- 1School of Basic Medical Sciences, Yunnan University of Chinese Medicine, Kunming, China
- 2Yunnan Key Laboratory of Integrated Traditional Chinese and Western Medicine for Chronic Disease in Prevention and Treatment, Yunnan University of Chinese Medicine, Kunming, China
- 3The First School of Clinical Medicine, Yunnan University of Chinese Medicine, Kunming, China
- 4Traditional Chinese Medicine Department, Tengchong People's Hospital, Tengchong, China
- 5Research Management Section, Kunming Municipal Hospital of Traditional Chinese Medicine, Kunming, China
- 6College of Traditional Chinese Medicine, Yunnan University of Chinese Medicine, Kunming, China
Background: Chronic viral infections, such as Human Immunodeficiency Virus (HIV), and their reactivation are considered potential contributing factors to Long-Corona Virus Disease (LC). However, research on the long-term sequelae of Long-COVID in individuals with HIV is limited.
Methods: We conducted a case-control study involving a total of 84 participants categorized into two groups: people living with HIV (PLWH) and people not living with HIV (PNLWH) within the six-month post-infection LC population. Differences in sequelae symptoms, cardiovascular biomarkers (VCAM-1, ICAM-1, and ACE2), Severe Acute Respiratory Syndrome Coronavirus 2 neutralization antibodies (SARS-CoV-2 nAb) and cytokines (IFN-γ, IL-6, and IL-17) were analyzed between the two groups.
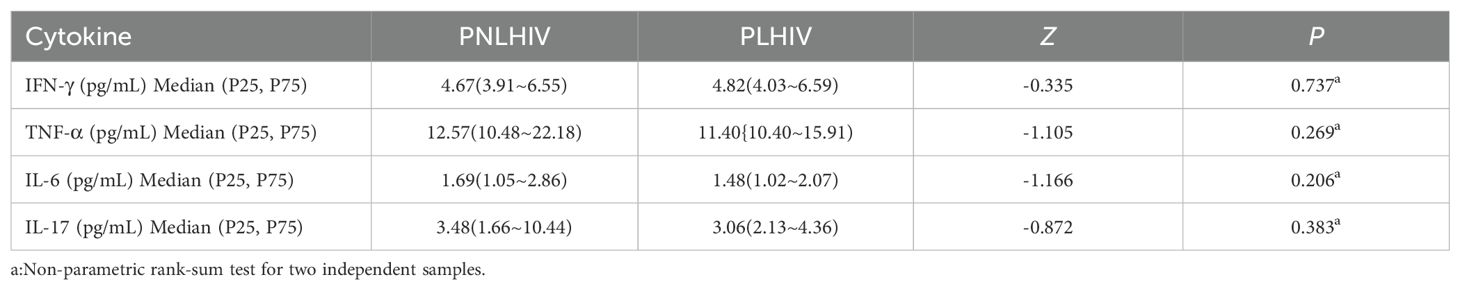
Results: After 6 months of infection, PLWH exhibited significantly higher serum levels of ACE2, VCAM-1, and ICAM-1 (P < 0.01, respectively) compared to PNLWH with COVID-19. Additionally, sequelae symptoms were more pronounced in PNLWH, and there were no differences in serum levels of IFN-γ, TNF-α, IL-6, and IL-17 between the two groups (P > 0.05, respectively).
Conclusion: PLWH had lower symptoms of LC and reduced frequency of symptoms, increased cardiovascular risk factors, and no differences in levels of inflammation or SARS-CoV-2 nAb levels when compared to PNLWH.
1 Introduction
Corona Virus Disease 2019 (COVID-19), caused by the highly contagious Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has resulted in a significant number of patients experiencing prolonged clinical symptoms for months after infection, a condition that is also known as “Long-COVID-19” or “Long-COVID” (LC) (1). This condition is characterized by persistent symptoms or delayed complications that manifest four weeks after the onset of the infection (2). As the number of recovered patients continues to rise, studies indicate that approximately 40.2% to 76% of patients, including those with mild acute illness, continue to face health issues six months post-infection (3). The long-term consequences of SARS-CoV-2 infection have now garnered increased attention (4). These enduring symptoms include fatigue, muscle weakness, insomnia, palpitations, chronic rhinitis, indigestion, chills, sore throat, and headaches (5). The potential mechanisms contributing to these symptoms include virus-specific cytopathic effects, inflammatory damage, immune responses to acute infection, and damage to organs and microvasculature (6). The presence of SARS-CoV-2 neutralization antibodies (SARS-CoV-2 nAb) has demonstrated a notable correlation with the severity spectrum of LC (7).
Recent studies have suggested that people living with Human Immunodeficiency Virus (PLWH) who are on effective antiretroviral therapy (ART) might face an elevated risk of developing LC (7, 8). However, information concerning this particular population is limited. Although cases of PLWH have been reported in early LC cohort studies, there is a lack of detailed characterisations of these individuals (9). In our study, we conducted a comparative analysis between PLWH and people not living with HIV (PNLWH) within the LC cohort. This analysis focused on differences in characteristics, vaccination status, common symptoms, cardiovascular biomarkers, and levels of inflammatory factors.
2 Materials and methods
2.1 Reagents
ACE2(EHC054 96Tests Lot.H230818-054a), VCAM-1(EHC123 96Tests Lot.H230818-123a), sICAM-1(EHC109 96Tests Lot.H230818-109a), IFN-γ (EHC102g 96Tests Lot.H230818-102a), IL-17(EHC170 96Tests Lot.H230818-170a), IL-6(EHC007 96Tests Lot.H230818-007a), and TNF-α( EHC103 96Tests Lot.H230818-103a) enzyme-linked immunosorbent assay (ELISA) kits were obtained from Neo Bio Science Technology Co, Ltd. (Shenzhen, China). SARS-CoV-2 nAb (RAS-N044 96Tests Lot.RA44-233W-19M) enzyme-linked immunosorbent assay (ELISA) kit was obtained from Acrobiosystems Co., Ltd. (Beijing, China).
2.2 Study design and participants
This comparative study was conducted during May and June 2023 at the Out-Patient Department, Tengchong People's Hospital, Yunnan Province, China. The definition of LC for our study adhered to the Centres for Disease Control and Prevention, considering signs, symptoms, and conditions persisting for 4 weeks or more after the initial phase of infection. Moreover, our study population also met the World Health Organization criteria, indicating symptoms lasting three or more months after the initial infection. Symptoms defined as LC encompassed respiratory (cough, dyspnea, chest pain), cardiovascular (easy fatigability, heart palpitations, chest tightness), neurological (dizziness, headache, sleep disturbance, loss of taste and/or smell), and musculoskeletal (joint pains, muscle pain) (10).
Inclusion criteria were set at 6 months after confirmed SARS-CoV-2 infection, 18-60 years of age, were willing to participate in the study, including survey completion, blood sample collection, and relevant laboratory testing. Individuals with severe hearing loss, impaired vision, or intellectual disability observed by the interviewers, or a major psychiatric illness were excluded. The researchers informed all enrollees about the study purpose and procedures. Identifiable information was kept confidential, and all participants provided written informed consent before screening for eligibility.
