- 1Gansu Provincial Hospital of TCM, Lanzhou, China
- 2The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
- 3The First Veterans Hospital of Sichuan Province, Chengdu, China
- 4Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background: To evaluate the methodological quality, report quality, and evidence quality of meta-analysis (MA) and systematic review (SR) on the efficacy of probiotics in the treatment of rheumatoid arthritis (RA).
Methods: Databases were used to identify eligible SRs/MAs until February 12, 2024. The methodological quality of the studies was assessed using AMSTAR-2 tool, the quality of the literature reports was scored using PRISMA checklists, and the quality of the evidence was graded using GRADE system.
Results: Seven reviews including 21 outcomes were included. Methodological quality of the included reviews was of general low, and the entries with poor scores were 2, 4, and 7. By PRISMA checklists, there were some reporting deficiencies, and quality problems were mainly reflected in the reporting registration and protocol, comprehensive search strategy and additional analysis. GRADE results elevated the quality of evidence to be low or very low overall.
Conclusions: Probiotics may have a therapeutic effect on RA, based on the evidence provided by the SRs/MAs in this overview. Nevertheless, there is still a lack of conclusive evidence due to methodological limitations in the included research. To make trustworthy judgments regarding the efficacy of probiotics in the treatment of RA, more large-scale, high-quality randomized controlled trials are still required.
1 Introduction
Rheumatoid arthritis (RA) is an autoimmune systemic inflammatory disease involving multiple joints in the human body (1). Failure to effectively block disease progression can lead to cartilage and bone erosion, ultimately leading to joint deformity (2). RA is the most common inflammatory disease, with a global prevalence of 0.5 to 1% (3). Although many factors have been reported that may have a significant impact on the development and progression of RA, the pathophysiologic mechanisms of RA remain unelucidated (4). Accumulating evidence reveals an association between gut microbe and RA (5). In contrast to normal rats, germ-free rats are more likely to develop RA and have more severe symptoms (6). In addition, patients with inflammatory bowel disease who have disturbed gut microbe are also more likely to exhibit joint inflammation (7). Furthermore, fasting and vegan diets have been associated with reduced RA activity attributed to altered gut microbiota (8, 9). Therefore, modulation of gut microbes has been recognized as a potential strategy for the treatment of patients with RA (10).
Probiotics have recently demonstrated encouraging outcomes when used as adjuvant therapy for treating RA (11). Probiotics are described as “living microorganisms that, when ingested in sufficient quantities, provide a health benefit to the host” because they can decrease the number of harmful bacteria by competing for nutrition and colonization sites (12). The effectiveness of probiotics for RA has been assessed in a number of overlapping systematic reviews (SRs) and meta-analyses (MAs) to date (13–19). Nevertheless, there has been inconsistent evidence from these SRs/MAs. Trustworthy evidence is produced by high-quality SRs/MAs, while low-quality SRs and MAs may unintentionally affect choices (20, 21). As a result, when several studies with similar findings are published, an overview of prior SRs/MAs on the subject is frequently required (22). The purpose of this study was to offer evidence for clinical decisions by methodically gathering, assessing, and synthesizing prior SRs/MAs on the use of probiotics in the treatment of RA.
2 Methods
2.1 Included and excluded criteria
The following inclusion criteria were taken into consideration: (a) SRs/MAs that used probiotics for RA were eligible; (b) patients with a clear diagnosis of RA; (c) studies comparing probiotics to placebo were eligible; (d) disease activity score (DAS), swollen joints count (SJC), level of C-reactive protein (CRP) and tumor necrosis factor-α (TNF-α) were used as outcomes. The eligibility of each article was established by consensus between the two reviewers. The following exclusion criteria were taken into consideration: (a) studies unrelated to the subject; (b) conference proceedings and protocols; (c) animal experiments.
