
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 15 July 2024
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1388274
This article is part of the Research Topic Case Reports in Autoimmune and Autoinflammatory Disorders View all 42 articles
Background: Acquired reactive perforating collagenosis (ARPC) poses a clinical challenge with an unclear pathogenesis. This disease has been frequently proven resistant to immunosuppressive treatments, significantly affecting the quality of life of patients. In this report, we highlight the efficacy of baricitinib as a viable option for maintenance therapy in ARPC.
Case summary: An 81-year-old woman presented to our hospital with recurrent pruritus and cup-like ulcerated lesions on her trunk and limbs persisting for 1 year. She exhibited limited response to oral antihistamines and topical steroids. Past medical history revealed a prolonged history of coronary heart disease and type 2 diabetes spanning several years to decades. Histopathological examination revealed cup-shaped depressions filled with necrotic inflammatory debris. In the dermis, a mixed inflammatory infiltrate composed of lymphocytes and histiocytes was observed. Van Gieson staining indicated the elimination of fibrous tissue extending from the dermis into the epidermis. Consequently, a diagnosis of ARPC was established. Due to the inadequate response to conventional treatments and the severe itching, we initiated baricitinib therapy for ARPC, resulting in gradual symptom improvement. Follow-up assessments showed no adverse reactions and normal laboratory findings.
Conclusion: The case report suggests that baricitinib might offer significant therapeutic benefits for ARPC.
Acquired reactive perforating collagenosis (ARPC) is a rare dermatosis often characterized by severe pruritus. First described in 1967 by Mehregan et al. (1), it typically presents as umbilicated hyperkeratotic papules or a dome-shaped lesion with a central crater, frequently affecting the extremities. The Koebner phenomenon has been observed in some patients. In addition, it is commonly associated with diabetes and chronic kidney disease (2). Despite having been recognized for many years, the pathogenesis and etiology of ARPC remain unknown. Moreover, there is a lack of clarity in the treatment consensus for ARPC. In this case report, we present a rare instance of ARPC associated with diabetes, which was effectively managed with baricitinib therapy.