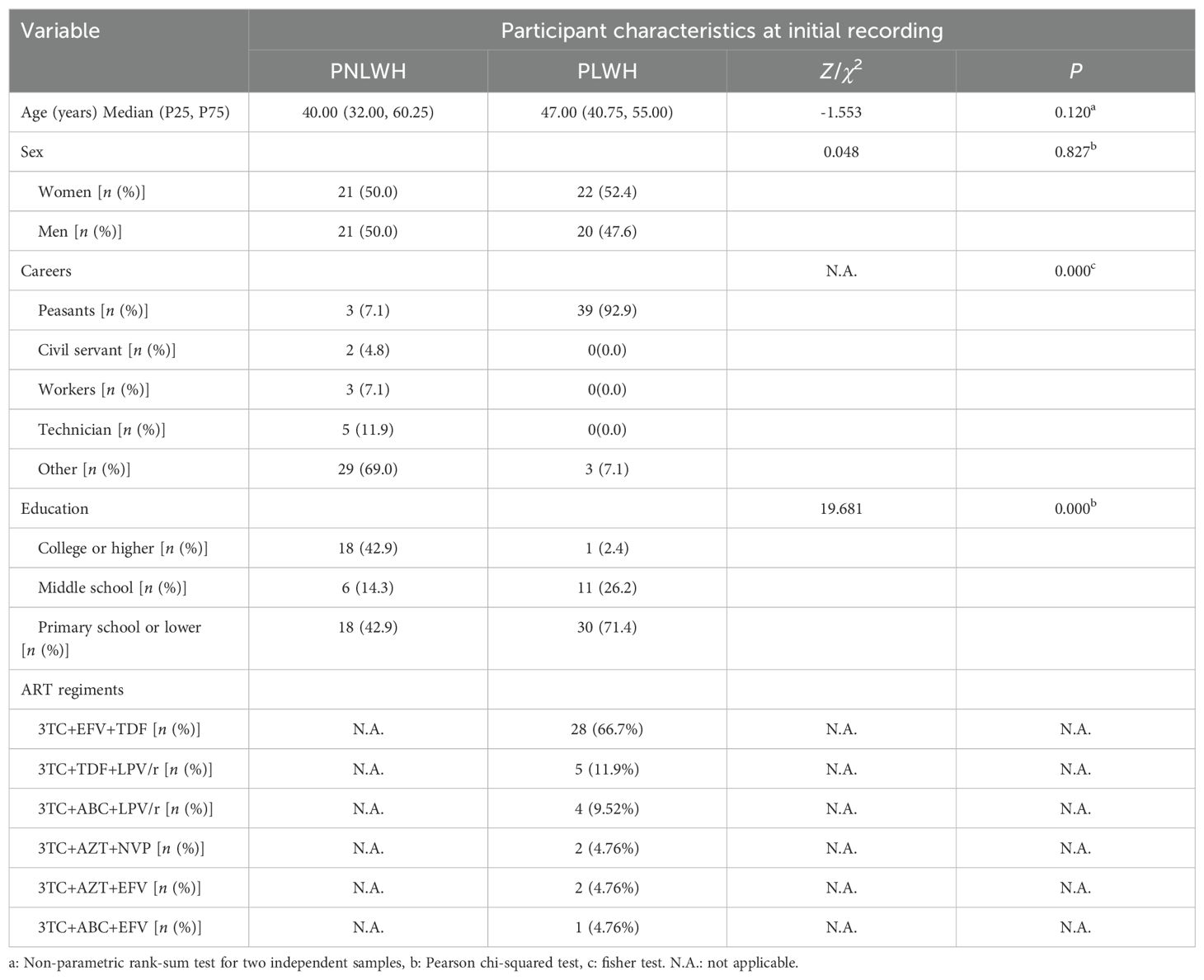
A total of 84 LC participants from Tengchong, Yunnan Province, China, were recruited based on the inclusion criteria, forming two groups: PLWH and PNLWH, all PLWH patients included in this study were undergoing ART (treatment regimens can be seen in Table 1) treatment during the course of the investigation and research, each group with 42 participants. The survey was conducted approximately 6 months after their initial nasopharyngeal swabs-positive test. Convalescent patients were asked to complete a questionnaire detailing self-reported symptoms related to LC at the specified time points in this study. Baseline characteristics for LC were documented, including age, sex, occupation, educational level. Probable Effect Factors Associated with Vaccine Hesitancy: COVID-19 Vaccination history, time from identification of contact with SARS-CoV-2 infected person to onset of symptoms, time to conversion to a negative nucleic acid test, and time to onset of symptoms. As part of the clinical study, a single venous blood draw will be conducted. This will occur during the sixth month of the initial positive nasopharyngeal swab test for COVID-19. The blood draw will be conducted on the morning of the day following the completion of this questionnaire survey. Under all fasting conditions, 2 milliliters of venous blood was collected at 8:00 a.m. The blood was centrifuged, serum was isolated, aliquoted at 250 µL, and frozen at -80°C. All samples were processed on the same day and kept frozen until use, and freeze/thaw cycles were avoided. The study received approval from the Research Ethics Commission of the First Affiliated Hospital of the Yunnan University of Chinese Medicine (NO. K [2020]015-02), and written informed consent was obtained from all patients (Figure 1).
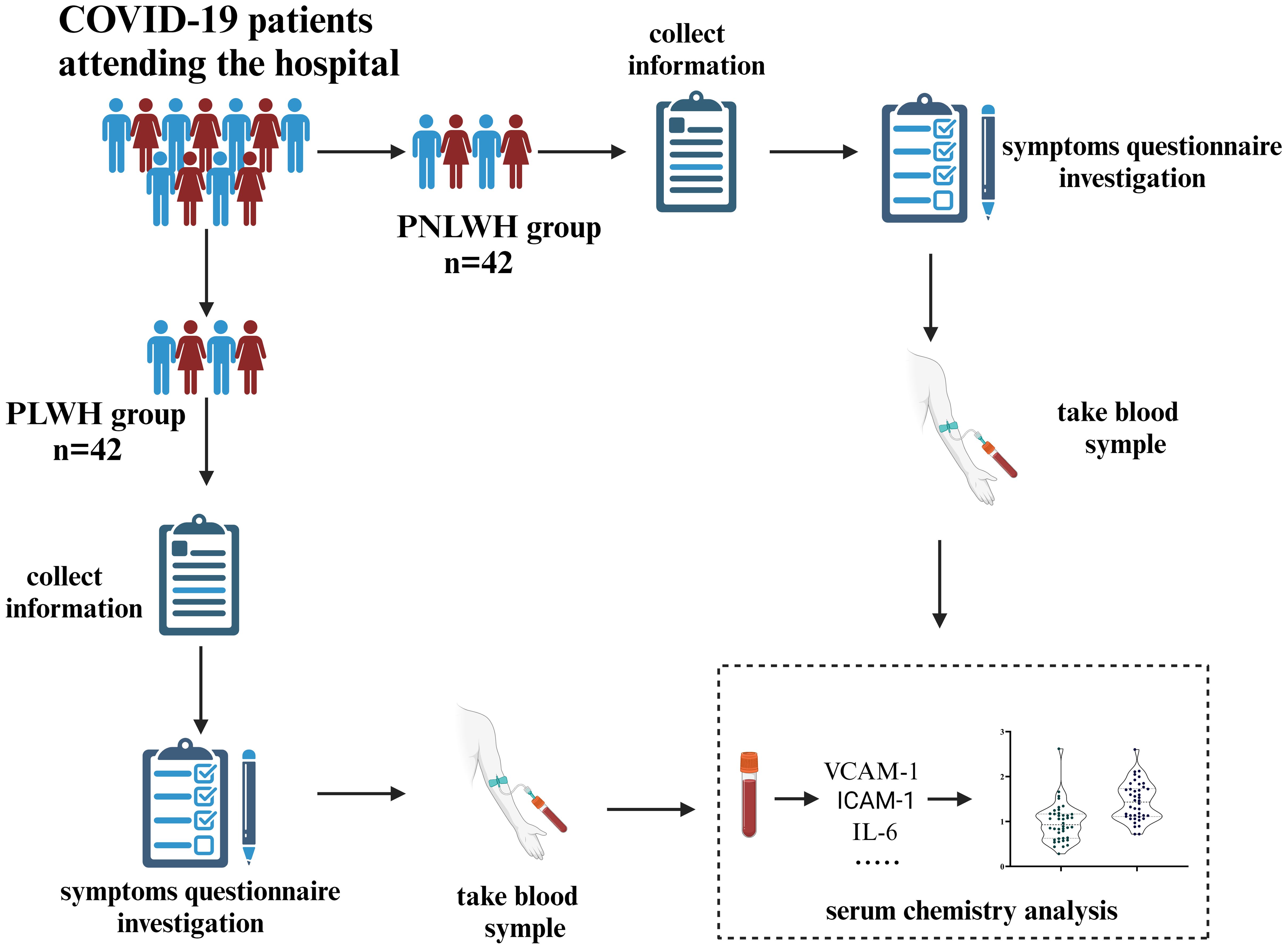
Figure 1. Flow chart of the study. 84 LC participants were invited and divided into PLWH and PNLWH groups, each with 42 participants. The survey, conducted about 6 months after a positive nasopharyngeal swab test, recorded patient characteristics via questionnaires. Venous blood samples were taken to assess SARS-CoV-2 nAb, cardiovascular biomarkers, and inflammatory factors.
