- 1State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Haihe Laboratory of Cell Ecosystem, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China
- 2Tianjin Institutes of Health Science, Tianjin, China
The optimal treatment for patients with severe aplastic anemia (SAA) who fail an initial course of antithymocyte globulin (ATG) plus cyclosporine has not yet been established. We compared the effectiveness of allogeneic hematopoietic stem cell transplantation (allo-HSCT) (n = 36) with repeated immunosuppressive therapy (IST) (n = 33) for relapsed/refractory SAA between 2007 and 2022. In the IST group, patients were retreated with ATG (n = 16) or high-dose cyclophosphamide (n = 17). The overall response rate was 57.6% at 6 months and 60.6% at 12 months. In the allo-HSCT group, patients received a transplant from a matched sibling donor (n = 6), matched unrelated donor (n = 7), or haploidentical donor (n = 23). All patients achieved neutrophil engraftment, and there were no cases of primary graft failure. The cumulative incidences (CIs) of grades II–IV and III–IV acute graft-versus-host disease (GVHD) were 36.1% ± 0.7% and 13.9% ± 0.3% at day +100, respectively. The 4-year CI of chronic GVHD (cGVHD) was 36.2% ± 0.7%, with moderate to severe cGVHD at 14.9% ± 0.4%. Compared with IST, HSCT recipients showed much higher hematologic recovery rate at 3, 6, and 12 months (63.9%, 83.3%, and 86.1%, respectively, p < 0.001). The estimated 4-year overall survival (OS) (79.8% ± 6.8% vs. 80.0% ± 7.3%, p = 0.957) was similar; however, the failure-free survival (FFS) was significantly better in the HSCT group (79.8% ± 6.8% vs. 56.6% ± 8.8%, p = 0.049). Of note, children in the HSCT cohort were all alive without treatment failures, exhibiting superior OS (100% vs. 50.0% ± 17.7%, p = 0.004) and FFS (100% vs. 50.0% ± 17.7%, p = 0.004) than children in the IST cohort. Subgroup analysis revealed that younger patients (age ≤ 35 years), especially children, and those with refractory SAA benefited more from HSCT. Therefore, for these patients, salvage HSCT may be more preferable than a second course of IST.
Introduction
Acquired severe aplastic anemia (SAA) is an immune-mediated hematopoietic stem cell disorder that presents with hypocellular marrow and pancytopenia (1). The first-line treatment options for SAA include allogeneic hematopoietic stem cell transplantation (allo-HSCT) and immunosuppressive therapy (IST) with antithymocyte globulin (ATG) and cyclosporine (CsA). The hematopoietic response rate to IST has been reported to be 70%–80% and the probability of survival at 5 years ranges from 60% to 85% (2–4). Despite improvement with IST, approximately one-third of SAA patients remain refractory to IST (1, 5) and 30%–40% may eventually relapse (3). The management of patients with refractory or relapsed SAA after IST represents a major challenge.
With advances in supportive care, donor selection, and conditioning regimen, the outcomes of alternative donor HSCT in patients with SAA have improved dramatically (6–8). HSCT from matched unrelated donor (MUD) or haploidentical donor (HID) has become an effective salvage treatment for IST failure. Xu et al. reported on the long-term outcomes of 287 SAA patients who underwent salvage HID-HSCT. For previously failed IST, 63 patients received ATG + CsA-based regimens, while the rest received CsA-based regimens. The estimated overall survival (OS) and failure-free survival (FFS) for the whole cohort at 9 years were 85.4% and 84.0%, respectively (9).
Salvage with a repeated course of ATG-based IST has also been employed in some patients. The response rates have varied significantly, ranging from 22% to 77%, and the response in the refractory setting is often inferior to that in the relapsed setting (10–12). Scheinberg et al. summarized the results of rabbit ATG (r-ATG) retreatment in patients with SAA who were refractory to or who had relapsed following horse ATG (h-ATG). The overall response rate (ORR) was 65% in relapsed patients; however, it was only 30% in refractory patients (10).
In addition, high-dose cyclophosphamide (HD-CTX) is highly immunosuppressive and has been used in both treatment-naïve and refractory/relapse SAA (13–16). An advantage of HD-CTX/CsA over ATG/CsA is that it is much cheaper, rendering it a reasonable alternative for those who cannot afford ATG-based therapy. A study reported a large cohort of SAA patients treated with HD-CTX, and confirmed its effectiveness. At 10 years, the OS was 88%, and the response rate was 71% (13). However, a small randomized study (17) combined HD-CTX (200 mg/kg) with CsA for SAA, and found a high rate of fungal infections and early mortality. To reduce the toxicity of CTX, we modified CTX dose to 120 mg/kg and deferred CsA to day 7 after the completion of CTX infusion. Our previous study observed no excess mortality and comparable outcomes between the HD-CTX group and the ATG group. Furthermore, the total medical cost of the HD-CTX group was much less than that of the ATG regimen (18).
Based on these findings, current therapeutic approaches for refractory/relapse SAA include salvage HSCT, a second course of ATG-based IST or HD-CTX. In addition, alemtuzumab (19) and the thrombopoietin receptor agonist (TPO-RA) eltrombopag (20, 21) are also effective in this setting of initial IST failure. However, data to determine the optimal second-line treatment are limited. Therefore, we conducted a retrospective study comparing the long-term efficacy of allo-HSCT with repeated IST as salvage therapy for relapsed/refractory SAA.
Method
Patient
The study was performed at the Institute of Hematology and Blood Diseases Hospital Chinese Academy of Medical Sciences. Between January 2007 and December 2022, 69 consecutive patients with relapsed/refractory SAA were enrolled. The detailed inclusion criteria were as follows: (1) age younger than 70 years; (2) diagnosis of SAA or very SAA (VSAA) (22) (congenital bone marrow disorders were excluded); (3) failure of an initial course of ATG plus CsA: TPO-RA was used together with ATG + CsA only in nine patients (eltrombopag in seven patients; hetrombopag in two patients). Refractory SAA was defined as lack of response with persistence of severe pancytopenia at least 3 months after IST. Relapse was considered if the patient had a previous response following IST and once more became transfusion dependent or met criteria for SAA (23); (4) salvaged with allo-HSCT or IST (ATG/CsA or HD-CTX/CsA). Allo-HSCT was indicated if patients were fit enough and had a matched sibling donor (MSD), MUD, or HID. If no suitable donor was available or patients refused HSCT, a second ATG or HD-CTX was applied. The choices of salvage treatment were made by patients and guardians and were also affected by their economic conditions. Patients with paroxysmal nocturnal hemoglobinuria (PNH) clones were also included in this analysis.
