- 1Phase 1 Clinical Trial Laboratory, Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 2Department of Scientific Research, Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 3Department of Clinical Pharmacy, Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Objectives: Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of non-small cell lung cancer (NSCLC). However, the application of ICIs can also cause treatment-related adverse events (trAEs) and immune-related adverse events (irAEs). This study was to evaluate both the irAEs and trAEs of different ICI strategies for NSCLC based on randomized clinical trials (RCTs). The study also examined real-world pharmacovigilance data from the Food and Drug Administration Adverse Event Reporting System (FAERS) regarding claimed ICI-associated AEs in clinical practice.
Methods: Based on Pubmed, Embase, Medline, and the Cochrane CENTRAL, we retrieved RCTs comparing ICIs with chemotherapy drugs or with different ICI regimens for the treatment of NSCLC up to October 20, 2023. Bayesian network meta-analysis (NMA) was performed using odds ratios (ORs) with 95% credible intervals (95%CrI). Separately, a retrospective pharmacovigilance study was performed based on FAERS database, extracting ICI-associated AEs in NSCLC patients between the first quarter (Q1) of 2004 and Q4 of 2023. The proportional reports reporting odds ratio was calculated to analyze the disproportionality.
Results: The NMA included 51 RCTs that involved a total of 26,958 patients with NSCLC. Based on the lowest risk of any trAEs, cemiplimab, tislelizumab, and durvalumab were ranked as the best. Among the agents associated with the lowest risk of grades 3-5 trAEs, tislelizumab, avelumab, and nivolumab were most likely to rank highest. As far as any or grades 3-5 irAEs are concerned, atezolizumab plus bevacizumab plus chemotherapy is considered the most safety option. However, it is associated with a high risk of grades 3-5 trAEs. As a result of FAERS pharmacovigilance data analysis, 9,420 AEs cases have been identified in 7,339 NSCLC patients treated with ICIs, and ICIs were related to statistically significant positive signal with 311 preferred terms (PTs), and comprehensively investigated and identified those AEs highly associated with ICIs. In total, 152 significant signals were associated with Nivolumab, with malignant neoplasm progression, death, and hypothyroidism being the most frequent PTs.
Conclusion: These findings revealed that ICIs differed in their safety profile. ICI treatment strategies can be improved and preventive methods can be developed for NSCLC patients based on our results.
1 Introduction
The prevalence and mortality of lung cancer are among the highest in the world (1). Nearly 85% of lung cancers are non-small cell lung cancers (NSCLC), and about 30% of patients diagnosed with NSCLC have locally advanced disease (stage III) (2). Over the past decade, immunotherapy has demonstrated promise when it comes to treat NSCLC. There has been a significant transformation in the environment of therapeutic for variety of cancer types due to immune checkpoint inhibitors (ICIs), including agents that target the programmed death-ligand 1 (PD-L1), programmed death-1 receptor (PD-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (3).
It has been demonstrated that ICIs are related to superior survival outcomes among patients with NSCLC in randomized clinical trials (RCTs) as compared to chemotherapy drugs that have been traditionally used. ICIs have proven to provide survival benefits (2), but they can also cause adverse events (AEs), including cutaneous reactions, including morbilliform, psoriasiform, lichenoid, eczematous, and rash (4, 5). Due to their ability to block the pathways that are involved in regulating the immune system, ICIs may associate with high risk of immune-related adverse events (irAEs) by inducing inflammation in the organs (6). ICI-associated irAEs can potentially involve multiple system organ classes (SOC), including the skin (eg, rash and pruritus) (7), gastrointestinal tract (eg, diarrhea and colitis) (8), endocrine (eg, hypothyroidism and hypophysitis) (9), lung (eg, pneumonitis) (7), and psychiatric disorders (eg, delirium) (10). Without proper management, irAEs can be severe and life-threatening and may result in treatment discontinuation or failure.
In previously published network meta-analysis (NMA), irAEs related to ICI therapy were primarily examined, but no studies have examined irAEs related to System Organ Classes (SOC) and treatment-related adverse events (trAEs) (11–14). Also, most of these published studies did not specifically evaluate the risk of trAEs associated with different ICI regimens, which may vary depending on the regimen. Furthermore, pharmacovigilance data from real-world clinical practice were not evaluated simultaneously. The use of ICIs has revolutionized cancer treatment standards and significantly enhanced patient prognoses. However, the utilization of these groundbreaking therapies has led to the observation and reporting of various types of adverse events, commonly known as irAEs (15). We conducted a NMA evaluating both the irAEs and trAEs due to no head-to-head RCTs comparing different ICI strategies. The study also examined real-world pharmacovigilance data from the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) regarding claimed ICI-associated AEs in clinical practice (16).
2 Methods
2.1 Study design
A NMA of RCTs was conducted to determine the risks associated with trAEs and irAEs in NSCLC patients receiving ICI therapy. The FAERS database was further analyzed retrospectively to determine the risk of AEs among NSCLC patients.
2.2 Systematic review procedures
2.2.1 Data sources and searches
This study was registered in PROSPERO (CRD42022324171). The NMA was performed according to the PRISMA checklist (17). Pubmed, Embase, and Cochrane CENTRAL were systematic searched, with no language restrictions, up to October 20, 2023.
2.2.2 Study selection and data extraction
We included RCTs that compared an ICI (eg, nivolumab, pembrolizumab) or a combination therapy of ICIs with (1) placebo, or (2) a chemotherapy drug (eg, carboplatin, cisplatin, pemetrexed, paclitaxel), or (3) a different ICI for patients with NSCLC. Data extraction was performed by two authors (XY L and HW X) independently. The third reviewer was responsible for adjudicating any disagreement between the two investigators (Y L). We obtained the following outcomes for each included study: (1) study information (ie, phase, design, source, and registered ID), (2) baseline characteristics (eg, sex, age, histology), and (3) interventions and outcomes (ie, population size, different treatment regimens, and different trAEs or irAEs number).
Two authors (HJ L and XY C) independently evaluated the risk-of-bias of included studies using the risk-of-bias tool 2.0 (18).
2.2.3 Outcome measures
In this study, the primary outcome was any trAEs or irAEs (severity grade 1-5), as well as severe trAEs or irAEs (severity grade 3-5) (19). There were five grades: mild-to-moderate AEs (grades 1 and 2), severe or medically significant but not immediately AEs (grade 3), life-threatening AEs (grade 4), and death-related AEs (grade 5) (20). In NMA, trAEs and irAEs were defined according to how each RCT reported its trAEs and irAEs. There was a strong association between trAEs and irAEs and the intervention treatment in the trial. Additionally, secondary outcomes included trAEs and irAEs specific to each SOC. AEs are standardized using the SOC of the Medical Dictionary for Regulatory Activities (MedDRA) terminology (21), which contains 27 SOCs. Consequently, MedDRA (version 25.0) was used to categorize AEs to their related SOC levels for each report.
2.2.4 Data Synthesis and statistical analysis
Data synthesis was performed using the “gemtc” package and a Bayesian NMA using R software (R Foundation for Statistical Computing, Vienna, Austria). Network plots of different treatment regimens were carried out using the “netmeta” package. We used a Markov chain Monte Carlo simulation using vague priors with four chains, with a burn-in period of 50,000 iterations, followed by 500,000 iterations. An odds ratio (OR) with a 95% credible interval (CrI) was reported as the effect estimate of the NMA. Matrix plots were used to illustrate the NMA estimates. To depict the ranking of the interventions, the surface under the cumulative ranking curve (SUCRA) was calculated. A narrative review was used when it was not possible to synthesize the data from the trials in an NMA. Among the treatments, the one associated with the lowest AE risk was ranked as the superior treatment regimen.
2.3 Pharmacovigilance study procedures
2.3.1 Data source
As part of our pharmacovigilance study, we examined AEs related to ICIs in patients with NSCLC using the FAERS database, which is a publicly accessible database of safety reports (22). ICIs and NSCLC were obtained as search items to search report data of ICIs and NSCLC from the FAERS between the decade quarter (Q1) of 2004 and Q4 of 2023. We included only cases in which ICIs for the therapy of NSCLC patients were used by the primary suspect (PS). The FAERS database also codes reported AEs according to preferred term (PT) codes from the MedDRA, which are logically categorized into five levels. PTs represent distinct descriptions of a single medical concept. The hierarchy also includes “high-level terms” (HLTs) and “high-level group terms” (HLGTs). SOCs are further divided into aetiologies, sites of presentation, and purposes of HLGTs. It is important to note that different PTs are classified into different SOCs, but unique primary SOC can be connected, a characteristic known as multiaxiality. MedDRA (version 25.0) was used and PTs with primary SOC = “Yes” were obtained and analyzed, thereby ensuring that the PTs analyzed were clinically relevant AEs.
