
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 18 April 2024
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1387503
This article is part of the Research Topic Inflammatory Disorders of the Oral Mucosa: from Stem Cell Biology to Clinical Management View all 6 articles
Background: The manifestations of bullous pemphigoid (BP) and herpes simplex virus (HSV) infection are similar in oral mucosa, and the laboratory detection of HSV has some limitations, making it difficult to identify the HSV infection in oral lesions of BP. In addition, the treatments for BP and HSV infection have contradictory aspects. Thus, it is important to identify the HSV infection in BP patients in time.
Objective: To identify the prevalence and clinical markers of HSV infection in oral lesions of BP.
Methods: This prospective cross-sectional descriptive analytical study was conducted on 42 BP patients with oral lesions. A total of 32 BP patients without oral lesions and 41 healthy individuals were enrolled as control groups. Polymerase chain reaction was used to detect HSV. Clinical and laboratory characteristics of patients with HSV infection were compared with those without infection.
Results: A total of 19 (45.2%) BP patients with oral lesions, none (0.0%) BP patients without oral lesions, and four (9.8%) healthy individuals were positive for HSV on oral mucosa. Among BP patients with oral lesions, the inconsistent activity between oral and skin lesions (p=0.001), absence of blister/blood blister in oral lesions (p=0.020), and pain for oral lesions (p=0.014) were more often seen in HSV-positive than HSV-negative BP patients; the dosage of glucocorticoid (p=0.023) and the accumulated glucocorticoid dosage in the last 2 weeks (2-week AGC dosage) (p=0.018) were higher in HSV-positive BP patients. Combining the above five variables as test variable, the AUC was 0.898 (p<0.001) with HSV infection as state variable in ROC analysis. The absence of blister/blood blister in oral lesions (p=0.030) and pain for oral lesions (p=0.038) were found to be independent predictors of HSV infection in multivariable analysis. A total of 14 (73.7%) HSV-positive BP patients were treated with 2-week famciclovir and the oral mucosa BPDAI scores significantly decreased (p<0.001).
Conclusion: HSV infection is common in BP oral lesions. The inconsistent activity between oral and skin lesions, absence of blister in oral lesions, pain for oral lesions, higher currently used glucocorticoid dosage, and higher 2-week AGC dosage in BP patients should alert physicians to HSV infection in oral lesions and treat them with 2-week famciclovir in time.
Bullous pemphigoid (BP) is an autoimmune subepidermal bullous dermatosis induced by autoantibodies against BP180 and BP230, the important components of the dermal–epidermal junction. It mainly manifests as tense blisters, urticarial plaques, erythema, and erosions on the skin with severe pruritus. Approximately 10%–20% of BP patients are accompanied by erosions or blisters on the mucosa, mainly on the oral mucosa (1–4).
Herpes simplex virus (HSV) has two subtypes, HSV-1 and HSV-2. HSV spreads widely among people, with 52%–91% of people reportedly HSV-1 seropositive and 4%–24% of people reportedly HSV-2 seropositive (5–8). The clinical manifestations of HSV infection are diverse, which include eczema herpetiform, herpetic gingivostomatitis, herpes labialis, genital herpes, and asymptomatic infection. HSV-1 mainly causes mucocutaneous infection outside the genitals, especially around the mouth or nose, and HSV-2 mainly causes mucocutaneous infection around the genital area (9).
On the skin, BP typically manifests as diffused urticarial plaques and blisters all around the body with severe pruritus, while HSV infection often appears as clustered vesicles and erosions around the mouth, nose, or vulva with pain, burning, pruritus, or tingling, which can distinguish the HSV infection from BP. However, on the oral mucosa, both BP and HSV infection present with erosions and blisters (1, 10). The similarity in manifestations between BP and HSV infection on oral mucosa makes it difficult to identify the HSV infection in oral lesions of BP patients.
The laboratory detection of HSV presents several limitations. HSV-specific IgG antibody tests have low specificity due to the high prevalence of HSV among people. Although a significant rise in the HSV-specific IgG antibody titer can provide diagnostic evidence for active HSV infection, it is less practical in clinical work because of the need for frequent follow-up visits. HSV-specific IgM antibody tests suffer from low sensitivity because of the short duration of IgM antibodies. Other methods, including viral culture, cytological smear, direct fluorescent antibody studies, tissue biopsy, and viral deoxyribonucleic acids (DNA) detection by polymerase chain reaction (PCR), are all challenging to practice and promote due to the high requirements for personnel, testing environment, and equipment (9). The limitations of HSV detection in clinical practice exacerbate the difficulty of identifying the HSV infection in oral lesions of BP patients in time.
The treatments for HSV infection and BP have some contradictions. HSV infection is mainly treated with antivirus drugs such as acyclovir, valacyclovir, and famciclovir. Maintaining an immunocompetent status is beneficial for suppressing HSV. Conversely, BP as an autoimmune disease is mainly treated by glucocorticoid or other immunosuppressants, which can, however, make BP patients immunocompromised and trigger HSV activation, prolong HSV shedding, and increase the risk of HSV dissemination (11). Hence, it is important to identify the HSV infection in BP patients in time.
Nikkels et al. found that there were 10% (2/20) of skin biopsies from BP patients positive for HSV by immunohistochemistry and PCR (12), while studies that explore the prevalence or clinical markers of HSV infection in oral lesions of BP are still absent.
In this study, we investigated the prevalence and clinical markers of HSV infection in oral lesions of BP patients to find out the importance of HSV infection among oral lesions of BP and provide some clinical clues for clinicians to identify HSV infection in time in oral lesions of BP, which could avoid the unnecessary adjustment of glucocorticoid or other immunosuppressants and add the antiviral treatment in time.
We conducted a prospective cross-sectional descriptive analytical study on BP patients with oral lesions who were admitted to our hospital from 1 January 2022 to 30 June 2023. BP patients without oral lesions and healthy individuals were enrolled as controls during the study. The Ethics Committee of the Chinese Academy of Medical Sciences, Hospital for Skin Diseases (2021-KY-060) approved the project. Written informed consents were obtained from all participants after a thorough explanation of study procedures and objectives.
Patients who were diagnosed with BP by clinical manifestation, biopsy, direct immunofluorescence, enzyme-linked immunosorbent assay or immunoblot, and indirect immunofluorescence using salt-split skin (13), and accompanied by oral lesions, regardless of the treatment status and the disease activity, were included in the study.
Patients were excluded from the study if they had met at least one of the following criteria: patients who could not exclude the diagnosis of mucous membrane pemphigoid; or patients who were accompanied by another autoimmune bullous disease, such as P200 pemphigoid, epidermolysis bullosa acquisita; or patients who were accompanied by other diseases that might affect the oral mucosa, such as Behet’s disease, oral lichen planus, Stevens–Johnson syndrome, and oral cancer.
