- 1Division of Maternal-Fetal Medicine, Department of Obstetrics, Gynecology & Reproductive Sciences, University of California (UCSF), San Francisco, San Francisco, CA, United States
- 2Center for Reproductive Sciences and Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California (UCSF), San Francisco, San Francisco, CA, United States
- 3Obstetrics & Gynecology Institute, Cleveland Clinic Foundation, Cleveland, OH, United States
- 4Department of Molecular, Cellular & Developmental Biology, David Geffen School of Medicine, UCLA, Los Angeles, CA, United States
- 5Department of Biochemistry and Molecular Medicine, University of California Davis Health, Sacramento, CA, United States
- 6Infectious Diseases Research Collaboration, Kampala, Uganda
- 7Department of Medicine, Makerere University, Kampala, Uganda
- 8Division of HIV, Global Medicine, and Infectious Diseases, Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
- 9Department of Molecular Immunology and Genetics, University of California, Los Angeles, Los Angeles, CA, United States
- 10Division of Experimental Medicine, Department of Medicine and Pediatrics, University of California, San Francisco, San Francisco, CA, United States
Introduction: Maternal intervillous monocytes (MIMs) and fetal Hofbauer cells (HBCs) are myeloid-derived immune cells at the maternal-fetal interface. Maternal reproductive history is associated with differential risk of pregnancy complications. The molecular phenotypes and roles of these distinct monocyte/macrophage populations and the influence of gravidity on these phenotypes has not been systematically investigated.
Methods: Here, we used RNA sequencing to study the transcriptional profiles of MIMs and HBCs in normal term pregnancies.
Results: Our analyses revealed distinct transcriptomes of MIMs and HBCs. Genes involved in differentiation and cell organization pathways were more highly expressed in MIMs vs. HBCs. In contrast, HBCs had higher expression of genes involved in inflammatory responses and cell surface receptor signaling. Maternal gravidity influenced monocyte programming, as expression of pro-inflammatory molecules was significantly higher in MIMs from multigravidae compared to primigravidae. In HBCs, multigravidae displayed enrichment of gene pathways involved in cell-cell signaling and differentiation.
Discussion: Our results demonstrated that MIMs and HBCs have highly divergent transcriptional signatures, reflecting their distinct origins, locations, functions, and roles in inflammatory responses. Furthermore, maternal gravidity influences the gene signatures of MIMs and HBCs, potentially modulating the interplay between tolerance and trained immunity. The phenomenon of reproductive immune memory may play a novel role in the differential susceptibility of primigravidae to pregnancy complications.
1 Introduction
The placenta is the site of maternal-fetal interactions that mediate nutrient, gas and waste exchange between mother and fetus. It also maintains immune tolerance critical for healthy pregnancy. A chimeric organ comprised of tree-like chorionic villous tissue of embryonic origin, the placenta derives its blood supply through maternal spiral arteries and maternal-fetal exchange occurs in the intervillous spaces and chorionic villi (1, 2).
From mid- to late gestation, the most abundant leukocyte in the placenta is the monocyte/macrophage (3, 4). Placental macrophages are critical for maintaining the tolerogenic environment that is required for a healthy pregnancy and defending against pathogens (5–7). Macrophages at the maternal-fetal interface include three distinct cell types differing in location and origin: 1) maternal decidual macrophages, 2) fetal Hofbauer cells (HBCs), and 3) maternal intervillous monocytes (MIMs) (8). Maternal decidual macrophages, located at the placental-uterine interface, are the most well characterized and have been shown to participate in angiogenesis, spiral artery remodeling, trophoblast invasion, apoptotic cell phagocytosis, and immunomodulation during normal pregnancies (9–11). Their overactivation can result in pathological conditions such as preeclampsia, fetal growth restriction, and stillbirth (12).
Despite their identification over a century ago (13–15), little is known regarding the phenotype and function of fetal Hofbauer cells (HBCs). They emerge from the yolk sac as early as the 18th day of gestation (16, 17). Later in pregnancy, they differentiate from fetal liver monocytes. HBCs remain at high levels throughout the pregnancy (18) as tissue-resident macrophages within the chorionic villous cores (19). Data suggest that HBCs participate in the immune response to exogenous pathogens (20–22), inflammatory responses to maternal disease (e.g. obesity, preeclampsia, and diabetes) (23–26), regulation of nutrient transport, placental vasculogenesis, and angiogenesis (27, 28).
In contrast to fetal-derived HBCs, MIMs are maternal bone marrow-derived monocytes in the peripheral circulation that enter and exit the intervillous space through maternal spiral arteries and veins, respectively. MIMs have been shown to exhibit increased influx to the intervillous space during spontaneous labor (29). They also play a significant role in systemic and placental inflammatory reactions (30–32).
MIMs and HBCs express the classic mononuclear phagocyte cell surface receptors, CD68 and CD14 (33). There is no consensus on specific markers to distinguish MIMs and HBCs, hence they have been discriminated from one another (and from decidual macrophages) chiefly by their anatomic localization at the maternal-fetal interface (34–36). Further investigation into the phenotype and role of these distinct placental mononuclear cells has been hindered by the difficulty of isolating pure populations of MIMs and HBCs, as they are located in close proximity.
Proper control of immune activation states at the maternal-fetal interface is required for successful pregnancies (37). M1 macrophages form the first-line in host defense against a variety of bacteria, protozoa, and viruses, as well as in anti-tumor immunity (38). In contrast, the M2 subset is characterized by diverse immunosuppressive activity, and includes wound-healing macrophages, IL-10 secreting regulatory macrophages, and tumor-associated macrophages (39). To date, there have been limited studies investigating the activation states of placental monocyte/macrophages. Although studies are sparse, HBCs have been shown to play an anti-inflammatory role in the placenta and may contribute to maternal-fetal tolerance. These studies have found that HBCs function predominately in the M2 activation state during normal pregnancy (18, 28, 40–44). Dysregulation of M1/M2 phenotypes in the placenta has been associated with pregnancy complications, such as atopic disease, gestational diabetes, preeclampsia, preterm birth, and fetal growth restriction as well as susceptibility to bacterial, protozoal and viral (CMV, HIV, Zika) infections (6, 15, 26, 45–48). For example, differential activation of M1/M2 ratios in HBCs has been found to be associated with birth weight in placental malaria (49).
Limited investigations have characterized monocyte/macrophage cells of the human placenta. In a targeted investigation, HBCs underwent dynamic changes in RNA expression of M1/M2 markers across gestation (50). Recent studies utilizing transcriptomic profiling have used a non-biased approach to characterize monocyte/macrophage populations using targeted (cell-selection) or non-targeted (bulk tissue) approaches (51). Targeted isolation of lung alveolar macrophages in different disease states revealed great diversity in macrophage activation states (52, 53). Other studies leveraging single cell RNA sequencing (scRNA-seq) methods on bulk tissue samples have also shown distinct populations of monocyte/macrophage cell types at the maternal-fetal interface, including cells in the placental villi, chorionic membranes, and the basal plate (54–57). These studies demonstrate the breadth of macrophage diversity across gestational ages and clinical states and suggest that mononuclear phagocytes may play a role in pregnancy-related health and complications.
Despite their close proximity at the maternal-fetal interface, it is important to distinguish MIMs and HBCs and not investigate them together as a single group of cells since MIMs are maternal monocytes originating from the peripheral circulation, whereas HBCs are fetal macrophages residing within the chorionic villi. We sought to compare the transcriptional profiles of MIMs and HBCs in normal term pregnancies to better understand the phenotypes of these cells at the maternal-fetal interface and to gain insight into their potential functions. We separated MIMs and HBCs and performed bulk RNA sequencing (RNA-seq) to characterize the two populations. We also investigated the influence of maternal gravidity on the activation states of maternal vs. fetal immune cells in term placentas. Together, these data provide a valuable analysis of these two cell populations in normal pregnancy against which alterations that occur in pregnancy complications can be interrogated.
2 Materials and methods
2.1 Study design
This is a nested case control study of pregnant patients that delivered from January to February 2015 who were enrolled in a randomized controlled trial of intermittent preventative treatment for malaria in pregnancy in Tororo, Uganda (ClinicalTrials.gov number, NCT02163447). Primigravida participants were compared to multigravida participants.
Eligibility criteria included healthy, HIV-negative patients ≥ 16 years of age between 12–20 weeks gestational age at the time of enrolment. Participants were enrolled from June through October 2014. Gestational age was confirmed by ultrasound. Patients were screened for malaria at the time of enrolment and monitored on a monthly basis throughout pregnancy and at delivery. All patients labored and delivered at term. Within 30 minutes of birth, placentas were gently rinsed twice with cold PBS to remove blood clots and debris. Maternal and fetal monocyte/macrophages were isolated as described below. All participants had no evidence of past or active placental malaria, confirmed by placental histopathology (Rogerson criteria) (58). Patient characteristics were described in Supplementary Table 1.