2.3.2 Signal mining
It is commonly used in pharmacovigilance studies as a method for evaluating potential associations between different types of AE and a particular drug, which can then be evaluated clinically through the assessment of individual case reports (23). Reporting odds ratio (ROR) reflects the likelihood of a particular drug being reported for a specific event of interest in comparison to the likelihood of reporting events for other drugs in the FAERS database (24), and ROR was calculated in this study to evaluate AE signals in ICI reports among NSCLC patients (10). Using full AE reports of NSCLC patients from the FAERS database as comparators, we conducted disproportionality analysis to evaluated possible relationship between AEs and ICIs in NSCLC patients by the ROR (25). Initially, we created a drug AE contingency table and then calculated ROR based on that table before performing the disproportionality analysis of ICI-related AE (26). It was determined that an AE signal was related to different ICI regimens if there were at least three reports of PT levels AEs, and the lower limit of the 95% CI of the ROR did not fall below one.
2.3.3 Descriptive analysis
In our study, we examined the clinical characteristics of NSCLC patients resulting from ICI-related AEs, including sex, age, age group, country, outcome, the most recent FDA acceptance year, the ICI regimens, the priority of the case, and the type of report. Death, disability, and life-threatening outcomes were reported as serious outcomes. Data importing and analysis were conducted using PostgreSQL (version 14) and R, based on previous published literature (26).
3 Results
3.1 Characteristics and quality of included studies
After searching the databases for 7,068 articles, 51 RCTs were included in the NMA, which included 26,958 patients (Supplementary Figure 1) (27–77). We summarize the detailed baseline characteristics of the included studies in Supplementary Table 1. A total of 34 studies showed a low risk of bias, while other studies had a ‘some concerns’ risk of bias in randomization and deviations from the intended intervention, as depicted in Supplementary Table 2.
3.2 Network meta-analysis outcomes
3.2.1 Primary outcomes: ranking the probability of treatment regimen
For any or grade 3-5 trAEs, network plots are displayed in Figure 1A and each comparison results of ORs and 95% Crls are revealed in Figure 2, and each rank probability of each ICI are shown in Figure 3. Regarding any trAEs, cemiplimab (Cemip), tislelizumab (Tisle), and durvalumab (Durva) were the lowest risk of any trAEs are likely to rank as the best following by pembrolizumab (Pem), avelumab (Ave), Durva plus tremelimumab (Durva-Treme), atezolizumab (Atezo) and nivolumab (Nivo), and there was a greater degree of safety than most other ICIs (ORs ranging between 0.01 and 0.61), whereas camrelizumab plus chemotherapy (Camre-Chemo) and sugemalimab plus chemotherapy (Suge-Chemo) were among the least safety drugs. In terms of grades 3-5 trAEs, Tisle, Ave, and Nivo were most likely to rank highest due to their association with the lowest risk of grades 3-5 trAEs following by Cemip, Atezo, Pem, sintilimab (Sinti), and Durva and they were more safety than most of other ICIs (ORs ranging between 0.04 and 0.74), whereas Durva-Treme-Chemo, Sinti-Chemo, and atezolizumab plus bevacizumab plus chemotherapy (Atezo-Bevac-Chemo) were among the least safety ICIs.
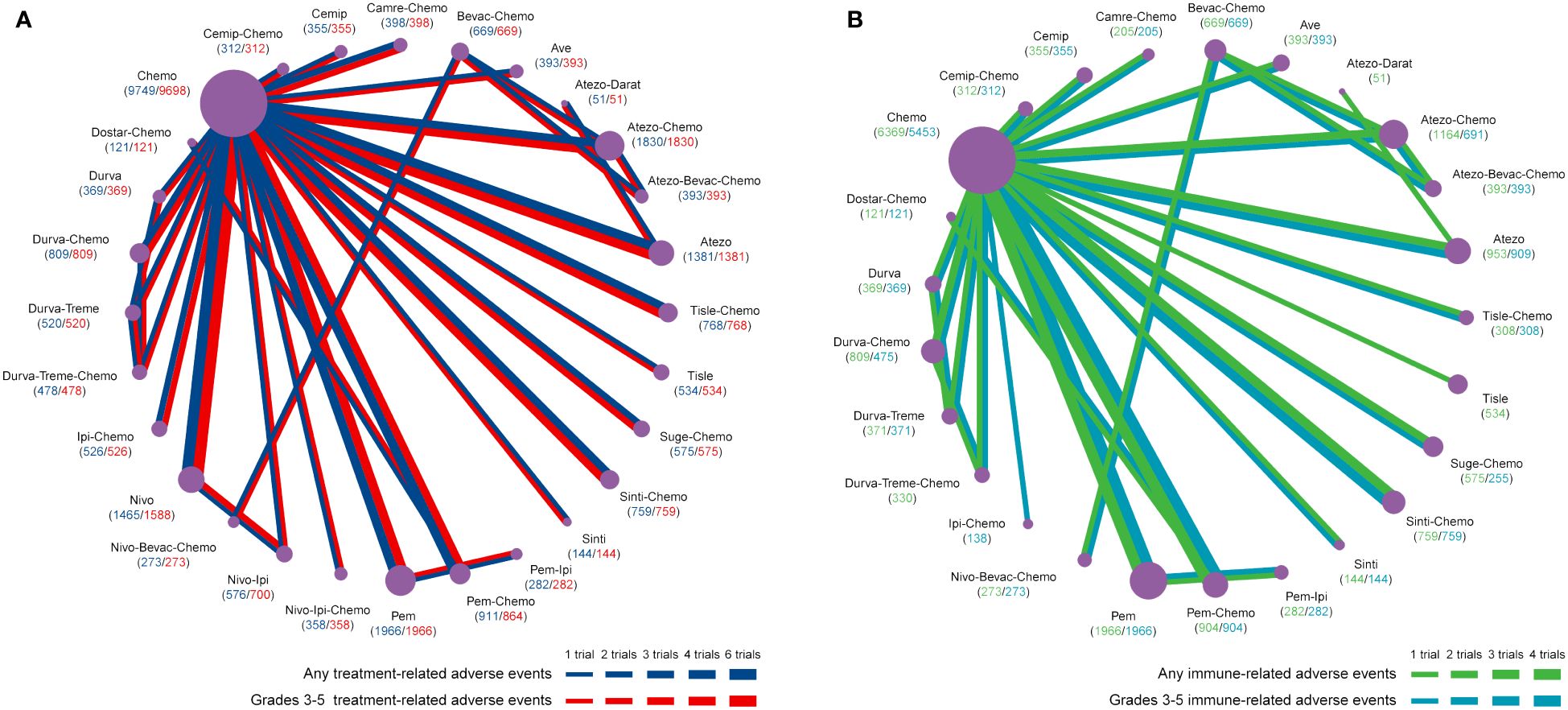
Figure 1 Network plots of adverse events of ICIs for NSCLC. (A) Comparisons were performed on any and grades 3-5 treatment-related adverse events. (B) Comparisons were performed on any and grade 3-5 immune-related adverse events. Atezo, atezolizumab; Ave, avelumab; Beva, bevacizumab; Camre, camrelizumab; Cemip, cemiplimab; Chemo, chemotherapy; Darat, daratumumab; Dostra, dostarlimab; Durva, durvalumab; ICI, immune checkpoint inhibitor; Ipi, ipilimumab; Nivo, nivolumab; NSCLC, non-small cell lung cancer; Pem, pembrolizumab; Sint, sintilimab; Sugema, sugemalimab; Tisle, tislelizumab; Treme, tremelimumab.
