- 1Division of Gastroenterology and Hepatology, Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
- 2Division of Rheumatology, Department of Internal Clinical Sciences, Anaesthesiologic and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy
- 3Laboratory of Microbiology and Virology, Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy
- 4The PhD National Programme in “Innovazione Nella Diagnosi, Prevenzione e Terapia Delle Infezioni a Rischio Epidemico-Pandemico”, Department of Medical Biotechnologies, University of Siena, Siena, Italy
- 5The PhD National Programme in One Health Approaches to Infectious Diseases and Life Science Research, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
- 6Division of Clinical Immunology, Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
- 7Department of Hematology, Sapienza University of Rome, Santa Maria Goretti Hospital, Latina, Italy
- 8Department of Molecular Medicine, Laboratory of Microbiology and Virology, Policlinico Umberto I Hospital, Sapienza University of Rome, Rome, Italy
- 9Istituto Superiore di Sanità, National Centre for Global Health, Rome, Italy
Mixed cryoglobulinemia vasculitis (MCV) is caused in ~90% of cases by chronic hepatitis C virus (HCVposMCV) and more rarely by hepatitis B virus (HBV) infection, or apparently noninfectious. HCVposMCV develops in only ~5% of patients with chronic hepatitis C (CHC), but risk factors other than female gender have not been identified so far. We conducted a retrospective case control study investigating whether past active HBV infection, defined by hepatitis B surface antigen (HBsAg) seroclearance and anti-core antibody (HBcAb) positivity, could be a risk factor for developing HCVposMCV. The prevalence of HBsAg seroclearance was 48% within 123 HCVposMCV patients and 29% within 257 CHC patients (p=0.0003). Multiple logistic regression including as variables gender, birth year, age at HBV testing, cirrhosis, and hepatocellular carcinoma, confirmed an association of HBsAg seroclearance with HCVposMCV [adjusted odds ratio (OR) 2.82, 95% confidence interval (95% CI) 1.73-4.59, p<0.0001]. Stratification by gender, however, showed that HBsAg seroclearance was associated with HCVposMCV in male [OR 4.63, 95% CI 2.27-9.48, p<0.0001] and not in female patients [OR 1.85, 95% 95% CI 0.94-3.66, p=0.076]. HBsAg seroclearance, and more likely occult HBV infection, is an independent risk factor for HCVposMCV in male CHC patients.
1 Introduction
Mixed cryoglobulinemia vasculitis (MCV) is a lymphoproliferative disorder of marginal zone B-cells, producing polyreactive IgM endowed with rheumatoid factor activity, which are nonmalignant but are prone to neoplastic transformation; the pathogenic IgM forms cryoprecipitable immune complexes with endogenous IgG that in vivo cause a small vessel vasculitis (1). About 90% of MCV cases are associated with chronic hepatitis C virus (HCV) infection (HCVposMCV), and 5% with surface antigen (HBsAg)-positive chronic hepatitis B virus (HBV) infection; in a minority of cases MCV is apparently not associated with infections (noninfectious MCV) (1, 2).
HCV and HBV can infect B-cells and both can cause, besides MCV, non-Hodgkin lymphoma (NHL) (3). HCV is widely believed to cause MCV and indolent NHL by continual antigenic stimulation, since both disorders can regress after the eradication of infection (1, 4); however, mutations of lymphoma-related genes concur to the pathogenesis of HCVposMCV (5).
Besides MCV and B-cell NHL, chronic HCV infection causes a spectrum of extrahepatic manifestations that include diabetes and cardiovascular disease, which is an important cause of mortality in infected people. Although HCV-associated B-cell lymphoproliferative disorders and cardiovascular disease are driven by different mechanisms, inflammation vs antigenic pressure and gene mutation, they share the characteristic of regressing after antiviral therapy as especially illustrated by the recent use of highly effective direct-acting antivirals (4, 6–8). While in the case of HCV-associated cardiovascular disease no risk factor have been identified except for those canonical such as diabetes, smoking and hypertension (9), the fact that only a minority of CHC patients develop HCVposMCV suggests specific risk factor(s) that, however, remain elusive.
It is estimated that, although serum cryoglobulins can be found in as high as 40% of subjects with chronic hepatitis C (CHC), only ~5% of them develop HCVposMCV, but the only risk factor for developing MCV identified so far is female gender (1). Concerning host factors, a genome-wide association study identified a segment on chromosome 6 near the NOTCH4 and MHC class II genes conferring 2.15 times the odds of having HCVposMCV within patients with CHC, but it was impossible to determine which one of the two genes determined this association (10). Concerning HCV-related factors, the claim that variations in a sequence of the hypervariable region 1 of the E2 protein were more prevalent in HCVposMCV than in CHC patients (11) was not confirmed by a later study (12). To our knowledge, no other risk factors for developing HCVposMCV have been suggested.