Clinical examinations of the skin and mucosa were performed carefully for each patient to assess the location, basement, type, and shape of the lesions, and the disease severity was assessed by bullous pemphigoid disease area index (BPDAI) (14).
The severity of oral lesions’ pain was assessed on a 3-grade scale: (i) mild, discomfort or pain with firm pressure that does not hinder eating or speaking; (ii) moderate, pain with gentle pressure that affects eating but not speaking or drinking; and (iii) severe, persistent pain without any pressure, which impedes speaking or drinking.
The disease activity of BP was divided into active and inactive. If the new lesions or pruritic symptoms cease to form and the established lesions begin to heal or even have disappeared, the disease will be judged as inactive; if not, the disease will be judged as active. In this study, we assessed the disease activity separately for oral and skin lesions. For BP patients, whose oral lesions were active but the skin lesions were inactive or whose oral lesions were inactive but the skin lesions were active, the disease activity would be defined as an inconsistent activity between oral and skin lesions.
Other clinical information of the patients, which included age, sex, comorbidities, duration of the skin and oral lesions up to the time of swabs taking, dosage and duration of the glucocorticoid usage when the swabs were taken, accumulated glucocorticoid dosage in the last 2 weeks (2-week AGC dosage), and other treatment information, was also collected and recorded.
Laboratory tests such as the titers of anti-BP180 NC16A antibody and anti-BP230 antibody, and the counts and percentages of immune cells, and the amount of albumin, globulin, total protein, and immunoglobulins in serum samples, were performed and collected when the patients were enrolled.
HSV-1 and HSV-2 were detected from the oral swabs of participants by PCR. For BP patients with oral lesions, swabs were taken from the oral lesions; for BP patients without oral lesions and healthy individuals, swabs were taken from the entire oral mucosa.
DNA was extracted from the swabs using DNA extraction buffer (Da An Gene, China) according to the manufacturer’s instructions. Five microliters of the extracted DNA was used as template in a final volume of 50 μl PCR mixture containing 25 μl of 2× GoTaq® Green Master Mix (Promega), 16 μl of nuclease-free water (Promega), and 2 μl of upstream and downstream primers (10 μmol/l, Sangon Biotech, China) for HSV-1, HSV-2, or β2-microglobulin. The sequences of the oligonucleotide primers used in PCR for HSV-1, HSV-2, and β2-microglobulin DNA detection are shown in Table 1 (15–17). Nuclease-free water (Promega) and HSV-1/HSV-2 positive DNA (provided by the sexually transmitted disease laboratory) were taken separately as negative and positive controls. The reaction was carried out in a DNA thermal cycler (GeneAmp® PCR System 9700, ABI, USA). Temperature cycling was as follows: 95°C for 5 min; 95°C over 45 s, 57°C over 45 s (HSV-1/HSV-2)/50°C over 45 s (β2-microglobulin), and 72°C over 1 min for 35 cycles; and 72°C for 5 min. The amplification products were separated by 2% agarose gel electrophoresis and visualized by subsequent 4S Red Plus Nucleic Acid Stain (Sangon Biotech, China) staining and UV radiation. In positive cases, a distinct and sharp band of the expected size was seen (Figure 1), and the results were verified by Sanger sequencing for the PCR product (Sangon Biotech, China).
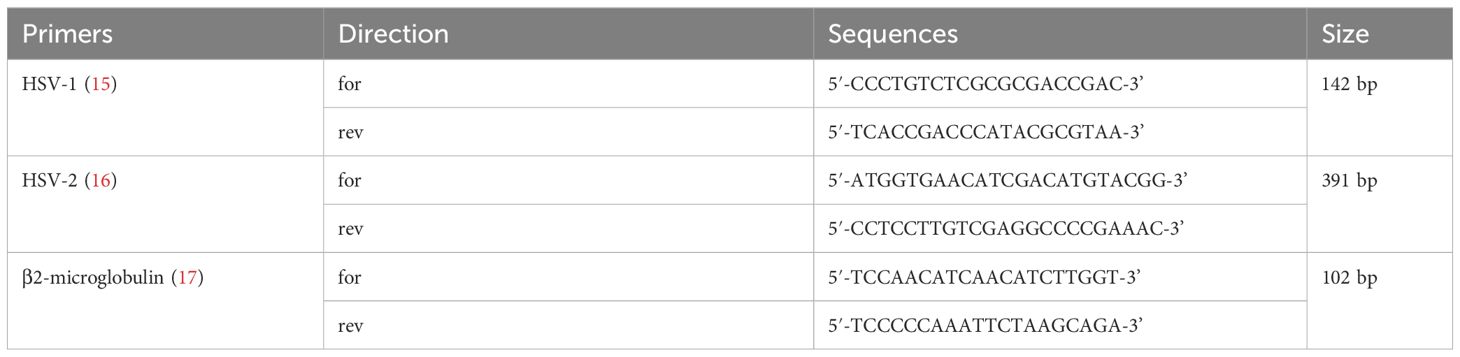
Table 1 Sequences of the oligonucleotide primers used in PCR for HSV-1, HSV-2, and β2-microglobulin DNA detection.