In September 2023, an 81-year-old woman was referred to the Department of Dermatology with a 1-year history of pruritic skin lesions. These lesions initially presented as umbilicated papules, but progressed to ulcerated plaques, predominantly on the trunk and limbs (Figure 1). The Koebner phenomenon was positive as new ulcerated plaques appeared on the normal skin after scratching. She had previously been diagnosed with eczema and had received antihistamines and topical corticosteroids without experiencing improvement in the pruritus and lesions. Her medical history included coronary heart disease for several years and type 2 diabetes mellitus (T2DM) for decades. At present, her T2DM is stable due to insulin treatment.
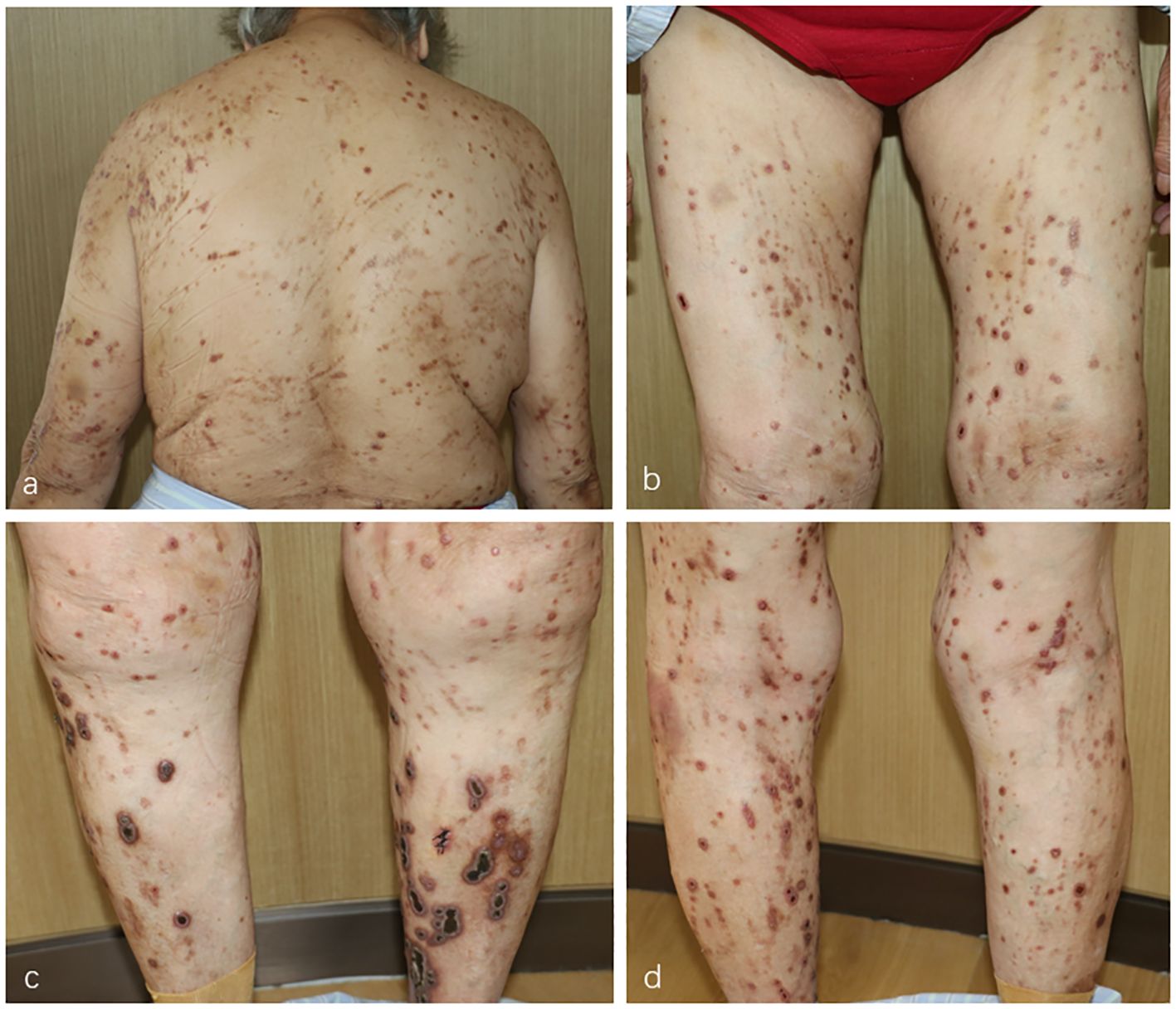
Figure 1 (A–D) Multiple umbilicated papules and nodules with a central keratotic crust on the patient’s trunk and limbs.
Upon admission, a series of serological tests, including acquired immunodeficiency syndrome (AIDS) screening; rapid plasma reagin test; hepatitis B virus serological markers; hepatitis C virus antibodies; T-SPOT; liver and kidney function tests; whole blood cell test; and C-reactive protein, anti-streptolysin “O,” antinuclear antibody, extractable nuclear antigen, and antineutrophil cytoplasmic antibody tests, yielded negative results, except for fasting blood glucose (7.89 mmol/L), glycosylated hemoglobin (8.5%), erythrocyte sedimentation (27 mm/h), and total IgE (682.6 kU/L).
A skin biopsy was subsequently performed, with the results of hematoxylin and eosin staining revealing a cup-shaped depression filled with necrotic inflammatory debris and a mixed inflammatory infiltrate composed of lymphocytes and histiocytes in the dermis. Elastica van Gieson staining showed fibrous tissue being ejected through the epidermis (Figure 2). In line with the clinical findings, a diagnosis of ARPC was considered. On the one hand, the patient’s itching was severe [Numeric Rating Scale (NRS) score of 9]; on the other hand, dupilumab did not achieve the expected effect in our other cases. Therefore, we initiated a therapeutic challenge with oral baricitinib at 2 mg QD. At 10 days after treatment, there was no significant improvement in her eruptions and itching. However, we recommended that the patient continue with the baricitinib regimen.
