
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 12 July 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1414136
This article is part of the Research Topic Overcoming Side Effects in Patients Undergoing Immunotherapies and Cell Therapies: A Deeper Evaluation of Advanced Therapeutic Medicinal Products View all articles
Introduction: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare yet life-threatening adverse events associated with immune checkpoint inhibitors (ICIs). This systematic review synthesizes the current literature to elucidate the clinical characteristics and outcomes of patients with ICI-related SJS/TEN.
Methods: We conducted a thorough search across databases including Embase, Web of Science, Cochrane, MEDLINE, Scopus, and PubMed. Selection criteria focused on reports of SJS/TEN among cancer patients treated with ICIs, analyzing clinical manifestations, therapeutic interventions, and outcomes.
Results: Our analysis included 47 articles involving 50 patients with ICI-related SJS/TEN. The cohort had a mean age of 63 years, with a slight male predominance (54%). Most patients had melanoma or non-small cell lung cancer. SJS/TEN typically occurred early, with a median onset of 23 days post-ICI initiation. Treatment primarily involved systemic corticosteroids and intravenous immunoglobulins. The overall mortality rate was 20%, higher for TEN at 32%, with infections and tumor progression as leading causes. Median time from onset to death was 28 days. Survivors experienced a median re-epithelization time of 30 days, positively correlated with the extent of epidermal detachment (rs = 0.639, p = 0.009). Deceased patients exhibited a significantly higher proportion of TEN (90% vs. 48%, p = 0.029) and a larger epidermal detachment area (90% vs. 30% of the body surface area [BSA], p = 0.005) compared to survivors. The combination therapy group showed a higher proportion of TEN compared to corticosteroid monotherapy or non-corticosteroid therapy groups (72% vs. 29% and 50%, p = 0.01), with no significant differences in mortality or re-epithelization time. Dual ICI therapy resulted in a higher TEN rate than single therapy (100% vs. 50%, p = 0.028). Among single ICI therapies, the sintilimab-treated group trended towards a higher TEN rate (75% vs. 40-50%, p = 0.417), a larger detachment area (90% vs. 30-48% of BSA, p = 0.172), and a longer re-epithelization time (44 vs. 14-28 days, p = 0.036) compared to other ICI groups, while mortality rates remained similar.
Conclusion: ICI-related SJS/TEN substantially impacts patient outcomes. Prospective clinical trials are critically needed to further clarify the pathogenesis and optimize therapeutic regimens.
Immune checkpoint inhibitors (ICIs) have markedly improved outcomes in advanced malignancies, particularly melanoma and non-small cell lung cancer, representing a paradigm shift in their management (1, 2). Nonetheless, the broad activation of the immune system by ICIs has precipitated a spectrum of immune-related adverse events (irAEs), with cutaneous irAEs occurring in 30-50% of treated individuals (3, 4). While most cutaneous irAEs are manageable, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) present as rare yet potentially fatal reactions, characterized by sudden widespread erythemas and skin peeling, often with accompanying mucositis and fever (5, 6). This disease continuum is classified into three types based on the extent of epidermal detachment: SJS affects less than 10% of the body surface area (BSA); TEN involves more than 30% of the BSA; and SJS/TEN overlap syndrome is defined by 10-30% of BSA involvement (5).
SJS/TEN has been reported with treatments including anti-programmed-death-1 (PD-1), anti-programmed cell death-ligand 1 (PD-L1), anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), and dual PD-1/CTLA-4 blockade (7, 8). ICI-related SJS/TEN can be fatal, primarily due to systemic infection, substantial hemorrhage, and organ failure (7). The National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) recommend permanently discontinuing ICIs, using corticosteroids and intravenous immunoglobulins (IVIG), alongside comprehensive supportive care for managing this condition (9, 10). However, these guidelines are founded more upon expert consensus than on evidence-based medicine. A meticulous and comprehensive review delineating the clinical characteristics and treatment outcomes for this patient population is lacking.
This systematic review aims to synthesize and critically evaluate the current literature on ICI-related SJS/TEN, shedding light on the clinical features and prognoses. Our goal is to provide evidence foundation that will inform future research, enhance clinical practices, and contribute to the development of refined management strategies.
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11) and meta-analyses of observational studies in epidemiology (MOOSE) guidelines (12).
An exhaustive search was performed by two independent reviewers (JZ and CPW) on March 14, 2024, utilizing the following databases: Embase, Web of Science, Cochrane, MEDLINE, Scopus, and PubMed. Detailed search terms and strategies are provided as Supplementary Table 1. Additionally, we conducted reference list searches of included studies and relevant review articles to retrieve all eligible studies.
Two reviewers (JZ and CPW) independently scrutinized the results, applying rigorous screening to titles, keywords, and abstracts before assessing full texts for relevance and inclusion criteria. Publications in languages other than English and unpublished conference abstracts were excluded. Articles were also omitted for reporting inaccurate outcomes, missing crucial data, or generalized cohort observations without individual patient details.
Patients were included if they developed SJS/TEN post-ICI therapy, adhering to the classification criteria established by Bastuji-Garin et al. (5), which include: widespread blistering on erythematous skin, mucosal involvement, and histopathological evidence of extensive keratinocyte death or epidermal necrosis with minimal inflammatory infiltration. A possible, probable, or definite association between ICI and SJS/TEN was assessed using the algorithm of drug causality for epidermal necrolysis (ALDEN) scoring system (13).
Any discrepancies arising during the selection process were resolved through consensus, or if necessary, by consulting a third reviewer (CXH).
Two authors (JZ and CPW) independently reviewed the full texts of articles considered potentially eligible, extracted the data onto an electronic form, and a third author (CXH) verified the information. The data included: (1) baseline characteristics such as age, gender, tumor types, ICI regimens, concurrent treatments, and accompanying irAEs; (2) clinical presentations, including the time to SJS/TEN onset, epidermal detachment area, fever or mucositis, histopathologic findings, and severity-of-illness score for toxic epidermal necrolysis (SCORTEN) (14); (3) management and outcomes of SJS/TEN, detailing the dosage and duration of corticosteroids and second-line treatments, acute mortality rate, time to death or re-epithelization, and objective tumor response to ICIs. Efforts to address missing data involved outreach to original authors and application of strategies by the Cochrane Handbook for Systematic Reviews of Interventions (15). Any disagreement was resolved by discussion with a fourth author (JL).
Two authors (JZ and CPW) evaluated the risk of bias in each study. The Methodological Quality and Synthesis of Case Series and Case Reports framework (16) was specifically employed for case series to evaluate bias in selection, ascertainment, causality, and reporting domains. Assessment was based on five binary criteria questions: (1) Did the patients in the study represent the full range of cases seen at the medical center? (2) Was the exposure adequately identified? (3) Was the outcome accurately determined? (4) Were other potential diagnoses ruled out? (5) Was all important information cited in the report? Each question was scored, with 5 indicating high quality, 4 moderate quality, and a score less than 3 indicative of low quality. Any disagreement was resolved by discussion with a third author (CXH).
Data were aggregated and presented using descriptive statistics. For normally distributed continuous variables, the mean ± standard deviation is presented. Non-normally distributed continuous variables are summarized using the median (interquartile range). Categorical variables are expressed as counts (percentages). Spearman’s rank correlation coefficient was used to assess the relationship between time to re-epithelization and other variables. For comparisons between two groups, the Student’s t-test was used for normally distributed continuous variables, while the Mann-Whitney U test was applied for non-normally distributed continuous variables. Fisher’s exact test was employed for categorical variables in two-group comparisons. For comparisons among three or more groups, one-way ANOVA was used for normally distributed continuous variables, the Kruskal-Wallis test for non-normally distributed continuous variables, and the Fisher-Freeman-Halton exact test for categorical variables. When overall differences were significant, post-hoc pairwise comparisons were conducted using Bonferroni correction to adjust for multiple comparisons. All statistical analyses were performed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA). A p-value < 0.05 was considered statistically significant for all tests, except where adjusted for multiple comparisons as noted. All statistical tests were two-sided. Specific statistical methods for each analysis are indicated in the respective tables and figures.
Our comprehensive search identified 2,205 articles, augmented by 30 additional records from reference lists. We excluded 543 duplicates, 1,500 records based on title and abstract screenings, and 28 that were not available in English. From the 164 articles assessed in full text, 117 were excluded for reasons including the presence of 46 conference abstracts, 11 articles with inadequate outcome data, 40 lacking individual patient data, 10 involving culprit drugs other than ICIs, and 10 describing ICI-related severe cutaneous adverse reactions other than SJS/TEN. Ultimately, 47 articles (8, 17–62) encompassing data from 50 patients met our inclusion criteria. The details of the selection procedure are presented in Figure 1.