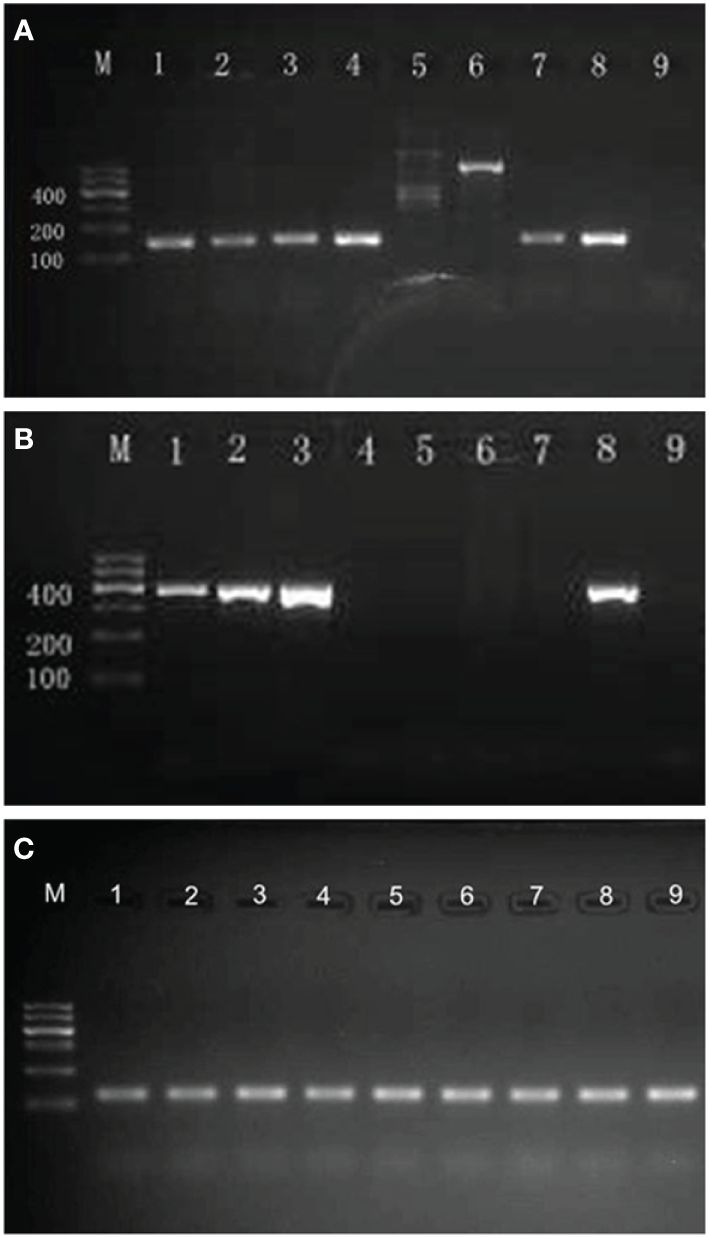
Figure 1 Results of agarose gel electrophoresis of PCR products. (A) Agarose gel electrophoresis of PCR products for HSV-1 DNA (142 bp). M, molecular weight marker (DNA ladder, 100 bp); lane 1–4,7, positive for HSV-1; lane 5,6, negative for HSV-1; lane 8, positive control for HSV-1; lane 9, negative control for HSV-1. (B) Agarose gel electrophoresis of PCR products for HSV-2 DNA (391 bp). M, molecular weight marker (DNA ladder, 100 bp); lanes 1–3, positive for HSV-2; lane 4–7, negative for HSV-2; lane 8, positive control for HSV-2; lane 9, negative control for HSV-2. (C) Agarose gel electrophoresis of PCR products for β2-microglobulin DNA (102 bp). M, molecular weight marker (DNA ladder, 100 bp); all samples were detected positive for β2-microglobulin DNA.
Participants who were detected positive for HSV were informed of the results by phone. The HSV-positive BP patients with oral lesions were treated with oral famciclovir 0.25g, three times daily for 2 weeks, with the dosage of glucocorticoid decreased or maintaining the existing treatments. The disease activity and BPDAI scores of oral lesions were assessed again by phone or clinic visit after the 2-week famciclovir treatment. The oral lesions were retested for HSV by PCR after 1 and 2 weeks of famciclovir treatment if the oral swabs were available. The HSV-positive participants without oral lesions were followed up by phone for oral lesions development 1 and 2 weeks after the oral swabs were taken.
Statistical analysis was performed using IBM SPSS 26.0 software. Descriptive statistics were used for categorical variables. Mean with standard deviation (SD) or median with interquartile range (IQR) was estimated for continuous variables. The chi-square test/Fisher’s exact test was used to compare the differences in categorical variables. The one-way ANOVA analysis, independent t-test, paired t-test, or Mann–Whitney U-test was used to compare the differences in continuous variables. Variables in the univariate analysis with a p<0.05 were taken up for multivariate analysis by binary regression analysis to identify the independent parameters of HSV infection. ROC analysis was conducted to figure out the clinical markers of HSV infection in oral lesions of BP. Results were considered statistically significant when the two-sided p<0.05.
In this study, we enrolled 42 BP patients with oral lesions as the lesion group, 32 BP patients without oral lesions as the non-lesion group, and 41 healthy individuals as the healthy control group.
The mean age (SD) of the participants was 67.4 (11.8) years in the lesion group, 65.6 (17.1) years in the non-lesion group, and 62.2 (9.8) years in the healthy control group. The male-to-female ratio of the three groups was 1.5:1, 2.2:1, and 1.2:1, respectively. Neither age (p=0.174) nor sex distribution (p=0.425) was statistically different among the three groups. Among all the participants, except for two patients in the lesion group accompanied by herpes labialis, all individuals did not have any suggestive symptoms of HSV infection.
In lesion and non-lesion groups, hypertension was the most common comorbidity, followed by diabetes. Glucocorticoid was the most commonly used drug, followed by immunosuppressants and tetracyclines. The comorbidity, treatment, and disease activity of skin lesions did not show significant differences between the lesion and non-lesion groups (Table 2).
Among the 42 BP patients in the lesion group, 22 (52.4%) patients were active in both oral lesions and skin lesions, three (7.1%) patients were inactive in both oral lesions and skin lesions, and 17 (40.5%) patients were active in oral lesions but inactive in skin lesions. A total of 30 (71.4%) patients developed skin lesions earlier than oral lesions, 11 (26.2%) patients developed skin lesions and oral lesions at the same time, and only one (2.4%) patient developed skin lesions later than oral lesions. The oral lesions of BP were mostly located on the buccal mucosa and then followed by the palate/posterior pharynx and gingiva. The median (IQR) oral mucosa BPDAI score of the enrolled BP patients in the lesion group was 5.0 (2.0–8.1), and about half of them were painful for oral lesions, but the pain was mostly mild (Table 3). Additionally, there were also four patients with lesions on extra-oral mucosa, including two patients with lesions on genital mucosa and two on nasal mucosa.