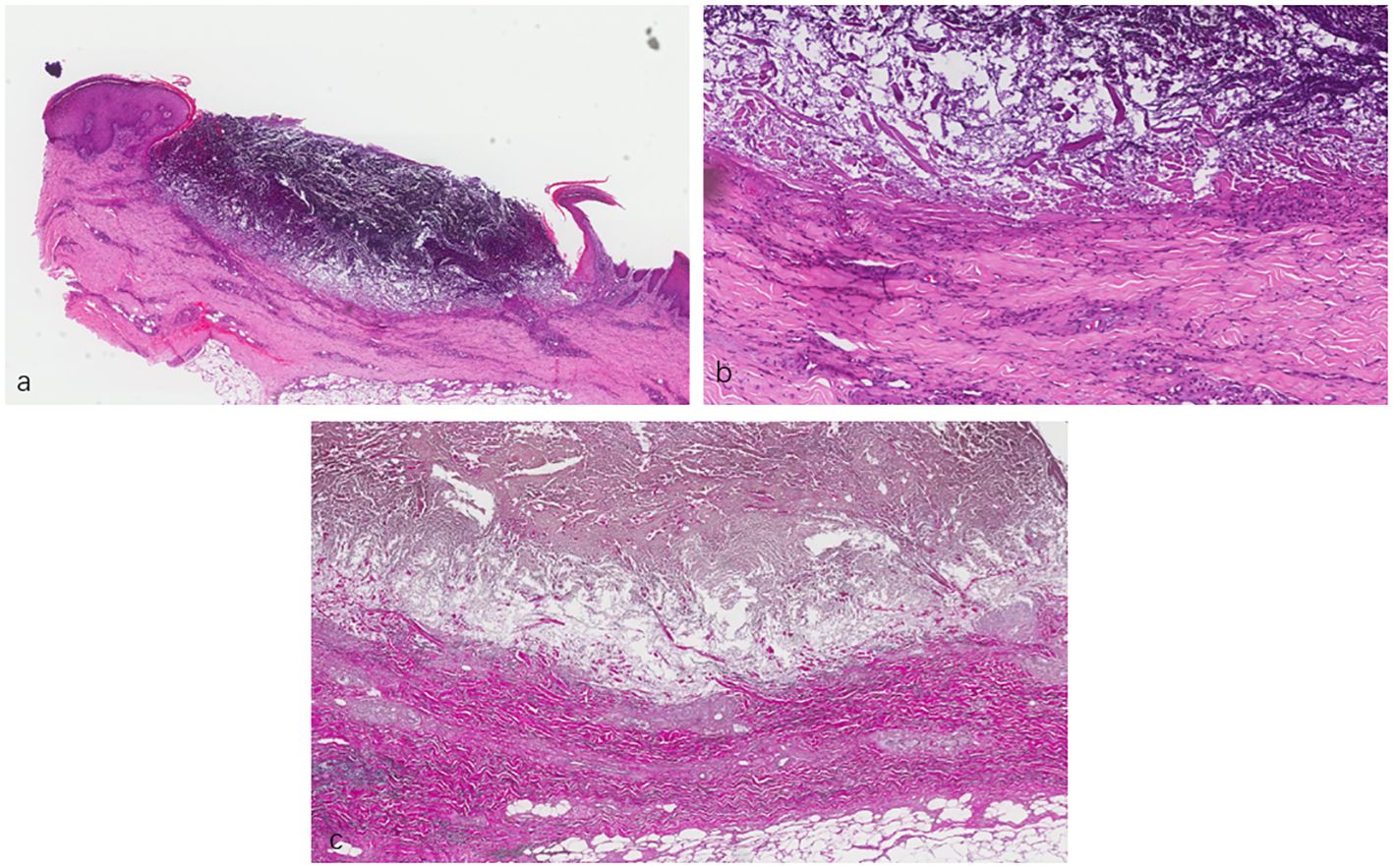
Figure 2 (A, B) Lesional skin biopsy showing a cup-shaped depression plugged with necrotic inflammatory debris. (C) Van Gieson staining showing elimination of fibrous tissue through the dermis into the epidermis. (A) ×40. (B) ×200. (C) ×400.
Surprisingly, her eruptions and itching showed complete improvement by the eighth week. At this point, the patient decided to discontinue the baricitinib treatment. Unfortunately, her lesions and itching gradually reappeared after discontinuation, prompting her to revisit the Department of Dermatology on December 28, 2023. During the physical examination, cup-like ulcerated lesions on her trunk and limbs were noted, along with multiple scar formations (Figure 3). The NRS score increased to 4 (Figure 4). Laboratory examinations during the second admission revealed negative results for the liver and kidney function tests, whole blood cell tests, and C-reactive protein, except for fasting blood glucose (15.54 mmol/L), glycosylated hemoglobin (10.2%), and total IgE (706.61 kU/L). With the patient’s consent, we resumed maintenance therapy with baricitinib at 2 mg QD. In April 2024, we conducted our last telephone follow-up of the patient, during which her eruptions and itching showed complete improvement.