Some studies have shown that hepatitis B surface antigen (HBsAg) seroclearance (13) and occult HBV infection (OBI) (14–16) are associated with an increased risk of developing B-cell lymphoproliferative disorders, and particularly diffuse large B-cell lymphoma (DLBCL). HBsAg seroclearance is commonly defined by negative HBsAg (HBsAgneg) and positive anti-core antibody (HBcAbpos) serology, and OBI by the presence of HBV DNA in serum and/or of replication-competent covalently closed circular HBV DNA (cccDNA) in tissues of HBsAgneg subjects (17). Currently available commercial assays detect plasma HBV DNA in ~1% of HBsAgnegHBcAbpos subjects intermittently and usually at low levels (17), whereas recent highly sensitive assays detect HBV DNA in up to 40% of these cases (18, 19); furthermore, HBV cccDNA was found in liver tissue in 62% of HBsAgnegHBcAbpos subjects (20). Therefore, the HBsAgnegHBcAbpos serology is commonly used as surrogate marker of OBI in clinical and epidemiological studies (17).
In this retrospective case control study, we investigated the prevalence of HBsAg seroclearance, defined by HBsAgnegHBcAbpos serology, in patients with HCVposMCV compared to patients with CHC. In addition, we explored the prevalence of HBsAg seroclearance in a small group of patients with noninfectious MCV compared to patients with B-cell NHL, rheumatoid arthritis, or miscellaneous diseases. Our results reveal an association of HBsAg seroclearance with the risk of developing HCVposMCV in male but not in female CHC patients and confirm female gender as an independent risk factor.
2 Methods
2.1 Study populations
This retrospective case control study was conducted at the Sapienza University of Rome Hospital, Rome, Italy. We compared the prevalence of HBsAg seroclearance (HBsAgnegHBcAbpos serology) in patients with HCVposMCV vs patients with CHC without symptoms or signs of vasculitis.
Two independent clinical research centers collected, blinded to each other, data concerning patients with HCVposMCV and CHC, respectively. Demographic, clinical and virological data of consecutive patients were obtained from internal medical records. The minimum requirements for inclusion in the study were the availability of full clinical information, negativity of HBsAg and of HIV serology, and availability of HBcAb serology. Serological and molecular assays for HBV, HCV and HIV were mostly done at the Central Laboratory of Virology of the Sapienza University Hospital, and rarely at other high-quality laboratories. The tertiary Referral Center for Mixed Cryoglobulinemia enrolled patients with HCVposMCV seen between 1997 and 2022; the Division of Gastroenterology and Hepatology enrolled patients with CHC seen between 1999 and 2022.
The diagnosis of CHC was based on the presence of anti-HCV antibodies and detectable viremia for at least 6 months after the first detection of infection in the absence of any clinical symptom or sign suggesting MCV at the time of diagnosis and throughout the follow-up. The diagnosis of HCVposMCV was based on the presence in CHC patients of serum cryoglobulins and of at least one of the following clinical items: purpura, peripheral neuropathy assessed by electroneurography, chronic skin ulcers, biopsy-proven cryoglobulinemic nephropathy.
The diagnosis of cirrhosis was established through blood test and ultrasound findings: reduced liver protein synthesis (prothrombin time, albumin, cholinesterase), liver stiffness evaluation by elastometry, ultrasonographic signs of portal hypertension (splenomegaly, ascites, portal vein collaterals), esophageal varices at endoscopy examination (21). In some cases, the diagnosis was made by liver biopsy. The diagnosis of hepatocellular carcinoma (HCC) was based on radiological hallmarks (contrast uptake in the arterial phase and washout in the venous/late phase) or liver biopsy according to the EASL guidelines (22). Cirrhosis was present in all patients at the first visit or previously, and none developed it during the follow-up.
A secondary analysis was conducted comparing noninfectious MCV (cases) versus other conditions: miscellaneous diseases, indolent B cell NHLs, and rheumatoid arthritis (controls) (see Supplementary Material).
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. All the laboratory studies were done as part of the routine workup of patients and therefore did not require specific ethical approval. Patient consent was waived since this was a retrospective and de-identified study.
2.2 Statistical analysis
The values are expressed as number and percent for categorical variables and as median and range for continuous variables. Birth year and age at HBV testing were adopted as time-related variables since other commonly used variables, such as age at infection or at disease onset, could not be clearly determined especially in CHC patients; birth year is of interest as it could cope with variations over time of HBV endemicity in the study area.
Univariate comparisons were done by the Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. Multivariate analysis was done by unconditional multiple logistic regression, and adjusted odds ratio (OR), 95% confidence interval (95% CI), and p-value are reported. The independent variables used for between-group comparisons were gender, birth year, age at latest HBV testing, HBcAb serology and, in the case of HCVposMCV vs CHC, the presence of cirrhosis and of hepatocellular carcinoma (HCC). Preliminary analysis of data revealed a significant inverse correlation between birth year and age at HBV serological testing, seemingly because elderly patients had undergone HBV tests later in life. Since collinearity between independent variables can distort the model development process in multivariate analysis, a stepwise selection procedure (bidirectional inclusion/elimination) was chosen to select variables to be included in the models. All statistical analyses were performed using the GraphPad Prism version 9.1.2 software or STATA Statistics/Data analysis version 16.1. A p-value of less than 0.05 was considered statistically significant.