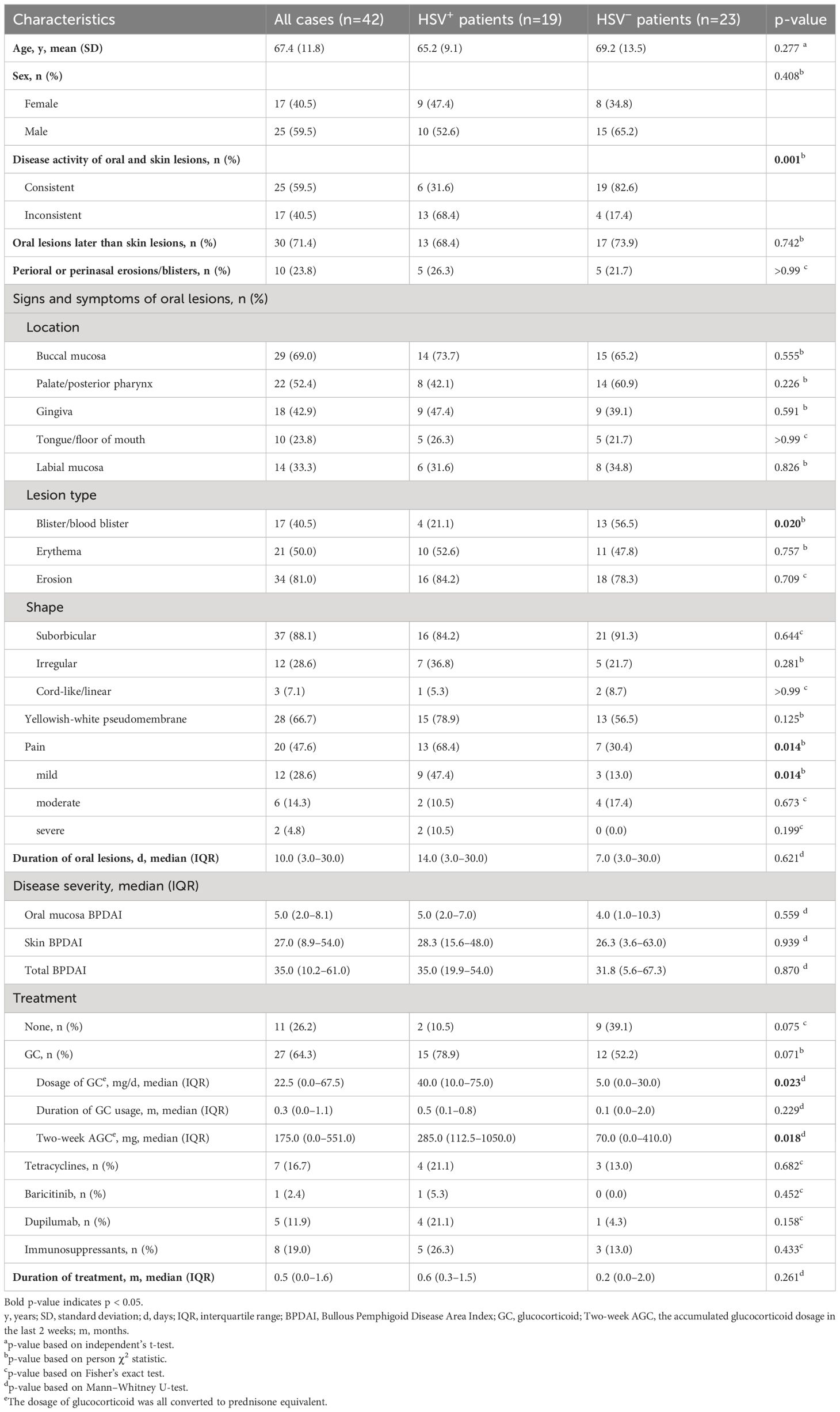
Table 3 Clinical characteristics of patients with HSV infection compared with those without HSV infection in the lesion group.
In the lesion group, HSV was positive in 19 (45.2%) cases detected by PCR, among whom 18 (42.9%) were positive for HSV-1 and one (2.4%) was positive for HSV-2. In the non-lesion group, none of the patients was positive for HSV. In the healthy control group, HSV was positive in four (9.8%) individuals, and all of them were positive for only HSV-1. For the positive rate of HSV, the lesion group was significantly higher than the non-lesion and healthy control groups (both p<0.001), while the latter two groups showed no statistical difference (p=0.126).
BP patients in the lesion group were divided into HSV-positive (19 cases) and HSV-negative (23 cases) subgroups according to the results of HSV detection. The clinical characteristics of the two subgroups were compared in many aspects, and the results are shown in Table 3. Compared to the HSV-negative subgroup, the inconsistent activity between oral and skin lesions (p=0.001), absence of blister/blood blister in oral lesions (p=0.020), and pain for oral lesions (p=0.014) were more often seen in the HSV-positive subgroup; the dosage of glucocorticoid (p=0.023) and 2-week AGC dosage (p=0.018) were higher in the HSV-positive subgroup. The age, sex distribution, development order of oral and skin lesions; proportion of perioral or perinasal lesions appearing; disease severity; duration of treatment and glucocorticoid usage; and location, shape, basement, and duration of oral lesions showed no statistical differences between the two subgroups (Table 3).
In addition to the clinical characteristics, the laboratory tests were also compared between HSV-positive and HSV-negative subgroups (Table 4). However, all the parameters that include the titer of BP180 autoantibody, positive rate, and titer of BP230 autoantibody, elevated rate of the count and percentage of immune cells in blood routine test, and the amount of total protein, albumin, globulin, and immunoglobulins in serum samples were not statistically different between the two subgroups (Table 4).
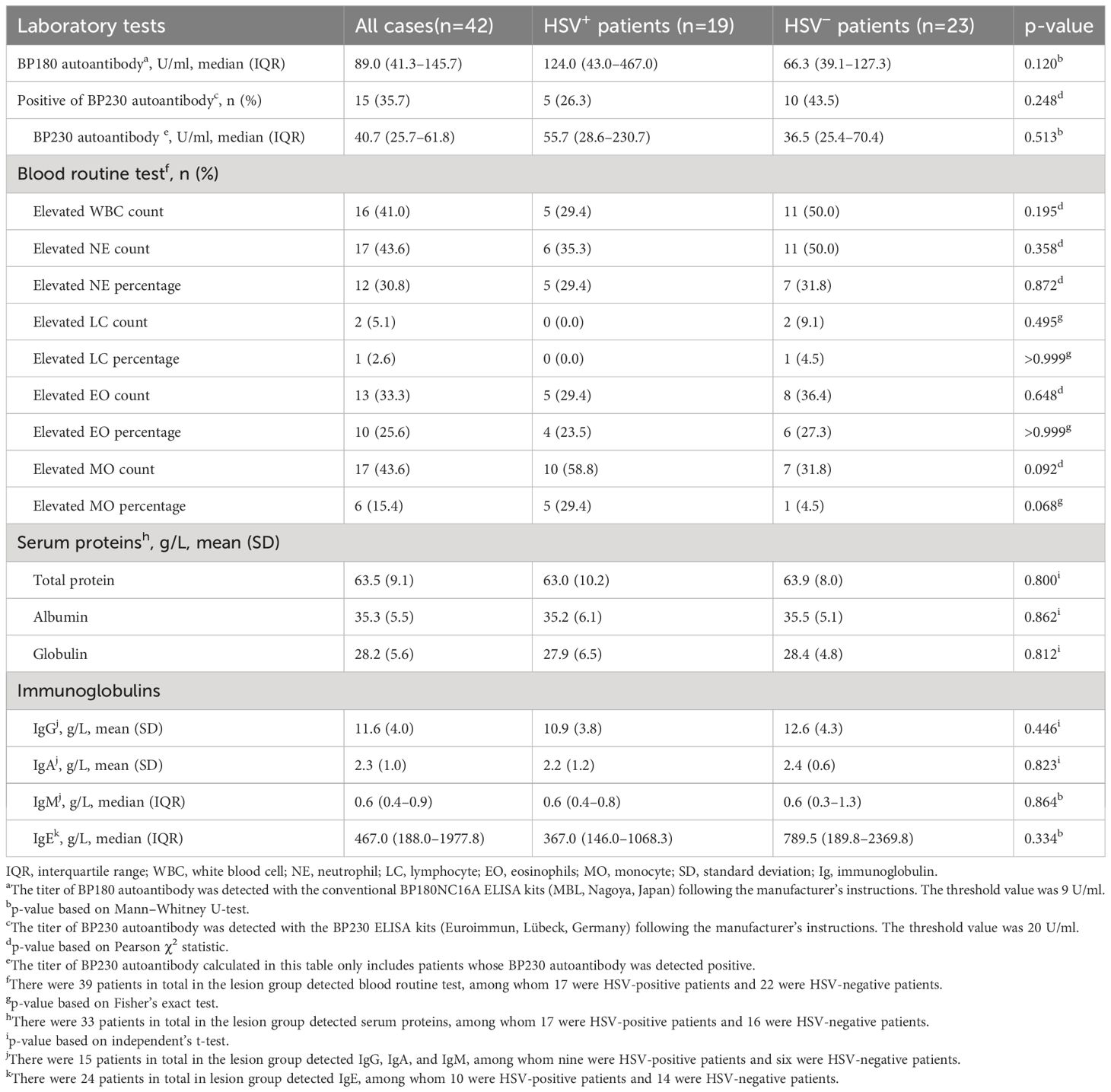
Table 4 Laboratory tests of patients with HSV infection compared with those without HSV infection in the lesion group.