2.2 Ethics statement
Written informed consent was obtained from all study participants. Ethical approval was obtained from the Uganda National Council of Science and Technology, the Makerere University School of Medicine Research and Ethics Committee, the Makerere University School of Biomedical Sciences Research and Ethics Committee, and the University of California, San Francisco.
2.3 Isolation of maternal intervillous monocytes
As described previously (59) with modifications, maternal blood was collected from the intervillous space by flushing spiral arteries from the maternal surface of the placenta with 30 ml of cold PBS, allowing fluid to passively drain via gravity for 15 minutes. Viable monocytes were isolated in a two-step process: 1) density gradient centrifugation using Ficoll; and 2) depletion of T cells, B cells, NK cells, dendritic cells, granulocytes, and erythrocytes using the Dynabeads Untouched Human Monocytes Kit (Invitrogen, 11350D) (Supplementary Figure 1).
2.4 Isolation of placental fetal Hofbauer cells
As previously reported (35) with modifications, placentas were washed with cold cytowash to remove excess blood prior to removal of amniotic membranes and decidual tissue. The remaining chorionic villi were minced into 5 mm-sized pieces and washed with cold PBS until clear. At least 50 g of villous tissue was treated with collagenase (0.075% collagenase, 0.04% DNase, 0.07% hyaluronidase, 3mM CaCl2) for 30 minutes in a 37°C water bath with gentle stirring. Immediately thereafter, the samples were centrifuged (1300 rpm) and the supernatant containing primarily syncytium was discarded. Undigested tissue was resuspended in cold cytowash, and digested with trypsin (0.125% trypsin, 0.02% DNase) for 60 minutes at 37°C, with gentle mixing every 10 minutes. After 60 minutes, additional collagenase was added and the digestion continued for another 30 minutes, also with gentle mixing every 10 minutes. The tissue was disaggregated by pipetting with a 5ml pipette, then filtered over 2x gauze then 1 mm and 90 μM sieves to remove undigested remnants. The cells were collected and washed 4 times with cold cytowash. The cell pellet was applied to a discontinuous Percoll gradient and the interface between 20% and 35% was collected. These cells were washed twice in cytowash, and HBCs were isolated by negative selection on Dynabeads coated with anti-EGFR to remove trophoblasts (Santa Cruz Biotechnology, sc-120) and anti-CD10 to remove fibroblasts (BioLegend, 312202; Goat anti-mouse IgG, Invitrogen, 110–33). The cells were collected, washed in PBS, and stored in RNAlater (Invitrogen) at -80°C (Supplementary Figure 1).
2.5 RNA isolation
RNA was extracted from isolated MIMs and HBCs using a RNeasy Micro Kit (Qiagen) following the standard manufacturer’s protocol. RNA quality was assessed using an Agilent 2100 BioAnalyzer (RIN > 7).
2.6 RNA sequencing
We profiled the transcriptomes of six paired samples of MIMs and HBCs by bulk RNA-seq at the UCLA Clinical Microarray Core Facility. cDNA and library construction were conducted using 1 µg RNA per sample and sequenced on an Illumina HiSeq 2500 to obtain ~30 million reads per sample.
2.7 Quantitative RT-PCR
We investigated expression levels of target genes to validate our profiling results and further explore relationships across a larger set of samples. In total, we examined 19 MIM and 15 HBC samples (Supplementary Table 1). These were a mixture of remaining samples from our RNA-seq analysis (MIMs, n=6; HBCs, n=2); and additional samples from independent placentas collected concurrently (MIMs, n=13; HBCs, n=13);. We converted purified RNA samples to cDNA using iSCRIPT Universal TaqMan (Bio-Rad), and performed qRT-PCR using TaqMan primers for selected targets (Supplementary Table 2) mixed with TaqMan Universal Master Mix II, no UNG (Life Technologies, Quant Studio 6). Reactions were carried out for 40 cycles. At least 3 technical replicates were analyzed for all comparisons. Differential expression between HBCs vs. MIMs was calculated via the ΔΔCT method. We normalized expression using the mean CT of housekeeping genes, GAPDH and ACTB.
2.8 Statistical analysis
For RNA-seq, FASTQ files were aligned to the human reference genome (GRCh37/hg19) using BWA (Burrows-Wheeler Alignment tool). Aligned BAM files were processed using htseqcount to obtain counts-per-million (CPM) values. Genes with CPM values > 0.5 in at least two samples were considered expressed above background. Data were further transformed using VOOM (60) and differentially expressed transcripts were identified using LIMMA. Genes missing a HGNC symbol were excluded in downstream analyses. In total, we examined 18,802 unique RNAs using this approach. We identified differentially expressed transcripts based on: 1) cell type of origin (MIMs vs. HBCs); or 2) cell type vs. gravidity. We defined differentially expressed transcripts between MIMs and HBCs as p<0.00001 (unadjusted); absolute FC > 4, false discovery rate <0.1%). In gravidity-based analyses, we considered genes expressed with high confidence in each cell type (average CPM > 0.5) and applied a cut-off of unadjusted p<0.05, absolute FC > 2. We conducted principal components analysis and hierarchical clustering of FC values using average linkage and Euclidean distance (pheatmap, RRID: SCR_016418) (61). Cell/tissue enrichment of the most abundant top 2% genes in each cell type was conducted using CTen (62). Functional enrichment analysis of Gene Ontology (GO) Biological Processes was evaluated via DAVID and a cut-off criteria of p<0.01; fold enrichment > 1.5, and minimum differentially expressed transcripts ≥10 (MIMs vs. HBCs) or ≥5 (cell type vs. gravidity) per GO term. Terms were grouped based on GO classifications. Raw and normalized data were deposited in the NCBI Gene Expression Omnibus (GSE244241).
For qRT-PCR, we employed Dunnett’s test (SSPS) to determine significant differences in expression (p<0.05). Relative FC values were expressed as average log2 ratios between MIMs and HBCs.
3 Results
3.1 Location of MIMs and HBCs at the maternal-fetal interface
We isolated paired MIMs and HBCs from human term placentas. Circulating MIMs are located in the intervillous space (IVS) of the placenta, where maternal blood circulates (Figure 1). HBCs reside in the chorionic villi (CV), which consists of an outer syncytiotrophoblast (STB) layer, an inner layer of cytotrophoblasts (CTBs), stroma, and fetal blood vessels. MIMs and HBCs were purified from the placental tissue as described.
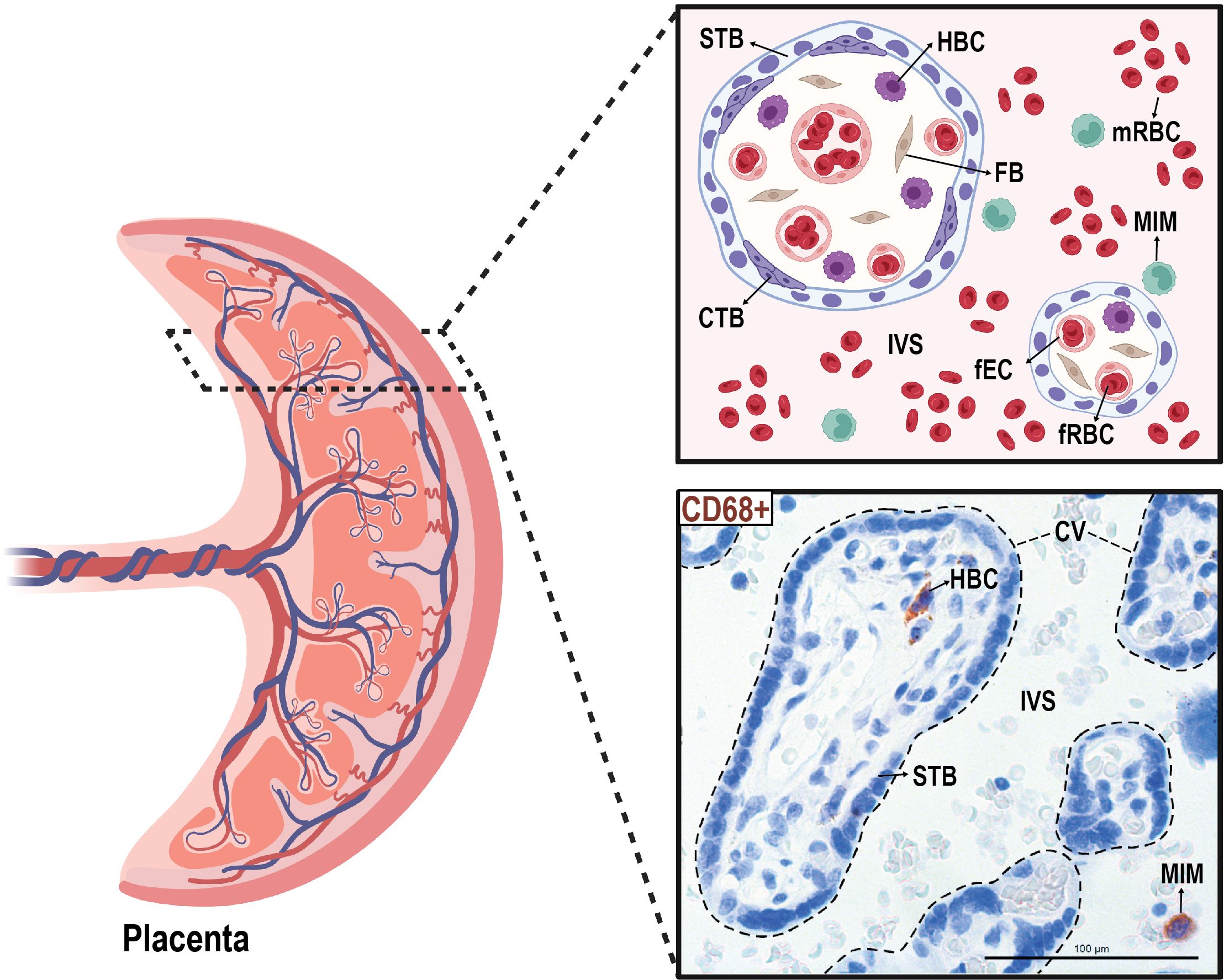
Figure 1 Anatomical localization of maternal intervillous monocytes (MIMs) and fetal Hofbauer cells (HBCs) within the placenta. MIMs and HBCs reside in unique placental compartments within close proximity. HBCs originate from the fetal yolk sac and reside in the chorionic villi (encircled with dashed lines). MIMs are derived from the maternal bone marrow and, ultimately, enter the placenta via the maternal spiral arteries that provide oxygenated blood to the intervillous space. MIMs and HBCs highly express CD68+ (brown) in histological cross-sections of the chorionic villi and intervillous space. CTB, cytotrophoblast; CV, chorionic villi; FB, fibroblast; fEC, fetal endothelial cell; fRBC, fetal red blood cell; HBC, Hofbauer cell; IVS, intervillous space; MIM, maternal intervillous monocyte; STB, syncytiotrophoblast. Created with BioRender.com.