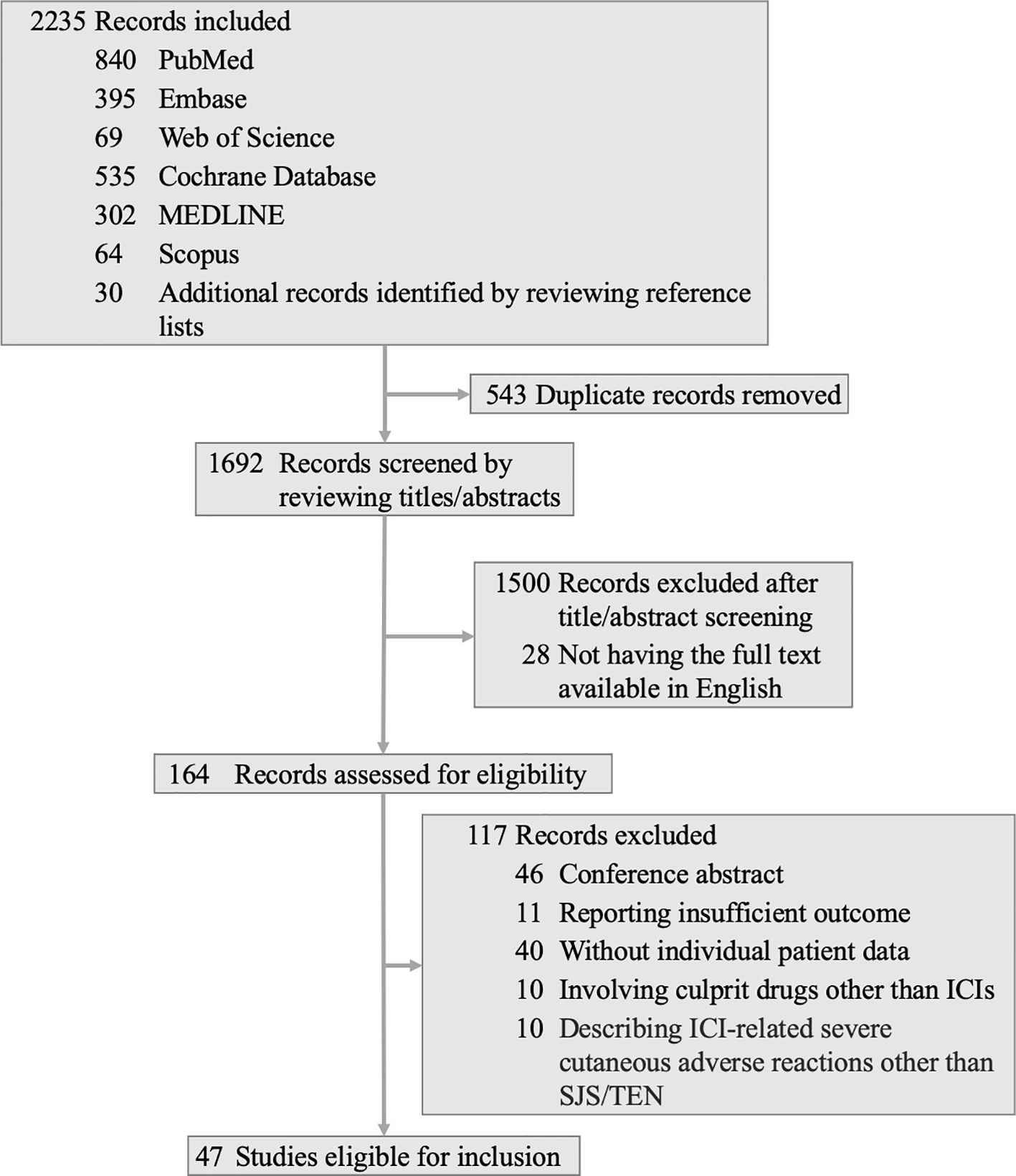
Figure 1 Flow diagram for search strategy and screening procedure. ICI, immune checkpoint inhibitor; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
We compiled comprehensive data on study and patient characteristics in Table 1 and Supplementary Table 2. The cohort included 18 patients (36%) with SJS, 28 (56%) with TEN, and 4 (8%) with SJS/TEN overlap syndrome. The mean age was 63 ± 14 years, with a slight male predominance (27 [54%]). The most common underlying tumors were non-small cell lung cancer (17 [34%]), melanoma (8 [16%]), and gastrointestinal cancer (5 [10%]). Most patients had stage IV disease (37 [74%]). PD-L1 expression was reported in 8 cases, with a median value of 30% (1%-80%).
ICI regimens were primarily anti-PD-1 (41 [82%]), followed by combination therapies of anti-PD-1 and anti-CTLA-4 (6 [12%]), anti-PD-L1 (2 [4%]), and anti-CTLA-4 (1 [2%]). The most frequently administered ICIs were pembrolizumab (20 [40%]), nivolumab (12 [24%]), and sintilimab (8 [16%]).
Concurrent medications within 4 weeks before SJS/TEN onset were reported in 22 cases (44%), including chemotherapy, radiotherapy, antibiotics, and targeted agents. Concomitant irAEs were noted in 8 patients (16%), presenting as hepatitis, myocarditis and/or myositis, and gastroenteritis.
The clinical characteristics of patients with ICI-related SJS/TEN are provided in Table 2 and Supplementary Table 3. SJS/TEN typically occurred after a median of 2 cycles (1-3) of ICI therapy, corresponding to a median latency period of 23 days (14-61) from ICI initiation to symptom onset. Preceding SJS/TEN, 6 patients (12%) developed grade 1-2 maculopapular rashes or lichenoid dermatitis, which partially or completely resolved with topical or systemic corticosteroids. Despite this resolution, the patients developed SJS/TEN upon continued ICI therapy.
The median epidermal detachment area peaked at 30% of BSA (15%-80%). Fever accompanied 17 cases (34%), with mucosal involvement observed in 42 cases (84%); oral mucosa was implicated in 39 cases (78%), ocular in 25 cases (50%), and genital in 15 cases (30%). Histopathological confirmation of SJS/TEN was reported in 40 cases (80%). SCORTEN were available for 23 cases (46%), with a median score of 3 (3-4), correlating with a predicted mortality rate of 32% (32%-62%).
Table 3 and Supplementary Table 4 provide a comprehensive overview of management and outcomes for patients with ICI-related SJS/TEN. Systemic corticosteroids were the mainstay of treatment, administered in 46 cases (92%) at a median prednisone equivalent dose of 1.9 (1.0-2.5) mg/kg/day for about 30 (25-53) days. IVIG was administered in 25 cases (50%), with a median cumulative dose of 2.0 (2.0-2.9) g/kg. Cyclosporine was used in 9 cases (18%), with an average dose of 3.9 ± 1.1 mg/kg/day and an average duration of 16 ± 6 days. Tumor necrosis factor-α (TNF-α) inhibitors were used in 6 cases (12%), including infliximab, adalimumab, and etanercept. Other treatments included methylprednisolone 0.5-1 g pulse therapy, plasma exchange, mycophenolate mofetil, and granulocyte colony-stimulating factor.
Of the 50 patients reviewed, 40 (80%) recovered, while 10 (20%) succumbed to SJS/TEN, with the mortality rate significantly higher in TEN than SJS or overlap syndrome (32% vs. 5%, p = 0.029). The median time from SJS/TEN onset to death was 28 (11-38) days. Infections and tumor progression were the most common causes of death.
The median duration from SJS/TEN onset to re-epithelization for survivors was 30 (24-34) days. A positive correlation was found between the re-epithelization time and the epidermal detachment area (rs = 0.639, p = 0.009), as depicted in Figure 2. Notably, no significant correlations were found between re-epithelization time and patient demographics, SCORTEN, or treatment modalities. Among the survivors of SJS/TEN, subsequent deaths within a mean duration of 95 days were attributed primarily to tumor progression and infections.
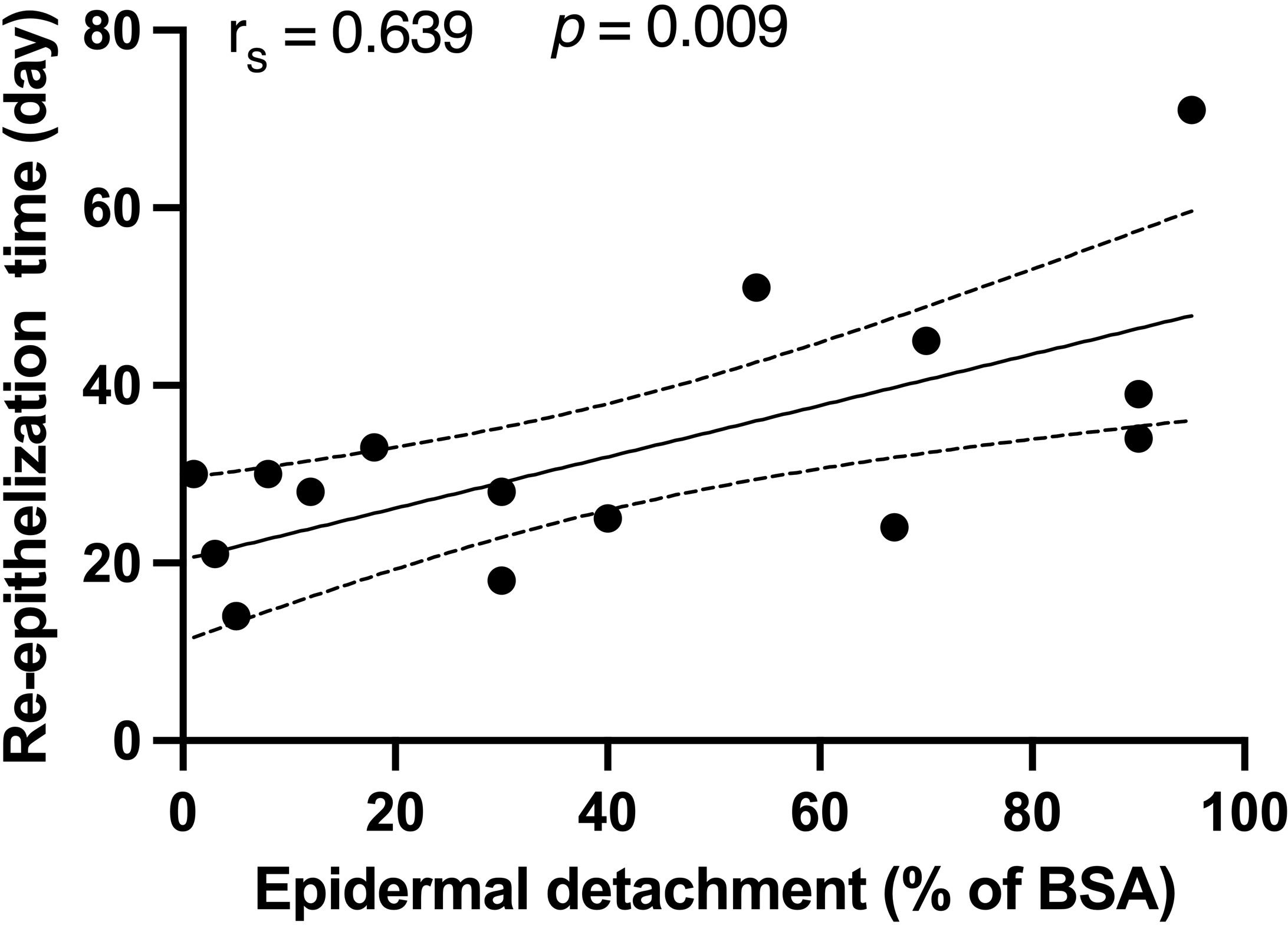
Figure 2 Correlations between the re-epithelization time and epidermal detachment area in recovered patients. Spearman’s rank correlation coefficient was used to assess the relationship between re-epithelization time and epidermal detachment area. BSA, body surface area.