3 Results
During the study period, 1997-2022, 123 cases with HCVposMCV and 257 with CHC (control group) were enrolled. HCVposMCV cases presented the following clinical items: purpura (82%), peripheral neuropathy assessed by electroneurography (63%), chronic skin ulcers (14%), biopsy-proven cryoglobulinemic nephropathy (9%). Cryoglobulins were classified as type 2 in 73% of cases; 10 patients (8%) had associated a B-cell NHL
Table 1 shows the univariate statistical comparison of demographic, clinical and virologic characteristics in total and gender stratified groups. Total HCVposMCV cases and CHC controls differed for all the variables tested except for HCV genotype distribution (not shown). HCVposMCV patients were mostly female and older than CHC patients; they had a lower percentage of cirrhosis and HCC, and 48% of them were HBcAb positive compared to 29% within CHC patients.
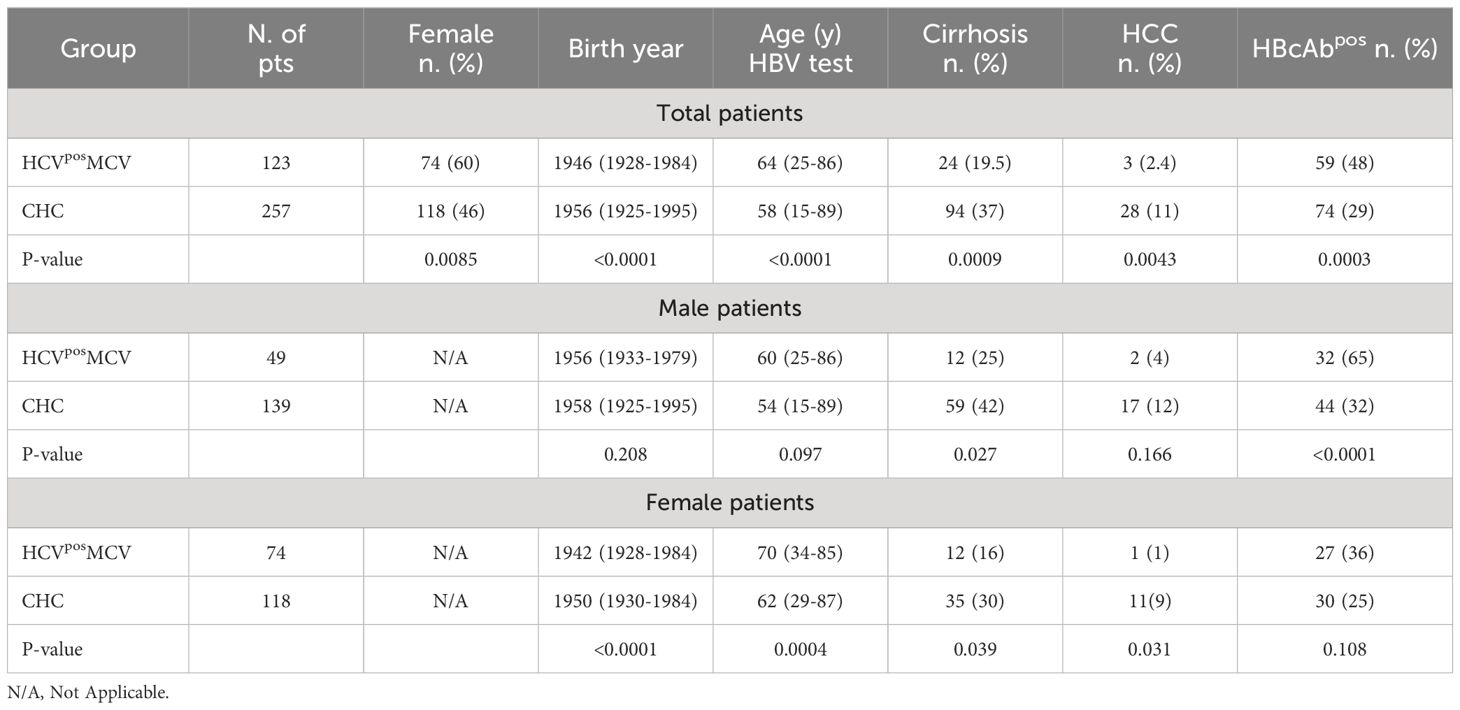
Table 1 Univariate analysis of HCVposMCV and CHC patients, total and stratified by gender. Continuous variables are expressed as median (range).
