
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 15 May 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1392499
This article is part of the Research Topic Exploring Novel Immunotherapy Targets and Combinational Immunotherapy in Breast and Gastrointestinal Cancers View all 12 articles
 Lei Yang1†
Lei Yang1† Xiujing Cui1†
Xiujing Cui1† Fengpeng Wu1
Fengpeng Wu1 Zifeng Chi1
Zifeng Chi1 Linlin Xiao1
Linlin Xiao1 Xuan Wang1
Xuan Wang1 Zezheng Liang1
Zezheng Liang1 Xiaoning Li1
Xiaoning Li1 Qiyao Yu2
Qiyao Yu2 Xueqin Lin1
Xueqin Lin1 Chao Gao1*‡
Chao Gao1*‡Background: Several studies have explored the effectiveness of PD-1/PD-L1 inhibitors combined with neoadjuvant chemoradiotherapy (nCRT) in the treatment of locally advanced rectal cancer(LARC), particularly in microsatellite stable(MSS) or mismatch repair proficient(pMMR) LARC patients. We undertook a single-arm systematic review to comprehensively evaluate the advantages and potential risks associated with the use of PD-1/PD-L1 inhibitors in conjunction with nCRT for patients diagnosed with locally advanced rectal cancer.
Methods: The PubMed, Embase, Cochrane Library, ClinicalTrials.gov, ASCO and ESMO were searched for related studies. The main outcomes were pathologic complete response (pCR), major pathological response (MPR), anal preservation, and adverse effects (AEs).
Results: Fourteen articles including 533 locally advanced rectal cancer (LARC) patients were analyzed. The pooled pCR, MPR, and anal preservation rates were 36%, 66% and 86%. Grade ≥3 adverse events occurred in 20%. Subgroup analysis showed that; dMMR/MSI-H had a pooled pCR (100%) and MPR (100%), pMMR/MSS had a pooled pCR (38%) and MPR (60%); the short-course radiotherapy and long-course radiotherapy had pooled pCR rates of 51% and 30%, respectively. The rates of pCR for the concurrent and sequential immuno-chemoradiotherapy subgroups at 30% and 40%, mirroring pCR rates for the PD-L1 and PD-1 inhibitor subgroups were 32% and 40%, respectively.
Conclusion: In cases of locally advanced rectal cancer, PD-1/PD-L1 inhibitors combined with neoadjuvant chemoradiotherapy have shown promising response rates and acceptable toxicity profiles. PD-1/PD-L1 inhibitors combined with neoadjuvant chemoradiotherapy hence has a positive outcome even in MSS LARC patients.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#myprospero, identifier CRD42023465380.
Neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) and then adjuvant therapy is considered the standard treatment model for locally advanced rectal cancer (LARC), it has been established that the application of conventional chemoradiotherapy (CRT) followed by radical surgical intervention can yield rates of pathological complete response (pCR) ranging from 10% to 15% (1, 2). However, the overall survival benefits of these preoperative treatment models are limited, with low anal preservation rates and high rates of distant metastasis (3).
In recent years, immune checkpoint inhibitors (ICIs) have emerged as effective treatments for a variety of cancers. KEYNOTE-177 results showed that immunotherapy significantly improved the prognosis of microsatellite instability-high (MSI-H)/deficient mismatch repair(dMMR) advanced colorectal cancer(CRC), and the FDA has approved the pembrolizumab as a standard first-line treatment for MSI-H advanced CRC (4). Although dMMR/MSI-H cancers represent less than 5% of metastatic CRC patients and 10% of early-stage CRC patients, the vast majority of patients have mismatch repair proficient (pMMR) or microsatellite stable (MSS) CRC, which belong to “cold tumors” that are not sensitive to immunotherapy (5, 6). These patients do not appear to benefit from immunotherapy alone (5). However, the NICHE study showed a favorable response to neoadjuvant nivolumab plus ipilimumab in 30% of patients with pMMR/MSS and all patients with dMMR/MSI-H colorectal cancer (7). The study firstly demonstrated the potential benefits of immune checkpoint inhibition in pMMR/MSS CRC. Therefore, many studies have adopted a regimen of nCRT combined with immunotherapy to improve the efficacy of neoadjuvant treatment in LARC patients.
Preclinical research, radiation therapy can cause the immune system to attack tumor cells, release tumor neoantigens, activate antitumor T cells, cause tumor-infiltrating T cells to aggregate, and upregulate PD-L1 expression in tumor tissues. The concomitant application of immunotherapy and radiation therapy could potentiate effectiveness (8, 9). These lay the theoretical foundation for the combination of immunotherapy and radiation, and propose a new treatment for LARC. Therefore, this systematic review evaluated the efficacy and safety of nCRT combined with immunotherapy for LARC patients. Further the pCR rates among different MMR/MSI states (dMMR/MSI-H, pMMR/MSS), different radiotherapy modes (long-course radiotherapy, short-course radiotherapy), different sequence of nCRT combined with immunotherapy (concurrent immunotherapy, sequential immunotherapy) and different immune checkpoint inhibitors (PD-1 inhibitor, PD-1 inhibitor) were compared in the subgroup analysis, in order to find the optimal combination therapy strategy for neoadjuvant therapy of LARC.
We conducted comprehensive searches of several online databases from inception to September 2023, which included PubMed, Embase, Cochrane Library, ClinicalTrials.gov, ASCO, and ESMO. Additional electronic searches were performed by conducting thorough investigations into the proceedings of two prominent international conferences, namely, the American Society of Clinical Oncology Annual Meeting and the European Society of Medical Oncology. This was done with consideration for the potential existence of unpublished research findings. The search terms used included “rectal cancer,” “neoadjuvant immunotherapy,” “PD-1,” “PD-L1,” and “neoadjuvant therapy.” Additionally, references from original research studies and literature reviews were also searched in order to ensure that no relevant study was overlooked.
The inclusion criteria were as follows: (1) studies involving patients with locally resectable advanced rectal cancer; (2) the primary focus is neoadjuvant chemoradiotherapy involving PD-1/PD-L1 immunosuppressants; and (3) the primary outcomes, including pathologic complete response (pCR), major pathological response (MPR), adverse events (AEs), and the rate of anal preservation, were either directly accessible or indirectly attainable in the study.
