- 1Department of Hematology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, China
- 2Department of Pediatrics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 3Department of Hematology, Hunan Children’s Hospital, Changsha, Hunan, China
- 4Department of Hematology, Wuhan Children’s Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 5Department of Hematology, Children’s Hospital of Soochow University, Suzhou, Jiangsu, China
- 6Department of Pediatric Hematology, First People’s Hospital of Chenzhou, Chenzhou, Hunan, China
- 7Department of Pediatrics, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
Background: For children with severe aplastic anemia, if the first immunosuppressive therapy (IST) fails, it is not recommended to choose a second IST. Therefore, for patients without matched sibling donor (MSD) and matched unrelated donor (MUD), haploidentical hematopoietic stem cell transplantation (Haplo-HSCT) can be chosen as a salvage treatment. This article aims to explore the comparison between upfront Haplo-HSCT and salvage Haplo-HSCT after IST.
Methods: 29 patients received salvage Haplo-HSCT, and 50 patients received upfront Haplo-HSCT. The two groups received Bu (Busulfan, 3.2mg/kg/d*2d on days -9 to-8), CY (Cyclophosphamide, 60mg/kg/d*2d on days -4 to-3), Flu (fludarabine, 40mg/m2/d*5d on days -9 to -5) and rabbit ATG (Anti-thymocyte globulin, total dose 10mg/kg divided into days -4 to -2).
Results: The OS of the salvage Haplo-HSCT group showed no difference to the upfront Haplo-HSCT group (80.2 ± 8.0% vs. 88.7 ± 4.8%, p=0.37). The FFS of the salvage Haplo-HSCT group also showed no difference to the frontline Haplo-HSCT group (75 ± 8.2% vs. 84.9 ± 5.3%, p=0.27). There was no significant difference in the incidence of other complications after transplantation between the two groups, except for thrombotic microangiopathy (TMA). In the grouping analysis by graft source, the incidence of II-IV aGVHD in patients using PBSC ± BM+UCB was lower than that in the PBSC ± BM group (p=0.010)
Conclusion: Upfront Haplo-HSCT and salvage Haplo-HSCT after IST in children with acquired severe aplastic anemia have similar survival outcomes. However, the risk of TMA increases after salvage Haplo-HSCT. This article provides some reference value for the treatment selection of patients. In addition, co-transplantation of umbilical cord blood may reduce the incidence of GVHD.
1 Introduction
The treatment of severe aplastic anemia (SAA) in children includes immunosuppressive therapy (IST) and hematopoietic stem cell transplantation (HSCT). IST mainly relies on anti-thymocyte globulin (ATG) and cyclosporine (CsA) as skeletons. According to the consensus in China, matched sibling donor (MSD) HSCT and matched unrelated donor (MUD) HSCT are recommended for patients under 50 years old. If the patient does not have MSD or MUD, haploidentical hematopoietic stem cell transplantation (Haplo-HSCT) can also be considered in well-experienced transplant centers (1). Similarly, the Japanese guidelines stratified the risk factors for SAA in children. In emergencies, patients with short telomere or PNH clones can choose Haplo-HSCT instead of IST (2). For pediatric patients who choose IST on the first line, there may be three outcomes: the first being responsive to IST without recurrence, the second being responsive to IST treatment then relapsed, and the third being consistently unresponsive to IST, defined as refractory. The response rates of retreatment with horse-ATG or rabbit ATG (r-ATG) in refractory or relapsed patients have varied significantly, from 22% to 77%. However, the average age of the subjects in these studies is mostly greater than 18 years old, and they are not solely targeted at pediatric patients (3–8). For children, Seiji Kojima et al.’s prospective study suggested that salvage alternative donor HSCT therapy is superior to repeated IST, and that pediatric patients are more susceptible to the currently used enhanced ATG therapy than adult patients, and a single ATG process is sufficient to identify their response to these immunosuppressive agents. In addition, IST increases the risk of clone evolution cannot be ignored. Overall, for children, if they fail to respond to an initial IST, it is not recommended to choose a second IST treatment (9).
When selecting the types of grafts for transplantation, G-CSF mobilized PBSC (G-PB) is a faster and more practical alternative to BM as a source of stem cells. However, G-CSF mobilized BM can also result in faster implantation of neutrophils and platelets. Furthermore, compared to PBSC, BM appears to result in fewer cases of acute and chronic GVHD (10). Recently, Haplo-HSCT combined with a third-party umbilical cord blood (UCB) unit has been used in clinical trials. UCB has fewer mature or primed T cells that are predominant in inducing GVHD compared with peripheral blood grafts. Additionally, UCB is a rich and rapidly accessible source of several immunomodulatory cells, such as regulatory T cells (Tregs) and mesenchymal stromal cells (MSCs), both of which have been found to be effective in controlling severe GVHD (11). However, because the number of stem cells derived from the donor is one order of magnitude larger than that of third-party cord blood, the cord blood will be rejected under normal circumstances, ultimately achieving donor chimerism.
Therefore, for patients without MSD and MUD, Haplo-HSCT can be a relatively good choice. We conducted a retrospective multicenter study to compare upfront Haplo-HSCT and salvage Haplo-HSCT after IST.
2 Methods
2.1 Patients
From December 12, 2015, to September 26, 2022, A total of 79 patients were included in our retrospective multicenter study, with data from 6 hospitals, namely First People’s Hospital of Chenzhou, Children’s Hospital of Soochow University, Wuhan Children’s Hospital, The First Affiliated Hospital of Zhengzhou University, Hunan Children’s Hospital, Guangzhou Women’s and Children’s Medical Center. All patients were diagnosed according to standard diagnostic methods (12) and excluded from MDS, inherited bone marrow failure syndrome, and all patients who underwent testing had negative PNH clones. Patients with any severe liver, heart, lung or kidney disease, or any active infection were excluded. Receiving upfront Haplo-HSCT is defined as not using IST before transplantation or only using CsA for less than three months (<3 months). Salvage Haplo-HSCT after IST is defined as using CsA and ATG or using CsA for more than three months (≥3 months) before transplantation. Twenty-nine patients received salvage Haplo-HSCT, and 50 patients received upfront Haplo-HSCT. The average age of the study subjects is 7.07 ± 3.07 years. The time from diagnosis to transplantation is 2.9 ± 7.5months in the upfront Haplo-HSCT group and 14.6 ± 19.3 months in the salvage Haplo-HSCT group. The median follow-up time is 30.46 ± 22.49 months. Patient characteristics are shown in Table 1.