Gender stratification (Table 1) revealed that HBsAgnegHBcAbpos serology was significantly (p<0.0001) more prevalent in males, but not in females, with HCVposMCV compared to gender-matched CHC controls. By contrast, significantly earlier birth year and older age at HBV testing compared to gender-matched CHC controls were observed in females and not in males with HCVposMCV. Furthermore, HCVposMCV females were born significantly earlier than males (Table 1), and the age at onset of symptoms/signs of MCV was significantly higher in females than in males [median 62 (range 32-85) vs 57 (range 30-83) years, p=0.038].
Within the HCVposMCV cohort, HBcAbpos serology was more prevalent in males than in females (p=0.003) while, remarkably, this gender difference was not observed within the CHC control cohort (p=0.33).
Protective levels of anti-HBs antibodies (HBsAbpos) were present in a similar proportion of HBcAbpos patients with HCVposMCV (28/59, 47%) or with CHC (32/74, 43%). HBcAbnegHBsAbpos serology was found in 25 patients (5/123 HCVposMCV and 20/257 CHC), all of whom were vaccinated. The prevalence of anti-HBs positivity was similar in HBcAbpos males and females with HCVposMCV (16/32 vs 17/27); however, the prevalence of anti-HBs positivity was lower within HBcAbpos males with HCVposMCV than in HBcAbpos males with CHC (16/32 vs 34/44, p=0.016), while this was not the case for HBcAbpos females (17/27 HCVposMCV vs 18/30 CHC).
HBV DNA was searched only in a small fraction of HBcAbpos subjects and was positive in none of 16 HCVposMCV and in 2/14 CHC patients tested.
Stepwise logistic regression analysis in total and gender-stratified populations is reported in Table 2. Within total populations, the ORs were 2.82 (95% CI 1.73-4.59) for HBcAb positivity and 0.52 (95% CI 0.29-0.93) for absence of cirrhosis. The association of HBcAb positivity with HCVposMCV was retained after excluding patients with cirrhosis by univariate (42/99 vs 42/165, p=0.006) and multivariate (OR 2.24, 95% CI 1.29-3.90, p=0.004) analysis (not shown). After gender stratification, HBcAb positivity was associated with HCVposMCV in males (OR 4.63, 95%CI 2.27-9.48, p<0.0001) but not in females.
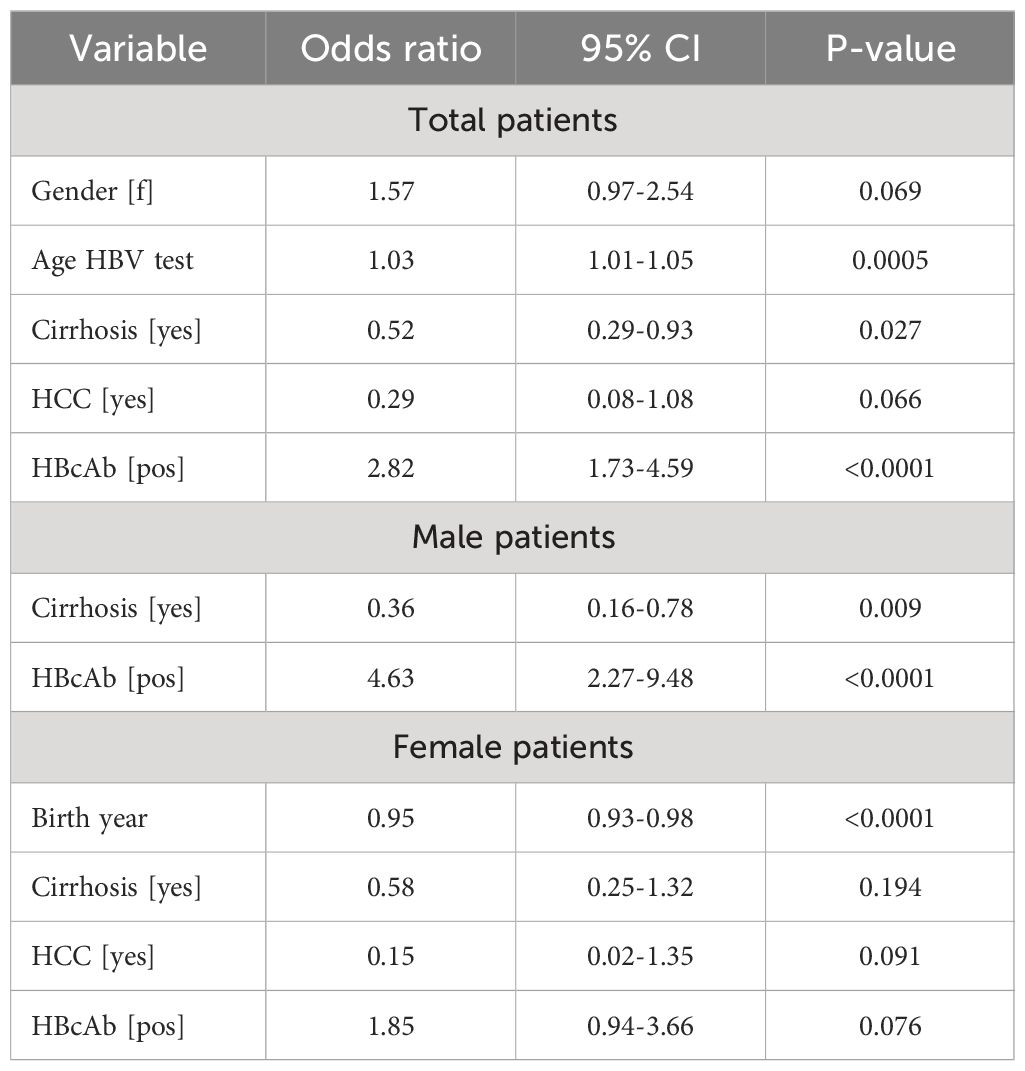
Table 2 Stepwise logistic regression analysis of HCVposMCV and CHC (control group) patients, total and stratified by gender.