Objective tumor response to ICIs was assessed in 27 patients, with only 4 (8%) showing a partial or complete response, while the rest experienced disease progression (15 [30%]) or stability (8 [16%]).
Deceased patients exhibited a significant higher proportion of TEN (90% vs. 48%, p = 0.029) and a larger area of epidermal detachment (90% vs. 30% of BSA, p = 0.005) compared to survivors. Other clinical characteristics and treatment regimens did not differ significantly between the two groups. For further details, please refer to Table 4.
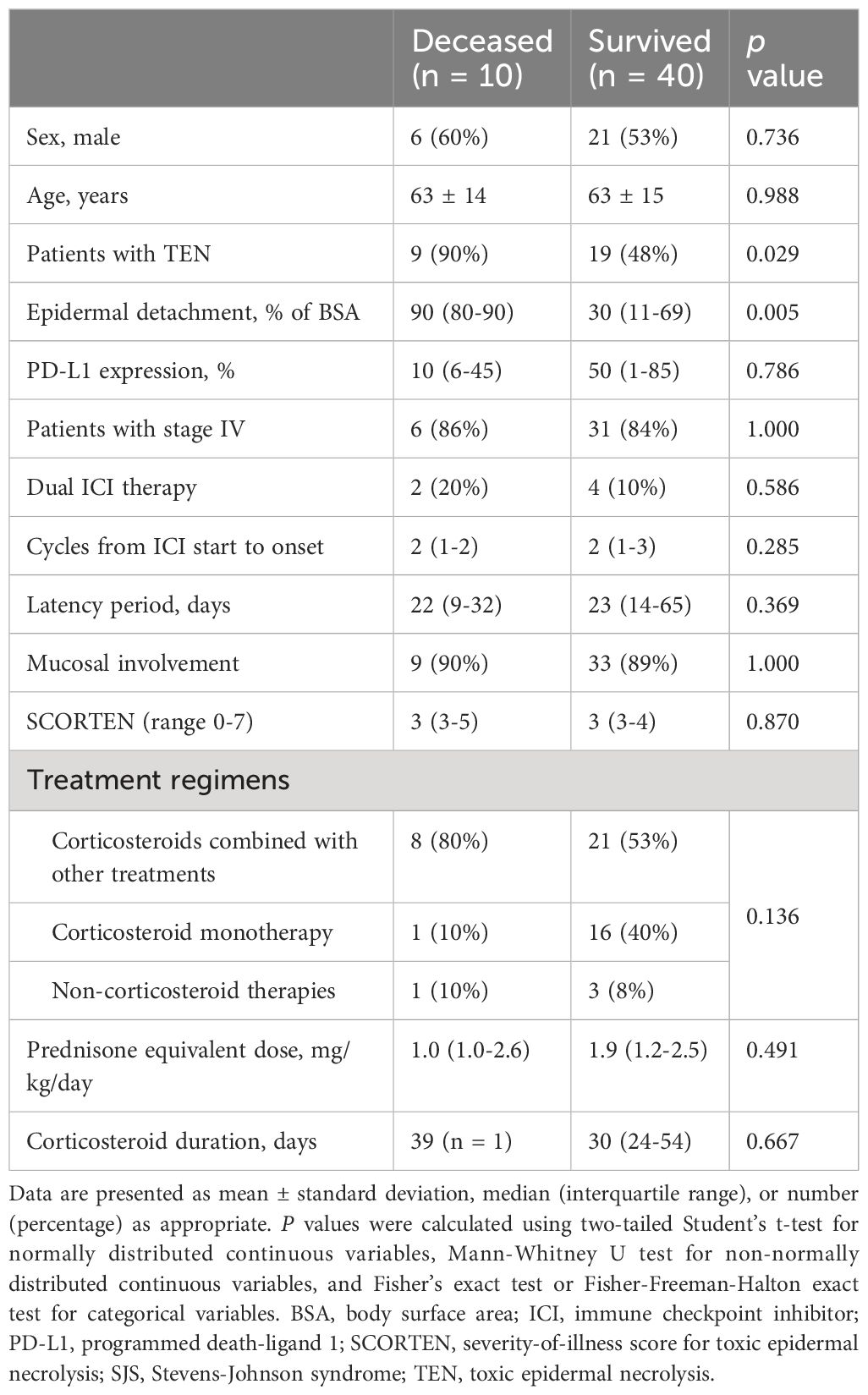
Table 4 Comparison of clinical characteristics and treatment regimens between deceased and survived patients with ICI-related SJS/TEN.
We divided the treatment regimens into three groups: corticosteroids combined with other therapies (including IVIG, cyclosporine, TNF-α inhibitors, etc.), corticosteroid monotherapy, and non-corticosteroid therapies. A significant difference was observed in the proportion of TEN patients among the three groups (p = 0.010). Notably, the combination therapy group had a significantly higher proportion of TEN patients compared to the corticosteroid monotherapy group (72% vs. 29%, p = 0.005). The combination therapy group also showed a larger epidermal detachment area (69%) compared to corticosteroid monotherapy (20%) and non-corticosteroid therapy (30%) groups, although this difference was not statistically significant. The mortality rates were 28%, 6%, and 25% in the combination therapy, corticosteroid monotherapy, and non-corticosteroid therapy groups, respectively (p = 0.136). The median re-epithelization time was 30, 30, and 25 days in the three groups, respectively (p = 0.537). See Table 5 for details.
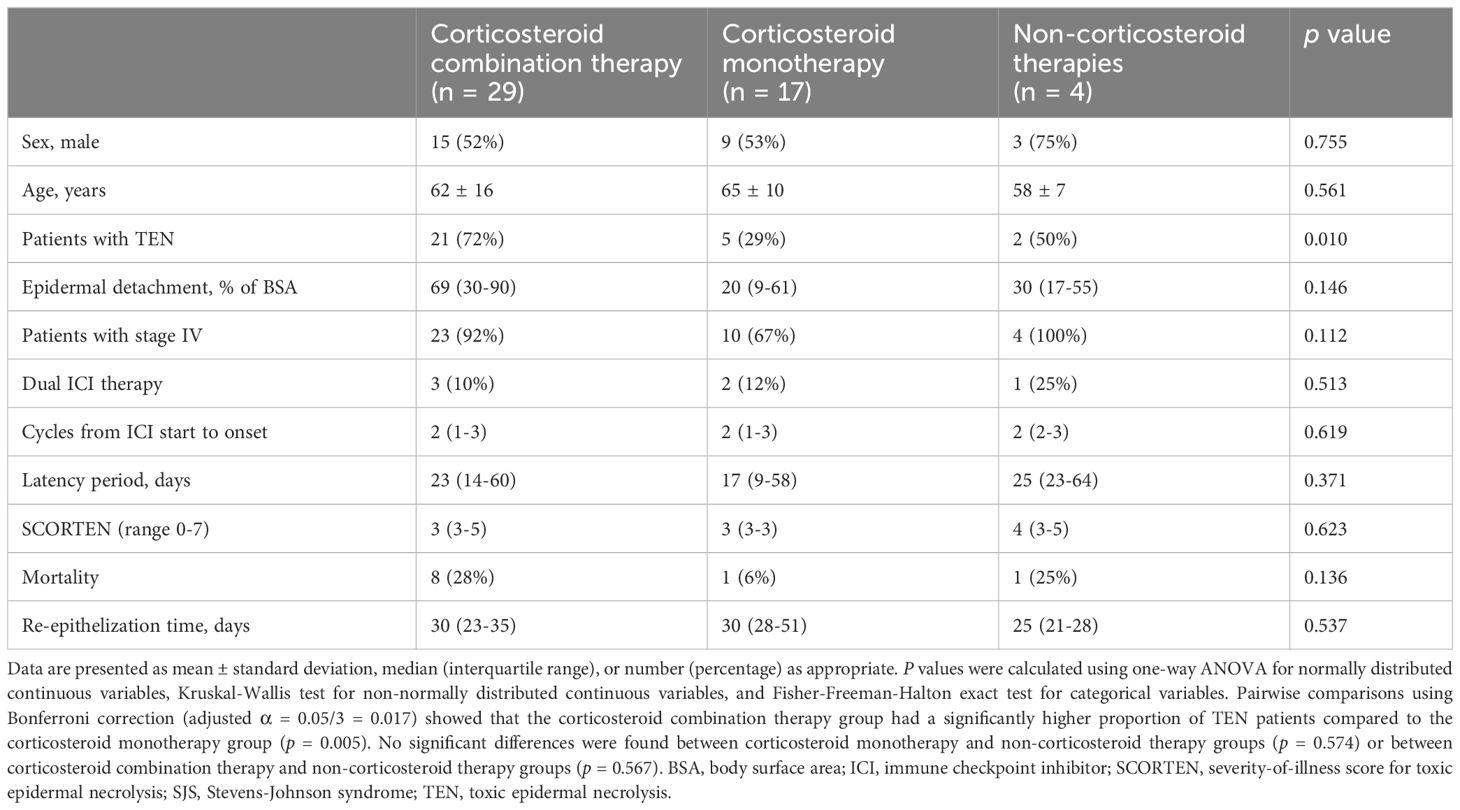
Table 5 Comparison of clinical characteristics and outcomes among patients with ICI-related SJS/TEN receiving different therapeutic regimens.
All patients receiving dual ICI therapy developed TEN, a significantly higher incidence (100%) compared to those receiving single ICI therapy (50%, p = 0.028). The dual ICI group also exhibited a more extensive epidermal detachment area (74% vs. 30% of BSA, p = 0.105) and a higher mortality rate (33% vs. 18%, p = 0.586) compared to single ICI group, though these differences were not statistically significant. Re-epithelization time was similar between groups (34 vs. 30 days, p = 0.456). Details are presented in Table 6.

Table 6 Comparison of clinical characteristics and outcomes between patients with ICI-related SJS/TEN receiving dual and single ICI therapy.