Furthermore, a multivariable model was established to identify the independent predictors of HSV infection in oral lesions of BP. In this model, HSV infection was set as the dependent variable and inconsistent activity between oral and skin lesions, absence of blister/blood blister in oral lesions, pain for oral lesions, dosage of glucocorticoid, and 2-week AGC dosage were set as independent variables. After adjustment, the absence of blister/blood blister in oral lesions (p=0.030) and pain for oral lesions (p=0.038) were found to be independently associated with HSV infection.
According to the above results, there were indeed some differences between BP patients with and without HSV infection in oral mucosa. To figure out whether these variables could serve as clinical markers to help indicate the HSV infection in oral lesions of BP, ROC analysis was done to calculate the prediction probability of the variables involved in the present study. First, the variables were analyzed individually for the area under the curve (AUC). The results showed that the inconsistent activity between oral and skin lesions (AUC=0.755, p=0.001), absence of blister/blood blister in oral lesions (AUC=0.677, p=0.035), pain for oral lesions (AUC=0.690, p=0.023), dosage of glucocorticoid (AUC=0.700, p=0.015, cut-off value=33.75mg/d prednisone equivalent with a sensitivity of 0.579 and specificity of 0.783), and 2-week AGC dosage (AUC=0.709, p=0.011, cut-off value=554mg prednisone equivalent with a sensitivity of 0.474 and specificity of 0.957) could significantly distinguish oral lesions of BP with HSV infection from those without HSV infection (Figures 2A–E). Then, all the aforementioned variables were combined into a single test variable to calculate AUC, resulting in an improved AUC of 0.898 with the highest Youden index of 0.678 (p<0.001) (Figures 2F, G).
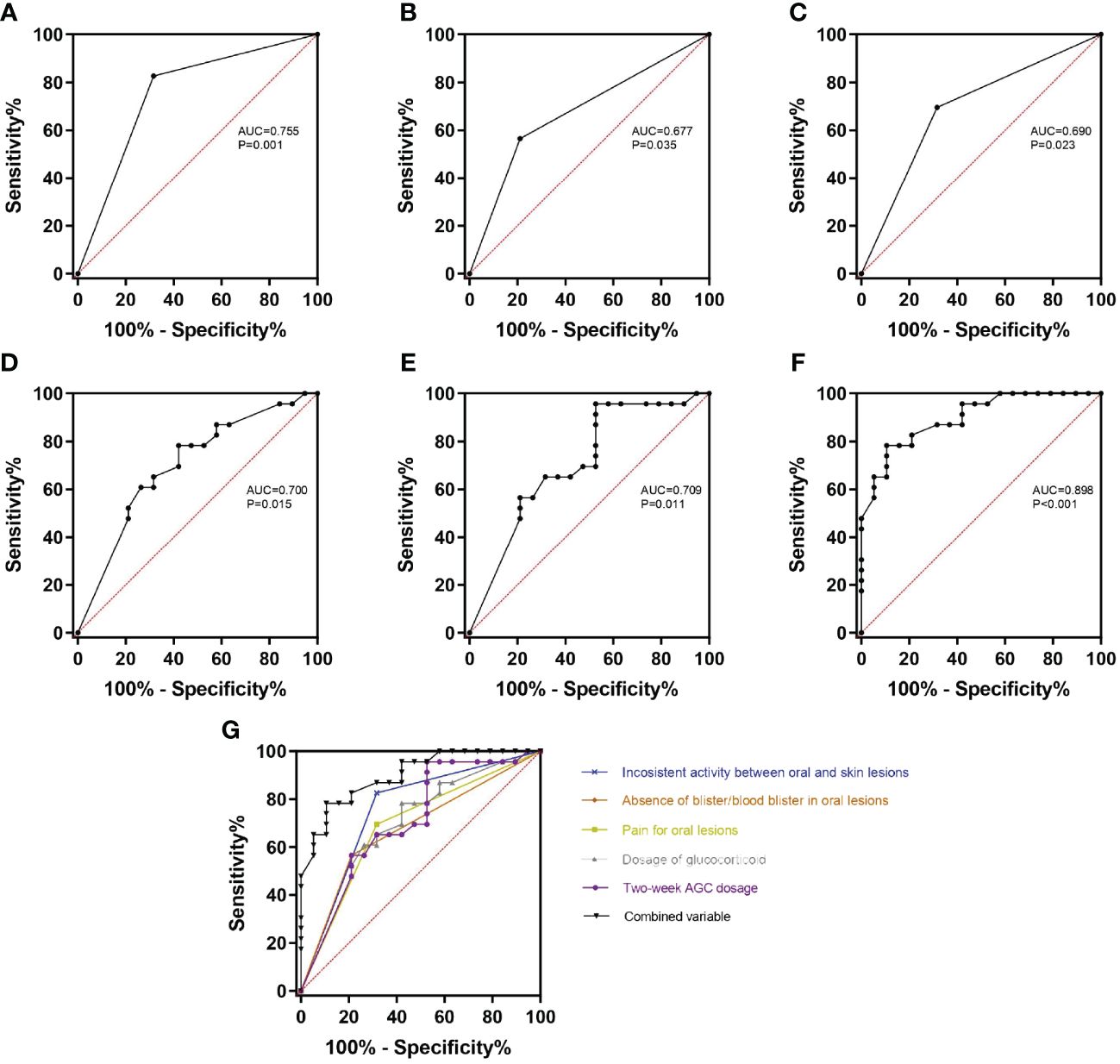
Figure 2 ROC curves of the variables with significant differences between HSV-positive and HSV-negative subgroups. (A–E) The inconsistent activity between oral and skin lesions (A), absence of blister/blood blister in oral lesions (B), pain for oral lesions (C), dosage of glucocorticoid (D), and 2-week AGC dosage (E) can significantly distinguish HSV-positive subgroup from HSV-negative subgroup (all p<0.05). (F) All the above variables were combined as the test variable, and the combined variable can get the highest diagnosis probability (AUC=0.898, p<0.001). (G) All the ROC curves were organized together to directly show that the combined variable has the biggest AUC. AUC, area under the curve; 2-week AGC dosage, accumulated glucocorticoid dosage in the last 2 weeks.