As a corollary to the study in HCVposMCV patients, we performed an exploratory study in a small group (n=39) of patients with noninfectious MCV, a much rarer disorder. Comparator groups were patients with miscellaneous diseases (n=376), indolent B-cell NHL (n=275), or rheumatoid arthritis (n=195); information on the study populations and on statistical analyses is provided in Supplementary Material. By stepwise logistic regression (Supplementary Table 1), the adjusted ORs for HBcAb positivity were 3.97 (95% CI 1.69-9.32, p=0.002) vs miscellaneous diseases, 3.63 (95% CI 1.66-7.91, p=0.001) vs indolent NHL, and 2.39 (95% CI 1.05-5.48, p=0.039) vs rheumatoid arthritis. The paucity of noninfectious MCV patients did not allow gender stratification.
4 Discussion
Patients with HCVposMCV or CHC differed for several of the variables tested. Females were more prevalent among HCVposMCV than CHC patients as known from several studies (23–25), and the prevalence of cirrhosis was lower within HCVposMCV patients as also reported in a large multicenter study from Italy comparing HCVposMCV and CHC patients with asymptomatic serum cryogobulins (25). A lower prevalence of cirrhosis in HCVposMCV than in CHC patients might be due to an earlier onset of vasculitis manifestations than of liver function decompensation, and to the fact that in our study the hepatology center collecting CHC patients is particularly devoted to the care of advanced liver disease.
Birth year and age at HBV testing are important variables since they impact on possible changes over time of HBV prevalence in the study area, on the high rate of false HBcAb positivity with earlier assays (17), and on the length of time of exposure to the risk of infection. The fact that male patients with HCVposMCV and CHC did not differ significantly for these variables reassures about confounding.
Gender preference for adverse outcomes associated with HBsAg seroclearance is not unprecedented. A retrospective cohort study (26) showed that male gender was an independent risk factor of developing HCC after HBsAg seroclearance whereas older age at seroclearance was a risk factor in females. The latter finding may recall our observation that, within the HCVposMCV cohort, females were born earlier and were older at the onset of vasculitis symptoms than males.
A possible explanation for the gender-related risk of HCC and HCVposMCV after HBsAg seroclearance might be a higher prevalence of OBI in males. Indeed, it has been argued (27) that in areas where the male/female ratio of HBV infection in blood donors is about 1 the prevalence of OBI in males ranges from 62% to 88%. A higher prevalence of OBI in males has been reported both in high endemic areas such as South Africa (28) and in medium-low endemic European countries (29).
It is reasonable to assume that the prevalence of HBcAb positivity may roughly parallel that of OBI (17), and that the increased risk of HCVposMCV in HBsAgneg/HBcAbpos CHC patients may depend, like in the case of HCC (30), on OBI rather than on HBsAg seroclearance itself. At the state of the art, it is difficult to determine the real prevalence of OBI, since serum HBV DNA is detected by commercial assays in ~1% of HBsAgnegHBcAbpos persons while highly sensitive assays and search of cccDNA in liver reveal a much higher prevalence (18–20). We noticed a lower frequency of anti-HBs positivity in HBcAbpos male patients with HCVposMCV rather than with CHC. It has been reported that the probability of having detectable serum HBV DNA is higher in HBsAgneg/HBcAbpos individuals who are anti-HBs negative (31, 32) and, interestingly, male sex and absence of anti-HBs are independent risk factors for reactivation of HBV infection in HBsAgnegHBcAbpos lymphoma patients treated with rituximab (33). Thus, these observations may suggest that absence of anti-HBs antibodies and male sex concur to increase the risk of OBI, and therefore of MCV, in HBsAg serocleared CHC patients.
Chronic infections by HBV or HCV cause HCC and B-cell NHL by mechanisms that include mutations of tumor-driver genes (5, 34–36). OBI is believed to cause oncogenic mutations in hepatocytes by mechanisms involving integration of viral DNA into the host genome and the production of potentially mutagenic proteins by cccDNA (30); the presence of putatively pro-oncogenic quasispecies of HBV cccDNA in tumor cells suggests a similar mechanism in OBI-associated DLBCL (16).
