- 1Clinical Laboratory, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, Zhejiang, China
- 2Clinical Laboratory, Nanxun District Hospital of Traditional Chinese Medicine, Huzhou, Zhejiang, China
- 3Clinical Laboratory, People’s Hospital of Anji, Huzhou, Zhejiang, China
Background: The efficiency of systemic immune-inflammation index (SII) in predicting prognosis of osteosarcoma (OSA) patients has been extensively analyzed, but no consistent findings are obtained. Therefore, this meta-analysis focused on identifying the precise prognostic value of SII for OSA.
Methods: We comprehensively searched electronic databases of PubMed, Embase, Web of Science, Cochrane Library, and China National Knowledge Infrastructure (CNKI) from inception to 24 February, 2024. Meanwhile, the efficiency of SII in predicting prognosis of OSA was evaluated by calculating pooled hazard ratios (HRs) as well as 95% confidence intervals (CIs). Additionally, the correlation of SII with the OSA clinicopathological characteristics was analyzed based on pooled odds ratios (ORs) and 95%CIs.
Results: Six studies with 1015 cases were enrolled into this work. According to the combined data, the higher SII was markedly related to poor overall survival (OS) (HR=2.01, 95%CI=1.30-3.09, p=0.002) and Enneking stage III (OR=2.21, 95%CI=1.11-4.39, p=0.024) of patients with OSA. Nonetheless, SII was not significantly related to gender, age, pathological fracture, tumor size, tumor location, tumor differentiation, and metastasis in patients with OSA.
Conclusions: In summary, the higher SII is markedly related to poor OS and advanced Enneking stage in OSA patients.
Systematic review registration: https://inplasy.com/inplasy-2024-7-0107/,
identifier INPLASY202470107.
Introduction
Osteosarcoma (OSA) shows the highest morbidity among primary malignant bone tumor among children and young adults (1). It exhibits the typical feature of formation of immature osteoid by tumor cells (2). All OSA patients have a median age of 20 years. The incidence of OSA in children and young adults worldwide varies between 2-3 per million, accounting for about 20%-30% of all primary bone tumors (3). In the past several decades, OSA patients have been treated by multiple strategies like surgery, radiotherapy, chemotherapy, gene therapy and immunotherapy (4). In spite of this, the prognosis of OSA remains poor. The 5-year survival rate in patients with localized OSA is about 60%, but that is only 20% in those developing metastases or recurrent disease (5). Around 10-15% of newly diagnosed OSA cases develop metastases, usually located in the lung (6). Therefore, it is of urgent necessary to develop novel and creditable markers for predicting prognosis of patients with OSA.
It has been widely reported that cancer-related inflammation and immune system have a critical effect on carcinogenesis, tumor growth, progression and metastasis (7, 8). Many hematological parameters including platelet-to-lymphocyte ratio (9), lymphocyte-to-monocyte ratio (10), prognostic nutritional index (PNI) (11), neutrophil-to-lymphocyte ratio (12), and systemic immune-inflammation index (SII) (13), are identified as efficient markers for predicting prognosis of various cancers. SII was developed in 2014 and reflects the general immune status of the body as an easy-to-access inflammatory parameter (14). SII is calculated as follows: SII= platelet number* neutrophil number/lymphocyte number (14). Notably, SII is previously reported to be significant for predicting prognosis of diverse solid tumors including rectal cancer (15), non-small cell lung cancer (16), breast cancer (17), renal cell carcinoma (18), and pancreatic neuroendocrine tumors (19). Meanwhile, the efficiency of SII in predicting prognosis of OSA patients is widely analyzed in numerous studies, but no consistent findings are obtained (20–25). For example, in some studies, the higher SII significantly predicted the prognosis of OSA patients (21, 22, 24, 25). Whereas in others, SII is not markedly related to the prognosis of OSA patients (23). Consequently, the present work was performed for identifying the accurate role of SII in predicting prognosis of OSA patients. Moreover, we investigated the correlation of SII with the OSA clinicopathological factors.
Materials and methods
Study guideline
The present work was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (26). This meta-analysis was registered in INPLASY under the registration number INPLASY202470107. This protocol can be available at https://inplasy.com/inplasy-2024-7-0107/.
Ethics statement
Ethical approval and informed consent were waived since the present work was conducted using previously published data.
Literature search
PubMed, Embase, Web of Science, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases were thoroughly retrieved between inception and 24 February, 2024 using the following search strategies (systemic immune-inflammatory index OR systemic-immune-inflammation index OR systemic immune-inflammation index) AND (osteosarcoma OR osteogenic sarcoma OR bone sarcoma). There was no limitation to publication language. The detailed search strategies for each database were shown in Supplementary File 1. Besides, we manually screened references in enrolled articles to identify more eligible studies.
Eligibility criteria
Studies conforming to criteria below were included: (1) the diagnosis of OSA was made pathologically; (2) the relation of SII with prognosis of OSA was reported; (3) hazard ratios (HRs) and 95% confidence intervals (CIs) were available or calculable using Tierney’s method (27); (4) the SII threshold was provided; and (5) there was no restriction of publication language. Studies below were excluded: (1) meeting abstracts, reviews, case reports, comments, and letters; (2) those did not provide survival data; and (3) animal studies.
Data collection and quality evaluation
Data were collected from qualified articles by two researchers (ZW and ZZ). Any disagreement between them was settled through discussion with a third researcher (ZJ) to reach a consensus. Data below were collected: first author, year, country, sample size, gender, age, study duration, study design, study center, Enneking stage, treatment, SII threshold, survival endpoints, survival analysis types, follow-up, and HRs with 95%CIs. Two independent reviewers (XW and ZZ) used Newcastle–Ottawa scale (NOS) to evaluate study quality (28). Notably, NOS evaluates study quality from 3 domains, selection, comparability, and outcome ascertainment. The total NOS score is 0-9 points, with ≥6 points indicating high-quality studies.
