
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 24 July 2024
Sec. Molecular Innate Immunity
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1405463
Introduction: Patients with systemic lupus erythematosus are prone to develop cardiovascular disease (CVD), and have increased morbidity and mortality.
Methods: We conducted a retrospective analysis on lupus nephritis patients to assess the occurrence and predictors of major adverse cardiovascular events (MACE). Data were collected from patients who underwent kidney biopsy between 2005 and 2020. Statistical analysis was performed to unveil correlations.
Results: 91 patients were analyzed in this period, with a mean age of 37.3 ± 12.3 years and 86% being female. The mean follow-up time was 62 ± 48 months. 15.38% of the patients underwent at least one MACE. Two patients deceased of CVD. Increased age (35.81 ± 11.14 vs 45.5 ± 15.11 years, p=0.012) entailed a higher occurrence of MACEs. Neutrophil count (5.15 ± 2.83 vs 7.3 ± 2.99 Giga/L, p=0.001) was higher, whereas diastolic blood pressure (DBP) was lower (89.51 ± 10.96 vs 78.43 ± 6.9 mmHg, p<0.001) at the time of the biopsy in patients with MACE. Age, neutrophil count, and DBP proved to be independent predictors of MACEs. We propose a new model (CANDE – Cardiovascular risk based on Age, Neutrophil count, and Diastolic blood pressure Estimation score) calculated from these variables, which predicts the probability of MACE occurrence.
Conclusion: This study underscores the importance of actively screening for cardiovascular risks in this vulnerable patient population. Age, neutrophil count, and diastolic blood pressure have been established as independent risk factors for MACE in lupus nephritis. The CANDE score derived from these parameters may serve as a prompt, cost-effective, and easily accessible estimation tool for assessing the likelihood of major adverse cardiovascular risk. These findings emphasize the necessity for comprehensive management strategies addressing both immune dysregulation and cardiovascular risk factors in systemic lupus erythematosus to mitigate adverse outcomes.
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by multiorgan involvement due to the dysregulation of both the innate and the adaptive immune systems. Renal involvement occurs in 30-50% and contributes vastly to mortality and morbidity. Cardiovascular (CV) risk increases dramatically in SLE and especially in patients with lupus nephritis. It is partly due to the increased level of inflammatory mediators and the accelerated progression of the traditional Framingham risk factors as well as the side effects of the immunosuppressive therapy (1). However, even with the elimination of the traditional risk factors, a drastic excess cardiovascular risk remains compared to the general population (2). This directs the attention towards searching for novel and more SLE-specific mechanisms and predictors of cardiovascular (CV) disease risk.
Our study aimed to find variables that would improve our understanding of the cardiovascular morbidity of patients with lupus nephritis. In this paper, we describe a novel risk prediction model that can be calculated at the time of the kidney biopsy for major cardiovascular events in lupus nephritis.
The study cohort comprised Caucasian individuals aged 18 years and above who underwent renal biopsy between 2005 and 2020 at a tertiary-care hospital in the Department of Internal Medicine and Oncology, Semmelweis University, Hungary. The histological processing of specimens was conducted in the Department of Pathology, Forensic and Insurance Medicine, Semmelweis University. The diagnosis of SLE was established based on the 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) after 2019, the 2012 Criteria for Systemic Lupus Erythematosus (SLICC) criteria between 2012 and 2019, and on the 1997 American College of Rheumatology (ACR) between 1997 and 2012 (3–5).
We collected comprehensive clinical data of the patients retrospectively. Patients underwent a physical examination and blood pressure determination. Our evaluation extended to the participants’ laboratory parameters, medication regimens, age, duration since the diagnosis of lupus, concurrent comorbidities, CV disease history, echocardiographic and electrocardiogram values, and smoking history.
The laboratory tests for immune serology were taken a maximum of three months preceding the biopsy, and regular laboratory parameters were assessed at the time of the biopsy. Immunosuppressive therapy was evaluated at the time of the biopsy and throughout the induction and maintenance phases. Cardiovascular medication use was registered contemporaneously with the biopsy. Blood pressure measurement was not standardized; it was performed using various automatic blood pressure monitors during hospital admission for the biopsy. Cigarette smoking status was determined through self-report.
Major adverse CV events (MACE) were defined as the composite of nonfatal myocardial infarction, hospitalization due to heart failure, coronary revascularization, stroke, and cardiovascular death. We evaluated MACE in the medical history from the time of the diagnosis of lupus and from the time of the biopsy.
Data were stored anonymized in an Excel (Microsoft, version 2016) file. Statistical analysis was conducted with IBM SPSS Statistics v28 software. Figures were formulated in GraphPad Prism 9.0.0 and IBM SPSS Statistics. To compare variables by MACE status, we used Chi-square tests or Fisher’s exact tests for categorical ones, and Mann-Whitney U-tests for continuous ones. To select variables for the multiple logistic regression, we built individual logistic regression models with each predictor that showed significant differences in univariate comparisons as independent and MACE as dependent variable. Given the relatively low number of participants in our dataset, we used these models to select one variable among interrelated factors for the final model based on the fit of the univariate model and the number of data points included. For the final multivariable prediction model, we included all variables selected using the above-described procedure, and then removed non-significant predictors one-by-one in a stepwise manner. For the final multivariable model, Receiver Operating Characteristic (ROC) curve was plotted, and area under the curve (AUC) was calculated to evaluate the model discrimination. Based on this model, we also developed a risk score using a previously established method to predict cardiovascular events in patients with lupus nephritis (6). Two-tailed p values <0.05 were considered statistically significant.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional and Institutional Committee of Science and Research Ethics of Semmelweis University, Budapest, Hungary (SE RKEB 225/2018). All analyses were performed in accordance with relevant guidelines and regulations, and informed consent was obtained from all subjects and/or their legal guardian(s) for further analyses at the time of the biopsies.
Between 2005 and 2020, 91 adult SLE patients underwent kidney biopsies. The male/female ratio was 14.3%/85.7%, with a mean age of 37.3 ± 12.3 years. The youngest patient was 18 years old, and the eldest patient was 74 years old. The mean follow-up time after the biopsy was 62 ± 48 months. 15.38% (14/91) of the patients suffered at least one major adverse cardiovascular event (MACE) following their lupus diagnosis. Of these, 8.79% (8 out of 91) had such events post-renal biopsy. In total, there were 18 events in 14 patients: three coronary revascularizations, five strokes, six hospitalizations due to heart failure, two acute myocardial infarcts, and two cardiovascular deaths (Table 1). Five patients suffered more than one MACE.
Patients with major adverse cardiovascular events were older (45.50 vs 35.81 years; p=0.012) (Figure 1A), had lower diastolic blood pressure (DBP) (78.42 vs 89.51 mmHg; p<0.001) (Figure 1B), higher leukocyte count (9.07 vs 6.99 Giga/liter; p=0.026) (Figure 1C), and absolute neutrophil count (7.30 vs 5.15 Giga/liter; p=0.01) (Figure 1D, Table 2, Supplementary Table S1). Neither leukocyte count nor the elevation of absolute neutrophil count was associated with steroid administration, or steroid dosage (r=0.097 p=0.375; r=0.110 p=0.315, respectively).
