- 1College of Bee Science and Biomedicine, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China
- 2National & Local United Engineering Laboratory of Natural Biotoxin, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China
- 3Apitherapy Research Institute of Fujian Agriculture and Forestry University, Fuzhou, Fujian, China
- 4Bee Pollination and Product Safety Research Laboratory, Apiculture Science Institute of Jilin Province, Jilin, Jilin, China
- 5Bee Research Institute, Heilongjiang Academy of Agricultural Sciences, Mudanjiang, Heilongjiang, China
Apis cerana is the original host of Vairimorpha (Nosema) ceranae, a widespread fungal parasite that causes bee nosemosis, which severely threatens the health of bee colonies and the sustainable development of the apiculture industry. To evaluate the impact of V. ceranae infection on A. c. cerana workers, V. ceranae spores were purified and used to inoculate newly emerged workers to evaluate the effects of V. ceranae infection. This was followed by an in-depth investigation of V. ceranae spore load and host sucrose solution consumption. Activities of four major antioxidant enzymes (SOD, PPO, CAT, and GST) were determined. Paraffin sections of the host midgut tissue were prepared and subjected to microscopic observation. The survival rates of V. ceranae-inoculated and uninoculated workers were analyzed. The results showed that spore load gradually increased and peaked at 12 dpi. The consumption of workers in the V. ceranae-inoculated group was extremely significant higher (P < 0.0001) than that of workers in the un-inoculated group. The results of antioxidant enzyme activity were suggestive of positive host defense via catalase (CAT) and glutathione-S-transferase (GST) in the middle stage of infection, as well as the negative fungal impact on superoxide dismutase (SOD) and polyphenol oxidase (PPO) at the whole stage of infection, reflecting the complex host-parasite interaction. Additionally, we observed a disruption in the structure of the host midgut epithelial cells. Moreover, the survival rate of workers in V. ceranae-inoculated groups was nearly always lower than that of workers in the uninoculated groups. These results demonstrate a consistent increase in spore load with the proliferation of V. ceranae, leading to persistent energetic stress and midgut epithelial cell structural damage to the host, ultimately resulting in a shortened lifespan for the host. Our findings enhance the current understanding of the interactions between A. cerana and V. ceranae as well as provide a solid basis for exploring the mechanisms underlying host response and V. ceranae infection.
1 Introduction
Vairimorpha (Nosema) ceranae is a fungal parasite that causes nosemosis, a chronic disease that is frequently observed in bee colonies worldwide (1). In 1996, Fries et al. (2). first identified V. ceranae in A. cerana colonies, and established A. cerana as the original host of this parasite. Since its initial discovery in Europe in 2006, V. ceranae has rapidly spread to other parts of the world, North America, South America, Asia, Africa, and likely other regions as well, likely facilitated by the global trade of bees, bee products, and the movement of beekeeping equipment (3).
V. ceranae is associated with a decline in bee health, reduced colony productivity, and colony collapse disorders (CCD) (4, 5). Over the past two decades, a substantial number of studies have been conducted to investigate the effect of V. ceranae infection on Apis mellifera, demonstrating that fungal invasion negatively influences various aspects of honeybee metabolism, behavior, physiology, and health (6–12). For instance, studies have shown that the V. ceranae infection cause metabolic stress, upregulates genes encoding α-glucosidase and trehalose transport enzymes (9), while downregulating trehalase and Glucose-Methanol-Choline oxidoreductase genes (11). These alterations, as confirmed by proteomics, resulted in reduced levels of energy supply proteins (8). The behavioral consequences of V. ceranae infection in A. mellifera are also noteworthy, as infected bees often display increased hunger and consume more sucrose to compensate for their impaired metabolic state. However, this increased food intake does not fully alleviate the underlying nutritional and energy deficits (6), and accelerated lipid loss in bees suggests that lipids may be mobilized as an emergency energy source (12). V. ceranae infection can damage the midgut epithelial cell structure, inhibit apoptosis and immunosuppression, and shorten the lifespan of A. mellifera workers (7, 10).
Through extensive interactions and long-term co-evolution, A. cerana and V. ceranae have evolved to coexist (13). Previous studies have investigated the effects of V. ceranae infections on A. cerana (14–17). Sinpoo et al. (14) found stronger immune responses and lower loads of Nosema apis and V. ceranae spores in workers of A. cerana compared to A. mellifera, suggesting a higher tolerance of A. cerana against microsporidian infection. Chantaphanwattana et al. (18) reported that V. ceranae proliferated more in A. mellifera, whereas A. cerana exhibited a stronger immune response and lower spore load, indicating a superior defense mechanism of A. cerana at the individual level. By contrasting the similarities and differences in V. ceranae infection of A. cerana and A. mellifera, a deeper understanding of the complex interactions between V. ceranae and bee hosts, as well as the factors responsible for coaptation between A. cerana and V. ceranae will be gained. In addition, novel and valuable insights from the aforementioned comparisons could guide the creation of targeted strategies to alleviate the adverse effects of fungal parasites on different bee species.
A. c. cerana is a bee species that is found in China and is widely used in beekeeping practice in several Asian countries (19). Previously, our team identified the miRNA response and miRNA-regulated network of A. c. cerana workers following V. ceranae invasion (20), and performed a comprehensive investigation of the profiles of highly expressed genes in both the host and microsporidian (21, 22). Recently, our group investigated the immune response of A. c. cerana workers to V. ceranae infection using a transcriptomic investigation and revealed that different cellular and humoral immune responses were used by A. c. cerana and A. mellifera ligustica workers to defend themselves against infection by V. Ceranae (23). Moreover, this study provides an opportunity for a deeper comparison of the effect of the same fungal parasite (V. ceranae) on two different bee species (A. cerana and A. mellifera), which will enrich our understanding of bee nosemosis and facilitate the development of biological drugs for nosemosis control.
Many aspects of the effect of V. ceranae on A. cerana are yet to be explored. Pathogenic infections in insects result in the generation of reactive oxygen species (ROS) that can kill and eliminate pathogens (24). However, excessive production of ROS in vivo induces oxidative stress, predominantly damaging various biological macromolecules, and ultimately resulting in cell death (25). Antioxidant enzymes reduce the oxidative response to balance ROS production and maintain homeostasis (26). Superoxide dismutase (SOD) catalyzes the disproportionation of superoxide to produce hydrogen peroxide and molecular oxygen, whereas catalase (CAT) participates in the decomposition of hydrogen peroxide into water and oxygen (27–29). Glutathione-S-transferase (GST) has been shown to exert function in metabolizing lipid peroxides (27, 30). Polyphenol oxidase (PPO) is an oxidoreductase that uses molecular oxygen to catalyze the oxidation of a wide range of phenolic compounds (31). Insects appear to use PPO in immune responses such as melanization to physically limit the spread of microbes (32). Whether V. ceranae infection affects the activities of major antioxidant enzymes in A. cerana remains largely unknown.
Currently, little is known regarding the effect of V. ceranae infection on A. cerana, hindering the understanding of the mechanisms underlying the interaction between V. ceranae and A. cerana and the development of novel strategies for the control of bee nosemosis. Here, to evaluate the impact of V. ceranae infection on A. c. cerana workers, V. ceranae spores were purified and used to inoculate newly emerged workers to evaluate the effects of V. ceranae infection. This was followed by an in-depth investigation of V. ceranae spore load and host sucrose solution consumption. Activities of four major antioxidant enzymes (SOD, PPO, CAT, and GST) were determined. Paraffin sections of the host midgut tissue were prepared and subjected to microscopic observation. The survival rates of V. ceranae-inoculated and uninoculated workers were analyzed. The findings from this study provide valuable experimental evidence for V. ceranae infection of A. c. cerana workers and contribute to the dissection of the host response, microsporidian infection, and host–microsporidian interaction.
2 Materials and methods
2.1 Spore extraction, artificial inoculation, and honeybee preparation for bioassay experiments
A. c. cerana workers were obtained from colonies in the teaching apiary of the College of Bee Science and Biomedicine, Fujian Agricultural and Forestry University, in October 2020. There were no specific bands amplified from V. apis, V. ceranae, or several viruses, such as SBV (Sacbrood Virus), DWV (Deformed Wing Virus), IAPV (Israeli Acute Paralysis Virus), BQCV (Black Queen Cell Virus), KBV (Kashmir Bee Virus), and CBPV (Chronic Bee Paralysis Virus) based on reverse transcription-polymerase chain reaction (RT-PCR) with specific corresponding primers, that eliminated the interference of pathogens on the samples.
V. ceranae-infected A. c. cerana workers were obtained from an apiary in Minhou, Fuzhou, China. V. ceranae spores were prepared using the discontinuous density gradient Percoll method, as described by Chen et al. (33). Briefly, (1) a total of 200 foragers were collected from an infected colony, the midgut tissues were dissected and then homogenized in distilled water, followed by filtration through four layers of sterile gauze and three cycles of centrifugation at 6000 × g for 5 min; (2) the supernatant was discarded as the spores remained in the sediment, and the resuspended pellet was then purified on a discontinuous Percoll gradient (Solarbio) consisting of 200 μL each of 25%, 50%, 75%, and 100% Percoll solution; (3) the spore suspension was overlaid onto the gradient and centrifuged at 18000 × g for 90 min at 4°C; (4) the spore pellet was isolated using a sterile syringe and then centrifuged again on a discontinuous Percoll gradient. Purified spores were examined by PCR using previously reported primers (34) and then preserved in our laboratory and at the China Common Microbial Strain Preservation and Management Center (Preservation number CGMCC: No. 28110). The spore concentration was determined using a CL kurt counter for the inoculation experiments.