Statistical analysis
The value of SII in predicting prognosis of OSA was analyzed by calculating combined HRs and 95%CIs. Moreover, the heterogeneity degree among enrolled studies was quantified by Cochran’s Q-test and Higgins I2 statistic. In the presence of obvious heterogeneity (I2>50% or p<0.010), we utilized a random-effects model; or else, we adopted a fixed-effects model. Additionally, the significance of SII for predicting prognosis of different subgroup OSA populations was explored by subgroup analysis. The correlation between SII and the OSA clinicopathological factors was investigated through pooling odds ratios (ORs) and 95%CIs. In the meantime, sensitivity analysis was also conducted for comparing the pooled effect when each study was excluded individually to determine whether a particular study is responsible for heterogeneity and to ensure results are stable. Publication bias was evaluated by using Begg’s funnel plot and Egger’s test. Stata version 12.0 (Statacorp, College Station, TX, USA) was employed for statistical analysis. p<0.05 stood for statistical significance.
Results
Process of literature search
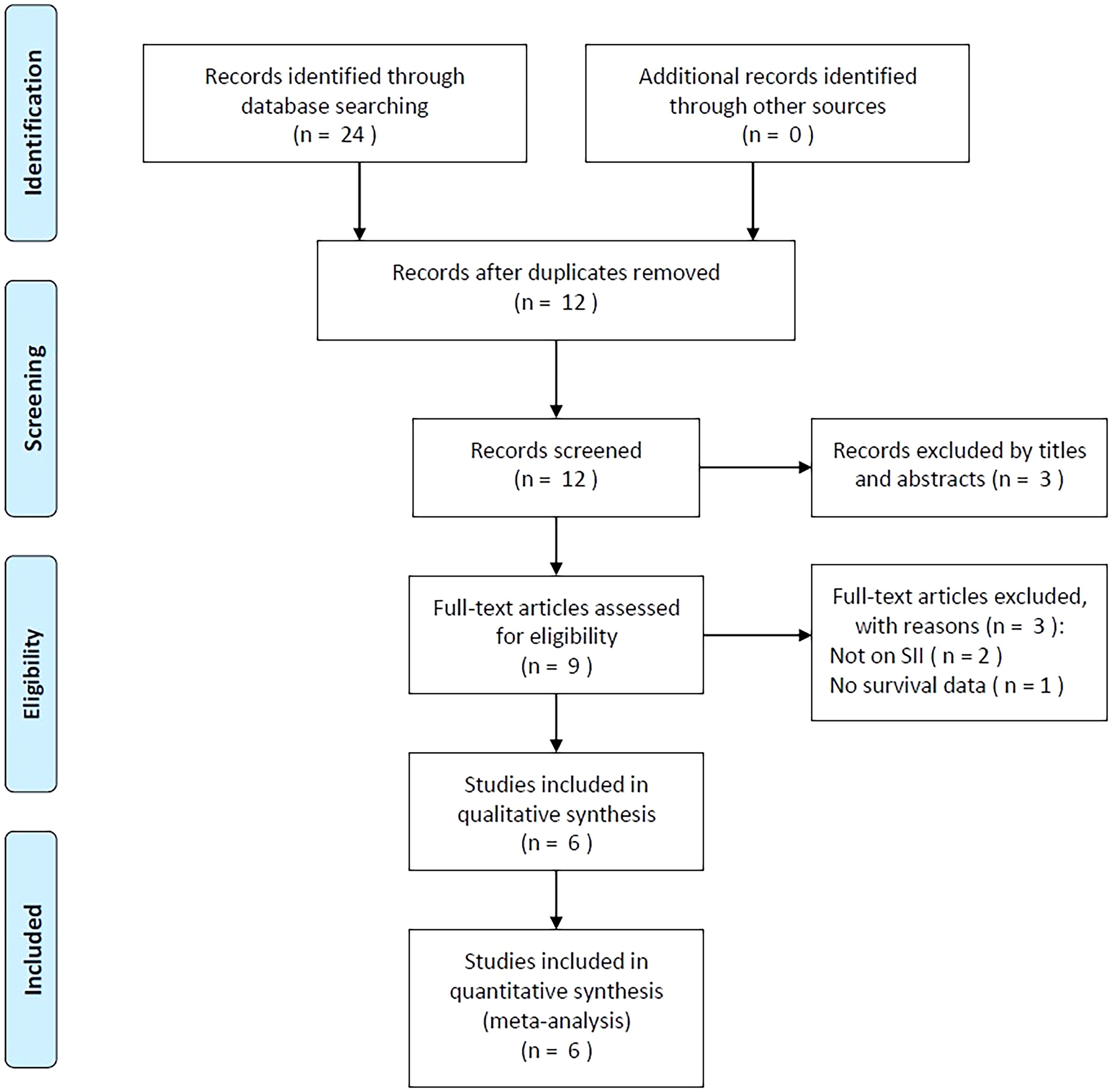
Initially, 24 studies were obtained through primary retrieval, among which, 12 were maintained when duplicates were eliminated (Figure 1). By title and abstract screening, we discarded three articles due to irrelevance. Full-texts of the rest 9 studies were examined, among which, three were eliminated due to irrelevance of SII (n=2) and unavailable survival data (n=1). Finally, six studies with 1015 patients (20–25) were enrolled into this work (Figure 1; Table 1).