2.3 SARS-CoV-2 nAb, inflammation and vascular biomarkers
Vascular blood biomarkers, including cardiovascular biomarkers (VCAM-1, ICAM-1, and ACE2), cytokines (IFN-γ, IL-6, and IL-17), were quantified using ELISA kits in accordance with the manufacturer's instructions.
2.4 Statistical analysis
The data analysis was conducted using SPSS 28.0. Normally distributed data was represented as mean ± SD, while non-normally distributed data was represented as Median (P25, P75). The Kolmogorov-Smirnov test was used to check for normal distribution, and independent samples t-tests were used for normally distributed data comparisons. The Mann-Whitney U test was used for non-normally distributed data comparisons. Count data was expressed as n(%) using the χ2 test. A significance level of P < 0.05 was considered statistically significant.
3 Results
3.1 Population demographic survey
The demographic and baseline characteristics of patients are shown in Table 1. No statistically significant difference in age and sex was observed between the PNLWH and PLWH groups (P > 0.05). (Table 1). Significant differences were observed in occupation and education (P < 0.01). The majority of participants in the PLWH group were employed as peasants, accounting for 92.9%, while in the PNLWH group, the majority had other occupations, constituting 69.0%. Additionally, the educational level in the PLWH group was generally lower, with 71.4% having completed primary school or lower, compared to the PNLWH group, where 42.9% had educational levels of primary school or lower (Table 1).
3.2 Probable effect factors associated with vaccine hesitancy
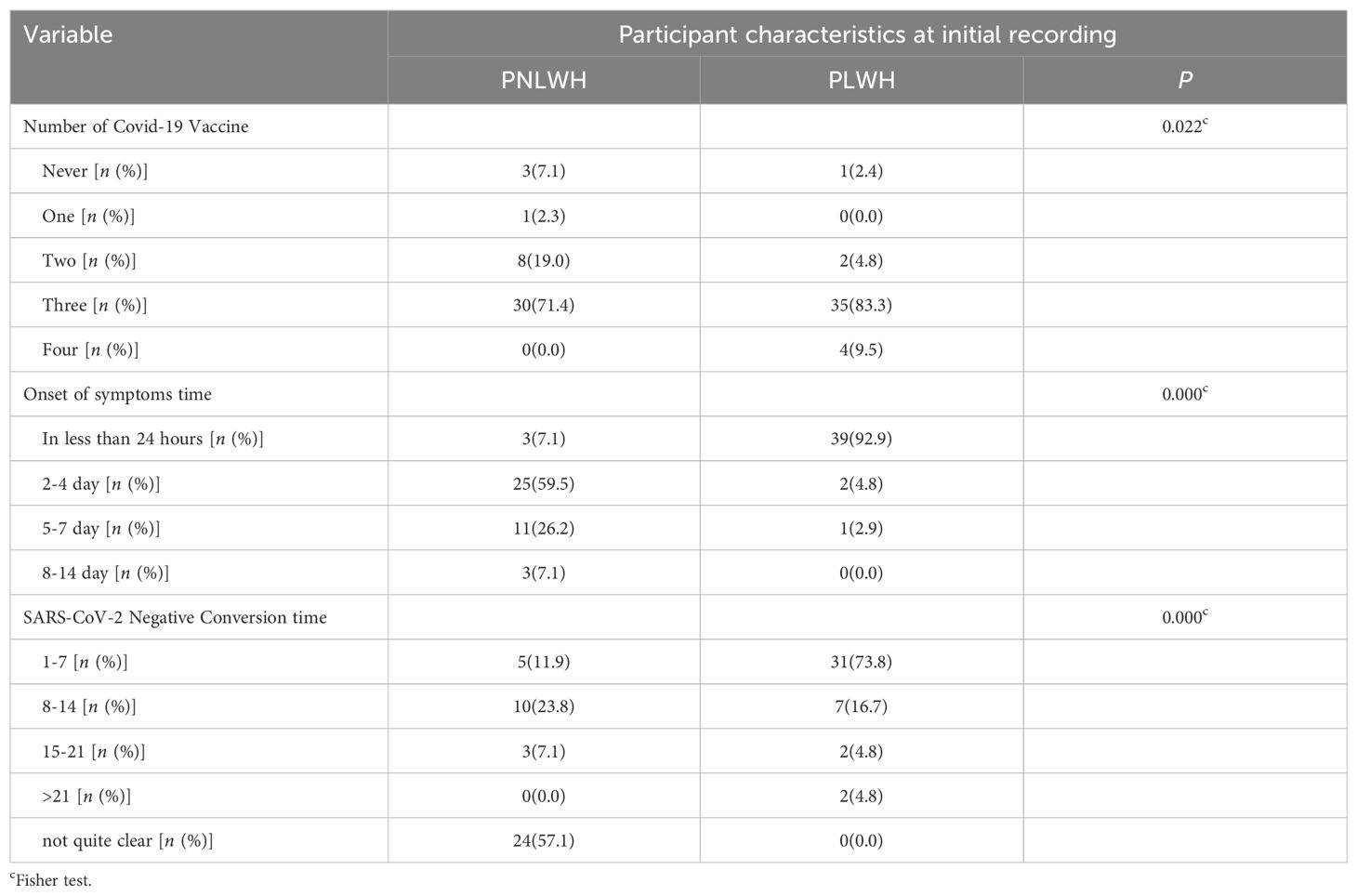
Significant differences were observed in the frequency of vaccination, onset of symptoms, and COVID-19 antigen-negative time between the PLWH and PNLWH groups (P < 0.05). Upon stratifying by the number of vaccine doses, the PNLWH group exhibited the highest proportion of vaccinated thrice (71.4%), followed by those vaccinated twice (19.0%). In the PLWH group, the majority also received three doses (83.3%), with the next highest group being those who received four doses (9.5%) (Table 2).
In the investigation of the time of the first symptoms after contact with SARS-CoV-2 infection individuals, notable differences were found between the PNLWH and PLWH groups. In the PNLWH group, the distribution of reported times was as follows: less than 24 hours (7.1%), 2–4 days (59.5%), 5–7 days (26.2%), and 8–14 days (7.1%). In contrast, the PLWH group reported the following distribution: less than 24 hours (92.9%), 2–4 days (4.8%), 5–7 days (2.9%), and 8–14 days (0%) (Table 2).
Moreover, a significant difference was observed in the time it took for the SARS-CoV-2 nucleic acid test (polymerase chain reaction) to transition from positive to negative between the PNLWH and PLWH groups (P < 0.01). Specifically, PNLWH in the group compared to the PLWH group: 1-7 days (11.9% vs 73.8%), 8–14 days (23.8% vs 16.7%), 15–21 days (7.1% vs 4.8%), more than 21 days (0% vs 4.8%), not quite clear (57.1% vs 0%) (Table 2).
3.3 SARS-CoV-2 nAb level
Upon conducting a comparative evaluation of serum SARS-CoV-2 nAb level between the PNLWH and PLWH groups, the analysis revealed no statistically significant differences (P >0.05) between the two cohorts (Figure 2).
