Low rates of headache and migraine associated with intravenous immunoglobulin infusion using a 15-minute rate escalation protocol in 123 patients with primary immunodeficiency
- 1Division of Allergy & Immunology, University of California, San Diego, CA, United States
- 2Global Medical Department, Bio Products Laboratory, Ltd., Elstree, United Kingdom
- 3Biostatistics Department, Atlantic Research Group, Charlottesville, VA, United States
by Geng B, Clark K, Evangelista M and Wolford E (2023). Front. Immunol. 13:1075527. doi: 10.3389/fimmu.2022.1075527
Error in figure/table caption
In the published article, there was an error in the caption for Figure 4 as published. The current caption to Figure 4 mistakenly describes the figure as depicting weeks on the x-axis when it is actually reporting number of infusions. The figure caption was displayed as “Incidence of product-related headache and migraine by infusion number, number of events (A) 5% IVIG formulation (108 patients for weeks 1–5, then 75 patients for weeks 6–18) (B) 10% IVIG formulation (48 patients)”. The corrected caption appears below.
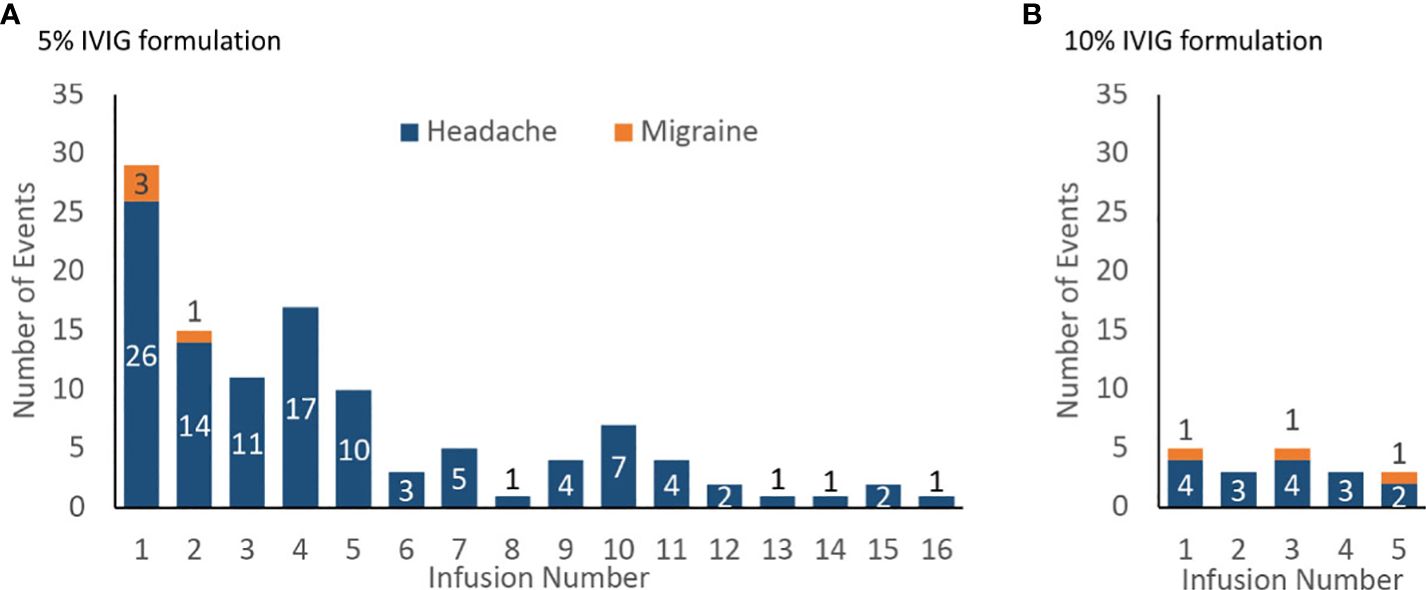
Figure 4 Incidence of product-related headache and migraine by infusion number, number of events (A) 5% IVIG formulation (108 patients for infusions 1–5, then 75 patients for infusions 6–18) (B) 10% IVIG formulation (48 patients).
Incidence of product-related headache and migraine by infusion number, number of events (A) 5% IVIG formulation (108 patients for infusions 1–5, then 75 patients for infusions 6–18) (B) 10% IVIG formulation (48 patients).
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: IVIg, headache, migraine, primary immunodefciencies, rate escalation, pooled
Citation: Geng B, Clark K, Evangelista M and Wolford E (2024) Corrigendum: Low rates of headache and migraine associated with intravenous immunoglobulin infusion using a 15-minute rate escalation protocol in 123 patients with primary immunodeficiency. Front. Immunol. 15:1430313. doi: 10.3389/fimmu.2024.1430313
Received: 09 May 2024; Accepted: 15 May 2024;
Published: 29 May 2024.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2024 Geng, Clark, Evangelista and Wolford. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kim Clark, a2ltLmNsYXJrQGtlZHJpb24uY29t
 Bob Geng
Bob Geng Kim Clark
Kim Clark Mark Evangelista3
Mark Evangelista3