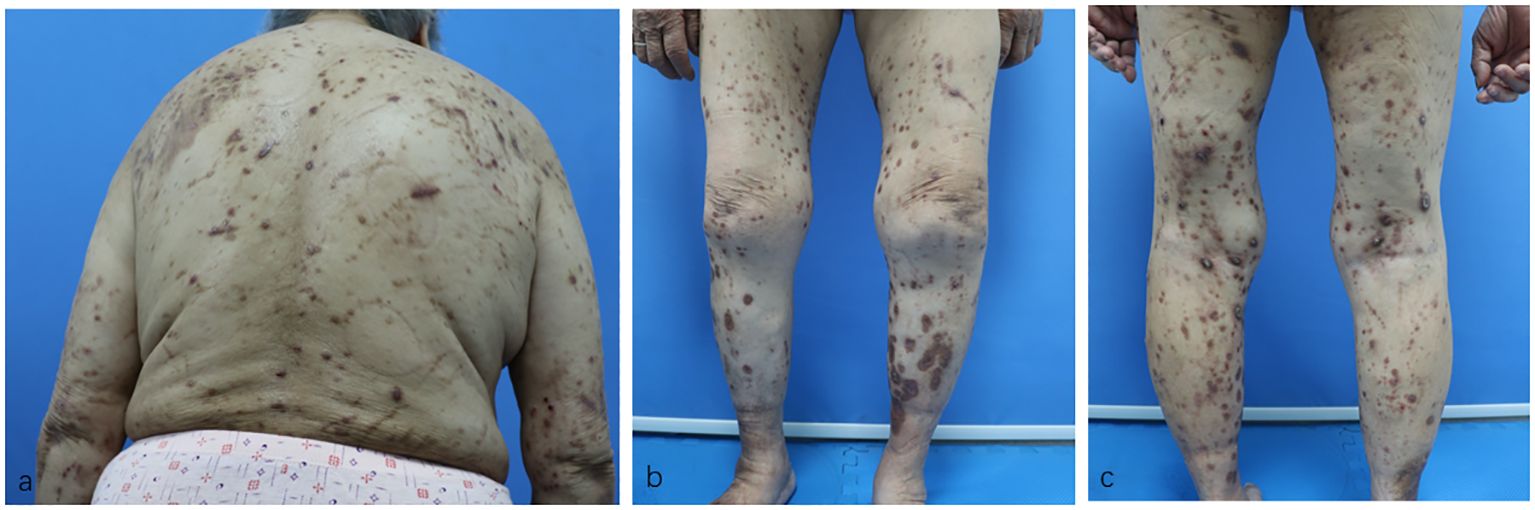
Figure 3 (A–C) Residual hyperpigmented lesions, atrophic scars, and a few superficial erosions on the patient’s trunk and limbs after baricitinib treatment.