Among patients receiving single ICI therapies, those treated with sintilimab had a higher proportion of TEN (75% vs. 40-50%) and a larger epidermal detachment area (90% vs. 30-48% of BSA) compared to those treated with other agents, although these differences were not statistically significant. Mortality rates were comparable across all groups. Notably, re-epithelization time was significantly longer in sintilimab group (44 vs. 14-28 days in other groups, p = 0.036). However, post-hoc pairwise comparisons revealed no significant differences between any two groups. This discrepancy may be attributed to the limited sample size, resulting in reduced statistical power and potential instability of the results. Details are presented in Table 7.
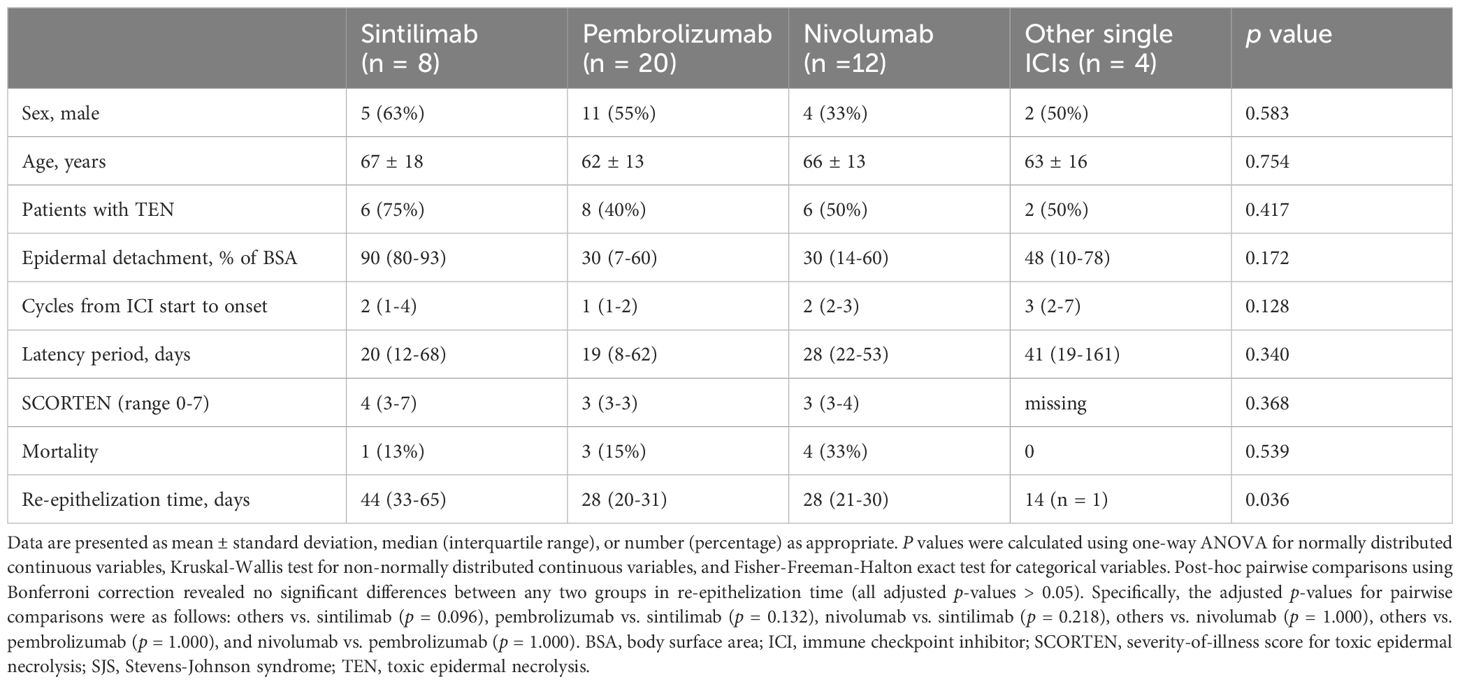
Table 7 Comparison of clinical characteristics and outcomes among patients with ICI-related SJS/TEN receiving different single ICI therapies.
Stage IV patients tended to have a higher proportion of TEN (60% vs. 29%), a larger epidermal detachment area (35% vs. 21% of BSA), and a shorter latency period (23 vs. 60 days) compared to patients with stage II or III, though these differences were not statistically significant. Both groups showed similar mortality rates (16% vs. 14%) and re-epithelization time (30 vs. 28 days). Details are presented in Table 8.
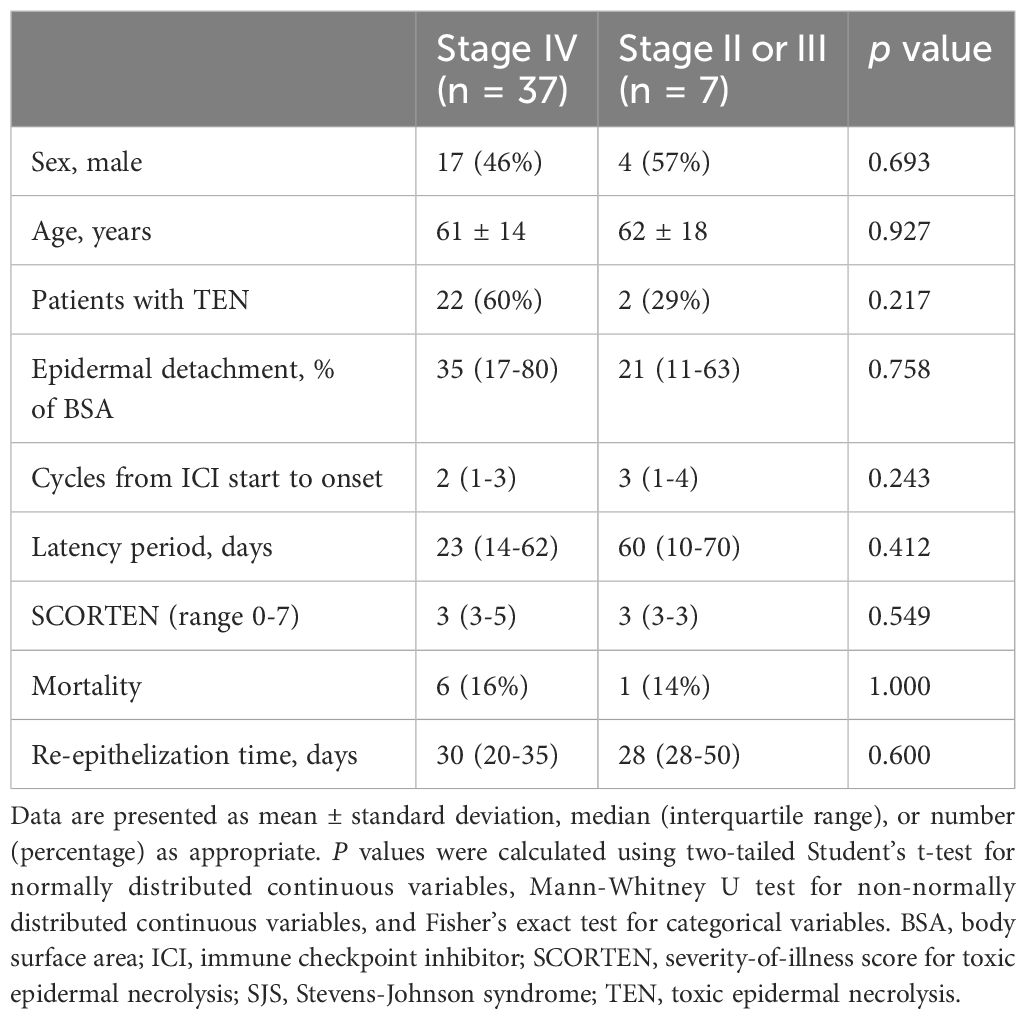
Table 8 Comparison of clinical characteristics and outcomes between stage IV and stage II/III patients with ICI-related SJS/TEN.
Our systematic review provides a comprehensive examination of the clinical profiles, treatment strategies, and outcomes for patients with ICI-related SJS/TEN. This cohort, characterized by a mean age of 63 years and a slight male predominance (54%), primarily comprised patients with melanoma and non-small cell lung cancer. SJS/TEN typically manifested early in the treatment course, with a median onset of 23 days post-ICI initiation, usually after 1-2 cycles. The mainstay of treatment involved systemic corticosteroids and IVIG. Despite these interventions, the overall mortality rate was 20%, with TEN cases showing a notably higher rate of 32%. Infections and tumor progression emerged as the primary causes of death, with a median time from SJS/TEN onset to death of 28 days. Survivors demonstrated a median re-epithelization time of 30 days, positively correlated with the extent of epidermal detachment. Notably, deceased patients exhibited a significantly higher proportion of TEN and a larger epidermal detachment area compared to survivors. There were no significant differences in mortality or re-epithelization time among different treatment groups. Dual ICI therapy resulted in a higher TEN rate compared to single ICI therapy. Among single ICI therapies, patients treated with sintilimab showed trends towards a higher TEN rate, larger detachment area, and longer re-epithelization time compared to other groups. However, mortality rates were comparable across different ICI regimens.
The clinical manifestations of ICI-related SJS/TEN in our study largely align with those triggered by traditional drugs, showing no significant gender difference in incidence, a relatively short latency period, and frequent occurrences of fever and mucositis (63, 64). The median latency period for ICI-related cases in our study was 23 days, falling within the classic 4-28 day range for SJS/TEN (63, 64). While mucosal involvement patterns in ICI-related SJS/TEN were similar to classic cases, the incidence was lower: oral mucosa was implicated in 78% of cases (vs. 90% in classic SJS/TEN), ocular in 50% (vs. 84%), and genital in 30% (vs. 60-70%) (65–68). This lower incidence might be due to underreporting or could indicate a distinctive feature of ICI-related cases. Notably, some patients with extensive epidermal detachment did not exhibit mucosal involvement, suggesting unique characteristics of ICI-related SJS/TEN (22, 36). A subset of patients initially developed mild maculopapular rashes or lichenoid dermatitis early in their ICI treatment. Despite initial improvement with corticosteroids, these conditions progressed to SJS/TEN with continued therapy (25, 47). This progression, uncommon in traditional drug-induced cases, underscores the need for heightened vigilance in monitoring patients exhibiting such symptoms after ICI therapy.