2.2 Strategy for searching
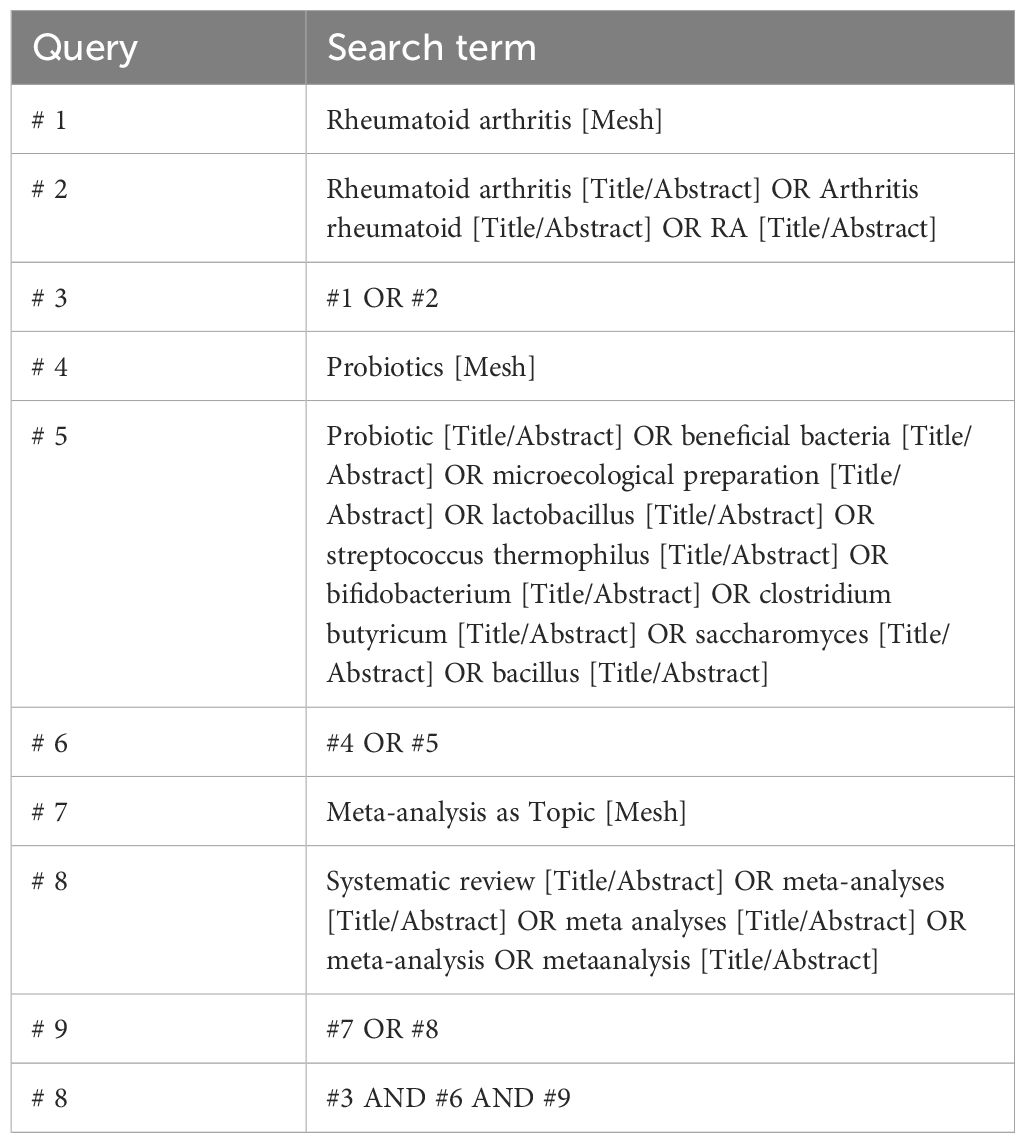
We comprehensively searched Embase, PubMed, Web of Science, Cochrane Library, and screened qualified SRs that had been released from database inception to February 12, 2024. We used a mix of free keywords and Mesh phrases to perform our search. The keywords mainly included RA, probiotics SA, and MA. The search strategy for PubMed is presented in Table 1, which was adjusted to adapt to different databases.
2.3 Data collection and extraction
Two reviewers independently conducted literature screening. All search results were imported into Endnote 20 to remove duplicates and inconsistent articles were removed based on the title and abstract. Finally, the full text was read out and eligible SRs were included. Two reviewers independently extracted the basic characteristics of eligible literature, including author, publication year, diagnostic criteria, sample size, intervention, comparison, and outcomes. Two reviewers crosschecked what was extracted, and consulted a third reviewer for any discrepancies.
2.4 Methodological evaluation
Using AMSTAR-2 tool (23), the methodological quality of the included SRs/MAs was evaluated independently by two investigators. A third investigator was consulted on any disagreement. Out of the 16 items that the tool evaluates, 7 are considered essential domains (items 2, 4, 7, 9, 11, 13, and 15). Three possibilities remain for the evaluation: “Yes,” “Partially Yes,” and “No.”
2.5 Reporting quality appraisal
Using PRISMA checklists (24), the reporting quality of the included SRs/MAs was evaluated independently by two investigators. A third investigator was consulted on any disagreement. Out of the 7 sections that the tool evaluates, 27 checklists are considered essential domains. Three possibilities remain for the evaluation: “Yes,” “Partially Yes,” and “No.”
2.6 Evidence quality evaluation
Using GRADE system (25), the evidence quality of the included SRs/MAs was evaluated independently by two investigators. A third investigator was consulted on any disagreement. The publication bias, bias risk, inconsistency, indirectness and imprecision are used to evaluate the GRADE scoring system. Four categories are used to group the results: high, moderate, low, and very low.