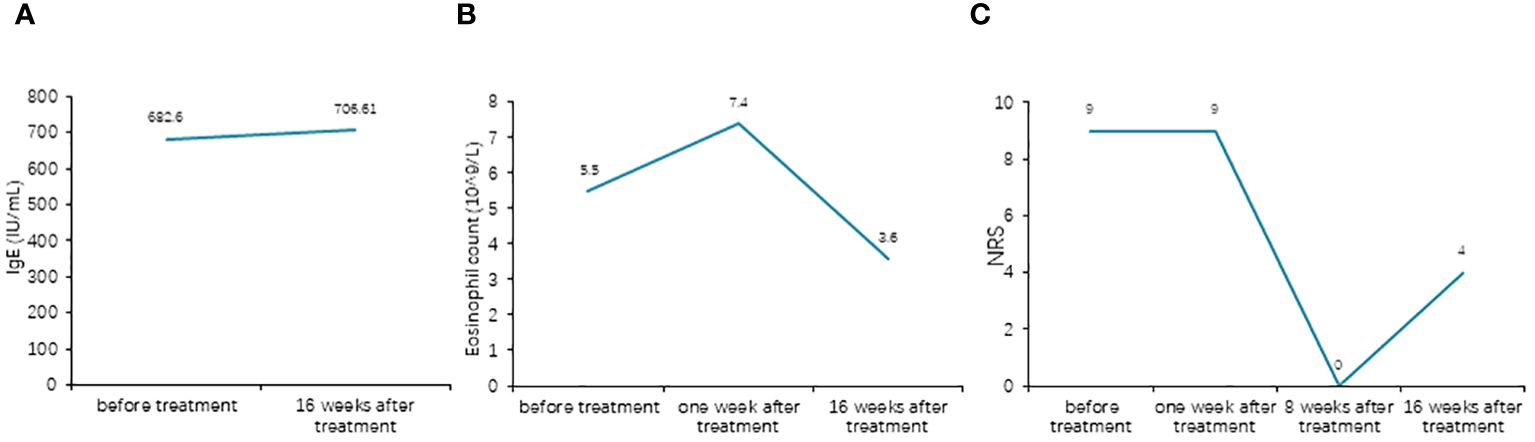
Figure 4 (A) Comparison of the total IgE levels before and after treatment. (B) Comparison of the eosinophil counts before and after treatment. (C) Comparison of the Numeric Rating Scale (NRS) scores before and after treatment.
ARPC is most frequently documented with concomitant T2DM and chronic kidney disease, as well as other systemic pathologies including malignancies, hypothyroidism, liver disorders, neurodermatitis, AIDS, and malignant hypertension (3). The giant variant typically presents as large (>1 cm) crateriform lesions. Central adherent keratotic plugs, severe pruritus, and erythematous halos are characteristics of this condition. Our patient exhibited a typical presentation of the “giant” variant of this disease, forming lesions with the presence of crusted plaques and central keratin plugs. Lesions often develop on the trunk or the limbs, areas easily accessible by hand (4). However, the treatment of perforating dermatoses is challenging. There is currently no consensus on treatment. Several therapeutic options have been reported as first-line therapy in ARPC, including topical corticoids, local and systemic retinoids, and UVB phototherapy, with variable results. Dynamic phototherapy, amitriptyline, and doxycycline have also been the subject of some studies (5, 6).
The etiology of ARPC is unknown; however, many theories have been posited for ARPC, tying superficial trauma of the skin to the elimination of biochemically altered type IV collagen fibers from the basement membrane. An early proposed mechanism for ARPC hypothesized that microcrystal-like deposits in the dermis biochemically altered the collagen fibers. Scratching then causes microtrauma, leading to the degradation of the basement membrane and the transepidermal elimination of collagen fibers (7). Furthermore, altered collagen may be induced by hypoxic states due to the vessel wall thickening associated with diabetic microvasculopathy. Allopurinol, a xanthine oxidase inhibitor, might decrease the elevated uric acid levels correlated with T2DM, reducing the crystal deposits within the dermis. However, recent studies have observed enhanced dermal infiltration of CD3+ T cells with a predominance of T helper 2 (Th2) cells in ARPC, similar to atopic dermatitis (AD). In addition, the cytokines interleukin 4 (IL-4) and IL-13, which act on neurons to promote itching, were also significantly upregulated in ARPC (8). Moreover, there is now an increasing amount of clinical data that support the use of dupilumab in ARPC (8, 9). These findings suggest that Th2-type inflammation is involved in the pathogenesis of ARPC. Our patient was successfully treated with a Janus kinase (JAK) inhibitor.
JAK inhibitors are a novel class of oral immunosuppressant medications licensed for the management of AD, psoriasis, rheumatoid arthritis, and alopecia areata. By inhibiting the action of four key tyrosine kinases, i.e., JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), they affect various cell lineages, including CD8-positive T cells, and suppress the interferon gamma and interleukin pathways (10). Baricitinib is a JAK1/JAK2 inhibitor used to treat severe rheumatoid arthritis, alopecia areata, and AD (11–13). More widespread cytokine perturbation via JAK inhibition is beneficial when the disease pathogenesis is complex and not well understood (14). In our case, baricitinib was significantly more effective than the conventional treatment for ARPC. Baricitinib reduced the lesion size and improved pruritus within 4 weeks of treatment. After 8 weeks of treatment, the patient achieved clearance of skin lesions and relief from pruritus, with no adverse events (AEs).