Enrolled study features
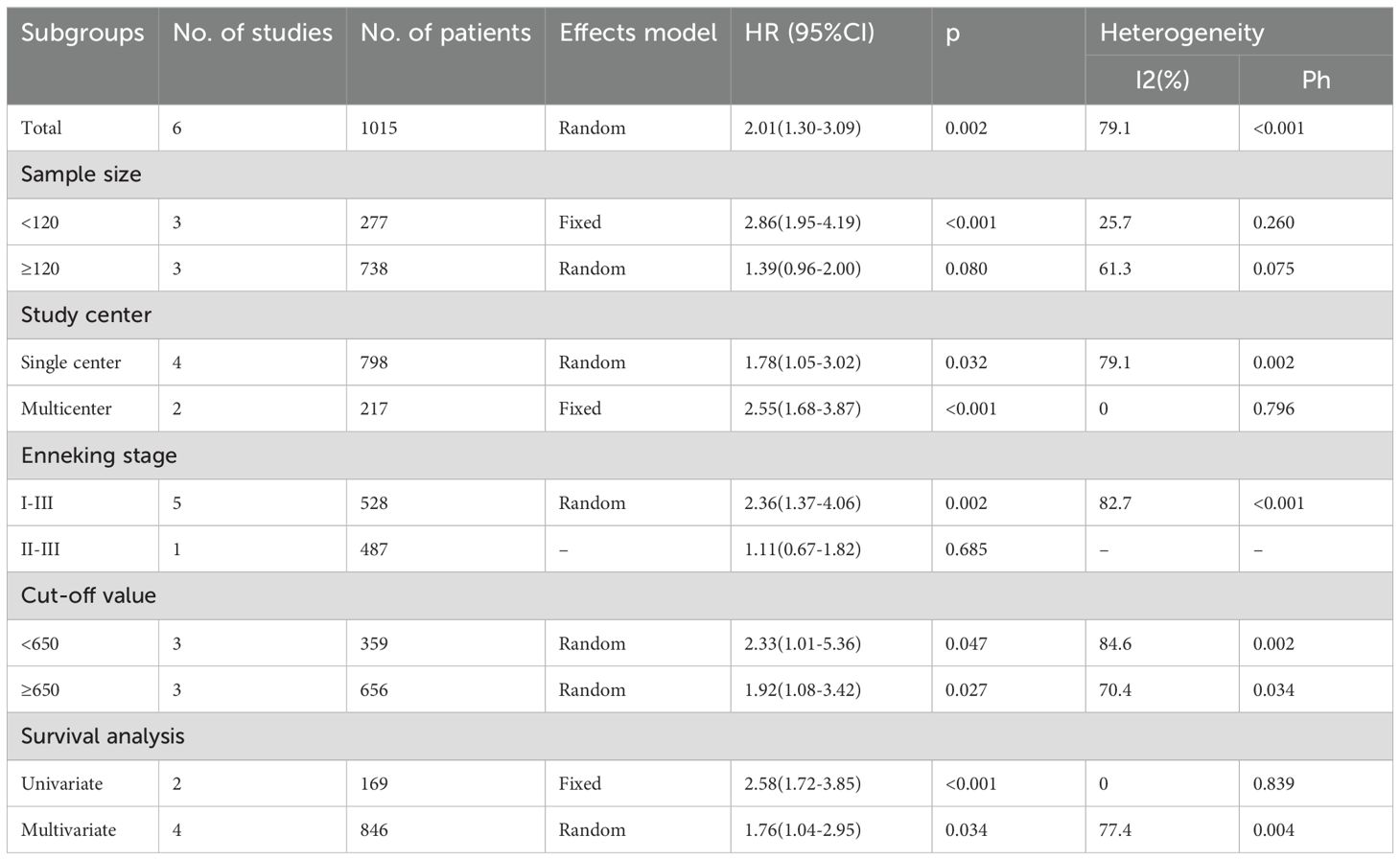
Table 1 displays basic study features (20–25). These studies were published during 2019-2023 and were all conducted in China. There were four (20–23) and two (24, 25) studies published in English and Chinese separately. All included studies were of retrospective design (20–25). The sample size was 77-487 (median, 116.5). There were four single center studies (20, 21, 23, 25) and two multicenter studies (22, 24). Five articles enrolled OSA patients of Enneking stage I-III (20–22, 24, 25), while one included those of Enneking stage II-III (23). All studies treated patients with mixed treatments including surgery, chemotherapy, and radiotherapy (20–25). The median SII threshold was 639.48 (range, 384.9-869.04). Each of our enrolled articles mentioned the relation of SII with overall survival (OS) in patients with OSA. In four articles, the HRs and 95%CIs were obtained through multivariate analysis (20, 22, 23, 25), while in another two articles, they were acquired through univariate analysis (21, 24). NOS scores ranged from 7 to 9, suggesting high-quality studies (Table 1).
SII and OS
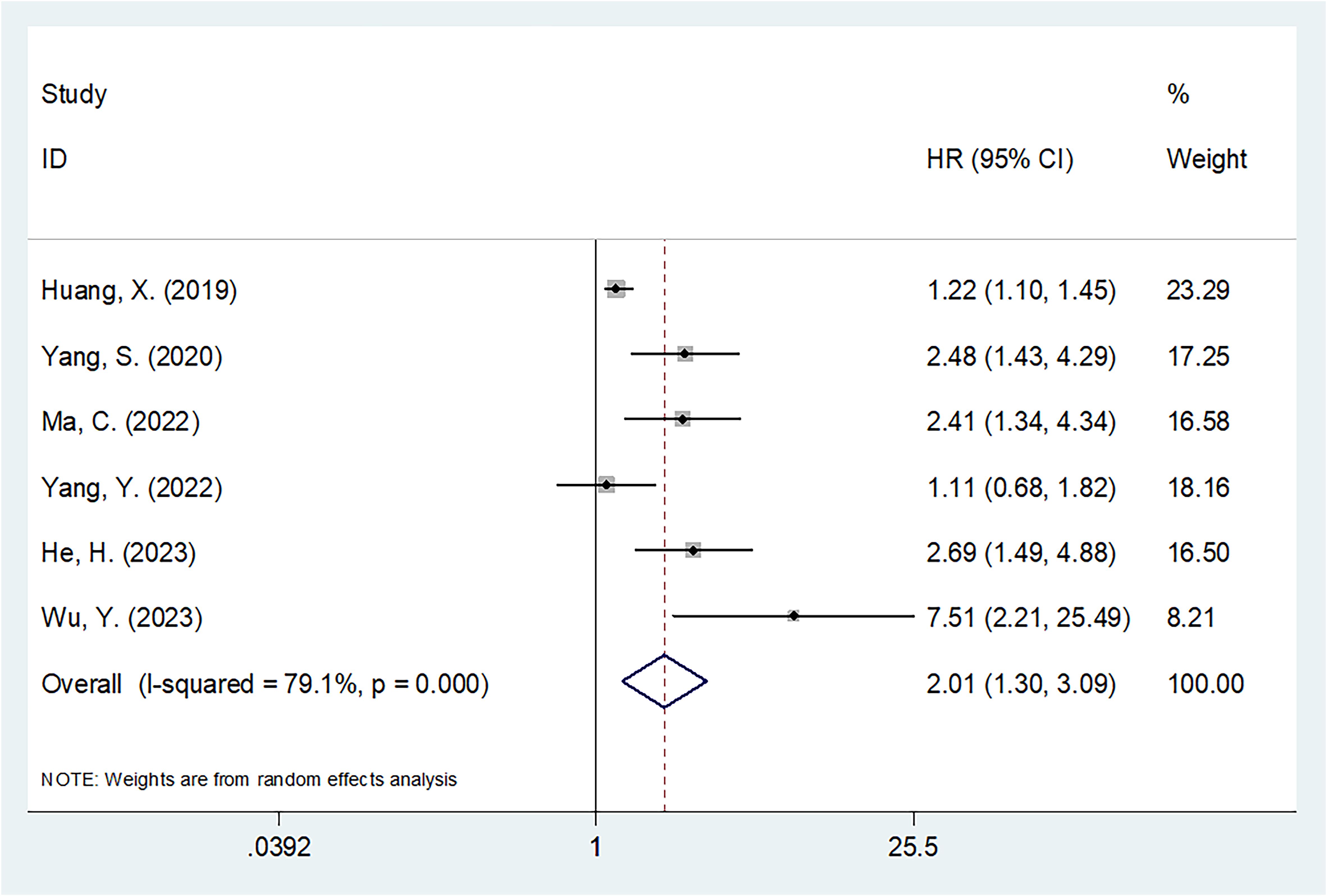
The six articles involving 1015 patients (20–25) reported the value of SII in predicting OS of OSA patients. Due to obvious heterogeneity (I2 = 79.1%, p<0.001), we used the random-effects model. HR=2.01, 95%CI=1.30-3.09, and p=0.002 were obtained from pooled results, suggesting that the higher SII was markedly related to dismal OS of OSA patients (Table 2; Figure 2). Upon subgroup analysis, SII still significantly predicted OS of OSA patients, irrespective of study center, threshold, and survival analysis types (p<0.02; Table 2). Additionally, SII was significantly related to dismal OS of OSA in subgroups below: sample size <120 (p<0.001) and patients with Enneking stage I-III (p=0.002) (Table 2).