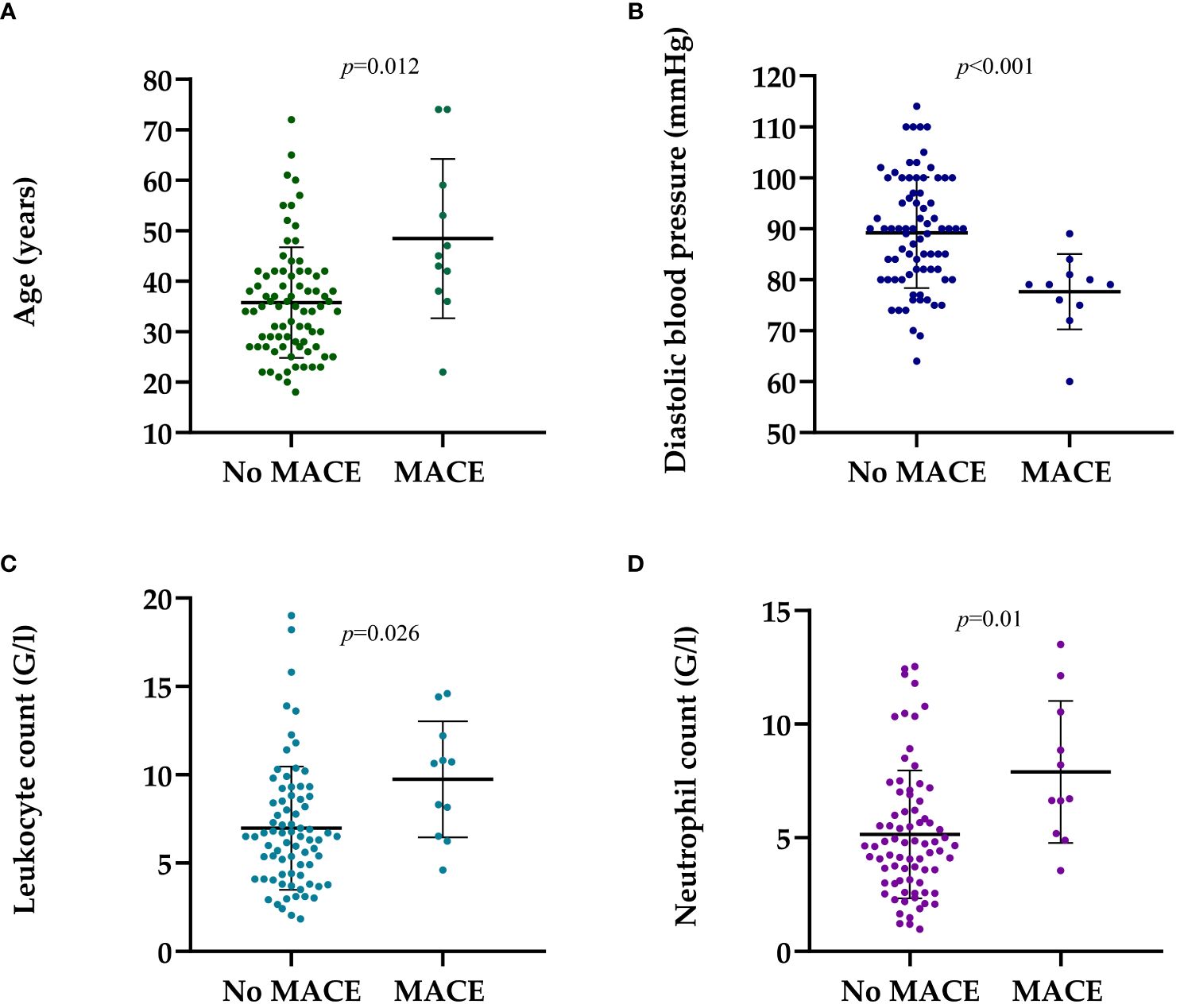
Figure 1 The dot-plots represent the parameters at the time of the kidney biopsy patients who had and had not MACE in medical history. (A) Mean (± SD) age of patients with MACE and no MACE was 45.50 ± 15.11 vs. 35.81 ± 11.74 years, p=0.012, respectively. (B) Mean (± SD) diastolic blood pressure in patients with MACE and no MACE history was 78.42 ± 6.90 vs. 89.51 ± 10.96 mmHg, p<0.001, respectively. (C) Mean (± SD) leukocyte count in patients with MACE and no MACE was 9.07 ± 3.25 vs. 6.99 ± 3.54 G/l, p=0.026, respectively. (D) Mean (± SD) neutrophil count in patients with MACE and no MACE was 7.30 ± 3.11 vs. 5.15 ± 2.85 G/L, p=0.01, respectively. Mann-Whitney U-test was used to analyze the differences between the groups. MACE, major adverse cardiovascular event; G/l, Giga/liter; mmHg, Millimeters of Mercury.
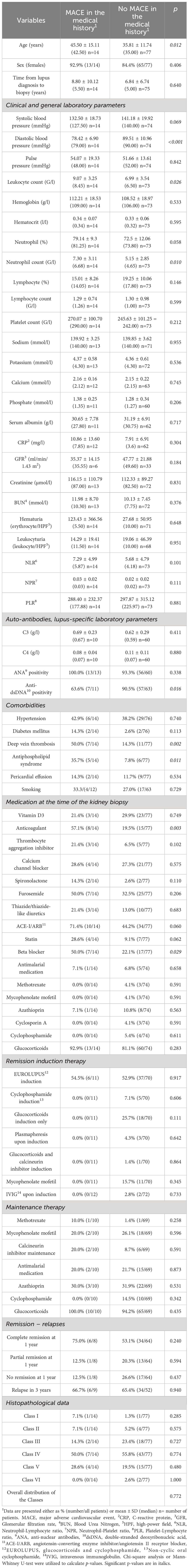
Table 2 Patient characteristics by major adverse cardiovascular event status for variables that have data for at least 75% of participants.
Patients with MACE were more often on anticoagulant (57.1% vs. 19.5%; p=0.003) (Figure 2A), and beta blocker (50.0% vs. 22.1%; p=0.029) medication at the time of renal biopsy (Figure 2B). Antihypertensive and diuretic medication use had no discernible influence on the on MACE risk (Supplementary Table S2).