Our study identified that about half of the patients with ICI-related SJS/TEN had received other medications within the month before the onset, including antibiotics and targeted drugs. These agents, such as allopurinol (69), sulfamethoxazole/trimethoprim (70), lenvatinib (24), and osimertinib (71), are well known to precipitate SJS/TEN. When SJS/TEN develops alongside ICI therapy and these medications, pinpointing the primary culprit drug becomes a substantial challenge. Sole reliance on clinical expertise or the ALDEN score might not yield a definitive determination of drug causality, which is pivotal in deciding the continuity of ICI treatment. Diagnostic approaches to ascertain the culprit drug in SJS/TEN encompass both in vivo and in vitro tests. In vivo assessments, like delayed intradermal tests or drug provocation tests, are generally contraindicated due to the life-threatening nature of SJS/TEN (72–74). In vitro assays such as lymphocyte transformation tests and enzyme-linked immunospot assays have exhibited high specificity and modest sensitivity in attributing causality to traditional drugs in the context of severe cutaneous adverse reactions (75–77). Nevertheless, their application for establishing a link between ICIs and SJS/TEN has not been documented. Additionally, the association between specific human leukocyte antigen (HLA) alleles and conventional drug-induced SJS/TEN is well-established, with strong correlations identified—such as HLA-B*58:01 with allopurinol-induced cases (78) and HLA-B*15:02 with carbamazepine-induced cases (79, 80). However, the potential link of certain HLA alleles with ICI-related SJS/TEN is yet to be elucidated.
In the diagnosis of ICI-related SJS/TEN, it is imperative to rule out other blistering dermatoses, including bullous lichenoid dermatitis, bullous radiation recall dermatitis, bullous pemphigoid, and paraneoplastic pemphigus. Each condition presents with distinct clinical manifestations, pathological findings, and carries a different prognosis, as outlined in Table 9 (81, 82). In cases presenting with atypical clinical features, an in-depth histopathological examination, supplemented by immunofluorescence studies and circulating autoantibody assays, is crucial to differentiate these conditions accurately. ICI-induced SJS/TEN requires permanent discontinuation of the culprit drug. In contrast, for other bullous dermatoses, the potential resumption of ICI therapy may be considered under careful evaluation (10).
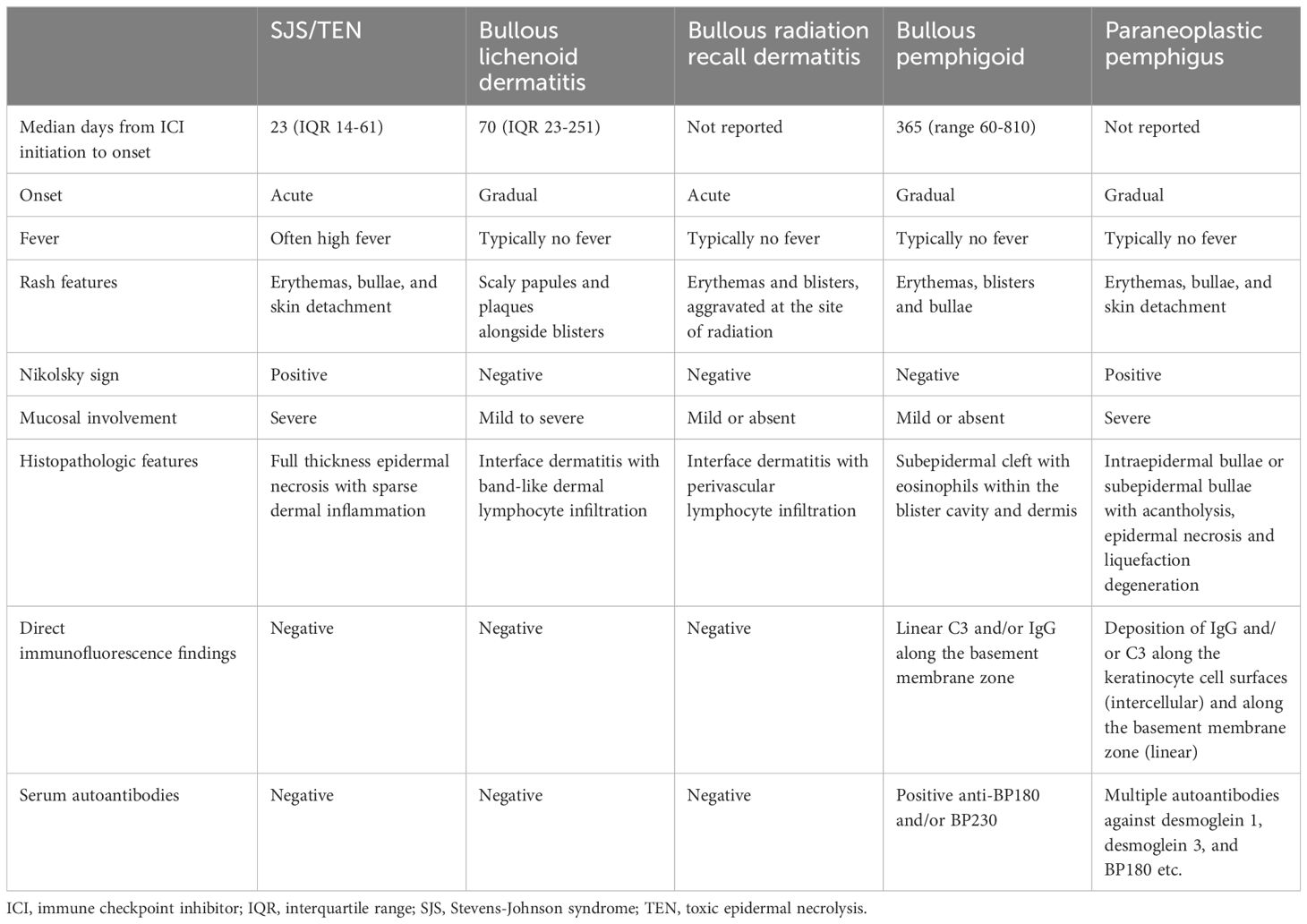
Table 9 Key points for the differential diagnosis of ICI-related SJS/TEN versus other blistering dermatoses.
While the role of corticosteroids in SJS/TEN treatment is debated, current guidelines still advocate for their use as the cornerstone of therapy in ICI-related cases, with methylprednisolone dosages suggested at 1-2 mg/kg/day (9, 10). For non-responders, adjunctive therapies such as IVIG, cyclosporine, or TNF-α inhibitors are considered (9, 10). Although the preference for second-line agents is not yet standardized, cyclosporine and TNF-α inhibitors are showing promising results (83–86). Considering the rapid progression of SJS/TEN—where peak skin detachment typically occurs within a median of 8 days—and the prognostic significance of the extent of skin loss, swift initiation of corticosteroids is crucial (87). This initial treatment may be effectively supplemented with TNF-α inhibitors or cyclosporine during the acute phase to halt the inflammatory cascade, limit epidermal necrosis, and expedite recovery. However, a prolonged course of corticosteroids may impede wound repair and elevate infection risks (88). It is therefore recommended to begin tapering corticosteroids as signs of recovery appear, such as the cessation of new erythema or blistering and the start of re-epithelization.
Our study found no significant differences in mortality rates or re-epithelization time among groups treated with combination corticosteroid therapy, corticosteroid monotherapy, or non-corticosteroid therapies. However, the combination therapy group showed a higher proportion of TEN cases and larger epidermal detachment area compared to the other groups. This observation likely reflects the tendency to employ more aggressive treatment strategies for patients with severe disease presentations. These findings underscore the urgent need for prospective, well-designed clinical trials to investigate the efficacy, safety, and optimal timing of these therapeutic approaches in managing ICI-related SJS/TEN.
The acute mortality rate for ICI-related SJS/TEN observed in our study was 20%, notably lower than both the SCORTEN-predicted mortality rate of 32% and reported mortality rates for cancer patients with SJS/TEN (89). This decrease in mortality may be attributed to enhanced recognition and prompt intervention by oncologists and dermatologists, coupled with advancements in supportive care (90). However, the mortality rate for TEN specifically persists at 32%, revealing resistance to current therapies among certain patients and highlighting the urgent need for more efficacious treatment approaches to enhance survival and prognosis. Our study found that the median time from onset to death for patients with ICI-related SJS/TEN was 28 days. Some surviving patients died from tumor progression or infection, on average, 95 days after onset. This timeline is consistent with that observed in classic SJS/TEN patients. In the RegiSCAR cohort study, Sekula et al. reported that most deaths in SJS/TEN occurred within 6 weeks, with mortality rates continuing to rise over the first year (91).
The predominant causes of death in our cohort were infections and tumor progression. Reported mortality predictors in SJS/TEN included factors such as advanced age, existing comorbidities, hematologic cancers, septicemia, pneumonia, and renal impairment (92). Sepsis has been pinpointed as the leading cause of death in these patients, with TEN, diabetes, and intensive care unit admission identified as key risk factors (93–95). Our study provides additional insights into prognostic factors specific to ICI-related cases. We found that deceased patients exhibited a significantly higher rate of TEN and larger epidermal detachment area compared to survivors. This finding suggests that the extent of epidermal detachment is a crucial determinant of prognosis in ICI-related cases, potentially due to the increased risk of infection and metabolic disturbances associated with extensive skin involvement. While SCORTEN remains the gold standard for prognostic evaluation in SJS/TEN (14), emerging research has identified potential biomarkers. Chemokine CCL27 and interleukin-15 are associated with disease severity and mortality (96, 97). Additionally, RIP3 expression may act as a diagnostic marker indicative of epidermal necrosis (98). These biomarkers, however, have not yet gained widespread clinical adoption and warrant further study.