2.2 Graft types
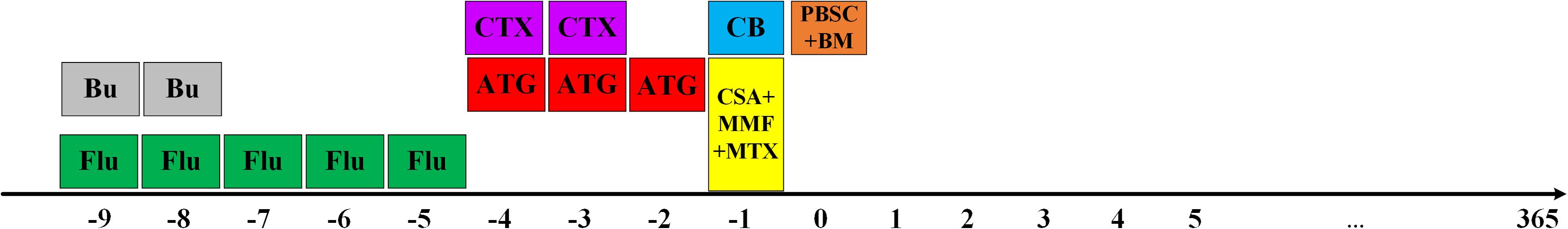
The selection of graft types includes PBSC ± BM or PBSC ± BM+UCB. To mobilize stem cells, rhG-CSF were subcutaneously injected into donors on days −4 to 0 (5 μg/kg/day). The target CD34+ should reach 2.5(10^6/kg), and the number of mononuclear cells was less than 10 (10^8/kg). The umbilical cord blood (UCB) units were obtained from the cord blood bank across China. The choice of UCB was according to HLA typing (from 6/6 to 4/6), cell count (from high to low), blood type (from matched to mismatched) and the patient’s preference. Days before the first and last stem cell infusion were designated by a minus (–) sign and a plus (+) sign, respectively. If choosing to use UCB in combination, a single UCB fusion was conducted on day-1 (Figure 1).
2.3 Conditioning regimen
The two groups received Bu (Busulfan, 3.2mg/kg/d*2d on days -9 to-8), CY (Cyclophosphamide, 60mg/kg/d*2d on days -4 to-3), Flu (fludarabine, 40mg/m2/d*5d on days -9 to -5) and rabbit ATG (Anti-thymocyte globulin, total dose 10mg/kg divided into days -4 to -2) (Figure 1).
2.4 GVHD prophylaxis
For prophylaxis of GVHD, CsA is routinely used intravenously from day-1, with the concentration maintained at 200-250ng/ml, and the dosage will be reduced after six months of use, and it will be stopped for about one year. In the case of liver GVHD, adjust to FK506 and keep the concentration between 8-12ng/ml. Mycophenolate mofetil (MMF) is routinely used, 30mg/kg per day, divided into twice or three times, until 30 days after transplantation. Methotrexate (MTX) is administered at a dosage of 15mg/m2 on day+1, 10mg/m2 on day+3, and day+6, respectively. G-CSF was used to increase the neutrophil count on day+5.
2.5 Definition of engraftment and evaluation of outcomes
Neutrophil engraftment is defined as ANC ≥0.5×10^9/L for three consecutive days, platelet engraftment is defined as platelets ≥20×10^9/L without transfusion for seven consecutive days, and red blood cell engraftment is defined as hemoglobin not less than 80g/L without transfusion. Graft failure is defined as the failure to achieve hematopoietic recovery after transplantation, with all or part of the hematopoietic cells derived from the recipient. Chimerism was detected by using fluorescence in situ hybridization probes to detect peripheral blood for sex-mismatched pairs or short tandem repeats of polymorphic DNA sequences for sex-matched pairs. After chimerism detection, only donor-type hematopoietic cells after allogeneic HSCT are defined as complete donor chimerism (13). To evaluate outcomes, three-year overall survival (OS) and failure-free survival (FFS) were estimated. The OS is calculated from the transplant date to the final follow-up date. The definition of FFS is the survival rate without treatment failure. Death, graft rejection, recurrence and the need for remedial treatment are considered as treatment failures (14).