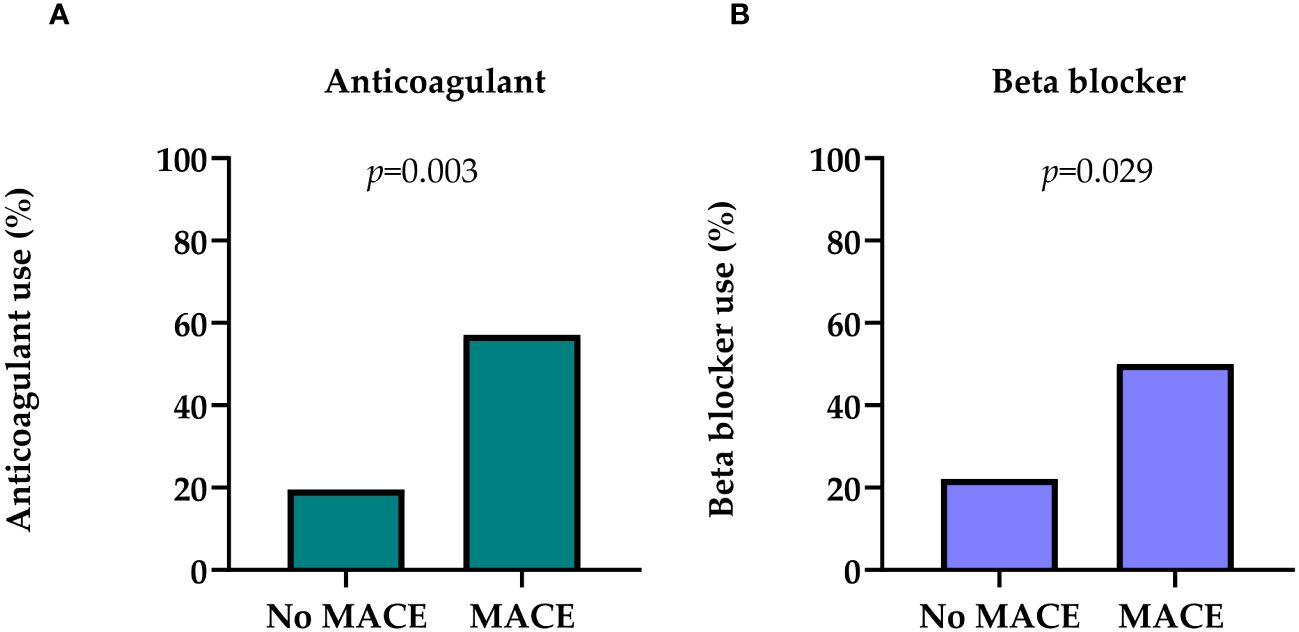
Figure 2 (A) Distribution of anticoagulant use in patients with MACE and no MACE in medical history. (B) Distribution of beta blocker use in patients with MACE and no MACE in the medical history. Chi square test was used to analyze the differences between the groups. MACE, major adverse cardiovascular event.
Patients with MACE less frequently had anti-dsDNA positivity (63.6% vs. 90.5%; p=0.016) (Figure 3A). Anti-dsDNA positivity was associated with lower absolute neutrophil count (5.08 vs 7.44 Giga/liter, p=0.035). Proteinuria did not show statistically significant impact on the occurrence of MACE (p=0.359).
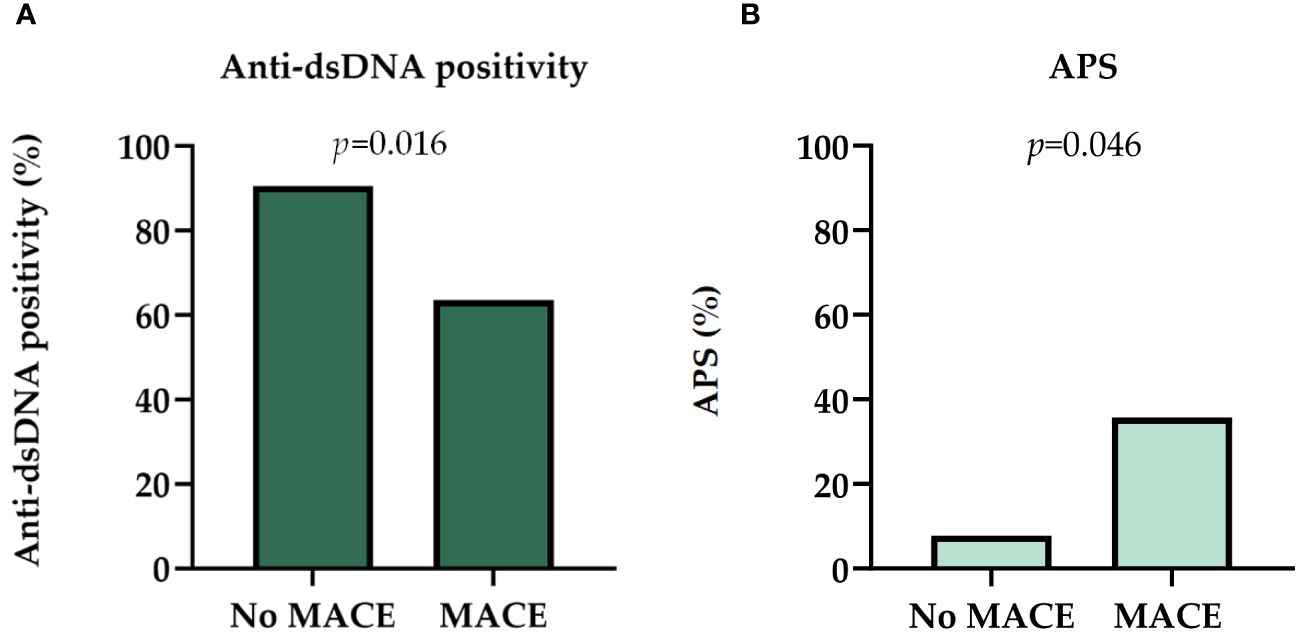
Figure 3 (A) Distribution of anti-dsDNA positivity in patients with MACE and no MACE at the time of the biopsy. (B) Distribution of antiphospholipid syndrome in patients at the time of the biopsy. Chi square test was used to analyze the differences between the groups. APS, antiphospholipid syndrome; dsDNA, double-stranded deoxyribonucleic acid; MACE, major adverse cardiovascular event.
Pulse pressure was non-significantly wider in individuals with MACE (54.07 vs 51.66 mmHg; p=0.842) (Table 2). Antiphospholipid syndrome (APS), as well as deep vein thrombosis in the medical history was more frequently present at the time of kidney biopsy among those with MACE events (35.7% vs. 7.8%; p=0.011; 50.0% vs. 14.3%, p=0.02) (Table 2, Supplementary Table S1, Figure 3B). However, it is important to note that all patients with APS were on anticoagulants, but not all anticoagulated patients had APS (Supplementary Figure S1).
Our analysis revealed no significant association between the occurrence of MACE and remission status (no remission: p=0.953, partial remission: p=0.790, 3-year relapse: p=0.953).
Univariate logistic regression indicated an association between MACE and older age (OR 1.059 per 1 year, p=0.011), lower DBP (OR 0.889 per 1 mmHg, p=0.002), higher absolute neutrophil count (OR 1.248 per 1 G/l, p=0.018), anticoagulant (OR 6.000, p=0.004) and beta blocker use (OR 3.529, p=0.036), absence of anti-dsDNA positivity (OR 0.184, p=0.026), presence of antiphospholipid syndrome (OR 6.574, p=0.007), and deep vein thrombosis (OR 6.000, p=0.004) (Table 3).
The subgroup analysis was constrained by the modest number of patients; however, the following associations may be noted.