Our data indicates that patients with ICI-related SJS/TEN typically experience re-epithelization within 30 days, a process closely linked to the degree of epidermal detachment. This duration is notably longer than the 12-17 days reported for classic SJS/TEN in the literature. For instance, Wang et al. reported re-epithelization times of 13.8 and 16.5 days for etanercept and corticosteroid groups, respectively, in a randomized controlled trial (83). Zhang et al., in a multicenter retrospective study, found times ranging from 12 to 13.5 days across different treatment groups (86). Krajewski et al.’s meta-regression analysis reported an average of 13 days (99). This discrepancy can be attributed to several factors. Firstly, our study defines re-epithelization time as the duration from SJS/TEN onset to complete skin healing, potentially encompassing a longer period compared to studies measuring from treatment initiation or using unclear definitions. Secondly, patients with ICI-related SJS/TEN are typically older, and often suffer from conditions such as anemia, hypoalbuminemia, or malnutrition, which may contribute to prolonged healing times.
Our study revealed that patients receiving dual ICI therapy experienced more severe SJS/TEN and showed a trend towards higher mortality rates compared to those on single ICI therapy. This finding aligns with broader observations in immunotherapy research, where multiple analyses have consistently shown that dual ICI therapy significantly increases the risk of grade ≥ 3 irAEs compared to single ICI therapy (100–102). Interestingly, our research also uncovered variations in adverse event profiles among different single ICI therapies. Notably, patients treated with sintilimab who developed SJS/TEN exhibited more severe disease manifestations and longer re-epithelization time compared to those on other ICIs, although mortality rates were not significantly elevated. While our findings focus on SJS/TEN severity, a phase II randomized controlled trial has reported a higher incidence of rash with sintilimab compared to pembrolizumab in advanced non-small cell lung cancer treatment (103). These findings suggest that even within the category of single ICI therapies, different agents may carry varying risks for severe cutaneous adverse reactions.
This systematic review has several limitations that merit attention. Firstly, the exclusion of non-English literature may have omitted relevant studies, potentially affecting the comprehensiveness of our analysis. Secondly, a substantial limitation is that almost all the included literature consists of case reports, which are subject to publication bias and may not represent the full spectrum of ICI-related SJS/TEN cases. Thirdly, critical data such as SCORTEN, detailed treatment regimens, and precise timing of death or recovery were inconsistently reported across case reports. These gaps in information could obscure a comprehensive understanding of the disease course and treatment efficacy. Lastly, the sample size, while considerable given the rarity of ICI-related SJS/TEN, remains relatively small, limiting the generalizability and statistical power of our findings. This constraint is particularly pronounced in our subgroup analyses, where some groups comprised only one or two cases, leading to potentially unstable statistical results and insufficient power to detect meaningful differences. Despite these limitations, our study provides valuable insights into the clinical characteristics, management, and outcomes of ICI-related SJS/TEN. However, the findings should be interpreted with caution, and future prospective studies with larger, more homogeneous cohorts are necessary to validate and expand upon these results.
Our systematic review illuminates the complex landscape of ICI-related SJS/TEN, highlighting its rarity yet serious nature within oncological settings. The findings underscore the importance of early recognition, accurate diagnosis, and multidisciplinary management to mitigate potentially fatal outcomes. Our analysis suggests potential differences in disease severity and outcomes based on treatment regimens and specific ICI agents, emphasizing the need for tailored monitoring approaches.
However, the study’s limitations, primarily the reliance on case reports and small sample sizes, necessitate cautious interpretation of these findings. These constraints underscore the critical need for prospective, well-designed clinical trials with larger cohorts and standardized reporting. Such studies are essential to validate our observations, elucidate underlying mechanisms, and enhance treatment protocols for ICI-related SJS/TEN.
In conclusion, while our study provides valuable insights into the current landscape of this severe adverse event, it also serves as a call to action for more robust research to advance our understanding and management of ICI-related SJS/TEN in cancer immunotherapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JZ: Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. CW: Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing. HZ: Writing – original draft, Writing – review & editing. CH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-252, 2022-PUMCH-C-054) and the National Key Clinical Specialty Project of China.
We acknowledge the assistance provided by AI-based language models during the manuscript preparation. This support involved ChatGPT 4, developed by OpenAI, and Claude, developed by Anthropic, which aided in providing translations, polishing text for clarity and coherence, and ensuring adherence to academic standards. These AI tools were used as assistive technologies to enhance the quality of our writing, while all scientific contents, analyses, and conclusions were solely determined by the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1414136/full#supplementary-material
ALDEN, algorithm of drug causality for epidermal necrolysis; BSA, body surface area; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; HLA, human leukocyte antigen; irAE, immune-related adverse event; ICI, immune checkpoint inhibitor; IVIG, intravenous immunoglobulins; PD-1, programmed-death-1; PD-L1, programmed cell death-ligand 1; SCORTEN, severity-of-illness score for toxic epidermal necrolysis; SJS, Stevens-Johnson syndrome; TNF-α, tumor necrosis factor-α; TEN, toxic epidermal necrolysis.
1. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. (2021) 398:1002–14. doi: 10.1016/S0140-6736(21)01206-X
2. Desai AP, Adashek JJ, Reuss JE, West HJ, Mansfield AS. Perioperative immune checkpoint inhibition in early-stage non-small cell lung cancer: A review. JAMA Oncol. (2023) 9:135–42. doi: 10.1001/jamaoncol.2022.5389
3. Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. (2020) 83:1255–68. doi: 10.1016/j.jaad.2020.03.132
4. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
5. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. (1993) 129:92–6. doi: 10.1001/archderm.1993.01680220104023
6. Alexandris D, Alevizopoulos N, Gakiopoulou H, Stavrinou N, Vourlakou C. Cutaneous Stevens Johnson – toxic epidermal necrolysis immunotherapy related toxicities in lung cancer patients. J Oncol Pharm Practice. (2022) 28:1276–82. doi: 10.1177/10781552221074623
7. Maloney NJ, Ravi V, Cheng K, Bach DQ, Worswick S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol. (2020) 59:e183–e8. doi: 10.1111/ijd.14811
8. Chen PY, Li ZY, Cai SQ. Case Report: Cadonilimab-related toxic epidermal necrolysis-like reactions successfully treated with supplemental Adalimumab. Front Immunol. (2023) 14:1188523. doi: 10.3389/fimmu.2023.1188523
9. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. (2019) 17:255–89. doi: 10.6004/jnccn.2019.0013
10. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–iv42. doi: 10.1093/annonc/mdx225
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Brooke BS, Schwartz TA, Pawlik TM. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. (2021) 156:787–8. doi: 10.1001/jamasurg.2021.0522
13. Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. (2010) 88:60–8. doi: 10.1038/clpt.2009.252
14. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. (2000) 115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x
15. Carpenter J, Rücker G, Schwarzer G. Assessing the sensitivity of meta-analysis to selection bias: a multiple imputation approach. Biometrics. (2011) 67:1066–72. doi: 10.1111/biom.2011.67.issue-3
16. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. (2018) 23:60–3. doi: 10.1136/bmjebm-2017-110853
17. Borg L, Buhagiar M, La Ferla E, Pisani D, Said J, Boffa MJ. Pembrolizumab-induced toxic epidermal necrolysis. Case Rep Oncol. (2022) 15:887–93. doi: 10.1159/000526931
18. Cai ZR, Lecours J, Adam JP, Marcil I, Blais N, Dallaire M, et al. Toxic epidermal necrolysis associated with pembrolizumab. J Oncol Pharm Practice. (2020) 26:1259–65. doi: 10.1177/1078155219890659
19. Chirasuthat P, Chayavichitsilp P. Atezolizumab-induced Stevens-Johnson syndrome in a patient with non-small cell lung carcinoma. Case Rep Dermatol. (2018) 10:198–202. doi: 10.1159/000492172
20. Chow KVC, O'Leary C, Paxton-Hall F, Lambie D, O'Byrne K. Pembrolizumab-induced toxic epidermal necrolysis: Case report. Oxford Med Case Rep. (2022) 2022:112–4. doi: 10.1093/omcr/omac025
21. Cui W, Cotter C, Sreter KB, Heelan K, Creamer D, Basu TN, et al. Case of fatal immune-related skin toxicity from sequential use of osimertinib after pembrolizumab: Lessons for drug sequencing in never-smoking non–Small-cell lung cancer. JCO Oncol Practice. (2020) 16:842–4. doi: 10.1200/OP.20.00489
22. Gallo Marin B, Oliva R, Kahn B, Borgovan T, Brooks BE, Massoud CM. Pembrolizumab-induced toxic epidermal necrolysis in a patient with metastatic esophageal adenocarcinoma. R I Med J 2013. (2022) 105:34–6.