The association of SII with the OSA clinicopathological features
Four studies with 436 patients (20–22, 25) provided the data on association of SII with the OSA clinicopathological characteristics. According to pooled data, an elevated SII was remarkably related to Enneking stage III in patients with OSA (OR=2.21, 95%CI=1.11-4.39, p=0.024; Table 3; Figure 3). However, SII was not significantly related to gender (OR=1.56, 95%CI=0.66-3.71, p=0.315), age (OR=1.34, 95%CI=0.91-1.99, p=0.141), pathological fracture (OR=1.38, 95%CI=0.85-2.24, p=0.189), tumor location (OR=1.81, 95%CI=0.91-3.62, p=0.092), tumor size (OR=2.18, 95%CI=0.88-5.37, p=0.092), tumor differentiation (OR=2.50, 95%CI=0.93-6.70, p=0.069), and metastasis (OR=2.44, 95%CI=0.69-8.59, p=0.164) of OSA patients (Table 3; Figures 3, 4).
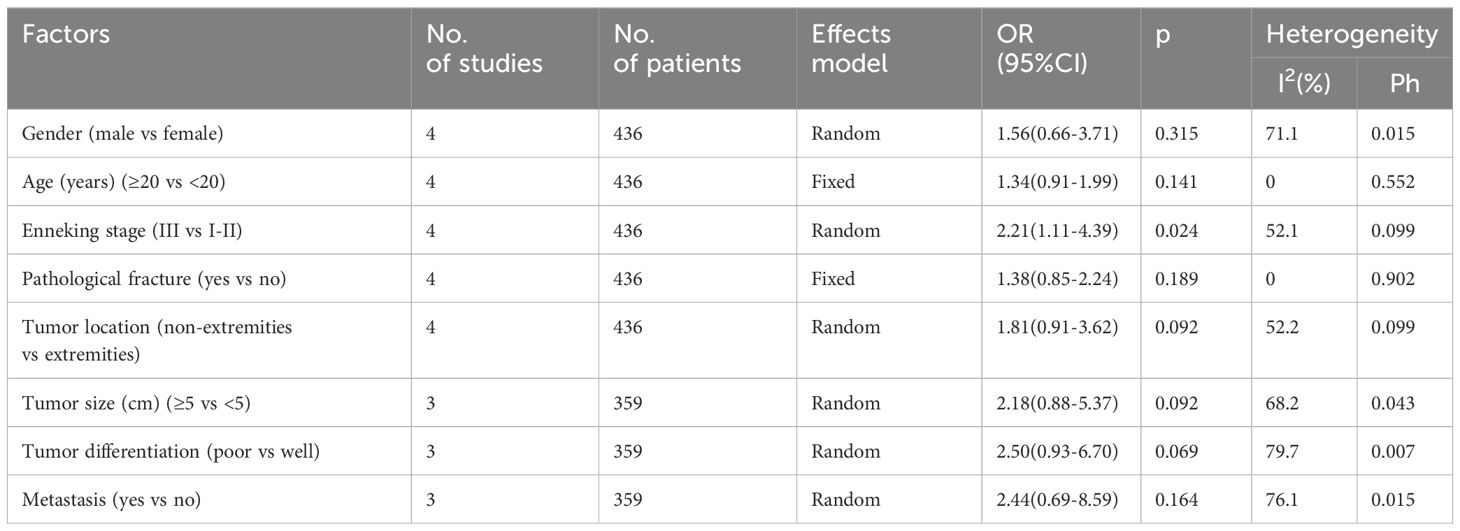
Table 3. The association between SII and clinicopathological features in patients with osteosarcoma.

Figure 3. Forest plots assessing the relationship between the SII and clinicopathological factors in OSA. (A) Gender (male vs female); (B) Age (years) (≥20 vs <20); (C) Enneking stage (III vs I-II); and (D) Pathological fracture (yes vs no).