2.6 Statistical methods
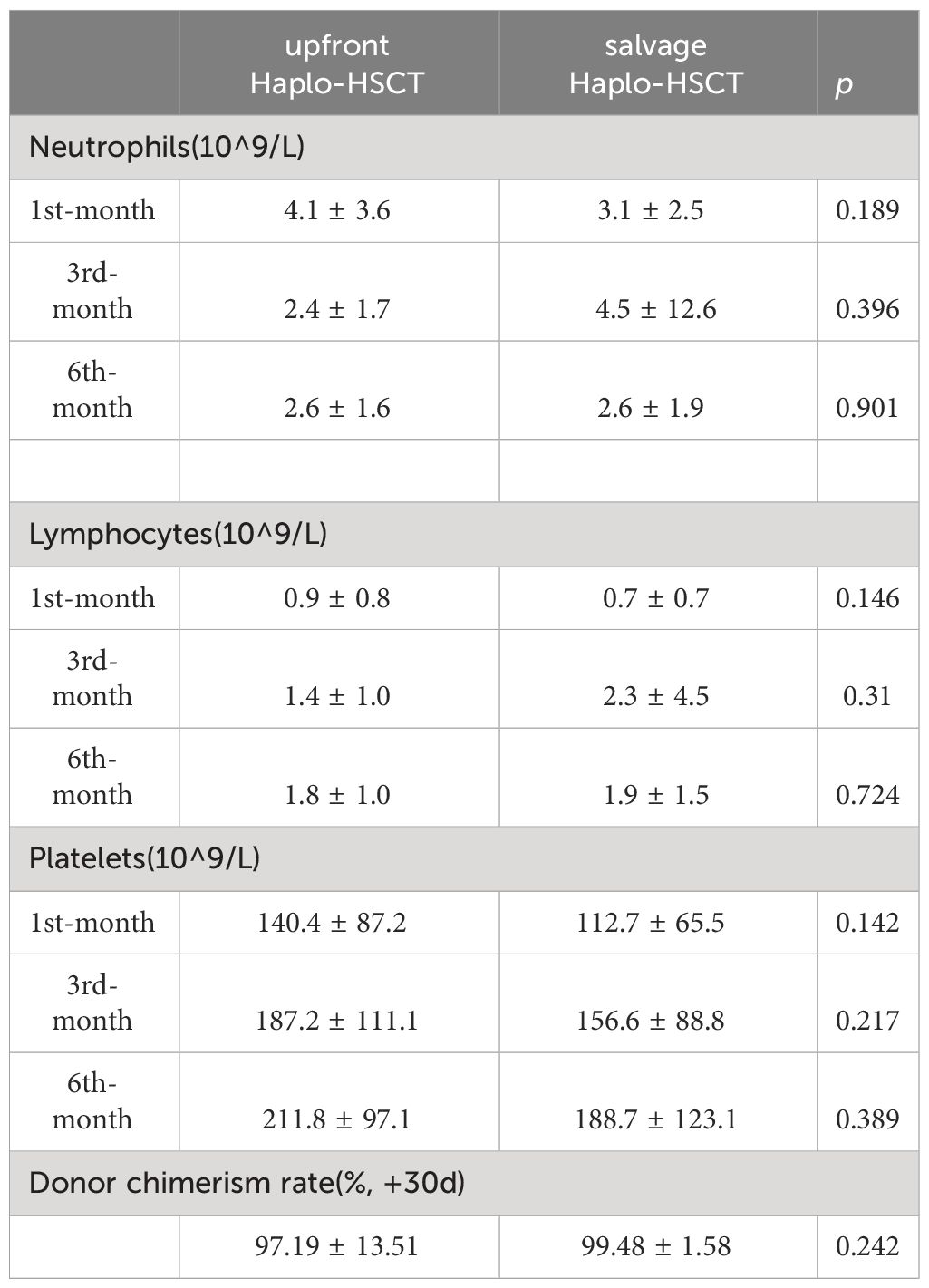
Continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile spacing, while categorical data are expressed as quantity (N) and percentage (%). Kruskal-Wallis test and analysis of variance (ANOVA)were used for continuous variables, and the Chi-square test or Fisher’s exact test were used for categorical variables. In addition, a subgroup analysis of graft types was also conducted. The relationship between independent variables and OS and FFS was analyzed using Cox regression. All independent variables are included in univariate analysis, and significant variables in univariate analysis are included in multivariate analysis. The probability of OS and FFS and survival curves are obtained by the Kaplan – Meier method. Log-rank test was used to compare the survival rate among groups. Compare the number of platelets, neutrophils, and lymphocytes in the first, third, and sixth month and donor chimerism rate at 1-month post-transplantation between the two groups to evaluate hematopoietic reconstitution. All statistical analyses were conducted using IBM SPSS version 25 and R project (4.2.2). A P-value of <0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
Table 1 shows the clinical characteristics of 79 patients. There were no significant differences between the two groups in terms of age, gender, donor age, gender, graft type, serum ferritin levels, average time of neutrophil and platelet implantation. The salvage Haplo-HSCT group was longer than the upfront Haplo-HSCT group in terms of time to diagnosis to transplantation before transplantation(14.6 ± 19.3 months VS 2.9 ± 7.5 months, p=0,004), and there are differences in the distribution of ABO matching between the two groups (p=0.002).
