- 1Bristol Haematology and Oncology Centre, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, United Kingdom
- 2Department of Clinical Oncology, Royal Cornwall Hospital, Truro, United Kingdom
- 3Department of Oncology, University College London Hospital and Mount Vernon Cancer Centre, Northwood, United Kingdom
- 4Oncology Medical Affairs Department, Sanofi-Aventis, Reading, United Kingdom
- 5OPEN Health, London, United Kingdom
Background: Cemiplimab was licensed in the United Kingdom (UK) in 2019 for the treatment of patients with locally advanced and metastatic CSCC not suitable for curative surgery or radiotherapy (advanced CSCC [aCSCC]). No UK multi-center studies have investigated the real-world experience of cemiplimab post marketing authorization in aCSCC.
Methods: This non-interventional retrospective study (10 UK centers) involved data collection from medical records of patients with aCSCC who initiated cemiplimab treatment between 2 July 2019 and 30 November 2020. The study period was a minimum of 12 and a maximum of 36 months post cemiplimab initiation. The primary objective was to describe the real-world clinical effectiveness of cemiplimab (primary outcome: overall response rate [ORR]).
Results: Of 105 patients, 70% (n=73/105) were male (median [range] age at index of 78.5 [55.4–93.2] years); most patients (63% [n=50/80]) had an Eastern Cooperative Oncology Group (ECOG) score of 1 and 62% (n=63/102) had metastatic disease. The ORR within 12 months was 42% (95% confidence interval [CI] 32%–51%) and the disease control rate was 62% (n=65/105). The median (95% CI) real-world progression-free survival and overall survival from index was 8.6 (6.0–18.7) and 21.0 (14.7–25.2) months, respectively. The median (range) number of cemiplimab infusions was 11.0 (1.0–44.0). Eighty-seven percent experienced no cemiplimab treatment interruptions; 13% (n=14/105) interrupted treatment due to immune-related adverse reactions (irARs) (47% [n=9/19] of treatment interruption events). Eighty-five percent (n=89/105) of patients had discontinued cemiplimab treatment by the end of the study; where reasons for discontinuation were recorded, 20% (n=17/87) discontinued due to the completion of their 2-year treatment course. Nineteen percent (n=20/105) of patients experienced irARs.
Conclusion: Effectiveness and safety data in this study are broadly similar to previous real-world studies of cemiplimab and the EMPOWER-CSCC1 clinical trial; with our cohort representing a broader population (included immunocompromised and transplant patients). Results support the use of cemiplimab for the treatment of aCSCC in a real-world setting.
1 Introduction
Patients with locally advanced cutaneous squamous cell carcinoma (laCSCC) or metastatic cutaneous squamous cell carcinoma (mCSCC) who are not candidates for curative therapies (herein described as advanced cutaneous squamous cell carcinoma [aCSCC]) have limited treatment options, with a poor prognosis and an impaired quality of life. Previous studies using chemotherapy or other systemic anti-cancer therapy have reported a median overall survival (OS) of approximately 8–15 months for this patient population (1–3).
Cemiplimab is a fully human immunoglobulin G4 (IgG4) monoclonal antibody that acts as an immune checkpoint inhibitor by binding to the programmed death-1 (PD-1) receptor, blocking the interaction between PD-1 and its ligands programmed death-ligand 1 and 2 (PD-L1 and PD-L2. Monoclonal antibodies such as cemiplimab have been shown to restore the cytotoxic capabilities of tumor antigen-specific T-cells in multiple cancers (4, 5).
Cemiplimab was granted a conditional marketing authorization by the European Commission in June 2019 as a monotherapy for the treatment of patients with aCSCC who are ineligible for curative surgery or curative radiation (6). In the National Health Service (NHS) in England, cemiplimab was initially available, pre-marketing authorization, via a Named Patient Scheme and was subsequently recommended by the National Institute for Health and Care Excellence (NICE) for reimbursement via the Cancer Drugs Fund (CDF) for advanced CSCC in July 2019 (7); followed by a recommendation for routine use in June 2022 (8). In Scotland, cemiplimab was accepted by the Scottish Medicines Consortium (SMC) in February 2020 for use on an interim basis, subject to ongoing evaluation and reassessment (9). Cemiplimab treatment is currently only reimbursed for a maximum of 2 years in England (8).
Cemiplimab is currently the first and only licensed systemic therapy for aCSCC in the UK (10, 11). Pembrolizumab, is also approved for the treatment of aCSCC in the United States (12), however it is not licensed in the UK for these patients.
The results of the EMPOWER-CSCC-1 study, a multicohort phase 2 study with 193 aCSCC patients treated with cemiplimab led to the approval of cemiplimab for aCSCC (6). The study demonstrated objective response rates of 44.9% to 50.8%, disease control rates (DCRs) ranging from 64.3% and 79.5% and a safety profile generally similar to other immune checkpoint inhibitors (12, 13). At final analysis (data cut-off: 1 March 2022), median OS had not been reached, with an estimated OS rate at 48 months of 61.8% (95% confidence interval [CI], 54.0−68.7) (14).
The generation of real-world evidence provides a broader picture of the performance of a treatment in a cohort of patients that is more reflective of routine clinical practice (15, 16).
To date, no UK multi-center studies have investigated the real-world clinical experience, effectiveness and safety outcomes of cemiplimab in aCSCC post marketing authorization. Herein, we address this evidence gap by collecting data on clinical outcomes, treatment patterns and pre-specified safety events in patients initiated on cemiplimab in routine UK clinical practice since its launch in July 2019 up to November 2020.
2 Study design and methods
2.1 Study design and setting
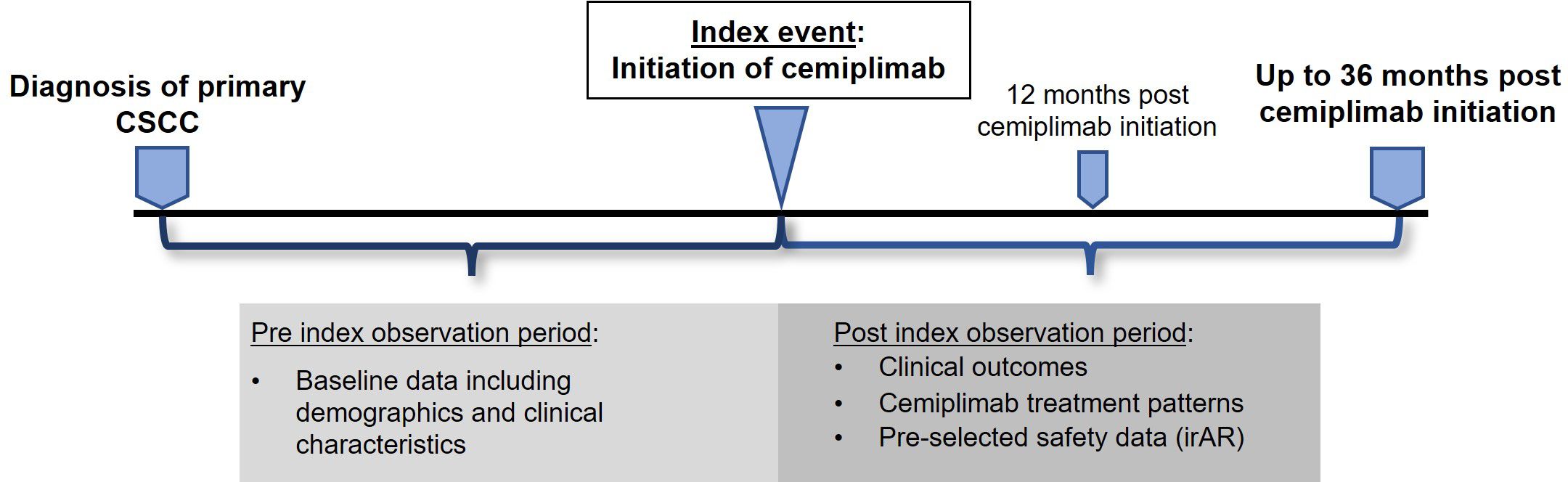
This was a UK, multi-center, non-interventional study with retrospective review of medical records of 105 patients with aCSCC across 10 centers. Health Research Authority (HRA) approval was obtained but no NHS Research Ethics Committee (REC) approval or patient consent was required (data collected within the NHS setting by members of the direct care team). The study design is summarized in Figure 1. The index event was the date of cemiplimab initiation, and the pre-index observation period was from the date of diagnosis of primary CSCC to the index date. The post-index observation period was a minimum of 12 and maximum of 36 months post-index for each patient (or until the date of death or the date of data collection if these occurred earlier than 36 months). Retrospective data collection took place between July 2022 and 31 October 2022 (follow-up of up to 36 months).