A total of 14 (73.7%) BP patients in the HSV-positive subgroup were added famciclovir (0.25g, three times a day for 2 weeks) with the dosage of glucocorticoid decreased or no change of the existing treatments. After 2 weeks of antiviral therapy, oral mucosa BPDAI scores significantly decreased [decreased oral mucosa BPDAI score, mean (SD), 3.9 (3.1), p<0.001]; 12 (85.7%) patients stopped developing new oral lesions, and eight (57.1%) patients got completely healed for oral lesions (Figure 3).
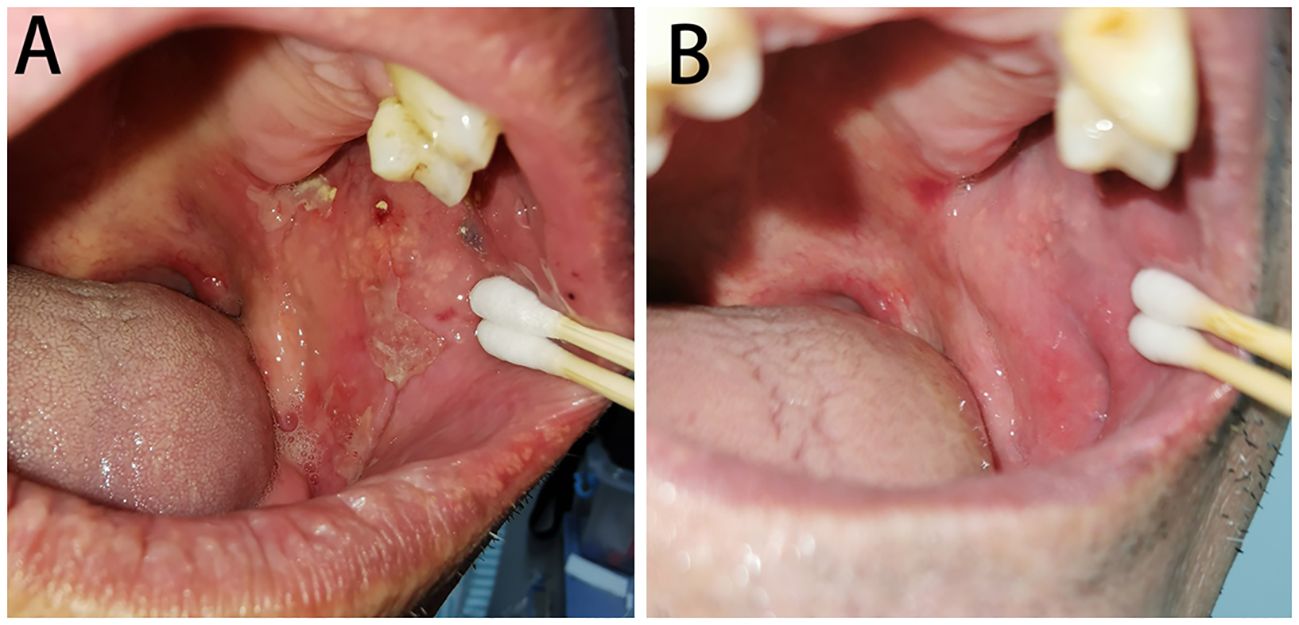
Figure 3 Oral lesions of BP with HSV infected before (A) and after (B) famciclovir therapy. (A) Several erythema and erosions covered with yellowish-white pseudomembranes distributed on the left buccal mucosa and were positive for HSV-1 by PCR. (B) The oral lesions almost completely healed after a 2-week treatment of famciclovir.
The other five (26.3%) BP patients in the HSV-positive subgroup were not added famciclovir because three were lost to follow-up and two refused to add famciclovir, since they could hardly feel discomfort with their oral lesions. For the two patients who refused to take oral famciclovir, although their existing oral lesions could heal within 1–2 weeks, new lesions continued to appear consistently, when followed up by phone 2 weeks later.
Six patients were retested for HSV on oral lesions after 1 week of famciclovir treatment, and the results showed four (66.7%) were still positive for HSV; another four patients were retested for HSV on oral lesions after 2 weeks of famciclovir treatment, and all patients were detected negative for HSV.
Although several studies have investigated the prevalence or clinical features of HSV infection in autoimmune bullous disease, they mainly focused on pemphigus vulgaris (PV). PV is the most common intraepidermal bullous dermatosis, which mainly manifests as erosions on the oral mucosa and can be accompanied by flaccid blisters and erosions on the skin sometimes (18). Konda et al. found that 23 out of 60 (38.3%) PV patients were positive for HSV, and male sex, the presence of fissures, hemorrhagic crusts, erosions with angulated margins, linear erosions, and raised erythrocyte sedimentation rate were significantly associated with HSV infection (19). However, they analyzed the clinical features of HSV infection in oral and skin lesions of PV simultaneously. The markedly different manifestations between oral and skin lesions of PV might have some influence on the results. Considering the significantly different characteristics between skin and oral lesions of PV and the difficulty of identifying HSV infection in oral lesions of PV, we carried out a prospective cross-sectional study targeted only oral lesions of PV and found that 17 out of 40 (42.5%) PV patients were positive for HSV in oral lesions and PV patients with HSV infection on oral lesions were more likely to have oral lesions located in the labial mucosa/posterior aspect of the pharynx, covered with pseudomembranes/ruptured blister walls, pain, in the active period, and with a higher pemphigus vulgaris area index score of oral lesions (20). In addition, Baum et al. found that PV patients with HSV-positive oral lesions had a higher C-reactive protein level, Pemphigus Disease Activity Index score, and shorter time to relapse (21). However, when it comes to the prevalence and clinical markers of HSV infection in BP patients, there was only one study that found that the positive rate of HSV in skin biopsies of BP patients was 10% (2/20) (12). No study had ever explored the prevalence or clinical markers of HSV infection in oral lesions of BP, which may be due to that oral lesions are more common in PV patients than BP patients.