3.2 Complications after transplantation
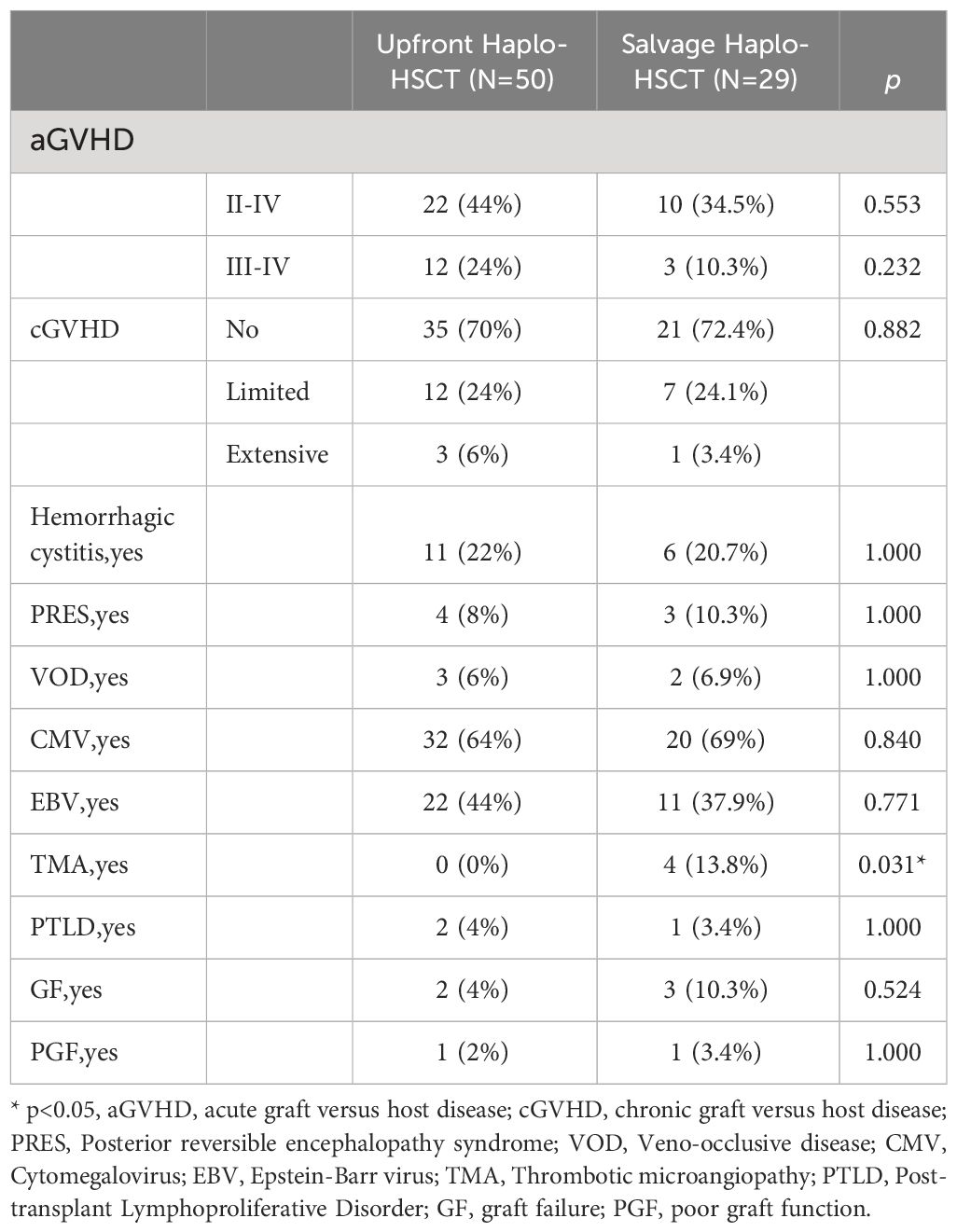
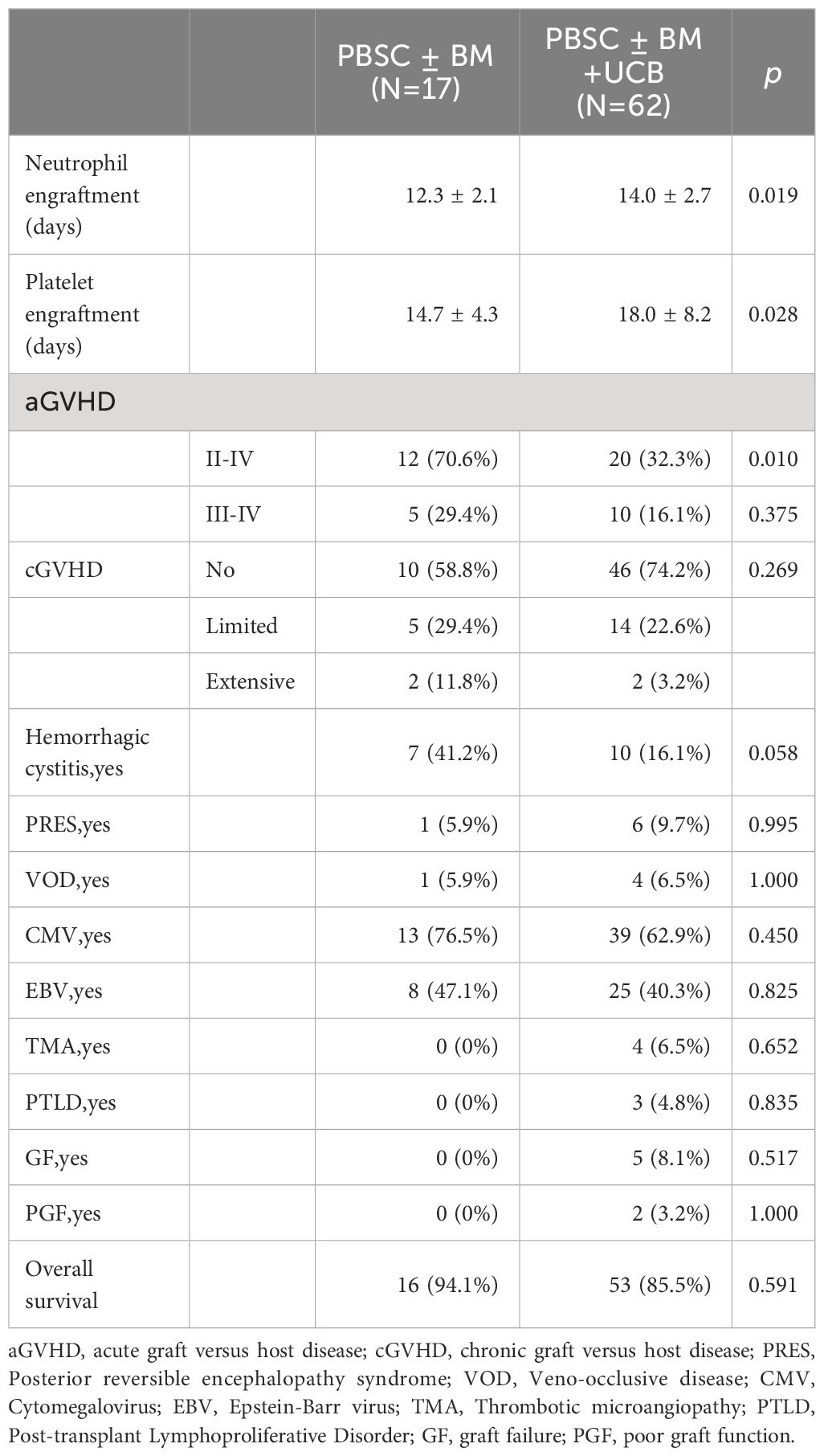
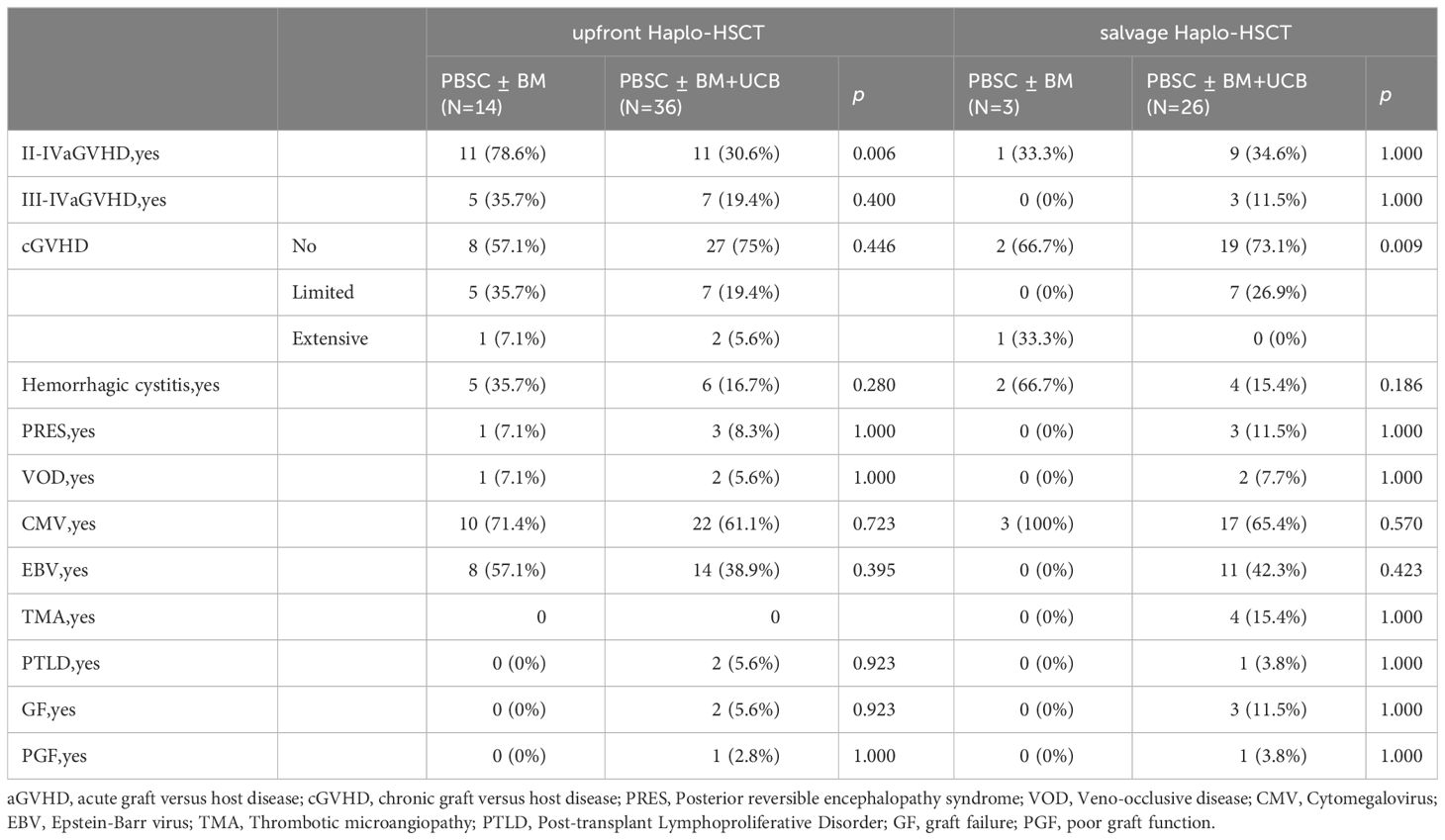
Only thrombotic microangiopathy (TMA) showed differences between the two groups, and the salvage Haplo-HSCT group had a higher probability of developing TMA (0% VS 13.8%, p=0.031, Table 2). The four patients who experienced TMA were all in the salvage Haplo-HCT group. Patient 1 developed TMA more than three months after transplantation, underwent plasma exchange, discontinued IST, and treatment with rituximab and basiliximab, but was unable to control the condition. Eventually, patient 1 died due to cytomegalovirus encephalitis, GVHD, and TMA. Patient 2 was cured under the treatment of rituximab, heparin, steroid hormones, and ruxolitinib. Patient 3 developed TMA on day +17 after transplantation and was cured after discontinuing cyclosporine. Patient 4 developed TMA at two months and eight months after transplantation and was treated with plasma exchange, rituximab, corticosteroids, basiliximab, and infliximab. Patient 4 died due to TMA, IV GVHD, pulmonary infection (Aspergillus), and cytomegalovirus infection one year after transplantation. There was no significant difference in other complications between the two groups, such as aGVHD, cGVHD, Posterior reversible encephalopathy syndrome, Veno-occlusive