The exclusion criteria were as follows: (1) the tumor is metastatic or unresectable; (2) neoadjuvant immunotherapy was not performed; (3) studies without usable data; (4) article type: review, comment, case report, and cell or animal study.
Most of the included trials were single-arm studies. Consequently, we evaluated the quality of the research using the methodological index for non-randomized studies (MINORS). Studies were classified as poor (0–12), or good (13–16) based on MINORS scores, in that order. Any disagreements were settled by agreement.
Two investigators retrieved the pertinent data from the eligible studies and noted the first author, the year of publication, the number of patients, the interventions, and the pCR rate of the included studies.
STATA/SE version 15.1 was used for all statistical analyses. A random-effect model was employed, and heterogeneity between studies was categorized as low (I2 < 50%) or high (I2 > 50%) using the Cochran Q chi-square test and I2 statistics. Sensitivity analysis was conducted by sequentially excluding studies contributing to high heterogeneity in pooled data.
As seen in Figure 1, following the screening of titles and abstracts, elimination of duplicates, and full-text review, 14 studies were ultimately included. The MINORS score system rated fourteen studies as good. Table 1 summarizes these details.
We analyzed pCR data from 14 studies involving 533 patients. The pooled pCR rate was 36% (95%CI:29%,43%) with minimal heterogeneity (I2 = 0%, P=0.471) (Figure 2A). A total of eleven studies reported on 382 patients in the MPR. With high heterogeneity (I2 = 92.7%, P=0.000), the pooled MPR rate was 66% (95%CI:50%,83%) (Figure 2B). Sensitivity analysis revealed that this meta-analysis’s pooled MPR rate was regarded as constant.
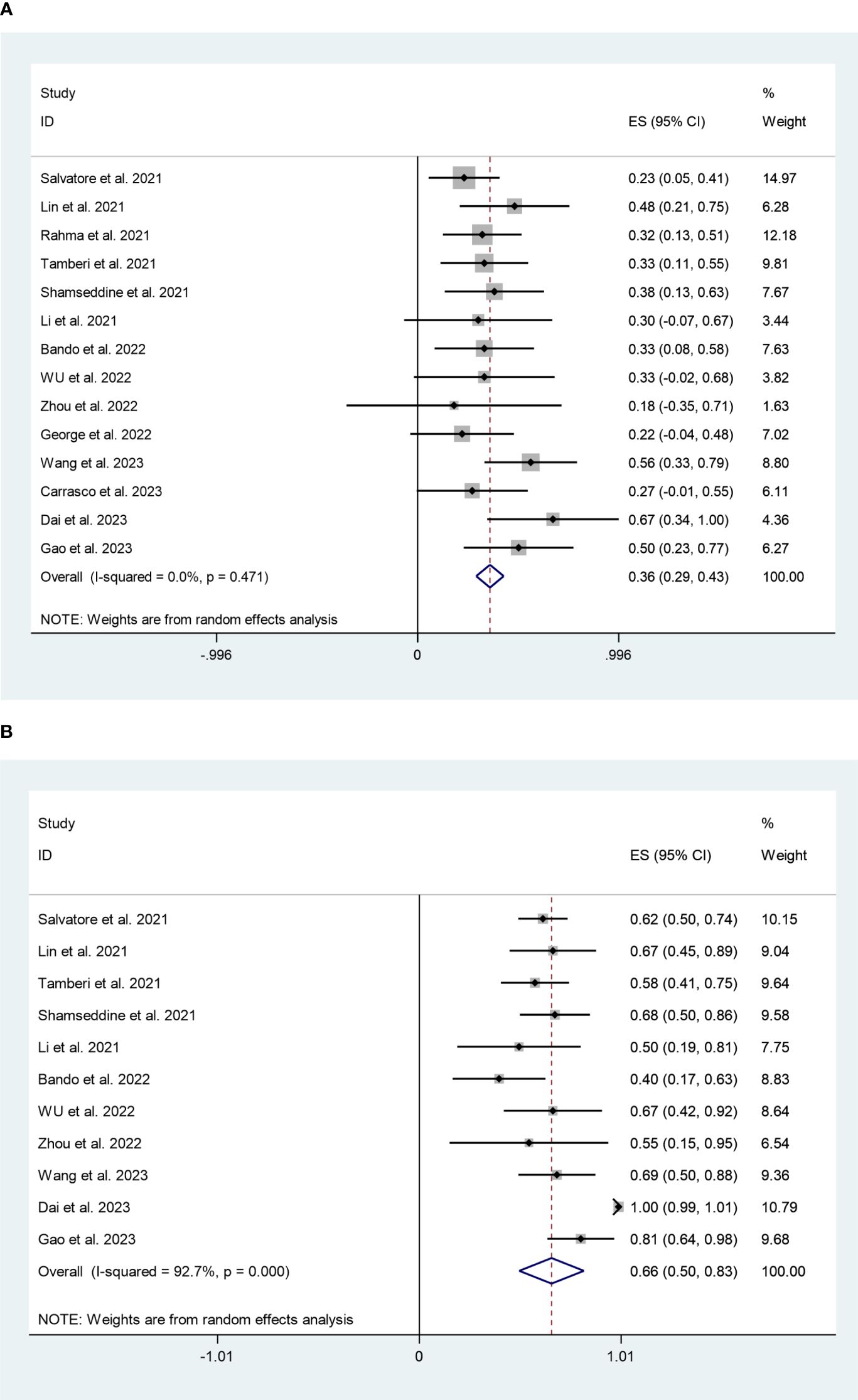
Figure 2 (A) Forest plots of pathological complete response rate. (B) Forest plots of major pathological response rate.
Five studies, including 136 participants, reported an anal preservation rate of 86% (95%CI:78%,94%), although with minimal heterogeneity (I2 = 33.9%, P=0.195) (Figure 3).
There was enough data from nine trials involving 377 patients to analyze the adverse effects grade ≥3 of PD-1/PD-L1 inhibitors plus nCRT; the pooled rate was 20% (95%CI:7%,33%) with minimal heterogeneity (I2 = 40.7%, P=0.096) (Figure 4).