Treatment protocol of HSCT
Patients were conditioned with a FAC or BFAC regimen as previously described (24). The FAC regimen consisted of fludarabine (150 mg/m2), CTX (120 mg/kg or 150 mg/kg), and r-ATG (Genzyme, Cambridge, MA, USA, 12.5 mg/kg) or porcine antihuman lymphocyte immunoglobulin (p-ALG, Wuhan Institute of Biological Products, China, 100 mg/kg). The BFAC regimen included intravenous busulfan (Bu, 6.4 mg/kg) on the basis of FAC. Generally, patients with longer disease duration, older age, or PNH clones received the augmented BFAC regimen. Consistent with our previous report (25), graft-versus-host disease (GVHD) prophylaxis regime consisted of CsA/tacrolimus + methotrexate ± mycophenolate mofetil.
Treatment protocol of IST
In the ATG group, patients were treated with r-ATG 3.0–3.5 mg/kg/day or p-ALG 20 mg/kg/day for five consecutive days (26). In the HD-CTX group, CTX was administered at a dosage of 30 mg/kg/day for four consecutive days (18). Oral CsA was started at an initial dose of 3 mg/kg/day on day 1 and day 11 in the ATG and CTX group, respectively. It was administered for at least 2 years, with subsequent adjustment according to whole blood CsA concentration of 100–200 ng/mL for adults and 100–150 ng/mL for children.
Definitions
The hematologic response was evaluated at 3, 6, and 12 months after IST. Complete response (CR) was defined as ANC ≥ 1.5×109/L, hemoglobin ≥100 g/L, and platelet count ≥100×109/L. Partial response (PR) was defined as transfusion independence with ANC > 0.5×109/L and no longer met the criteria for severe disease (27). The overall response (OR) included both CR and PR. If blood counts did not meet the criteria of PR or CR, it was assessed as no response (NR).
After HSCT, neutrophil and platelet engraftment, primary and secondary graft failure (GF), and poor graft function (PGF) were defined as previously described (28–30). OS was defined as the time from the initiation of second-line treatment to the last follow-up or death. FFS was defined as survival with response. Death, NR by 6 months and beyond, disease progression requiring clinical intervention, clonal evolution, and relapse were considered treatment failures for IST (31). Death and primary or secondary GF were regarded as failure events for HSCT. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were graded according to the international criteria (32, 33). GVHD-free, failure-free survival (GFFS) was defined as survival without grades III–IV aGVHD, extensive cGVHD, or treatment failures.
Statistical analysis
The probabilities of OS, FFS, and GFFS were estimated using the Kaplan–Meier method, with differences compared by the log-rank test. Variables with p-values < 0.1 in univariate analysis were evaluated in multivariate analysis using the Cox proportional hazard regression model. In addition, factors including salvage therapy (HSCT or repeated IST), disease course, and status (refractory/relapse) were also incorporated into the model based on previous findings (12, 34). Cumulative incidences (CIs) of engraftment and GVHD were estimated in the competing risk model; death was considered as a competing risk. Differences in the distribution of various parameters were compared using chi-square or Student’s t-test as appropriate. p-values < 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS version 22.0 and the R software (version 3.4.3).
Results
Baseline characteristics
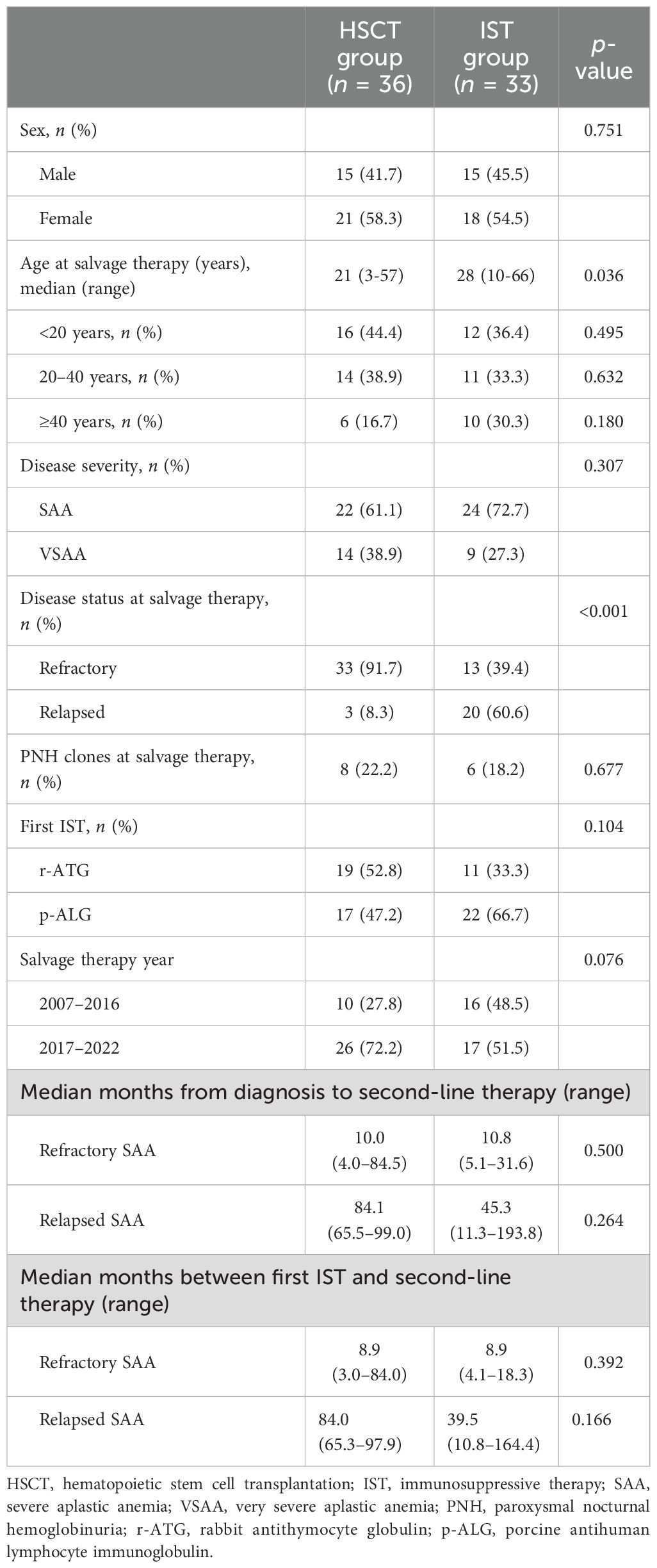
A total of 69 patients were enrolled in the study. Patient characteristics are presented in Table 1. In the HSCT group, patients received a transplant from MSD (n = 6), MUD (n = 7), or HID (n = 23). In the second IST group, patients were retreated with ATG (r-ATG, n = 7; p-ALG, n = 9) or HD-CTX (n = 17). Patients in the HSCT cohort were younger (median age at 21 years) than those in the IST cohort (median age at 28 years) (p = 0.036). The majority (91.7%, 33/36) of HSCT recipients were refractory SAA, compared to 39.4% (13/33) in the IST group (p < 0.001). There were no significant differences in sex ratio, disease duration, severity of disease (SAA/VSAA), or the presence of PNH clones at salvage therapy between the two cohorts.