Before artificial inoculation, the prepared microsporidian spores were observed microscopically and examined using PCR with specific primers for V. ceranae (F: 5′-CGGATAAAAGAGTCCGTTACC-3′; R: 5′-TGAGCAGGGTTCTAGGGAT-3′) (17, 20) and V. apis (F: 5′-CCATTGCCGGATAAGAGAGT-3′; R: 5′-CACGCATTGCTGCATCATTGAC-3′), as previously described by Chen et al. (34). The inoculation and rearing of A. c. cerana workers were performed according to a previously established protocol (20). At 24 h after emergence, members of the treatment group (n = 35) were each immobilized and fed 5 μL of 50% (w/v) sucrose solution containing 1 × 106 V. ceranae spores by using a pipette, whereas members of the control group (n = 35) were fed 5 μL of sucrose solution without V. ceranae spores. The treatment (or control) group contained six plastic cages. All workers were reared in an incubator at 34 ± 0.5°C and 60–70% RH (relative humidity). After 24 h, the honeybees were fed a feeder containing 4 mL of 50% (w/v) sucrose solution, and the feeders were replaced daily throughout the experiment. Each cage was carefully checked, and dead honeybees were removed daily. Three biological replicates were used in each experiment.
The prepared workers from V. ceranae- and uninoculated groups underwent spore load measurement (n = 3), antioxidant enzyme activity examination (n = 3), hematoxylin and eosin staining (HE) of paraffin sections (n = 3).
2.2 Measurement of spore load in midguts of the workers
During the V. ceranae infection process, 1–12 days post inoculation (dpi), the spores in the midguts of the workers were extracted every 24 h based on the standard method described by Fries et al. (35) and Naree et al. (16), followed by calculation under an optical microscope (CSOIF, Shanghai, China), according to the procedure described by Cantwell (36). In brief, (1) after inoculation with V. ceranae, a single worker in the treatment group was collected from the cage daily, and their midgut tissues were then dissected using a clean ophthalmic forced and transferred into a sterile 1.5-mL Eppendorf (EP) tube; (2) 100 μL of sterile water was added to the EP tube, and then full grinding with an automatic high throughput tissue grinder (Meibi, Zhejiang, China) was performed; (3) using a micropipette, 20 μL of the aforementioned solution was carefully observed with a hemocytometer plate (Qiujing, Shanghai, China) under an optical microscope (CSOIF, Shanghai, China), after which spores were counted.
2.3 Detection of host average sucrose solution consumption
According to the method described in Section 2.1, separate experimental workers were prepared for the purpose of statistical analysis of sucrose solution consumption and survival rates. The daily average consumption of sucrose solution by each worker in the V. ceranae- and uninoculated groups was analyzed using a previously described method (37, 38). The feeder containing 4 mL of sucrose solution was weighed and the weight was recorded as A. To avoid errors due to repeated changes in feeders, the feeder was weighed every 24 h thereafter and the weight was recorded as B. The sucrose solution in the feeders was replaced daily. The numbers of survivors in both V. ceranae- and un-inoculated groups were counted. The average sucrose solution consumption per worker per day was calculated according to the following formula: (B-A)/number of survivors in the V. ceranae-inoculated group (or uninoculated group). Each biological replicate was performed three times, n = 35.
2.4 Examination of the antioxidant enzyme activity
At 1–12 dpi, three workers were randomly selected from V. ceranae- and un-inoculated groups, the midgut tissues were carefully dissected and then added into a sterile EP tube containing 180 µL of saline followed by fully ground with a high-throughput tissue grinder (MEIBI, Hangzhou, China), respectively. The grinding fluid was centrifugation at 5000 × g for 5 min, and the supernatant was then transferred to a sterile EP tube, stored at −80°C. Following the corresponding instructions, the activities of SOD and PPO were examined using the Insect SOD ELISA Kit (MLBIO, Shanghai, China) and Insect PPO ELISA Kit (MLBIO, Shanghai, China), respectively, whereas those of CAT and GST were determined using the Insect CAT ELISA Kit (MLBIO, Shanghai, China) and Insect GST ELISA Kit (MLBIO, Shanghai, China), respectively. The experiment included three biological replicas. Specific antioxidant enzyme activity was expressed as units of enzyme activity per milligram of protein.
2.5 Paraffin sectioning, HE staining, and microscopic observation of midgut tissues of workers
At 7, 8, 9, and 10 dpi, three workers were randomly selected from V. ceranae- and uninoculated groups, and the midgut tissues were carefully dissected. Next, according to the method described by Xing et al. (23), midgut tissue from one of the workers was fixed with 4% paraformaldehyde (PFA). By using an embedding center (Junjie, Wuhan, China) and a microtome (Leica, Nussloch, Germany), paraffin sections of midgut tissues were stained with HE staining by Shanghai Sangon Biological Engineering Co. Ltd., and then detected under an optical microscope with digital camera (SOPTOP, Shanghai, China).
2.6 Survival rate of workers
Using the same experimental group as in Section 2.3, before replacing the sucrose solution every 24 hours, the survival status of workers in both the V. ceranae-inoculated group and the uninoculated group was recorded, and dead ones were removed. This recording was continued until 20 dpi. Subsequently, the daily survival rates for the both groups were calculated on basis of the formula: (number of living worker bees in the treatment or control group)/105.
2.7 Statistics
Statistical analysis of the data was performed using SPSS software. 21 (IBM, Armonk, NY, USA) and GraphPad Prism 6.0 software (GraphPad, San Diego, CA, USA). To analyze significant differences among multiple groups of data, either One-way ANOVA (followed by Tukey’s post-hoc test), Two-way ANOVA (followed by Bonferroni’s post-hoc test), multiple t tests was performed on the spore load and average sucrose solution consumption, and activity of SOD, PPO, GST and CAT using the SPSS software. Data are presented as the mean ± standard deviation (SD). Tukey’s test and the significant difference letter marker method were used to compare the number of spore loads between the 2 d. The log-rank (Mantel–Cox) test was used to analyze the host survival rate.
3 Results
3.1 Dynamics of V. ceranae spore load in the midguts of A. c. cerana workers
After discontinuous Percoll gradient centrifugation, oval spores with high refraction were observed using an optical microscope (OM) (Figure 1A). In addition, the expected fragment (76 bp) could be amplified from the purified spores with specific primers for V. ceranae but could not be amplified from sterile water and spores with V. apis specific primers (Figure 1B). These results confirmed that the prepared spores were V. ceranae spores.
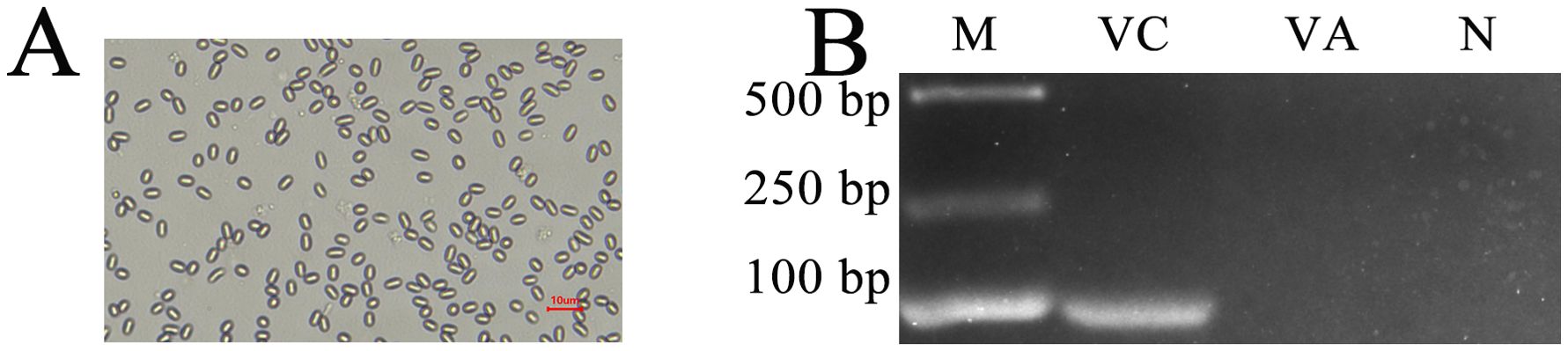
Figure 1. (A) Microscopic observation of V. ceranae spores derived from Percoll discontinuous density centrifugation (400× amplification). (B) Agarose gel electrophoresis for PCR amplification products from purified spores. Lane M: DNA marker; Lane VC: specific primers for V. ceranae; Lane VA: specific primers for V. apis; Lane N: sterile water (negative control).