23. Goldinger SM, Stieger P, Meier B, Micaletto S, Contassot E, French LE, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. (2016) 22:4023–9. doi: 10.1158/1078-0432.CCR-15-2872
24. Gong Y, Mao J, Liu M, Gao J. A case of toxic epidermal necrolysis associated with lenvatinib and sintilimab therapy for intrahepatic cholangiocarcinoma. J Int Med Res. (2023) 51:3000605231173556. doi: 10.1177/03000605231173556
25. Gopee NH, Gourley AM, Oliphant TJ, Hampton PJ. Toxic epidermal necrolysis occurring with immune checkpoint inhibitors. Dermatol Online J. (2020) 26:13030/qt8fc428f6. doi: 10.5070/D3268049884
26. Gracia-Cazaña T, Padgett E, Calderero V, Oncins R. Nivolumab-associated Stevens-Johnson syndrome in a patient with lung cancer. Dermatol Online J. (2021) 27:13030/qt2897t6dq. doi: 10.5070/D3273052777
27. Griffin LL, Cove-Smith L, Alachkar H, Radford JA, Brooke R, Linton KM. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep. (2018) 4:229–31. doi: 10.1016/j.jdcr.2017.09.028
28. Haratake N, Tagawa T, Hirai F, Toyokawa G, Miyazaki R, Maehara Y. Stevens-Johnson syndrome induced by pembrolizumab in a lung cancer patient. J Thorac Oncol. (2018) 13:1798–9. doi: 10.1016/j.jtho.2018.05.031
29. Hsu T-J, Liu K-L. Stevens-Johnson syndrome and toxic epidermal necrolysis related to immune checkpoint inhibitors: Two cases and literature review. DERMATOLOGICA SINICA. (2020) 38:236–9. doi: 10.4103/ds.ds_24_20
30. Huang KK, Han SS, He LY, Yang LL, Liang BY, Zhen QY, et al. Combination therapy (toripalimab and lenvatinib)-associated toxic epidermal necrolysis in a patient with metastatic liver cancer: A case report. World J Clin Cases. (2022) 10:3478–84. doi: 10.12998/wjcc.v10.i11.3478
31. Huang Y, Zhu L, Ma X, Hong Y, Su X, Lai W, et al. A case of sintilimab-induced SJS/TEN : Dermatologic adverse reactions associated with programmed cell death protein-1 inhibitors. DERMATOLOGIC Ther. (2022) 35:e15663. doi: 10.1111/dth.15663
32. Ito J, Fujimoto D, Nakamura A, Nagano T, Uehara K, Imai Y, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer. (2017) 109:58–61. doi: 10.1016/j.lungcan.2017.04.020
33. Kian W, Zemel M, Elobra F, Sharb AA, Levitas D, Assabag Y, et al. Intravenous immunoglobulin efficacy on pembrolizumab induced severe toxic epidermal necrolysis. Anti-Cancer Drugs. (2022) 33:E738–E40. doi: 10.1097/CAD.0000000000001162
34. Kim MC, Khan HN. Nivolumab-induced toxic epidermal necrolysis: rare but fatal complication of immune checkpoint inhibitor therapy. Cureus. (2021) 13:e15017. doi: 10.7759/cureus.15017
35. Konstantina T, Konstantinos R, Anastasios K, Anastasia M, Eleni L, Ioannis S, et al. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer. (2019) 135:29–32. doi: 10.1016/j.lungcan.2019.06.015
36. Koshizuka K, Sakurai D, Sunagane M, Mita Y, Hamasaki S, Suzuki T, et al. Toxic epidermal necrolysis associated with nivolumab treatment for head and neck cancer. Clin Case Rep. (2021) 9:848–52. doi: 10.1002/ccr3.3695
37. Kubicki SL WM, Patel AB. Toxic epidermal necrolysis during cotherapy with ipilimumab and nivolumab. J Immunother Precis Oncol. (2018) 1:78–81. doi: 10.4103/JIPO.JIPO_7_18
38. Kumar R, Bhandari S. Pembrolizumab induced toxic epidermal necrolysis. Curr Problems Cancer. (2020) 44:100478. doi: 10.1016/j.currproblcancer.2019.05.001
39. Li G, Gong S, Wang N, Yao X. Toxic epidermal necrolysis induced by sintilimab in a patient with advanced non-small cell lung cancer and comorbid pulmonary tuberculosis: A case report. Front Immunol. (2022) 13:989966. doi: 10.3389/fimmu.2022.989966
40. Li X, Li G, Chen D, Su L, Wang RP, Zhou Y. Case Report: sintilimab-induced Stevens-Johnson Syndrome in a patient with advanced lung adenocarcinoma. Front Oncol. (2023) 13:912168. doi: 10.3389/fonc.2023.912168
41. Logan IT, Zaman S, Hussein L, Perrett CM. Combination therapy of ipilimumab and nivolumab-associated toxic epidermal necrolysis (TEN) in a patient with metastatic melanoma: A case report and literature review. J OF Immunother. (2020) 43:89–92. doi: 10.1097/CJI.0000000000000302
42. Lye YL, Shan B, Jia CH, Liu J, Hou J, Du WL, et al. Toxic epidermal necrolysis induced by sintilimab: A case report. Ann Dermatol. (2023) 35:S100–S2. doi: 10.5021/ad.21.072
43. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. (2016) 39:149–52. doi: 10.1097/CJI.0000000000000112
44. Oguri T, Sasada S, Shimizu S, Shigematsu R, Tsuchiya Y, Ishioka K, et al. A case of Guillain-Barré Syndrome and Stevens-Johnson syndrome/toxic epidermal necrosis overlap after pembrolizumab treatment. J Invest Med High Impact Case Rep. (2021) 9:23247096211037462. doi: 10.1177/23247096211037462
45. Rodríguez-Otero N, Chamorro-Pérez J, Fernández-Lozano C, Elías-Sáenz I, Berná-Rico E, de Nicolás-Ruanes B, et al. Nivolumab-induced Stevens-Johnson syndrome: Not only due to PD-1 inhibition. J Allergy Clin Immunology: In Practice. (2023) 11:2936–8.e1. doi: 10.1016/j.jaip.2023.06.008
46. Pathria M MJ, Trufant J. A case of Stevens–Johnson syndrome in a patient on ipilimumab. Int J Case Rep Imag. (2016) 7:300–2. doi: 10.5348/ijcri-201651-CR-10639
47. Potts J, Lee RR, Hilliard CA. Lichenoid dermatitis preceding Stevens-Johnson syndrome in a patient treated with nivolumab. BMJ Case Rep. (2022) 15:e251233. doi: 10.1136/bcr-2022-251233
48. Robinson S, Saleh J, Curry J, Mudaliar K. Pembrolizumab-induced Stevens-Johnson syndrome/toxic epidermal necrolysis in a patient with metastatic cervical squamous cell carcinoma: A case report. Am J Dermatopathol. (2020) 42:292–6. doi: 10.1097/DAD.0000000000001527
49. Ryu S, Jun I, Kim T-I, Seo KY, Kim EK. Pembrolizumab-induced Stevens-Johnson syndrome with severe ocular complications. Ocular Immunol inflammation. (2022) 30:1533–5. doi: 10.1080/09273948.2021.1896006
50. Saad E, Adhikari P, Antala D, Abdulrahman A, Begiashvili V, Mohamed K, et al. Steven-Johnson syndrome: A rare but serious adverse event of nivolumab use in a patient with metastatic gastric adenocarcinoma. J Med Cases. (2022) 13:449–55. doi: 10.14740/jmc3992
51. Salati M, Pifferi M, Baldessari C, Bertolini F, Tomasello C, Cascinu S, et al. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann OF Oncol. (2018) 29:283–4. doi: 10.1093/annonc/mdx640
52. Sandhu M, Kc B, Bhandari J, Gambhir HS, Farah R. Pembrolizumab-associated Stevens-Johnson syndrome in a patient with metastatic non-small cell lung cancer: A case report. Cureus. (2023) 15:e41439. doi: 10.7759/cureus.41439
53. Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J OF CANCER. (2017) 81:237–9. doi: 10.1016/j.ejca.2017.03.026
54. Sommerfelt H, Sandvik LF, Bachmann IM, Brekke RL, Svendsen HL, Guttormsen AB, et al. Toxic epidermal necrolysis after immune checkpoint inhibition, case report, and review of the literature. Acta ONCOLOGICA. (2022) 61:1295–9. doi: 10.1080/0284186X.2022.2119099
55. Vivar KL, Deschaine M, Messina J, Divine JM, Rabionet A, Patel N, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J cutaneous pathol. (2017) 44:381–4. doi: 10.1111/cup.12876
56. Watanabe Y, Yamaguchi Y, Takamura N, Takahashi Y, Aihara M. Toxic epidermal necrolysis accompanied by several immune-related adverse events developed after discontinuation of nivolumab. Eur J OF CANCER. (2020) 131:1–4. doi: 10.1016/j.ejca.2020.02.044
57. Wu JY, Kang K, Yi J, Yang B. Pembrolizumab-induced Stevens-Johnson syndrome in advanced squamous cell carcinoma of the lung: A case report and review of literature. World J Clin Cases. (2022) 10:6110–8. doi: 10.12998/wjcc.v10.i18.6110
58. Yang H, Ma Q, Sun Y, Zhang K, Xing Y, Li H. Case Report: Toxic epidermal necrolysis associated with sintilimab in a patient with relapsed thymic carcinoma. Front Oncol. (2022) 12:1065137. doi: 10.3389/fonc.2022.1065137
59. Zhang L, Shen L, Lu Y, Xue J. Cancer immunotherapy and toxic epidermal necrolysis. BMJ SUPPORTIVE PALLIATIVE Care. (2020) 10:314–5. doi: 10.1136/bmjspcare-2019-002135
60. Zhang J, Zhang P, Xu Q-Y, Zhu Y-T, Chen W, Ji C. Pembrolizumab associated Stevens-Johnson syndrome with porokeratosis in a patient in the setting of primary hepatocellular carcinoma. Australas J OF Dermatol. (2022) 63:E71–E4. doi: 10.1111/ajd.13704
61. Zhang L, Wu Z. Adalimumab for sintilimab-induced toxic epidermal necrolysis in a patient with metastatic gastric Malignancy: A case report and literature review. Clinical Cosmetic Investigational Dermatol. (2023) 16:457–61. doi: 10.2147/CCID.S401286
62. Zhao Y, Cao Y, Wang X, Qian T. Treatment of PD-1 inhibitor-associated toxic epidermal necrolysis: A case report and brief review. OncoTargets Ther. (2022) 15:345–51. doi: 10.2147/OTT.S353743
63. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. EuroSCAR-study J Invest Dermatol. (2008) 128:35–44. doi: 10.1038/sj.jid.5701033
64. Lee HY, Dunant A, Sekula P, Mockenhaupt M, Wolkenstein P, Valeyrie-Allanore L, et al. The role of prior corticosteroid use on the clinical course of Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control analysis of patients selected from the multinational EuroSCAR and RegiSCAR studies. Br J Dermatol. (2012) 167:555–62. doi: 10.1111/j.1365-2133.2012.11074.x
65. Bequignon E, Duong TA, Sbidian E, Valeyrie-Allanore L, Ingen-Housz-Oro S, Chatelin V, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: ear, nose, and throat description at acute stage and after remission. JAMA Dermatol. (2015) 151:302–7. doi: 10.1001/jamadermatol.2014.4844
66. Morales ME, Purdue GF, Verity SM, Arnoldo BD, Blomquist PH. Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthalmol. (2010) 150:505–10.e1. doi: 10.1016/j.ajo.2010.04.026
67. Gulanikar A, Abrol A, Sagar S. Study of genital manifestations of Stevens Johnson Syndrome/Toxic Epidermal Necrolysis. Indian J Sex Transm Dis AIDS. (2022) 43:39–42. doi: 10.4103/ijstd.IJSTD_61_19
68. Meneux E, Wolkenstein P, Haddad B, Roujeau JC, Revuz J, Paniel BJ. Vulvovaginal involvement in toxic epidermal necrolysis: a retrospective study of 40 cases. Obstet Gynecol. (1998) 91:283–7. doi: 10.1016/S0029-7844(97)00596-6
69. Stamp LK, Chapman PT. Allopurinol hypersensitivity: Pathogenesis and prevention. Best Pract Res Clin Rheumatol. (2020) 34:101501. doi: 10.1016/j.berh.2020.101501
70. Fukasawa T, Urushihara H, Takahashi H, Okura T, Kawakami K. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antibiotic use: A case-crossover study. J Allergy Clin Immunol Pract. (2023) 11:3463–72. doi: 10.1016/j.jaip.2023.07.012
71. Sato I, Mizuno H, Kataoka N, Kunimatsu Y, Tachibana Y, Sugimoto T, et al. Osimertinib-associated toxic epidermal necrolysis in a lung cancer patient harboring an EGFR mutation-A case report and a review of the literature. Medicina (Kaunas). (2020) 56:403. doi: 10.3390/medicina56080403
72. Rodríguez-Pérez R, de Las Vecillas L, Cabañas R, Bellón T. Tools for etiologic diagnosis of drug-induced allergic conditions. Int J Mol Sci. (2023) 24:12577. doi: 10.3390/ijms241612577
73. Mayorga C, Sanz ML, Gamboa P, Garcia-Aviles MC, Fernandez J, Torres MJ. In vitro methods for diagnosing nonimmediate hypersensitivity reactions to drugs. J Investig Allergol Clin Immunol. (2013) 23:213–25.