In dMMR/MSI-H subgroup, the pooled pCR rate was 100% (95%CI:97%,103%) with negligible heterogeneity (I2 = 0%, P=0.370) (Figure 5A). While, the pMMR/MSS subgroup had a pooled pCR rate of 38% (95%CI:28%,49%) with negligible heterogeneity (I2 = 0%, P=0.579) (Figure 5A). Additionally, as shown in Figure 5B, the subgroup analysis revealed that the pooled MPR rates for the dMMR/MSI-H and pMMR/MSS subgroups were 100% (95%CI:97%,103%, I2 = 0%, P=0.370) and 60% (95%CI:46%,74%, I2 = 54.6%, P=0.040). Sensitivity analysis revealed that this meta-analysis’s pooled MPR rate was regarded as constant.
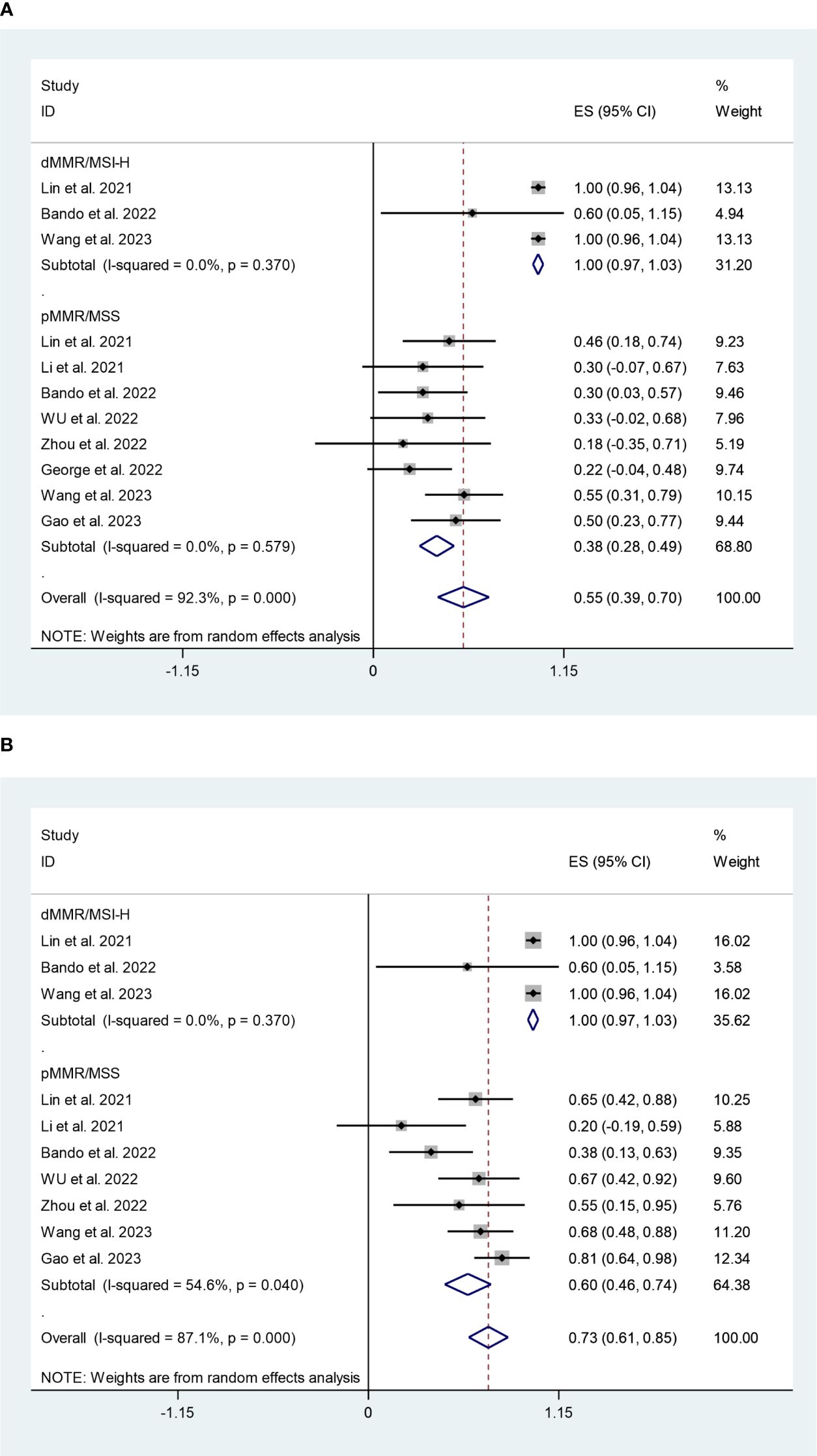
Figure 5 (A) Forest plots of mismatch repair status based on pathological complete response rate. (B) Forest plots of mismatch repair status based on major pathological response rate.
The subgroup analysis revealed that the combined pCR rates for the long-course radiotherapy (LCRT) and short-course radiotherapy (SCRT) subgroups were 30% (95%CI:22%,38%, I2 = 0%, P=0.941), 51% (95%CI:38%,64%, I2 = 0%, P=0.526) (Figure 6).
The concurrent and sequential immuno-CRT subgroups had pCR rates of 30% (95%CI:20%,41%, I2 = 0%, P=0.563) and 40% (95%CI:31%,49%, I2 = 0%, P=0.443) respectively, according to the subgroup analysis (Figure 7).
The PD-L1 and PD-1 inhibitors subgroups had pCR rates of 32% (95%CI:21%,43%, I2 = 21.5%, P=0.272) and 40% (95%CI:30%,50%, I2 = 0%, P=0.687) respectively, according to the subgroup analysis (Figure 8A). In terms of safety, the adverse effects grade ≥3 of PD-L1 and PD-1 inhibitors were 10% (95%CI:-4%,24%, I2 = 0%, P=0.951) and 18% (95%CI:1%,34%, I2 = 0%, P=0.835), respectively (Figure 8B).