To the best of our knowledge, this case marks the inaugural utilization of baricitinib in the treatment of ARPC. This case underscores the efficacy of baricitinib for ARPC and advocates its consideration in instances of conventional treatment-resistant ARPC. While certain studies have indicated the involvement of type 2 inflammation in the pathogenesis of ARPC, further clinical and foundational research is imperative to corroborate this conclusion.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JZ: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. YD: Resources, Writing – review & editing. YC: Data curation, Resources, Writing – review & editing. YS: Project administration, Resources, Writing – review & editing. YG: Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mehregan AH, Schwartz OD, Livingood CS. Reactive perforating collagenosis. Arch Dermatol. (1967) 96:277–82. doi: 10.1001/archderm.1967.01610030055009
2. Kawakami T, Akiyama M, Ishida-Yamamoto A, Nakano H, Mitoma C, Yoneda K, et al. Clinical practice guide for the treatment of perforating dermatosis. J Dermatol. (2020) 47:1374–82. doi: 10.1111/1346-8138.15647
3. Faver IR, Daoud MS, Su WP. Acquired reactive perforating collagenosis: report of six cases and review of the literature. J Am Acad Dermatol. (1994) 30:575–80. doi: 10.1016/S0190-9622(94)70065-6
4. Hoque SR, Ameen M, Holden CA. Acquired reactive perforating collagenosis: four patients with a giant variant treated with allopurinol. Br J Dermatol. (2006) 154:759–62. doi: 10.1111/j.1365-2133.2005.07111.x
5. Yong A, Chong WS, Tey HL. Effective treatment of uremic pruritus and acquired perforating dermatosis with amitriptyline. Australas J Dermatol. (2014) 55:54–7. doi: 10.1111/ajd.12026
6. Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. (2012) 28:50–2. doi: 10.1111/j.1600-0781.2011.00634.x
7. Haftek M, Euvrard S, Kanitakis J, Delawari E, Schmitt D. Acquired perforating dermatosis of diabetes mellitus and renal failure: further ultrastructural clues to its pathogenesis. J Cutan Pathol. (1993) 20:350–5. doi: 10.1111/j.1600-0560.1993.tb01274.x
8. Ben L, Yibei W, Xiaoyan W, Xinyu Z, Ruzeng X, Zhenying Z. Dupilumab improve acquired reactive perforating collagenosis characterized by type 2 inflammation. Front Immunol. (2023) 10:1240262. doi: 10.3389/fimmu.2023.1240262
9. Ying Y, Shuang C, Zhen-Ying Z. Dupilumab may be an alternative option in the treatment of acquired reactive perforating collagenosis combined with AD. Immun Inflammation Dis. (2022) 10:e574. doi: 10.1002/iid3.574
10. Yamaoka K, Saharinen P, Pesu M, Holt VE 3rd, Silvennoinen O, O’Shea JJ. The janus kinases (Jaks). Genome Biol. (2004) 5:253. doi: 10.1186/gb-2004-5-12-253
11. Mogul A, Corsi K, McAuliffe L. Baricitinib: The second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother. (2019) 53:947–53. doi: 10.1177/1060028019839650
12. Andrew M, Matthew H. Baricitinib in alopecia areata. N Engl J Med. (2022) 386:1751–2. doi: 10.1056/NEJMe2203440
13. Simpson EL, Lacour JP, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. (2020) 183:242–55. doi: 10.1111/bjd.18898
Keywords: acquired reactive perforating collagenosis, baricitinib, case report, diabetes, Th2-type inflammation
Citation: Zheng J, Ding Y, Chen Y, Shi Y and Gao Y (2024) Effectiveness of baricitinib in acquired reactive perforating collagenosis: a case report. Front. Immunol. 15:1388274. doi: 10.3389/fimmu.2024.1388274
Received: 19 February 2024; Accepted: 21 June 2024;
Published: 15 July 2024.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Esraah Alharris, University of Al-Qadisiyah, IraqCopyright © 2024 Zheng, Ding, Chen, Shi and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlu Gao, Z2FveXVubHVAMTI2LmNvbQ==; Yuling Shi, c2hpeXVsaW5nMTk3M0AxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.