Recent knowledge on gene mutations in monoclonal B-cells of HCVposMCV patients, although limited, may provide clues to explain a pathogenetic role of OBI in this disease. Although considered non-neoplastic, clonal B-cells of HCVposMCV patients harbor mutations of several lymphoma-related genes (5). These genes regulate NFκB and NOTCH signaling and cell proliferation (KLF2, TNFAIP3, NOTCH1), chromatin organization (HIST1H2BE, EP300), cell communication (FAT1, PTPRD), and V(D)J mutation (KLHL6). Remarkably, some of these genes (KLF2, TNFAIP3, NOTCH1, HIST1H2BE) are recurrently mutated in HBsAgpos DLBCL (36) and in HCV-associated B-cell NHL (5, 35). OBI increases the risk of HCC in CHC patients, indicating a synergistic effect that may include additive mutagenesis (30); thus, it can be speculated that HBV cccDNA in rogue B-cells of subjects with CHC may cooperate with HCV in inducing gene mutations that drive dysregulated clonal expansion and eventually HCVposMCV. These mutations are seemingly non-transforming, since in most patients HCVposMCV regresses after the clearance of HCV by therapy with interferon (1) or with direct-acting antivirals (8, 37).
Our preliminary data suggest that HBsAg seroclearance can also be associated with the risk of noninfectious MCV. In this regard, it is of interest that also in this disorder the clonally expanded rogue B-cells harbor mutations of lymphoma-related genes (CARD11, TNFAIP3, CCND3, ID3, BTG2, KLHL6) (38). These genes are also mutated in HBsAgpos DLBCL (36) and in HCV-associated B-cell NHL (5, 35). Remarkably, two genes mutated in noninfectious MCV (TNFAIP3 and KLHL6) are also mutated in HCVposMCV (5). The KLHL6 gene is particularly interesting, since in noninfectious MCV it has been shown that its mutation dysregulates V(D)J somatic mutation, leading to the generation in rogue B cells of an “iconic” pathogenic antibody carrying the WA public idiotype (38). The WA idiotype is expressed by clonal B-cells of more than half of patients with noninfectious MCV (38) or with HCVposMCV (39), suggesting KLHL6 mutation as one possible pathogenetic link between these disorders.
To conclude, we first acknowledge that our study suffers from limitations: it was carried in a low HBsAg endemic area (central Italy), asymptomatic cryoglobulins were not investigated in CHC patients, and serum HBV DNA was searched only in a fraction of patients. Nevertheless, our data robustly support an association of HBsAg seroclearance with the risk of developing HCVposMCV in male CHC patients; to the best of our knowledge, this is the first exogenous risk factor for HCVposMCV submitted so far. Studies in different HBV endemic areas and in-depth investigation of the role of OBI are warranted. Also, the possibility that OBI might be a risk factor for “noninfectious” MCV should be pursued by further studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
AM: Methodology, Writing – review & editing, Data curation, Investigation. VF: Data curation, Investigation, Methodology, Writing – review & editing. LC: Data curation, Investigation, Writing – review & editing. PR: Data curation, Investigation, Writing – review & editing. AF: Data curation, Investigation, Writing – review & editing. MV: Data curation, Writing – review & editing, Supervision. AP: Data curation, Supervision, Writing – review & editing. FS: Data curation, Supervision, Writing – review & editing. AS: Data curation, Supervision, Writing – review & editing, Methodology. GA: Data curation, Supervision, Writing – review & editing, Methodology. SB: Data curation, Supervision, Writing – review & editing, Funding acquisition. MT: Data curation, Methodology, Supervision, Writing – review & editing, Formal analysis, Software. FC: Data curation, Methodology, Supervision, Writing – review & editing. MC: Methodology, Supervision, Writing – review & editing, Conceptualization, Formal analysis, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Ministry of Education, Universities and Research grant PRIN 2017ATZ2YK, and by co-funding from NextGeneration EU (in the context of the National Recovery and Resilience Plan) Investment PE8-Project Age-It “Ageing Well in an Ageing Society” (DM 1557 11.10.2022) and the Extended Partnership initiative on Emerging Infectious Diseases (project no. PE00000007, INF-ACT). The NextGeneration EU funding source had no role in the study design,
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1411146/full#supplementary-material