2.2 Patients
The population comprised adult (≥18 years) patients with aCSCC treated with cemiplimab as part of routine clinical care in the UK in participating centers. Patients treated with ≥ 1 dose of cemiplimab, initiated between 2 July 2019 and 30 November 2020, were eligible for inclusion. Patients known to have opted out of participation in any research study during the post-index observation period were excluded.
2.3 Study objectives and outcomes
The primary objective was to describe the real-world clinical effectiveness of cemiplimab in patients with aCSCC treated in routine clinical practice. The secondary objectives included patient demographics and clinical characteristics, treatment patterns and safety events in patients who received cemiplimab for the treatment of aCSCC as part of routine clinical practice. The primary outcome was the overall response rate (ORR) within 12 months post-initiation of cemiplimab. Secondary clinical effectiveness outcomes included: ORR within the observation period, best response, DCR, time to response (best response of CR and PR), duration of response (DoR), duration of treatment (DoT), real-world progression-free survival (rwPFS), and overall survival (OS). Safety events collected included immune-related adverse reactions (irARs) of any grade, treatment interruptions due to irARs and duration of treatment interruptions.
2.4 Data sources and data collection
Data were collected retrospectively from medical records by a member of the direct care team, using a bespoke pseudonymized electronic case report form (eCRF). Patient demographics and clinical characteristics were collected during the pre-index observation period; clinical outcomes, treatment patterns and pre-specified safety event data were collected during the post-index observation period. Real-world responses (i.e., partial response [PR] or complete response [CR], stable disease or progression) were taken as documented in the medical records. Where the documented response did not align with generally understood definitions of response as outlined previously (17), responses were classified by the centers investigator based on their interpretation of the information available in the medical records.
2.5 Statistical methods
2.5.1 Data analysis
Statistical analyses were conducted using Stata V14 (StataCorp LLC) and Microsoft Excel. Data were pooled for analysis and are presented as descriptive statistics of central tendency (median; arithmetic mean or geometric mean) and dispersion (interquartile range [IQR] and/or range; standard deviation [SD] and/or 95% CI), and/or frequencies and percentages.
Responses were based on an assessment according to routine practice, as documented in medical records. The ORR within 12 months post cemiplimab initiation was analyzed as the proportion of patients who achieved either a CR or PR during this time period and presented as frequency and percentage, with two-sided 95% CI for the percentage (also provided for CR and PR). DCR represents the proportion of patients with a CR, PR or SD. OS and rwPFS were analyzed from the date of initiation of cemiplimab using the Kaplan-Meier (KM) method. For KM analysis of OS, the event was defined as death (all causes), reported as median (95% CI). Patients not recorded as having died and those lost to follow-up (LTFU) were censored on the date they were last known to be alive. For KM analysis of rwPFS, the event was defined as the date of disease progression (as first documented in medical records after initiation according to radiological progression or the date when the patient discontinued cemiplimab due to progression), or date of death (all causes). Patients alive and without disease progression, and those LTFU, were censored on the date their disease was last known not to have progressed. The time to PR, CR and best response was evaluated from the date of cemiplimab initiation until the first documentation of a PR or CR. Best response was defined as the most favorable (i.e., complete response > partial response > stable disease > progressive disease) response recorded at any point for a patient within the given time window (6 months, 12 months, 36 months). Response was also assessed in the form of a swimmer plot, according to best response during the observation period against OS (as per KM analysis), and as a Sankey plot. Patients in the following four categories were defined as immunocompromised: 1) patients previously receiving solid organ transplant or allogeneic stem cell transplant; 2) patients with significant autoimmune disease treated with immunosuppressants; 3) patients with concurrent malignancies other than CSCC (e.g., including, but not limited to, hematological malignancies); 4) patients with infection (e.g., including, but not limited to, human immunodeficiency virus [HIV], Hepatitis B and Hepatitis C).
This was a descriptive study; hence, no sample size estimation or power calculation was conducted. The target sample size of 80-100 patients was based on estimates of the precision (95% confidence limits) of the study primary outcome, based on previously published objective response rate data (12, 18).