Patients with a history of coronary revascularization demonstrated an elevated neutrophil-platelet ratio (0.06 vs 0.02; p=0.02) (Supplementary Table S3). Patients with a stroke were older (56.20 vs. 36.20 years; p=0.017) and had lower DBP (78.00 vs 88.34 mmHg; p=0.018) (Supplementary Table S4). Patients with hospitalization due to heart failure were more frequently smokers (78.0% vs 25.4%; p=0.031) and had higher C-reactive protein level (18.13 vs 7.52 mg/L; p=0.021) (Supplementary Table S5). Myocardial infarction occurred in two cases and two patients died of cardiovascular causes in this period (Supplementary Tables S6, S7).
Among interrelated factors (such as neutrophil count/anti-dsDNA positivity and deep vein thrombosis/antiphospholipid syndrome/anticoagulant use), one variable was selected based on the fit of the univariate model and the number of available data points. For the final multivariable prediction model, we included all variables selected using the above-described procedure (age, DBP, neutrophil count, beta blocker and anticoagulant use). Subsequently, non-significant predictors were systematically removed in a stepwise manner (Supplementary Figure S2). Finally, among the variables that showed a significant association with MACE based on univariate logistic regressions, only lower diastolic blood pressure, higher neutrophil count, and older age at the time of the biopsy remained as independent risk factors for MACE (Table 4). Using these findings, we devised a scoring system (the CANDE score – Cardiovascular risk-based on Age, Neutrophil count, and Diastolic blood pressure Estimation Score) to assess cardiovascular risk in patients with lupus nephritis. This system is designed to predict the probability of major adverse cardiovascular events (MACE) at the time of renal biopsy.

Table 4 Independent risk factors for major adverse cardiovascular events based on multivariable logistic regression that provide the basis of the CANDE (Cardiovascular risk –based on Age, Neutrophil count, and Diastolic blood pressure Estimation) score.
The rounded beta values (coefficients) from the final multivariable logistic regression (Table 4) were used as weighting factors for the calculation of the CANDE score. To simplify the calculation, the effect of age was calculated for 10-year intervals. The CANDE-score is a linear combination of the values of the features and their corresponding weights. By applying logistic regression with the CANDE score as the independent variable, we demonstrated that a 1-point higher CANDE score is associated with a 13.7% increase of MACE risk (p<0.001) (Table 4). The score has showed good calibration (Hosmer-Lemeshow test X2 = 2.322, p=0.970).
The logistic regression model provides a framework for estimating these probabilities to calculate the absolute risk of MACE directly for each patient based on their specific CANDE score (points). By using the intercept (β0) and the coefficient (β), we can transform the individual point scores into probabilities. To facilitate better understanding and practical application, we have formulated a risk assessment table (Figure 4, Supplementary Figure S2).
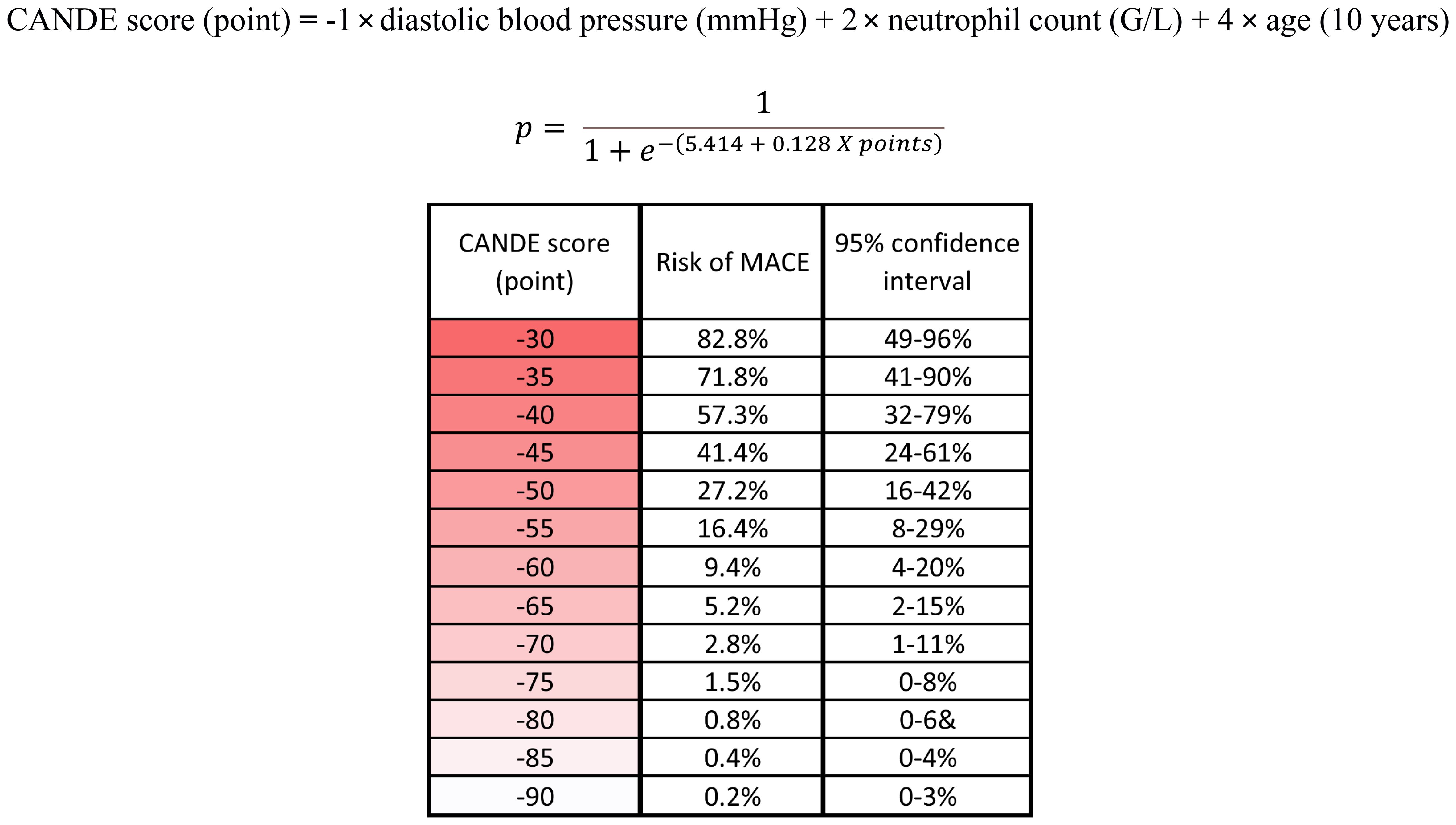
Figure 4 The rounded beta values (coefficients) from the final multivariable logistic regression were used as weighting factors to calculate the CANDE score. The logistic regression model provides a framework for estimating these probabilities to calculate the absolute risk of MACE directly for each patient based on their specific CANDE score (points). The risk assessment table facilitates the practical use of the score. MACE, major adverse cardiovascular event; G/l, Giga/liter; mmHg, Millimeters of Mercury.