The spore counting results suggested that the spore load of V. ceranae in the midguts of workers presented an overall elevation trend (Figure 2). In detail, as compared with initial spore load (1 × 106), the V. ceranae spore load in the midguts of the workers continuously increased from 1 dpi (3.5 ± 0.87 × 106) to 4 dpi (52.83 ± 2.75 × 106), from 5 dpi (72 ± 13.08 × 106) to 8 dpi (48.53 ± 11.533 × 107), and from 9 dpi (42.68 ± 9.42 × 107) to 12 dpi (10.51 ± 0.83 × 108) (Figure 2); however, the spore load was decreased from 8 to 9 dpi. In addition, the quantity of spores reached the peak at 12 dpi (Figure 2).
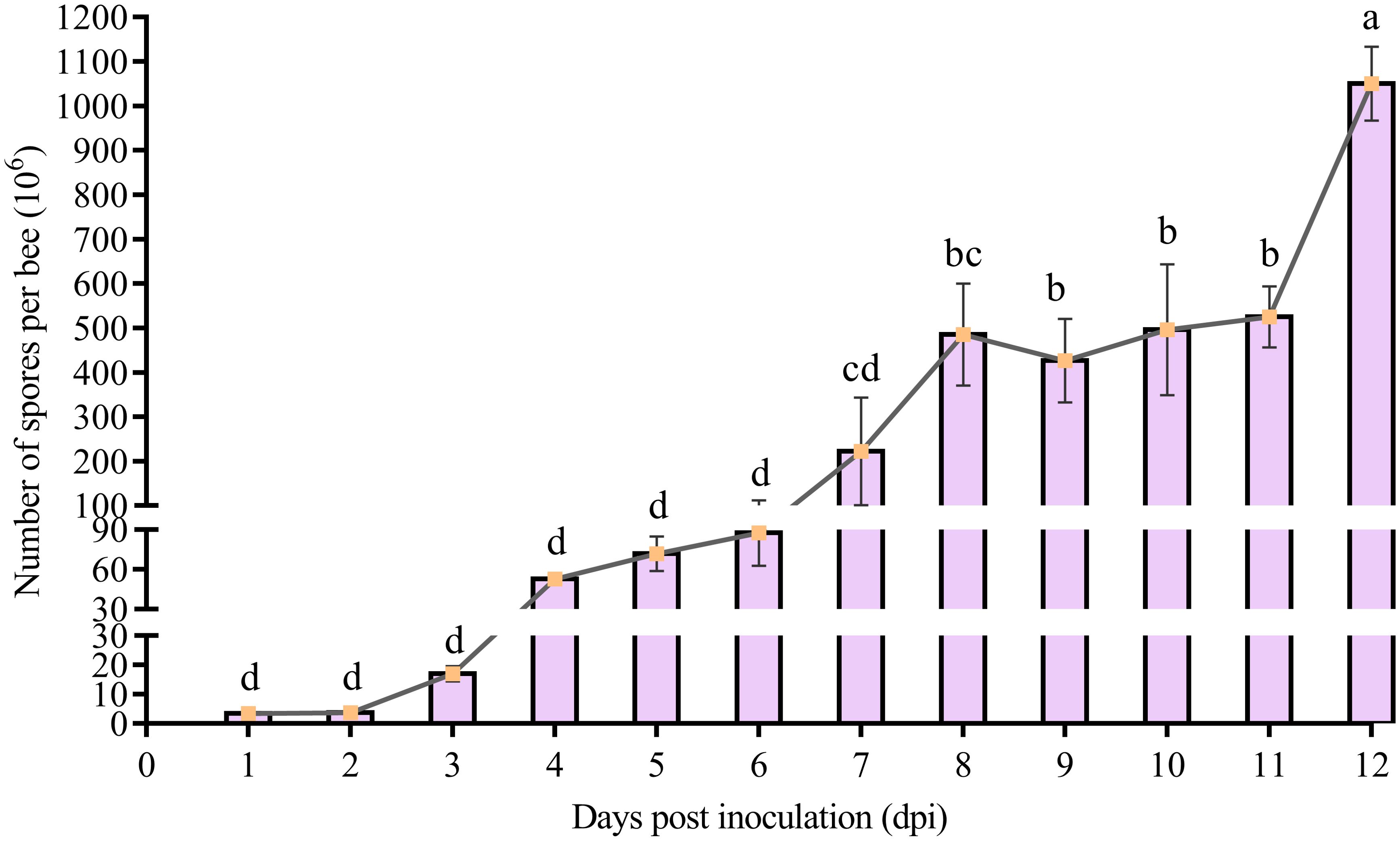
Figure 2. Spore load of V. ceranae in the midguts of A. c. cerana workers after inoculation (n=3). Whiskers indicate the mean ± SD. Data were analyzed using the One-way ANOVA (Tukey’s post-hoc test). The same lowercase letters above the curve indicate no significant difference (P > 0.05), whereas different lowercase letters above the curve indicate statistically significant differences (P < 0.05).
3.2 Influence of V. ceranae infection on average sucrose solution consumption of A. c. cerana workers
The average sucrose consumption of V. ceranae-inoculated and un-inoculated workers was 0.0308 ± 0.0080 g/d and 0.0371 ± 0.0107 g/d, respectively. The consumption of workers in the V. ceranae-inoculated group was extremely significant higher (P<0.0001) than that of workers in the un-inoculated group, significant difference in the average sucrose solution consumption between the two groups was observed at 5 (P = 0.0244), 8 dpi (P = 0.0005), 10 dpi (P = 0.0443), 11 dpi (P = 0.0497), 12 dpi (P = 0.0243), 13 dpi (P = 0.0286) and 20 dpi (P = 0.0018) (Figure 3).
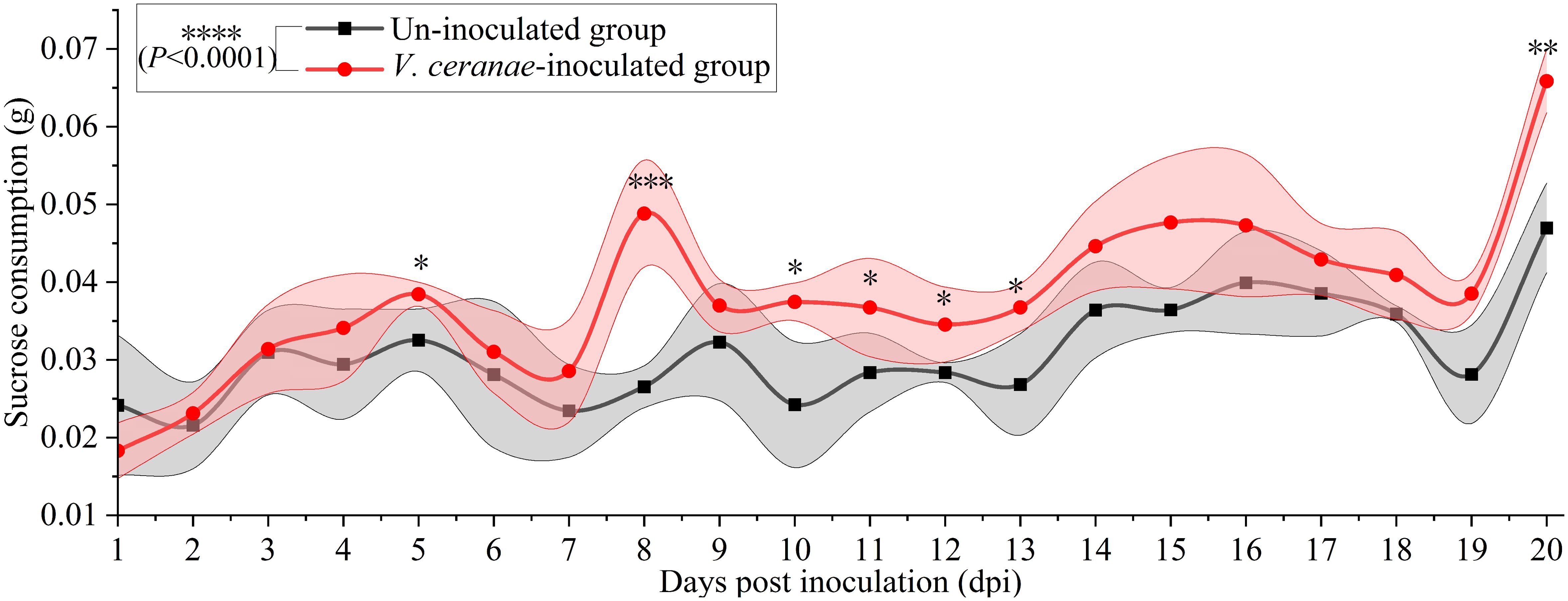
Figure 3. Average sucrose solution consumption of V. ceranae-inoculated and un-inoculated workers (n=105). Data were showed as mean ± SD, that analyzed using the Two-way ANOVA (Bonferroni’s post-hoc test), multiple t tests; * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001, **** indicates P < 0.0001.
3.3 Impact of V. ceranae infection on SOD and PPO activities in A. c. cerana midguts of the workers
Compared to that in the uninoculated group, the SOD activity in the V. ceranae-inoculated group was decreased at 2 (P = 0.2736)–10 dpi (P = 0.0399), with a significant difference observed at 5 dpi (P = 0.0097), 6 dpi (P = 0.0434), 9 dpi (P = 0.0015), and 10 dpi (Figure 4A). The PPO activity was consistently lower in the V. ceranae-inoculated group in comparison to that in the un-inoculated group at 2 (P = 0.2249)–12 (P = 0.5391) dpi, although no statistically significant difference was observed between these two groups (Figure 4B).