74. Romano A, Caubet JC. Antibiotic allergies in children and adults: from clinical symptoms to skin testing diagnosis. J Allergy Clin Immunol Pract. (2014) 2:3–12. doi: 10.1016/j.jaip.2013.11.006
75. Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clin Exp Allergy. (2013) 43:1027–37. doi: 10.1111/cea.12145
76. Klaewsongkram J, Buranapraditkun S, Thantiworasit P, Rerknimitr P, Tuchinda P, Chularojanamontri L, et al. The role of in vitro detection of drug-specific mediator-releasing cells to diagnose different phenotypes of severe cutaneous adverse reactions. Allergy Asthma Immunol Res. (2021) 13:896–907. doi: 10.4168/aair.2021.13.6.896
77. Chu MT, Wang CW, Chang WC, Chen CB, Chung WH, Hung SI. Granulysin-based lymphocyte activation test for evaluating drug causality in antiepileptics-induced severe cutaneous adverse reactions. J Invest Dermatol. (2021) 141:1461–72.e10. doi: 10.1016/j.jid.2020.11.027
78. Wong CS, Yeung CK, Chan CY, Yap DY, Tang SC, Cheung BM, et al. HLA-B*58:01 screening to prevent allopurinol-induced severe cutaneous adverse reactions in Chinese patients with chronic kidney disease. Arch Dermatol Res. (2022) 314:651–9. doi: 10.1007/s00403-021-02258-3
79. Chen Z, Liew D, Kwan P. Effects of a HLA-B*15:02 screening policy on antiepileptic drug use and severe skin reactions. Neurology. (2014) 83:2077–84. doi: 10.1212/WNL.0000000000001034
80. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. (2004) 428:486. doi: 10.1038/428486a
81. Kawsar A, Edwards C, Patel P, Heywood RM, Gupta A, Mann J, et al. Checkpoint inhibitor-associated bullous cutaneous immune-related adverse events: a multicentre observational study. Br J Dermatol. (2022) 187:981–7. doi: 10.1111/bjd.21836
82. Jacoby TV, Chang MS, Thompson LL, Foreman RK, Reynolds KL, Chen ST. Histopathologically-confirmed lichenoid eruptions from immune checkpoint inhibitor therapy: a retrospective cohort analysis. Br J Dermatol. (2021) 185:1254–6. doi: 10.1111/bjd.20698
83. Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. (2018) 128:985–96. doi: 10.1172/JCI93349
84. Jacobsen A, Olabi B, Langley A, Beecker J, Mutter E, Shelley A, et al. Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst Rev. (2022) 3:Cd013130. doi: 10.1002/14651858.CD013130.pub2
85. Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. JAMA Dermatol. (2017) 153:514–22. doi: 10.1001/jamadermatol.2016.5668
86. Zhang J, Lu CW, Chen CB, Wang CW, Chen WT, Cheng B, et al. Evaluation of combination therapy with etanercept and systemic corticosteroids for Stevens-Johnson syndrome and toxic epidermal necrolysis: A multicenter observational study. J Allergy Clin Immunol Pract. (2022) 10:1295–304.e6. doi: 10.1016/j.jaip.2022.01.038
87. Heng YK, Lee HY, Roujeau JC. Epidermal necrolysis: 60 years of errors and advances. Br J Dermatol. (2015) 173:1250–4. doi: 10.1111/bjd.13989
88. Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg. (1986) 204:503–12. doi: 10.1097/00000658-198611000-00001
89. Wu J, Lee YY, Su SC, Wu TS, Kao KC, Huang CC, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis in patients with Malignancies. Br J Dermatol. (2015) 173:1224–31. doi: 10.1111/bjd.14052
90. Bettuzzi T, Penso L, de Prost N, Hemery F, Hua C, Colin A, et al. Trends in mortality rates for Stevens-Johnson syndrome and toxic epidermal necrolysis: experience of a single centre in France between 1997 and 2017. Br J Dermatol. (2020) 182:247–8. doi: 10.1111/bjd.18360
91. Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. (2013) 133:1197–204. doi: 10.1038/jid.2012.510
92. Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. (2016) 136:1387–97. doi: 10.1016/j.jid.2016.03.023
93. Saito Y, Abe R. New insights into the diagnosis and management of Stevens-Johnson syndrome and toxic epidermal necrolysis. Curr Opin Allergy Clin Immunol. (2023) 23:271–8. doi: 10.1097/ACI.0000000000000914
94. Sunaga Y, Hama N, Ochiai H, Kokaze A, Lee ES, Watanabe H, et al. Risk factors for sepsis and effects of pretreatment with systemic steroid therapy for underlying condition in SJS/TEN patients: Results of a nationwide cross-sectional survey in 489 Japanese patients. J Dermatol Sci. (2022) 107:75–81. doi: 10.1016/j.jdermsci.2022.07.004
95. Brüggen MC, Le ST, Walsh S, Toussi A, de Prost N, Ranki A, et al. Supportive care in the acute phase of Stevens-Johnson syndrome and toxic epidermal necrolysis: an international, multidisciplinary Delphi-based consensus. Br J Dermatol. (2021) 185:616–26. doi: 10.1111/bjd.19893
96. Su SC, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen CB, et al. Interleukin-15 is associated with severity and mortality in Stevens-Johnson syndrome/toxic epidermal necrolysis. J Invest Dermatol. (2017) 137:1065–73. doi: 10.1016/j.jid.2016.11.034
97. Wang F, Ye Y, Luo ZY, Gao Q, Luo DQ, Zhang X. Diverse expression of TNF-α and CCL27 in serum and blister of Stevens-Johnson syndrome/toxic epidermal necrolysis. Clin Transl Allergy. (2018) 8:12. doi: 10.1186/s13601-018-0199-6
98. Hasegawa A, Shinkuma S, Hayashi R, Hama N, Watanabe H, Kinoshita M, et al. RIP3 as a diagnostic and severity marker for Stevens-Johnson syndrome and toxic epidermal necrolysis. J Allergy Clin Immunol Pract. (2020) 8:1768–71.e7. doi: 10.1016/j.jaip.2020.01.006
99. Krajewski A, Maciejewska-Markiewicz D, Jakubczyk K, Markowska M, Strużyna J, Mądry R, et al. Impact of multiple medical interventions on mortality, length of hospital stay and reepithelialization time in Toxic Epidermal Necrolysis, Steven-Johnsons Syndrome, and TEN/SJS Overlap - Metanalysis and metaregression of observational studies. Burns. (2022) 48:263–80. doi: 10.1016/j.burns.2021.11.004
100. Chen Y, Han H, Cheng J, Cheng Q, Zhu S, Zhan P, et al. Efficacy and safety of anti-PD-1/PD-L1-based dual immunotherapies versus PD-1/PD-L1 inhibitor alone in patients with advanced solid tumor: a systematic review and meta-analysis. Cancer Immunol Immunother. (2024) 73:155. doi: 10.1007/s00262-024-03734-1
101. Mangla A, Lee C, Mirsky MM, Wang M, Rothermel LD, Hoehn R, et al. Neoadjuvant dual checkpoint inhibitors vs anti-PD1 therapy in high-risk resectable melanoma: A pooled analysis. JAMA Oncol. (2024) 10:612–20. doi: 10.1001/jamaoncol.2023.7333
102. Yan YD, Cui JJ, Fu J, Su YJ, Chen XY, Gu ZC, et al. A network comparison on safety profiling of immune checkpoint inhibitors in advanced lung cancer. Front Immunol. (2021) 12:760737. doi: 10.3389/fimmu.2021.760737
103. Maggie Liu SY, Huang J, Deng JY, Xu CR, Yan HH, Yang MY, et al. PD-L1 expression guidance on sintilimab versus pembrolizumab with or without platinum-doublet chemotherapy in untreated patients with advanced non-small cell lung cancer (CTONG1901): A phase 2, randomized, controlled trial. Sci Bull (Beijing). (2024) 69:535–43. doi: 10.1016/j.scib.2023.12.046
Keywords: immune checkpoint inhibitor, immune-related adverse events, irAE, severe cutaneous adverse reaction, Stevens-Johnson syndrome, toxic epidermal necrolysis
Citation: Zhou J, Wang C-P, Li J, Zhang H-L and He C-X (2024) Stevens-Johnson syndrome and toxic epidermal necrolysis associated with immune checkpoint inhibitors: a systematic review. Front. Immunol. 15:1414136. doi: 10.3389/fimmu.2024.1414136
Received: 08 April 2024; Accepted: 01 July 2024;
Published: 12 July 2024.
Edited by:
Igor Age Kos, Universitätsklinikum des Saarlandes, GermanyReviewed by:
Katrin Schaper-Gerhardt, Johannes Wesling Klinik, GermanyCopyright © 2024 Zhou, Wang, Li, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Xia He, aGN4cHVtY2hAZm94bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.