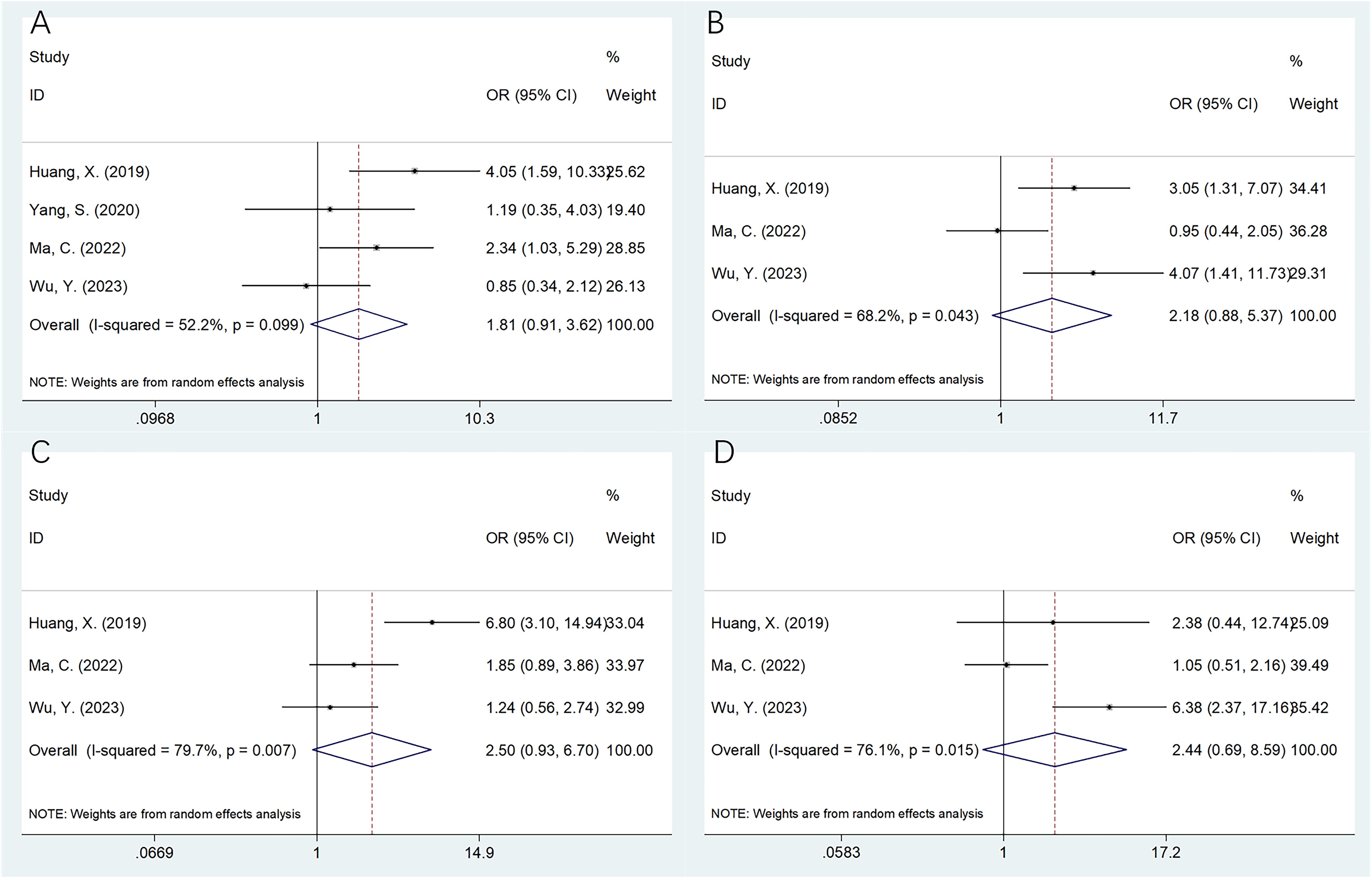
Figure 4. Forest plots assessing the relationship between the SII and clinicopathological factors in OSA. (A) Tumor location (non‐extremities vs extremities); (B) Tumor size (cm) (≥5 vs <5); (C) Tumor differentiation (poor vs well); and (D) Metastasis (yes vs no).
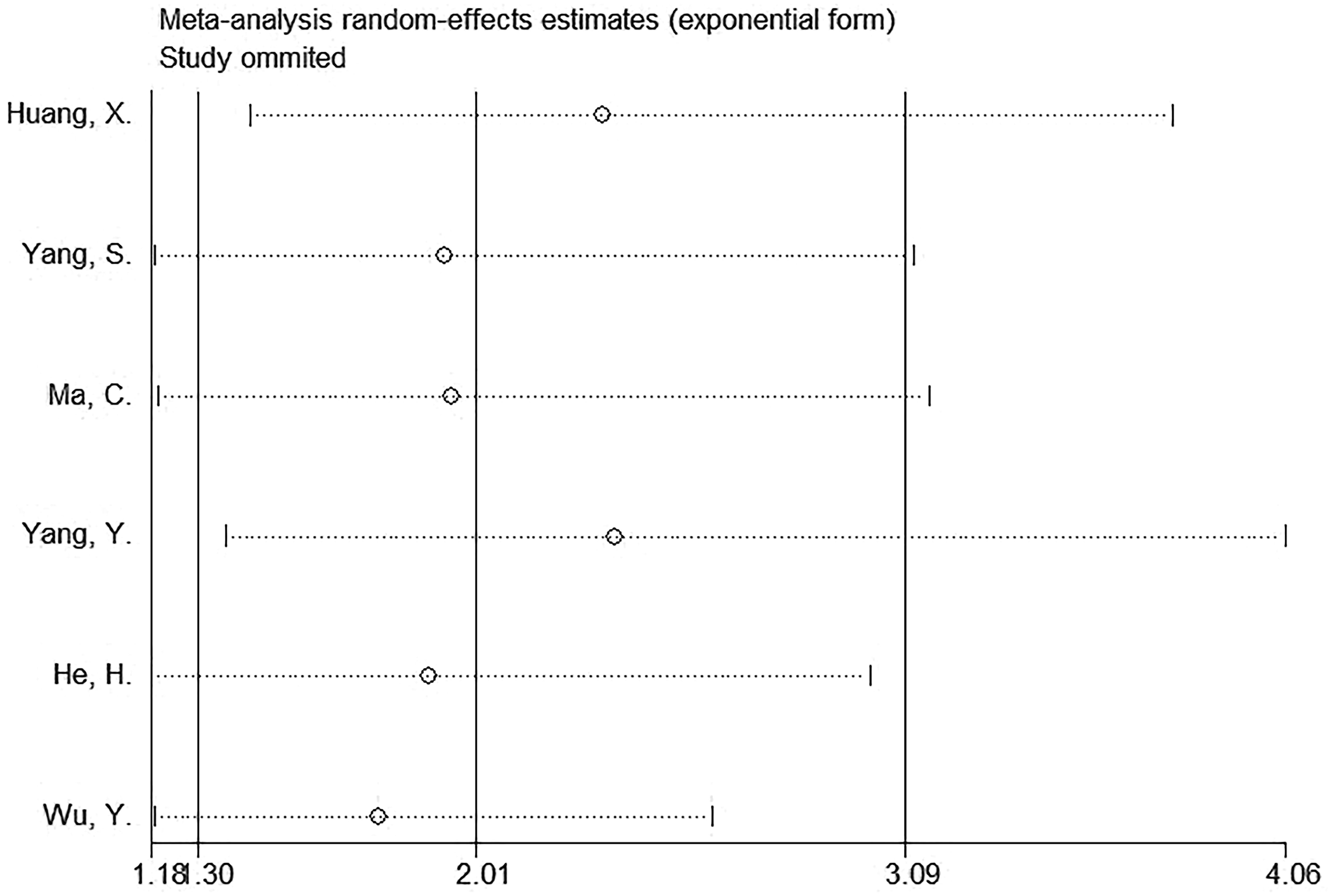
Sensitivity analysis
Sensitivity analysis suggested that the observed effect size for the relationship of SII with OS was not affected by any single study (Figure 5), which indicated the stability of the results in this work.