3 Results
3.1 Selection of literature
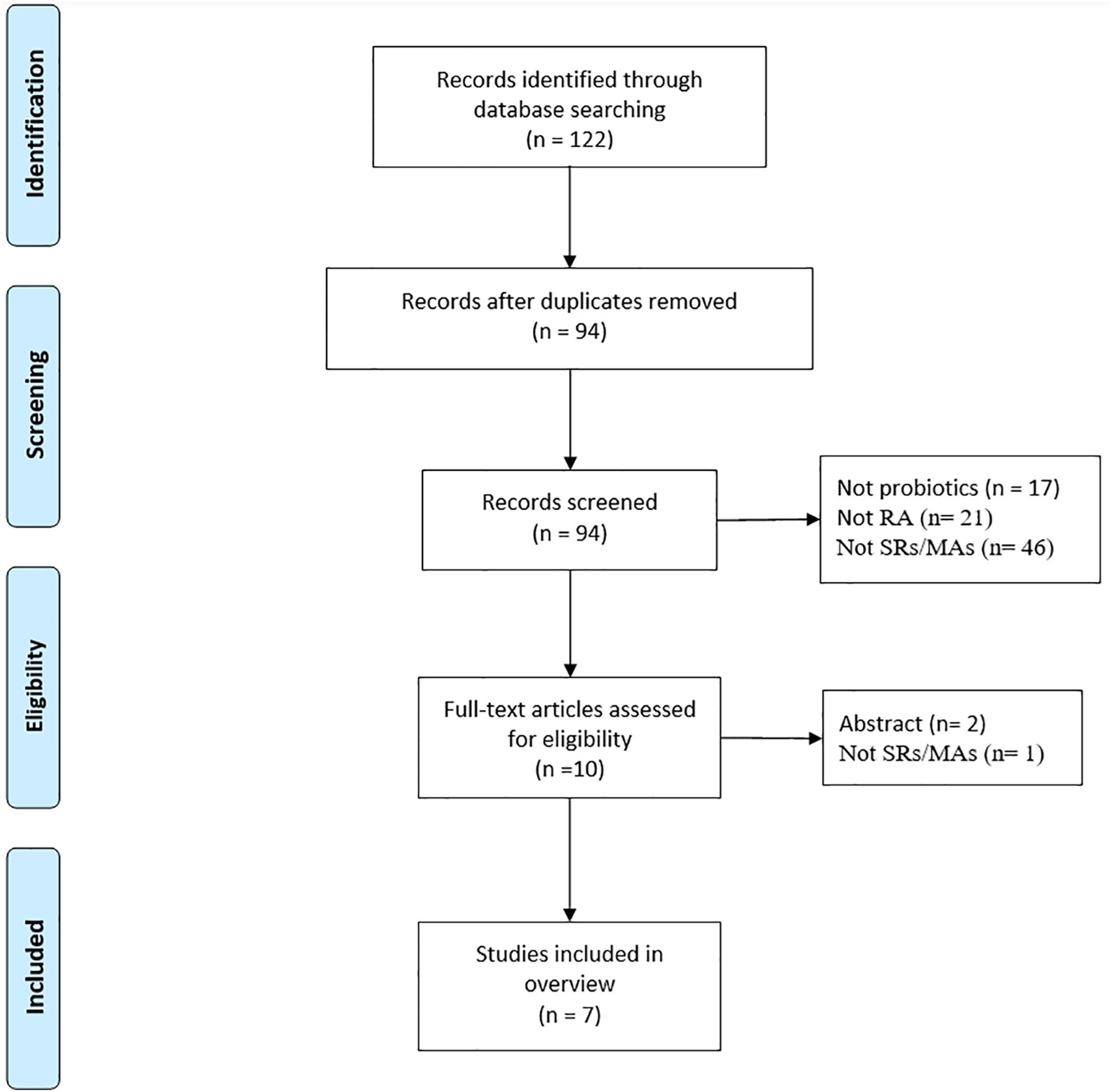
A total of 122 studies were retrieved, and 94 studies were obtained after excluding duplicate studies. After reading the titles and abstract, 10 literatures were obtained. Of the remaining studies, 3 studies were excluded: conference abstracts (n = 2), not SR/MA (n = 1). Ultimately, this review includes 12 SR-MAs (13–19) that satisfied the inclusion requirements. The literature screening process is illustrated in Figure 1.