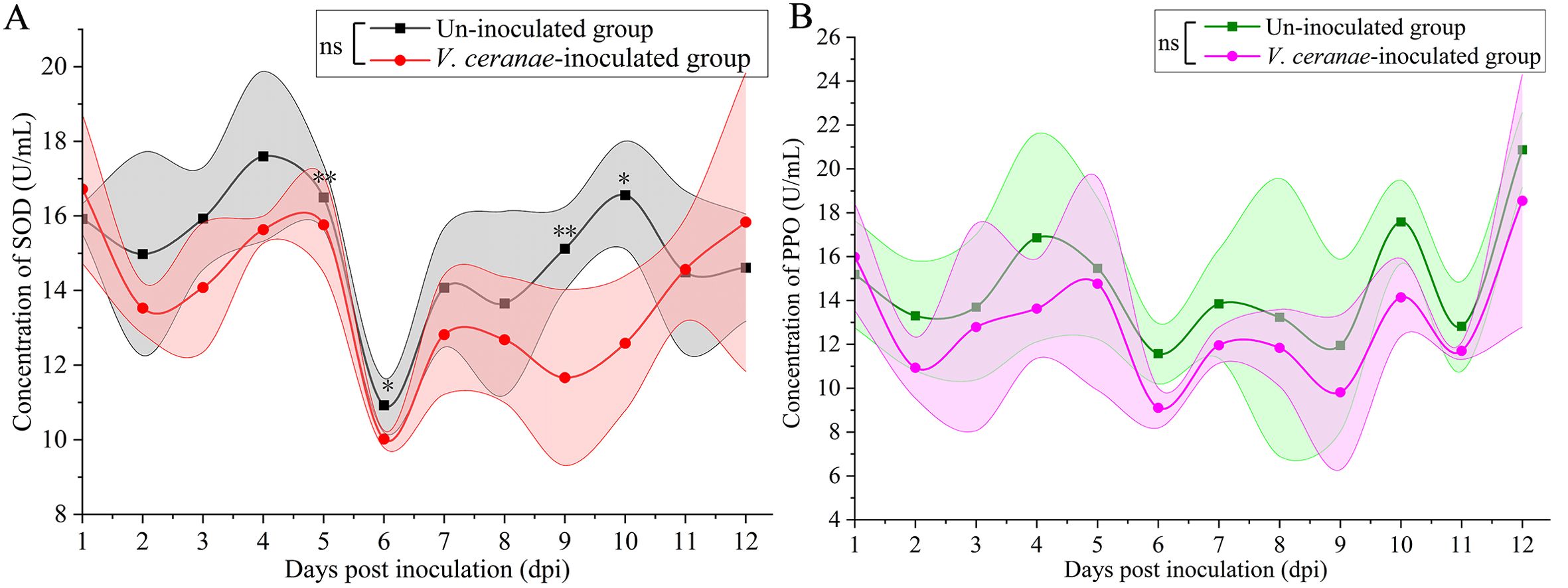
Figure 4. Detection of SOD (A) and PPO (B) activities in midguts of V. ceranae- and un-inoculated workers (n=3). Data were showed as mean ± SD, that analyzed using the Two-way ANOVA (Bonferroni’s post-hoc test), multiple t tests; ns indicates nonsignificance, * indicates P < 0.05, ** indicates P < 0.01.
3.4 Impact of V. ceranae infection on CAT and GST activities in the midguts of A. c. cerana workers
CAT activity was reduced in the V. ceranae-inoculated group in comparison with that in the un-inoculated group at 1 (P = 0.9209)–3 dpi (P = 0.0981), (Figure 5A); conversely, then was elevated in the V. ceranae-inoculated group at 4 (P = 0.1596)–7 (P = 0.1954) dpi; CAT activity was again downregulated in the host midgut following V. ceranae inoculation at 8 (P = 0.3183)–12 dpi (P = 0.4700), with a significant difference observed at 10 dpi (P = 0.0478) (Figure 5A). Additionally, GST activity was up-regulated at 5 (P = 0.5171)–6 dpi (P = 0.3692) and then reduced at 7 (P = 0.8196)–10 dpi (P = 0.0070), followed by an increase at 11 dpi (P = 0.1391), with a significant difference observed at 9 dpi (P = 0.0229), and 10 dpi (P = 0.0070) (Figure 5B).
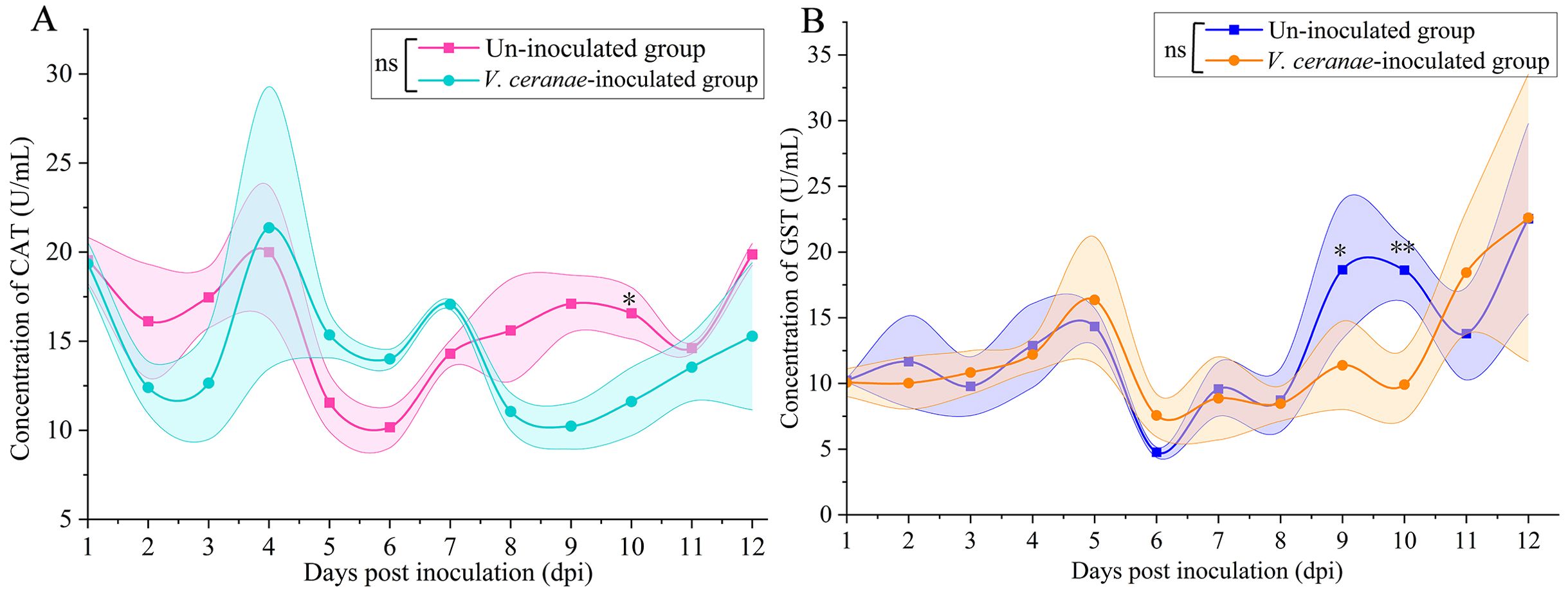
Figure 5. Detection of CAT (A) and GST (B) activities in midguts of V. ceranae- and un-inoculated workers (n=3). Data were showed as mean ± SD, that analyzed using the Two-way ANOVA (Bonferroni’s post-hoc test), multiple t tests; ns indicates nonsignificance, * indicates P < 0.05, ** indicates P < 0.01.
3.5 Influence of V. ceranae infection on midgut epithelial cell structure of A. c. cerana workers
Microscopic observation of paraffin sections revealed darkly stained microsporidia in the midgut epithelial cells of V. ceranae-inoculated workers at 7–10 dpi (Figures 6C, D, G, H, K, L O, P), whereas no V. ceranae was detected in the midgut epithelial cells of uninoculated workers (Figures 6A, B, E, F, I, J, M, N). The number of V. ceranae spores in host cells gradually increased with increasing infection time (Figures 6C, D, G, H, K, L O, P).