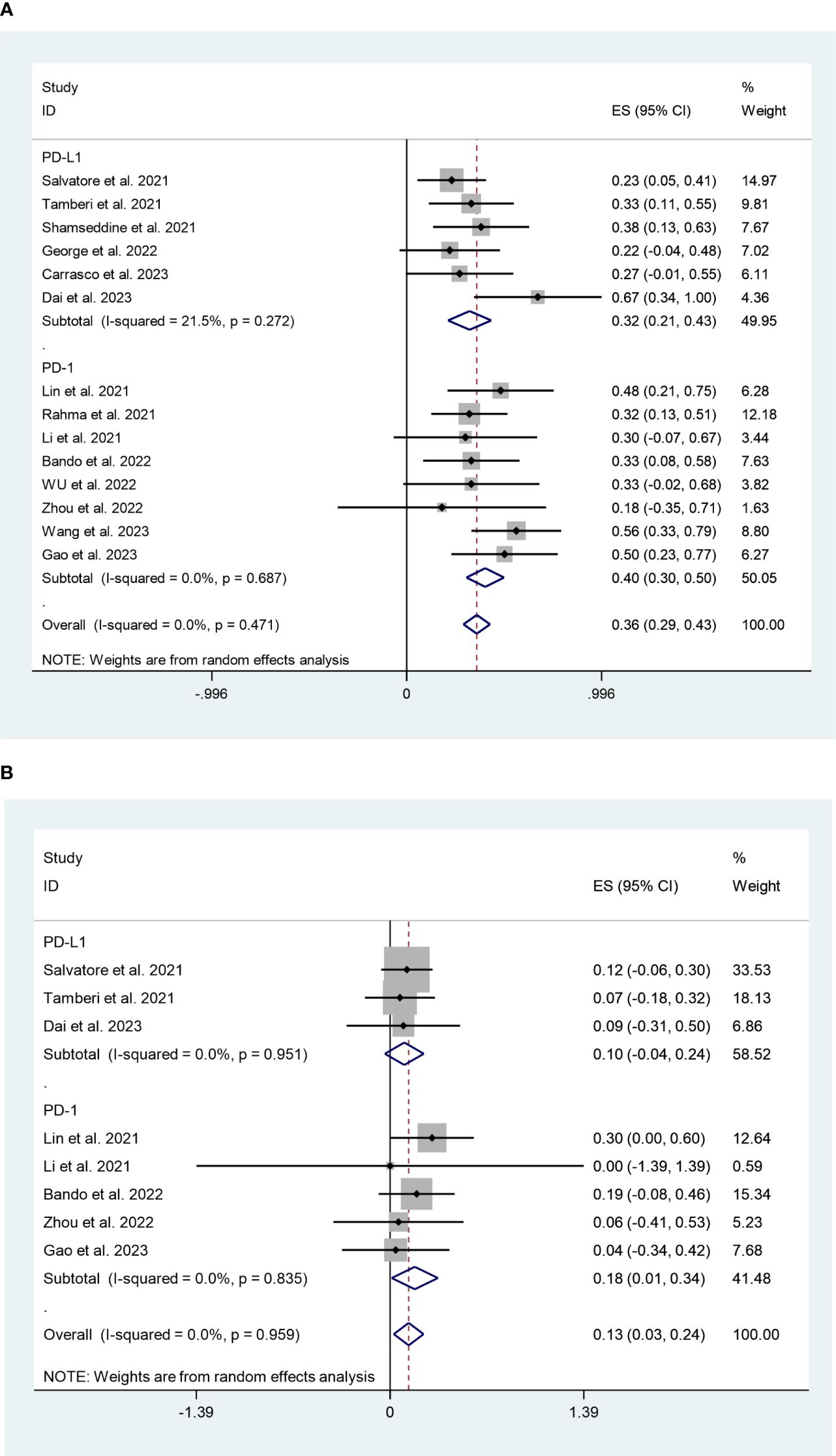
Figure 8 (A) Forest plots of PD-1/PD-L1 inhibitors based on pathological complete response rate. (B) Forest plots of PD-1/PD-L1 inhibitors based on grade ≥3 effects.
Analysis using the Egger and Begg tests revealed no evidence of publication bias (P=0.522 and 0.661, respectively).
To our knowledge, this study is one of the earliest report to investigate the safety and efficacy of nCRT when combined with PD-1/PD-L1 inhibitors in patients with locally advanced rectal cancer. Positive outcomes and tolerance to the neoadjuvant PD-1/PD-L1 inhibitors treatment were shown by the results. This systematic review revealed that 36% and 66% of LARC patients treated with nCRT combined with PD-1/PD-L1 inhibitors achieved pCR and MPR, respectively. In comparison, the pCR rate for traditional neoadjuvant chemoradiotherapy is approximately 15% (1), underscoring the potential benefits of adding PD-1/PD-L1 inhibitors. Furthermore, the systematic review showed that this therapeutic method did not significantly raise the incidence of major adverse events (AEs); the pooled anal preservation rate was 86%, and the grade ≥3 toxicity was 20%. Consequently, the combination of PD-1/PD-L1 inhibitors and nCRT emerges as a promising therapeutic strategy that warrants further investigation.
Around 6% of rectal adenocarcinomas are mismatch-repair deficient (24, 25). In the case of individuals diagnosed with metastatic colorectal cancer with dMMR, the utilization of immune checkpoint inhibitors as a first-line treatment had exhibited remarkable effectiveness. This is evidenced by objective response rates falling within the range of 33% to 55%, substantial and clinically significant sustainability of treatment response, and an extension of the overall survival period (26, 27). According to Zhang et al., two MSI-H patients with LARC who received nivolumab as a neoadjuvant therapy saw complete response (CR) (28). Furthermore, the NICHE study explored a combination therapy of nivolumab and the ipilimumab in early-stage (II-III) CRC patients, showed 100% pathological response in patients with dMMR tumors. Allowing immunotherapy for LARC to be initiated in the preoperative stage (7). Our study also revealed that the MSI-H/dMMR LARC subgroup had a pooled pCR rate of 100%,suggesting that nCRT combined with immunotherapy leads to superior outcomes in patients with dMMR/MSI-H LARC.
A majority of LARC patients had MSS tumors, however, MSS LARC patients, had no responses to immunotherapy in Keynote-016 and Keynote-028, the single-agent ICI provided very little benefit to MSS tumors (5, 29). The 30 patients in the pMMR/MSI-L cohort responded pathologically to nivolumab+ipilimumab in the NICHE trial by 30%, with a 10% pCR rate, marking the first instance of immune checkpoint inhibition efficacy in pMMR/MSS patients (7). As a result, research had begun into combinations treatment that enhance the effectiveness of immunologic therapy in this subtype (30, 31). Currently researchers are primarily focused on how ICIs and nCRT together can boost efficacy in MSS LARC patients by overcoming the “immune-cold” tumor microenvironment.