Publication bias
We employed Begg’s test and Egger’s test for evaluating the possible publication bias. From Figure 6, funnel plots showed symmetry. Moreover, the results (p=0.124 and 0.178 upon Begg’s and Egger’s tests separately) demonstrated the absence of significant publication bias (Figure 6).
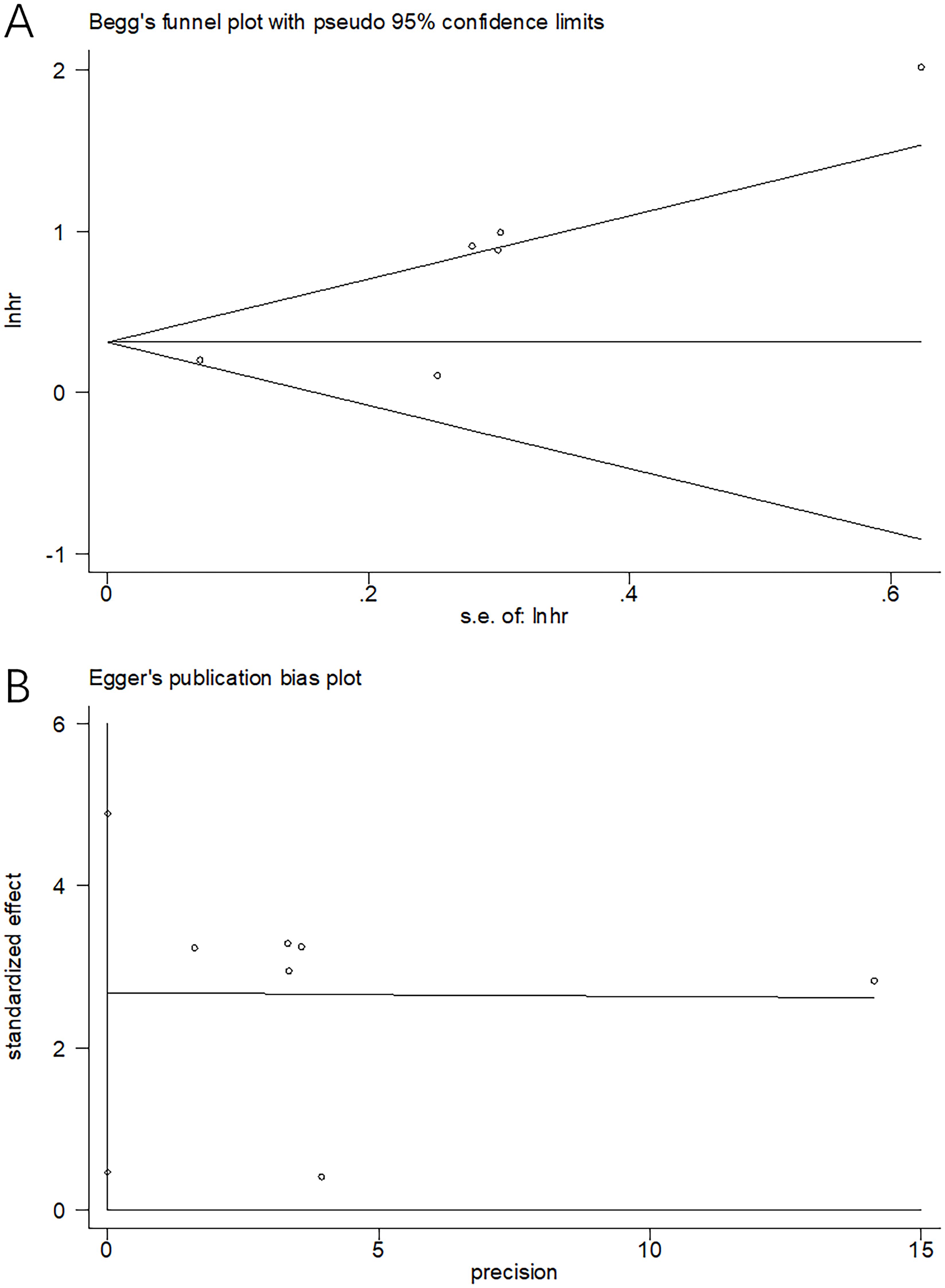
Figure 6. Publication bias tests. (A) Begg’s test for OS, p=0.124; (B) Egger’s test for OS, p=0.178.
Discussion
According to prior reports, the effect of SII on predicting prognosis of OSA patients remains controversial. In this work, information was synthesized in six articles involving 1015 patients for identifying the precise effect of SII on predicting OSA prognosis. In this meta-analysis, an elevated SII significantly predicted OS of OSA patients. Moreover, the higher SII was markedly related to advanced Enneking stage in OSA. SII served as the cheap and reliable prognostic marker for OSA patients. As far as we know, this meta-analysis is the first to investigate the effect of SII on predicting OSA prognosis.