3 Results
3.1 Patient demographics and clinical characteristics
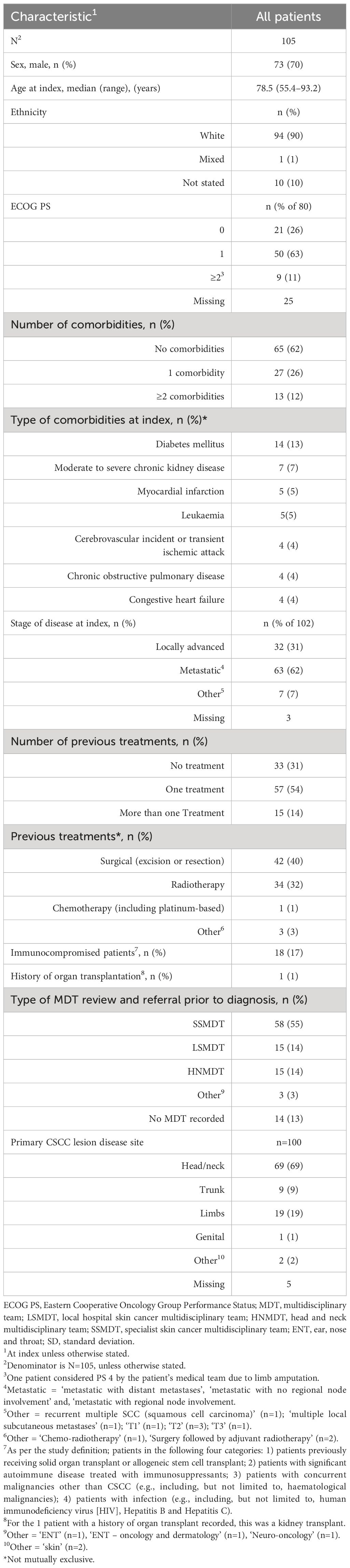
Baseline demographics and clinical characteristics are summarized in Table 1. A total of 105 patients with aCSCC were included in this study. The median age at index was 78.5 years and 70% were male. Ninety percent of patients (n=94/105) were White, 89% (n=71/80) had Eastern Cooperative Oncology Group Performance Status (ECOG PS) scores of 0–1 and 11% (n=9/80) had scores ≥2. Sixty-two percent (n=65/105) of patients had no comorbidities, 26% (n=27/105) had 1 comorbidity and 12% (n=13/105) had ≥2. Overall, 17% (n=18/105) were immunocompromised and 1% (n=1/105) had a history of an organ transplant. Diabetes mellitus and chronic kidney disease (moderate to severe) were the two most common comorbidities followed by myocardial infarction and leukemia. Most patients were recorded as having either metastatic (including regional nodal and distant metastases) (62% [n=63/102]) or locally advanced (31% [n=32/102]) disease at baseline. The most common primary CSCC lesion disease sites were head/neck (69% [n=69/100]) and limbs (19% [n=19/100]). The median time from diagnosis of primary disease to diagnosis of aCSCC was 38.1 weeks (range, 0.0-487.0). Over half of the patients (55% [n=58/105]) were referred to a specialist skin cancer multidisciplinary team (SSMDT), 14% (n=15/105) were referred to a local hospital skin cancer multidisciplinary team (LSMDT) and 14% (n=15/105) to a head and neck multidisciplinary team (HNMDT). Sixty-nine percent (n=72/105) of patients had received at least one prior treatment, with 14% (n=15/105) of patients receiving more than one prior treatment. The most common prior treatment was surgery (excision or resection; 40% [n=42/105]), followed by radiotherapy (32% [34/105]).
3.2 Real-world effectiveness of cemiplimab
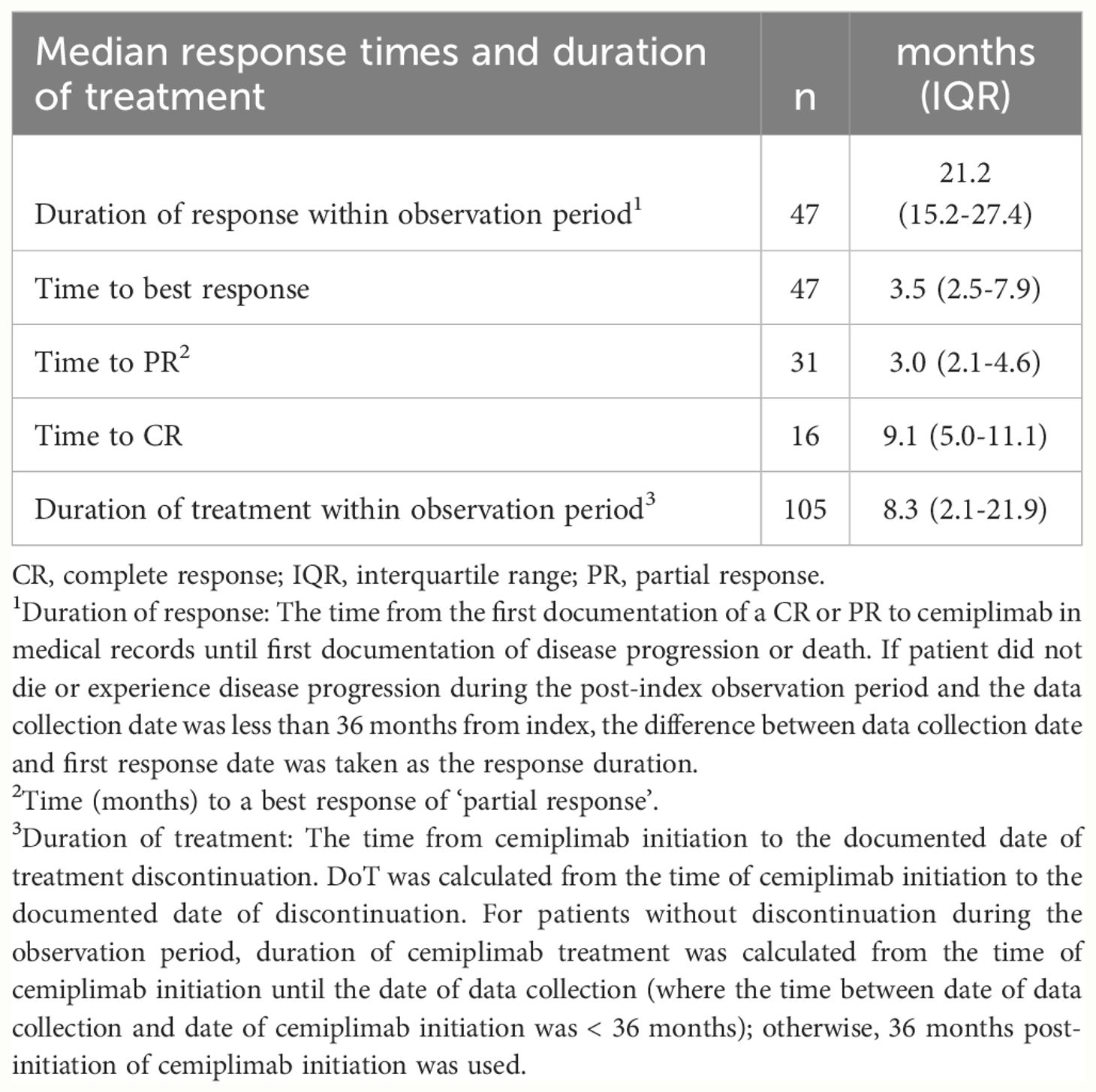
Real-world best response rates increased numerically over time, with ORR within 6-, 12- and up to 36-months post-initiation of cemiplimab being 38% (95% CI, 29%–47%; n=40/105), 42% (95% CI, 32%–51%; n=44/105), and 45% (95% CI, 35%–54%; n=47/105), respectively (Figure 2. This included a numerical increase in CR rate as best response from 5% (n=5/105) to 12% (n=13/105) between 6 months and 12 months (Figure 2). Best response to cemiplimab is also summarized in a Sankey plot (Figure 3) and in a Swimmer plot (Figure 4). By the end of the observation period, most patients had a documented assessment of treatment response, leading to a numerical increase in both the ORR (42% vs. 45%) and the proportion of patients achieving a CR (12% vs 15%) between 12 months and the end of the observation period (up to 36 months). A numerical increase in the proportion of patients with SD (20% [n=21/105] vs 21% [n=22/105]) and PD (14% [n=15/105] vs. 16% [n=17/105]) was also observed. A DCR of 56% (n=59/105), 62% (n=65/105) and 66% (n=69/105) was achieved within 6-, 12- and 36-months post-index, respectively (Figure 2). The median time to PR was 3.0 (IQR, 2.1–4.6) months and to CR was 9.1 (IQR, 5.0–11.1) months and the median time to a best response (CR or PR) was 3.5 (IQR, 2.5–7.9) months (Table 2). The median DoT was 8.3 (IQR, 2.1–21.9) months, however, for responding patients, long DoR were observed with a median DoR of 21.2 (IQR: 15.2–27.4) months (Table 2). The median rwPFS observed was 8.6 (95% CI, 6.0–18.7) months (Figure 5A) with a median OS of 21.0 (95% CI, 14.7–25.2) months for the study population (Figure 5B). Additional rwPFS, OS and ORR rates stratified by immunocompromised status are included in the supplementary information (Supplementary Tables S3–S5, Supplementary Figures S1, S2).