Preclinical research, radiation therapy could cause the immune system to attack tumor cells, release tumor neoantigens, activate antitumor T cells, cause tumor-infiltrating T cells to aggregate, and upregulate PD-L1 expression in tumor tissues (32, 33). The concomitant application of immunotherapy and radiation therapy appeared to potentiate effectiveness (34, 35). The observation of distant effects in clinical research involving immunotherapy and radiotherapy was seen as compelling evidence that the latter enhances the body’s immune response against tumors. These form the theoretical foundation for the combination of immunotherapy and radiation (36, 37).
We examined the effectiveness of nCRT plus PD-1/PD-L1 inhibitors for pMMR/MSS patients with LARC based on the aforementioned research and theories, the pCR and MPR rates were 38% and 60%, respectively. Therefore, radiation therapy is anticipated to make MSS LARC patients more sensitive to immunotherapy. Future research should focus on studies can be conducted to find markers that can clearly predict therapeutic efficacy.
Positive PD-L1 expression and high TMB were reliable indicators of neoadjuvant effectiveness, according to the VOLTAGE-A research (16). Similarly Lin et al. found that compared to patients with CPS <1/TMB <10, the pCR of patients with PD-L1 CPS ≥1/TMB ≥10 was substantially greater (11). Neoadjuvant chemoradiotherapy combined with immunotherapy had significantly improved the rate of pathological remission in LARC patients, however, special attention was still needed for LARC patients with high risk of disease progression after surgery. Therefore, exploring the effect of the addition of immunotherapy on tumor regression grade(TRG) and searching more reliable clinico-pathological biomarkers could improve the evaluation performance of postoperative prognosis and assist in planning postoperative treatment. Sert et al. used the TRG system and neoadjuvant rectal score(NARS) to assess the postoperative pathological response of LARC patients receiving nCRT, compared the value of TRG system and NARS in determining the prognosis, and found that the NARS may be a more favorable tool for predicting overall survival(OS) and metastasis free survival(MFS) (38). In addition, the watch and wait (W&W) strategy may be safe for LARC patients in complete clinical remission (cCR), it was challenging to accurately select these patients, therefore minimizing diagnostic differences among endoscopists was crucial. Wang et al. found that the deep learning algorithms of endoscopic images could accurately assessed the response of LARC to neoadjuvant therapy, and help to exactly select the best candidates for W&W strategies (39). Although the clinical strategy of nCRT combined with immunotherapy had great potential, there were still many areas that need to be improved. Artificial intelligence and its subtypes, especially deep learning(DL) played an increasing role in the diagnosis and prognosis of LARC, DL protocols seemed to help physicians optimize the strategy of neoadjuvant treatment of LARC by improving diagnostic accuracy, increasing clinician experience, and minimizing diagnostic subjectivity among physicians (40, 41).
It’s possible that the proinflammatory effects of RT and ICIs will produce intolerable toxicity. According to a meta-analysis, 68.3% of grade 3 or above adverse events were treatment-related side effects for immunotherapy plus chemotherapy. Additionally, the rate of grade 3 or higher adverse events in radiotherapy combinations with immunotherapy was 12.4% (42). Our finding demonstrated that PD-1/PD-L1 inhibitors plus nCRT did not increase the occurrence of significant adverse events. The pooled rates of ≥3 AEs were 20%. Moreover the surgical site infection(SSI) was one of the most common adverse events following rectal cancer procedures, among which sepsis was a life-threatening complication. Mulita et al. found that in rectal cancer patients the incidence of SSI was 21.8%, the postoperative sepsis was 12.77%, and advanced age, diabetes, cardiovascular disease and American Society of Anesthesiologists(ASA) class >2 are important factors affecting the occurrence of SSI and postoperative sepsis (43, 44). Interestingly, the studies included in this systematic review showed that immunotherapy combined with nCRT did not increase the incidence of SSI and postoperative sepsis, and there were no documented surgery-related deaths (15–17, 20, 23). This allows us to posit that PD-1/PD-L1 inhibitors and CRT combination therapy has a favorable safety profile.
In evaluating radiotherapy dosing and segmentation, previous studies have shown that short-course radiotherapy followed by double-drug chemotherapy holds advantages over long-course concurrent chemoradiotherapy in terms of tumor regression and overall survival (2, 45). This raises the question of whether its combination with immunotherapy could further enhance these outcomes. Our findings show that, while the pCR rates for PD-1/PD-L1 inhibitors with long-course radiotherapy(LCRT) and short-course radiotherapy(SCRT) were 30% and 51%, respectively. The aforementioned data demonstrated compared to LCRT, SCRT with immunotherapy presenting notably higher pCR rates. The study of different fractionation modes combined with immunotherapy in colorectal cancer found that the results of radiotherapy combined with immunosuppressants at three biologic effect doses of 16.4Gy×1, 8Gy×3 and 2Gy×18 showed that 8Gy×3 was the best segmentation mode, which significantly increased the number of tumor-infiltrating lymphocytes and the expression of PD-L1 and TIGIT, and could most effectively control tumor development (90% complete response) after combining ICI (46). In the same animal model, Morisada et al. showed that short-course hypofractionation could achieve better local and ectopic anti-tumor effects compared with conventional fractionation, such as increasing tumor-specific CD8+ T cells in draining lymph nodes, and concluded that ICI combined with hypofractionated radiotherapy was more effective than conventional fractionation to break immune tolerance and achieve stronger anti-tumor immune effects (47). In addition, it has been found that short-course hypofractionated radiotherapy can effectively inhibit tumor growth and recruit T lymphocytes in draining lymph nodes, maintain low levels of suppressor T cells and high levels of effector T cells, and at the same time, short-course hypofractionated radiotherapy can increase the levels of IL-8, IL-6, TNFa and other factors, and has a stronger effect of promoting dendritic cell (DC) maturation and activation, thereby increasing the efficacy of immunotherapy (48, 49). At present, most clinical studies use SBRT combined with immunotherapy, with a small number of SBRT fractions and a large single dose, taking into account the effect of tumor local control(LC) and promoting immune function. This study is the first to show that the perioperative SCRT combination with immunochemotherapy for LARC has a higher pCR rate than LCRT.