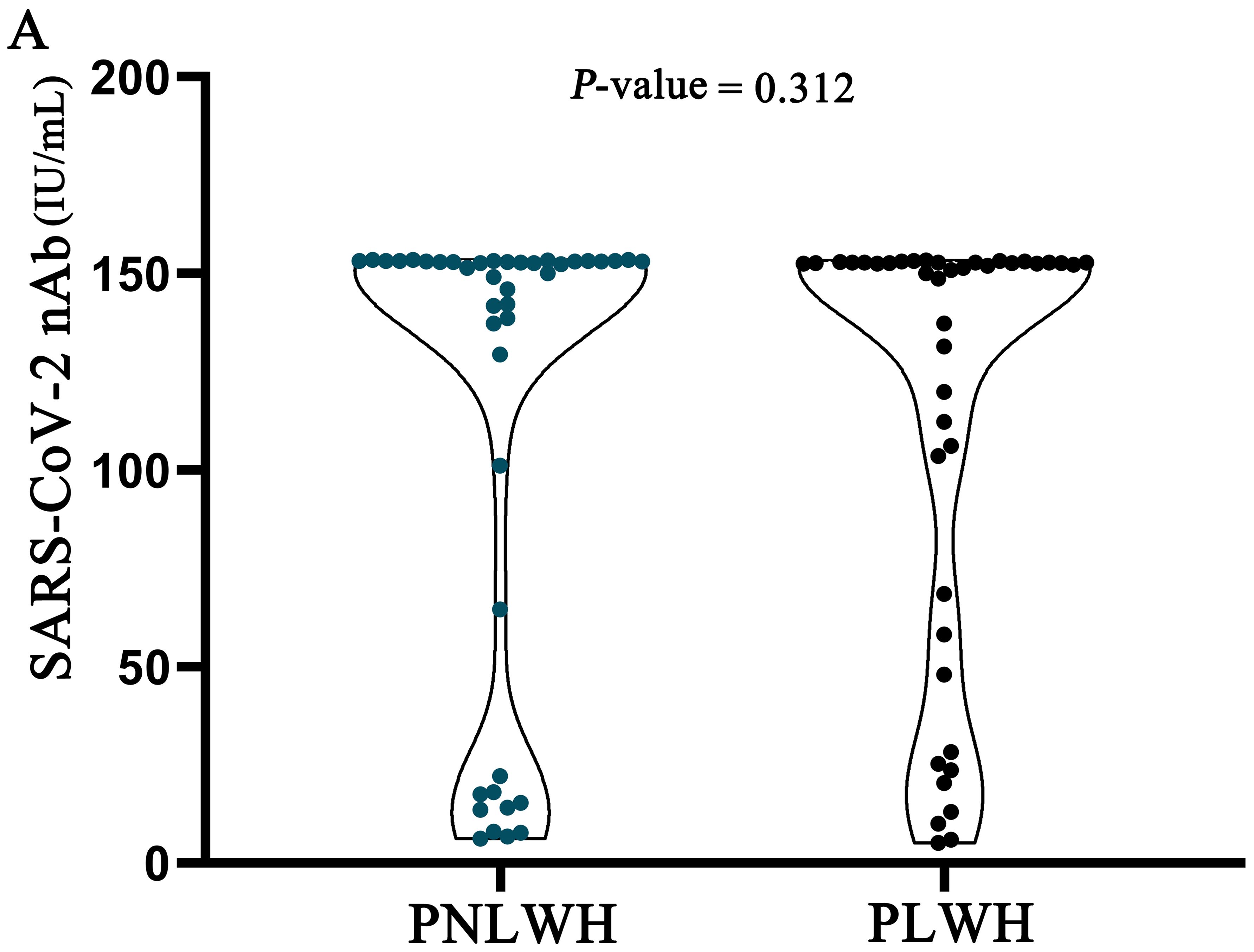
Figure 2. SARS-CoV-2 nAb level were statistically analysed in two groups of LC patients. There was no difference in SARS-CoV-2 nAb level between the two groups (P>0.05).
3.4 Sequelae symptoms
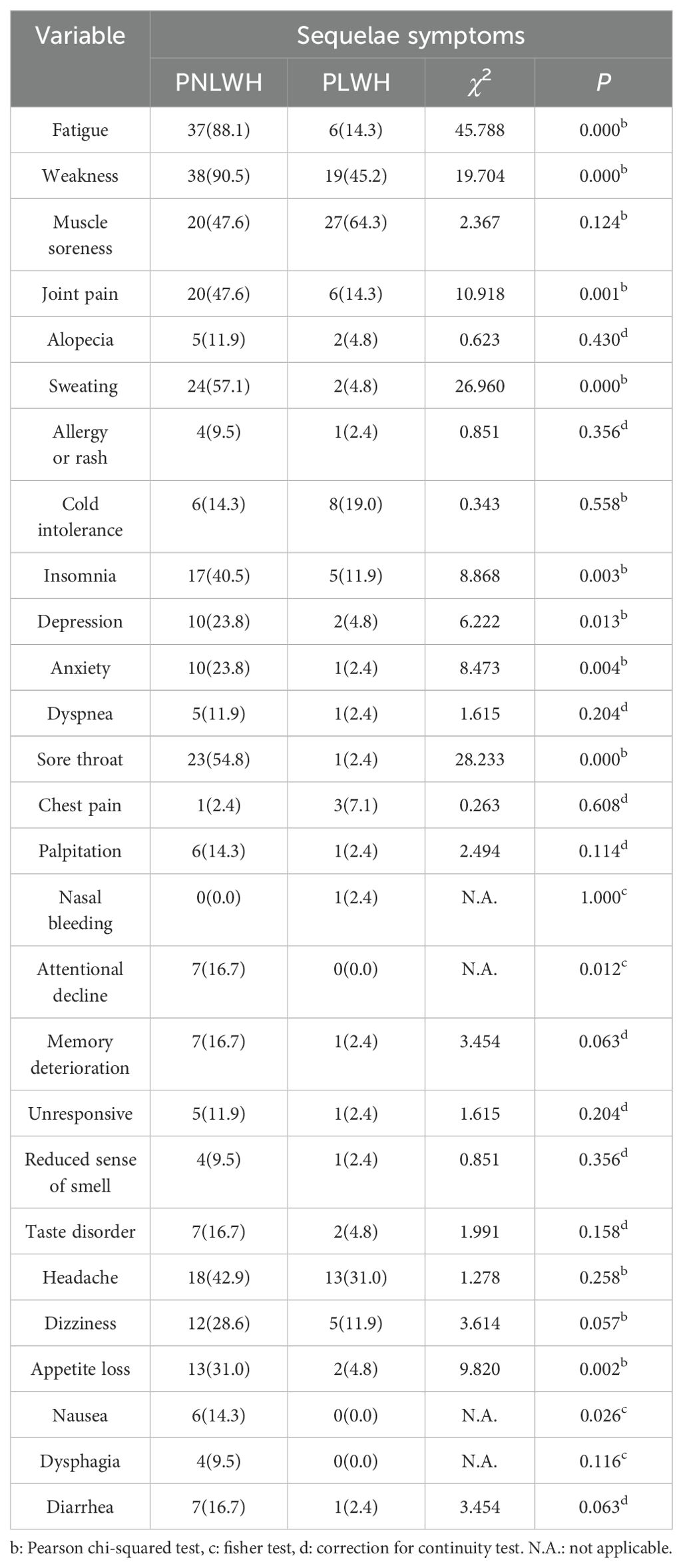
Following 6 months of SARS-CoV-2 infection, the LC was present in all participants. The table shows that the PNLWH group generally showed more LC symptoms than the PLWH group. Notably, significant differences were observed in the following symptoms, with higher prevalence in the PNLWH group: fatigue (88.1% vs 14.3%), weakness (90.5% vs 45.2%), joint pain (47.6% vs 14.3%), sweating (57.1% vs 4.8%), insomnia (40.5% vs 11.9%), anxiety (23.8% vs 2.4%), sore throat (54.8% vs 2.4%), appetite loss (31.0% vs 4.8%) (P < 0.01, respectively), depression (23.8% vs 4.8%), attentional decline (16.7% vs 0.0%) and nausea (14.3% vs 0.0%) (P < 0.05, respectively). Some of these symptoms do not have statistical significance: muscle soreness (47.6% vs 64.3%), alopecia (11.9% vs 4.8 %), allergy or rash (9.5% vs 2.4%), cold intolerance (14.3% vs 19.0%), dyspnea (11.9% vs 2.4%), chest pain (2.4% vs 7.1%), palpitation (14.3% vs 2.4%), nasal bleeding (0.0% vs 2.4%), memory deterioration (16.7% vs 2.4%), unresponsive (11.9% vs 2.4%), reduced sense of smell (9.5% vs 2.4%), taste disorder (16.7% vs 4.8%), headache (42.9% vs 31.0%), dizziness (28.6% vs 11.9%), dysphagia (9.5% vs 0.0%), diarrhea (16.7% vs 2.4%) (P < 0.05) (Table 3).