3.2 Study characteristics
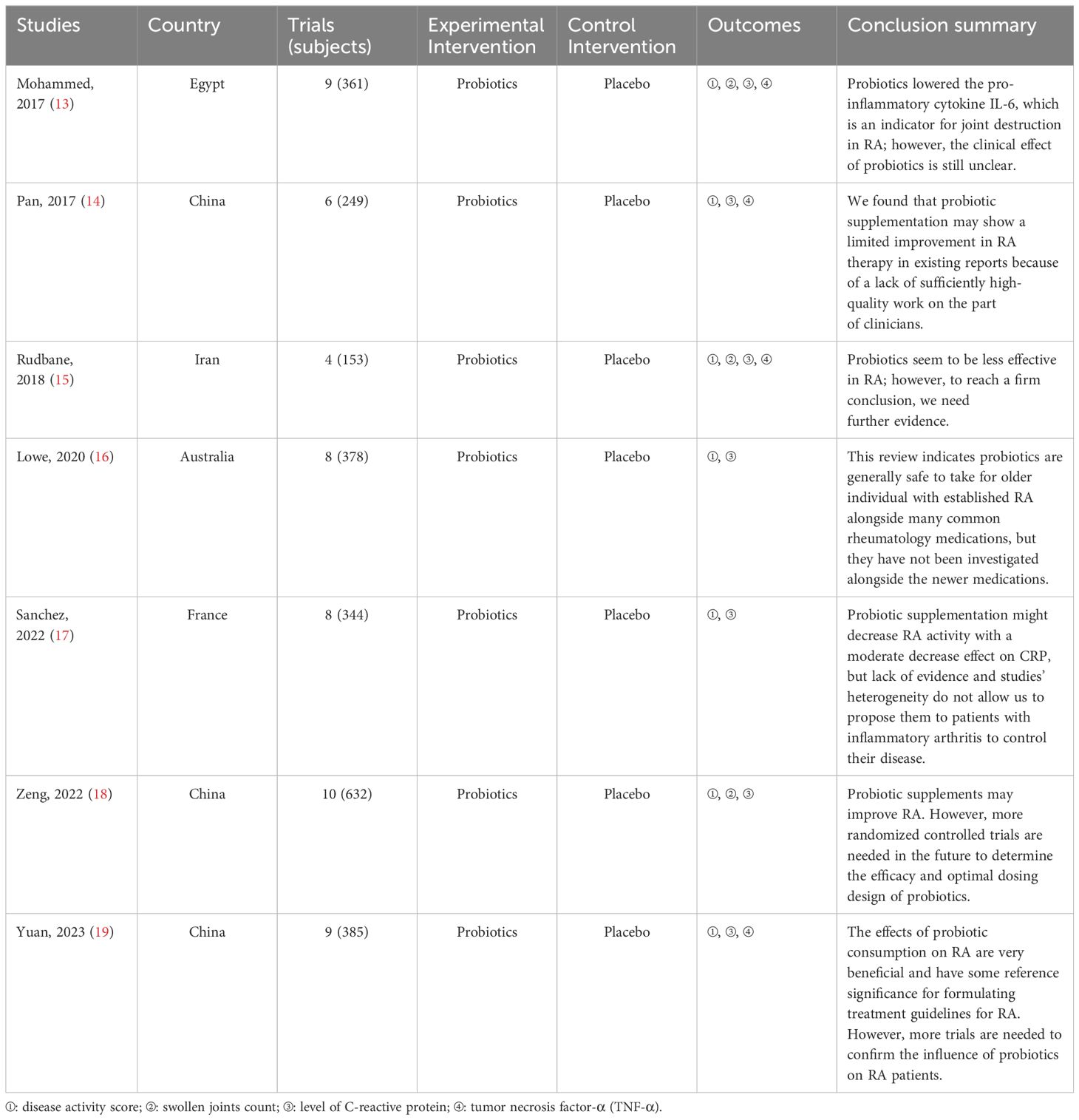
A summary of the data extracted from the seven SRs/MAs is provided in Table 2. These include the SRs/MAs published between 2017 and 2023. All reviews were published in English. The number of trails included in these SRs varied widely, ranging from 4 to 10, and the total number of participants ranged from 153 to 632. Interventions in the therapy group were probiotics, whereas placebo was used in the control group. Almost all SRs/MAs reached a positive conclusion.
3.3 Methodological evaluation
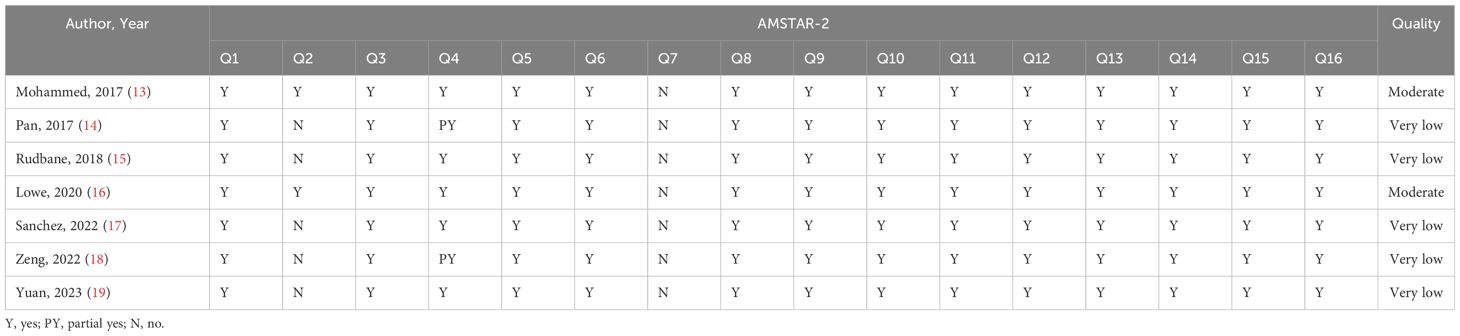
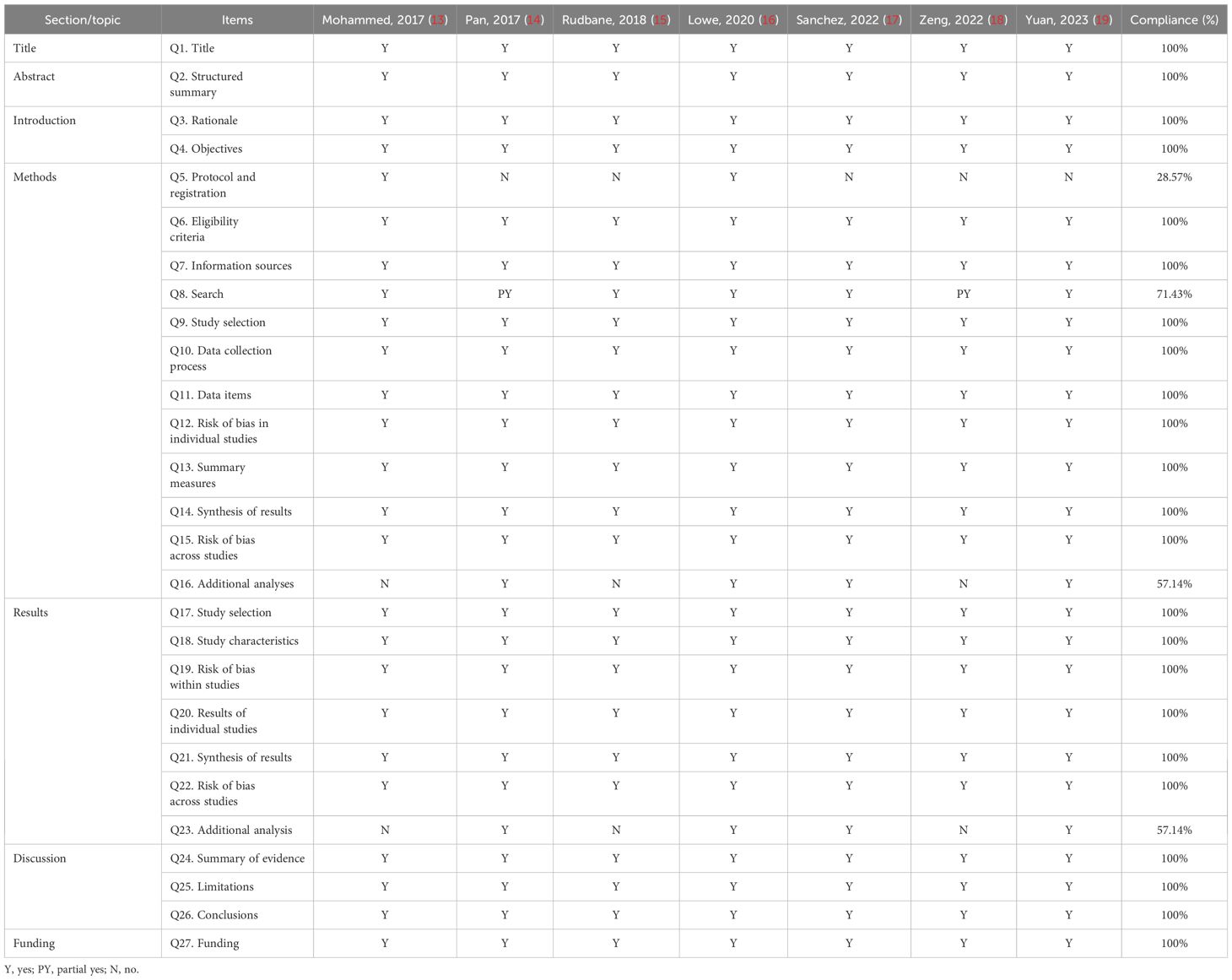
AMASTAR-2 was used to assess the methodological quality, two included studies were rated as moderate quality, and the remaining studies ware rated as critically low quality. The key factors affecting the quality of the studies included items 2 (only two reviews registered a protocol), 4 (only two reviews used a comprehensive literature search strategy), and 7 (no reviews provided a list of excluded studies and justified the exclusions). The detailed results are presented in Table 3.
3.4 Reporting quality appraisal
PRISMA was used to assess the reporting quality, and overall, the quality of reporting remains not fully satisfactory. The key factors affecting the quality of the studies included Q5 (only two reviews registered a protocol), Q8 (only two reviews used a comprehensive literature search strategy), Q16 (three reviews provided an additional analysis), and Q23 (three reviews provided an additional analysis). The detailed results are presented in Table 4.