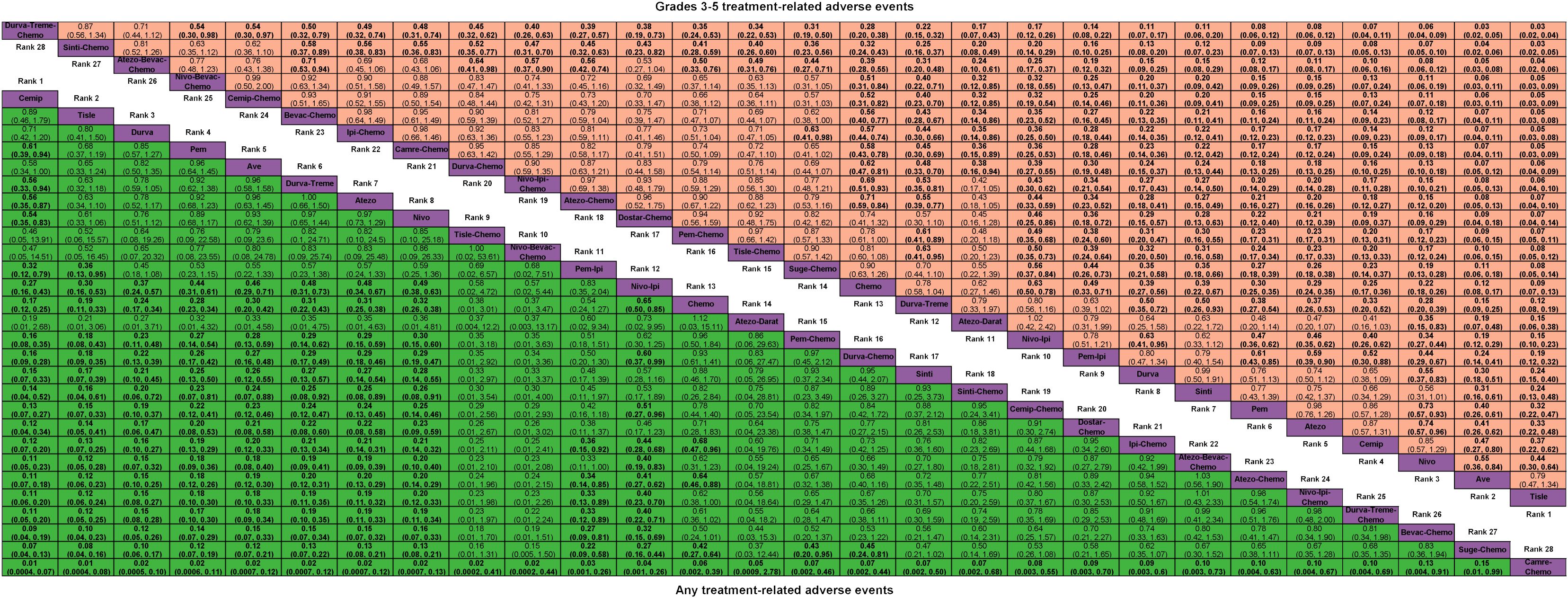
Figure 2 Odds ratio (95% CrI) of any and grades 3-5 treatment-related adverse events associated with each treatment regimen. Atezo, atezolizumab; Ave, avelumab; Beva, bevacizumab; Camre, camrelizumab; Cemip, cemiplimab; Chemo, chemotherapy; CrI, credible interval; Darat, daratumumab; Dostra, dostarlimab; Durva, durvalumab; Ipi, ipilimumab; Nivo, nivolumab; Pem, pembrolizumab; Sint, sintilimab; Sugema, sugemalimab; Tisle, tislelizumab; Treme, tremelimumab.
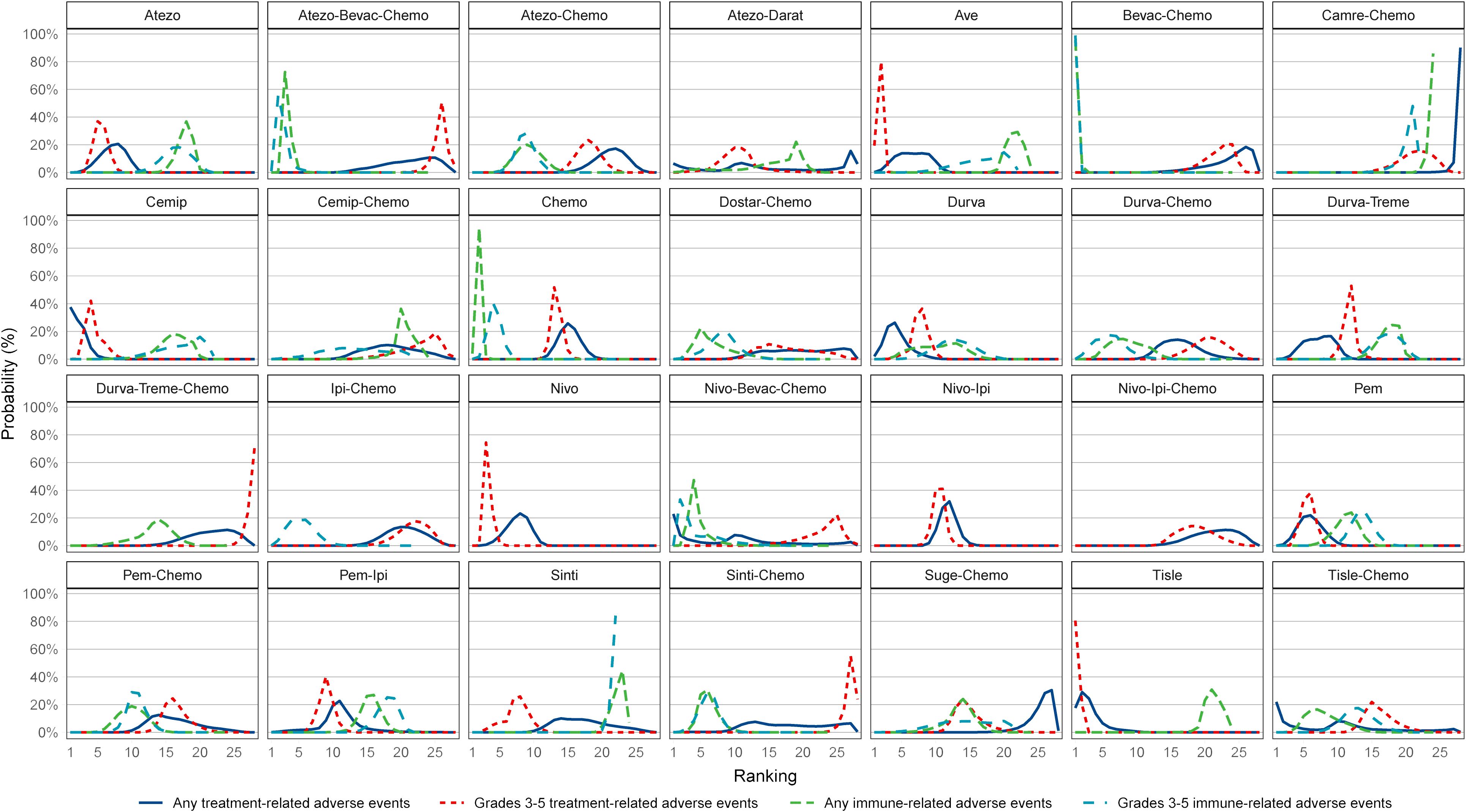
Figure 3 Ranking of the probability of being the best treatment regimen. Atezo, atezolizumab; Ave, avelumab; Beva, bevacizumab; Camre, camrelizumab; Cemip, cemiplimab; Chemo, chemotherapy; CrI, credible interval; Darat, daratumumab; Dostra, dostarlimab; Durva, durvalumab; Ipi, ipilimumab; Nivo, nivolumab; Pem, pembrolizumab; Sint, sintilimab; Sugema, sugemalimab; Tisle, tislelizumab; Treme, tremelimumab.
Network plots of any or grade 3-5 irAEs are displayed in Figure 1B and each comparison results of ORs and 95% Crls are displayed in Figure 4, and each rank probability of each ICI are displayed in Figure 3. Regarding any irAEs, Bevac-Chemo, Chemo, and Atezo-Bevac-Chemo associated with the lowest risk and provided greater safety than most of the other ICIs (ORs ranging between 0.0003 and 0.57), they were most likely to rank as the best, whereas Camre-Chemo, Sinti, and Ave were among the least safety ICIs. In terms of grades 3-5 irAEs, Bevac-Chemo, Atezo-Bevac-Chemo, and Chemo associated with the lowest risk and were safer than most other ICIs (ORs ranging between 0.0001 and 0.40), they were most likely to rank as the best, whereas Sinti, Camre-Chemo, and Pem plus ipilimumab (Pem-Ipi) were among the least safety ICIs.
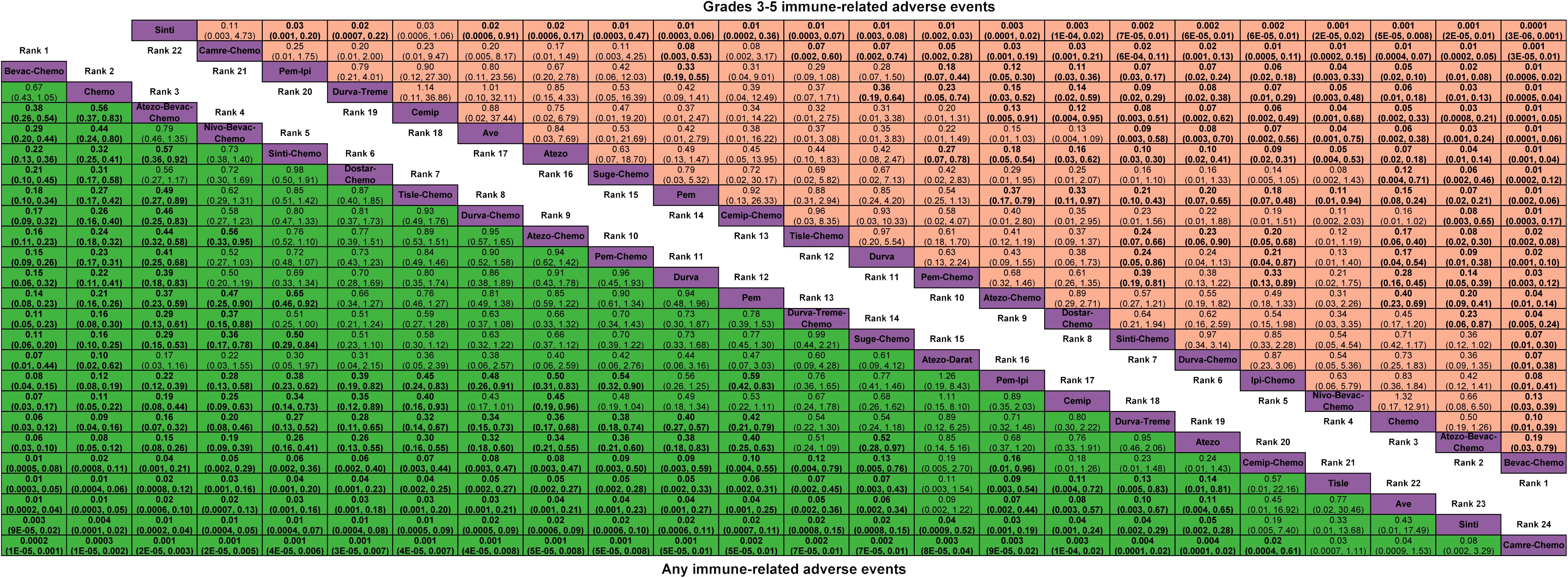
Figure 4 Odds ratio (95% CrI) of any and grades 3-5 immune-related adverse events associated with each treatment regimen. Atezo, atezolizumab; Ave, avelumab; Beva, bevacizumab; Camre, camrelizumab; Cemip, cemiplimab; Chemo, chemotherapy; CrI, credible interval; Darat, daratumumab; Dostra, dostarlimab; Durva, durvalumab; Ipi, ipilimumab; Nivo, nivolumab; Pem, pembrolizumab; Sint, sintilimab; Sugema, sugemalimab; Tisle, tislelizumab; Treme, tremelimumab.