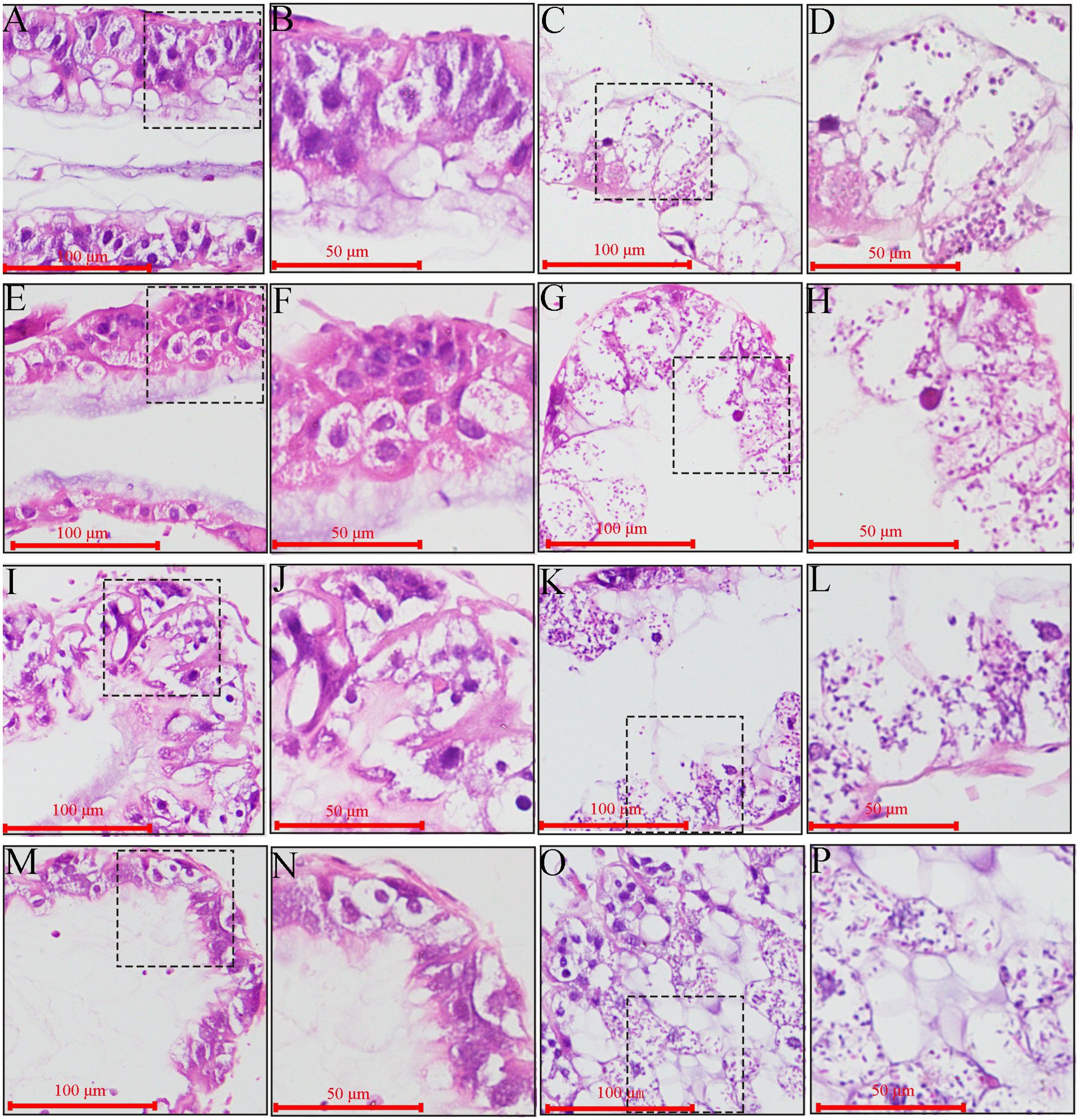
Figure 6. Microscopic observation of paraffin midgut sections of un-inoculated and V. ceranae-inoculated A. c. cerana workers. (A, B) Midgut tissue of un-inoculated worker at 7 dpi without spores; (C, D) Midgut tissue of inoculated worker at 7 dpi with V. ceranae spores; (E, F) Midgut tissue of un-inoculated worker at 8 dpi without spores; (G, H) Midgut tissue of inoculated worker at 8 dpi with V. ceranae spores; (I, J) Midgut tissue of un-inoculated worker at 9 dpi without spores; (K, L) Midgut tissue of inoculated worker at 9 dpi with V. ceranae spores; (M, N) Midgut tissue of un-inoculated worker at 10 dpi without spores; (O, P) Midgut tissue of inoculated worker at 10 dpi with V. ceranae spores. (A, C, E, G, I, K, M, O) were microscopic fields under 200× amplification, whereas (B, D, F, H, J, N, P) were microscopic fields under 400× amplification. Black dashed boxes show the selected region for observation under 200× amplification.
In addition, the columnar and goblet cells were closely arranged, the epithelial cells were orderly arranged, the periesophageal membrane was clearly visible (Figures 6A, B, E, F, I, J, M, N), the outlines of columnar and goblet cells of V. ceranae-inoculated workers were arranged loosely and became unclear, and the periesophageal membrane was invisible (Figures 6C, D, G, H, K, L O, P). Moreover, as the infection progressed, the epithelial cells fell off the midgut wall and entered the intestinal lumen, together with the nuclei, cytoplasm, and various types of V. ceranae spores (Figure 6).
3.6 Influence of V. ceranae infection on lifespan of A. c. cerana workers
The survival rate statistics indicated that the survival rates of workers in both the V. ceranae-inoculated and uninoculated groups at 1–5 dpi were high, and there was little difference between the groups (Figure 7). Survival rates in both groups began to decrease at 5 dpi (Figure 7). At 5–11 dpi, the survival rate of workers in the V. ceranae-inoculated group was lower than that in the un-inoculated group, whereas the survival rate of workers in the V. ceranae-inoculated group was significantly lower than that in the uninoculated group at 11–20 dpi (Figure 7).
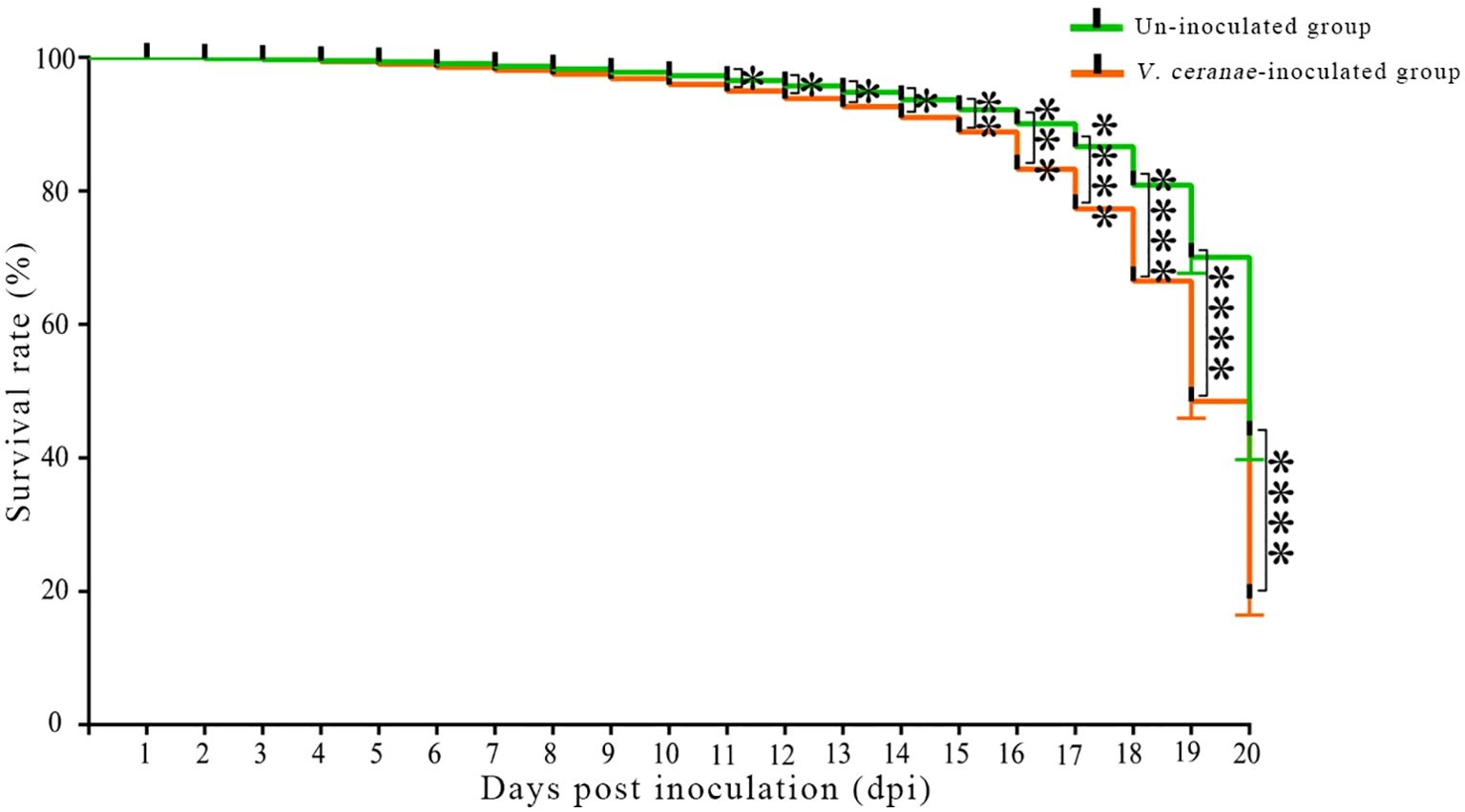
Figure 7. Survival rate of A. c. cerana workers after un-inoculation and inoculation with V. ceranae spores (n=3). Data were analyzed using the Log-rank (Mantel-Cox) test; * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001, and **** indicates P < 0.0001.