Outcomes of HSCT
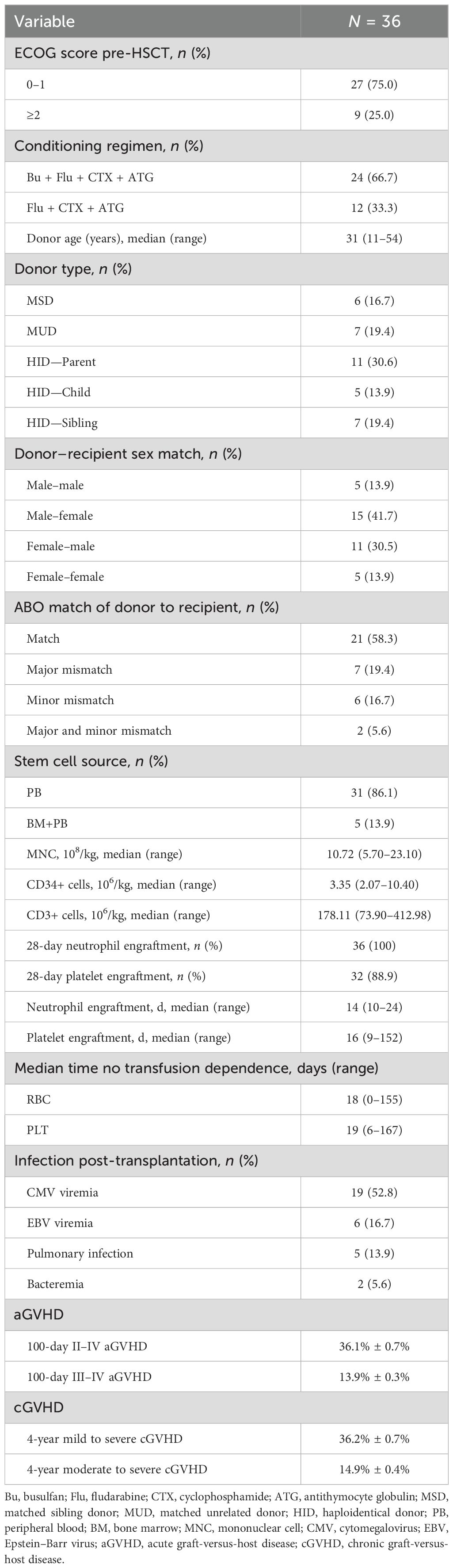
Patient and donor characteristics are shown in Table 2. All 36 patients survived for more than 28 days and achieved neutrophil engraftment with a median of 14 (10–24) days. A total of 32 patients (88.9%) achieved platelet engraftment with a median time of 16 (9–152) days. Most patients (31/36) received grafts from peripheral blood (PB), and only 5 received a combination of bone marrow (BM) and PB. The median infused mononuclear cell, CD34+, and CD3+ dose was 10.72 × 108/kg, 3.35 × 106/kg, and 178.11 × 106/kg, respectively. No cases of primary or secondary GF were observed. One patient developed primary PGF, but refused further intervention and died 8.6 months post-transplant. One patient experienced secondary PGF, received CD34-selected PB stem cells from the original donor, and achieved complete trilineage recovery at 7 months after boost infusion. At 3, 6, and 12 months after HSCT, 63.9%, 83.3%, and 86.1% of the recipients had achieved normal blood routine, respectively.
The CI of grades II–IV and III–IV aGVHD on day +100 was 36.1% ± 0.7% and 13.9% ± 0.3%, respectively. A total of 33 patients survived for more than 100 days and were evaluable for cGVHD. The 4-year CI of cGVHD was 36.2% ± 0.7%, and that of moderate to severe cGVHD was 14.9% ± 0.4%. In univariate analysis, patients with higher graft CD34+ cell infusion (>3.35×106/kg) had higher rates of extensive cGVHD (32.8% vs. 5.6%, p = 0.045). No other factor was identified on the incidence of GVHD.
A total of 29 patients are alive with a median follow-up of 45.6 (5.9–166.9) months. The causes of death included severe pneumonia (n = 3), intracranial hemorrhage (n = 1), Aeromonas hydrophila bacteremia (n = 1), poor graft function (n = 1), and suicide (n = 1).