3.5 Evidence quality evaluation
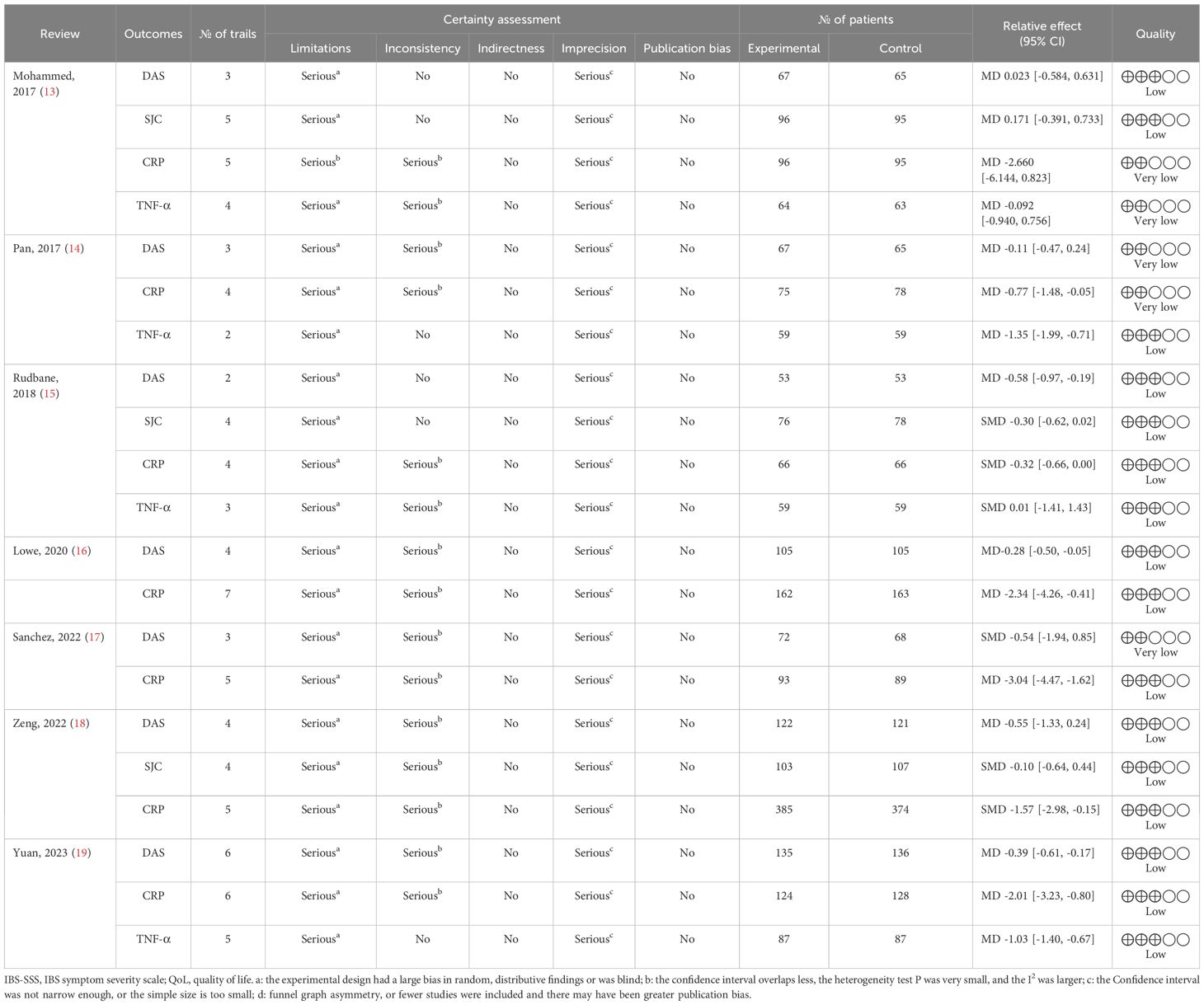
The seven SRs included 21 outcomes related to the treatment of RA with probiotics. The results showed that 16 (19.05%) were rated as low quality and 5 (80.95%) as critically low quality. The risk of bias (21/21, 100%), imprecision (21/21,100%) and inconsistency (15/21,71.43%) were the main factors in obtaining the results. The details are presented in Table 5.
4 Discussion
The level of evidence from SRs is considered to be the highest (26, 27), provided that the SRs stand up to the process of producing evidence.
4.1 A definitive conclusion cannot be reached
In the seven included articles, all literatures reported positive results, which indicated the prospect of treatment of RA with probiotics. However, due to the low methodological quality and evidence quality of the included SRs/MAs, the results of these studies should be treated with caution. At present, due to factors such as the pathogenesis of RA and the lack of objective diagnostic criteria for RA, the long-term clinical efficacy evaluation of RA is not satisfactory, and more studies are needed in the future to conduct in-depth analysis and discussion on the above issues. In addition, the existing clinical studies also have shortcomings, such as small sample size, short follow-up time, and inconsistent efficacy evaluation. There is still insufficient evidence to confirm the superiority of probiotics treatment for RA. In the future, it is necessary to improve the quality of the original trails and conduct more prospective clinical studies with large samples and multiple centers to provide more high-quality evidence-based evidence for clinical workers to apply probiotics treatment for RA.