3.5 Cardiovascular biomarker levels
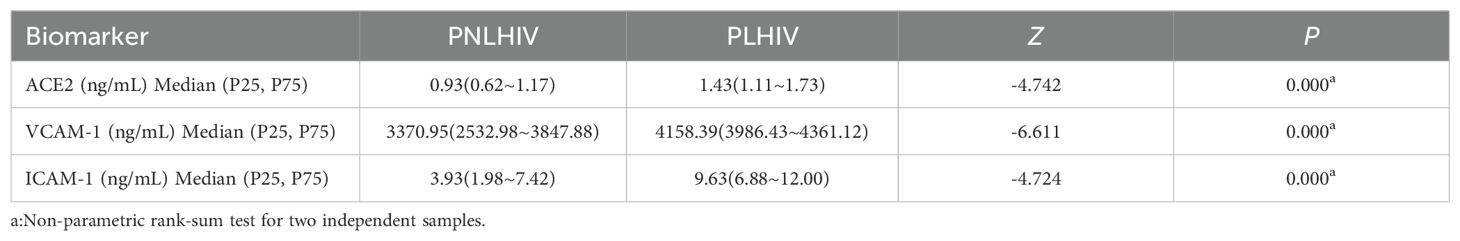
The levels of the leading three biomarkers, VCAM-1, ICAM-1, and ACE-2, were compared between the PNLWH and PLWH within the LC outpatient population. The results demonstrate a distinct difference in the vascular biomarkers profile of the PNLWH and PLWH in the LC cohort. The median blood serum levels of ACE-2 (Figure 3A), VCAM-1 (Figure 3B), and ICAM-1 (Figure 3C) were lower in PNLWH patients than in PLWH (P < 0.01, respectively) (Table 4).
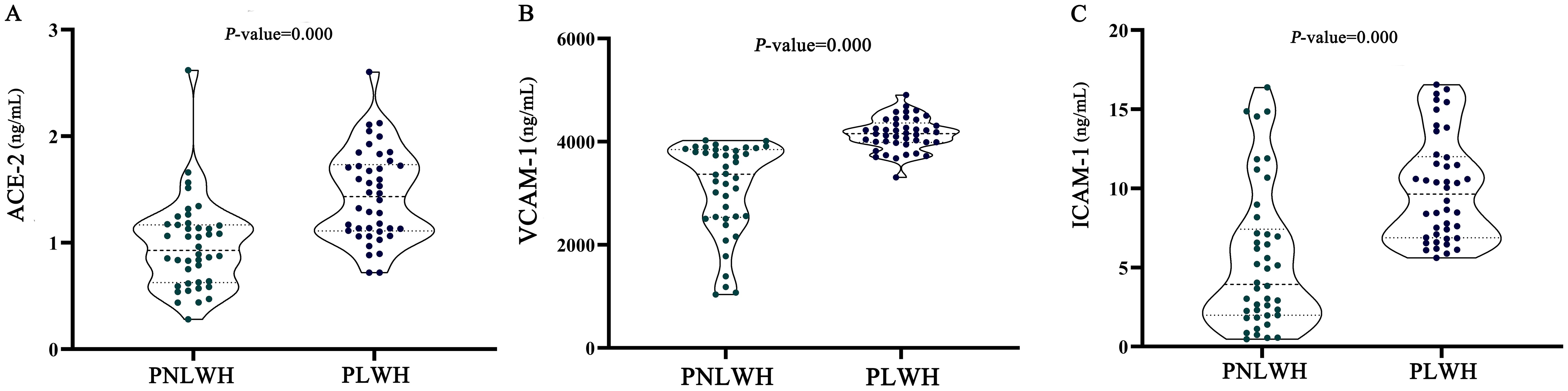
Figure 3. Statistical analysis of cardiovascular biomarker levels. ACE-2 were significantly lower in the group of PLWH compared to the group of PNLWH. P < 0.01, respectively. (A). VCAM-1 were significantly lower in the group of PLWH compared to the group of PNLWH. P < 0.01, respectively. (B). ICAM-1 were significantly lower in the group of PLWH compared to the group of PNLWH. P < 0.01, respectively. (C).
3.6 Cytokine levels
For the inflammatory response, the levels of pro-inflammatory cytokine IFN-γ, TNF-α, IL-6, IL-17 were measured by ELISA. The median blood serum levels of IFN-γ (Figure 4A), TNF-α (Figure 4C), IL-6 (Figure 4B), IL-17 (Figure 4D) showed no significant differences between PNLWH and PLWH. (P > 0.05) (Table 5).
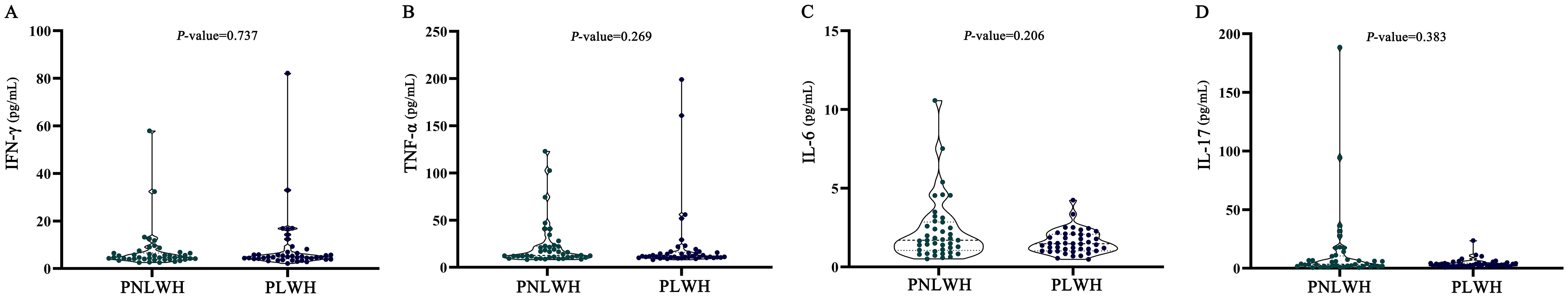
Figure 4. Statistical analysis of cytokine levels. There were no significant differences in IFN-γ levels between PLWH and PNLWH groups (P > 0.05). (A). There were no significant differences in TNF-α levels between PLWH and PNLWH groups (P > 0.05). (B). There were no significant differences in IL-6 levels between PLWH and PNLWH groups (P > 0.05). (C). There were no significant differences in IL-17 levels between PLWH and PNLWH groups (P > 0.05). (D).
4 Discussion
PLWH represent a vulnerable population that warrants special attention and potentially increased risks of post-COVID-19 complications and LC (11). Some inflammatory markers may be elevated in HIV-positive individuals (10). However, up-to-date data on this subject remains exceedingly limited. Early cohort reports hint at the presence of PLWH in cases of LC, but existing studies have not sufficiently outlined the characteristics of this subgroup (9). In response to this gap, our study seeks to evaluate the demographic features, comorbidities, and clinical presentations specific to PLWH treated with ART of LC. Furthermore, we conducted a comprehensive analysis comparing cardiovascular biomarkers, cytokine levels, and SARS-CoV-2 nAb levels during the 6-month post-infection recovery period in PLWH and PNLWH. This approach aims to enhance our understanding of the biological differences between these groups.