3.2 Differences in gene expression between MIMs and HBCs
We conducted mRNA profiling of MIMs and HBCs. The distribution of average gene counts was similar in the two cell types for all placental samples (Figure 2A). The top 2% of abundant genes in MIMs and HBCs (319 genes; dashed line; Figure 2A) were significantly enriched for macrophage-progenitor signatures (myeloid CD33+ and monocyte CD14+) as determined by cell type enrichment analysis (Figure 2B). Genes abundantly expressed in MIMs were highly enriched for expression profiles associated with placental tissue, suggesting that the placental reference gene expression database may include genes of maternal blood origin. The profiles of abundant genes in MIMs and HBCs were enriched for signatures of whole blood, which includes both monocytes and myeloid cells. Additionally, these profiles were akin to other organs with high levels of tissue-resident macrophages.
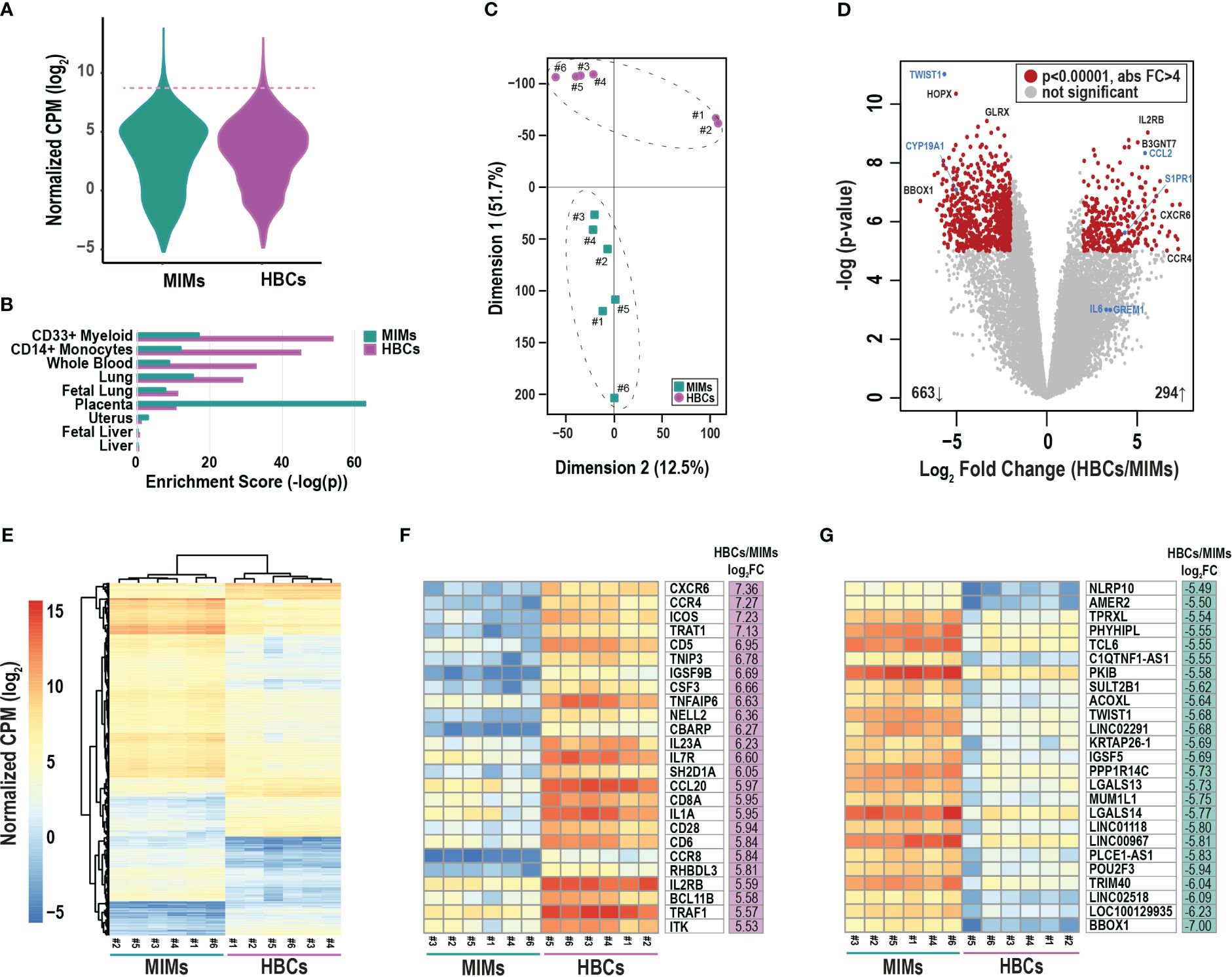
Figure 2 Differentially expressed genes between MIMs and HBCs. (A) Distribution of average gene counts [log2 normalized counts per million (CPM)] in MIMs and HBCs. (B) Cell/tissue enrichment of the most abundant genes in each cell type (top 2%, dashed line in panel A). (C) Multidimensional scaling plot of MIM (teal squares) and HBC (purple circles) transcriptomes. Paired MIMs and HBCs denoted by sample IDs. (D) Volcano plot displaying significance (negative log p-value) and fold difference in expression (log2). Red dots signify differentially expressed genes between the two cell populations (p<0.00001; absolute FC > 4). Genes labeled in blue were validated via qRT-PCR. (E) Hierarchical clustering plot displaying absolute mRNA expression of differentially expressed genes. Expression of the top 25 most up- (F) or down- (G) regulated genes in terms of fold change (FC) in HBCs vs. MIMs.
Principal component analysis of all transcripts clearly separated MIMs from HBCs with Dimension 1 accounting for 51.7% of the variability (Figure 2C). LIMMA identified 976 differentially expressed (Figure 2D; red circles) transcripts between the two cell types (p<0.00001, absolute FC > 4). Within this subset, 663 genes (69%) were upregulated in MIMs whereas 294 genes (31%) were upregulated in HBCs. Hierarchical clustering of differentially expressed genes also highlighted the distinct profiles of these two cell types and confirmed the similarity of samples within each cell type (Figure 2E). The 25 most upregulated genes in HBCs vs. MIMs included molecules involved in inflammatory responses (CXCR6, CCR4, CCL20, CD6, CD28, IL1A, TNFAIP6; Figure 2F). The 25 most upregulated genes in MIMs vs. HBCs included molecules linked to IL-6-regulated cytokine pathways (NLRP10, TWIST1) and macromolecule metabolism (PKIB, PPP1R14C, POU2F3, TRIM40, TWIST1; Figure 2G). Overall, our analyses demonstrate distinct transcriptomic signatures between these two cell types.