Our systematic review found higher pCR rates following the sequential (40%) immuno-CRT compared to concurrent immuno-CRT (30%). As for the timing of radiotherapy combined with immunotherapy, from the mechanism of action, it has been suggested that immune checkpoint inhibitors can not only activate killer T cells, but also normalize tumor blood vessels, reduce the state of tumor hypoxia, and increase the possibility of tumor sensitivity to radiotherapy (50). Therefore, immunoinduction followed by radiotherapy may improve the efficacy of radiotherapy, but the use of this model requires screening out the population sensitive to immunotherapy in advance. On the other hand, radiotherapy can activate the body’s immunity through the release of tumor antigen, so radiotherapy sequential immunotherapy may be more promising. However, the exposure of tumor antigen in the human body after release has a certain timeliness, and it will be cleared by the body after too long, which also poses challenges for the timing of immunotherapy intervention. As for which of the above two forms is better, there is no definite answer (51). This is mainly due to the difference in the target of immune checkpoint inhibitors and the difference in the clinical stage of patients. Therefore, it is necessary to explore the most appropriate combination therapy according to the drug and the patient’s own situation.
A meta-analysis showed that PD-1 inhibitors had an OS advantage over PD-L1 inhibitors (HR0.75), regardless of monotherapy (HR0.78) or combination chemotherapy (HR0.68), and PD-1 inhibitors also had better progression-free survival(PFS). The OS advantage of PD-1 versus PD-L1 inhibitors appeared to be more pronounced in combination therapy (HR0.67) than immunomonotherapy (HR0.85) (52). The results of this study showed that the pCR rate of PD-L1 inhibitors and PD-1 inhibitors was respectively 32% and 40%. Chemoradiotherapy can increase the expression of PD-L1 in tumor cells, leading to the synergy of immune drugs and chemoradiotherapy, and additional PD-L1 expression may offset T cell activation through excessive consumption of PD-L1 antibodies, thus causing this difference (53). And with PD-L1 inhibitors, the tumors escaped the anti-tumor immune response via the PD-1/PD-L2 axis. On the other hand, PD-L1 inhibition also played an important role in blocking the interaction between PD-L1 and CD80, so that CD80 preferentially binded to CTLA-4 on the surface of T cells, and the binding of CTLA-4 and CD80 negatively regulated the activation of T lymphocytes, which can only be achieved by PD-L1 antibodies (54). Research by Chen et al. indicated that exosomes in malignant melanoma also express PD-L1, which can directly bind to PD-1 on the surface of T cells and inhibit T cell function, and can also bind to PD-L1 monoclonal antibody.PD-L1 monoclonal antibodies may be neutralized by exosomes PD-L1 before they have a role to play (55). In terms of safety, our study found that PD-1 subgroup(18%) had a higher incidence of adverse events than PD-L1 subgroup(10%), and PD-1/PD-L2 may play an important role in mediating lung immune tolerance. PD mice experiment showed that PD-1inhibitors could undermine PD-1/PD-L2 combined with other partners such as repulsive guidance molecule b (RGMb) the interaction of balance, and result in adverse events (56), which may provide a useful guide for clinicians.
This systematic review has various limitations. First, approximately 50% of the data were included from active studies or abstracted from conferences, resulting in a limited number of patients and incomplete clinical data. Therefore, a large number of research are still needed to confirm the significance of nCRT combined with immunotherapy in LARC patients. Second, heterogeneity is further exacerbated by differences in immunotherapeutic drugs, patient characteristics, and study designs, the high heterogeneity in the pooled MPR rate is a concern, although slight improvement was observed in the subgroup analysis. Third, nCRT combined with immunotherapy is a relatively new treatment modality, up to now only one of the included studies had long-term survival data. Rahma et al. found that nCRT plus pembrolizumab was associated with a statistically significant improvement in 3yr OS compared with nCRT (95% vs 87%, P=0.04), but not in DFS. Since only one study reported long-term efficacy, there were insufficient direct comparative data on time-to-event outcomes, such as OS and DFS, in order to evaluate the survival outcomes of patients with LARC who received nCRT combined with immunotherapy. Pending the release of data on OS and DPS in subsequent studies, we will continue to analyze whether this treatment strategy will benefit long-term survival in LARC patients. Fourth, the predominance of single-arm, phase II clinical trials with limited sample sizes in the existing clinical research, which could lead to over- reporting of the efficacy of these neoadjuvant regimens. Despite these limitations, we found that there was increased efficacy when adding PD-1/PD-L1 inhibitors to nCRT for LARC patients, and our findings suggested possible directions for future treatment strategies.