The investigation reveals a notable discrepancy in occupational distribution and educational attainment between the two groups. Predominantly, PLWH are employed in agriculture, signifying their classification within the lower-income bracket in China. This demographic faces significant life stress, a factor that existing research indicates correlates positively with an increased risk of HIV infection (12). Furthermore, there is a prevailing trend of lower educational levels, primarily at the elementary or below level, among the PLWH, suggesting a negative correlation between HIV infection and individual education levels. Typically, individuals with limited education lack fundamental knowledge regarding HIV prevention, transmission, risks, and mitigation strategies (13).
The high vaccination rates among the participants, reaching 92.9% in the PNLWH group and 97.6% in the PLWH group, underscore substantial compliance with public health guidelines. Notably, there is a significant disparity in vaccination frequency between PLWH and PNLWH. In the PNLWH group, 19.0% received two doses, and 71.4% received three doses. In contrast, the PLWH group reported higher numbers, with 83.3% receiving three doses and 9.5% receiving four doses. This suggests that PLWHs are more focused on personal protection and are more willing to be vaccinated. Compelling evidence from existing research analysis has shown that vaccination is strongly correlated with a reduced incidence of LC. A comprehensive survey, controlling for factors such as gender, demographics, and medical history, indicates that vaccination significantly decreases the risk of LC (14). Particularly, individuals who have completed the full vaccination schedule exhibit a markedly lower risk of LC (15). Even receiving just two doses of the vaccine has been shown to reduce the likelihood of developing LC (16). However, for patients who have received at least one dose of the COVID-19 vaccine, there is currently no available data to conclusively determine whether an increase in the number of doses administered continues to proportionally decrease the risk of developing LC. Additionally, there is a lack of research data to thoroughly analyze this disparity's potential impact on the Post-Acute Sequelae of SARS-CoV-2 infection (17).
SARS-CoV-2 nAb represent a pivotal element in the immune response against the novel coronavirus, serving as a primary defense mechanism by specifically targeting and inhibiting the virus's ability to enter host cells (18). It is generated in response to the presence of the SARS-CoV-2 spike protein, either through natural infection or vaccination. This defense response primary mode of action involves binding to the receptor-binding domain of the spike protein, effectively obstructing the virus's interaction with the ACE2 receptor, which is crucial for viral entry into human cells (19–21). A cross-sectional study found that individuals with LC exhibited significantly lower neutralizing antibody levels compared to those without LC during the same post-SARS-CoV-2 infection period (22). Studies have shown that a broad spectrum of comprehensive antibody responses predicts the onset of various long-term COVID-19 symptoms, with this phenomenon being more pronounced in certain long-term symptoms (such as gastrointestinal and neurological symptoms) (23). Additionally, some studies have shown that HIV-positive individuals who have had COVID-19 exhibit abnormal specific T lymphocyte (T-cell) responses and elevated levels of certain inflammatory markers, making them more susceptible to developing LC. However, these studies did not clearly specify whether the HIV-positive participants were receiving antiretroviral therapy (ART) (7). The presence of comprehensive antibodies also indicates a past infection with SARS-CoV-2 over a period of time. In this study, all patients tested positive for SARS-CoV-2 nAb. However, there was no significant difference in the levels of SARS-CoV-2 nAb between the two groups.
Compared to PNLWH, PLWH exhibit an acute phase of COVID-19 symptom with earlier onset (92.9% within 24 h vs 7.1%), potentially linked to an increased susceptibility to pulmonary infections, even in the presence of ART (24). Studies have indicated that HIV significantly impacts bronchial and alveolar epithelia, reducing resistance to external viral infections (25, 26). This study also highlights a significant difference in awareness of the timing when the nucleic acid test turned negative between PLWH and PNLWH. In the PNLWH group, 57.1% of participants were unaware of the time when their nucleic acid test turned negative, whereas in the PLWH group, all participants could accurately report this time. This disparity may indicate a heightened level of attention that PLWH devotes to monitoring their health status and treatment progression. Moreover, there is currently no conclusive evidence that an extended duration of nucleic acid test negativity significantly increases the risk of developing LC.
Concerns have arisen about the potential increased severity of LC in PLWH. A retrospective study revealed that PLWH are 1.75 times more likely to experience LC symptoms at least 9 months post-diagnosis compared to those PNLWH (8). Interestingly, our study discovered that PLWH undergoing ART displayed milder LC symptoms compared to PNLWH. This observation may be linked to the use of ART contributing to a better COVID-19 prognosis in individuals co-infected with CoV-HIV (27). And other research has found that HIV-infected individuals undergoing ART (antiretroviral therapy) do not show significant differences in the incidence or severity of COVID-19 compared to those without HIV infection (28). The rates of hospitalization and disease progression are not worse than those in the general population (29), suggesting that ART may play a protective role in maintaining immune function (30, 31). Notably, during the period from the onset of LC symptoms to the time of this survey, none of the participants experienced severe symptoms, and all had a favorable prognosis. However, further support for this perspective is warranted through large-scale clinical observations and randomized controlled trials.
Despite COVID-19 primarily affecting the respiratory system, it is accompanied by significant cardiovascular complications (CVCs). The roles of ACE2 within the cardiovascular and immune systems are crucial for maintaining homeostasis (32). The primary pathways leading to the development of CVCs and the recently recognized LC are hypothesized to result directly from the viral S protein/ACE2 axis, downregulation of ACE2, and the subsequent damage caused by the immune response (34). The literature on how ACE2 levels regulate the pathogenesis of COVID-19 is marked by conflicting perspectives. While numerous authors contend that ACE2 is a fundamental contributor to the risk factors for severe COVID-19 (33, 34), an emerging body of literature argues that ACE2 upregulation serves as a protective factor for SARS-CoV-2 outcomes, primarily due to its role in counteracting the potent vasoconstrictive effect of angiotensin II (33, 35). Consequently, ACE2 expression may exhibit paradoxical effects, simultaneously assisting SARS-CoV-2 infection while limiting viral pathogenicity. Our findings indicate that PLWH on ART has a relatively higher level of serum ACE2 than PNLWH in patients with LC. Nevertheless, further clinical research with larger population size and an analysis of whether ACE2 elevation is a protective or aggravating factor is necessary to establish the evidence found in this preliminary study.
ICAM-1 and VCAM-1 belong to the immunoglobulin family of CAM and play crucial roles in regulating the strong adherence of leukocytes to endothelial cells, particularly in various acute or chronic inflammation diseases (36). ICAM-1 and VCAM-1 primarily facilitate the transmigration of the leukocytes within the cell, and elevated levels in the blood lead to the cell membrane forming clusters and colonies. Following monocyte accumulation, additional inflammatory cytokines and fat cells adhere to the cell surface, narrowing the blood vessel. This process directly impacts blood flow and may contribute to diseases such as atherosclerosis, myocardial infarction, hypertension, and stroke, among others (37). ICAM-1 and VCAM-1 contribute to vascular inflammation, and these markers have been widely utilized as indicators of endothelial dysfunction, demonstrating a significant association with cardiovascular disease (CVD) risk and mortality in the general population (37). HIV infection is linked to an increased risk of CVD, a leading cause of death among HIV-positive individuals receiving effective ART (38). ART, particularly protease inhibitors (PI), is a common HIV treatment that may be associated with this heightened risk. Studies have suggested an increased risk of atherosclerotic CVD among PLWH with LC who are on a PI-based regimen (39). However, this process is primarily thought to be triggered by impaired lipid metabolism and endothelial dysfunction (40, 41). Moreover, research indicates that compared with non-PI treatments, patients in the boosted-PI group exhibit more evidence of dyslipidaemia. Contrastingly, VCAM-1 levels were higher (42). In this study, we investigated ICAM-1 and VCAM-1, which have evidence of association with CVD in LC patients with and without HIV. As anticipated, significantly elevated levels of ICAM-1 and VCAM-1 were observed in PLWH with LC, compared with those without HIV. This elevation may be linked to an increased risk of atherosclerotic CVD among PLWH, with LC receiving a PI-based regimen.