The higher SII is the result of higher platelet number, higher neutrophil number, and/or lower lymphocyte number. The precise mechanisms of the association between SII and prognosis of OSA are not fully elucidated yet. Notably, the value of SII in predicting OSA prognosis is interpreted from several aspects. First, several studies have reported that platelets may protect cancer cells from the immune system’s cytotoxicity (29). In tumor cells, cytokines may stimulate megakaryocytes to produce platelets, resulting in an increase in platelet count (30). Platelets can also facilitate epithelial-mesenchymal transition (EMT) in cancer cells by directly contacting them or indirectly secreting prostaglandin E2 and growth factors (29). Second, during tumorigenesis, neutrophils contribute to the proliferation, invasion, and migration of tumor cells as well as tumor immunosuppression (31). Through the secretion of chemokines and cytokines, neutrophils are able to directly affect tumor cells or have an indirect effect on other tumor microenvironment (TME) components (32). These include vascular endothelial growth factor, transforming growth factor-beta, matrix metalloproteinases, interleukin-6 (IL-6), and IL-8 (33). Third, lymphocytes are crucial to anti-tumor cell-mediated responses. Lymphocytes can migrate into the TME and evolve into tumor-infiltrating lymphocytes (TILs), which can suppress the proliferation and migration of tumors through apoptosis (34, 35). Therefore, a higher SII can serve as the reasonable marker for predicting OSA prognosis.
Recently, SII is widely reported in meta-analysis with significant value in predicting prognosis of different solid tumors (36–40). According to Yang et al. in their meta-analysis involving 30 studies, the higher SII levels before treatment were related to poor OS and recurrence−free survival (RFS) of gastric cancer (36). Zhang and colleagues reported in their meta-analysis with 3464 patients that the higher SII was remarkably related to poor OS and DFS, low differentiation degree and advanced stage of oral squamous cell carcinoma (37). As mentioned in one meta-analysis enrolling 8133 patients with prostate cancer, a high SII was dramatically associated with poor OS, and worse progression-free survival/biochemical recurrence-free survival (PFS/bRFS) (38). In the meta-analysis comprising 2169 patients, Zeng et al. reported that the higher SII served as the effective marker to predict OS and PFS of nasopharyngeal carcinoma (39). Based on a meta-analysis with 1402 patients, the high SII was related to dismal OS of cholangiocarcinoma patients undergoing invasive surgery (40). Our meta-analysis findings were consistent with those in other cancer types. Notably, tumor necrosis rate is an important index for cancer treatment in patients with OSA (41, 42). A tumor necrosis rate > 90% usually indicates necrosis of tumor tissue, which showed inhibition of the blood supply to tumor tissue (41). In this meta-analysis, the association between SII and tumor necrosis rate was not analyzed due to limited information in included studies. The relationship between SII and tumor necrosis rate should be explored in future studies.
There were some limitations in the present work. First, this study had a small sample size. We just recruited six studies with 1015 patients, although we searched the most recent literature and did not restrict publication language. Second, all included studies were from China. In this regard, the findings in this work are more applicable for Chinese OSA populations. While the value of SII in the prognosis of OSA in other regions needs to be explored. Third, only retrospective studies were enrolled, which might lead to selection bias. Fourth, we only analyzed the prognostic value of SII for OS in this meta-analysis. The association between SII and other survival endpoints such as RFS, PFS, and DFS etc. was not investigated for OSA patients. We actually did not exclude RFS, PFS, and DFS in eligibility criteria, they were not included because of limited data provided in eligible studies. The correlation between SII and RFS, PFS, and DFS in OSA needs to be explored in future studies. Considering the above limitations, large-scale multi-regional prospective studies should be conducted for validation.
Conclusions
In summary, according to our meta-analysis results, the higher SII is remarkably related to poor OS of OSA patients. Additionally, the elevated SII is also significantly related to advanced Enneking stage in OSA. SII is the candidate prognostic marker of OSA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XW: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. ZW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. ZZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft. ZJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1416068/full#supplementary-material
Abbreviations
SII, systemic immune-inflammation index; OSA, osteosarcoma; CNKI, China National Knowledge Infrastructure; HR, hazard ratio; CI, confidence interval; OR, odds ratio; OS, overall survival; PNI, prognostic nutritional index; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle–Ottawa scale; EMT, epithelial-mesenchymal transition; TME, tumor microenvironment; TILs, tumor-infiltrating lymphocytes; RFS, recurrence−free survival; PFS, progression-free survival.
References
1. Sadykova LR, Ntekim AI, Muyangwa-Semenova M, Rutland CS, Jeyapalan JN, Blatt N, et al. Epidemiology and risk factors of osteosarcoma. Cancer Invest. (2020) 38:259–69. doi: 10.1080/07357907.2020.1768401
2. Belayneh R, Fourman MS, Bhogal S, Weiss KR. Update on osteosarcoma. Curr Oncol Rep. (2021) 23:71. doi: 10.1007/s11912-021-01053-7
3. Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer (Oxford England: 1990). (2019) 109:36–50. doi: 10.1016/j.ejca.2018.11.027
4. Zhao X, Wu Q, Gong X, Liu J, Ma Y. Osteosarcoma: a review of current and future therapeutic approaches. BioMed Eng Online. (2021) 20:24. doi: 10.1186/s12938-021-00860-0
5. Gorlick R, Janeway K, Lessnick S, Randall RL, Marina N. Children's Oncology Group's 2013 blueprint for research: bone tumors. Pediatr Blood Cancer. (2013) 60:1009–15. doi: 10.1002/pbc.24429
6. Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. New Engl J Med. (2021) 385:2066–76. doi: 10.1056/NEJMra2103423
7. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
8. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
9. Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol. (2015) 22:4363–70. doi: 10.1245/s10434-015-4518-z
10. Lin ZX, Ruan DY, Li Y, Wu DH, Ma XK, Chen J, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. (2015) 21:10898–906. doi: 10.3748/wjg.v21.i38.10898
11. Feng Z, Wen H, Ju X, Bi R, Chen X, Yang W, et al. The preoperative prognostic nutritional index is a predictive and prognostic factor of high-grade serous ovarian cancer. BMC Cancer. (2018) 18:883. doi: 10.1186/s12885-018-4732-8
12. Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. (2018) 23:634–40. doi: 10.1007/s10147-018-1250-2
13. He K, Si L, Pan X, Sun L, Wang Y, Lu J, et al. Preoperative systemic immune-inflammation index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol. (2022) 12:829689. doi: 10.3389/fonc.2022.829689
14. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
15. Yu X, Jiang W, Dong X, Yan B, Xu S, Lin Z, et al. Nomograms integrating the collagen signature and systemic immune-inflammation index for predicting prognosis in rectal cancer patients. BJS Open. (2024) 8(2):zrae014. doi: 10.1093/bjsopen/zrae014
16. Wei B, Zhang Y, Shi K, Jin X, Qian K, Zhang P, et al. Predictive value of systemic immune-inflammation index in the high-grade subtypes components of small-sized lung adenocarcinoma. J Cardiothorac Surg. (2024) 19:39. doi: 10.1186/s13019-024-02528-x
17. Pang J, Ding N, Yin N, Xiao Z. Systemic immune-inflammation index as a prognostic marker in HER2-positive breast cancer patients undergoing trastuzumab therapy. Sci Rep. (2024) 14:6578. doi: 10.1038/s41598-024-57343-0
18. Monteiro FSM, Fiala O, Massari F, Myint ZW, Kopecky J, Kucharz J, et al. Systemic immune-inflammation index in patients treated with first-line immune combinations for metastatic renal cell carcinoma: insights from the ARON-1 study. Clin genitourinary Cancer. (2024) 22:305–14.e3. doi: 10.1016/j.clgc.2023.11.013
19. Chen G, Liu L, Tan C, Tan Q, Chen Y, An X, et al. Prognostic significance of systemic immune-inflammation index in patients with nonfunction pancreatic neuroendocrine tumor undergoing surgical resection. Cancer Med. (2024) 13:e7114. doi: 10.1002/cam4.7114
20. Huang X, Hu H, Zhang W, Shao Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. (2019) 234:18408–14. doi: 10.1002/jcp.28476
21. Yang S, Wu C, Wang L, Shan D, Chen B. Pretreatment inflammatory indexes as prognostic predictors for survival in osteosarcoma patients. Int J Clin Exp Pathol. (2020) 13:515–24.