Outcomes of repeated IST
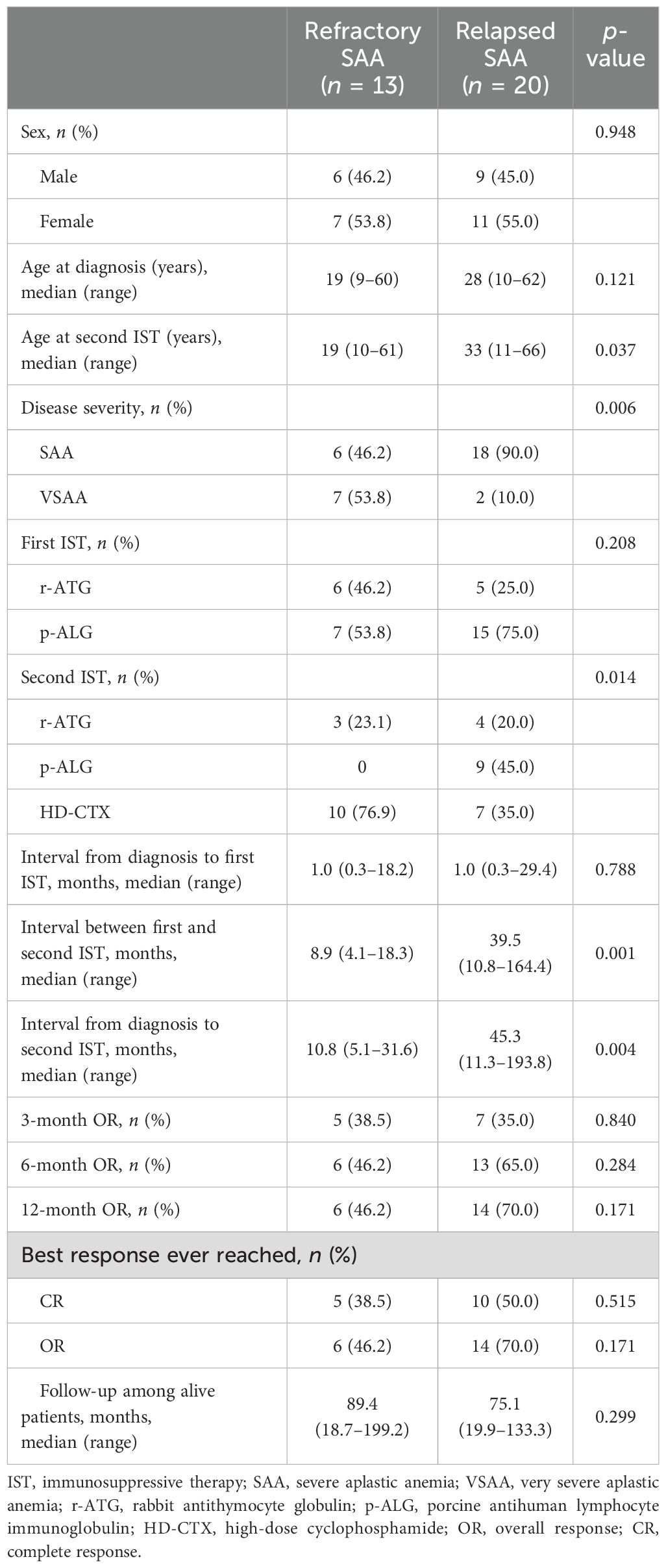
The characteristics of patients and outcomes of repeated IST are summarized in Table 3. The median interval between the first and second course of IST was 8.9 (4.1–18.3) months for refractory setting. The median time from the initial ATG to relapse was 36.0 (5.0–132.0) months; the median time between the first and second course of IST was 39.5 (10.8–164.4) months for relapse setting. At 3 months after the initiation of the second IST, response was observed in 12 patients, including 11 with PR and 1 with CR. By 6 months, 29 cases were evaluated, with 4 achieving CR and 15 achieving PR. The ORR among all patients was 57.6% (19/33), being higher in relapsed patients than in those with refractory SAA (65.0% vs. 46.2%), although the difference was not statistically significant due to the limited patient cohort (p = 0.284). In addition, patients salvaged with repeated IST pre- or post-2017 showed comparable response rates (68.8% vs. 47.1%, p = 0.208). Ten patients improved between 6 and 12 months, and the ORR was 60.6% (20/33) at 12 months, including 13 CR and 7 PR. Hematologic responses in the second ATG group and HD-CTX group showed no significant differences in ORRs at 3 months (37.5% vs. 35.3%, p = 0.895), 6 months (50.0% vs. 64.7%, p = 0.393), and 12 months (56.3% vs. 64.7%, p = 0.619) (Supplementary Table 1).
The median follow-up was 81.6 (18.7–199.2) months among alive patients from the start of second IST. A total of six patients died, and causes of death included pulmonary infection (n = 3) and central nervous system infection (n = 1). Moreover, two patients received a second course of ATG from the same course and died of severe allergic reaction within 1 week. Two CR patients relapsed 43 and 59 months again, and successfully rescued with the third IST. No secondary clonal disorders were observed.
Survival outcomes
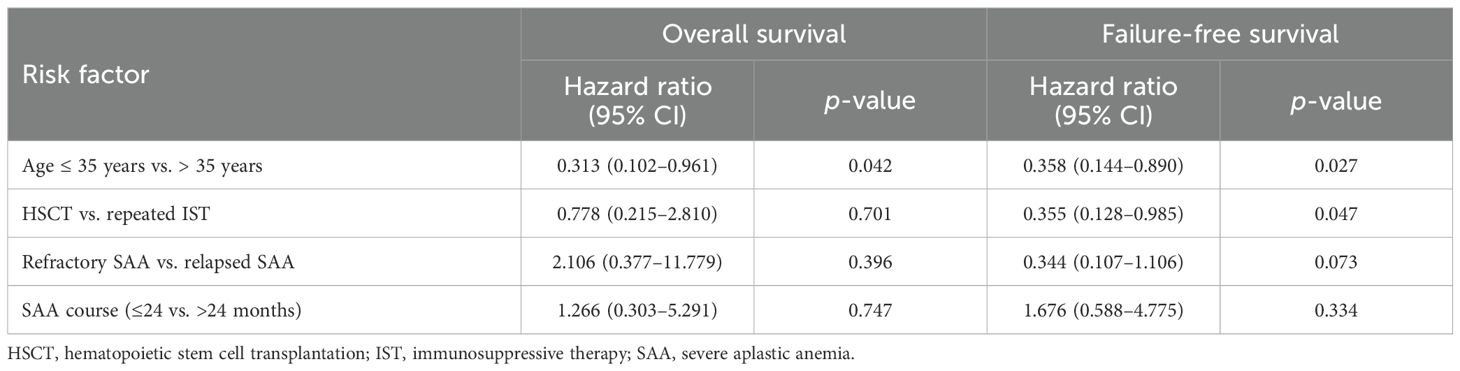
The estimated 4-year OS and FFS for the entire cohort were 80.0% ± 5.0% and 68.3% ± 5.8%, respectively. The 4-year CI of treatment-related mortality (TRM) was 20.0% ± 5.0%. In univariate analysis, younger patients (≤35 years) demonstrated better FFS (77.2% ± 6.1% vs. 43.7% ± 11.9%, p = 0.007) and a trend toward improved OS (85.5% ± 5.1% vs. 63.7% ± 11.9%, p = 0.057). Compared with repeated IST, salvage allo-HSCT offered a comparable OS (79.8% ± 6.8% vs. 80.0% ± 7.3%, p = 0.957) but a significantly higher FFS (79.8% ± 6.8% vs. 56.6% ± 8.8%, p = 0.049) (Figure 1). Further analysis revealed that the FFS of HSCT was clearly better than that of second ATG (79.8% vs. 49.2%, p = 0.018), but comparable to that of HD-CTX (79.8% vs. 64.7%, p = 0.295). Multivariate analysis identified age ≤ 35 years as a favorable factor for both the OS [hazard ratio (HR) 0.313, 95% CI 0.102–0.961] and FFS (HR 0.358, 95% CI 0.144–0.890). In addition, the choice of allo-HSCT was an independent factor for superior FFS (HR 0.355, 95% CI 0.128–0.985) (Table 4).