Figure 2 Real-world ORR, best response and DCR within 6-, 12- and 36 months post-index. Real-world best response within 6- 12- and 36 months post-index is shown. In addition to a complete response, partial response or stable disease, additional categories were also present: The number of patients with progressive disease (6 months: 12% [n=13/105]; 12 months: 14% [n=15/105]; 36 months: 16% [n=17/105]), inconclusive responses (6 months: 1% [n=1/105]; 12 months: 1% [n=1/105]; 36 months: 3% [n=3/105]), no documented response within the time window (6 months: 15% [n=16/105]; 12 months: 8% [n=8/105]; 36 months: 0% [n=0/105]), Died or discontinued treatment before 12 weeks (i.e., prior to first response scan) without a recorded response (6 months: 14% [n=15/105]; 12 months: 14% [n=15/105]; 36 months: 14% [n=15/105]) and other (1% [n=1/105] for all timepoints). One patient stopped treatment 6 months post-initiation without a recorded treatment response. The disease control rate (DCR) and overall response rate (ORR) within 6-, 12- and 36 months is also shown.
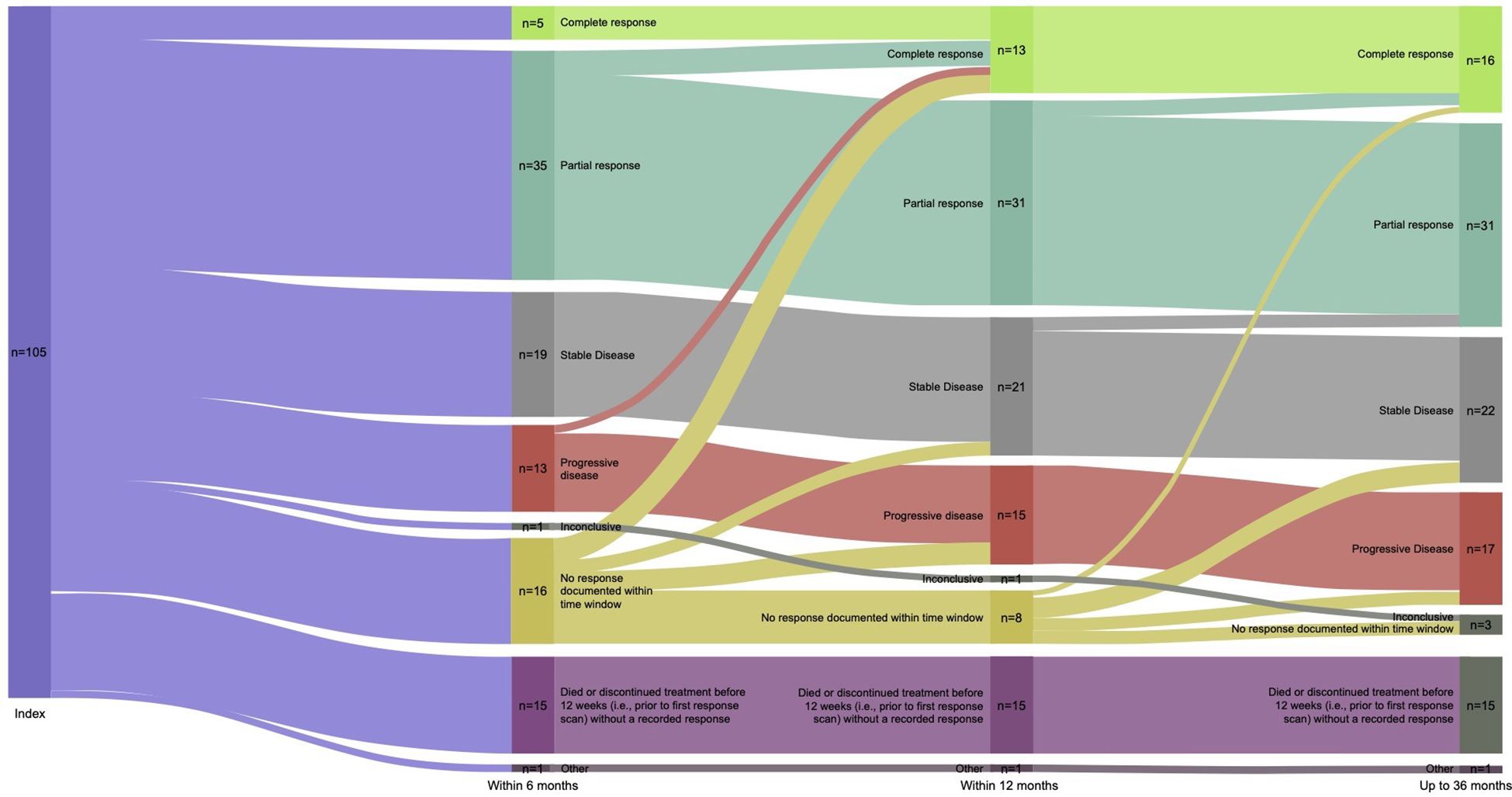
Figure 3 Sankey plot of response to cemiplimab. A Sankey plot of the best response to cemiplimab within 6-, 12- and 36 months is shown, depicting change in best response for patients at each timepoint. This is the best recorded response leading up to each timepoint, patients cannot be categorized into a less favorable response category.