3.3 Validation of genes differentially expressed in MIMs vs. HBCs
To validate the RNA-seq results (Figure 2D, blue marked genes, Supplementary Table 3), we used quantitative RT-PCR to assess the expression of selected genes that were highly differentially expressed (TWIST1, CYP19A1, CCL2, S1PR1; p<0.00001) and two genes with a more modest difference (GREM1, p=0.003; IL6, p=0.002). Using a larger sample set, which included additional placentas, we confirmed the expression of CCL2 and S1PR1 to be significantly higher in HBCs vs. MIMs and expression of CYP19A1 and TWIST1 to be significantly higher in MIMs vs. HBCs (Figure 3A). Expression of IL6 and GREM1 was increased in HBCs vs. MIMs. Overall, the magnitude and directionality of expression differences assayed by the two platforms were strongly positively correlated (R2 = 0.8541, Figure 3B).
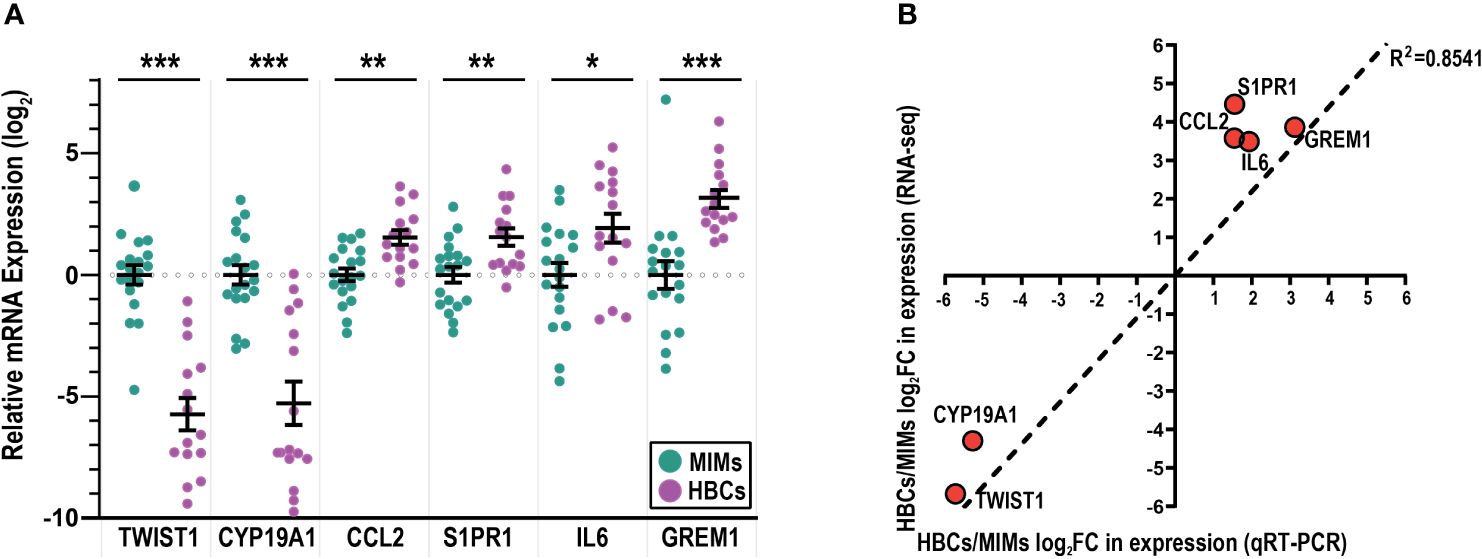
Figure 3 mRNA validation of differentially expressed genes between MIMs and HBCs. (A) Relative expression of genes identified to be differentially expressed between HBCs (purple circle) and MIMs (teal circle) via qRT-PCR. Expression values (ΔΔCT) were normalized to housekeeping genes (GAPDH, ACTB) and adjusted by the average MIM expression. Asterisks indicate significant differences between MIMs and HBCs (***p<0.0005; **p<0.005; *p<0.05). Bars reflect mean and standard error (SE). (B) Correlation plot between RNA-seq and qRT-PCR expression levels of HBC/MIM fold change values in log2 format. Regression line signifies relationship between fold change differences of MIMs and HBCs quantified via RNA-seq vs. qRT-PCR (slope = 1095; R2 = 0.8541).
3.4 Mapping differentially expressed genes to biologic processes
We evaluated differentially expressed genes between MIMs and HBCs for enrichment of biological processes (Figure 4A). Fifty GO terms were identified as overrepresented (Supplementary Table 4) and included processes related to cell motility, adhesion, inflammation, cell death, signal transduction, communication, metabolism, and organization. MIMs had greater expression of genes related to cytoskeleton organization, intracellular signal transduction and cell differentiation. In contrast, HBCs tended toward increased expression of specific biological processes related to inflammation, single organismal cell-cell adhesion and cell surface receptor signaling pathways.

Figure 4 Functional enrichment analysis of differentially expressed genes between MIMs and HBCs. (A) Selected enriched biological processes (criteria: p<0.01, number of differentially expressed genes associated with enriched term ≥ 10) and the number of differentially expressed genes with higher expression in MIMs or HBCs in each category. (B) Hierarchical clustering of differentially expressed genes associated with inflammatory response pathway (GO:0006954). (C) Relative expression of M1/M2 markers in HBCs vs. MIMs. A panel of 31 genes associated with pro- (M1) and anti- (M2) inflammatory activation states were identified based on current literature. Out of this panel, 15 genes were differentially expressed between MIMs and HBCs (average log2count > 0, p<0.01 and absolute log2FC > 1).
Further exploration of a subset of genes involved in the inflammatory response (Figure 4B) confirmed robust differences in gene expression between MIMs and HBCs. Those with known functions in macrophage biology that were more highly expressed in MIMs compared to HBCs included CYP19A1, TGM2, SNAP23 and VAMP8. Macrophage-associated genes that were more highly expressed in HBCs vs. MIMs were KDM6B, NFKB1, CXCL3, CCL20, IL1A, CCL2, CSF1, IL10, IL23A, CCR7 and IRAK2. Overall, these findings demonstrate that MIMs and HBCs have different transcriptional profiles and suggest potential drivers of differences in the function and behavior of these cells.
3.5 Expression of M1/M2 markers in MIMs and HBCs
We compiled a panel of thirty-one molecules associated with pro- and anti-inflammatory states (M1 vs. M2, respectively) based on published data (Supplementary Table 5) (37, 40, 63–68). We relaxed our cut-off criteria (p<0.01, absolute log2FC > 1, average log counts > 0) to explore the expression profiles of these markers in MIMs and HBCs. Fifteen genes were differentially expressed between MIMs and HBCs. Of these, 13 were more highly expressed in HBCs and included M1 and M2 markers (Figure 4C). The average fold-increase of these molecules was 19.6 (range 4.5 to 75). Examples of the largest differences between MIMs and HBCs included IL23A (75-fold), IL1A (61.8-fold), and VEGFA (18.8-fold). Two M2 markers, TGM2 (13.2-fold) and VEGFB (2.1-fold), were the only markers that were more highly expressed in MIMs vs. HBCs.
3.6 Gene expression differences by gravidity
We next assessed differences in gene expression in MIMs and HBCs between primigravida and multigravida pregnancies. Gravidity influenced the expression of 120 and 95 genes in MIMs and HBCs, respectively (p<0.05; absolute FC > 2; Figure 5A). Overlap in these subsets was limited to five genes: C15ORF48, ZNF135, OR2B11, MSC, and LINC01291. Hierarchical clustering showed that in MIMs, 85% (158/186) of the differentially expressed genes were more highly expressed in multigravidae (Figure 5B). In HBCs, differentially expressed genes were equally distributed in the up- and down-regulated categories (50.6% and 49.4%, respectively).