4 Discussion
There is an ongoing debate and uncertainty surrounding the precise lifecycle duration of V. ceranae across different hosts. In A. mellifera, all intracellular stages of the life cycle of V. ceranae were observed at 3 dpi inside the worker’s epithelial cells using transmission electron microscopy, suggesting that the time for V. ceranae to complete the developmental cycle was 3 d (39). Based on other lines heterologous lepidopteran cell line IPL-LD-65Y in vitro, Gisder et al. reported that the life cycle of N. apis, the sister species of V. ceranae, is approximately 4 d (40). Although A. cerana is the original host of V. ceranae, its life cycle of V. ceranae in the midgut epithelium cells of A. cerana is unknown. Here, we observed a continuous increase in spore load from 1–12 dpi (Figure 2), indicative of sustained proliferation of V. ceranae within host cells. Additionally, the number of spores increased 10-fold every 4 d (Figure 2), suggesting that the life cycle of V. ceranae in the midgut epithelial cells of A. cerana workers was approximately 4 d, which differs from reports on A. mellifera. Intriguingly, we observed the presence of spores (3.5 ± 0.87 × 106) as early as 1 dpi. Similar phenomenon was observed in previous studies, namely that by 3 h post inoculation, mature spores can be observed in the ventricular lumen of infected A. mellifera workers (39). We hypothesized that the detected V. ceranae spores at 1 dpi contained spores that did not infect the bee host cells or empty spores. However, further studies are needed to verify this hypothesis.
Nosema spp. have lost mitochondria during evolution, and thus must acquire energetic substrates directly from hosts for maintenance, growth, and reproduction (41, 42). Intestinal lesions caused by V. ceranae proliferation may decrease the absorptive capacity of honeybees and cause starvation symptoms, such as impoverishment of hypopharyngeal protein secretions (8). Chronic nutrient and energy stress suppress the innate immune system (43). After V. ceranae infection, workers of A. mellifera consumed a significantly higher amount of sucrose solution over the 24-h period tested (6). Here, we observed that the sucrose solution consumption of V. ceranae-inoculated workers was nearly always higher than that of uninoculated workers from 1–20 dpi, which indicated that the workers attempted to compensate for the imposed energy stress by consuming more sucrose solution. This is consistent with the results of a previous study on A. mellifera workers infected with V. ceranae (44). In summary, these results demonstrate that V. ceranae infection results in energy stress in both A. mellifera and A. cerana workers. In addition, the chronic stress caused by V. ceranae infection is likely to negatively influence foraging behavior, nutritional balance, immune response, and other important aspects of A. cerana workers, thus deserving more attention.
Insects maintain homeostasis in the body by neutralizing ROS and xenobiotics through the activation of various metabolic pathways that help protect cellular components from oxidative stress (26, 45). The antioxidant enzyme system, comprising SOD, PPO, CAT, and GST, is a critical physiological system for resisting oxidative stress in insects (46, 47). After infection with pathogens, the insect immune system stimulates the production of ROS, including superoxide radicals, to kill the invaders (24). CAT efficiently breaks down toxic hydrogen peroxide into harmless water and oxygen molecules (48). GST protects cells from oxidative stress by conjugating glutathione to ROS and other electrophilic compounds (49). It has been previously reported that GST is linked to oxidative stress response in the midgut of Drosophila melanogaster larvae (50). In the present study, CAT activity in the V. ceranae-inoculated group increased significantly at 5, 6, and 7 dpi (Figure 5A), whereas GST activity was significantly elevated at 6 dpi (Figure 5B). We inferred that the host enhanced the levels of CAT and GST activities in response to V. ceranae-caused oxidative stress in the middle stage of the infection process, thereby maintaining homeostasis of the internal environment. SOD catalyzes the dismutation of superoxide radicals into hydrogen peroxide and oxygen, thereby regulating ROS levels and preventing oxidative damage to cells (51). A significant decline in the activities of both SOD and GST was detected at 10 dpi, and CAT activity was reduced at both 9 and 10 dpi (Figures 4A, 5). These results suggest that these three critical antioxidant enzymes are inhibited in the host midgut. This phenomenon may be attributed to the escalating spore load in the late infection stage, leading to a prolonged intensification of V. ceranae stress in the host and consequently suppressing the activities of SOD, CAT, and GST. PPO is involved in the melanization process, a common immune response in insects that involves the production of dark and pigmented structures that can trap and kill invading pathogens or parasites. In addition to its role in melanization, it simultaneously induces cellular and humoral immunity (52). Roberts and Hughes (53) found a negative correlation between PPO levels in the midguts of A. mellifera workers and V. ceranae infection intensity. Intriguingly, although the level of PPO activity in the V. ceranae-inoculated group was always lower than that in the uninoculated group, there was no significant difference between the groups (Figure 4B), indicating that PPO activity in the host midguts was not affected by fungal invasion. It is inferred that, as the host of origin of V. ceranae, A. cerana workers potentially evolved a strategy to counteract the negative influence of microsporidians on PPO activity. Further studies are required to explore these underlying mechanisms. Insects exhibit dynamic changes in GST and CAT in response to pathogen infection. For instance, in the larvae of Holotrichia parallela, following joint infection of entomopathogenic nematode and Bacillus thuringiensis, there was an initial increase in GST and CAT activity during the early stage, followed by a decrease in activity in the later stage (54). In this study, the contrasting alterations observed in GST and CAT levels between the middle and late stages of V. ceranae infection could potentially be ascribed to the insects’ initial augmentation of antioxidant enzyme activity as a mechanism of defense against the fungal invasion. Conversely, a decline in enzyme activity may occur in the later stage as a result of energy depletion or other physiological adjustments.
In a previous study, Higes et al. (39) detected that, 7 d after V. ceranae infection, the majority of epithelial cells showed evidence of degeneration, such as the presence of vacuoles in the cytoplasm or a brightly stained nucleus. Few unaltered cells were also observed, and either at the tips or at the bottom of the epithelial folds, there were cells containing V. ceranae intracellular stages. Here, we observed that the number of V. ceranae in the A. cerana worker midgut epithelial cells increased from 7 to 10 dpi (Figure 6), which is consistent with the findings of Higes et al. (39) Collectively, these results offer a solid basis for further investigations of V. ceranae infection in A. cerana workers, such as functional studies on fungal virulence factor-associated genes and the development of an RNAi-based control strategy.
V. ceranae infection damaged the structure of midgut epithelial cells in A. mellifera workers (7). Based on microscopic observations by optical microscopy and transmission electron microscopy, Fries et al. (2) first reported that V. ceranae-infected ventriculus cells of A. cerana workers were shed into or burst open into the gut lumen where mature spores were released, and described the size of the spores. Darkly stained microsporidia were clearly detected in the midgut epithelial cells of V. ceranae-inoculated workers at 7–10 dpi (Figure 6). Additionally, the boundaries of the midgut epithelial cells in V. ceranae-inoculated workers were blurred, and the nuclei almost disappeared, with nucleic acid substances dispersed around the cytoplasm (Figure 6). These results suggest that V. ceranae infection also causes serious damage to the structure of the midgut epithelial cells of A. c. cerana workers. Collectively, the findings from previous studies (2) and this study offer experimental evidence for the damage caused by V. ceranae infection to the structure of A. c. cerana worker midgut epithelial cells, laying the foundation for further investigation of the pathogenesis of bee nosemosis.
Paris et al. (55) assessed the survival rate and eating behavior of A. mellifera workers infected with V. ceranae for 1–22 d, and the results showed that the survival rate of infected workers was significantly lower than that of uninfected workers. We previously observed that the mortality rate of A. mellifera workers inoculated with V. ceranae spores gradually increased over time, and that the mortality rate of V. ceranae-inoculated workers was significantly higher than that of uninoculated workers at 7 and 10 dpi (33). It has been documented that V. ceranae infection significantly increased the mortality rate of A. cerana workers (56). In this study, the survival rates of workers in both the V. ceranae- and un-inoculated groups were high at 1–5 dpi (Figure 7), implying that V. ceranae had little influence on host longevity at the early stage of infection. However, at 5–20 dpi (Figure 7), the survival rate of V. ceranae-inoculated workers was always lower than that of uninoculated workers, and the difference was significant at 11–20 dpi, indicating that V. ceranae infection shortened the lifespan of A. cerana workers, similar to the findings of Huang et al. (56). In conclusion, V. ceranae can negatively affect the midgut epithelial cell structure and lifespan of A. c. cerana workers during infection.
Collectively, the findings of the present study indicate that the spore load continuously increases as V. ceranae proliferates in the midgut of A. c. cerana workers, giving rise to continuous energetic stress for the host, apparent damage to the host midgut epithelial cell structure, induction of CAT and GST activities in the host midgut at the middle stage, and inhibition of the activities of SOD, CAT, and GST at the late stage of infection, which ultimately shortens the host lifespan (Figure 8).