22. Ma C, Yu R, Li J, Guo J, Xu J, Wang X, et al. Preoperative prognostic nutritional index and systemic immune-inflammation index predict survival outcomes in osteosarcoma: A comparison between young and elderly patients. J Surg Oncol. (2022) 125:754–65. doi: 10.1002/jso.26757
23. Yang Y, Zhou C, Ma X. Use of nomogram on nutritional assessment indicators to predict clinical outcomes in patients undergoing surgical resection for high-grade osteosarcoma. Nutr Cancer. (2022) 74:3564–73. doi: 10.1080/01635581.2022.2081342
24. He H, Liu Y, Zhang R, Li J, Peng G. Value of controlling nutritional status score and related indicators in predicting the prognosis of osteosarcoma patients. Electron J Metab Nutr Cancer. (2023) 10:813–9. doi: 10.16689/j.cnki.cn11-9349/r.2023.06.018
25. Wu Y, Chen X, Shi T. Relationship between systemic immune inflammation index and prognosis of osteosarcoma patients and construction of prediction model. West China Med J. (2023) 38:1468–73. doi: 10.7507/1002-0179.202308074
26. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
27. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
28. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
29. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. (2011) 20:576–90. doi: 10.1016/j.ccr.2011.09.009
30. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. (2011) 11:123–34. doi: 10.1038/nrc3004
31. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. (2013) 13:159–75. doi: 10.1038/nri3399
32. Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. (2016) 273:312–28. doi: 10.1111/imr.12444
33. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. (2016) 4:83–91. doi: 10.1158/2326-6066.CIR-15-0313
34. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. (2004) 21:137–48. doi: 10.1016/j.immuni.2004.07.017
35. Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. (2015) 16:807–20. doi: 10.1080/15384047.2015.1040960
36. Yang X, Wu C. Systemic immune inflammation index and gastric cancer prognosis: A systematic review and meta−analysis. Exp Ther Med. (2024) 27:122. doi: 10.3892/etm.2024.12410
37. Zhang J, Dai S. Prognostic and clinicopathological role of pretreatment systemic immune-inflammation index in patients with oral squamous cell carcinoma: a meta-analysis. Front Oncol. (2023) 13:1303132. doi: 10.3389/fonc.2023.1303132
38. Zhang B, Xu T. Prognostic significance of pretreatment systemic immune-inflammation index in patients with prostate cancer: a meta-analysis. World J Surg Oncol. (2023) 21:2. doi: 10.1186/s12957-022-02878-7
39. Zeng Z, Xu S, Wang D, Qin G. Prognostic significance of systemic immune-inflammation index in patients with nasopharyngeal carcinoma: a meta-analysis. Syst Rev. (2022) 11:247. doi: 10.1186/s13643-022-02123-y
40. Liu XC, Jiang YP, Sun XG, Zhao JJ, Zhang LY, Jing X. Prognostic significance of the systemic immune-inflammation index in patients with cholangiocarcinoma: A meta-analysis. Front Oncol. (2022) 12:938549. doi: 10.3389/fonc.2022.938549
41. Yang Y, Li Y, Niu X, Liu W. Imaging evaluation of blood supply changes after chemotherapy of osteosarcoma and its correlation with tumor necrosis rate. J Bone Oncol. (2023) 41:100492. doi: 10.1016/j.jbo.2023.100492
42. Fidan AK, Uçmak G, Demirel BB, Efetürk H, Öztürk İ, Demirtaş Şenlik S, et al. The relation between staging fluorine-18 fluorodeoxyglucose positron emission tomog- raphy/computed tomography metabolic parameters and tumor necrosis rate in pediatric osteosarcoma patients. Turk J Med Sci. (2021) 51:1115–22. doi: 10.3906/sag-2004-358
Keywords: systemic immune-inflammation index, meta-analysis, osteosarcoma, evidence-based medicine, prognosis
Citation: Wang X, Wu Z, Zhang Z and Jiang Z (2024) Prognostic and clinicopathological value of systemic immune-inflammation index in patients with osteosarcoma: a meta-analysis. Front. Immunol. 15:1416068. doi: 10.3389/fimmu.2024.1416068
Received: 11 April 2024; Accepted: 29 July 2024;
Published: 15 August 2024.
Edited by:
Takaji Matsutani, Maruho, JapanReviewed by:
Abbas Karimi, Tabriz University of Medical Sciences, IranZhenzhen Han, Anhui Medical University, China
Copyright © 2024 Wang, Wu, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziwei Jiang, amlhbmc3NTMxMjAyNEAxNjMuY29t
 Xiaoyan Wang1
Xiaoyan Wang1 Ziwei Jiang
Ziwei Jiang