These data suggested that nCRT combined with immunotherapy can improve MPR rate and pCR rate of pMMR/MSS locally advanced rectal cancer, while patients with dMMR/MSI-H had a higher pathological response rate. Preoperative immunotherapy combined with chemoradiotherapy can improve anal retention in patients with low rectal cancer, without increasing the incidence of adverse events. In addition, short-course radiotherapy, PD-1 inhibitors, and sequential immuno-CRT were associated with higher pCR rates compared to long-course radiotherapy, PD-L1 inhibitors, and concurrent immune CRT. In conclusion, this study provides favorable supporting evidence for neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
LY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XC: Data curation, Project administration, Software, Supervision, Visualization, Writing – review & editing. FW: Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. ZC: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – review & editing. LX: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. XW: Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – review & editing. ZL: Conceptualization, Methodology, Project administration, Software, Writing – review & editing. XNL: Writing – review & editing. QY: Data curation, Software, Writing – review & editing. XQL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. CG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Natural Science Foundation of Hebei Province (H2020206311), the Central Guiding Local Science and Technology Development Fund Project (Hebei Science and Technology Department Project) (226Z7712G) and the Government funded Clinical Medicine Excellent Talents Project of Hebei Provincial Department of Finance (2022-180).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1392499/full#supplementary-material
1. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. (2016) 34:3300–7. doi: 10.1200/JCO.2016.66.6198
2. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:29–42. doi: 10.1016/S1470-2045(20)30555-6
3. Fleming CA, Cullinane C, Lynch N, Killeen S, Coffey JC, Peirce CB. Urogenital function following robotic and laparoscopic rectal cancer surgery: meta-analysis. Br J Surg. (2021) 108:128–37. doi: 10.1093/bjs/znaa067
4. Andre T, Amonkar M, Norquist JM, Shiu K, Kim TW, Jensen BV, et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol. (2021) 22:665–77. doi: 10.1016/S1470-2045(21)00064-4
5. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
6. Haraldsdottir S. Microsatellite instability testing using next-generation sequencing data and therapy implications. JCO Precis Oncol. (2017) 1:1–4. doi: 10.1200/PO.17.00189
7. Chalabi M, Fanchi LF, Dijkstra KK, van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. (2020) 26:566–76. doi: 10.1038/s41591-020-0805-8
8. Slone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma2. JNCI: J Natl Cancer Institute. (1979) 63:1229–35. doi: 10.1186/s12967-021-02928-w
9. Boustani J, Lecoester B, Baude J, Latour C, Adotevi O, Mirjolet C, et al. Anti-PD-1/anti-PD-L1 drugs and radiation therapy: combinations and optimization strategies. Cancers. (2021) 13(19):4893. doi: 10.3390/cancers13194893
10. Salvatore L, Bensi M, Corallo S, Bergamo F, Pellegrini I, Rasola C, et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): The AVANA study. J Clin Oncol. (2021) 39:3511–1. doi: 10.1200/JCO.2021.39.15_suppl.3511
11. Lin Z, Cai M, Zhang P, Li G, Liu T, Li X, et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer. (2021) 9:e003554. doi: 10.1136/jitc-2021-003554
12. Rahma OE, Yothers G, Hong TS, Russell MM, You YN, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. (2021) 7:1225–30. doi: 10.1001/jamaoncol.2021.1683
13. Tamberi S, Grassi E, Zingaretti C, Papiani G, PINI S, Corbelli J, et al. A phase II study of capecitabine plus concomitant radiation therapy followed by durvalumab (MEDI4736) as preoperative treatment in rectal cancer: PANDORA study final results. J Clin Oncol. (2022) 40:LBA3513–LBA3513. doi: 10.1200/JCO.2022.40.17_suppl.LBA3513
14. Shamseddine A, Zeidan YH, Bouferraa Y, Turfa R, Kattan J, Mukherji D, et al. SO-30 Efficacy and safety of neoadjuvant short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced rectal adenocarcinoma: Averectal study. Radiat Oncol. (2021) 15:233. doi: 10.1016/j.annonc.2021.05.054
15. Li YJ, Zhang L, Dong QS, Cai Y, Zhang YZ, Wang L, et al. [Short-term outcome of programmed cell death protein1 (PD-1) antibody combined with total neoadjuvant chemoradiotherapy in the treatment of locally advanced middle-low rectal cancer with high risk factors]. Zhonghua wei chang wai ke za zhi = Chin J Gastrointestinal Surg. (2021) 24:998–1007. doi: 10.3760/cma.j.cn441530-20210927-00386
16. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin Cancer Res. (2022) 28:1136–46. doi: 10.1158/1078-0432.CCR-21-3213
17. Wu A, Li Y, Ji D, Zhang L, Zhang X, Cai Y, et al. PKUCH 04 trial: Total neoadjuvant chemoradiation combined with neoadjuvant PD-1 blockade for pMMR/MSS locally advanced middle to low rectal cancer. J Clin Oncol. (2022) 40:3609–9. doi: 10.1200/JCO.2022.40.16_suppl.3609
18. Zhou L, Yu G, Shen Y, Ding H, Zheng K, Wen R, et al. Safety and efficacy of programmed death-1 inhibitor combined with neoadjuvant chemoradiotherapy in the treatment of microsatellite stable extremely low rectal cancer. Chin J Gastrointestinal Surg. (2022) 25(3):250–6. doi: 10.3760/cma.j.cn441530-20211207-00487
19. George TJ, Yothers G, Jacobs SA, Finley GG, Wade JL, Rocha Lima CMSP, et al. Phase II study of durvalumab following neoadjuvant chemoRT in operable rectal cancer: NSABP FR-2. J Clin Oncol. (2022) 40:99–9. doi: 10.1200/JCO.2022.40.4_suppl.099
20. Wang Y, Shen L, Wan J, Zhang H, Wu R, Wang J, et al. Short-course radiotherapy combined with CAPOX and Toripalimab for the total neoadjuvant therapy of locally advanced rectal cancer: a randomized, prospective, multicentre, double-arm, phase II trial (TORCH). BMC Cancer. (2022) 22:274. doi: 10.1186/s12885-022-09348-z
21. Carrasco J, Beniuga G, Jouret-Mourin A, Baldin P, Sinapi I, Schroeder D, et al. 599P R-IMMUNE: A phase Ib/II study to evaluate safety and efficacy of atezolizumab combined with radio-chemotherapy in a preoperative setting for patients with locally advanced rectal cancer (LARC). Ann Oncol. (2023) 34:S432. doi: 10.1016/j.annonc.2023.09.1790
22. Dai S, Wang F, Shen Y, Shi L, Xu L, Lai C, et al. Efficacy and safety of neoadjuvant preoperative short-course radiation followed by envafolimab plus CAPEOX in microsatellite stable (MSS)/mismatch repair proficient (pMMR) locally advanced rectal cancer. J Clin Oncol. (2023) 41:134–4. doi: 10.1200/JCO.2023.41.4_suppl.134