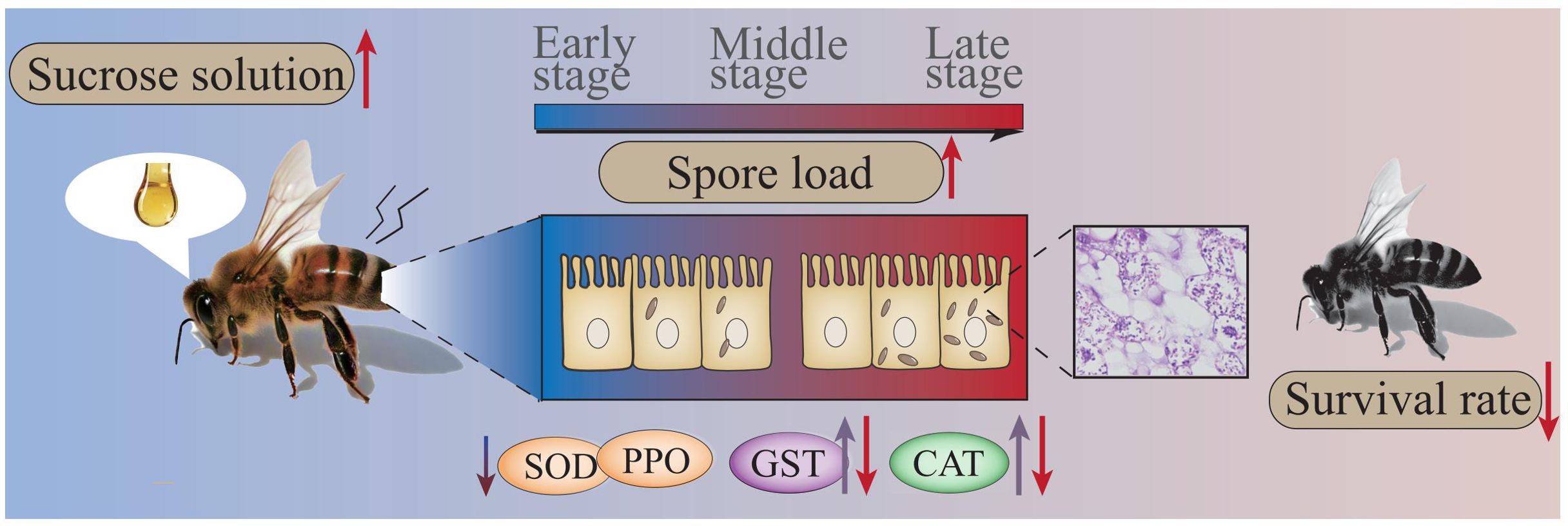
Figure 8. Hypothetical schematic diagram of the impact of V. ceranae infection on A. c. cerana worker.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Laboratory Animals Ethics and Welfare Committee of College of Animal Sciences, Fujian Agriculture and Forestry University (PZCASFAFU24018). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XF: Methodology, Software, Validation, Writing – original draft. HaZ: Methodology, Software, Validation, Writing – original draft. HeZ: Conceptualization, Methodology, Software, Validation, Writing – original draft. SD: Software, Validation, Writing – original draft. JQ: Data curation, Methodology, Writing – original draft. YS: Formal analysis, Writing – original draft. KL: Formal analysis, Writing – original draft. HJ: Data curation, Writing – original draft. YW: Data curation, Writing – original draft. YL: Data curation, Writing – original draft. DZ: Data curation, Visualization, Writing – original draft. ZF: Formal analysis, Methodology, Visualization, Writing – review & editing. DC: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. RG: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (32172792, 32372943), the Earmarked Fund for China Agriculture Research System (CARS-44-KXJ7), the Natural Science Foundation of Fujian Province (2022J01133, 2023J01447), the Master Supervisor Team Fund of Fujian Agriculture and Forestry University (RG), the Scientific and Technical Innovation Fund of Fujian Agriculture and Forestry University (KFb22060XA).
Acknowledgments
All authors thank the reviewers and editors for their constructive comments and recommendations. RG appreciates the great love from his adorable daughter.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Klee J, Besana AM, Genersch E, Gisder S, Nanetti A, Tam DQ, et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J Invertebr Pathol. (2007) 96:1–10. doi: 10.1016/j.jip.2007.02.014
2. Fries I, Feng F, da Silva AD, Slemenda SB, Pieniazek NJ. Nosema ceranae n. sp.(Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur J Protistol. (1996) 32:356–65. doi: 10.1016/S0932-4739(96)80059-9
3. Boncristiani H, Ellis JD, Bustamante T, Graham J, Jack C, Kimmel C, et al. World honey bee health: the global distribution of western honey bee (Apis mellifera L.) pests and pathogens. Bee World. (2020) 98:1–5. doi: 10.1080/0005772X.2020.180033
4. Higes M, Martín-Hernández R, Botías C, Bailón EG, González-Porto AV, Barrios L, et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol. (2008) 10:2659–69. doi: 10.1111/j.1462-2920.2008.01687.x
5. Paxton R. Does infection by Nosema ceranae cause “Colony Collapse Disorder” in honey bees (Apis mellifera)? J Apicultural Res. (2010) 49:80–84. doi: 10.3896/IBRA.1.49.1.11
6. Mayack C, Naug D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J Invertebr Pathol. (2009) 100:185–8. doi: 10.1016/j.jip.2008.12.001
7. Garcıa-Palencia P, Martın-Hernández R, González-Porto AV, Marin P, Meana A, Higes MJ. Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-workers honey bees (Apis mellifera). J Apic Res. (2010) 49:278–83. doi: 10.3896/IBRA.1.49.3.08
8. Vidau C, Panek J, Texier C, Biron DG, Belzunces LP, Le Gall M, et al. Differential proteomic analysis of midguts from Nosema ceranae-infected honeybees reveals manipulation of key host functions. J Invertebr Pathol. (2014) 121:89–96. doi: 10.1016/j.jip.2014.07.002
9. Dussaubat C, Brunet JL, Higes M, Colbourne JK, Lopez J, Choi JH, et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS One. (2012) 7:e37017. doi: 10.1371/journal.pone.0037017
10. Goblirsch M, Huang ZY, Spivak M. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS One. (2013) 8:e58165. doi: 10.1371/journal.pone.0058165
11. Aufauvre J, Misme-Aucouturier B, Viguès B, Texier C, Delbac F, Blot N. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS One. (2014) 9:e91686. doi: 10.1371/journal.pone.0091686
12. Li W, Chen Y, Cook SC. Chronic Nosema ceranae infection inflicts comprehensive and persistent immunosuppression and accelerated lipid loss in host Apis mellifera honey bees. Int J Parasitol. (2018) 48:433–44. doi: 10.1016/j.ijpara.2017.11.004
13. Li J, Qin H, Wu J, Sadd BM, Wang X, Evans JD, et al. The prevalence of parasites and pathogens in Asian honeybees Apis cerana in China. PLoS One. (2012) 7:e47955. doi: 10.1371/journal.pone.0047955
14. Sinpoo C, Paxton RJ, Disayathanoowat T, Krongdang S, Chantawannakul P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J Insect Physiol. (2017) 105:1–8. doi: 10.1016/j.jinsphys.2017.12.010
15. Wu Y, Zheng Y, Chen Y, Chen G, Zheng H, Hu F. Apis cerana gut microbiota contribute to host health though stimulating host immune system and strengthening host resistance to Nosema ceranae. R Soc Open Sci. (2020) 7:192100. doi: 10.1098/rsos.192100
16. Naree S, Benbow ME, Suwannapong G, Ellis JD. Mitigating Nosema ceranae infection in western honey bee (Apis mellifera) workers using propolis collected from honey bee and stingless bee (Tetrigona apicalis) hives. J Invertebr Pathol. (2021) 185:107666. doi: 10.1016/j.jip.2021.107666
17. Fan Y, Wang J, Yu K, Zhang W, Cai Z, Sun M, et al. Comparative transcriptome investigation of Nosema ceranae infecting eastern honey bee workers. Insects. (2022) 13:241. doi: 10.3390/insects13030241
18. Chantaphanwattana T, Houdelet C, Sinpoo C, Voisin SN, Bocquet M, Disayathanoowat T, et al. Proteomics and immune response differences in Apis mellifera and Apis cerana inoculated with three Nosema ceranae isolates. J Proteome Res. (2023) 22:2030–43. doi: 10.1021/acs.jproteome.3c00095
19. Lin Z, Page P, Li L, Qin Y, Zhang Y, Hu F, et al. Go east for better honey bee health: Apis cerana is faster at hygienic behavior than A. mellifera. PLoS One. (2016) 11:e0162647. doi: 10.1371/journal.pone.0162647
20. Chen D, Du Y, Chen H, Fan Y, Fan X, Zhu Z, et al. Comparative identification of microRNAs in Apis cerana cerana workers' midguts in response to Nosema ceranae invasion. Insects. (2019) 10:258. doi: 10.3390/insects10090258
21. Fu ZM, Zhou DD, Chen HZ, Geng SH, Chen DF, Zheng YZ. et al., Analysis of highly expressed genes in Apis cerana cerana workers' midguts responding to Nocema ceranae stress. J Sichuan Uni(Natural Sci Edition). (2020) 57:191–8. doi: 10.3969/i.issn.0490-6756.2020.01.029
22. Xiong CL, Chen HZ, Geng SH, Zhou NH, Zhou DD, Zhu ZW, et al. Expression profile of high-expressing genes and its potential role during Apis cerana cerana infected by Nosema ceranae. J Sichuan Uni (Natural Sci Edition). (2020) 57:596–604. doi: 10.3969/i.issn0490-6756.2020.03.030
23. Xing W, Zhou D, Long Q, Sun M, Guo R, Wang L. Immune response of eastern honeybee worker to Nosema ceranae infection revealed by transcriptomic investigation. Insects. (2021) 12:728. doi: 10.3390/insects12080728
24. Ouali R, Vieira LR, Salmon D, Bousbata S. Rhodnius prolixus hemolymph immuno-physiology: deciphering the systemic immune response triggered by trypanosoma cruzi establishment in the vector using quantitative proteomics. Cells. (2022) 11:1449. doi: 10.3390/cells11091449