CANDE score was applicable either in the group where MACE was examined across the entire medical history (OR 1.137; p<0.001), and in the subset where MACE was observed following the renal biopsy (OR 1.081; p=0.01) (Table 4). The ROC curve analysis found an AUC of 0.866 (95% CI: 0.768-0.965), with a sensitivity of 0.786 and specificity of 0.819 at the optimal cut-off value of -53.73 (Figure 5).
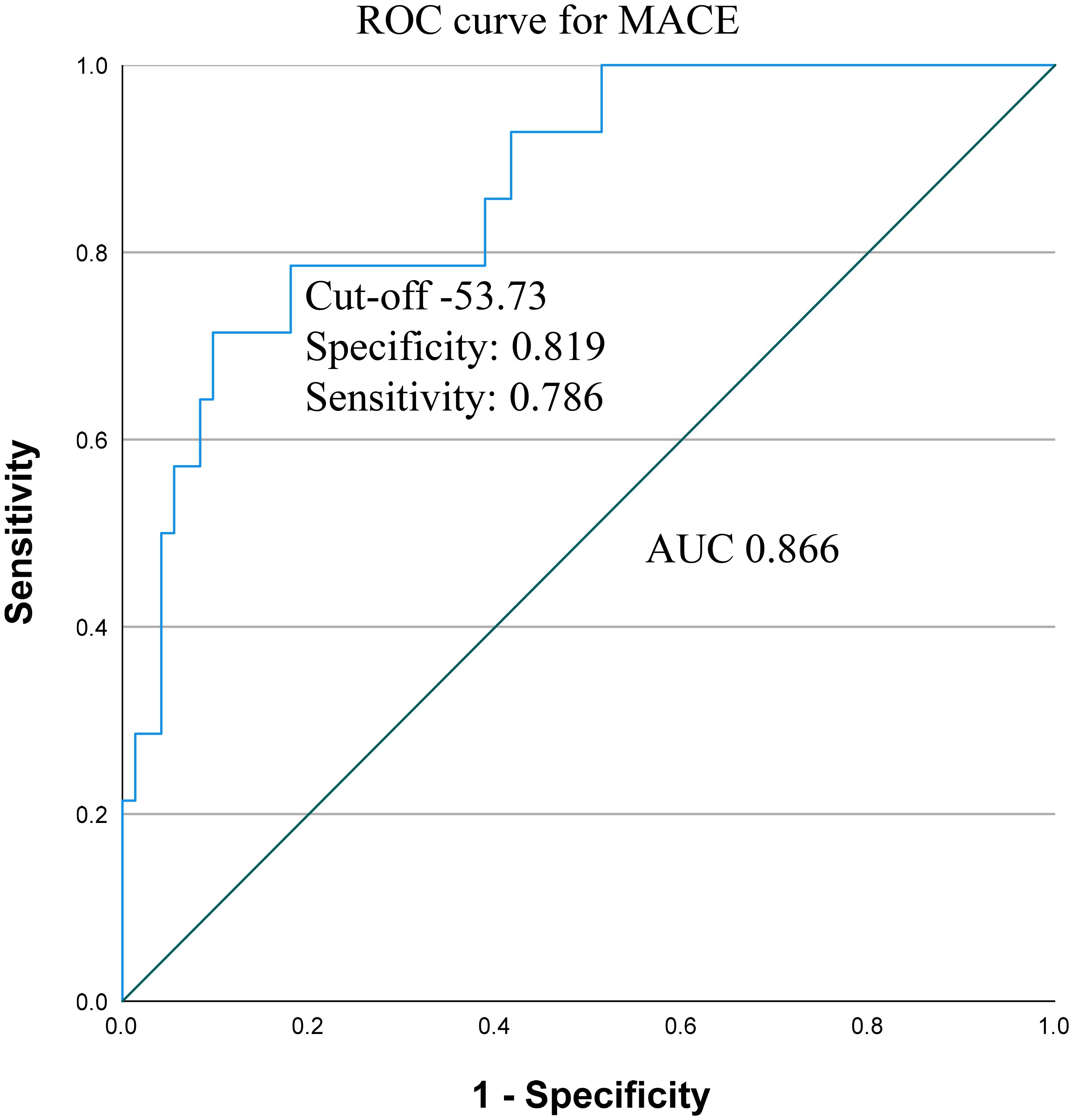
Figure 5 Receiver operating characteristic (ROC) curve and area under the curve (AUC) with cut-off value, sensitivity and specificity for CANDE score predicting MACE. MACE, major adverse cardiovascular event.
Our 16-year retrospective cohort study provides comprehensive insights into CV risk factors associated with lupus nephritis at the time of the biopsy. Lower diastolic blood pressure, higher neutrophil count, and age proved to be independent risk factors for MACEs. Based on a multivariate logistic regression we derived the CANDE score, a tool facilitating the prognostication of MACE in patients diagnosed with lupus nephritis. The CANDE score may become a fast, cheap, and readily available estimation tool at the time of the biopsy to evaluate major adverse cardiovascular risks. This study emphasizes the significance of screening for cardiovascular risks in this particularly susceptible patient population.
While, a conclusion for causality cannot be driven from a prediction model, the main components of our tool are plausibly associated with the risk of CV complications. Diastolic hypotension has been substantiated as an independent risk factor for incident heart failure (7). Whereas cardiovascular risk monotonously increases with higher systolic blood pressure (SBP), the relationship between DBP and cardiovascular diseases has a nonlinear J-shaped association (8). In accordance with findings from NHANES III and the Framingham Heart studies, pulse pressure (PP) tends to rise as DBP decreases during the 6thand 7th decades of life. This phenomenon is attributed to arterial stiffness induced by atherosclerosis (9, 10). The stiffening of arteries leads to diminished elastic recoil, decreased arterial compliance, and a subsequent reduction in DBP. Over time, compromised arterial compliance contributes to increased afterload and an elevation in SBP, thereby further expanding the PP, which is associated with adverse cardiovascular and renal outcomes (11). The consequently greater afterload amplifies myocardial oxygen demand, ultimately culminating in myocardial ischemia and the onset of both systolic and diastolic dysfunction (7).
Moreover, DBP governs left coronary perfusion gradient. During diastole, as the myocardium relaxes, the extravascular compression on the left coronaries is alleviated, allowing them to regain complete patency. The patency of the left coronary arteries is contingent upon aortic diastolic pressure. Consequently, coronary perfusion is determined by the difference between the aortic diastolic blood pressure and the left ventricular end-diastolic pressure (12). A decline in DBP reduces coronary blood flow due to the diminished perfusion gradient, leading to myocardial hypoxia and subsequent impairment of contractile function.
These correlations are particularly evident in lupus nephritis patients who exhibit a heightened susceptibility to atherosclerosis, arterial stiffness, coronary artery diseases, and left ventricular hypertrophy as opposed to the general population or individuals with SLE lacking renal involvement (13).
Despite the absence of isolated diastolic hypotension (with DBP ranging from 60-114 mmHg) or significant PP variations within this study, our findings suggest that the marginally lower DBP observed in patients with MACE may indicate an underlying accelerated atherosclerosis, notwithstanding the relatively young age of these individuals and the patient population at large. This finding also emphasizes the need for comprehensive screening and prevention measures in this patient group.