As the first study that explored the prevalence and clinical markers of HSV infection in oral lesions of BP patients, first, we found that nearly half (45.2%) of the patients were positive for HSV in oral lesions, which suggested that HSV infection was common in BP oral lesions and should further arouse the attention that we paid on HSV infection among oral lesions of BP. For this part of the work, we used PCR to detect HSV, which had both a high specificity and sensitivity for HSV detection but could not rule out the possibility of asymptomatic shedding of HSV. To figure out this problem, we enrolled 41 healthy individuals and 32 BP patients without oral lesions as control groups. In the healthy control group, we found that there were 9.8% of healthy individuals detected positive for HSV without any signs or history of HSV infection. In our previous study, the rate of HSV asymptomatic shedding in oral mucosa of healthy individuals was 4.8% (20), which was similar to the 4.7% reported by Tateishi et al. (22) and a little lower than the rate in this study. There were studies showing that the prevalence of HSV infection and asymptomatic shedding increased with age (9, 22). The relatively older age of healthy individuals in our study may explain this difference. In the non-lesion group, none of the BP patients without oral lesions was detected positive for HSV. It was worth noticing that there were two special BP patients who did not have oral lesions at the first time, so we initially enrolled them into the non-lesion group and found that both patients were detected positive for HSV. Interestingly, they developed oral lesions 3 and 5 days later separately, so thereafter, we moved them into the lesion group and retested the HSV on oral lesions, which were still positive. These results suggested that the asymptomatic shedding of HSV in oral mucosa indeed existed in healthy individuals but was rare in BP patients. The usage of immunosuppressants and the ruptured barrier of oral mucosa in BP may play a part in this result.
Second, we found that BP patients in the HSV-positive subgroup were more likely to have inconsistent activity between oral and skin lesions, absence of blister/blood blister in oral lesions, pain for oral lesions, higher dosage of glucocorticoid, and higher 2-week AGC dosage compared with those in the HSV-negative subgroup, and the prediction probability of the combined features for HSV infection in oral lesions of BP was 0.898, which suggested that the parameters could serve as good clinical markers of HSV infection in oral lesions of BP and were helpful in identifying the HSV infection in BP oral lesions in time. There used to be two case reports showing that the HSV infection was likely to be related to the exacerbation of BP (23, 24). The characteristic of inconsistent activity between oral and skin lesions in the HSV-positive subgroup suggested that the influence of oral HSV infection on BP might be topical. It only induced activeness or treatment refractoriness of oral lesions instead of skin lesions. The similar titer of BP180 autoantibody between HSV-positive and HSV-negative subgroups further verified this conclusion. Numerous studies indicate that glucocorticoids can suppress both the innate and adaptive immunity of people (25), which makes glucocorticoids one of the risks of making HSV activated (26–28). There were also some studies showing that glucocorticoids can directly induce HSV reactivation by stimulating the glucocorticoid receptor (11, 29, 30). These can explain the higher dosage of glucocorticoid and higher 2-week AGC dosage in the HSV-positive subgroup.
Third, we observed that the oral mucosa BPDAI scores significantly decreased, and oral lesions of most HSV-positive patients got inactive or even completely healed after famciclovir treatment without the dosage of glucocorticoid increased, which suggested that the early antiviral treatment was good for the control of BP oral lesions and further emphasized the importance of identifying HSV infection in BP oral lesions and adding the anti-viral treatment in time.
Finally, on the one hand, we noticed that four of the six patients were still positive for HSV after 1 week of famciclovir treatment, and all four patients became negative for HSV after 2 weeks of famciclovir treatment. On the other hand, most of the BP patients are in an immunosuppression status with the long-time use of glucocorticoid or other immunosuppressants. Thus, compared with 1 week of antiviral therapy for recurrent HSV infection, 2 weeks of antiviral therapy is more recommended for HSV infection in oral lesions of BP.
There were also several limitations in our study. First, partial laboratory tests were lacking in the lesion group (Table 4), which might have some influence on the results. Second, the sample size was not so big, and a multicenter study with a larger sample size is needed to be done to further confirm these results. Finally, although we have set up control groups to show that the asymptomatic shedding of HSV in BP oral mucosa was rare, the direct evidence of active HSV infection, which includes the histological pathology result or viral culture, was absent.
To conclude, HSV infection is common in oral lesions of BP. The inconsistent activity between oral and skin lesions, absence of blister/blood blister in oral lesions, pain for oral lesions, higher currently used glucocorticoid dosage, and higher 2-week AGC dosage in BP patients should alert the physicians to the possibility of HSV infection in oral lesions and prescribe them with 2-week famciclovir in time.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Chinese Academy of Medical Sciences, Hospital for Skin Diseases (2021-KY-060). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HZ: Writing – review & editing, Writing – original draft, Software, Resources, Methodology, Formal analysis, Data curation. MY: Writing – review & editing, Methodology, Formal analysis, Data curation. GL: Writing – review & editing, Visualization, Resources, Data curation. SL: Writing – review & editing, Supervision, Resources, Methodology, Investigation, Data curation. CZ: Writing – review & editing, Resources, Data curation. KJ: Writing – review & editing, Resources, Data curation. SF: Writing – review & editing, Supervision, Resources, Investigation, Funding acquisition, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-059), Scientific Research Project of Jiangsu Provincial Health Commission (ZD2021035), National Key R&D Program of China (2022YFC3601800), and Clinical and Translational Medicine Research Project of Chinese Academy of Medical Sciences (2023-I2M-C&T-B-112).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BP, bullous pemphigoid; HSV, herpes simplex virus; DNA, deoxyribonucleic acids; PCR, polymerase chain reaction; BPDAI, bullous pemphigoid disease area index; 2-week AGC dosage, accumulated glucocorticoid dosage in the last 2 weeks; SD, standard deviation; IQR, interquartile range; AUC, area under the curve; SD, standard deviation; PV, pemphigus vulgaris.
1. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. (2013) 381:320–32. doi: 10.1016/S0140-6736(12)61140-4
2. Joly P, Baricault S, Sparsa A, Bernard P, Bédane C, Duvert-Lehembre S, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. (2012) 132:1998–2004. doi: 10.1038/jid.2012.35
3. Della TR, Combescure C, Cortés B, Marazza G, Beltraminelli H, Naldi L, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. (2012) 167:1111–7. doi: 10.1111/j.1365-2133.2012.11108.x
4. Di Zenzo G, Thoma-Uszynski S, Fontao L, Calabresi V, Hofmann SC, Hellmark T, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. (2008) 128:415–26. doi: 10.1016/j.clim.2008.04.012
5. Klavs I, Kojouharova M, Kortbeek T, Kriz B, Prosenc K, Roubalova K, et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect. (2004) 80:185–91. doi: 10.1136/sti.2003.005850
6. Li YY, Hidaka Y, Kino Y, Mori R. Seroepidemiology of herpes simplex virus type 1 in Yanji, Jilin, China. Microbiol Immunol. (1990) 34:551–5. doi: 10.1111/j.1348-0421.1990.tb03171.x
7. Davidovici BB, Green M, Marouni MJ, Bassal R, Pimenta JM, Cohen D. Seroprevalence of herpes simplex virus 1 and 2 and correlates of infection in Israel. J Infect. (2006) 52:367–73. doi: 10.1016/j.jinf.2005.08.005
8. Olsson J, Kok E, Adolfsson R, Lövheim H, Elgh F. Herpes virus seroepidemiology in the adult Swedish population. Immun Ageing. (2017) 14:10. doi: 10.1186/s12979-017-0093-4
9. Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. (2007) 57:737–763, 764-766. doi: 10.1016/j.jaad.2007.06.027
10. Boitsaniuk SI, Levkiv MO, Fedoniuk LY, Kuzniak NB, Bambuliak AV. ACUTE HERPETIC STOMATITIS: CLINICAL MANIFESTATIONS, DIAGNOSTICS AND TREATMENT STRATEGIES. Wiad Lek. (2022) 75:318–23. doi: 10.36740/WLek202201229
11. Goswami P, Ives AM, Abbott A, Bertke AS. Stress hormones epinephrine and corticosterone selectively reactivate HSV-1 and HSV-2 in sympathetic and sensory neurons. Viruses. (2022) 14:1115. doi: 10.3390/v14051115
12. Nikkels AF, Delvenne P, Herfs M, Pierard GE. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. (2008) 9:163–8. doi: 10.2165/00128071-200809030-00004
13. Montagnon CM, Tolkachjov SN, Murrell DF, Camilleri MJ, Lehman JS. Subepithelial autoimmune blistering dermatoses: Clinical features and diagnosis. J Am Acad Dermatol. (2021) 85:1–14. doi: 10.1016/j.jaad.2020.11.076
14. Montagnon CM, Lehman JS, Murrell DF, Camilleri MJ, Tolkachjov SN. Subepithelial autoimmune bullous dermatoses disease activity assessment and therapy. J Am Acad Dermatol. (2021) 85:18–27. doi: 10.1016/j.jaad.2020.05.161
15. Lucotte G, Bathelier C, Lespiaux V, Bali C, Champenois T. Detection and genotyping of herpes simplex virus types 1 and 2 by polymerase chain reaction. Mol Cell Probes. (1995) 9:287–90. doi: 10.1016/s0890-8508(95)91508-7
16. Zhang J, Su M, Ma Y, Liu J, Yu W, Ding Z. Detection of herpes simplex virus infection by polymerase chain reaction. J Fourth Military Med University. (2000) 21:939. doi: 10.3321/j.issn:1000-2790.2000.08.044
17. Zaravinos A, Bizakis J, Spandidos DA. Prevalence of human papilloma virus and human herpes virus types 1-7 in human nasal polyposis. J Med Virol. (2009) 81:1613–9. doi: 10.1002/jmv.21534
19. Konda D, Chandrashekar L, Dhodapkar R, Ganesh RN, Thappa DM. Clinical markers of herpes simplex virus infection in patients with pemphigus vulgaris. J Am Acad Dermatol. (2023) 88:587–92. doi: 10.1016/j.jaad.2019.06.002
20. Zhang H, Wang Y, Li S, Yu M, Feng S. Prevalence and clinical features of herpes simplex virus infection in oral lesions of pemphigus vulgaris: A prospective, cross-sectional study. J Am Acad Dermatol. (2022) 87:1201–3. doi: 10.1016/j.jaad.2022.03.015
21. Baum S, Atar I, Coster D, Dovrat S, Solomon M, Sprecher E, et al. Relationship between pemphigus vulgaris severity and PCR-positive herpes simplex virus. Acta Derm Venereol. (2022) 102:adv00703. doi: 10.2340/actadv.v102.917
22. Tateishi K, Toh Y, Minagawa H, Tashiro H. Detection of herpes simplex virus (HSV) in the saliva from 1,000 oral surgery outpatients by the polymerase chain reaction (PCR) and virus isolation. J Oral Pathol Med. (1994) 23:80–4. doi: 10.1111/j.1600-0714.1994.tb00261.x
23. Tani N, Yoshida Y, Goto H, Ishii N, Hashimoto T, Yamamoto O. Bullous pemphigoid exacerbation associated with reactivation of herpes simplex virus infection. Eur J Dermatol. (2020) 30:429–31. doi: 10.1684/ejd.2020.3809
24. Grattan CE, Small D, Kennedy CT, Scully C. Oral herpes simplex infection in bullous pemphigoid. Oral Surg Oral Med Oral Pathol. (1986) 61:40–3. doi: 10.1016/0030-4220(86)90200-8
25. Galati A, Brown ES, Bove R, Vaidya A, Gelfand J. Glucocorticoids for therapeutic immunosuppression: Clinical pearls for the practicing neurologist. J Neurol Sci. (2021) 430:120004. doi: 10.1016/j.jns.2021.120004
26. Sainz B, Loutsch JM, Marquart ME, Hill JM. Stress-associated immunomodulation and herpes simplex virus infections. Med Hypotheses. (2001) 56:348–56. doi: 10.1054/mehy.2000.1219
27. Zahwa H, Yorty JL, Bonneau RH. Elevated maternal corticosterone during lactation hinders the neonatal adaptive immune response to herpes simplex virus (HSV) infection. Brain Behav Immun. (2008) 22:339–53. doi: 10.1016/j.bbi.2007.08.012
28. Jusufbegovic D, Schaal S. QUIESCENT HERPES SIMPLEX KERATITIS REACTIVATION AFTER INTRAVITREAL INJECTION OF DEXAMETHASONE IMPLANT. Retin cases Brief Rep. (2017) 11:296–7. doi: 10.1097/ICB.0000000000000376
29. Ives AM, Bertke AS. Stress hormones epinephrine and corticosterone selectively modulate herpes simplex virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory, neurons. J Virol. (2017) 91:e00582-17. doi: 10.1128/JVI.00582-17
30. Ostler JB, Harrison KS, Schroeder K, Thunuguntla P, Jones C. The glucocorticoid receptor (GR) stimulates herpes simplex virus 1 productive infection, in part because the infected cell protein 0 (ICP0) promoter is cooperatively transactivated by the GR and Krüppel-like transcription factor 15. J Virol. (2019) 93:e02063-18. doi: 10.1128/JVI.02063-18
Keywords: Bullous pemphigoid, autoimmune subepidermal bullous disease, oral lesions, HSV, infection, prevalence, clinical markers
Citation: Zhang H, Yu M, Liang G, Li S, Zhao C, Jing K and Feng S (2024) Prevalence and clinical markers of herpes simplex virus infection in oral lesions of bullous pemphigoid. Front. Immunol. 15:1387503. doi: 10.3389/fimmu.2024.1387503
Received: 17 February 2024; Accepted: 01 April 2024;
Published: 18 April 2024.
Edited by:
Alessia Paganelli, Santa Maria Nuova Hospital, ItalyReviewed by:
Alfonso Motolese, AUSL Romagna, ItalyCopyright © 2024 Zhang, Yu, Liang, Li, Zhao, Jing and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suying Feng, ZmVuZ3N5QHB1bWNkZXJtLmNhbXMuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.