disease, Cytomegalovirus infection, Epstein-Barr virus infection, Post-transplant Lymphoproliferative Disorder, Graft failure and Poor graft function. In the upfront Haplo-HSCT group, 12 patients (24%) developed grade III-IV acute graft versus host disease (aGVHD), and 3 patients (6.0%) developed intensive chronic graft versus host disease(cGVHD). In the salvage Haplo-HSCT group, the incidence of III-IV aGVHD and intensive cGVHD was 10.3% and 3.4%, respectively (Table 2). In the grouping analysis by graft source, the incidence of II-IV aGVHD in patients using PBSC ± BM+UCB was lower than that in the PBSC ± BM group (32.3% vs 70.6%, p=0.010, Table 3). In subgroup analysis of graft types (Table 4), for the upfront Haplo-HSCT group, the incidence of II-IV aGVHD in patients using PBSC ± BM+UCB as the graft source was lower than that in the PBSC ± BM group (30.6% vs 78.6%, p=0.006). For the salvage Haplo-HSCT group, using PBSC ± BM+UCB as the graft source can reduce the severity of cGVHD (p=0.009).
3.3 Engraftment and hematopoietic reconstruction
There was no significant difference in the average time of neutrophil and platelet implantation between the two groups (p>0.05, Table 1). Two patients (4%) in the upfront Haplo-HSCT group experienced graft failure, while three patients (10.3%) in the salvage Haplo-HSCT group experienced graft failure (p=0.524, Table 2). Table 5 reflects the hematopoietic reconstruction situation of the two groups, and overall, the speed of hematopoietic reconstruction in the salvage Haplo-HSCT group is equal to that in the upfront Haplo-HSCT group.