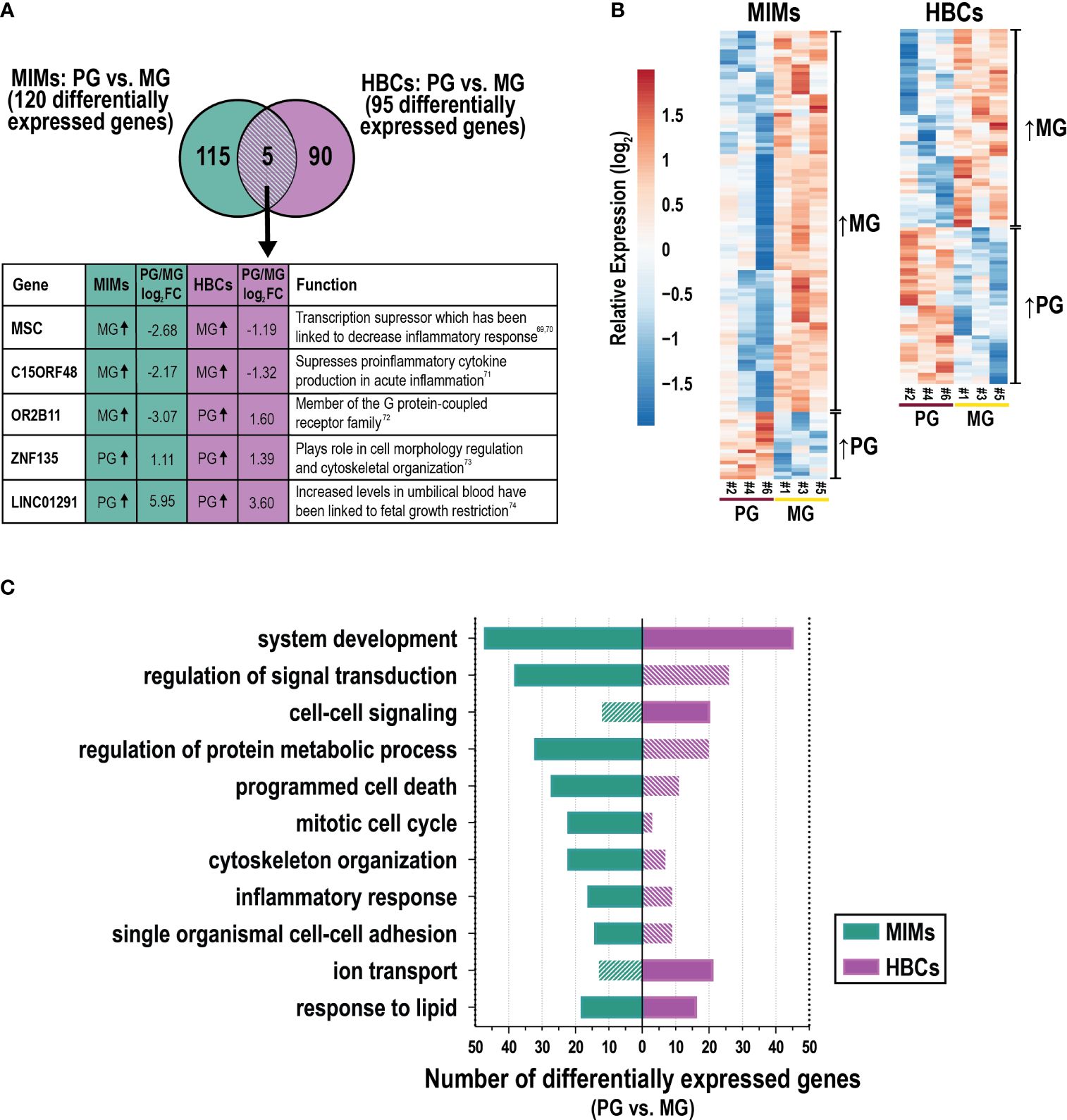
Figure 5 The influence of gravidity on gene expression in MIMs and HBCs. (A) Genes identified to be differentially expressed due to gravidity [primigravida (PG) or multigravida (MG) placentas] in the two cell types (p<0.05; absolute FC > 2). Five differentially expressed genes in common between MIMs and HBCs due to gravidity are shown in the box with their previously described functions (69–74). (B) Relative expression (based on average expression of primigravidae) of genes significantly influenced by gravidity in MIMs or HBCs. Hierarchical clustering denotes genes higher in multigravidae vs. primigravidae or vice versa in each cell type. (C) Select enriched biological processes associated with gravidity in MIMs or HBCs (p<0.01; number of differentially expressed genes ≥6 in any cell type). Filled bars indicate significance (p<0.01). Hatched bars indicate non-significance.
GO analyses of these data is shown in Supplementary Table 6. Figure 5C depicts a portion of the results for each cell type with statistically significant biological processes denoted by solid color bars. System development and response to lipids were the only processes that were significantly differentially expressed in association with gravidity in both cell types. MIM genes that were differentially expressed as a function of gravidity were involved in inflammation (e.g., inflammatory response, response to cytokines); migration/adhesion (e.g., single organismal cell-cell adhesion, leukocyte migration); cell cycle processes (e.g., mitotic cell cycle); protein metabolism; response to lipids; programmed cell death; signal transduction; system development and organizational pathways (e.g., cytoskeleton) (Figure 5C). Cytokine response genes influenced by gravidity (n=20) included IL1A, IFNB1, F3, CXCL3, CCL20, and CXCL2. Genes in this category were upregulated in multigravidae vs. primigravidae. By comparison, HBC genes that were differentially expressed as a function of gravidity were involved in system development, cell-cell signaling, ion transport, response to lipids and positive regulation of cell proliferation. There was a notable lack of inflammatory response pathways. Individual genes that were differentially expressed between multigravidae and primigravidae, included transcription factors (e.g., WNT5B), ion transporter genes (e.g., KCNMA1) and major cytokines (e.g., CCL13 and IFNG). Overall, gravidity was associated with changes in transcription profiles of MIMs and HBCs, commonly modulating genes related to system development, response to lipids, and inflammatory response in both cell types. Our analyses of placental macrophages from two distinct origins suggest inherent programming influenced by gravidity status.
4 Discussion
We purified MIM and HBC populations from two placental compartments in the setting of uncomplicated term deliveries. RNA sequencing revealed stark differences in the transcriptional signatures of these two cell populations. Both MIMs and HBCs expressed diverse combinations of M1 and M2 subtype markers (Figure 3C); interestingly, HBCs expressed significantly higher levels of both M1 and M2 markers than MIMs. We found distinct transcriptional profiles of MIMs and HBCs with mixed M1/M2 gene expression, lending credence to ongoing discussions that the M1/M2 dichotomy (33) does not fully capture the heterogeneous profiles of placental immune cells. Furthermore, much of what is known about macrophage function has been developed through in vitro stimulation experiments that may not accurately reflect the in vivo microenvironment. Unbiased “-omics” approaches may reveal the broad spectrum of activation states of these cells at the maternal-fetal interface. Overall, our findings highlight the divergent phenotypes of MIMs and HBCs in healthy pregnancies at term, which likely reflects their separate origins and adaptability to changing microenvironments in the distinct compartments they occupy throughout pregnancy.
Most studies to date have suggested that HBCs predominantly exhibit an anti-inflammatory, M2-like phenotype (15, 28, 40–44). However, our data demonstrate that HBCs exhibit greater phenotypic heterogeneity than previously described. HBCs expressed both M1 (e.g. IL6 and IL23A) and M2 (e.g. IL10 and VEGFA) markers at high levels (Figure 4C). This is consistent with limited functional analyses of HBCs showing that they express high levels of toll-like receptors and secrete the pro-inflammatory cytokines IL-6 and IL-8 in response to inflammatory stimuli (75). Other studies have demonstrated phagocytic activity of HBCs across all trimesters (21, 35, 36, 76). Comparison of cytokine profiles of HBCs from early and late gestation has shown increased expression of inflammatory mediators as pregnancy progresses (77). The broad expression of M1 markers in our study also suggests that there may be other pro-inflammatory functional roles for HBCs in normal term placentas. Future studies of HBCs in different disease states, especially leveraging single cell approaches, will help to further elucidate the full spectrum of HBC functional states.
Our results showed that TWIST1 was 51-fold more highly expressed in MIMs compared to HBCs, which was confirmed by qRT-PCR. Expression of this molecule has been linked to the downregulation of NFKB mediated pro-inflammatory cytokine production in both murine and human macrophages (78, 79). In vitro studies investigating human monocyte derived macrophages revealed that TWIST1 downregulated cytokine responses to NOD2, a sensor of bacterial peptidoglycan, through an epigenetic mechanism that enabled the formation of immune memory responses (80, 81). Thus, an anti-inflammatory phenotype of MIMs may be regulated by epigenetic modifications. Other differentially expressed regulators (e.g., NLRP10 and SLUT2B1) may be similarly regulated.
Most investigations focusing on MIMs to date been limited to various disease states such as malaria, preeclampsia, and obesity (24, 30, 49). We found 13-fold higher expression of TGM2 and 5-fold higher expression of SNAP23 in MIMs compared to HBCs. TGM2 is an anti-inflammatory macrophage marker in both human and mouse (82). In mouse studies, TGM2 plays a protective role in LPS-induced apoptosis of macrophages (83). SNAP23 regulates phagosome formation and maturation in macrophages as well as TLR4 transport upon LPS stimulation (84, 85). We also found that MIMs are mixture of M1/M2 subtypes at term (57, 86). It is possible that the upregulation of biological processes related to metabolism is indicative of a transition that occurs at parturition (29). More studies are needed to fully understand the phenotypic changes that occur during normal pregnancy and birth, as well as in the context of pregnancy complications such as preterm birth.