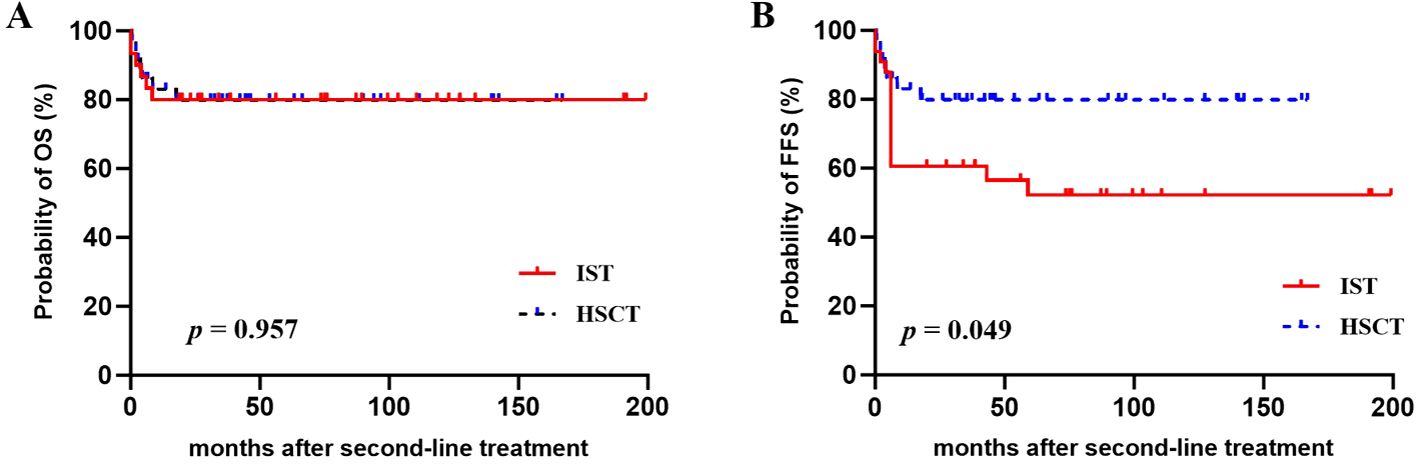
Figure 1. (A) Overall survival (OS) and (B) failure-free survival (FFS) after second-line treatments with repeated IST or salvage HSCT.
We further analyzed survival outcomes for the HSCT group and the IST group, respectively. In the HSCT cohort (Supplementary Table 2), older patients (>35 years) showed a significantly lower 4-year OS (38.1% vs. 89.2%, p = 0.002) and FFS (38.1% vs. 89.2%, p = 0.002) when compared with younger patients. In addition, OS and FFS were worse in recipients conditioned with the BFAC regimen than with the FAC regimen. It was noteworthy that compared to the FAC regimen, patients in the BFAC cohort were older (26 years vs. 14 years, p = 0.022) and had a longer disease course (13 months vs. 9 months, p = 0.039), which might contribute to their inferior survival outcomes. The GFFS was also calculated in the HSCT group, which was considered to be an alternative marker of quality of survival. The estimated 4-year GFFS was 71.4% ± 7.7%; poor GFFS was observed in MUD (42.9%) transplants as compared to MSD (83.3%) and HID (77.4%) transplants (p = 0.049), and also in older patients (>35 years) (38.1% vs. 78.8%, p = 0.052). Because of the limited HSCT cohort size, we did not perform further multivariate analysis. In the IST cohort, none of the assessed variables [age, disease course, disease status (refractory/relapse), retreated with second ATG or HD-CTX, and salvage therapy year (pre- or post-2017)] were detected to affect the survival.
Analysis of subgroup
As we identified age ≤ 35 years as an independent prognostic factor, we stratified patients into two age groups: ≤35 years and >35 years (Figures 2A–D). For patients aged ≤35 years, salvage HSCT provided a similar 4-year OS (89.2% ± 5.9% vs. 80.8% ± 8.9%, p = 0.343) but a far better FFS (89.2% ± 5.9% vs. 62.9% ± 10.5%, p = 0.023) than a second IST. For patients aged >35 years, no significant differences in survival were observed between the two salvage therapies.

Figure 2. Subgroup analysis of survival outcomes following repeated IST or salvage HSCT. (A–D) The overall survival (OS) and failure-free survival (FFS) for patients with two age stratifications (≤35 years, >35 years). (E–H) The OS and FFS for children and adults, respectively. (I, J) The OS and FFS in patients with refractory SAA.
In addition, considering our study included both children (n = 21) and adults (n = 48), we analyzed survival outcomes between the two groups (Figures 2E–H). The 4-year OS was 81.0% ± 8.6% for children and 79.6% ± 6.1% for the adult cohort (p = 0.960). The 4-year FFS was 81.0% ± 8.6% for children and 62.5% ± 7.4% for the adult cohort (p = 0.167). Of note, children in the HSCT group were all alive without treatment failures, yielding a significantly higher 4-year OS (100% vs. 50.0% ± 17.7%, p = 0.004) and FFS (100% vs. 50.0% ± 17.7%, p = 0.004) than children in the IST group. For adults, however, outcomes of transplants and repeated IST were not significantly different.
We also conducted subgroup analysis in 46 patients with refractory SAA, and found this group benefited more from HSCT than repeated IST. At 6 months after second-line treatment, the CR rate was 81.8% in the HSCT cohort, while it was only 7.7% in the IST cohort (p < 0.001). The 4-year OS was not statistically different (81.5% vs. 69.2%, p = 0.398); however, the FFS was far better for patients salvaged with HSCT than with IST (81.5% vs. 46.2%, p = 0.032) (Figures 2I, J).