Atherosclerotic plaques are multifaceted formations of vascular and immune cells, manifesting concomitantly with chronic inflammation. Subclinical atherosclerosis is detected in 25-56% of SLE patients, who also exhibit a substantial progression compared to the general population (10% vs 5% per year) (14). Given that immune dysregulation is the hallmark of SLE, it substantively contributes to atherosclerosis. Our study substantiates the pivotal role played by neutrophils in the occurrence of MACEs.
Innate immune dysregulation, with a particular focus on neutrophil granulocytes, significantly contributes to the pathogenesis of cardiovascular complications in SLE patients. Neutrophil granulocytes are the most abundant and rapidly responsive immune cells in the circulation. While their antimicrobial arsenal is robust, it carries several deleterious consequences, including direct organ damage and the release of autoantigens. Of particular interest are low-density granulocytes (LDGs), an abnormal subset of neutrophils in SLE and present as a highly proinflammatory phenotype. Besides the enhanced inflammatory cytokine contribution to accelerated atherosclerosis, LDG cells are more susceptible to spontaneous neutrophil extracellular trap (NET) formation and mitochondrial reactive oxygen species production (15). Overall, NETosis entails an exceptionally high level of inflammatory mediator release, contributing to atherosclerosis.
NETs may also instigate both arterial and venous thrombotic and thromboembolic events. NETs have the potential to provide a scaffold for thrombocyte aggregation and the consolidation of thrombi. Concurrently, local hypoxia induces the release of endothelial procoagulant factors, intensifying the prothrombotic milieu (16). These findings underscore the critical role of innate immune dysregulation, particularly involving neutrophils and NETs, in driving cardiovascular complications in SLE patients.
Sustained glucocorticoid use increases the risk of cardiovascular diseases, contributing to the emergence of major risk factors, including dyslipidemia, obesity, diabetes, and hypertension. Glucocorticoids exert anti-insulin effects, increasing lipolysis and fatty acid release while impeding the uptake and storage of glucose as glycogen while endorsing gluconeogenesis and glycogenolysis. Glucocorticoids stimulate proteolysis, elevating the concentration of amino acids. Additionally, they diminish the translocation of glucose transporters to the cell surface (17). Ultimately, these metabolic alterations culminate in obesity, especially in visceral adiposity, which is recognized as a significant cardiovascular risk factor (18).
Glucocorticoids contribute to impaired vasodilation, increased contractility, and plasma volume expansion, thereby compromising blood pressure regulation and favoring hypertension, leading to cardiac hypertrophy (19).
Thromboembolic complications are more frequent in glucocorticoid administration. Cortisol amplifies procoagulant factors, hematocrit, and viscosity, leading to endothelial dysfunction. These alterations collectively predispose individuals to a state of hypercoagulability (20). Numerous SLE cohort analyses have indicated a correlation between higher cumulative glucocorticoid doses and greater incidence of cardiovascular events (21–24). Our study was not structured to calculate cumulative steroid and other immunosuppressive (ISU) doses. Thus, we posit that this might be one of the reasons why the use of ISUs did not influence MACE occurrence in our study. Moreover, many subjects in our cohort were undergoing low-dose glucocorticoid treatment both at entry and during the maintenance phase, precluding a control group for meaningful comparison.
Anti-dsDNA positivity is recognized as a cardiovascular risk factor in SLE patients, forging connections to increased inflammatory mediators, endothelial dysfunction, and enhanced atherosclerosis. Furthermore, anti-dsDNA positivity is associated with an augmentation of NET-derived molecules such as cell-free nucleosomes, neutrophil elastase, and myeloperoxidase. Thus, anti-dsDNA positivity is linked to a higher cardiovascular risk in general (25, 26). Despite these previous observations, our study revealed an association wherein anti-dsDNA positivity correlated with fewer MACE cases. Moreover, neutrophil count was higher in the anti-dsDNA negative cases. The latter finding correlates with the preceding studies; anti-dsDNA expedited the rate of the apoptosis in neutrophils (27–29). It is important to note that measurement methods varied over the years, and it prevented us from seeking precise correlations in antibody titers, potentially impacting the results. However, the underpinning of our opposing results still necessitates comprehensive elucidation.
Antiphospholipid syndrome (APS) is a significant predisposing factor in atherosclerosis, myocardial infarction, stroke, and valvular heart disease. Antiphospholipid antibodies bind to endothelial β2-glycoprotein1 receptors (β2-GP1) and exert endothelial dysfunction via various mechanisms. By inhibiting the endothelial nitric oxide synthase, they counteract several endothelial regulatory mechanisms such as leukocyte adhesion, endothelial cell proliferation, vascular permeability, and smooth muscle cell growth. APS antibodies upregulate leukocyte adhesion molecule expression, concurrently inducing endothelin-1 and tissue factor, thereby promoting thrombocyte aggregation (30). Notably, anti-β2-GP1 and anticardiolipin antibodies mediate the uptake of oxidized low-density lipoproteins by macrophages, underscoring a proatherogenic effect (31, 32).
In addition, valvular involvement emerges as a relatively common cardiac manifestation of APS, occurring in 15-30% of the patients. This involvement typically manifests as valvular thickening, dysfunction, and vegetation, predominantly affecting the atrial aspect of the mitral valve or the vascular surface of the aortic valve. Although the precise pathomechanism remains elusive, it is postulated that anti-β2-GP1 targets the valvular endothelial β2-GP1, eliciting endothelial dysfunction and complement fixation (33).
In conjunction with this evidence, our findings align with the compelling data indicating a higher prevalence of MACE with concurrent APS. This congruence strengthens our current knowledge and highlights the significance of our findings in understanding the broader cardiovascular implications of APS.
Although cardiovascular risk increases vastly in patients with SLE, particularly those with renal involvement, there currently exists no specific guidelines for primary cardiovascular prevention tailored to SLE patients except for APS [ (34–36). General considerations include crucial aspects such as smoking cessation, diabetes mellitus control, and pursuing an active life (37). The administration of statin therapy in SLE patients is advised to adhere to the guidelines established by the American Heart Association and American College of Cardiology’s guidelines (38). Patients maintaining a blood pressure of 130-139/80-89 mmHg or above over a span of two years face a significantly higher chance of atherosclerosis compared to their normotensive counterparts (39). Although there is no specific recommendation for an antihypertensive regimen tailored to lupus patients, angiotensin convertase enzyme inhibitors (ACE-I) are generally used in SLE patients. The LUMINA study suggested that ACE-Is delayed the onset of renal complications and decreased the activity in SLE, potentially serving as a means of primary prevention (40). Furthermore, ACE-I effectively reduces proteinuria, a pivotal cardiovascular risk factor (41). Consequently, the European Alliance of Associations for Rheumatology (EULAR) and European Renal Association (ERA) advocate the implementation of renin-angiotensin-aldosterone blockade, irrespective of lupus nephritis (36, 37, 42). It is noteworthy, however, that in our study only 50.6% of the patients with a confirmed SLE were on ACE-I/ARB at the time of the biopsy, while 9.8% of them with hypertension received no ACE-I/ARB. These findings challenge the alignment of current practices with recommendations and suggest a gap between idealized guidelines and real-world clinical scenarios.