3.4 Survival
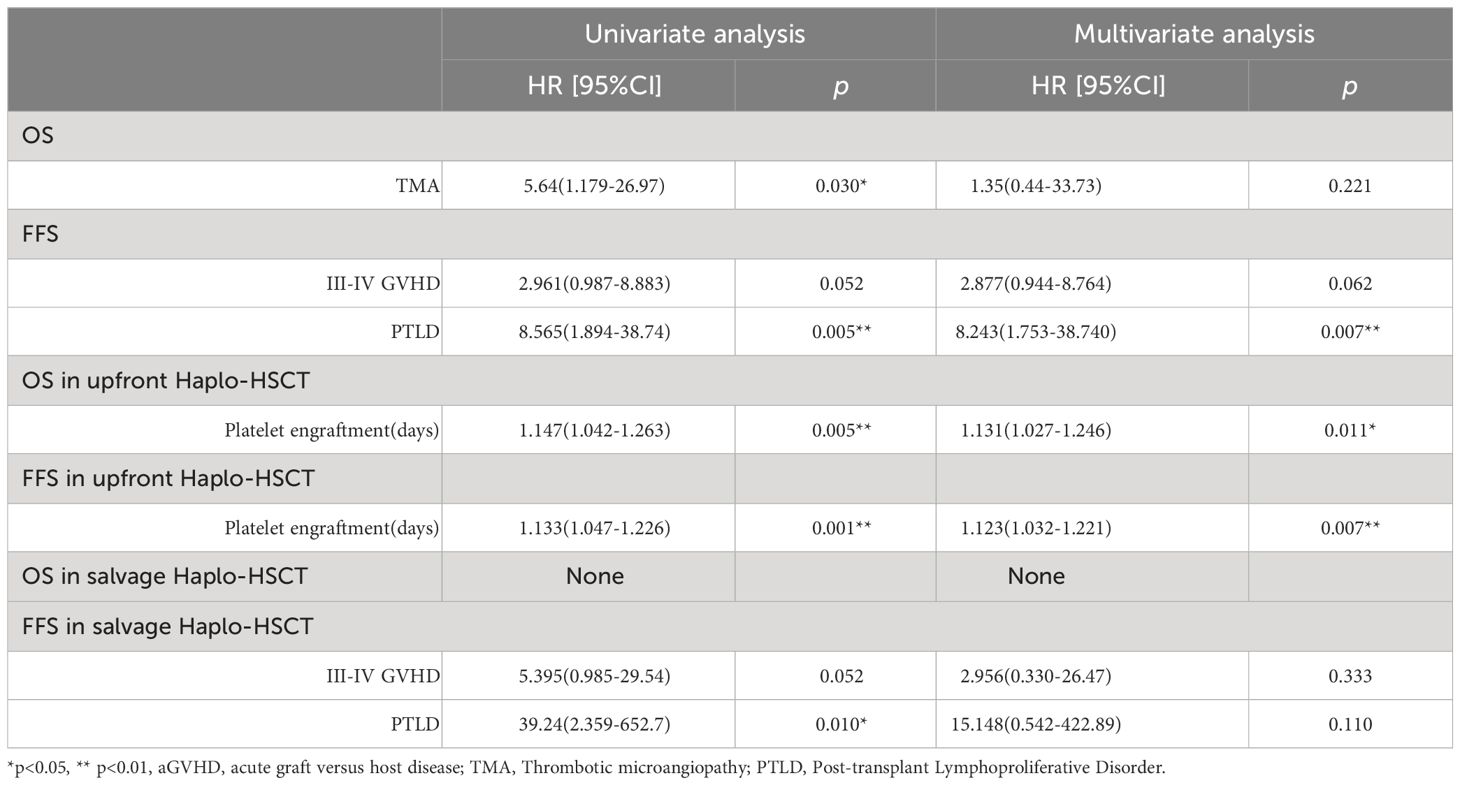
The OS of the salvage Haplo-HSCT group showed no difference to the frontline Haplo-HSCT group (80.2 ± 8.0% vs. 88.7 ± 4.8%, p=0.37) (Figure 2A). The FFS of the salvage Haplo-HSCT group also showed no difference to the frontline Haplo-HSCT group (75 ± 8.2% vs 84.9 ± 5.3%, p=0.27) (Figure 2B). Of the 79 patients, a total of 10 died due to infection (n=3), multiple organ failure (n=3), hemorrhagic shock (n=2), cerebral hemorrhage (n=1), and TMA(n=1). In univariate and multivariate Cox regression, only statistically significant independent variables were listed, and the results showed that the occurrence of Post-transplant Lymphoproliferative Disorder (PTLD) is a risk factor for FFS. For the upfront Haplo-HSCT group, prolonged platelet implantation time is a risk factor for OS and FFS (Table 6).

Figure 2 Kaplan-Meier estimates of (A) OS of upfront Haplo-HSCT and salvage Haplo-HSCT; (B) FFS of upfront Haplo-HSCT and salvage Haplo-HSCT.
4 Discussion
Previous studies have shown that the OS of first-line Haplo-HSCT is comparable to IST, but the FFS and health-related quality of life of Haplo-HSCT are superior to IST treatment (15). However, due to the high risk of transplantation, patients may prefer to prioritize IST treatment. For patients who have failed IST treatment and do not have a matching donor, salvage Haplo-HSCT can be chosen. However, there are currently few studies that directly compare the survival outcomes of upfront Haplo-HSCT and salvage Haplo-HSCT. Therefore, our study aims to compare upfront Haplo-HSCT and salvage Haplo-HSCT post-IST, providing a basis for the selection of treatment for children with SAA. In our study, the baseline features of the two groups of patients were almost matched, making the comparison of results between the two groups more reliable.