Studies have demonstrated that immune abnormalities and inflammation may persist after severe COVID-19, with highly activated myeloid cells, pro-inflammatory cytokines, and persistently activated T-cells detected 8–12 months after COVID-19 (43–46). The highlighted inflammatory mediators released by immune cells include Interferon-α, IFN-γ, Interleukin-1β, IL-6, Interleukin-12, IL-17, Interleukin-18, Interleukin-33, TNF-α and Transforming Growth Factor-β with altered levels associated with various clinical features of COVID-19 (47–49). However, our study found no significant differences in IFN-γ, TNF-α, IL-6, and IL-17 serum levels between PNLWH and PLWH with LC. Contrary to this, studies have reported that the anti-inflammatory effects of immunosuppression could potentially be protective in mitigating poor outcomes in COVID-19 patients with cytokine storm-related complications (27). Several studies indicate an overall asymptomatic or mild COVID-19 in immunocompromised patients, encompassing children undergoing anticancer therapy (50), users of immunosuppressive chronic drugs (51), transplant recipients (52), and PLWH (53). Moreover, other studies suggest that ART has been demonstrated to improve inflammation markers in individuals with HIV, although not normalized; the effects of various regimens may vary and be complex. Specifically, PI-based regimens have shown efficacy in improving inflammation markers in individuals with HIV and reducing systemic inflammation associated with HIV infection (54, 55). These findings suggest differential effects of chronic viral coinfections on the likelihood of developing LC.
5 Conclusions
In this study, the majority of individuals infected with HIV are involved in agriculture, reflecting a predominant occupational choice. Furthermore, there is a noticeable trend of lower educational levels, primarily at an elementary level or below. It is noteworthy that PLWH had a higher rate of completion for three or more vaccinations, indicating a heightened focus on personal protection and a greater willingness to be vaccinated among HIV patients. Within the PNLWH group, 57.1% of participants were unaware of their exact seroconversion timing, while in the PLWH group, all participants could accurately report their seroconversion timeline. This difference might signify an elevated level of attention that PLWH pays to their health status and treatment progression. There are concerns about the potentially greater severity of the LC in PLWH. Interestingly, our study revealed that PLWH undergoing ART exhibited milder LC symptoms than PNLWH. This observation may be attributed to the use of antiretroviral therapy (ART), which has been shown to improve the prognosis of COVID-19 in individuals co-infected with HIV and coronavirus. Moreover, the cardiovascular disease-related risk factors, including ACE2, VCAM-1, and ICAM-1, were significantly higher in patients with PLWH. This might be attributed to the long-term effects of PI on these patients’ cardiovascular systems. Future research with larger sample sizes and randomized controlled trials is crucial to validate and extend these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Research Ethics Commission of the First Affiliated Hospital of the Yunnan University of Chinese Medicine (NO. K [2020]015-02). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QL: Writing – review & editing, Formal analysis, Investigation. YM: Writing – original draft, Investigation. PH: Investigation, Writing – original draft. DC: Data curation, Writing – original draft. TZ: Data curation, Writing – original draft. XW: Data curation, Investigation, Writing – original draft. YX: Data curation, Investigation, Writing – original draft. PL: Data curation, Investigation, Writing – original draft. WW: Funding acquisition, Project administration, Supervision, Writing – review & editing. ZW: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work were supported by National Science Foundation of China (No. 82260930); Yunnan Provincial Science and Technology Department Science and Technology Program Key Research and Development Project (No.202103AC100005); Yunnan Provincial Science and Technology Department Traditional Chinese Medicine Joint Special Project-surface Project (No.202101AZ070001-020, NO.202001AZ070001-035); Basic Research General Project of Yunnan Provincial Science and Technology Department (No.202101AT070244); National Traditional Chinese Medicine Multidisciplinary Innovation Team Project (No. ZYYCXTD-D-202201); Special Project of SARS-CoV-2 Infection in Traditional Chinese Medicine of the State Administration of Traditional Chinese Medicine No. 2023ZYCCYJ02-24. Yunnan University of Traditional Chinese Medicine Joint University-Institute Fund for Diannan School of Medicine (No. XYLH202329).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Centers for Disease Control and Prevention. Post-Covid Conditions. In: Information for Healthcare Providers (2021) Atlanta, GA, USA: U.S. Department of Health and Human Services. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html.
2. Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. (2021) 4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568
3. Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. (2021) 27:1507–13. doi: 10.1016/j.cmi.2021.05.033
4. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
5. Huang Y, Pinto MD, Borelli JL, Asgari Mehrabadi M, Abrahim HL, Dutt N, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler looking for clarity in the haze of the pandemic. Clin Nurs Res. (2022) 31:1390–8. doi: 10.1177/10547738221125632
6. Ortona E, Malorni W. Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur Respir J. (2022) 59:2102245. doi: 10.1183/13993003.02245-2021
7. Peluso MJ, Spinelli MA, Deveau TM, Forman CA, Munter SE, Mathur S, et al. Postacute sequelae and adaptive immune responses in people with HIV recovering from SARS-COV-2 infection. Aids. (2022) 36:F7–F16. doi: 10.1097/qad.0000000000003338
8. Kingery JR, Safford MM, Martin P, Lau JD, Rajan M, Wehmeyer GT, et al. Health status, persistent symptoms, and effort intolerance one year after acute COVID-19 infection. J Gen Intern Med. (2022) 37:1218–25. doi: 10.1007/s11606-021-07379-z
9. Peluso MJ, Kelly JD, Lu S, Goldberg SA, Davidson MC, Mathur S, et al. Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of SARS-coV-2 infection: cohort description and approaches for measurement. Open Forum Infect Dis. (2022) 9:ofab640. doi: 10.1093/ofid/ofab640
10. Kamanzi P, Mulundu G, Mutale K, Mumba C, Ngalamika O. HIV and inflammatory markers are associated with persistent COVID-19 symptoms. Immun Inflammation Dis. (2023) 11:e859. doi: 10.1002/iid3.859
11. Peluso MJ, Antar AA. Long COVID in people living with HIV. Curr Opin. (2023) 18:126–34. doi: 10.1097/COH.0000000000000789
12. Stadtler H, Shaw G, Neigh GN. Mini-review: Elucidating the psychological, physical, and sex-based interactions between HIV infection and stress. Neurosci Lett. (2021) 747:135698. doi: 10.1016/j.neulet.2021.135698
13. Jukes M, Simmons S, Bundy D. Education and vulnerability: the role of schools in protecting young women and girls from HIV in southern Africa. Aids. (2008) 22:S41–56. doi: 10.1097/01.aids.0000341776.71253.04
14. Brannock MD, Chew RF, Preiss AJ, Hadley EC, Redfield S, McMurry JA, et al. Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program. Nat Commun. (2023) 14:2914. doi: 10.1101/2022.10.06.22280795
15. Fatima S, Ismail M, Ejaz T, Shah Z, Fatima S, Shahzaib M, et al. Association between long COVID and vaccination: A 12-month follow-up study in a low-to middle-income country. PloS One. (2023) 18:e0294780. doi: 10.1371/journal.pone.0294780
16. Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine. (2022) 53:101624. doi: 10.1016/j.eclinm.2022.101624