We also explored the relationship between maternal gravidity and gene expression in MIMs and HBCs (Figure 5). In both cell types, increased gravidity was associated with differential expression of genes related to multiple pathways, including metabolism, development, and cell signaling. However, only five genes were differentially expressed in both MIMs and HBCs as a function of gravidity. Interestingly MSC and C15ORF48 were both more highly expressed in multigravidae compared to primigravidae; both genes have been linked to suppression of pro-inflammatory response (69–71). Our analyses suggest a novel influence of gravidity on the gene signatures of MIMs and HBCs. As many pregnancy complications, such as preeclampsia, are more common in primigravidae, we hypothesize that epigenetic mechanisms involved in gene regulation and programming shape the gene signatures and innate memory of MIMs, which may work to facilitate success of subsequent pregnancies. Furthermore, the epigenetic profiles of MIMs and other placental immune cells in multigravid environments could also affect the gene signatures of fetal HBCs. Though multiple studies have delved into the vast epigenetic landscape that shapes normal and disease states in pregnancy (31, 87, 88), future work is needed to determine how gravidity influences MIMs and HBCs throughout pregnancy in this context.
Our profiles of maternal-derived MIMs and fetal-derived HBCs are consistent with recent work from Vento-Tormo et al. that identified distinct subsets of placental macrophage populations using scRNA-seq (56). Many of the most highly differentially expressed genes we reported for MIMs and HBCs were also identified by their study (Supplementary Figure 2). The differences between the markers we report and the scRNA-seq study could be due to differences in the methods of analyses. We directly compared purified MIMs and HBCs vs. the Vento-Tormo et al. study that profiled all cells at the maternal-fetal interface, including MIMs and HBCs. Another significant difference was the gestational age of sampling. Vento-Tormo, et al. analyzed first trimester placentas vs. in our study we focused on term samples.
Thomas et al. have recently proposed that earlier studies on HBCs have been contaminated by a population of maternal myeloid cells they termed placental associated maternal macrophages (PAMMs) that localize to the placental surface (36). PAMM contamination is unlikely in our study, as our HBC purification technique discarded the first two rounds of tissue digestion containing the syncytial layer and any associated PAMMs. Our methods combined with those described by Thomas et al. may simplify identification and isolation of HBCs for future transcriptomic, epigenetic and functional studies.
Our study had limitations. The limited sample size, particularly after further stratification by gravidity, constitutes one of our main limitations. Due to limited resources at our study site, we were not able to conduct confirmatory flow cytometry assays on our purified MIMs and HBCs to further characterize these cell types. The prevalence of M1 genes and pro-inflammatory markers in MIMs and HBCs could be due to parturition, which is a pro-inflammatory state. Studies suggest that maternal macrophages exhibiting an M1 phenotype promote cervical ripening, uterine contractions, and delivery (57, 89, 90). It is also possible that the switch to an M1-subtype helps protect the fetus and mother from infection following amniotic sac rupture and prevents placental retention. Also, studies report an increase in activated monocytes in preterm labor as compared to normal pregnancies (91), additional evidence that activated macrophages may play a significant role during parturition. Finally, this study was focused on placentas from African patients with likely lifetime exposures to malaria and many other infectious agents that are endemic to the region. Thus, the results might not be fully representative of those in other populations. Finally, our study is limited to MIMs and HBCs within the placenta. As MIMs are peripheral monocytes that enter and exit the placental intervillous spaces, the analysis of peripheral monocytes in parallel may provide further insights into the local responses of MIMs within the placenta.
The primary strengths of our study were the isolation and purification of MIMs and HBCs using a targeted cell approach based on placental anatomy and the comparisons of samples from primigravidae and multigravidae. Our technique can be implemented with limited resources, enabling further studies on MIMs and HBCs in under-resourced settings. We demonstrated that maternal and fetal myeloid cells at the maternal-fetal interface exhibit diverse gene signatures, with greater transcriptional heterogeneity than previously reported. Our results also suggest a novel influence of maternal gravidity on the transcriptional states of both MIMs and HBCs. Understanding the interactions between these two cell populations is critical to uncover mechanisms of immunoregulation in the context of reproductive memory and placental disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the Uganda National Council of Science and Technology, the Makerere University School of Medicine Research and Ethics Committee, the Makerere University School of Biomedical Sciences Research and Ethics Committee and the University of California, San Francisco. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NO: Investigation, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. JFR: Investigation, Methodology, Data curation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. SB: Investigation, Validation, Visualization, Writing – review & editing. MYK: Investigation, Data curation, Formal analysis, Writing – review & editing. MRA: Investigation, Visualization, Writing – original draft, Writing – review & editing. JA: Investigation, Visualization, Writing – review & editing. DJM: Investigation, Data curation, Writing – review & editing. MRK: Funding acquisition, Project administration, Supervision, Writing – review & editing. AK: Project administration, Supervision, Data curation, Writing – review & editing. GD: Funding acquisition, Project administration, Data curation, Supervision, Writing – review & editing. PJR: Methodology, Supervision, Writing – review & editing. GC: Conceptualization, Methodology, Writing – review & editing. MEF: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. SJF: Conceptualization, Methodology, Formal analysis, Writing – review & editing. SLG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institutes of Allergy and Infectious Diseases grant K08AI141728 (SLG), National Center for Advancing Translational Science grant UL1TR000124 (SLG), National Institutes of Allergy and Infectious Diseases grant K24AI113002 (MEF), Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Scientist Development Program grant K12HD000849 (SLG), Eunice Kennedy Shriver National Institute of Child Health & Human Development grant P01HD059454 (GD), and Burroughs Wellcome Fund for the Reproductive Scientist Development Program (SLG).
Acknowledgments
We thank the patients who participated in this study. We also thank John Ategeka and Samuel Wamala for technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1384361/full#supplementary-material
References
1. Erlebacher A, Fisher SJ. Baby’s first organ. Sci Am. (2017) 317:46–53. doi: 10.1038/scientificamerican1017–46
2. Labarrere CA, DiCarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P, et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol. (2017) 216:287.e1–287.e16. doi: 10.1016/j.ajog.2016.12.029
3. Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. (2010) 88:625–33. doi: 10.1189/jlb.1209796
4. Toothaker JM, Olaloye O, McCourt BT, McCourt CC, Silva TN, Case RM, et al. Immune landscape of human placental villi using single-cell analysis. Development. (2022) 149:dev200013. doi: 10.1242/dev.200013
5. Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest. (2013) 123:3472–87. doi: 10.1172/JCI60561
6. Jena MK, Nayak N, Chen K, Nayak NR. Role of macrophages in pregnancy and related complications. Arch Immunol Ther Exp (Warsz). (2019) 67:295–309. doi: 10.1007/s00005–019-00552–7
7. Tang M-X, Hu X-H, Liu Z-Z, Kwak-Kim J, Liao A-H. What are the roles of macrophages and monocytes in human pregnancy? J Reprod Immunol. (2015) 112:73–80. doi: 10.1016/j.jri.2015.08.001
8. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional differentiation. Front Immunol. (2014) 5:514. doi: 10.3389/fimmu.2014.00514
9. Houser BL. Decidual macrophages and their roles at the maternal-fetal interface. Yale J Biol Med. (2012) 85:105–18.
10. Lash GE, Pitman H, Morgan HL, Innes BA, Agwu CN, Bulmer JN. Decidual macrophages: key regulators of vascular remodeling in human pregnancy. J Leukoc Biol. (2016) 100:315–25. doi: 10.1189/jlb.1A0815-351R
11. Vondra S, Höbler A-L, Lackner AI, Raffetseder J, Mihalic ZN, Vogel A, et al. The human placenta shapes the phenotype of decidual macrophages. Cell Rep. (2023) 42:111977. doi: 10.1016/j.celrep.2022.111977
12. Ning F, Liu H, Lash GE. The role of decidual macrophages during normal and pathological pregnancy. Am J Reprod Immunol. (2016) 75:298–309. doi: 10.1111/aji.12477
13. Castellucci M, Kaufmann P. “Hofbauer Cells.,”. In: Pathology of the Human Placenta. Springer New York, New York, NY (1990). p. 71–80. doi: 10.1007/978–1-4757–4193-3_4
14. Kastschenko N. Das menschliche Chorionepithel und dessen Rolle bei der Histogenèse der Placenta. Arch Anat Physiol. (1885), 451–80.