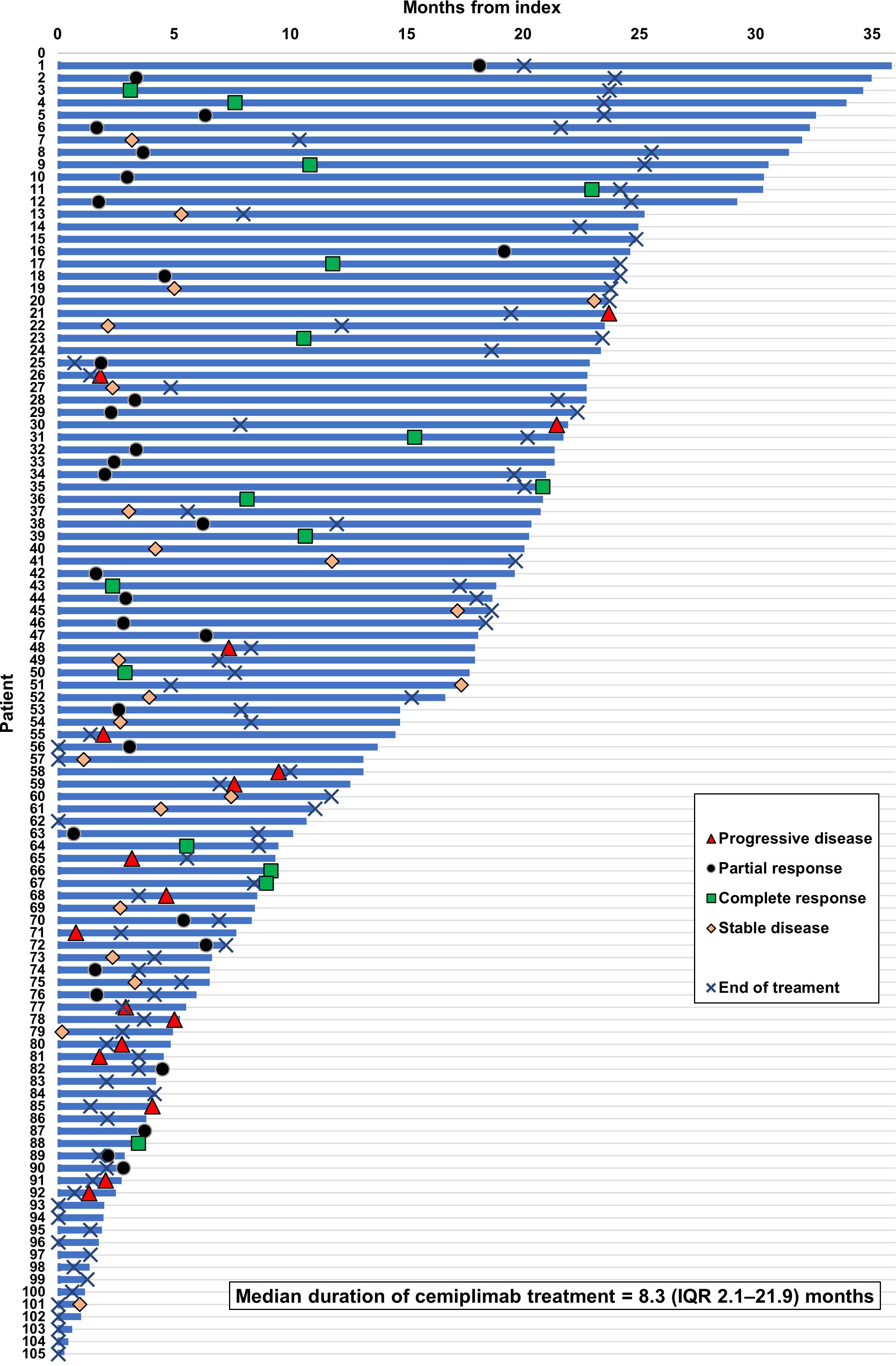
Figure 4 Swimmer plot of response to cemiplimab. Swimmer plot shows OS (calculated as time until death, LTFU or last contact within observation), with treatment responses of CR, PR or PD plotted according to when they occurred during the observation period. The median duration of treatment was 8.3 (IQR 2.1–21.9) months.
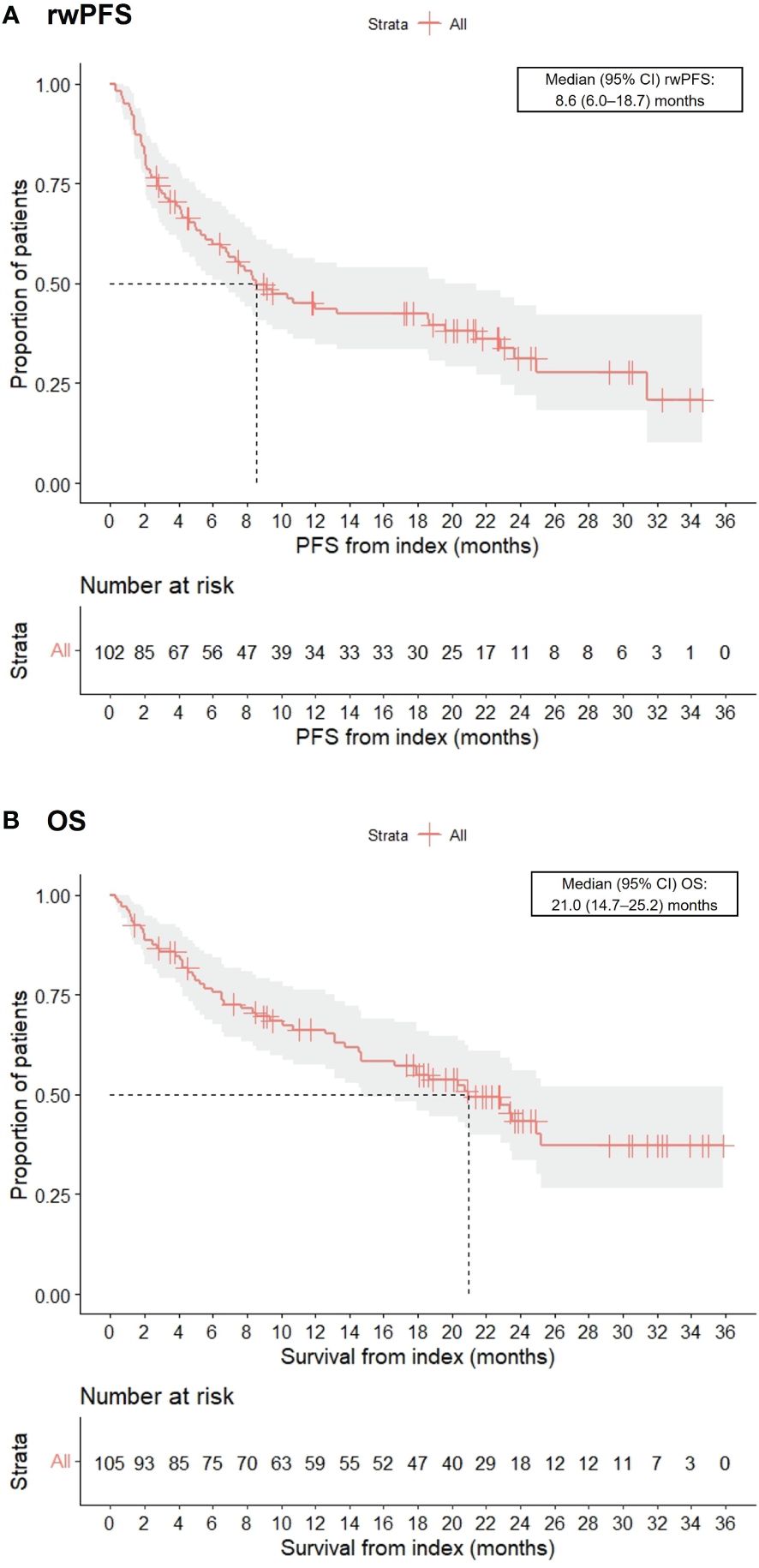
Figure 5 Kaplan-Meier charts of real-world progression-free survival (rwPFS) (A) and overall survival (OS) (B).
