600E subclonal mutation. J Hematol Oncol. (2022) 15:109. doi: 10.1186/s13045-022-01330-3
107. Reyes KR, Liu Y-C, Huang C-Y, Banerjee R, Martin T, Shah N, et al. Clinical outcomes and salvage therapies in patients with relapsed/refractory multiple myeloma following progression on BCMA-targeted CAR-T therapy. Blood. (2022) 140:617–9. doi: 10.1182/blood-2022-160401
108. Reyes KR, Liu Y-C, Huang C-Y, Banerjee R, Martin T, Wong SW, et al. Salvage therapies including retreatment with BCMA-directed approaches after BCMA CAR-T relapses for multiple myeloma. Blood Adv. (2024) 8:2207–16. doi: 10.1182/bloodadvances.2023012066
109. Puertas B, Fernández-Sánchez A, Alejo E, Rey-Bua B, Martín-López AÁ, Pérez López E, et al. A research center’s experience of T-cell redirecting therapies in triple-class refractory multiple myeloma. Blood Adv. (2024) 8:3478–87. doi: 10.1182/bloodadvances.2024012773
110. Liu R, Yang R, Xu X, Zhao W, Wang F, Zhang W, et al. Outcomes in patients with multiple myeloma receiving salvage treatment after BCMA-specific CAR-T therapy: A retrospective analysis of LEGEND-2. Br J Haematol. (2024) 204:1780–9. doi: 10.1111/bjh.19340
111. Larson RC, Kann MC, Graham C, Mount CW, Castano AP, Lee W-H, et al. Anti-TACI single and dual-targeting CAR T cells overcome BCMA antigen loss in multiple myeloma. Nat Commun. (2023) 14:7509. doi: 10.1038/s41467-023-43416-7
112. Sun F, Cheng Y, Wanchai V, Guo W, Mery D, Xu H, et al. Bispecific BCMA/CD24 CAR-T cells control multiple myeloma growth. Nat Commun. (2024) 15:615. doi: 10.1038/s41467-024-44873-4
113. Fernández de Larrea C, Staehr M, Lopez AV, Ng KY, Chen Y, Godfrey WD, et al. Defining an optimal dual-targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA escape–driven relapse in multiple myeloma. Blood Cancer Discovery. (2020) 1:146–54. doi: 10.1158/2643-3230.BCD-20-0020
114. Roders N, Nakid-Cordero C, Raineri F, Fayon M, Abecassis A, Choisy C, et al. Dual chimeric antigen receptor T cells targeting CD38 and SLAMF7 with independent signaling demonstrate preclinical efficacy and safety in multiple myeloma. Cancer Immunol Res. (2024) 12:478–90. doi: 10.1158/2326-6066.CIR-23-0839
115. Feng Y, Liu X, Li X, Zhou Y, Song Z, Zhang J, et al. Novel BCMA-OR-CD38 tandem-dual chimeric antigen receptor T cells robustly control multiple myeloma. Oncoimmunology. (2021) 10:1959102. doi: 10.1080/2162402X.2021.1959102
116. Li H, Li J, Wu J, Shi Z, Gao Y, Song W, et al. A second-generation CD38-CAR-T cell for the treatment of multiple myeloma. Cancer Med. (2023) 12:10804–15. doi: 10.1002/cam4.5818
117. Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase 1 trial of autologous CAR T Cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. (2019) 7:100–12. doi: 10.1158/2326-6066.CIR-18-0307
118. Jiang D, Huang H, Qin H, Tang K, Shi X, Zhu T, et al. Chimeric antigen receptor T cells targeting FcRH5 provide robust tumour-specific responses in murine xenograft models of multiple myeloma. Nat Commun. (2023) 14:3642. doi: 10.1038/s41467-023-39395-4
119. Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting Malignancy-associated κ light chains. J Clin Invest. (2016) 126:2588–96. doi: 10.1172/JCI86000
120. Cho S-F, Xing L, Anderson KC, Tai Y-T. Promising antigens for the new frontier of targeted immunotherapy in multiple myeloma. Cancers (Basel). (2021) 13:6136. doi: 10.3390/cancers13236136
121. Zhao G, Wei R, Feng L, Wu Y, He F, Xiao M, et al. Lenalidomide enhances the efficacy of anti-BCMA CAR-T treatment in relapsed/refractory multiple myeloma: a case report and revies of the literature. Cancer Immunol Immunother. (2021) 71:39–44. doi: 10.1007/s00262-021-02959-8
122. Zhao J, Yang H, Ge J, Li L, Yao Q, He S, et al. Pomalidomide improves the effectiveness of CAR-T treatment in the relapsed and refractory multiple myeloma or B-cell leukemia/lymphoma with extramedullary disease. Blood Sci. (2024) 6:e00184. doi: 10.1097/BS9.0000000000000184
123. Yan Y, Tu Y, Wu D-P, Li X. BCMA CAR-T-cell therapy in combination with long-term pomalidomide is a safe and effective treatment for relapsed/refractory multiple myeloma. Blood. (2023) 142:2116. doi: 10.1182/blood-2023-181573
Keywords: multiple myeloma, BCMA CAR-T, relapse, salvage treatment, T-cell -engaging therapy
Citation: Fu B, Liu R, Gao G, Lin Z and He A (2024) Mechanisms and salvage treatments in patients with multiple myeloma relapsed post-BCMA CAR-T cell therapy. Front. Immunol. 15:1433774. doi: 10.3389/fimmu.2024.1433774
Received: 16 May 2024; Accepted: 07 October 2024;
Published: 22 October 2024.
Edited by:
Muhammad Salman Faisal, University at Buffalo, United StatesReviewed by:
Larry D. Anderson Jr., University of Texas Southwestern Medical Center, United StatesCopyright © 2024 Fu, Liu, Gao, Lin and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aili He, aGVhaWxpQHhqdHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.