3.2.2 Secondary outcomes: ranking the probability of treatment regimen
The ranking probability of an ICI treatment regimen having the lowest risk is dependent on the SOC, as well as on the irAE and trAE. We describe herein the secondary outcomes that are statistically significant. We evaluated trAEs using 13 SOCs and irAEs using seven SOCs in this study. Network plots of secondary outcomes are shown in Supplementary Figures 2, 3 and each comparison results of ORs and 95% Crls are shown in Supplementary Figures 4, 5, and each rank probability of each ICI regimen are shown in Supplementary Figures 6, 7.
First, we evaluated the trAEs of different SOCs. Sinti, Cemip, and Atezo-Chemo were ranked as having the lowest risk of blood and lymphatic system disorders (ORs ranging between 0.0002 and 0.64), whereas Durva-Treme-Chemo was the least safe. Chemo had the lowest risk of endocrine disorders, followed by Suge-Chemo and Atezo-Chemo (ORs ranging between 0.008 and 0.26), whereas Nivo-Ipi-Chemo and Nivo-Ipi was the lowest safety regimen. There was the lowest risk of gastrointestinal disorders related to Durva, Nivo, Durva-Treme, and Pem (ORs ranging from 0.02 to 0.63), whereas Pem-Chemo appeared to be among the most unsafe ICI regimens. The lowest-ranking ICIs in terms of general disorders and administration site conditions were Cemip, Nivo, Ave, and Durva (ORs ranging from 0.18 to 0.69), while Durva-Treme-Chemo was considered one of the least safe ICIs. It was noted that Atezo, Nivo-Ipi, and Tisle were ranked as the lowest risk ICIs in terms of investigations (ORs ranging from 0.002 to 0.42) while Cemip-Chemo was ranked as the least safe ICI. Among the ICIs tested for metabolism and nutrition disorders, Tisle, Durva, and Cemip displayed the lowest risks (ORs ranging from 0.15 to 0.59), whereas Tisle-Chemo presented the lowest level of safety. It was found that Ave had the lowest risk of musculoskeletal and connective tissue disorders (ORs between 0.01 and 0.30), whereas Atezo-Chemo had the least safety. In terms of nervous system disorders, Pem, Cemip and Nivo were most likely to rank as the lowest risk (ORs ranging between 0.02 and 0.49), whereas Atezo-Chemo was among the least safety ICIs. Pem-Chemo has the lowest risk of respiratory, thoracic, and mediastinal disorders (ORs ranging from 0.01 to 0.78), whereas Pem-Ipi has the greatest risk. Ave, Tisle and Atezo were most likely to rank as the lowest risk ICIs in terms of skin and subcutaneous tissue disorders (ORs ranging from 0.0004 to 0.05), whereas Nivo-Ipi-Chemo ranked among the lowest in safety terms. Ave (ORs ranging between 0.01 and 0.15), was the ICI regimen associated with the lowest risks of infections and infestations, whereas Atezo-Darat was among the least safe.
Second, we evaluated the irAEs of different SOC. Among the ICIs, Durva was associated with a low risk of endocrine disorders, however, most of the comparisons did not reveal any significant difference, whereas Nivo-Ipi-Chemo was associated with a low safety profile. There was a low rate of gastrointestinal disorders associated with Nivo (ORs ranging between 0.01 and 0.64) while Nivo-Ipi-Chemo was one of the least safely associated ICIs. Chemo and Nivo were associated with the least risk of hepatobiliary disorders, however, most comparisons did not result in a significant difference, whereas Camre-Chemo was associated with the least safety. Pem was related to the lowest risk of injury, poisoning, and procedural complications (ORs ranging between 0.04, 0.37), whereas Atezo-Chemo was related to the lowest risk of safety. The lowest risk of investigations was reported for Chemo, followed by Atezo-Chemo and Sinti-Chemo (ORs ranging from 0.01 to 0.55), whereas Camre-Chemo had the lowest safety rating. As far as respiratory, thoracic, and mediastinal disorders are concerned, Chemo was the best performing ICI, followed by Durva (ORs varying between 0.04 and 0.48), while Camre-Chemo was the least safe. Chemo had the lowest incidence of skin and subcutaneous tissue disorders, followed by Tisle-Chemo and Pem-Chemo (ORs ranging from 0.01 to 0.48), whereas Nivo-Ipi-Chemo had the lowest incidence.
3.2.3 Heterogeneity and inconsistency
The results of this study indicate that there is favorable transitivity and consistency across the included trials, which allows for direct and indirect comparisons to be made. Based on the Q test and the I2 statistic, it was found that most of the heterogeneity and inconsistency were minimal (I2 0%) or low (I2 < 25%) across the included studies (Supplementary Figure 8).
3.3 Pharmacovigilance analysis
3.3.1 Adverse events among ICI NSCLC patients in the FDA adverse events reporting system
FAERS contained 23,941 reports related to ICI immunotherapy for NSCLC patients (Supplementary Table 3). Statistics on AEs in patients treated with ICIs in cases with NSCLC were obtained after excluding cases that occurred as a result of concomitant medications, adverse reactions and related treatment indications. A relatively constant number of cases were reported each year. Patients with NSCLC experienced AEs differently according to their ICI treatment strategy. Considering the proportion of AEs following various ICI treatment strategies, it appears that ICIs may contribute to a significant portion of AEs.
3.3.2 Scanning for ICI-related adverse events
Based on the reports of ICIs in NSCLC patients, we counted the types and the number of AEs (Supplementary Table 4). Malignant neoplasm progression (N = 3,337, 5.27%), death (N = 1,978, 3.12%), pneumonitis (N = 1,093, 1.73%), pneumonia (N = 1,087, 1.72%), pyrexia (N = 1,042, 1.65%) were the five most categories of AEs. Using the full AEs of NSCLC patients in the FAERS database as a comparator, we calculated the ROR of PTs with no less than three cases (N = 3,532) in the AEs. NSCLC patients with PTs that met the aforementioned conditions were considered to have ICI-related AEs. ICI reports with 9,420 AEs related to ICI were reviewed for further analysis in NSCLC patients (N = 7,339). We found that 6 types of ICIs either monotherapy or in combination related to a statistically significant positive signal in 311 PTs and 23 different SOCs, after filtering by our criteria for a valid signal (Figure 5). In cases involving ICI-related AEs, the most common concomitant AEs were neoplasms benign, malignant and unspecified, respiratory, thoracic and mediastinal disorders, and general disorders and administration site conditions. Only 0.72% of ICI-related AEs occurred in conjunction with immune system disorders (Figure 5A). Furthermore, malignant neoplasm progression (17.82%), death (11.64%), and pneumonitis (5.98%) were among the top 10% of ICI-related AEs (Figure 5B). In addition, a comparison of the ROR of different ICI monotherapy or combination therapy for different ICI-associated AEs is shown in Figure 5C. Based on the disproportionality analysis, there were 152 significant safety signals for Nivo, 98 for Durva, 89 for Pem, 15 for Atezo, 3 for Nivo-Ipi, and 1 for Cemip within the PTs, and those 311 PTs linked to 23 SOCs. As a result of disproportionality analysis of PTs linked to SOCs, there were 22 significant safety signals for Nivo and 20 for Durva, 21 for Pem, 12 for Atezo, 2 for Nivo-Ipi and 1 for Cemip. Upon further analysis of the detected safety PTs signals, it was determined that the following AEs were most frequently reported: for Nivo, malignant neoplasm progression (N = 1,439), death (N = 858) and hypothyroidism (N = 196); for Durva, radiation pneumonitis (N = 601) and malignant neoplasm progression (N = 469); for Pem, immune-mediated adverse reaction (N = 86); for Atezo, disease progression (N = 104). SOC signals were most frequently associated with the following: for Nivo, neoplasms benign, malignant and unspecified (N = 1,509) and general disorders and administration site conditions (N = 970); for Durva, neoplasms benign, malignant and unspecified (N = 782) and respiratory, thoracic and mediastinal disorders (N = 644); for Pem, endocrine disorders (N = 167); for Atezo, general disorders and administration site conditions (N = 109).