4.2 Research deficiencies to be improved
No SR was rated as high quality according to AMSTAR-2 tool, and no SR reported all 27 entries according to PRISMA. The risk of bias was high of the included SRs for the following reasons: no comprehensive retrieval strategy was adopted, failure to provide a list of excluded documents with reasons, evaluation and discussion of publication bias and evidence credibility, insufficient analysis of sources of heterogeneity and their impact on results, and lack of reporting and discussion of registration options and funding sources. According to the Cochrane handbook, all systematic evaluations should report the registered protocol and registration number in the methodology section (28). Unfortunately, none of the included studies declared that they had registered the protocol and provided the registration number, so we determined that they did not have a prior registered protocol, which increases the risk of bias while decreasing the transparency of the study (29). In addition, additional analyses, such as subgroup analysis, sensitivity analysis, and publication bias assessment, were not performed in the included studies when performing MA, which means that the efficacy of different probiotics for different populations of RA has not been precisely explored, and the results of current SRs are not necessarily robust and are likely to be altered by further analysis (30). According to GRADE evaluation results, all included outcomes were low or extremely low, indicating that the conclusions of the included SRs/MAs are likely to be significantly different from the actual situation. The main factors contributing to the evidence downgrade were risk of bias, imprecision and inconsistency. Almost all the original SR/MA-included trials had some defects in randomization, hiding blindness, and follow-up, and the risk of publication bias was high. Small sample sizes trails with a lack of randomization, blinding, and allocation concealment cause evidence quality to range from moderate to very low. To guarantee the availability of evidence, future SRs/MAs must be planned and carried out strictly in accordance with AMSTAR-2 and PRISMA.
4.3 Strengths and limitations
Insofar as we are aware, this study offers the first thorough evaluation and synopsis of the evidence bolstering probiotic usage in RA. But there are some limitations that need to be recognized. First, the outcomes of various original studies included in the relevant SR/MA are different, and the number of original studies and sample size of some outcome indicators are small, which can lead to low evidence strength of outcome indicators. Furthermore, due to the large difference in the outcome indicators of the included studies, quantitative combination and analysis were not carried out in this study, and only the study was described according to the conclusion of the original text.
5 Conclusion
Probiotics may have a therapeutic effect on RA, based on the evidence provided by the SRs/MAs in this overview. Nevertheless, there is still a lack of conclusive evidence due to methodological limitations in the included research. To make trustworthy judgments regarding the efficacy of probiotics in the treatment of RA, more large-scale, high-quality randomized controlled trials are still required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
WL: Conceptualization, Writing – original draft. YZ: Conceptualization, Data curation, Writing – original draft. DG: Conceptualization, Methodology, Writing – original draft. RG: Conceptualization, Data curation, Writing – original draft. JY: Conceptualization, Data curation, Writing – original draft. HY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was received by Implementation plan of high level key discipline construction project of traditional Chinese medicine of the State Administration of traditional Chinese Medicine, China (No.[2022]226); Science and Technology Program of Lanzhou, China (2022-ZD-73); Administration of Traditional Chinese Medicine of Gansu Province, China(GZKP-2022-17); the Project of Director of Gansu Famous Doctors Studio, China (No. [2022] 50); The Key Talent Project of Gansu Province(No. [2023]20): Development and transformation of clinical talent cultivation based on the needle knife medical platform; National Natural Science Foundation of China (No. 82274481); Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2023Y9230).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smith MH, Berman JR. What is rheumatoid arthritis? JAMA. (2022) 327:1194. doi: 10.1001/jama.2022.0786
2. Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. (2005) 53:191–209; quiz 210–2. doi: 10.1016/j.jaad.2004.07.023
3. Lopez-Corbeto M, Martínez-Mateu S, Pluma A, Ferrer R, López-Lasanta M, De Agustín JJ, et al. The ovarian reserve as measured by the anti-Müllerian hormone is not diminished in patients with rheumatoid arthritis compared to the healthy population. Clin Exp Rheumatol. (2021) 39:337–43. doi: 10.55563/clinexprheumatol/73txen
4. Zhao J, Guo S, Schrodi SJ, He D. Molecular and cellular heterogeneity in rheumatoid arthritis: mechanisms and clinical implications. Front Immunol. (2021) 12:790122. doi: 10.3389/fimmu.2021.790122
5. Bergot AS, Giri R, Thomas R. The microbiome and rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2019) 33:101497. doi: 10.1016/j.berh.2020.101497
6. Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. (2011) 7:569–78. doi: 10.1038/nrrheum.2011.121
7. Saarela M, Lähteenmäki L, Crittenden R, Salminen S, Mattila-Sandholm T. Gut bacteria and health foods–the European perspective. Int J Food Microbiol. (2002) 78:99–117. doi: 10.1016/S0168-1605(02)00235-0
8. Peltonen R, Nenonen M, Helve T, Hänninen O, Toivanen P, Eerola E. Faecal microbial flora and disease activity in rheumatoid arthritis during a vegan diet. Br J Rheumatol. (1997) 36:64–8. doi: 10.1093/rheumatology/36.1.64