Discussion
Patients with refractory/relapse SAA are at increased risk of death from infection and hemorrhage and later clonal evolution. Outcomes remain poor for them and the best option is not consensual. In the current study, we conducted a long-term follow-up of 69 patients with SAA who were rescued with HSCT or repeated intensified IST after failure of the first ATG-base IST. Compared with repeated IST, we observed that HSCT provided a superior FFS in both univariate and multivariate analysis. Subgroup analyses revealed that younger patients (age ≤ 35 years) and patients with refractory SAA benefited more from HSCT.
In our repeated IST cohort, two patients received a second ATG from the same source and died of severe allergic reactions within 1 week. In a prospective multicenter trial (35), likewise, 3 out of 21 patients developed an anaphylactoid reaction to the same source h-ATG and could not complete the second IST. Furthermore, Tichelli et al. found that serum sickness occurred earlier after repeated ATG as compared to initial exposure (36). Therefore, anaphylactic reactions and serum sickness are worth noting in the second course of ATG. In the HD-CTX group, however, patients showed better tolerability with no excess mortality, which might be attributed to the reduction dose of CTX and the deferral administration of CsA in our modified regimen. Although the FFS of second ATG was inferior to salvage HSCT, the other regimen of IST, HD-CTX plus CsA, achieved an FFS that was comparable to HSCT. However, owing to the limited numbers, the results need to be confirmed in a larger study.
For patients who have relapsed after the initial IST, a prior response implies an immune-mediated pathogenetic mechanism, and thus, 50%–65% of them can be successfully salvaged with a second IST (10). For patients with refractory SAA, the lack of response may due to non-immune-based pathophysiology, extreme hematopoietic stem cell exhaustion, or inadequate immunosuppression (10, 37). Several studies have reported unsatisfactory responses to a further course of IST in a refractory setting (12, 19, 35, 38). In our IST cohort, the response rate at 6 months was 57.6%, and it was also lower in refractory patients than in relapsed patients (46.2% vs. 65.0%). In the subgroup analysis of refractory SAA, we found that repeated IST provided a significantly lower CR rate at 6 months (7.7% vs. 81.8%, p < 0.001) and FFS at 4 years (46.2% vs. 81.5%, p = 0.032) when compared with HSCT. Therefore, it is reasonable to consider HSCT instead of a second IST for refractory patients.
Favorable outcomes of HSCT post-IST failure have been investigated in many studies. Kosaka and colleagues compared the efficacy of repeated IST and alternative donor HSCT in children who failed previous IST and found that HSCT provided a better chance of FFS at 5 years than a second course of IST (83.9% vs. 9.5%, p = 0.001) (35). The long-term outcomes in the HSCT cohort of our study were also encouraging, with an estimated 4-year OS and FFS of 79.8% ± 6.8%. Notably, all children in the HSCT cohort were alive without treatment failures, showing a 4-year OS and FFS of 100%, which is significantly higher than that of children in the IST cohort. In addition, HSCT offered a superior FFS than repeated IST for patients aged ≤35 years, while no such survival advantage was observed in patients aged >35 years. These results suggested that younger patients, especially children, benefited more from HSCT than a second IST.
Graft failure is a major concern for transplant patients with refractory/relapse SAA, as they are often heavily transfused and have a longer course of disease. In our research, over half (63.9%) of the recipients underwent alternative donor HSCT. Of note, no primary or secondary graft failure was observed. The encouraging outcome might be partly attributed to the application of the intensified BFAC conditioning regimen in the majority (24/36) of patients. As previously reported (39, 40), adding BU or low-dose total body irradiation for augmented conditioning facilitated engraftment in SAA patients. Hashem et al. also showed that application of a more intense conditioning was successful in overcoming the high rates of GF (8). However, as we mentioned above, recipients conditioned with the BFAC regimen were older and had a longer disease course than recipients conditioned with the FAC regimen. Several studies (41–43) have demonstrated that older age and a longer SAA history were negative predictors of survival. Consequently, patients in the BFAC group showed inferior survival, which was consistent with our previous report (24).
Several studies (44, 45) have demonstrated higher rates of GVHD in the salvage cohort compared to frontline MSD-HSCT. A significantly high proportion of alternative donor HSCT in the salvage setting might contribute to this disparity. Our results showed acceptable GVHD; the 100-day grades II–IV aGVHD was 36.1%, and 4-year cGVHD was 36.2%. The rates were comparable to our previous studies involving HID-HSCT for SAA (24, 46), reported II–IV aGVHD rates of 35%–38.4%, and cGVHD rates of 23%–35.3%. The 4-year GFFS was 71.4% ± 7.7%, which was not inferior to our recent data (47) reporting the rates of 61.2%–67.6% in 260 AA patients. In conclusion, our data presented the long-term outcomes of allo-HSCT and repeated IST in the salvage setting for patients with refractory/relapse SAA. Compared with IST, HSCT exhibited clear advantages in rapid complete hematopoietic recovery and superior FFS, especially in younger patients (≤35 years) and refractory setting. Therefore, for these patients, salvage HSCT may be more preferable than a second course of IST. We acknowledge several limitations of our study, including its retrospective nature and a relatively small sample size from a single center. Further prospective, multicenter studies in large cohorts are warranted to validate our results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the ethical committee of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences. We have obtained written informed consent from the patient or patient’s parent/guardian.