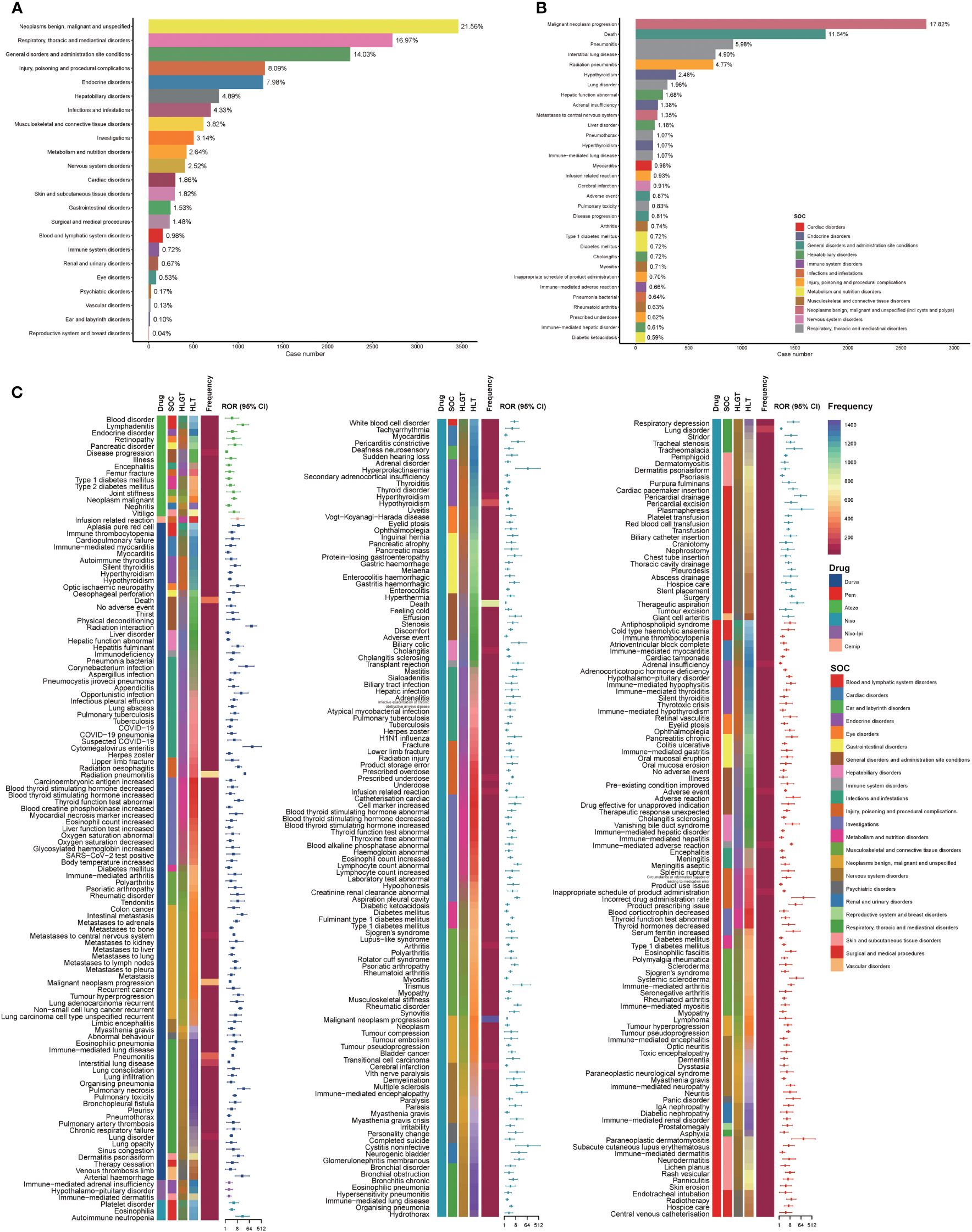
Figure 5 Scanning for ICI-related adverse events based on the FAERS database. (A) Bar plot shows the statistics of SOC regarding PTs of adverse events. The percentage values labeled in the figure represent the proportion of cases with such adverse events out of the total ICI-related AE cases. (B) Bar plot shows the statistics of the top 10% PTs of adverse events. The color indicates the SOC of the corresponding PT. The percentage values labeled in the figure represent the proportion of cases with such adverse events out of the total ICI-related adverse event cases. (C) The heatmap and forest plot shows the ROR for 311 adverse events (with cases no less than 3 and the lower limit of the 95% CI of the ROR exceeds one) in the FAERS database under different ICI treatment strategies (including Atezo, Ave, Durva, Nivo, Nivo-Ipi). The color indicates the ICI drugs, SOC, HLGT, HLT of the corresponding PT, and the frequency of PTs. This figure also depicted the hierarchical relationship of PTs for categories of ICI-related AEs in MedDRA. Due to the limitation of the figure, the legends of HLT and HLGT were provided in Supplementary Figure 9. Atezo, atezolizumab; Ave, avelumab; CI, confidence interval; Durva, durvalumab; FAERS, FDA Adverse Event Reporting System; HLGT, high level group term; HLT, high level term; ICI, immune checkpoint inhibitor; Ipi, ipilimumab; Nivo, nivolumab; PT, preferred term; ROR, reporting odds ratio; SOC, systemic organ class.
3.3.3 Descriptive analysis of cases with ICI-related adverse events and comparison between the fatal and non-fatal groups
The clinical characteristics of the ICI-related AEs reported in the FAERS database were analyzed statistically following the screening of the reports of ICIs in the FAERS database (N = 7,339) (Table 1). Data from 5,391 cases reports showed that the median age of the patients was 69 years old (interquartile range [IQR] 61-75). There are a large number of reported male cases (N =4,517, 69%) and most of cases are reported from Japan (N = 2,814, 38%). 37.63% (N =2,762) Cases occurred fatal related outcomes.
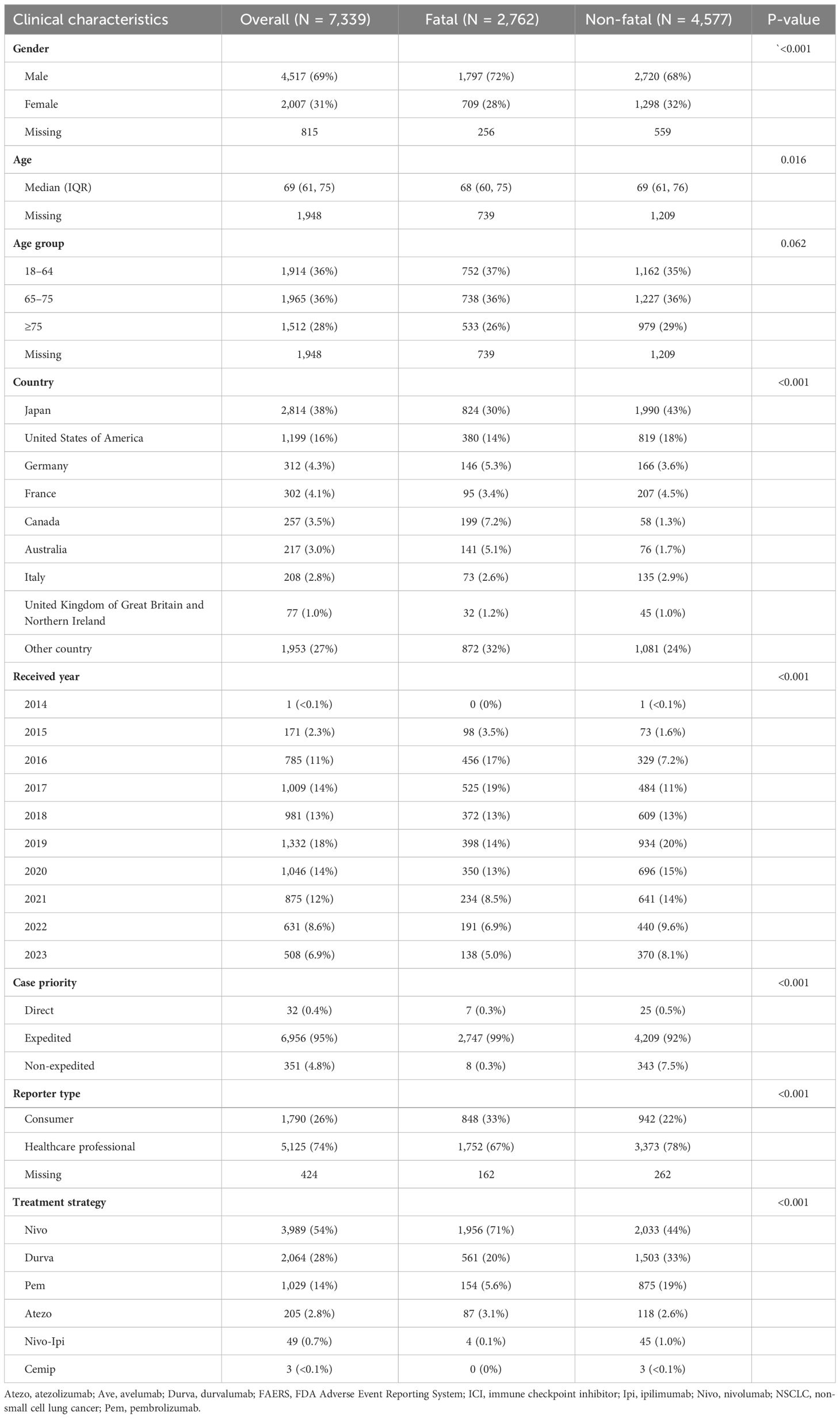
Table 1 Characteristics of reports with ICI-related adverse events of NSCLC patients sourced from the FAERS database.