Despite the existence of well-defined risk factors in SLE, the efficacy of primary prevention in this patient cohort is rendered inefficient for several reasons. Given that SLE patients are predominantly of a younger demographic, the conventional risk factors are frequently disregarded and neglected. The lack of testing for established cardiovascular risk factors (e.g., LDL-cholesterol, BMI, diabetes) further highlights the general unawareness within this population. Additionally, there is a shortage of comprehensive literature reviewing specific preventive measures, and guidelines fail to encompass the entirety of the interdisciplinary facets of this disease. This inadequacy is exacerbated by the fact that most landmark trials exclude lupus nephritis patients from the studies. Furthermore, this deficiency is mirrored in the clinical approach, where physicians often gravitate towards addressing monodisciplinary or urgent multidisciplinary concerns, forgetting about long-term, non-specialized issues.
Many established cardiovascular risk assessment tools, such as Framingham Risk Score, SCORE, and Atherosclerotic Cardiovascular Disease (ASCVD) Score, consider predominantly traditional risk elements, neglecting the nuanced impact of a proinflammatory cytokine burden or the extended steroid use. In contrast, the QRISK3 calculator forecasts cardiovascular risk in SLE patients more accurately by incorporating the interplay of both traditional and non-traditional CV risk factors, such as chronic kidney disease and SLE, into consideration (43). The CANDE score is the first lupus nephritis-focused CV risk calculator among these tools. Its unique attribute lies in its applicability at the time of the biopsy, requiring expeditious clinical assessment and an easily accessible laboratory test to prognosticate the potential incidence of major adverse cardiovascular events.
The limitations of our study need to be acknowledged. The analysis confined to a modest sized patient cohort, intrinsically constrained the study’s robustness. While our scrutiny focused on patients undergoing kidney biopsy in our center, a portion of their subsequent care transpired in external institutions, consequently limiting the scope of accessible data. This constraint was further exacerbated by the retrospective nature of our data collection and the single-center focus. Recognizing the impact of race on SLE severity, regrettably, the population of Hungary did not provide an encompassing analysis, it was limited only to Caucasian patients.
Yet, after its external validation, our newly proposed risk assessment score may emerge as an inexpensive and easily accessible tool for patients with lupus nephritis. Hereby we advocate fellow researchers to undertake independent validation studies, to strengthen and enhance the utility of the scoring system in clinical practice.
This study underscores the importance of actively screening for cardiovascular risks within this notably vulnerable patient population. Age, neutrophil count, and diastolic blood pressure have been established as independent risk factors for MACE in lupus nephritis. The CANDE score derived from these parameters, after external validation, may serve as a prompt, cost-effective, and readily accessible estimation tool at the time of the biopsy for assessing the likelihood of major adverse cardiovascular risk.
The data analyzed in this study is subject to the following licenses/restrictions: The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons. Requests to access these datasets should be directed to NL, bGVkby5ub3JhQHNlbW1lbHdlaXMuaHU=.
The studies involving humans were approved by Regional and Institutional Committee of Science and Research Ethics of Semmelweis University, Budapest, Hungary. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
AM: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. MJ: Investigation, Writing – review & editing. KB: Investigation, Writing – review & editing. AGT: Formal analysis, Writing – review & editing. AT: Resources, Writing – review & editing. NL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. NL and this work were supported by the National Research, Development and Innovation Office, Hungary. Grant number: FK_142911. The open-access publication was supported by Semmelweis University, Budapest, Hungary. AGT was supported by the UK Medical Research Council (S011676), the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund (2021 Thematic Excellence Programme funding scheme, TKP2021-NKTA-47), and the Horizon Europe Framework Programme (HORIZON-HLTH 2023-TOOL-05-03/101136305). The sponsors had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
We would like to thank the Department of Pathology, Forensic and Insurance Medicine, Semmelweis University, especially Deján Dobi, Attila Fintha, and Magdolna Kardos for the evaluation of the kidney biopsy samples during this period.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1405463/full#supplementary-material
1. Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev. (2013) 9:15–9. doi: 10.2174/157340313805076304
2. Skamra C, Ramsey-Goldman R. Management of cardiovascular complications in systemic lupus erythematosus. Int J Clin Rheumtol. (2010) 5:75–100. doi: 10.2217/ijr.09.73
3. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheumatol. (1997) 40:1725. doi: 10.1002/art.1780400928
4. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:1400–12. doi: 10.1002/art.40930
5. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2012) 64:2677–86. doi: 10.1002/art.34473
6. Sullivan LM, Massaro JM, D'Agostino RB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. (2004) 23:1631–60. doi: 10.1002/sim.1742
7. Guichard JL, Desai RV, Ahmed MI, Mujib M, Fonarow GC, Feller MA, et al. Isolated diastolic hypotension and incident heart failure in older adults. Hypertension. (2011) 58:895–901. doi: 10.1161/HYPERTENSIONAHA.111.178178
8. Birrane JP, Foschi M, Sacco S, McEvoy JW. Another nail in the coffin of causality for the diastolic blood pressure J curve. Hypertension. (2022) 79:794–7. doi: 10.1161/HYPERTENSIONAHA.122.18997
9. Burt VL, Harris T. The third National Health and Nutrition Examination Survey: contributing data on aging and health. Gerontologist. (1994) 34:486–90. doi: 10.1093/geront/34.4.486
10. Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. (1999) 100:354–60. doi: 10.1161/01.CIR.100.4.354
11. Tang KS, Medeiros ED, Shah AD. Wide pulse pressure: A clinical review. J Clin Hypertens (Greenwich). (2020) 22:1960–7. doi: 10.1111/jch.14051
12. Ramanathan T, Skinner H. Coronary blood flow. Continuing Educ Anaesthesia Crit Care Pain. (2005) 5:61–4. doi: 10.1093/bjaceaccp/mki012
13. Sun EY, Alvarez C, Sheikh SZ. Association of lupus nephritis with coronary artery disease by ISN/RPS classification: results from a large real-world lupus population. ACR Open Rheumatol. (2019) 1:244–50. doi: 10.1002/acr2.1035
14. Roman MJ, Crow MK, Lockshin MD, Devereux RB, Paget SA, Sammaritano L, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheumatol. (2007) 56:3412–9. doi: 10.1002/art.22924