In terms of complications after transplantation, there was only a difference in the incidence of TMA between the two groups, and the salvage Haplo-HSCT group had a higher incidence of TMA, which may be related to the longer use of cyclosporine before transplantation. Previous studies have shown that cyclosporine may damage vascular endothelium, and exposure to prior calcineurin inhibitors is a risk factor for transplant-associated TMA (16, 17). In the past few years, the probability of developing III - IV aGVHD in patients with Haplo-HSCT based on G-CSF and ATG protocols was 4.9-29.6% (18), the incidence of aGVHD in our groups remains relatively low. Our study showed no significant difference in the frequency of aGVHD and cGVHD between the two groups, which is like the study by Zhu et al., who also mainly used Haplo-HSCT for salvage treatment. They concluded that the incidence of II-IV aGVHD for frontline Haplo-HSCT and salvage Haplo-HSCT was comparable (19). However, another study targeting pediatric patients, with matched family donor (MFD) as the main source of salvage treatment, found that the cumulative incidence of II-IV aGVHD of HSCT post IST failure was higher than MFD HSCT (25.0 ± 5.0% vs 8.0 ± 1.0%, P<0.0001). The five-year cumulative incidence of cGVHD was also higher in HSCT post-IST compared to MFD (20.0 ± 4.0% vs 6.0± 2.0%, P<0.0001) (20). Another study focuses on teenagers, cumulative incidence of aGVHD was similar in MFD transplants and transplants post failed IST(P=0.18). cGVHD was higher in transplants post failed IST than in MFD HSCT (P=0.0009) (21). In addition, our research findings suggest that co-transplantation with cord blood may reduce the incidence of GVHD, which is consistent with previous research findings. A study by Wu et al. included 91 children (average age 1-16 years old) based on the “Beijing protocol”, and the conclusion was that the incidence of GVHD in the haplo-cord group is lower compared to the haplo group (11).
In our study, hematopoietic reconstitution in the salvage Haplo-HSCT group was almost equal to the front-line Haplo-HSCT group. In multivariate analysis, the occurrence of PTLD is an adverse factor for FFS, but there is no difference in the frequency of occurrence between the two groups. Therefore, based on the above situation, there is no statistically significant difference in OS and FFS between the two groups. Zhu et al.’s study showed that the EFS was lower in the salvage HSCT group compared with the Haplo-HSCT group (41.7 ± 14.2% versus 80.0 ± 8.9%; P=0.046) (19) Marsh, Judith et al.’s research suggested that OS was comparable between 86% in the MFD HSCT group, 90% in patients given front-line IST alone, and 78% in transplantation post failed front-line IST (P=0.14). EFS in the same groups was respectively 83%, 64% and 71% (P=0.04) (21). Wu D et al.’s research showed that the estimated OS and FFS of salvage Haplo-HSCT at three years was 76.30 ± 9.70%, and the median age of research subjects was 26 years (10–54 years) (22). Yoo, K H et al.’s research suggested that the estimated EFS of the frontline HSCT group was higher than that of the salvage HSCT group (91.3% vs 50.9%, P = 0.015) (23). On the contrary, Xu et al. believed that there was no significant difference in II-IV aGVHD (P=0.699), cGVHD (P=0.916), OS (P=0.698), and FFS (P=0.899) between the two groups for children receiving upfront Haplo-HSCT or salvage Haplo-HSCT (24). Therefore, we believe that for experienced transplant centers, patients with generally acceptable conditions, and voluntarily, patients without MSD and MUD can actively receive upfront Haplo-HSCT. Of course, salvage Haplo-HSCT after IST failure is advisable, but it carries a higher risk of TMA. In addition, for well-experienced transplant centers, choosing upfront Haplo-HSCT can reduce the drug side effects of IST and avoid the risk of anxiety for parents and worsening infection when IST is ineffective for a long time.
However, our research has some limitations. Firstly, the sample size of the salvage treatment group is small. Secondly, as this study is retrospective, there may be other possible factors that have yet to be included in the analysis. In future research, we need a well-designed prospective study to verify our findings and increase our sample size.
5 Conclusion
Upfront Haplo-HSCT and salvage Haplo-HSCT after IST in children with acquired severe aplastic anemia have similar survival outcomes. However, the risk of TMA increases after salvage Haplo-HSCT. This article provides some reference value for the treatment selection of patients. In addition, co-transplantation of umbilical cord blood may reduce the incidence of GVHD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Guangzhou Women and Children’s Medical Research Ethics Committee, and the committee’s reference number is 27601[2019]. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
DL: Formal analysis, Writing – original draft. YQ: Data curation, Writing – original draft. DW: Data curation, Writing – review & editing. BZ: Data curation, Writing – review & editing. MS: Data curation, Writing – review & editing. HX: Data curation, Writing – review & editing. JL: Data curation, Writing – review & editing. RY: Data curation, Writing – review & editing. MZ: Methodology, Supervision, Writing – review & editing. HL: Funding acquisition, Methodology, Writing – original draft. HJ: Formal analysis, Funding acquisition, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by Guangzhou Municipal Health Commission High Tech, Major, and Characteristic Clinical Research Projects(2019TS56).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. (2021) 14:145. doi: 10.1186/s13045-021-01159-2
2. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. (2018) 20:67. doi: 10.1007/s11912-018-0716-8
3. Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anemia. Br J Hematol. (2006) 133:622–7. doi: 10.1111/j.1365-2141.2006.06098.x
4. Di Bona E, Rodeghiero F, Bruno B, Gabbas A, Foa P, Locasciulli A, et al. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anemia patients unresponsive to a first course of intensive immunosuppressive therapy. Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Br J Hematol. (1999) 107:330–4. doi: 10.1046/j.1365-2141.1999.01693.x