9. Müller H, de Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol. (2001) 30:1–10.
10. Zhao T, Wei Y, Zhu Y, Xie Z, Hai Q, Li Z, et al. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front Immunol. (2022) 13:1007165. doi: 10.3389/fimmu.2022.1007165
11. Bungau SG, Behl T, Singh A, Sehgal A, Singh S, Chigurupati S, et al. Targeting probiotics in rheumatoid arthritis. Nutrients. (2021) 13:3376. doi: 10.3390/nu13103376
12. Ferro M, Charneca S, Dourado E, Guerreiro CS, Fonseca JE. Probiotic supplementation for rheumatoid arthritis: A promising adjuvant therapy in the gut microbiome era. Front Pharmacol. (2021) 12:711788. doi: 10.3389/fphar.2021.711788
13. Mohammed AT, Khattab M, Ahmed AM, Turk T, Sakr N, M Khalil A, et al. The therapeutic effect of probiotics on rheumatoid arthritis: a systematic review and meta-analysis of randomized control trials. Clin Rheumatol. (2017) 36:2697–707. doi: 10.1007/s10067-017-3814-3
14. Pan H, Li R, Li T, Wang J, Liu L. Whether probiotic supplementation benefits rheumatoid arthritis patients: A systematic review and meta-analysis. Engineering. (2017) 3:115–21. doi: 10.1016/J.ENG.2017.01.006
15. Aqaeinezhad Rudbane SM, Rahmdel S, Abdollahzadeh SM, Zare M, Bazrafshan A, Mazloomi SM. The efficacy of probiotic supplementation in rheumatoid arthritis: a meta-analysis of randomized, controlled trials. Inflammopharmacology. (2018) 26:67–76. doi: 10.1007/s10787-017-0436-y
16. Lowe JR, Briggs AM, Whittle S, Stephenson MD. A systematic review of the effects of probiotic administration in inflammatory arthritis. Complement Ther Clin Pract. (2020) 40:101207. doi: 10.1016/j.ctcp.2020.101207
17. Sanchez P, Letarouilly JG, Nguyen Y, Sigaux J, Barnetche T, Czernichow S, et al. Efficacy of probiotics in rheumatoid arthritis and spondyloarthritis: A systematic review and meta-analysis of randomized controlled trials. Nutrients. (2022) 14:354. doi: 10.3390/nu14020354
18. Zeng L, Deng Y, He Q, Yang K, Li J, Xiang W, et al. Safety and efficacy of probiotic supplementation in 8 types of inflammatory arthritis: A systematic review and meta-analysis of 34 randomized controlled trials. Front Immunol. (2022) 13:961325. doi: 10.3389/fimmu.2022.961325
19. Yuan Y, Ji W, Lin Z, Gan K. Benefits of probiotics in rheumatoid arthritis patients: A systematic review and meta-analysis. Trop J Pharm Res. (2023) 22:399–406. doi: 10.4314/tjpr.v22i2.24
20. Huang J, Zhang J, Wang Y, Ma J, Yang X, Guo X, et al. Scientific evidence of chinese herbal medicine (Gegen qinlian decoction) in the treatment of ulcerative colitis. Gastroenterol Res Pract. (2022) 2022:7942845. doi: 10.1155/2022/7942845
21. Huang J, Lu M, Zheng Y, Ma J, Ma X, Wang Y, et al. Quality of evidence supporting the role of acupuncture for the treatment of irritable bowel syndrome. Pain Res Manag. (2021) 2021:2752246. doi: 10.1155/2021/2752246
22. Saunders H, Gallagher-Ford L, Kvist T, Vehviläinen-Julkunen K. Practicing healthcare professionals' Evidence-based practice competencies: an overview of systematic reviews. Worldviews Evid Based Nurs. (2019) 16:176–85. doi: 10.1111/wvn.12363
23. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
25. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
26. Yang K, Zhang J, Zhao L, Cheng L, Li Y, Kang Y, et al. An umbrella review of Lianhua Qingwen combined with Western medicine for the treatment of coronavirus disease 2019. Acupuncture Herbal Med. (2022) 2:143−151. doi: 10.1097/HM9.0000000000000041
27. Chen Z, Jiang T, Peng Y, Qiang X, Yang F, Hu H, et al. Acupuncture and moxibustion treating lower urinary tract symptoms due to benign prostatic hyperplasia: a systematic review and network meta-analysis. Acupuncture Herbal Med. (2022) 2:84−90. doi: 10.1097/HM9.0000000000000029
28. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858
29. Huang J, Liu H, Chen J, Cai X, Huang Y. The effectiveness of tai chi in patients with breast cancer: an overview of systematic reviews and meta-analyses. J Pain Symptom Manage. (2021) 61:1052–9. doi: 10.1016/j.jpainsymman.2020.10.007
Keywords: rheumatoid arthritis, probiotics, evidence, treatment, adjunctive therapy
Citation: Li W, Zhang Y, Guo D, Gong R, Yuan J and Yang H (2024) Quality of evidence supporting the role of probiotics for rheumatoid arthritis: an overview of systematic reviews. Front. Immunol. 15:1397716. doi: 10.3389/fimmu.2024.1397716
Received: 08 March 2024; Accepted: 13 May 2024;
Published: 30 May 2024.
Edited by:
Jesús Muñoz-Rojas, Meritorious Autonomous University of Puebla, MexicoReviewed by:
Liliana Lopez Pliego, Benemerita Universidad Autonoma de Puebla, MexicoAndrés Corral Lugo, Carlos III Health Institute (ISCIII), Spain
Copyright © 2024 Li, Zhang, Guo, Gong, Yuan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijun Yang, eWhqdGNtMTY5OTlAMTYzLmNvbQ==
 Weiqing Li1
Weiqing Li1 Huijun Yang
Huijun Yang