Based on the proportion of ICI-related fatal related outcomes. A comparison of the outcomes of the fatal and non-fatal groups may provide further information regarding how to improve fatal related outcome in NSCLC patients. Compared with females, there is a greater proportion of males in the fatal group (P < 0.001). A significant difference exists between the fatal and non-fatal groups in the proportion of cases treated with different types of ICIs on a monotherapy or combination basis (P < 0.001), and Nivo and Atezo was related to increased risk of death. The age group of the fatal and non-fatal groups did not differ significantly (P = 0.062, respectively).
4 Discussion
AEs have been reported following increased ICI use, prompting additional investigations. This study is the first to analyze AEs associated with ICIs in NSCLC in a comprehensive manner, using data from both clinical trials and FAERS real-world data. A total of 26,958 patients from 51 RCTs for NSCLC were included in NMA. Through disproportionality analysis of the full list of AEs reported in NSCLC patients of FAERS database, we identified AEs that were highly related to the ICI regimens, and evaluated the characteristics of NSCLC patents of these events.
The NMA revealed several key findings related to ICI regimens and the risk of AEs among patients with NSCLC. First, Cemip and Tisle are related to the lowest risk of any or grades 3-5 trAEs. The Durva-Treme-Chemo regimen, on the other hand, was associated with higher risks than the other ICI regimens. Second, Atezo-Bevac-Chemo was related to a low risk of any or grades 3-5 irAE, but was related to grades 3-5 trAEs. There were higher risks associated with Sinti and Camre-Chemo than with the other ICI regimens. Third, as a result of the comparison of ICI regimens related AEs by different SOCs, different regimens were ranked based on the risk of trAEs or irAEs. For trAEs, Sinti was related to a lower risk of blood and lymphatic system disorders; Chemo was related to a reduced risk of endocrine disorders; A lower risk of gastrointestinal disorders was associated with Durva; As a result of Cemip, a lower risk of general disorders and administration site conditions was observed. There was a lower risk of investigations associated with Atezo; Tisle was related to a reduced risk of metabolism and nutrition disorders; It has been demonstrated that Ave reduces the risk of musculoskeletal and connective tissue disorders, skin and subcutaneous tissue disorders; It was found that Pem was related to a lower risk of nervous system disorders; Pem-Chemo is related to reduced risk of respiratory, thoracic, and mediastinal disorders. For irAEs, Durva was related to the lowest risk of endocrine disorders; Nivo was related to the lowest risk of gastrointestinal disorders and hepatobiliary disorders; Pem was related to the lowest risk of injury, poisoning and procedural complications; Chemo was related to the lowest risk of investigations, respiratory, thoracic and mediastinal disorders, and skin and subcutaneous tissue disorders.
Insights were gained from the disproportionality analysis. As a result of FAERS pharmacovigilance data analysis, 9,420 cases of AEs were associated with ICI treatment in 7,339 patients with NSCLC. There were 152 significant signals associated with Nivo, with malignant neoplasm progression, death and hypothyroidism being the most frequent PTs; For Durva, there were 98 significant signals, and radiation pneumonitis and malignant neoplasm progression were the most frequent PTs; A total of 89 significant signals were observed in the Pem, and immune-mediated adverse reactions are the most common PTs.
As for SOCs, Nivo was associated with 23 SOCs, which were associated with different PTs, with the most frequent SOCs being neoplasms benign, malignant and unspecified and general disorders and administration site conditions; Durva was associated with 20 SOCs, and the most common SOCs are neoplasms benign, malignant and unspecified and respiratory, thoracic and mediastinal disorders; Pem was linked to 21 SOCs, and the most frequency SOCs is endocrine disorders.
As previously reported, Cemip had the best safety profile among all ICIs for any-grade trAEs as indicated by previous studies (78), and ICIs and chemotherapy are known to increase the incidence of trAEs of grades 3-5, particularly when antiangiogenesis drugs are administered simultaneously (79), however, irAEs showed contrary results. In this study, ICI regimens were extensively compared and the risk of trAE and irAE was examined. However, this study has several different respects from previous published studies. For instance, previous studies provided limited evidence on the different type of ICIs, such as PD1 or PD-L1 (12, 13), limited evidence on only irAEs results and lack evidence of the novel ICIs, such as Cemip, or number of included RCTs (11, 14). In this study, we included recently published RCTs that evaluated novel ICIs, such as Cemip and Dostar, and the present study ranked the all evaluated treatment options in the RCTs. In previous studies, NSCLC treatment options were ranked differently from other cancers.
Oncologists, emergency department physicians, critical care providers, and other specialists need to be aware of the most serious toxic effects of ICIs used across cancer types. Immune-mediated damage to normal tissue is the most common presentation of AEs in various organ systems. There is a possibility that the differences in the risk of AEs may be related to the different mechanisms of action of each medication and to the combined use of ICIs (80). Further examination and verification of whether ICIs are associated with these AEs is necessary by conducting additional clinical studies. Furthermore, due to the high risk of irAEs, the clinical use of ICIs should be approached with particular care. In some published guidelines on managing irAEs, it is recommended that a rigorous clinical exam should be conducted prior to initiating ICI to assess the baseline (81, 82). Those with high incidences or poor prognoses should be closely monitored for ICI-associated AEs after immunotherapy has been decided upon. Clinical decisions regarding ICI treatment should be based on the findings presented in the present study. It is important that clinicians determine the duration and type of immunosuppressive treatment depending on the severity of the irAE, and consider whether to reintroduce ICIs after discontinuing them. This approach emphasizes the need to diagnose and treat each patient individually (19).
We believe that this is the first study of its kind to examine trAEs and irAEs associated with different ICI regimens for NSCLC, combining real-world data from the FAERS database with pharmacovigilance studies. The safety profile of ICIs in NSCLC has been analyzed in a full landscape of this study.
4.1 Limitations
There are some limitations of this study. First, there were a couple of differences in the terms used to describe AEs in the RCTs. Based on our review, we found that the grading system and terminology used to report AEs are consistent and compatible. Furthermore, with the increase in the number of NSCLC patients receiving ICI therapy, awareness of AEs may have increased. Moreover, those AEs have been matched to their corresponding MedDRA codes at the PT level. Second, there were a wide range of CrIs in this study. This is because there were only a few studies, only small sample sizes in some included trials, and different reporting standards. Third, based on the AEs reported in the RCTs, we were limited in our ability to rank a treatment regimen in terms of its probability of having the lowest risk of each AE. Several treatment options and SOCs could not be evaluated and ranked due to the limited number of available trials. Third, FAERS database is a system for reporting spontaneous incidents around the world. There are many factors that contribute to the selection bias of the data, including ethnicity and geographic location, the timing of drug approvals and market penetration, and understanding of specific AEs. Due to these limitations, we could neither calculate the incidence of ICI-related AEs nor obtain a causal association between ICIs and AEs. In addition, we were unable to obtain more detailed information and characteristic about the reports, and the specific time of death of NSCLC patients. We were unable to conduct further competitive risk analyses between ICIs and AEs. Fourth, we have difficulty extracting all the available anticancer regimens from the FAERS database individually and setting them as the comparator of the disproportionality analysis. It is the wide variety of anticancer regimens, including ICIs, targeted therapy regimens, chemotherapeutics, etc. There may be some indication bias as a result of this, which may lead to false positive associations being established. Last, AEs have only been reported for a few ICIs in the FAERS database, and no AEs have been reported for the novel ICIs. It is reasonable to assume that the NMA of this study can provide this evidence in light of these limitations.