3.3 Cemiplimab treatment patterns
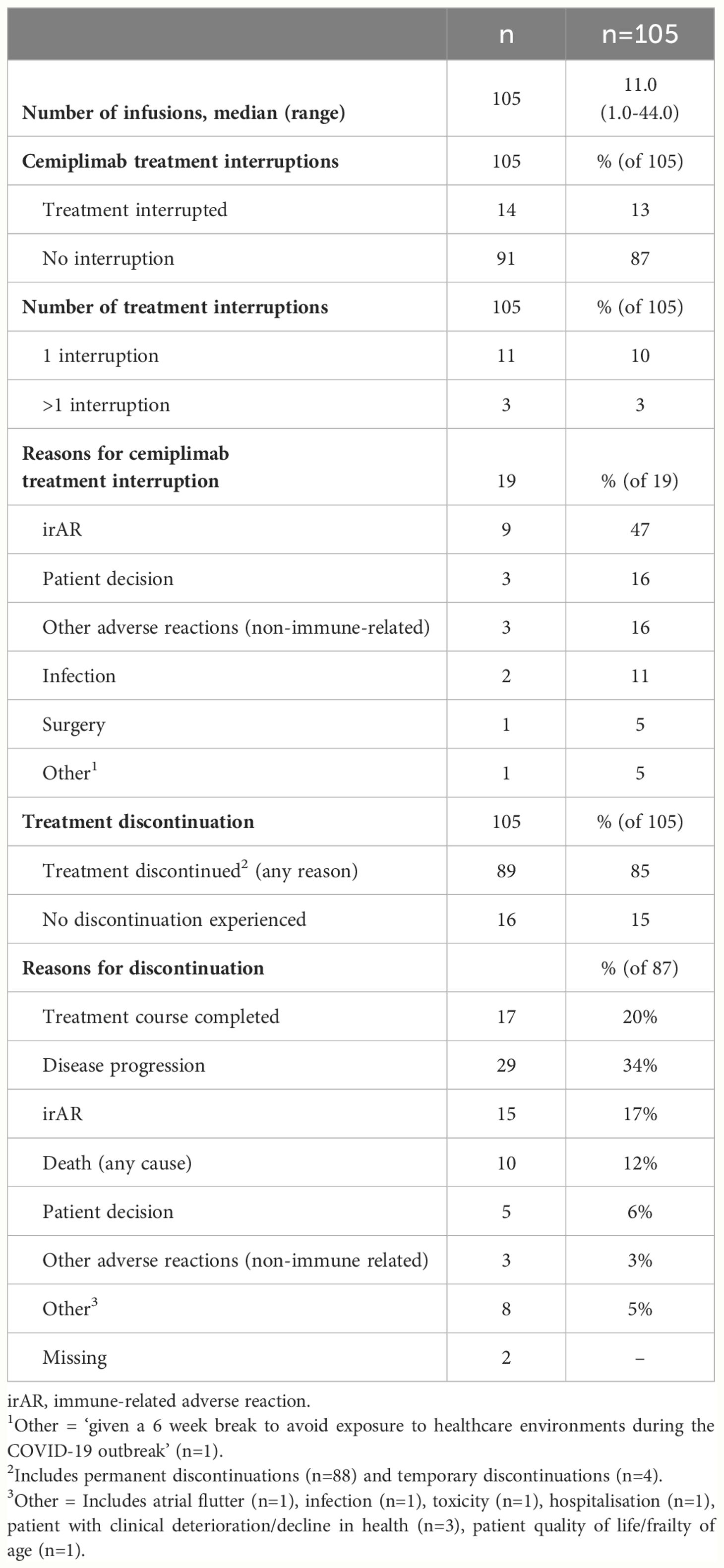
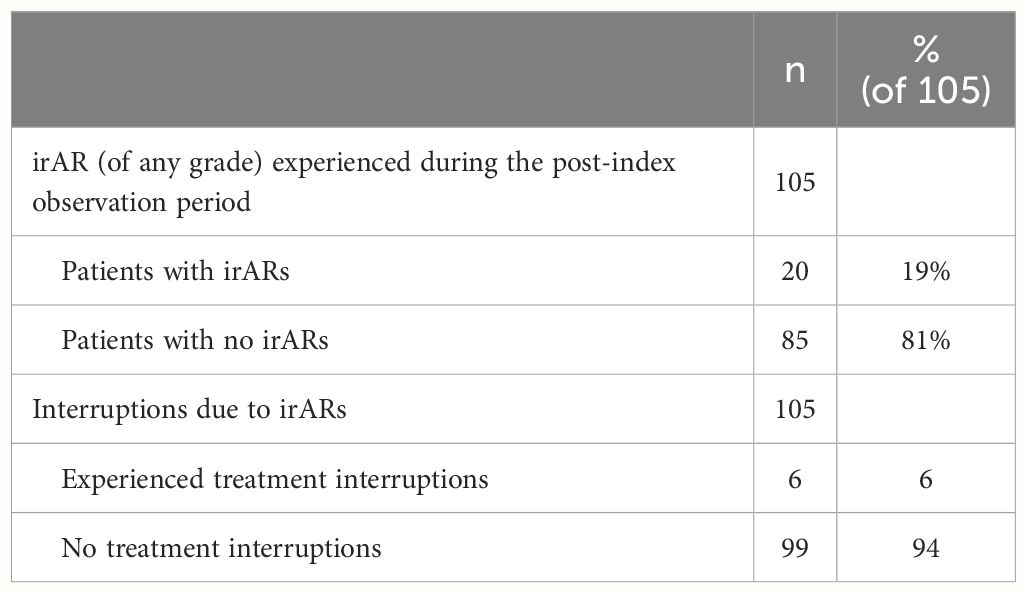
Cemiplimab treatment patterns are summarized in Table 3. The median (range) number of cemiplimab infusions during the observation period was 11.0 (1.0–44.0). A total of 13% (n=14/105) of patients experienced cemiplimab treatment interruptions, which included 47% (n=9/19) of treatment interruption events that were due to an irAR. Other interruptions included patient decision and other adverse reaction (non-immune related) in 16% (n=3/19) respectively. A total of 85% (n=89/105) of patients discontinued cemiplimab treatment (any reason). For patients with discontinuation data available (n=87), 34% (n=29/87) discontinued due to disease progression and 20% (n=17/87) discontinued due to the completion of their 2-year treatment course. Within the study period, 12% (n=10/87) of patients were recorded to have discontinued due to death (any cause). Additional information on irARs and cemiplimab treatment interruptions in immunocompromised patients can be found in the supplementary information (Supplementary Tables S6, S7).
3.4 Real-world cemiplimab safety
A total of 19% (n=20/105) of patients experienced irARs of any grade during the post-index observation period (Table 4). Additionally, 6% (n=6/105) of patients (with a total of 9 interruption events) also had treatment interruptions due to irARs. The median (range) duration of interruption was 22 days (21-57). A summary of the management of irARs is summarized in Supplementary Table S1 and Supplementary Table S2.
4 Discussion
To our knowledge, REACT-CEMI is the first multi-center UK real-world study to investigate the early clinical experience, effectiveness and safety of cemiplimab for the treatment of aCSCC post marketing authorization. The results provide important insights into UK practice and they outline the early management of aCSCC and the clinical characteristics of these patients. Furthermore, results provide a real-world outlook on the early outcomes of aCSCC patients who received cemiplimab within the first 2 years post marketing authorization, and patients who initiated cemiplimab during the coronavirus disease-2019 (COVID-19) pandemic.