5. Marsh JC, Hows JM, Bryett KA, Al-Hashimi S, Fairhead SM, Gordon-Smith EC. Survival after antilymphocyte globulin therapy for aplastic anemia depends on disease severity. Blood. (1987) 70:1046–52. doi: 10.1182/blood.V70.4.1046.1046
6. Means RT Jr., Krantz SB, Dessypris EN, Lukens JN, Niblack GD, Greer JP, et al. Re-treatment of aplastic anemia with antithymocyte globulin or antilymphocyte serum. Am J Med. (1988) 84:678–82. doi: 10.1016/0002-9343(88)90104-0
7. Stein RS, Means RT Jr., Krantz SB, Flexner JM, Greer JP. Treatment of aplastic anemia with an investigational antilymphocyte serum prepared in rabbits. Am J Med Sci. (1994) 308:338–43. doi: 10.1097/00000441-199412000-00005
8. Tichelli A, Passweg J, Nissen C, Bargetzi M, Hoffmann T, Wodnar-Filipowicz A, et al. Repeated treatment with horse antilymphocyte globulin for severe aplastic anemia. Br J Hematol. (1998) 100:393–400. doi: 10.1046/j.1365-2141.1998.00578.x
9. Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood. (2008) 111:1054–9. doi: 10.1182/blood-2007-08-099168
10. Deotare U, Al-Dawsari G, Couban S, Lipton JH. G-CSF-primed bone marrow as a source of stem cells for allografting: revisiting the concept. Bone marrow Transplantat. (2015) 50:1150–6. doi: 10.1038/bmt.2015.80
11. Yao D, Tian Y, Li J, Li B, Lu J, Ling J, et al. Association between haploidentical hematopoietic stem cell transplantation combined with an umbilical cord blood unit and graft-versus-host disease in pediatric patients with acquired severe aplastic anemia. Ther Adv Hematol. (2022) 13:20406207221134409. doi: 10.1177/20406207221134409
12. Camitta BM, Rappeport JM, Parkman R, Nathan DG. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. (1975) 45:355–63. doi: 10.1182/blood.V45.3.355.355
13. Liu Z, Zhang Y, Xiao H, Yao Z, Zhang H, Liu Q, et al. Cotransplantation of bone marrow-derived mesenchymal stem cells in haploidentical hematopoietic stem cell transplantation in patients with severe aplastic anemia: an interim summary for a multicenter phase II trial results. Bone marrow Transplantat. (2017) 52:704–10. doi: 10.1038/bmt.2016.347
14. Yagasaki H, Takahashi Y, Hama A, Kudo K, Nishio N, Muramatsu H, et al. Comparison of matched-sibling donor BMT and unrelated donor BMT in children and adolescent with acquired severe aplastic anemia. Bone marrow Transplantat. (2010) 45:1508–13. doi: 10.1038/bmt.2009.378
15. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Jin S, et al. Comparison of efficacy and health-related quality of life of first-line haploidentical hematopoietic stem cell transplantation with unrelated cord blood infusion and first-line immunosuppressive therapy for acquired severe aplastic anemia. Leukemia. (2020) 34:3359–69. doi: 10.1038/s41375-020-0933-7
16. Higham CS, Collins G, Shimano KA, Melton A, Kharbanda S, Winestone LE, et al. Transplant-associated thrombotic microangiopathy in pediatric patients: pre-HSCT risk stratification and prophylaxis. Blood advances. (2021) 5:2106–14. doi: 10.1182/bloodadvances.2020003988
17. Teoh CW, Riedl Khursigara M, Ortiz-Sandoval CG, Park JW, Li J, Bohorquez-Hernandez A, et al. The loss of glycocalyx integrity impairs complement factor H binding and contributes to cyclosporine-induced endothelial cell injury. Front Med. (2023) 10:891513. doi: 10.3389/fmed.2023.891513
18. Xu ZL, Huang XJ. Haploidentical stem cell transplantation for aplastic anemia: the current advances and future challenges. Bone marrow Transplantat. (2021) 56:779–85. doi: 10.1038/s41409-020-01169-7
19. Yang S, Yuan X, Ma R, Jiang L, Guo J, Zang Y, et al. Comparison of outcomes of frontline immunosuppressive therapy and frontline haploidentical hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an HLA-matched sibling donor. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplantat. (2019) 25:975–80. doi: 10.1016/j.bbmt.2019.01.017
20. Dufour C, Pillon M, Sociè G, Rovò A, Carraro E, Bacigalupo A, et al. Outcome of aplastic anemia in children. A study by the severe aplastic anemia and pediatric disease working parties of the European group blood and bone marrow transplant. Br J Hematol. (2015) 169:565–73. doi: 10.1111/bjh.13297
21. Dufour C, Pillon M, Passweg J, Socié G, Bacigalupo A, Franceschetto G, et al. Outcome of aplastic anemia in adolescence: a survey of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. (2014) 99:1574–81. doi: 10.3324/haematol.2014.106096
22. Liu L, Wang X, Jin S, Hao L, Zhang Y, Zhang X, et al. Haploidentical hematopoietic stem cell transplantation for nonresponders to immunosuppressive therapy against acquired severe aplastic anemia. Bone marrow Transplantat. (2016) 51:424–7. doi: 10.1038/bmt.2015.249
23. Choi YB, Yi ES, Lee JW, Sung KW, Koo HH, Yoo KH. Immunosuppressive therapy versus alternative donor hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an HLA-matched familial donor. Bone marrow Transplantat. (2017) 52:47–52. doi: 10.1038/bmt.2016.223
Keywords: upfront Haplo-HSCT, salvage Haplo-HSCT, IST, SAA, children, multicenter study
Citation: Luo D, Qu Y, Wang D, Zhang B, Sun M, Xiong H, Lu J, Yang R, Zhao M, Liu H and Jiang H (2024) Comparison of upfront haploidentical hematopoietic stem cell transplantation and salvage haploidentical hematopoietic stem cell transplantation after immunosuppressive therapy in children with acquired severe aplastic anemia - a multicenter study. Front. Immunol. 15:1384640. doi: 10.3389/fimmu.2024.1384640
Received: 12 February 2024; Accepted: 10 April 2024;
Published: 24 April 2024.
Edited by:
Robert James Hayashi, Washington University in St. Louis, United StatesReviewed by:
Shalini Shenoy, Washington University in St. Louis, United StatesEhud Even-Or, Hadassah Medical Center, Israel
Copyright © 2024 Luo, Qu, Wang, Zhang, Sun, Xiong, Lu, Yang, Zhao, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Jiang, amlhbmdfaHVhMThAc2luYS5jbg==
 Danqi Luo
Danqi Luo Yuhua Qu1
Yuhua Qu1 Dao Wang
Dao Wang Ming Sun
Ming Sun Hao Xiong
Hao Xiong Jun Lu
Jun Lu Mingyi Zhao
Mingyi Zhao Haiyan Liu
Haiyan Liu Hua Jiang
Hua Jiang