References
2. Terrier B, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, et al. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. (2012) 119:5996–6004. doi: 10.1182/blood-2011-12-396028
3. Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. (2011) 117:1792–8. doi: 10.1182/blood-2010-06-275818
4. Arcaini L, Besson C, Frigeni M, Fontaine H, Goldaniga M, Casato M, et al. Interferon-free antiviral treatment in B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood. (2016) 128:2527–32. doi: 10.1182/blood-2016-05-714667
5. DeFrancesco I, Visentini M, Zibellini S, Minafò YA, Rattotti S, Ferretti VV, et al. Mutational and immunogenetic landscape of HCV-associated B-cell lymphoproliferative disorders. Am J Hematol. (2021) 96:E210–4. doi: 10.1002/ajh.26167
6. Androutsakos T, Mouziouras D, Katelani S, Psichogiou M, Sfikakis PP, Protogerou AD, et al. A comparative study on the presence and reversibility of subclinical arterial damage in HCV-infected individuals and matched controls. Viruses. (2023) 15:1374. doi: 10.3390/v15061374
7. Cespiati A, Coelho Rodrigues I, Santos I, Policarpo S, Carvalhana S, Fracanzani AL, et al. Effect of HCV eradication by DAAs on liver steatosis, carotid atherosclerosis, and associated metabolic comorbidities: A systematic review. Liver Int. (2024) 44:1075–92. doi: 10.1111/liv.15876
8. Cacoub P, Comarmond C, Vieira M, Régnier P, Saadoun D. HCV-related lymphoproliferative disorders in the direct-acting antiviral era: From mixed cryoglobulinaemia to B-cell lymphoma. J Hepatol. (2022) 76:174–85. doi: 10.1016/j.jhep.2021.09.023
9. Petta S, Maida M, Macaluso FS, Barbara M, Licata A, Craxì A, et al. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational sudies. Gastroenterology. (2016) 150:145–55. doi: 10.1053/j.gastro.2015.09.007
10. Zignego AL, Wojcik GL, Cacoub P, Visentini M, Casato M, Mangia A, et al. Genome-wide association study of hepatitis C virus- and cryoglobulin-related vasculitis. Genes Immun. (2014) 15:500–5. doi: 10.1038/gene.2014.41
11. Gerotto M, Dal Pero F, Loffreda S, Bianchi FB, Alberti A, Lenzi M. A 385 insertion in the hypervariable region 1 of hepatitis C virus E2 envelope protein is found in some patients with mixed cryoglobulinemia type 2. Blood. (2001) 98:2657–63. doi: 10.1182/blood.V98.9.2657
12. Bianchettin G, Bonaccini C, Oliva R, Tramontano A, Cividini A, Casato M, et al. Analysis of hepatitis C virus hypervariable region 1 sequence from cryoglobulinemic patients and associated controls. J Virol. (2007) 81:4564–71. doi: 10.1128/JVI.02104-06
13. Zhou X, Pan H, Yang P, Ye P, Cao H, Zhou H. Both chronic HBV infection and naturally acquired HBV immunity confer increased risks of B-cell non-Hodgkin lymphoma. BMC Cancer. (2019) 19:477. doi: 10.1186/s12885-019-5718-x
14. Chen MH, Hsiao LT, Chiou TJ, Liu JH, Gau JP, Teng HW, et al. High prevalence of occult hepatitis B virus infection in patients with B cell non-Hodgkin's lymphoma. Ann Hematol. (2008) 87:475–80. doi: 10.1007/s00277-008-0469-9
15. Tajima K, Takahashi N, Ishizawa K, Murai K, Akagi T, Noji H, et al. High prevalence of diffuse large B-cell lymphoma in occult hepatitis B virus-infected patients in the Tohoku district in Eastern Japan. J Med Virol. (2016) 88:2206–10. doi: 10.1002/jmv.24584
16. Sinha M, Sundar K, Premalata CS, Asati V, Murali A, Bajpai AK, et al. Pro-oncogenic, intra host viral quasispecies in diffuse large B cell lymphoma patients with occult Hepatitis B Virus infection. Sci Rep. (2019) 9:14516. doi: 10.1038/s41598-019-51157-1
17. Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. (2019) 71:397–408. doi: 10.1016/j.jhep.2019.03.034
18. Wang S, Li H, Kou Z, Ren F, Jin Y, Yang L, et al. Highly sensitive and specific detection of hepatitis B virus DNA and drug resistance mutations utilizing the PCR-based CRISPR-Cas13a system. Clin Microbiol Infect. (2021) 27:443–50. doi: 10.1016/j.cmi.2020.04.018
19. Piermatteo L, Scutari R, Chirichiello R, Alkhatib M, Malagnino V, Bertoli A, et al. Droplet digital PCR assay as an innovative and promising highly sensitive assay to unveil residual and cryptic HBV replication in peripheral compartment. Methods. (2022) 201:74–81. doi: 10.1016/j.ymeth.2021.05.011
20. Raimondo G, Navarra G, Mondello S, Costantino L, Colloredo G, Cucinotta E, et al. Occult hepatitis B virus in liver tissue of individuals without hepatic disease. J Hepatol. (2008) 48:743–6. doi: 10.1016/j.jhep.2008.01.023
21. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. (2021) 75:659–89. doi: 10.1016/j.jhep.2021.05.025
22. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
23. Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. (2004) 33:355–74. doi: 10.1016/j.semarthrit.2003.10.001
24. Trejo O, Ramos-Casals M, García-Carrasco M, Yagüe J, Jiménez S, de la Red G, et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Med (Baltimore). (2001) 80:252–62. doi: 10.1097/00005792-200107000-00004
25. Kondili LA, Monti M, Quaranta MG, Gragnani L, Panetta V, Brancaccio G, et al. A prospective study of direct-acting antiviral effectiveness and relapse risk in HCV cryoglobulinemic vasculitis by the Italian PITER cohort. Hepatology. (2022) 76:220–32. doi: 10.1002/hep.32281
26. Yip TC, Chan HL, Wong VW, Tse YK, Lam KL, Wong GL. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol. (2017) 67:902–8. doi: 10.1016/j.jhep.2017.06.019
27. Allain JP. Global epidemiology of occult HBV infection. Ann Blood. (2017) 2:7. doi: 10.21037/aob.2017.06.01
28. Allain JP, Belkhiri D, Vermeulen M, Crookes R, Cable R, Amiri A, et al. Characterization of occult hepatitis B virus strains in South African blood donors. Hepatology. (2009) 49:1868–76. doi: 10.1002/hep.22879
29. Candotti D, Grabarczyk P, Ghiazza P, Roig R, Casamitjana N, Iudicone P, et al. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. J Hepatol. (2008) 49:537–47. doi: 10.1016/j.jhep.2008.04.017
30. Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol. (2020) 73:952–64. doi: 10.1016/j.jhep.2020.05.042
31. Iizuka H, Ohmura K, Ishijima A, Satoh K, Tanaka T, Tsuda F, et al. Correlation between anti-HBc titers and HBV DNA in blood units without detectable HBsAg. Vox Sang. (1992) 63:107–11. doi: 10.1111/j.1423-0410.1992.tb02495.x
32. Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. (2008) 48:1001–26. doi: 10.1111/j.1537-2995.2008.01701.x
33. Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. (2009) 27:605–11. doi: 10.1200/JCO.2008.18.0182
34. Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. (2016) 64:S84–S101. doi: 10.1016/j.jhep.2016.02.021
35. Armand M, Degaud M, Tesson B, Laurent C, Vavasseur M, Parisot M, et al. Exploring the genetic landscape of HCV-related B-cell lymphomas using whole exome sequencing. Leukemia. (2023) 37:1388–91. doi: 10.1038/s41375-023-01868-2
36. Ren W, Ye X, Su H, Li W, Liu D, Pirmoradian M, et al. Genetic landscape of hepatitis B virus-associated diffuse large B-cell lymphoma. Blood. (2018) 131:2670–81. doi: 10.1182/blood-2017-11-817601
37. Gragnani L, Visentini M, Fognani E, Urraro T, De Santis A, Petraccia L, et al. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology. (2016) 64:1473–82. doi: 10.1002/hep.28753
38. Singh M, Jackson KJL, Wang JJ, Schofield P, Field MA, Koppstein D, et al. Lymphoma driver mutations in the pathogenic evolution of an iconic human autoantibody. Cell. (2020) 180:878–894.e19. doi: 10.1016/j.cell.2020.01.029
Keywords: HBsAg seroclearance, occult HBV infection, chronic hepatitis C, mixed cryoglobulinemia vasculitis, risk factor
Citation: Morrone A, Fiorilli V, Cinti L, Roberto P, Ferri AL, Visentini M, Pulsoni A, Spinelli FR, De Santis A, Antonelli G, Basili S, Tosti ME, Conti F and Casato M (2024) Surface antigen serocleared hepatitis B virus infection increases the risk of mixed cryoglobulinemia vasculitis in male patients with chronic hepatitis C. Front. Immunol. 15:1411146. doi: 10.3389/fimmu.2024.1411146
Received: 02 April 2024; Accepted: 27 June 2024;
Published: 11 July 2024.
Edited by:
Roberto Paganelli, Institute for Advanced Biologic Therapies, ItalyReviewed by:
Theodoros Androutsakos, National and Kapodistrian University of Athens, GreeceValentina Svicher, University of Rome Tor Vergata, Italy
Copyright © 2024 Morrone, Fiorilli, Cinti, Roberto, Ferri, Visentini, Pulsoni, Spinelli, De Santis, Antonelli, Basili, Tosti, Conti and Casato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milvia Casato, bWlsdmlhLmNhc2F0b0B1bmlyb21hMS5pdA==
†These authors share first authorship
 Anna Morrone
Anna Morrone Valerio Fiorilli
Valerio Fiorilli Lilia Cinti3,4
Lilia Cinti3,4 Piergiorgio Roberto
Piergiorgio Roberto Alejandro L. Ferri
Alejandro L. Ferri Marcella Visentini
Marcella Visentini Francesca Romana Spinelli
Francesca Romana Spinelli Adriano De Santis
Adriano De Santis Guido Antonelli
Guido Antonelli Stefania Basili
Stefania Basili Maria Elena Tosti
Maria Elena Tosti Fabrizio Conti
Fabrizio Conti Milvia Casato
Milvia Casato