The primary objective of the study was to describe the real-world clinical effectiveness of cemiplimab in patients with advanced CSCC treated in routine clinical practice. As part of this, ORR within 12 months of cemiplimab initiation, was assessed as the primary outcome of the study, with 42% of patients responding to treatment at 12 months. This percentage relatively increased to 45% over the whole study period which was driven by a numerical increase in both CR and PR rates over time as patient responses deepened (Figure 3). These responses were marginally higher than those reported for cemiplimab in the UK early access programme (19), and in line with previously reported responses as part of a French and Italian early access schemes (20, 21). More recent real-world studies have investigated the use of cemiplimab and pembrolizumab in aCSCC with higher response rates reported (22, 23). The phase 2 EMPOWER-CSCC-1 trial reported objective response rates of 46.4% to 50.8%, which are broadly similar to the ORRs observed in this study within 12- and 36-months post cemiplimab initiation. In addition, the DoR reported in the current study was shorter than that reported in the EMPOWER-CSCC-1 trial. This could be attributed to the shorter follow-up period and the real-world setting of the current study.
The overall DCR (up to 36 months) in our study (66%) was also broadly similar to the DCRs reported in the Italian study and the NPS (21). However, the follow-up for the Italian study was shorter, at 9 months. Our results demonstrated a median rwPFS of 8.6 months and a median OS of 21 months. These compare favorably against the UK and French early access studies (19, 20) but are shorter than the median PFS and OS reported in the EMPOWER-CSCC1 trial and the recent Australian real-world evidence study (23). The reasons for this could be that, firstly, our data are reflective of early clinical experience with potentially immature treatment and referral pathways. In addition, the COVID-19 pandemic was ongoing during the observation period, therefore we cannot rule out that this may have had an impact on delaying or commencing treatment. Indeed, a range of studies have highlighted the negative impact of COVID-19 on CSCC treatment accessibility and diagnosis (24, 25). For instance, a retrospective Serbian study demonstrated that CSCC patients post-pandemic exhibited statistically significant increases in the largest tumor diameter and had an increased rate of invasive disease (25). Further to this, an Italian study reported that the reduced level of access to medical care during the COVID-19 pandemic led to a documented diagnostic delay (24).
The current study represents a more diverse patient population, in terms of age, comorbidities, ECOG score and immunocompromized status. Some of these patients, such as patients with immunocompromized status or with transplant history (representing 18% of patients in the present study) or with certain comorbidities, would have been excluded from the EMPOWER study. In terms of the patient demographics and clinical characteristics, based on the age of the patients included in the study, it was evident that this was an elderly cohort; consistent with other real-world studies and the natural history of CSCC generally (26).
Progression of CSCC is often fast, hence, a timely referral plays a crucial role in outcomes for patients (19). Whilst most patients (87%) were referred via an MDT prior to diagnosis, 13% of patients had no MDT recorded. Furthermore, while most patients were seen at an SSMDT (55%), approximately 1 in 8 patients were seen by other MDTs.
Overall, cemiplimab appeared to be well tolerated; eighty-seven percent of patients had no treatment interruptions and a total of 19% (n=20/105) experienced irARs. In 17% of patients (n=15/87), irARs led to cemiplimab treatment discontinuation. This is broadly consistent with the rate of irARs seen in other retrospective real-world studies of cemiplimab in aCSCC (27).
Limitations of this study included the retrospective study design based on secondary use of data. As such, the results are dependent on the completeness and quality of the medical records and the reliability of the abstraction of data from them. From a statistical perspective, this was a descriptive study, hence, no analyses to control for confounding were conducted. In addition, response/progression assessments were real-world estimates and were impacted by the frequency by which assessments were made in routine clinical practice. Where real-world response/progression outcomes were not clearly documented in the medical records, these were retrospectively classified by the center investigator based on the available information, where possible, which could have led to potential bias in the absence of blinded centralized review.
The results are expected to be broadly generalizable to the wider source population. Firstly, in relation to the study center selection, centers were selected based on post-marketing authorisation use of cemiplimab which included centers with early experience with cemiplimab across England, Wales and Scotland. Due to this, results obtained are likely to be representative of early use of cemiplimab and representative of UK practice. As cemiplimab reimbursement is restricted to 2 years in the UK, experience of this treatment in the UK may differ from other healthcare settings with longer reimbursement.
This study is the first description of the early real-world clinical experience of cemiplimab for the treatment of aCSCC in a multi-center UK clinical setting. The ORR observed in the present study was consistent with other real-world studies of cemiplimab conducted globally, including the Named Patient Scheme conducted in the UK and the EMPOWER-CSCC1 trial. The results of this study support the use of cemiplimab for the treatment of aCSCC in a real-world setting and will help inform clinical decisions.
Data availability statement
The datasets presented in this article are not readily available because the original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author. Requests to access the datasets should be directed to YW1hcm5hdGguY2hhbGxhcGFsbGlAdWhidy5uaHMudWs=.
Ethics statement
The requirement of ethical approval was waived by Health Research Authority (HRA) for the studies involving humans, because this is not required for a non-interventional study with retrospective review of medical records. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a non-interventional study with retrospective review of medical records.
Author contributions
AC: Conceptualization, Writing – original draft, Writing – review & editing. GS: Writing – original draft, Writing – review & editing. HS: Writing – original draft, Writing – review & editing. PD: Conceptualization, Writing – original draft, Writing – review & editing. JL-B: Conceptualization, Writing – original draft, Writing – review & editing. EO: Writing – original draft, Writing – review & editing. SK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received funding from Sanofi.
Acknowledgments
The authors would like to acknowledge the contribution of all REACT-CEMI investigators and their site support teams, including, Dr. Ricky Frazer (Velindre Cancer Centre, Cardiff), Dr. Rob Metcalf (The Christie NHS Foundation Trust, Manchester), Dr. Steven Watkins (Queen Elizabeth Hospital Birmingham), Dr. Kate Fife (Addenbrookes Hospital, Cambridge), Dr. Oliver Donnelly (Queen Alexandra Hospital, Portsmouth), Dr. Stefano Schipani (The Beatson West of Scotland Cancer Centre) and Dr Kent Yip (Ipswich Hospital). Additionally, they would like to thank OPEN VIE (doing business as OPEN Health), a healthcare consultancy company (funded by Sanofi), for their help and involvement in the study design, study implementation, data collection, data analysis, interpretation, medical writing support and submission of this manuscript. Lastly, the authors would like to thank the patients whose data were included in this study and their families.
Conflict of interest
Author AC: Speaker fees and meeting support from Astellas, Bayer, Janssen, Sanofi, Eusa, Pfizer, Eisai Ltd. Advisory boards for Regeneron, Merck, Amgen and Sanofi. Author GS: Speaker fees received from Bristol Myers Squibb, Sanofi and AstraZeneca. Meeting support and honoraria received from Takeda, Sanofi, Bristol Myers Squibb and AstraZeneca. Author HS: Speaker fees received from Bristol Myers Squibb, Merck, Sharp & Dohme, Sanofi and Novartis. Meeting support from Bristol Myers Squibb and Merck, Sharp & Dohme. Honoraria/advisory boards for Bristol Myers Squibb, Merck, Sharp & Dohme, Regeneron, Novartis, Immunocore and CDR-Life. Author EO was employed by the company OPEN VIE doing business as OPEN Health. Authors PD, JL-B, and SK were employed by the company Sanofi at the time of the study.