5 Conclusions
In conclusion, comprehensive investigation and identification of AEs associated with ICIs was conducted using data from clinical trials and the FAERS database through NMA and disproportionality analyses. Given that cemip and tisle are associated with low trAE risks, they may be the preferred ICI regimens for NSCLC. There was a low risk of any or grades 3-5 irAEs with Atezo-Bevac-Chemo, whereas a high risk of grades 3-5 trAEs was associated with it. Those with autoimmune diseases or immunosuppressive medications may find this finding particularly important. There should be close monitoring and caution when using Durva-Treme-Chemo, Sinti, and Camre-Chemo. While the safety profiles of the ICIs differed, Nivo had the most significant signals, and we found several significant safety signals, including malignant neoplasm progression, death, and pneumonia. Based on the findings of this study, clinicians will be able to better understand potential AEs and be able to intervene early if they occur.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. HX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. HL: Conceptualization, Formal analysis, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. XC: Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. YL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Drug Safety Research Project of Guangxi Zhuang Autonomous Region (Guiyaojiankezhishu[2023]017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1396752/full#supplementary-material
References
1. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). (2021) 41:1037–48. doi: 10.1002/cac2.12197
2. Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang T, et al. Management of locally advanced non-small cell lung cancer: state of the art and future directions. Cancer Commun (Lond). (2023) 44:23–46. doi: 10.1002/cac2.12505
3. Johnson DB, Reynolds KL, Sullivan RJ, Balko JM, Patrinely JR, Cappelli LC, et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. (2020) 21:e398–404. doi: 10.1016/s1470-2045(20)30107-8
4. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. Bmj. (2018) 363:k4226. doi: 10.1136/bmj.k4226
5. Ji HH, Tang XW, Dong Z, Song L, Jia YT. Adverse event profiles of anti-Ctla-4 and anti-Pd-1 monoclonal antibodies alone or in combination: analysis of spontaneous reports submitted to Faers. Clin Drug Investig. (2019) 39:319–30. doi: 10.1007/s40261-018-0735-0
6. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
7. Berner F, Flatz L. Autoimmunity in immune checkpoint inhibitor-induced immune-related adverse events: A focus on autoimmune skin toxicity and pneumonitis. Immunol Rev. (2023) 318:37–50. doi: 10.1111/imr.13258
8. Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. (2017) 6:e1344805. doi: 10.1080/2162402x.2017.1344805
9. Grouthier V, Lebrun-Vignes B, Moey M, Johnson DB, Moslehi JJ, Salem JE, et al. Immune checkpoint inhibitor-associated primary adrenal insufficiency: who vigibase report analysis. Oncologist. (2020) 25:696–701. doi: 10.1634/theoncologist.2019-0555
10. Zhou C, Peng S, Lin A, Jiang A, Peng Y, Gu T, et al. Psychiatric disorders associated with immune checkpoint inhibitors: A pharmacovigilance analysis of the Fda adverse event reporting system (Faers) database. EClinicalMedicine. (2023) 59:101967. doi: 10.1016/j.eclinm.2023.101967
11. Zhang W, Gu J, Bian C, Huang G. Immune-related adverse events associated with immune checkpoint inhibitors for advanced non-small cell lung cancer: A network meta-analysis of randomized clinical trials. Front Pharmacol. (2021) 12:686876. doi: 10.3389/fphar.2021.686876
12. Zhou C, Li M, Wang Z, An D, Li B. Adverse events of immunotherapy in non-small cell lung cancer: A systematic review and network meta-analysis. Int Immunopharmacol. (2022) 102:108353. doi: 10.1016/j.intimp.2021.108353
13. Chen CY, Huang CH, Chen WC, Huang MS, Wei YF. Comparative safety of immune checkpoint inhibitors and chemotherapy in advanced non-small cell lung cancer: A systematic review and network meta-analysis. Int Immunopharmacol. (2022) 108:108848. doi: 10.1016/j.intimp.2022.108848
14. Gu J, Shi L, Jiang X, Wen J, Zheng X, Cai H, et al. Severe immune-related adverse events of immune checkpoint inhibitors for advanced non-small cell lung cancer: A network meta-analysis of randomized clinical trials. Cancer Immunol Immunother. (2022) 71:2239–54. doi: 10.1007/s00262-022-03140-5
15. Shalata W, Yakobson A, Cohen AY, Goldstein I, Saleh OA, Dudnik Y, et al. Unexpected adverse events of immune checkpoint inhibitors. Life (Basel). (2023) 13:1657. doi: 10.3390/life13081657
16. Sarangdhar M, Tabar S, Schmidt C, Kushwaha A, Shah K, Dahlquist JE, et al. Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat Biotechnol. (2016) 34:697–700. doi: 10.1038/nbt.3623
17. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The prisma extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/m14-2385
18. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/jco.2017.77.6385
20. National Cancer Institute. Common Terminology Criteria for Adverse Events (Ctcae) V5.0. Available online at: https://Ctep.Cancer.Gov/Protocoldevelopment/Electronic_Applications/Ctc.Htm#Ctc_50 (Accessed November 12, 2023).
21. Medical Dictionary for Regulatory Activities. Welcome to Meddra. Medical Dictionary for Regulatory Activities (2023). Available online at: https://www.Meddra.Org/ (Accessed November 11, 2023).
22. Li H, Sun X, Sun D, Zhao J, Xu Z, Zhao P, et al. Thromboembolic events associated with immune checkpoint inhibitors: A real-world study of data from the food and drug administration adverse event reporting system (Faers) database. Int Immunopharmacol. (2021) 98:107818. doi: 10.1016/j.intimp.2021.107818
23. Caster O, Aoki Y, Gattepaille LM, Grundmark B. Disproportionality analysis for pharmacovigilance signal detection in small databases or subsets: recommendations for limiting false-positive associations. Drug Saf. (2020) 43:479–87. doi: 10.1007/s40264-020-00911-w
24. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. (2004) 13:519–23. doi: 10.1002/pds.1001
25. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the Fda adverse event reporting system. PloS One. (2011) 6:e28124. doi: 10.1371/journal.pone.0028124
26. Banda JM, Evans L, Vanguri RS, Tatonetti NP, Ryan PB, Shah NH. A curated and standardized adverse drug event resource to accelerate drug safety research. Sci Data. (2016) 3:160026. doi: 10.1038/sdata.2016.26
27. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (Checkmate 9la): an international, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/s1470-2045(20)30641-0
28. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
29. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage iv or recurrent non-small-cell lung cancer. N Engl J Med. (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
30. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
31. Chang J, Wu YL, Lu S, Wang J, Mok T, Zhang L, et al. Three-year follow-up and patient-reported outcomes from checkmate 078: nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer. Lung Cancer. (2022) 165:71–81. doi: 10.1016/j.lungcan.2021.12.009
32. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
33. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with Pd-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. (2021) 397:592–604. doi: 10.1016/s0140-6736(21)00228-2
34. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of Pd-L1-selected patients with Nsclc. N Engl J Med. (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
35. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (Impower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:924–37. doi: 10.1016/s1470-2045(19)30167-6
36. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous Nsclc (Impower131): results from a randomized phase iii trial. J Thorac Oncol. (2020) 15:1351–60. doi: 10.1016/j.jtho.2020.03.028
37. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous Nsclc: results from the randomized phase 3 Impower132 trial. J Thorac Oncol. (2021) 16:653–64. doi: 10.1016/j.jtho.2020.11.025
38. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous Nsclc. N Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
39. Park K, Özgüroğlu M, Vansteenkiste J, Spigel D, Yang JCH, Ishii H, et al. Avelumab versus docetaxel in patients with platinum-treated advanced Nsclc: 2-year follow-up from the javelin lung 200 phase 3 trial. J Thorac Oncol. (2021) 16:1369–78. doi: 10.1016/j.jtho.2021.03.009
40. Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, et al. Five year survival update from keynote-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced Nsclc. J Thorac Oncol. (2021) 16:1718–32. doi: 10.1016/j.jtho.2021.05.001
41. Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. 24-Month Overall Survival from Keynote-021 Cohort G: Pemetrexed and Carboplatin with or without Pembrolizumab as First-Line Therapy For advanced Nonsquamous Non-Small Cell Lung cancer. J Thorac Oncol. (2019) 14:124–9. doi: 10.1016/j.jtho.2018.08.004
42. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of keynote-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with Pd-L1 tumor proportion score of 50% or greater. J Clin Oncol. (2019) 37:537–46. doi: 10.1200/jco.18.00149
43. Zhou C, Feng J, Ma S, Chen H, Ma Z, Huang C, et al. 1262p randomized, open-label phase iii study of pembrolizumab (Pembro) vs docetaxel (Doce) in patients (Pts) with previously treated Nsclc with pd-L1 tumour proportion score (Tps) ≥1%: keynote-033. Ann Oncol. (2020) 31:S816. doi: 10.1016/j.annonc.2020.08.1576
44. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, Pd-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (Keynote-042): A randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/s0140-6736(18)32409-7
45. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from keynote-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. (2020) 38:1505–17. doi: 10.1200/jco.19.03136
46. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous Nsclc: protocol-specified final analysis of keynote-407. J Thorac Oncol. (2020) 15:1657–69. doi: 10.1016/j.jtho.2020.06.015
47. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with Pd-L1 tumor proportion score ≥ 50%: randomized, double-blind phase iii keynote-598 study. J Clin Oncol. (2021) 39:2327–38. doi: 10.1200/jco.20.03579
48. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the mystic phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:661–74. doi: 10.1001/jamaoncol.2020.0237
49. Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, et al. Updated efficacy analysis including secondary population results for oak: A randomized phase iii study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. (2018) 13:1156–70. doi: 10.1016/j.jtho.2018.04.039
50. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
51. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (Poplar): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. doi: 10.1016/s0140-6736(16)00587-0
52. Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, et al. Phase iii trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. (2017) 35:3449–57. doi: 10.1200/jco.2016.71.7629
53. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (Camel): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. (2021) 9:305–14. doi: 10.1016/s2213-2600(20)30365-9
54. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous Nsclc (Camel-Sq): A phase 3 trial. J Thorac Oncol. (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
55. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous Nsclc (Rationale 304): A randomized phase 3 trial. J Thorac Oncol. (2021) 16:1512–22. doi: 10.1016/j.jtho.2021.05.005
56. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous nsclc: A randomized, double-blind, phase 3 study (Oncology program by innovent anti-Pd-1-11). J Thorac Oncol. (2020) 15:1636–46. doi: 10.1016/j.jtho.2020.07.014
57. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous Nsclc: results from a randomized, double-blind, phase 3 trial (Orient-12). J Thorac Oncol. (2021) 16:1501–11. doi: 10.1016/j.jtho.2021.04.011
58. Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage Iiib/Iv non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase ii study. J Clin Oncol. (2012) 30:2046–54. doi: 10.1200/jco.2011.38.4032
59. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
60. Leighl NB, Laurie SA, Goss GD, Hughes BGM, Stockler M, Tsao MS, et al. Cctg Br34: A randomized phase 2 trial of durvalumab and tremelimumab with or without platinum-based chemotherapy in patients with metastatic Nsclc. J Thorac Oncol. (2022) 17:434–45. doi: 10.1016/j.jtho.2021.10.023
61. Gettinger SN, Redman MW, Bazhenova L, Hirsch FR, Mack PC, Schwartz LH, et al. Nivolumab plus ipilimumab vs nivolumab for previously treated patients with stage iv squamous cell lung cancer: the lung-map S1400i phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:1368–77. doi: 10.1001/jamaoncol.2021.2209
62. Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage iii non-small-cell lung cancer in China (Gemstone-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:209–19. doi: 10.1016/s1470-2045(21)00630-6
63. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (Gemstone-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. (2022) 23:220–33. doi: 10.1016/s1470-2045(21)00650-1
64. Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. (2021) 32:1137–47. doi: 10.1016/j.annonc.2021.06.004
65. Pillai RN, Ramalingam SS, Thayu M, Lorenzini P, Alvarez Arias DA, Moy C, et al. Daratumumab plus atezolizumab in previously treated advanced or metastatic Nsclc: brief report on a randomized, open-label, phase 1b/2 study (Luc2001 Jnj-54767414). JTO Clin Res Rep. (2021) 2:100104. doi: 10.1016/j.jtocrr.2020.100104
66. Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: the prolung phase 2 randomized clinical trial. JAMA Oncol. (2020) 6:856–64. doi: 10.1001/jamaoncol.2020.0409
67. Makharadze T, Gogishvili M, Melkadze T, Baramidze A, Giorgadze D, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in advanced Nsclc: 2-year follow-up from the phase 3 empower-lung 3 part 2 trial. J Thorac Oncol. (2023) 18:755–68. doi: 10.1016/j.jtho.2023.03.008
68. Hayashi H, Sugawara S, Fukuda Y, Fujimoto D, Miura S, Ota K, et al. A randomized phase ii study comparing nivolumab with carboplatin-pemetrexed for Egfr-mutated Nsclc with resistance to Egfr tyrosine kinase inhibitors (Wjog8515l). Clin Cancer Res. (2022) 28:893–902. doi: 10.1158/1078-0432.Ccr-21-3194
69. Jung HA, Park S, Choi YL, Lee SH, Ahn JS, Ahn MJ, et al. Continuation of pembrolizumab with additional chemotherapy after progression with Pd-1/Pd-L1 inhibitor monotherapy in patients with advanced Nsclc: A randomized, placebo-controlled phase ii study. Clin Cancer Res. (2022) 28:2321–8. doi: 10.1158/1078-0432.Ccr-21-3646
70. Lai GGY, Yeo JC, Jain A, Zhou S, Pang M, Alvarez JJS, et al. A randomized phase 2 trial of nivolumab versus nivolumab-ipilimumab combination in Egfr-mutant Nsclc. JTO Clin Res Rep. (2022) 3:100416. doi: 10.1016/j.jtocrr.2022.100416
71. Shi Y, Wu L, Yu X, Xing P, Wang Y, Zhou J, et al. Sintilimab versus docetaxel as second-line treatment in advanced or metastatic squamous non-small-cell lung cancer: an open-label, randomized controlled phase 3 trial (Orient-3). Cancer Commun (Lond). (2022) 42:1314–30. doi: 10.1002/cac2.12385
72. Zhou C, Huang D, Fan Y, Yu X, Liu Y, Shu Y, et al. Tislelizumab versus docetaxel in patients with previously treated advanced Nsclc (Rationale-303): A phase 3, open-label, randomized controlled trial. J Thorac Oncol. (2023) 18:93–105. doi: 10.1016/j.jtho.2022.09.217
73. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: A multicenter randomized phase iii trial (Choice-01). J Clin Oncol. (2023) 41:651–63. doi: 10.1200/jco.22.00727
74. Lu S, Wu L, Jian H, Cheng Y, Wang Q, Fang J, et al. Sintilimab plus chemotherapy for patients with Egfr-mutated non-squamous non-small-cell lung cancer with disease progression after Egfr tyrosine-kinase inhibitor therapy (Orient-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. (2023) 11:624–36. doi: 10.1016/s2213-2600(23)00135-2
75. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase iii Poseidon study. J Clin Oncol. (2023) 41:1213–27. doi: 10.1200/jco.22.00975
76. Lee SM, Schulz C, Prabhash K, Kowalski D, Szczesna A, Han B, et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (Ipsos): A phase 3, global, multicentre, open-label, randomised controlled study. Lancet. (2023) 402:451–63. doi: 10.1016/s0140-6736(23)00774-2
77. Lim SM, Peters S, Ortega Granados AL, Pinto GDJ, Fuentes CS, Lo Russo G, et al. Dostarlimab or pembrolizumab plus chemotherapy in previously untreated metastatic non-squamous non-small cell lung cancer: the randomized Perla phase ii trial. Nat Commun. (2023) 14:7301. doi: 10.1038/s41467-023-42900-4
78. Li Y, Liang X, Li H, Chen X. Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without Pd-L1 selection: A systematic review and network meta-analysis. Chin Med J (Engl). (2023) 136:2156–65. doi: 10.1097/cm9.0000000000002750
79. Liu L, Bai H, Wang C, Seery S, Wang Z, Duan J, et al. Efficacy and safety of first-line immunotherapy combinations for advanced Nsclc: A systematic review and network meta-analysis. J Thorac Oncol. (2021) 16:1099–117. doi: 10.1016/j.jtho.2021.03.016
80. Khan Z, Hammer C, Guardino E, Chandler GS, Albert ML. Mechanisms of immune-related adverse events associated with immune checkpoint blockade: using germline genetics to develop a personalized approach. Genome Med. (2019) 11:39. doi: 10.1186/s13073-019-0652-8
81. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: Asco guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/jco.21.01440
82. Majem M, García-Martínez E, Martinez M, Muñoz-Couselo E, Rodriguez-Abreu D, Alvarez R, et al. Seom clinical guideline for the management of immune-related adverse events in patients treated with immune checkpoint inhibitors (2019). Clin Transl Oncol. (2020) 22:213–22. doi: 10.1007/s12094-019-02273-x
Keywords: non-small cell lung cancer, pharmacovigilance, immune checkpoint inhibitors, real-world study, FAERS
Citation: Liang X, Xiao H, Li H, Chen X and Li Y (2024) Adverse events associated with immune checkpoint inhibitors in non-small cell lung cancer: a safety analysis of clinical trials and FDA pharmacovigilance system. Front. Immunol. 15:1396752. doi: 10.3389/fimmu.2024.1396752
Received: 06 March 2024; Accepted: 17 April 2024;
Published: 30 April 2024.
Edited by:
Alessandro Poggi, San Martino Hospital (IRCCS), ItalyReviewed by:
Walid Shalata, Soroka Medical Center, IsraelHanping Wang, Peking Union Medical College Hospital (CAMS), China
Copyright © 2024 Liang, Xiao, Li, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li, bGl5YW4yMDEwMjAxMEBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work
 Xueyan Liang
Xueyan Liang Hewei Xiao2†
Hewei Xiao2† Xiaoyu Chen
Xiaoyu Chen Yan Li
Yan Li