Author contributions
LZ: Writing – original draft. JL: Writing – original draft. WL: Writing – review & editing. XYZ: Writing – review & editing. SC: Writing – review & editing. YS: Writing – review & editing. MeH: Writing – review & editing. XLZ: Writing – review & editing. MG: Writing – review & editing. JW: Writing – review & editing. YH: Writing – original draft. EJ: Writing – review & editing. MiH: Writing – review & editing. FZ: Writing – original draft. SF: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-017 and 2021-I2M-C&T-B-080), a Tianjin Municipal Science and Technology Commission Grant (21JCZDJC01170), the Haihe Laboratory of Cell Ecosystem Innovation Fund (22HHXBSS00036), National Nature Science Foundation of China (82170217), Clinical Research Project of Tianjin Society of Hematology and Regenerative Medicine (2022 TSHRM08003), and the Special Fund Project for Science and Technology Innovation Strategy of Nanjing (YKK21273).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1425076/full#supplementary-material
References
1. Bacigalupo A. How I treat acquired aplastic anemia. Blood. (2017) 129:1428–36. doi: 10.1182/blood-2016-08-693481
2. Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. New Engl J Med. (2017) 376:1540–50. doi: 10.1056/NEJMoa1613878
3. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. (2012) 120:1185–96. doi: 10.1182/blood-2011-12-274019
4. Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. New Engl J Med. (2011) 365:430–8. doi: 10.1056/NEJMoa1103975
5. Young NS, Longo DL. Aplastic anemia. New Engl J Med. (2018) 379:1643–56. doi: 10.1056/NEJMra1413485
6. Dufour C, Veys P, Carraro E, Bhatnagar N, Pillon M, Wynn R, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the uk paediatric bmt working party, paediatric diseases working party and severe aplastic anaemia working P. Br J Haematology. (2015) 171:585–94. doi: 10.1111/bjh.13614
7. Xu L-P, Jin S, Wang S-Q, Xia L-H, Bai H, Gao S-J, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. (2017) 10:25. doi: 10.1186/s13045-017-0398-y
8. Hashem H, Rihani R, Shanap MA, Khattab E, Tbakhi A, Sultan I. Novel conditioning regimen for upfront haploidentical hematopoietic cell transplantation in children with severe aplastic anemia and donor-specific anti-hla antibodies. Bone marrow Transplant. (2022) 57:304–5. doi: 10.1038/s41409-021-01536-y
9. Xu LP, Xu ZL, Wang SQ, Wu DP, Gao SJ, Yang JM, et al. Long-term follow-up of haploidentical transplantation in relapsed/refractory severe aplastic anemia: A multicenter prospective study. Sci Bull. (2022) 67:963–70. doi: 10.1016/j.scib.2022.01.024
10. Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematology. (2006) 133:622–7. doi: 10.1111/j.1365-2141.2006.06098.x
11. Di Bona E, Rodeghiero F, Bruno B, Gabbas A, Foa P, Locasciulli A, et al. Rabbit antithymocyte globulin (R-atg) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Br J Haematology. (1999) 107:330–4. doi: 10.1046/j.1365-2141.1999.01693.x
12. Clé DV, Atta EH, Dias DS, Lima CB, Bonduel M, Sciuccati G, et al. Repeat course of rabbit antithymocyte globulin as salvage following initial therapy with rabbit antithymocyte globulin in acquired aplastic anemia. Haematologica. (2015) 100:e345–7. doi: 10.3324/haematol.2015.123760
13. Brodsky RA, Chen AR, Dorr D, Fuchs EJ, Huff CA, Luznik L, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. (2010) 115:2136–41. doi: 10.1182/blood-2009-06-225375
14. Brodsky RA, Chen AR, Brodsky I, Jones RJ. High-dose cyclophosphamide as salvage therapy for severe aplastic anemia. Exp Hematol. (2004) 32:435–40. doi: 10.1016/j.exphem.2004.02.002
15. Jaime-Pérez JC, González-Llano O, Gómez-Almaguer D. High-dose cyclophosphamide in the treatment of severe aplastic anemia in children. Am J Hematol. (2001) 66:71. doi: 10.1002/1096-8652(200101)66:1<71::aid-ajh1019>3.0.co;2-d
16. Brodsky RA, Sensenbrenner LL, Smith BD, Dorr D, Seaman PJ, Lee SM, et al. Durable treatment-free remission after high-dose cyclophosphamide therapy for previously untreated severe aplastic anemia. Ann Internal Med. (2001) 135:477–83. doi: 10.7326/0003-4819-135-7-200110020-00006
17. Tisdale JF, Dunn DE, Geller N, Plante M, Nunez O, Dunbar CE, et al. High-dose cyclophosphamide in severe aplastic anaemia: A randomised trial. Lancet. (2000) 356:1554–9. doi: 10.1016/s0140-6736(00)03126-3
18. Zhang F, Zhang L, Jing L, Zhou K, Wang H, Peng G, et al. High-dose cyclophosphamide compared with antithymocyte globulin for treatment of acquired severe aplastic anemia. Exp Hematol. (2013) 41:328–34. doi: 10.1016/j.exphem.2013.01.001
19. Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Wu CO, Young NS. Activity of alemtuzumab monotherapy in treatment-naive, relapsed, and refractory severe acquired aplastic anemia. Blood. (2012) 119:345–54. doi: 10.1182/blood-2011-05-352328
20. Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. (2014) 123:1818–25. doi: 10.1182/blood-2013-10-534743
21. Ruan J, Zuo W, Chen M, Yang C, Han B. Eltrombopag is effective in patients with relapse/refractory aplastic anemia—Report from a single center in China. Ann Hematol. (2020) 99:2755–61. doi: 10.1007/s00277-020-04266-1
22. Camitta Bm, Thomas Ed, Nathan Dg, Santos G, Gordon-Smith Ec, Gale Rp, et al. Severe aplastic anemia: A prospective study of the effect of early marrow transplantation on acute mortality. Blood. (1976) 48:63–70. doi: 10.1182/blood-2016-09-738534
23. DeZern AE, Eapen M, Wu J, Talano J-A, Solh M, Dávila Saldaña BJ, et al. Haploidentical bone marrow transplantation in patients with relapsed or refractory severe aplastic anaemia in the USA (Bmt ctn 1502): A multicentre, single-arm, phase 2 trial. Lancet Haematology. (2022) 9:e660–e9. doi: 10.1016/s2352-3026(22)00206-x
24. Zhang Y, Huo J, Liu L, Shen Y, Chen J, Zhang T, et al. Comparison of hematopoietic stem cell transplantation outcomes using matched sibling donors, haploidentical donors, and immunosuppressive therapy for patients with acquired aplastic anemia. Front Immunol. (2022) 13:837335. doi: 10.3389/fimmu.2022.837335
25. Chen X, Wei J, Huang Y, He Y, Yang D, Zhang R, et al. Effect of antithymocyte globulin source on outcomes of hla-matched sibling allogeneic hematopoietic stem cell transplantation for patients with severe aplastic anemia. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplant. (2018) 24:86–90. doi: 10.1016/j.bbmt.2017.10.007
26. Liu X, Yang W, Zhang L, Jing L, Ye L, Zhou K, et al. Development and validation of early death risk score model for emergency status prediction in very severe aplastic anemia. Front Immunol. (2023) 14:1175048. doi: 10.3389/fimmu.2023.1175048
27. Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. (2016) 172:187–207. doi: 10.1111/bjh.13853
28. Esteves I, Bonfim C, Pasquini R, Funke V, Pereira NF, Rocha V, et al. Haploidentical bmt and post-transplant cy for severe aplastic anemia: A multicenter retrospective study. Bone Marrow Transplant. (2015) 50:685–9. doi: 10.1038/bmt.2015.20
29. Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. (1989) 73:606–13. doi: 10.1182/blood.V73.2.606.606
30. Kharfan-Dabaja MA, Kumar A, Ayala E, Aljurf M, Nishihori T, Marsh R, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: A report on behalf of the american society for transplantation and cellular therapy. Transplant Cell Ther. (2021) 27:642–9. doi: 10.1016/j.jtct.2021.04.007
31. Choi YB, Yi ES, Lee JW, Sung KW, Koo HH, Yoo KH. Immunosuppressive therapy versus alternative donor hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an hla-matched familial donor. Bone Marrow Transplant. (2016) 52:47–52. doi: 10.1038/bmt.2016.223
32. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute gvhd grading. Bone Marrow Transplant. (1995) 15:825–8.
33. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplant. (2005) 11:945–56. doi: 10.1016/j.bbmt.2005.09.004
34. Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: A report from the european group for blood and marrow transplantation (Ebmt). Haematologica. (2007) 92:11–8. doi: 10.3324/haematol.10075
35. Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood. (2008) 111:1054–9. doi: 10.1182/blood-2007-08-099168
36. Tichelli A, Passweg J, Nissen C, Bargetzi M, Hoffmann T, Wodnar-Filipowicz A, et al. Repeated treatment with horse antilymphocyte globulin for severe aplastic anaemia. Br J haematology. (1998) 100:393–400. doi: 10.1046/j.1365-2141.1998.00578.x
37. Marsh JCW, Kulasekararaj AG. Management of the refractory aplastic anemia patient: what are the options? Blood. (2013) 122:3561–7. doi: 10.1182/blood-2013-05-498279
38. Scheinberg P, Townsley D, Dumitriu B, Scheinberg P, Weinstein B, Rios O, et al. Horse antithymocyte globulin as salvage therapy after rabbit antithymocyte globulin for severe aplastic anemia. Am J Hematol. (2014) 89:467–9. doi: 10.1002/ajh.23669
39. Dulley FL, Vigorito AC, Aranha FJ, Sturaro D, Ruiz MA, Saboya R, et al. Addition of low-dose busulfan to cyclophosphamide in aplastic anemia patients prior to allogeneic bone marrow transplantation to reduce rejection. Bone Marrow Transplant. (2004) 33:9–13. doi: 10.1038/sj.bmt.1704325
40. Arcuri LJ, Nabhan SK, Cunha R, Nichele S, Ribeiro AAF, Fernandes JF, et al. Impact of cd34 cell dose and conditioning regimen on outcomes after haploidentical donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for relapsed/refractory severe aplastic anemia. Biol Blood Marrow Transplant. (2020) 26:2311–7. doi: 10.1016/j.bbmt.2020.09.007
41. Bacigalupo A, Brand R, Oneto R, Bruno B, Socié G, Passweg J, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy–the european group for blood and marrow transplantation experience. Semin Hematol. (2000) 37:69–80. doi: 10.1016/s0037-1963(00)90031-3
42. Xu LP, Wang SQ, Ma YR, Gao SJ, Cheng YF, Zhang YY, et al. Who is the best haploidentical donor for acquired severe aplastic anemia? Experience from a multicenter study. J Hematol Oncol. (2019) 12:87. doi: 10.1186/s13045-019-0775-9
43. Bacigalupo A, Socié G, Hamladji RM, Aljurf M, Maschan A, Kyrcz-Krzemien S, et al. Current outcome of hla identical sibling versus unrelated donor transplants in severe aplastic anemia: an ebmt analysis. Haematologica. (2015) 100:696–702. doi: 10.3324/haematol.2014.115345
44. Xu L-P, Wang S-Q, Wu D-P, Wang J-M, Gao S-J, Jiang M, et al. Haplo-identical transplantation for acquired severe aplastic anaemia in a multicentre prospective study. Br J Haematology. (2016) 175:265–74. doi: 10.1111/bjh.14225
45. Dufour C, Pillon M, Passweg J, Socie G, Bacigalupo A, Franceschetto G, et al. Outcome of aplastic anemia in adolescence: A survey of the severe aplastic anemia working party of the european group for blood and marrow transplantation. Haematologica. (2014) 99:1574–81. doi: 10.3324/haematol.2014.106096
46. Zhang Y, Zhang GX, Pang AM, Yang DL, Zhang RL, Zhai WH, et al. [Clinical analysis of 76 patients with severe aplastic anemia treated with haploid hematopoietic stem cell transplantation]. Zhonghua xueyexue zazhi. (2023) 44:202–10. doi: 10.3760/cma.j.issn.0253-2727.2023.03.005
Keywords: severe aplastic anemia, relapse, refractory, hematopoietic stem cell transplantation, immunosuppressive therapy
Citation: Zhang L, Li J, Liang W, Zhang X, Chen S, Shi Y, Hao M, Zhao X, Gong M, Wei J, He Y, Jiang E, Han M, Zhang F and Feng S (2024) Comparison of hematopoietic stem cell transplantation and repeated intensified immunosuppressive therapy as second-line treatment for relapsed/refractory severe aplastic anemia. Front. Immunol. 15:1425076. doi: 10.3389/fimmu.2024.1425076
Received: 29 April 2024; Accepted: 29 July 2024;
Published: 16 August 2024.
Edited by:
Robert James Hayashi, Washington University in St. Louis, United StatesReviewed by:
Hasan Hashem, King Hussein Cancer Center, JordanFan Lin, Peking University People’s Hospital, China
Copyright © 2024 Zhang, Li, Liang, Zhang, Chen, Shi, Hao, Zhao, Gong, Wei, He, Jiang, Han, Zhang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sizhou Feng, c3pmZW5nQGloY2Ftcy5hYy5jbg==; Fengkui Zhang, Zmt6aGFuZ0BpaGNhbXMuYWMuY24=; Yi He, aGV5aUBpaGNhbXMuYWMuY24=
†These authors have contributed equally to this work and share first authorship
 Lining Zhang1,2†
Lining Zhang1,2† Jianping Li
Jianping Li Xiaoyu Zhang
Xiaoyu Zhang Shulian Chen
Shulian Chen Yuanyuan Shi
Yuanyuan Shi Xiaoli Zhao
Xiaoli Zhao Yi He
Yi He Erlie Jiang
Erlie Jiang Mingzhe Han
Mingzhe Han Sizhou Feng
Sizhou Feng