25. Wijesinghe WAJP, Jeon YJ, Ramasamy P, Wahid MEA, Vairappan CS. Anticancer activity and mediation of apoptosis in human HL-60 leukaemia cells by edible sea cucumber (Holothuria edulis) extract. Food Chem. (2013) 139:326–31. doi: 10.1016/j.foodchem.2013.01.058
26. Felton GW, Summers CB. Antioxidant systems in insects. Arch Insect Biochem Physiol. (1995) 29:187–97. doi: 10.1002/arch.940290208
27. Ahmad S, Duval DL, Weinhold LC, Pardini RS. Cabbage looper antioxidant enzymes: tissue specificity. Insect Biochem. (1991) 21:563–72. doi: 10.1016/0020-1790(91)90111-Q
28. Felton GW, Duffey SS. Protective action of midgut catalase in lepidopteran larvae against oxidative plant defenses. J Chem Ecol. (1991) 17:1715–32. doi: 10.1007/BF00993724
29. Ahmad S. Ecology. Biochemical defence of pro-oxidant plant allelochemicals by herbivorous insects. Biochem Syst Ecol. (1992) 20:269–96. doi: 10.1016/0305-1978(92)90040-K
30. Dubovskiy IM, Martemyanov VV, Vorontsova YL, Rantala MJ, Gryzanova EV, Glupov VV. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp Biochem Physiol C Toxicol Pharmacol. (2008) 148:1–5. doi: 10.1016/j.cbpc.2008.02.003
31. Mayer AMJP. Polyphenol oxidases in plants-recent progress. Phytochemistry. (1986) 26:11–20. doi: 10.1016/S0031-9422(00)81472-7
32. Cerenius L, Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. (2004) 198:116–26. doi: 10.1111/j.0105-2896.2004.00116.x
33. Chen D, Chen H, Du Y, Zhou D, Geng S, Wang H, et al. Genome-Wide identification of long non-coding RNAs and their regulatory networks involved in Apis mellifera ligustica response to Nosema ceranae Infection. Insects. (2019) 10:245. doi: 10.3390/insects10080245
34. Chen Y, Evans JD, Smith IB, Pettis JS. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J Invertebr Pathol. (2008) 97:186–8. doi: 10.1016/j.jip.2007.07.010
35. Fries I, Chauzat M-P, Chen Y-P, Doublet V, Genersch E, Gisder S, et al. Standard methods for Nosema research. J Apicult Res. (2013) 52:1–28. doi: 10.3896/IBRA.1.52.1.14
37. Di N, Hladun KR, Zhang K, Liu TX, Trumble JT. Laboratory bioassays on the impact of cadmium, copper and lead on the development and survival of honeybee (Apis mellifera L.) larvae and foragers. Chemosphere. (2016) 152:530–8. doi: 10.1016/j.chemosphere.2016.03.033
38. Liu YJ, Qiao NH, Diao QY, Jing Z, Vukanti R, Dai PL, et al. Thiacloprid exposure perturbs the gut microbiota and reduces the survival status in honeybees. J Hazard Mater. (2020) 389:121818. doi: 10.1016/j.jhazmat.2019.121818
39. Higes M, Garcia-Palencia P, Martin-Hernandez R, Meana A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J Invertebr Pathol. (2007) 94:211–7. doi: 10.1016/j.jip.2006.11.001
40. Gisder S, Mockel N, Linde A, Genersch E. A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee-pathogenic microsporidia. Environ Microbiol. (2011) 13:404–13. doi: 10.1111/j.1462-2920.2010.02346.x
41. Burri L, Williams BA, Bursac D, Lithgow T, Keeling PJ. Microsporidian mitosomes retain elements of the general mitochondrial targeting system. Proc Natl Acad Sci U S A. (2006) 103:15916–20. doi: 10.1073/pnas.0604109103
42. Tsaousis AD, Kunji ER, Goldberg AV, Lucocq JM, Hirt RP, Embley TM. A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature. (2008) 453:553–6. doi: 10.1038/nature06903
43. Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. (2014) 58:193–210. doi: 10.1007/s12026-014-8517-0
44. Martin-Hernandez R, Botias C, Barrios L, Martinez-Salvador A, Meana A, Mayack C, et al. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol Res. (2011) 109:605–12. doi: 10.1007/s00436-011-2292-9
45. Wu L, Xu W, Li H, Dong B, Geng H, Jin J, et al. Vitamin C attenuates oxidative stress, inflammation, and apoptosis induced by acute hypoxia through the Nrf2/Keap1 signaling pathway in Gibel Carp (Carassius gibelio). Antioxidants (Basel). (2022) 11:935. doi: 10.3390/antiox11050935
46. Yang LH, Huang H, Wang JJ. Antioxidant responses of citrus red mite, Panonychus citri (McGregor) (Acari: Tetranychidae), exposed to thermal stress. J Insect Physiol. (2010) 56:1871–6. doi: 10.1016/j.jinsphys.2010.08.006
47. Durak R, Dampc J, Kula-Maximenko M, Molon M, Durak T. Changes in antioxidative, oxidoreductive and detoxification enzymes during development of aphids and temperature increase. Antioxidants (Basel). (2021) 10:1181. doi: 10.3390/antiox10081181
48. Zhao H, Yi X, Hu Z, Hu M, Chen S, Muhammad RU, et al. RNAi-mediated knockdown of catalase causes cell cycle arrest in SL-1 cells and results in low survival rate of Spodoptera litura (Fabricius). PLoS One. (2013) 8:e59527. doi: 10.1371/journal.pone.0059527
49. Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. (2005) 45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857
50. Li HM, Buczkowski G, Mittapalli O, Xie J, Wu J, Westerman R, et al. Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol Biol. (2008) 17:325–39. doi: 10.1111/j.1365-2583.2008.00808.x
51. Ishihara Y, Takemoto T, Ishida A, Yamazaki T. Protective actions of 17beta-estradiol and progesterone on oxidative neuronal injury induced by organometallic compounds. Oxid Med Cell Longev. (2015) 2015:343706. doi: 10.1155/2015/343706
52. Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. (2007) 25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615
53. Roberts KE, Hughes WO. Immunosenescence and resistance to parasite infection in the honey bee, Apis mellifera. J Invertebr Pathol. (2014) 121:1–6. doi: 10.1016/j.jip.2014.06.004
54. Li ET, Wu HJ, Wang ZM, Li KB, Zhang S, Cao YZ, et al. PI3K/Akt/CncC signaling pathway mediates the response to EPN-Bt infection in Holotrichia parallela larvae. Pest Manag Sci. (2023) 79:1660–73. doi: 10.1002/ps.7337
55. Paris L, Alaoui HE, Delbac F, Diogon M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr Opin Insect Sci. (2018) 26:149–54. doi: 10.1016/j.cois.2018.02.017
Keywords: honeybee, Apis cerana, Vairimorpha (Nosema) ceranae, host-parasite interaction, antioxidant enzyme
Citation: Fan X, Zhao H, Zang H, Dong S, Qiu J, Song Y, Li K, Jiang H, Wu Y, Lü Y, Zhou D, Fu Z, Chen D and Guo R (2024) Extensive influence of microsporidian infection on sucrose solution consumption, antioxidant enzyme activity, cell structure, and lifespan of Asian honeybees. Front. Immunol. 15:1404766. doi: 10.3389/fimmu.2024.1404766
Received: 21 March 2024; Accepted: 30 October 2024;
Published: 19 November 2024.
Edited by:
Guojun Wu, Rutgers, The State University of New Jersey, United StatesReviewed by:
Esmaeil Amiri, Mississippi State University, United StatesFrancisco José Reynaldi, National Scientific and Technical Research Council (CONICET), Argentina
Copyright © 2024 Fan, Zhao, Zang, Dong, Qiu, Song, Li, Jiang, Wu, Lü, Zhou, Fu, Chen and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Guo, cnVpZ3VvQGZhZnUuZWR1LmNu
†These authors have contributed equally to this work
 Xiaoxue Fan
Xiaoxue Fan Haodong Zhao1†
Haodong Zhao1† Jianfeng Qiu
Jianfeng Qiu Ying Wu
Ying Wu Yang Lü
Yang Lü Dafu Chen
Dafu Chen Rui Guo
Rui Guo