The authors declare that this study received funding from Sanofi. The funder had the following involvement in the study: Study conceptualization, data interpretation, supported manuscript draft writing and review/editing. The funder was not involved in formal data analysis or visualization.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1408667/full#supplementary-material
References
1. Galbiati D, Cavalieri S, Alfieri S, Resteghini C, Bergamini C, Orlandi E, et al. Activity of platinum and cetuximab in cutaneous squamous cell cancer not amenable to curative treatment. Drugs Context. (2019) 8:212611. doi: 10.7573/17404398
2. Foote MC, McGrath M, Guminski A, Hughes BGM, Meakin J, Thomson D, et al. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann Oncol. (2014) 25:2047–52. doi: 10.1093/annonc/mdu368
3. Samstein RM, Ho AL, Lee NY, Barker CA. Locally advanced and unresectable cutaneous squamous cell carcinoma: outcomes of concurrent cetuximab and radiotherapy. J Skin Cancer. (2014) 2014:284582. doi: 10.1155/2014/284582
4. Burova E, Hermann A, Waite J, Potocky T, Lai V, Hong S, et al. Characterization of the anti-PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. (2017) 16:861–70. doi: 10.1158/1535-7163.MCT-16-0665
5. Wang Y, Gu T, Tian X, Li W, Zhao R, Yang W, et al. A small molecule antagonist of PD-1/PD-L1 interactions acts as an immune checkpoint inhibitor for NSCLC and melanoma immunotherapy. Front Immunol. (2021) 12:654463. doi: 10.3389/fimmu.2021.654463
6. Libtayo. European Medicines Agency. (2023). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/libtayo.
7. Cemiplimab for treating metastatic or locally advanced cutaneous squamous cell carcinoma (TA592). National Institute for Health and Care Excellence. (2019). Available at: https://www.nice.org.uk/guidance/ta592.
8. Cemiplimab for treating advanced cutaneous squamous cell carcinoma. National Institute for Health and Care Excellence. (2022). Available at: https://www.nice.org.uk/guidance/ta802.
9. Scottish Medicines Consortium. cemiplimab (Libtayo). Available online at: https://www.scottishmedicines.org.uk/medicines-advice/cemiplimab-libtayo-full-smc2216/.
10. Ahmed SR, Petersen E, Patel R, Migden MR. Cemiplimab-rwlc as first and only treatment for advanced cutaneous squamous cell carcinoma. Expert Rev Clin Pharmacol. (2019) 12:947–51. doi: 10.1080/17512433.2019.1665026
11. Boutros A, Cecchi F, Tanda ET, Croce E, Gili R, Arecco L, et al. Immunotherapy for the treatment of cutaneous squamous cell carcinoma. Front Oncol. (2021) 11:733917. doi: 10.3389/fonc.2021.733917
12. Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. (2020) 8:e000775. doi: 10.1136/jitc-2020-000775
13. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. (2018) 379:341–51. doi: 10.1056/NEJMoa1805131
14. Migden MR, Schmults C, Khushanlani N. 814P Phase II study of cemiplimab in patients with advanced cutaneous squamous cell carcinoma (CSCC): Final analysis from EMPOWER-CSCC-1 groups 1, 2 and 3. Ann Oncol. (2022) 33:S918–9. doi: 10.1016/j.annonc.2022.07.940
15. Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc. (2018) 11:295–304. doi: 10.2147/JMDH
16. Peris K, Piccerillo A, Del Regno L, Di Stefani A. Treatment approaches of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereology. (2022) 36:19–22. doi: 10.1111/jdv.17400
17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
18. LIBTAYO 350 mg concentrate for solution for infusion - Summary of Product Characteristics (SmPC) - (emc). Available online at: https://www.medicines.org.uk/emc/product/10438.
19. Challapalli A, Watkins S, Cogill G, Stewart G, Ellis S, Sykes A, et al. Cemiplimab in advanced cutaneous squamous cell carcinoma: the UK experience from the Named Patient Scheme. J Eur Acad Dermatol Venereol. (2022) 36:e590–2. doi: 10.1111/jdv.18082
20. Hober C, Fredeau L, Pham-Ledard A, Boubaya M, Herms F, Celerier P, et al. Cemiplimab for locally advanced and metastatic cutaneous squamous-cell carcinomas: Real-life experience from the french CAREPI study group. Cancers (Basel). (2021) 13:3547. doi: 10.3390/cancers13143547
21. Baggi A, Quaglino P, Rubatto M, Depenni R, Guida M, Ascierto PA, et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur J Cancer. (2021) 157:250–8. doi: 10.1016/j.ejca.2021.08.018
22. Koch Hein EC, Vilbert M, Hirsch I, Fernando Ribeiro M, Muniz TP, Fournier C, et al. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: Real-world experience from a Canadian comprehensive cancer centre. Cancers (Basel). (2023) 15:4312. doi: 10.3390/cancers15174312
23. McLean L, Lim A, Bressel M, Lee J, Ladwa R, Guminski A, et al. (2023)., in: Immunotherapy for locally advanced and metastatic cutaneous squamous-cell carcinomas in a real-world Australian cohort, (Rome, Italy). p. 267. Available at: https://eado2023.com/wp-content/uploads/2023/04/Abstract-Band_EADO2023_Stand-21-04-2023-kl.pdf.
24. Cariti C, Merli M, Avallone G, Rubatto M, Marra E, Fava P, et al. Melanoma management during the COVID-19 pandemic emergency: A literature review and single-center experience. Cancers (Basel). (2021) 13:6071. doi: 10.3390/cancers13236071
25. Jović M, Marinković M, Suđecki B, Jurišić M, Bukumirić Z, Jovanović M, et al. COVID-19 and cutaneous squamous cell carcinoma-impact of the pandemic on unequal access to healthcare. Healthcare (Basel). (2023) 11:1994. doi: 10.3390/healthcare11141994
26. Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. (2001) 344:975–83. doi: 10.1056/NEJM200103293441306
Keywords: cemiplimab, CSCC, real-world, skin cancer, United Kingdom
Citation: Challapalli A, Stewart G, Shaw H, Davies PJ, Lopez-Baez JC, Ottley EC and Kelly S (2024) Real-world evidence study on the early use of cemiplimab in the UK: REACT-CEMI (Real World evidence of advanced CSCC treatment with cemiplimab). Front. Immunol. 15:1408667. doi: 10.3389/fimmu.2024.1408667
Received: 28 March 2024; Accepted: 17 June 2024;
Published: 12 July 2024.
Edited by:
Selma Ugurel, Essen University Hospital, GermanyReviewed by:
Thilo Gambichler, University Hospital of the Ruhr, GermanyAnn W. Silk, Dana–Farber Cancer Institute, United States
Tess Van Meerhaeghe, Université Libre de Bruxelles, Belgium
Copyright © 2024 Challapalli, Stewart, Shaw, Davies, Lopez-Baez, Ottley and Kelly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amarnath Challapalli, YW1hcm5hdGguY2hhbGxhcGFsbGlAdWhidy5uaHMudWs=
 Amarnath Challapalli
Amarnath Challapalli Grant Stewart2
Grant Stewart2 Edward C. Ottley
Edward C. Ottley