17. Spinelli MA. SARS-CoV-2 vaccination in people with HIV. Lancet HIV. (2021) 8:e455–6. doi: 10.1016/S2352-3018(21)00128-4
18. Suryadevara N, Shrihari S, Gilchuk P, VanBlargan LA, Binshtein E, Zost SJ, et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell. (2021) 184:2316–31. doi: 10.1016/j.cell.2021.03.029
19. Baum A, Ajithdoss D, Copin R, Zhou A, Lanza K, Negron N, et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. (2020) 370:1110–5. doi: 10.1126/science.abe2402
20. Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. (2020) 182:73–84. doi: 10.1016/j.cell.2020.05.025
21. Hansen J, Baum A, Pascal KE, Russo V, Giordano S, Wloga E, et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. (2020) 369:1010–4. doi: 10.1126/science.abd0827
22. Jansen EB, Ostadgavahi AT, Hewins B, Buchanan R, Thivierge BM, Sganzerla Martinez G, et al. PASC (Post Acute Sequelae of COVID-19) is associated with decreased neutralizing antibody titers in both biological sexes and increased ANG-2 and GM-CSF in females[J. Sci Rep. (2024) 14:9854. doi: 10.1038/s41598-024-60089-4
23. Buck AM, Deitchman AN, Takahashi S, Lu S, Goldberg SA, Bodansky A, et al. The breadth of the neutralizing antibody response to original SARS-CoV-2 infection is linked to the presence of Long COVID symptoms. J Med Virol. (2023) 95:e29216. doi: 10.1002/jmv.v95.11
24. Garcia Garrido HM, Mak AMR, Wit F, Wong GWM, Knol MJ, Vollaard A, et al. Incidence and risk factors for invasive pneumococcal disease and community-acquired pneumonia in human immunodeficiency virus–infected individuals in a high-income setting. Clin Infect Dis. (2020) 71:41–50. doi: 10.1093/cid/ciz728
25. Lassiter C, Fan X, Joshi PC, Jacob BA, Sutliff RL, Jones DP, et al. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther. (2009) 6:1–12. doi: 10.1186/1742-6405-6-1
26. Xu S, Vucic EA, Shaipanich T, Lam S, Lam W, Montaner JS, et al. Decreased telomere length in the small airway epithelium suggests accelerated aging in the lungs of persons living with human immunodeficiency virus (HIV). Respir Res. (2018) 19:1–4. doi: 10.1186/s12931-018-0821-0
27. Romanelli A, Mascolo S. Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: A therapeutical hypothesis. Am J Transplant. (2020) 20:1947–8. doi: 10.1111/ajt.15905
28. Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: A cohort study. Ann Intern Med. (2020) 173:536–41. doi: 10.7326/M20-3689
29. Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO clinical characterization protocol (UK): A prospective observational study. Clin Infect Dis. (2021) 73:e2095–106. doi: 10.1093/cid/ciaa1605
30. Saberian M, Bagheri A, Mokhtari M, Aramin H, Saeedi R, Zare M, et al. Outcomes of COVID-19 in people living with HIV: A review and meta-analysis. J Acquir Immune Defic Syndr. (2021) 87:291–8. doi: 10.1097/QAI.0000000000002679
31. Harter G, Spinner CD, Roider J, Bickel M, Krznaric I, Grunwald S, et al. COVID-19 in people living with human immunodeficiency virus: A case series of 33 patients. Infection. (2020) 48:647–51. doi: 10.1007/s15010-020-01438-z
32. Cooper SL, Boyle E, Jefferson SR, Heslop CR, Mohan P, Mohanraj GG, et al. Role of the renin–angiotensin–aldosterone and kinin–kallikrein systems in the cardiovascular complications of COVID-19 and long COVID. Int J Mol Sci. (2021) 22:8255. doi: 10.3390/ijms22158255
33. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection. Lancet Respir Med. (2020) 8:e21. doi: 10.1016/S2213-2600(20)30116-8
34. Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. (2020) 55:2000688. doi: 10.1183/13993003.00688-2020
35. Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. (2020) 43:648–54. doi: 10.1038/s41440-020-0455-8
36. Sans M, Panés J, Ardite E, Elizalde JI, Arce Y, Elena M, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. (1999) 116:874–83. doi: 10.1016/S0016-5085(99)70070-3
37. Singh V, Kaur R, Kumari P, Pasricha C, Singh R. ICAM-1 and VCAM-1: gatekeepers in various inflammatory and cardiovascular disorders. Clin Chim Acta. (2023) 548:117487. doi: 10.1016/j.cca.2023.117487
38. Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. Aids. (2013) 27:973–9. doi: 10.1097/QAD.0b013e32835cae9c
39. Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. (2007) 356:1723–35. doi: 10.1056/NEJMoa062744
40. Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. (1998) 351:1881–3. doi: 10.1016/s0140-6736(98)03391-1
41. Glesby MJ. Coronary heart disease in HIV-infected patients. Curr HIV/AIDS Rep. (2005) 2:68–73. doi: 10.1007/s11904-005-0021-7
42. Ounjaijean S, Kulprachakarn K, Aurpibul L, Kaewpoowat Q, Boonyapranai K, Chaiwarith R, et al. Cardiovascular risks in Asian HIV-infected patients receiving boosted-protease inhibitor-based antiretroviral treatment. J Infect Dev Ctries. (2021) 15:289–96. doi: 10.3855/jidc.12864
43. Bergamaschi L, Mescia F, Turner L, Hanson AL, Kotagiri P, Dunmore BJ, et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. (2021) 54:1257–75. doi: 10.1016/j.immuni.2021.05.010
44. Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier C, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. (2022) 23:210–6. doi: 10.1038/s41590-021-01113-x
45. Santopaolo M, Gregorova M, Hamilton F, Arnold D, Long A, Lacey A, et al. Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months. Elife. (2023) 12:e85009. doi: 10.7554/eLife.85009
46. Taeschler P, Adamo S, Deng Y, Cervia C, Zurbuchen Y, Chevrier S, et al. T-cell recovery and evidence of persistent immune activation 12 months after severe COVID-19. Allergy. (2022) 77:2468–81. doi: 10.1111/all.15372
47. Chang SH, Minn D, Kim SW, Kim YK. Inflammatory markers and cytokines in moderate and critical cases of COVID-19. Clin Lab. (2021) 9:2115. doi: 10.7754/Clin.Lab.2021.210142
48. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/s0140-6736(20)30183-5
49. Queiroz MAF, Neves P, Lima SS, Lopes JDC, Torres M, Vallinoto I, et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol. (2022) 12:922422. doi: 10.3389/fcimb.2022.922422
50. Hrusak O, Kalina T, Wolf J, Balduzzi A, Provenzi M, Rizzari C, et al. Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment. Eur J Cancer. (2020) 132:11–6. doi: 10.1016/j.ejca.2020.03.021
51. Han Y, Jiang M, Xia D, He L, Lv X, Liao X, et al. COVID-19 in a patient with long-term use of glucocorticoids: A study of a familial cluster. Clin Immunol. (2020) 214:108413. doi: 10.1016/j.clim.2020.108413
52. Bussalino E, De Maria A, Russo R, Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient with SARS-CoV-2 pneumonia: A case report. Am J Transplant. (2020) 20:1922–4. doi: 10.1111/ajt.15920
53. Calza L, Bon I, Tadolini M, Borderi M, Colangeli V, Badia L, et al. COVID-19 in patients with HIV-1 infection: a single-centre experience in northern Italy. Infection. (2021) 49:333–7. doi: 10.1007/s15010-020-01492-7
54. Arenas-Pinto A. Systemic inflammation and residual viraemia in HIV-positive adults on protease inhibitor monotherapy: a cross-sectional study. BMC Infect Dis. (2015) 15:138. doi: 10.1186/s12879-015-0889-9
Keywords: COVID-19, human immunodeficiency virus, Long-COVID, symptoms, cardiovascular biomarkers
Citation: Li Q, Ma Y, He P, Chen D, Zhang T, Wang X, Xu Y, Li P, Wen W and Wang Z (2024) Long COVID symptoms 6 months after acute infection among people living with HIV and people not living with HIV. Front. Immunol. 15:1430214. doi: 10.3389/fimmu.2024.1430214
Received: 01 June 2024; Accepted: 25 October 2024;
Published: 28 November 2024.
Edited by:
Helen Carolyn Steel, University of Pretoria, South AfricaReviewed by:
Debashis Dutta, University of Nebraska Medical Center, United StatesMieke Adri Van Der Mescht, University of Pretoria, South Africa
Copyright © 2024 Li, Ma, He, Chen, Zhang, Wang, Xu, Li, Wen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weibo Wen, d2Vud2VpYm8yMDIwQDE2My5jb20=; Zefeng Wang, MTY1NTI4NDY0QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qin Li
Qin Li Yijie Ma3†
Yijie Ma3† Tingrui Zhang
Tingrui Zhang