15. Juha M, Molnár A, Jakus Z, Ledó N. NETosis: an emerging therapeutic target in renal diseases. Review. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1253667
16. Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. (2014) 66:2532–44. doi: 10.1002/art.38703
17. Di Dalmazi G, Pagotto U, Pasquali R, Vicennati V. Glucocorticoids and type 2 diabetes: from physiology to pathology. J Nutr Metab. (2012) 2012:525093. doi: 10.1155/2012/525093
18. Ruiz-Castell M, Samouda H, Bocquet V, Fagherazzi G, Stranges S, Huiart L. Estimated visceral adiposity is associated with risk of cardiometabolic conditions in a population based study. Sci Rep. (2021) 11:9121. doi: 10.1038/s41598-021-88587-9
19. Liu B, Zhang TN, Knight JK, Goodwin JE. The glucocorticoid receptor in cardiovascular health and disease. Cells. (2019) 8. doi: 10.3390/cells8101227
20. Coelho MC, Santos CV, Vieira Neto L, Gadelha MR. Adverse effects of glucocorticoids: coagulopathy. Eur J Endocrinol. (2015) 173:M11–21. doi: 10.1530/EJE-15-0198
21. Jung JY, Kim HA, Lee HY, Suh CH. Body mass index and glucocorticoid dose contribute to subclinical atherosclerosis in Korean patients with systemic lupus erythematosus: A prospective 4 year follow-up study. Int J Rheum Dis. (2019) 22:1410–8. doi: 10.1111/1756-185X.13588
22. Ajeganova S, Gustafsson T, Lindberg L, Hafström I, Frostegård J. Similar progression of carotid intima-media thickness in 7-year surveillance of patients with mild SLE and controls, but this progression is still promoted by dyslipidaemia, lower HDL levels, hypertension, history of lupus nephritis and a higher prednisolone usage in patients. Lupus Sci Med. (2020) 7:e000362. doi: 10.1136/lupus-2020-eurolupus.201
23. Haque S, Gordon C, Isenberg D, Rahman A, Lanyon P, Bell A, et al. Risk factors for clinical coronary heart disease in systemic lupus erythematosus: the lupus and atherosclerosis evaluation of risk (LASER) study. J Rheumatol. (2010) 37:322–9. doi: 10.3899/jrheum.090306
24. Svenungsson E, Jensen-Urstad K, Heimbürger M, Silveira A, Hamsten A, de Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. (2001) 104:1887–93. doi: 10.1161/hc4101.097518
25. Patiño-Trives AM, Pérez-Sánchez C, Pérez-Sánchez L, Luque-Tévar M, Ábalos-Aguilera MC, Alcaide-Ruggiero L, et al. Anti-dsDNA antibodies increase the cardiovascular risk in systemic lupus erythematosus promoting a distinctive immune and vascular activation. Arterioscler Thromb Vasc Biol. (2021) 41:2417–30. doi: 10.1161/ATVBAHA.121.315928
26. Langseth MS, Helseth R, Ritschel V, Hansen CH, Andersen GØ, Eritsland J, et al. Double-stranded DNA and NETs components in relation to clinical outcome after ST-elevation myocardial infarction. Sci Rep. (2020) 10:5007. doi: 10.1038/s41598-020-61971-7
27. Armstrong DJ, Crockard AD, Wisdom BG, Whitehead EM, Bell AL. Accelerated apoptosis in SLE neutrophils cultured with anti-dsDNA antibody isolated from SLE patient serum: a pilot study. Rheumatol Int. (2006) 27:153–6. doi: 10.1007/s00296-006-0219-z
28. Kozyr AV, Sashchenko LP, Kolesnikov AV, Zelenova NA, Khaidukov SV, Ignatova AN, et al. Anti-DNA autoantibodies reveal toxicity to tumor cell lines. Immunol Lett. (2002) 80:41–7. doi: 10.1016/S0165-2478(01)00308-X
29. Hsieh SC, Sun KH, Tsai CY, Tsai YY, Tsai ST, Huang DF, et al. Monoclonal anti-double stranded DNA antibody is a leucocyte-binding protein to up-regulate interleukin-8 gene expression and elicit apoptosis of normal human polymorphonuclear neutrophils. Rheumatol (Oxford). (2001) 40:851–8. doi: 10.1093/rheumatology/40.8.851
30. Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J Clin Invest. (2011) 121:120–31. doi: 10.1172/JCI39828
31. Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of beta 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin Exp Immunol. (1997) 107:569–73. doi: 10.1046/j.1365-2249.1997.d01-948.x
32. Kobayashi K, Matsuura E, Liu Q, Furukawa J, Kaihara K, Inagaki J, et al. A specific ligand for beta(2)-glycoprotein I mediates autoantibody-dependent uptake of oxidized low density lipoprotein by macrophages. J Lipid Res. (2001) 42:697–709. doi: 10.1016/S0022-2275(20)31631-X
33. Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. Antiphospholipid syndrome: role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J Am Coll Cardiol. (2017) 69:2317–30. doi: 10.1016/j.jacc.2017.02.058
34. Drosos GC, Vedder D, Houben E, Boekel L, Atzeni F, Badreh S, et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. (2022) 81:768–79. doi: 10.1136/annrheumdis-2021-221733
35. Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. (2019) 78:1296–304. doi: 10.1136/annrheumdis-2019-215213
36. Avasare R, Drexler Y, Caster DJ, Mitrofanova A, Jefferson JA. Management of lupus nephritis: new treatments and updated guidelines. Kidney360. (2023) 4:1503–11. doi: 10.34067/KID.0000000000000230
37. Rovin BH, Ayoub IM, Chan TM, Liu Z-H, Mejía-Vilet JM, Floege J. KDIGO 2024 clinical practice guideline for the management of LUPUS NEPHRITIS. Kidney Int. (2024) 105:S1–s69. doi: 10.1016/j.kint.2023.09.002
38. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2018) 73:3168–209. doi: 10.1016/j.jacc.2018.11.002
39. Tselios K, Gladman DD, Su J, Urowitz M. Impact of the new American College of Cardiology/American Heart Association definition of hypertension on atherosclerotic vascular events in systemic lupus erythematosus. Ann Rheum Dis. (2020) 79:612–7. doi: 10.1136/annrheumdis-2019-216764
40. Durán-Barragán S, McGwin G Jr., Vilá LM, Reveille JD, Alarcón GS. Angiotensin-converting enzyme inhibitors delay the occurrence of renal involvement and are associated with a decreased risk of disease activity in patients with systemic lupus erythematosus–results from LUMINA (LIX): a multiethnic US cohort. Rheumatol (Oxford). (2008) 47:1093–6. doi: 10.1093/rheumatology/ken208
41. Agrawal V, Marinescu V, Agarwal M, McCullough PA. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. (2009) 6:301–11. doi: 10.1038/nrcardio.2009.11
42. Fanouriakis A, Kostopoulou M, Cheema K, Anders H-J, Aringer M, Bajema I, et al. Update of the joint european league against rheumatism and european renal association-european dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. (2019) 79:713–23. doi: 10.1136/annrheumdis-2020-216924
Keywords: kidney biopsy, cardiovascular risk, lupus nephritis, prediction tool, major adverse cardiovascular event, systemic lupus erythematosus
Citation: Molnár A, Juha M, Bulajcsík K, Tabák ÁG, Tislér A and Ledó N (2024) Proposal of a novel cardiovascular risk prediction score in lupus nephritis. Front. Immunol. 15:1405463. doi: 10.3389/fimmu.2024.1405463
Received: 22 April 2024; Accepted: 08 July 2024;
Published: 24 July 2024.
Edited by:
Reza Akbarzadeh, University of Lübeck, GermanyCopyright © 2024 Molnár, Juha, Bulajcsík, Tabák, Tislér and Ledó. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nóra Ledó, bGVkby5ub3JhQHNlbW1lbHdlaXMuaHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.