23. Gao J, Zhang X, Yang Z, Zhang J, Bai Z, Deng W, et al. Interim result of phase II, prospective, single-arm trial of long-course chemoradiotherapy combined with concurrent tislelizumab in locally advanced rectal cancer. Front Oncol. (2023) 13:1057947. doi: 10.3389/fonc.2023.1057947
24. Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen H, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. (2017) 2017:1–15. doi: 10.1200/PO.17.00073
25. Overman MJ, Ernstoff MS, Morse MA. Where we stand with immunotherapy in colorectal cancer: deficient mismatch repair, proficient mismatch repair, and toxicity management. Am Soc Clin Oncol Educ Book. Am Soc Clin Oncol Annu Meeting. (2018) 38:239–47. doi: 10.1200/EDBK_200821
26. Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. (2014) 2:831–8. doi: 10.1158/2326-6066.CIR-14-0069
27. Reynders K, Illidge T, Siva S, Chang JY, de Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. (2015) 41:503–10. doi: 10.1016/j.ctrv.2015.03.011
28. Zhang J, Cai J, Deng Y, Wang H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology. (2019) 8:e1663108. doi: 10.1080/2162402X.2019.1663108
29. O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PloS One. (2017) 12:e0189848. doi: 10.1371/journal.pone.0189848
30. Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. (2019) 20:849–61. doi: 10.1016/S1470-2045(19)30027-0
31. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. (2020) 38:2053–61. doi: 10.1200/JCO.19.03296
32. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol (Baltimore Md 1950). (2005) 174:7516–23. doi: 10.4049/jimmunol.174.12.7516
33. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. (2015) 520:373–7. doi: 10.1038/nature14292
34. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. (2014) 74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258
35. Dovedi SJ, Illidge TM. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology. (2015) 4:e1016709. doi: 10.1080/2162402X.2015.1016709
36. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. New Engl J Med. (2012) 366:925–31. doi: 10.1056/NEJMoa1112824
37. Brix N, Tiefenthaller A, Anders H, Belka C, Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev. (2017) 280:249–79. doi: 10.1111/imr.12573
38. Sert F, Ozturk M, Yalman D, Ozkok S. Does neoadjuvant rectal score predict treatment outcomes better than the all grading systems used in neoadjuvantly treated rectal cancer? Asian J Surg. (2023) 46:4378–84. doi: 10.1016/j.asjsur.2022.10.010
39. Wang A, Zhou J, Wang G, Zhang B, Xin H, Zhou H. Deep learning of endoscopic features for the assessment of neoadjuvant therapy response in locally advanced rectal cancer. Asian J Surg. (2023) 46:3568–74. doi: 10.1016/j.asjsur.2023.03.165
40. Bousis D, Verras G, Bouchagier K, Antzoulas A, Panagiotopoulos I, Katinioti A, et al. The role of deep learning in diagnosing colorectal cancer. Przeglad Gastroenterologiczny. (2023) 18:266–73. doi: 10.5114/pg.2023.129494
41. Chlorogiannis DD, Verras G, Tzelepi V, Chlorogiannis A, Apostolos A, Kotis K, et al. Tissue classification and diagnosis of colorectal cancer histopathology images using deep learning algorithms. Is the time ripe for clinical practice implementation? Przeglad Gastroenterologiczny. (2023) 18:353–67. doi: 10.5114/pg.2023.130337
42. Zhou X, Yao Z, Bai H, Duan J, Wang Z, Wang X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. (2021) 22:1265–74. doi: 10.1016/S1470-2045(21)00333-8
43. Panos G, Mulita F, Akinosoglou K, Liolis E, Kaplanis C, Tchabashvili L, et al. Risk of surgical site infections after colorectal surgery and the most frequent pathogens isolated: a prospective single-centre observational study. Medicinski Glasnik. (2021) 18:438–43. doi: 10.17392/1348-21
44. Mulita F, Liolis E, Akinosoglou K, Tchabashvili L, Maroulis I, Kaplanis C, et al. Postoperative sepsis after colorectal surgery: a prospective single-center observational study and review of the literature. Przeglad Gastroenterologiczny. (2022) 17:47–51. doi: 10.5114/pg.2021.106083
45. Jin J, Tang Y, Hu C, Jiang L, Jiang J, Li N, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. (2022) 40:1681–92. doi: 10.1200/JCO.21.01667
46. Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J Immunother Cancer. (2019) 7:160. doi: 10.1186/s40425-019-0634-9
47. Morisada M, Clavijo PE, Moore E, Sun L, Chamberlin M, van Waes C, et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. (2018) 7:e1395996. doi: 10.1080/2162402X.2017.1395996
48. John-Aryankalayil M, Palayoor ST, Cerna D, Simone CBN, Falduto MT, Magnuson SR, et al. Fractionated radiation therapy can induce a molecular profile for therapeutic targeting. Radiat Res. (2010) 174:446–58. doi: 10.1667/RR2105.1
49. Kulzer L, Rubner Y, Deloch L, Allgäuer A, Frey B, Fietkau R, et al. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J Immunotoxicol. (2014) 11:328–36. doi: 10.3109/1547691X.2014.880533
50. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. (2017) 20:409–26. doi: 10.1007/s10456-017-9562-9
51. Yang H, Jin T, Li M, Xue J, Lu B. Synergistic effect of immunotherapy and radiotherapy in non-small cell lung cancer: current clinical trials and prospective challenges. Precis Clin Med. (2019) 2:57–70. doi: 10.1093/pcmedi/pbz004
52. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: A systematic review and meta-analysis. JAMA Oncol. (2020) 6:375–84. doi: 10.1001/jamaoncol.2019.5367
53. Fournel L, Wu Z, Stadler N, Damotte D, Lococo F, Boulle G, et al. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. (2019) 464:5–14. doi: 10.1016/j.canlet.2019.08.005
54. Herbst RS, Soria J, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. (2014) 515:563–7. doi: 10.1038/nature14011
55. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. (2018) 560:382–6. doi: 10.1038/s41586-018-0392-8
Keywords: PD-1/PD-L1 inhibitors, neoadjuvant immunotherapy, rectal cancer (RC), systematic review, neoadjuvant chemoradiotherapy
Citation: Yang L, Cui X, Wu F, Chi Z, Xiao L, Wang X, Liang Z, Li X, Yu Q, Lin X and Gao C (2024) The efficacy and safety of neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer patients: a systematic review. Front. Immunol. 15:1392499. doi: 10.3389/fimmu.2024.1392499
Received: 27 February 2024; Accepted: 23 April 2024;
Published: 15 May 2024.
Edited by:
Ping Wang, Michigan State University, United StatesReviewed by:
Ahmed Zarea, Michigan State University, United StatesCopyright © 2024 Yang, Cui, Wu, Chi, Xiao, Wang, Liang, Li, Yu, Lin and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Gao, NDgwMDA5ODZAaGVibXUuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Chao Gao, orcid.org/0000-0003-1173-5881
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.