15. Reyes L, Golos TG. Hofbauer cells: their role in healthy and complicated pregnancy. Front Immunol. (2018) 9:2628. doi: 10.3389/fimmu.2018.02628
16. Barcena A, Muench MO, Kapidzic M, Fisher SJ. A new role for the human placenta as a hematopoietic site throughout gestation. Reprod Sci. (2009) 16:178–87. doi: 10.1177/1933719108327621
17. Van Handel B, Prashad SL, Hassanzadeh-Kiabi N, Huang A, Magnusson M, Atanassova B, et al. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood. (2010) 116:3321–30. doi: 10.1182/blood-2010–04-279489
18. Tang Z, Abrahams VM, Mor G, Guller S. Placental Hofbauer cells and complications of pregnancy: Hofbauer cells and pregnancy complications. Ann N Y Acad Sci. (2011) 1221:103–8. doi: 10.1111/j.1749-6632.2010.05932.x
19. Demir R, Erbengi T. Some new findings about hofbauer cells in the chorionic villi of the human placenta. Cells Tissues Organs. (1984) 119:18–26. doi: 10.1159/000145857
20. Bokun V, Moore JJ, Moore R, Smallcombe CC, Harford TJ, Rezaee F, et al. Respiratory syncytial virus exhibits differential tropism for distinct human placental cell types with Hofbauer cells acting as a permissive reservoir for infection. PloS One. (2019) 14:e0225767. doi: 10.1371/journal.pone.0225767
21. Azari S, Johnson LJ, Webb A, Kozlowski SM, Zhang X, Rood K, et al. Hofbauer Cells Spread Listeria monocytogenes among Placental Cells and Undergo Pro-Inflammatory Reprogramming while Retaining Production of Tolerogenic Factors. mBio. (2021) 12:e01849–21. doi: 10.1128/mBio.01849–21
22. Pantazi P, Kaforou M, Tang Z, Abrahams VM, McArdle A, Guller S, et al. Placental macrophage responses to viral and bacterial ligands and the influence of fetal sex. iScience. (2022) 25:105653. doi: 10.1016/j.isci.2022.105653
23. Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. (2008) 29:274–81. doi: 10.1016/j.placenta.2007.12.010
24. Edlow AG, Glass RM, Smith CJ, Tran PK, James K, Bilbo S. Placental macrophages: A window into fetal microglial function in maternal obesity. Int J Dev Neurosci. (2019) 77:60–8. doi: 10.1016/j.ijdevneu.2018.11.004
25. Mercnik MH, Schliefsteiner C, Fluhr H, Wadsack C. Placental macrophages present distinct polarization pattern and effector functions depending on clinical onset of preeclampsia. Front Immunol. (2023) 13:1095879. doi: 10.3389/fimmu.2022.1095879
26. Sisino G, Bouckenooghe T, Aurientis S, Fontaine P, Storme L, Vambergue A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochim Biophys Acta BBA - Mol Basis Dis. (2013) 1832:1959–68. doi: 10.1016/j.bbadis.2013.07.009
27. Seval Y, Korgun ET, Demir R. Hofbauer cells in early human placenta: possible implications in vasculogenesis and angiogenesis. Placenta. (2007) 28:841–5. doi: 10.1016/j.placenta.2007.01.010
28. Zulu MZ, Martinez FO, Gordon S, Gray CM. The elusive role of placental macrophages: the hofbauer cell. J Innate Immun. (2019) 11:447–56. doi: 10.1159/000497416
29. Vikberg S, Lindau R, Solders M, Raffetseder J, Budhwar S, Ernerudh J, et al. Labour promotes systemic mobilisation of monocytes, T cell activation and local secretion of chemotactic factors in the intervillous space of the placenta. Front Immunol. (2023) 14:1129261. doi: 10.3389/fimmu.2023.1129261
30. Basu S, Leahy P, Challier J-C, Minium J, Catalano P, Hauguel-de Mouzon S. Molecular phenotype of monocytes at the maternal–fetal interface. Am J Obstet Gynecol. (2011) 205:265.e1–8. doi: 10.1016/j.ajog.2011.06.037
31. Kim SY, Romero R, Tarca AL, Bhatti G, Kim CJ, Lee J, et al. Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reprod Immunol. (2012) 68:8–27. doi: 10.1111/j.1600-0897.2012.01108.x
32. Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation: MONOCYTE EDUCATION BY TROPHOBLAST EXOSOMES. Am J Reprod Immunol. (2011) 65:65–77. doi: 10.1111/aji.2010.65.issue-1
33. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
34. Vinnars M-TN, Rindsjö E, Ghazi S, Sundberg A, Papadogiannakis N. The number of CD68 + (Hofbauer) cells is decreased in placentas with chorioamnionitis and with advancing gestational age. Pediatr Dev Pathol. (2010) 13:300–4. doi: 10.2350/09-03-0632-OA.1
35. Tang Z, Tadesse S, Norwitz E, Mor G, Abrahams VM, Guller S. Isolation of hofbauer cells from human term placentas with high yield and purity: ISOLATION OF PLACENTAL HOFBAUER CELLS. Am J Reprod Immunol. (2011) 66:336–48. doi: 10.1111/aji.2011.66.issue-4
36. Thomas JR, Appios A, Zhao X, Dutkiewicz R, Donde M, Lee CYC, et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J Exp Med. (2021) 218:e20200891. doi: 10.1084/jem.20200891
37. Zhang Y-H, He M, Wang Y, Liao A-H. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. (2017) 8:120. doi: 10.3389/fimmu.2017.00120
38. Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. (2015) 72:4111–26. doi: 10.1007/s00018-015-1995-y
39. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling: Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. (2013) 229:176–85. doi: 10.1002/path.4133
40. Schliefsteiner C, Peinhaupt M, Kopp S, Lögl J, Lang-Olip I, Hiden U, et al. Human placental hofbauer cells maintain an anti-inflammatory M2 phenotype despite the presence of gestational diabetes mellitus. Front Immunol. (2017) 8:888. doi: 10.3389/fimmu.2017.00888
41. Schliefsteiner C, Ibesich S, Wadsack C. Placental hofbauer cell polarization resists inflammatory cues in vitro. Int J Mol Sci. (2020) 21:736. doi: 10.3390/ijms21030736
42. Joerink M, Rindsjö E, van Riel B, Alm J, Papadogiannakis N. Placental macrophage (Hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta. (2011) 32:380–5. doi: 10.1016/j.placenta.2011.02.003
43. Loegl J, Hiden U, Nussbaumer E, Schliefsteiner C, Cvitic S, Lang I, et al. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction. (2016) 152:447–55. doi: 10.1530/REP-16–0159
44. Tang Z, Niven-Fairchild T, Tadesse S, Norwitz ER, Buhimschi CS, Buhimschi IA, et al. Glucocorticoids enhance CD163 expression in placental hofbauer cells. Endocrinology. (2013) 154:471–82. doi: 10.1210/en.2012–1575
45. Renaud SJ, Graham CH. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol Invest. (2008) 37:535–64. doi: 10.1080/08820130802191375
46. Ma Y, Ye Y, Zhang J, Ruan C-C, Gao P-J. Immune imbalance is associated with the development of preeclampsia. Med (Baltimore). (2019) 98:e15080. doi: 10.1097/MD.0000000000015080
47. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) 122:787–95. doi: 10.1172/JCI59643
48. Yao Y, Xu X-H, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. (2019) 10:792. doi: 10.3389/fimmu.2019.00792
49. Gaw SL, Hromatka BS, Ngeleza S, Buarpung S, Ozarslan N, Tshefu A, et al. Differential activation of fetal hofbauer cells in primigravidas is associated with decreased birth weight in symptomatic placental malaria. Malar Res Treat. (2019) 2019:1–10. doi: 10.1155/2019/1378174
50. Swieboda D, Johnson EL, Beaver J, Haddad L, Enninga EAL, Hathcock M, et al. Baby’s first macrophage: temporal regulation of hofbauer cell phenotype influences ligand-mediated innate immune responses across gestation. J Immunol. (2020) 204:2380–91. doi: 10.4049/jimmunol.1901185
51. Beyer M, Mallmann MR, Xue J, Staratschek-Jox A, Vorholt D, Krebs W, et al. High-resolution transcriptome of human macrophages. PloS One. (2012) 7:e45466. doi: 10.1371/journal.pone.0045466
52. Goenka A, Prise IE, Connolly E, Fernandez-Soto P, Morgan D, Cavet JS, et al. Infant alveolar macrophages are unable to effectively contain mycobacterium tuberculosis. Front Immunol. (2020) 11:486. doi: 10.3389/fimmu.2020.00486
53. Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. (2014) 40:274–88. doi: 10.1016/j.immuni.2014.01.006
54. Pavličev M, Wagner GP, Chavan AR, Owens K, Maziarz J, Dunn-Fletcher C, et al. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res. (2017) 27:349–61. doi: 10.1101/gr.207597.116
55. Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A, et al. A single-cell survey of the human first-trimester placenta and decidua. Sci Adv. (2018) 4:eaau4788. doi: 10.1126/sciadv.aau4788
56. Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature. (2018) 563:347–53. doi: 10.1038/s41586–018-0698–6
57. Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife. (2019) 8:e52004. doi: 10.7554/eLife.52004
58. Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. (2007) 7:105–17. doi: 10.1016/S1473–3099(07)70022–1
59. Hunkapiller NM, Fisher SJ. “Chapter 12 Placental Remodeling of the Uterine Vasculature.,”. In: Methods in Enzymology. Amsterdam: Elsevier (2008). p. 281–302. doi: 10.1016/S0076–6879(08)03012–7
60. Law CW, Chen Y, Shi W, Smyth GK. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. (2014) 15:R29. doi: 10.1186/gb-2014–15-2-r29
61. Pearson KL III. On lines and planes of closest fit to systems of points in space. Lond Edinb Dublin Philos Mag J Sci. (1901) 2:559–72. doi: 10.1080/14786440109462720
62. Shoemaker JE, Lopes TJ, Ghosh S, Matsuoka Y, Kawaoka Y, Kitano H. CTen: a web-based platform for identifying enriched cell types from heterogeneous microarray data. BMC Genomics. (2012) 13:460. doi: 10.1186/1471–2164-13–460
63. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
64. Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal–maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. (2011) 187:3671–82. doi: 10.4049/jimmunol.1100130
65. Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PloS One. (2008) 3:e2078. doi: 10.1371/journal.pone.0002078
66. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. (2014) 6. doi: 10.12703/P6–13
67. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. (2014) 5:491. doi: 10.3389/fimmu.2014.00491
68. He L, Jhong J-H, Chen Q, Huang K-Y, Strittmatter K, Kreuzer J, et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. (2021) 37:109955. doi: 10.1016/j.celrep.2021.109955
69. Yan J, Yu J, Yuan S, Tang W, Ma W, Yang X, et al. Musculin is highly enriched in Th17 and IL-22-producing ILC3s and restrains pro-inflammatory cytokines in murine colitis. Eur J Immunol. (2021) 51:995–8. doi: 10.1002/eji.202048573
70. Yu J, Liu Y, Zhang W, Yang X, Tang W, Liang H, et al. Musculin deficiency aggravates colonic injury and inflammation in mice with inflammatory bowel disease. Inflammation. (2020) 43:1455–63. doi: 10.1007/s10753-020-01223-y
71. Lee CQE, Kerouanton B, Chothani S, Zhang S, Chen Y, Mantri CK, et al. Coding and non-coding roles of MOCCI (C15ORF48) coordinate to regulate host inflammation and immunity. Nat Commun. (2021) 12:2130. doi: 10.1038/s41467–021-22397–5
72. Gregory SG, Barlow KF, McLay KE, Kaul R, Swarbreck D, Dunham A, et al. The DNA sequence and biological annotation of human chromosome 1. Nature. (2006) 441:315–21. doi: 10.1038/nature04727
73. Bai SW, Herrera-Abreu MT, Rohn JL, Racine V, Tajadura V, Suryavanshi N, et al. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. (2011) 9:54. doi: 10.1186/1741–7007-9–54
74. Wang G, Yu J, Yang Y, Liu X, Zhao X, Guo X, et al. Whole-transcriptome sequencing uncovers core regulatory modules and gene signatures of human fetal growth restriction. Clin Transl Med. (2020) 9:e9. doi: 10.1186/s40169–020-0259–0
75. Young OM, Tang Z, Niven-Fairchild T, Tadesse S, Krikun G, Norwitz ER, et al. Toll-like receptor-mediated responses by placental hofbauer cells (HBCs): A potential pro-inflammatory role for fetal M2 macrophages. Am J Reprod Immunol. (2015) 73:22–35. doi: 10.1111/aji.12336
76. Matsubara S, Takayama T, Yamada T, Usui R, Izumi A, Watanabe T, et al. Hofbauer Cell Activation and its increased Glucose-6-Phosphate Dehydrogenase Activity in Second Trimester-spontaneous Abortion: an Ultrastructural Dual Staining Enzyme-cytochemical Study: HOFBAUER G6PD IN SPONTANEOUS ABORTION. Am J Reprod Immunol. (2003) 49:202–9. doi: 10.1034/j.1600-0897.2003.01180.x
77. Pavlov OV, Selutin AV, Pavlova OM, Selkov SA. Two patterns of cytokine production by placental macrophages. Placenta. (2020) 91:1–10. doi: 10.1016/j.placenta.2020.01.005
78. Šošić D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-κB activity. Cell. (2003) 112:169–80. doi: 10.1016/S0092–8674(03)00002–3
79. Sharif MN, Šošić D, Rothlin CV, Kelly E, Lemke G, Olson EN, et al. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. (2006) 203:1891–901. doi: 10.1084/jem.20051725
80. Zheng S, Hedl M, Abraham C. Twist1 and Twist2 Contribute to Cytokine Downregulation following Chronic NOD2 Stimulation of Human Macrophages through the Coordinated Regulation of Transcriptional Repressors and Activators. J Immunol. (2015) 195:217–26. doi: 10.4049/jimmunol.1402808
81. Sun R, Hedl M, Abraham C. Twist1 and twist2 induce human macrophage memory upon chronic innate receptor treatment by HDAC-mediated deacetylation of cytokine promoters. J Immunol. (2019) 202:3297–308. doi: 10.4049/jimmunol.1800757
82. Abdelaziz MH, Abdelwahab SF, Wan J, Cai W, Huixuan W, Jianjun C, et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J Transl Med. (2020) 18:58. doi: 10.1186/s12967-020-02251-w
83. Zhang S, Fu B, Xiong Y, Zhao Q, Xu S, Lin X, et al. Tgm2 alleviates LPS-induced apoptosis by inhibiting JNK/BCL-2 signaling pathway through interacting with Aga in macrophages. Int Immunopharmacol. (2021) 101:108178. doi: 10.1016/j.intimp.2021.108178
84. Hatsuzawa K, Sakurai C. Regulatory mechanism of SNAP23 in phagosome formation and maturation. Yonago Acta Med. (2020) 63:135–45. doi: 10.33160/yam.2020.08.001
85. Kinoshita D, Sakurai C, Morita M, Tsunematsu M, Hori N, Hatsuzawa K. Syntaxin 11 regulates the stimulus-dependent transport of Toll-like receptor 4 to the plasma membrane by cooperating with SNAP-23 in macrophages. Mol Biol Cell. (2019) 30:1085–97. doi: 10.1091/mbc.E18–10-0653
86. Wang H, He M, Hou Y, Chen S, Zhang X, Zhang M, et al. Role of decidual CD14+ macrophages in the homeostasis of maternal–fetal interface and the differentiation capacity of the cells during pregnancy and parturition. Placenta. (2016) 38:76–83. doi: 10.1016/j.placenta.2015.12.001
87. Apicella C, Ruano CSM, Méhats C, Miralles F, Vaiman D. The role of epigenetics in placental development and the etiology of preeclampsia. Int J Mol Sci. (2019) 20:2837. doi: 10.3390/ijms20112837
88. Liu Y, Fan X, Wang R, Lu X, Dang Y-L, Wang H, et al. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. (2018) 28:819–32. doi: 10.1038/s41422-018-0066-y
89. Huang Q, Jin X, Li P, Zheng Z, Jiang Y, Liu H. Elevated inflammatory mediators from the maternal-fetal interface to fetal circulation during labor. Cytokine. (2021) 148:155707. doi: 10.1016/j.cyto.2021.155707
90. Shan Y, Shen S, Long J, Tang Z, Wu C, Ni X. Term and preterm birth initiation is associated with the macrophages shifting to M1 polarization in gestational tissues in mice. Biology. (2022) 11:1759. doi: 10.3390/biology11121759
Keywords: placenta, myeloid cells, monocyte, Hofbauer cell, pregnancy, gravidity, RNA-Seq, transcriptomics
Citation: Ozarslan N, Robinson JF, Buarpung S, Kim MY, Ansbro MR, Akram J, Montoya DJ, Kamya MR, Kakuru A, Dorsey G, Rosenthal PJ, Cheng G, Feeney ME, Fisher SJ and Gaw SL (2024) Gravidity influences distinct transcriptional profiles of maternal and fetal placental macrophages at term. Front. Immunol. 15:1384361. doi: 10.3389/fimmu.2024.1384361
Received: 09 February 2024; Accepted: 31 May 2024;
Published: 26 June 2024.
Edited by:
Benjamin G. Dewals, Fonds National de la Recherche Scientifique (FNRS), BelgiumReviewed by:
William Horsnell, University of Cape Town, South AfricaMichael Eikmans, Leiden University Medical Center (LUMC), Netherlands
Copyright © 2024 Ozarslan, Robinson, Buarpung, Kim, Ansbro, Akram, Montoya, Kamya, Kakuru, Dorsey, Rosenthal, Cheng, Feeney, Fisher and Gaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie L. Gaw, U3RlcGhhbmllLkdhd0B1Y3NmLmVkdQ==
 Nida Ozarslan
Nida Ozarslan Joshua F. Robinson
Joshua F. Robinson Sirirak Buarpung1,2
Sirirak Buarpung1,2 Dennis J. Montoya
Dennis J. Montoya Genhong Cheng
Genhong Cheng Margaret E. Feeney
Margaret E. Feeney Stephanie L. Gaw
Stephanie L. Gaw