
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 10 April 2024
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1383343
Hydroxychloroquine (HCQ) is used as a traditional disease-modifying antirheumatic drugs (DMARDs), for the treatment of autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). However, it can cause serious adverse reactions, including hyperpigmentation of the skin and bull’s-eye macular lesions. Here, we present a case of HCQ-induced hyperpigmentation of the skin and bull’s-eye macular lesions in a patient who received HCQ for RA. A 65-year-old female patient developed blurred vision and hyperpigmentation of multiple areas of skin over the body for one month after 3 years of HCQ treatment for RA. Based on clinical presentation, ophthalmological examination and dermatopathological biopsy, a diagnosis of drug-induced cutaneous hyperpigmentation and bullous maculopathy of the right eye was made. After discontinuation of HCQ and treatment with iguratimod tablets, the hyperpigmentation of the patient ‘s skin was gradually reduced, and the symptoms of blurred vision were not significantly improved. We also reviewed the available literature on HCQ-induced cutaneous hyperpigmentation and bull’s-eye macular lesions and described the clinical features of HCQ-induced cutaneous hyperpigmentation and bull’s-eye macular lesions. In conclusion, clinicians should be aware of early cutaneous symptoms and HCQ-associated ophthalmotoxicity in patients with rheumatic diseases on HCQ sulphate and should actively monitor patients, have them undergo regular ophthalmological examinations and give appropriate treatment to prevent exacerbation of symptoms.
Hydroxychloroquine (HCQ) is a 4-aminoquinoline antimalarial drug. It is commonly used as a sulfate, namely HCQ sulfate. Its antimalarial effect is the same as that of chloroquine, but its toxicity is only half that of chloroquine. In addition, HCQ sulfate also has anti-inflammatory, immunomodulatory and anticoagulant effects (1–4), so it is widely used in clinical treatment of rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren’s syndrome, skin diseases, etc., and the adverse reactions are gradually increasing (5, 6). There are fewer reports on HCQ-induced hyperpigmentation of the skin and macular lesions in the bull’s eye. This article analyses the literature related to HCQ sulfate-induced hyperpigmentation of the skin and macular lesions in the bull’s eye in conjunction with the literature review by taking a case of HCQ sulfate-induced hyperpigmentation and macular lesions in the bull’s eye as an example in order to warn the clinic to fully understand the adverse effects of HCQ.
We assessed a 65-year-old female patient with RA in December 2023 who had been diagnosed with RA 10 years earlier and was now feeling pain in both shoulders, wrists, and finger joints of both hands with mild limitation of movement. The patient was treated with HCQ (400mg/d) and low-dose prednisolone (5 mg/d) for 3 years. The patient had no other comorbidities or medications, and for the past month felt blurred vision and noticed a darkening of the skin color. Our physical examination revealed excessive skin pigmentation in many parts of the body, especially in the head and face, neck, upper limbs and lower limbs (Figure 1). The patient attributed this to chronic ultraviolet light exposure.
We performed relevant laboratory tests on the patient, which showed an elevated erythrocyte sedimentation rate of 61.00 mm/h (normal, 0-20 mm/L), an elevated rheumatoid factor of 43.15 IL/ml (normal, 0-20 IL/ml), and an elevated anti-cyclic citrullinated peptide antibody of > 500.00 U/mL (normal, 0-20 U/mL). We performed an ophthalmological examination of the patient because he had symptoms of blurred vision and because studies have shown that HCQ can cause retinopathy (7, 8). The visual field examination results showed that the visual field of the right eye showed a visual field defect outside the range of 45° above and 25° on the nasal side, and the light sensitivity of the remaining visual field decreased significantly. Compared with the dark spots around the physiological blind spots of the left eye, the photosensitivity decreased widely outside the range of 40°, and the photosensitivity decreased scattered within the range of 40°. Therefore, we performed optical coherence tomography (OCT) (Figure 2) and fundus screening (Figures 3A-D) on the patient. The results of OCT examination showed that the retinal pigment epithelium (RPE) layer in the macular area of the right eye was disordered and uneven, and irregular mass uplift was seen. The reflection of the ellipsoid zone and the IS/OS layer was interrupted and discontinuous. The RPE layer on the temporal side of the macular showed localized choroidal depression. The macular morphology was irregular, and the thickness of the macular fovea was significantly thinner (Figure 2A). However, the results of OCT of the left eye did not show any significant abnormality (Figure 2B). The results of fundus screening showed that the right eye had a round-like lesion in the macular area, about 2.5*3PD in size. The lesion was uneven yellow-white, and the center was dark brown. In conjunction with the OCT findings, a bull’s eye macular lesion was considered.
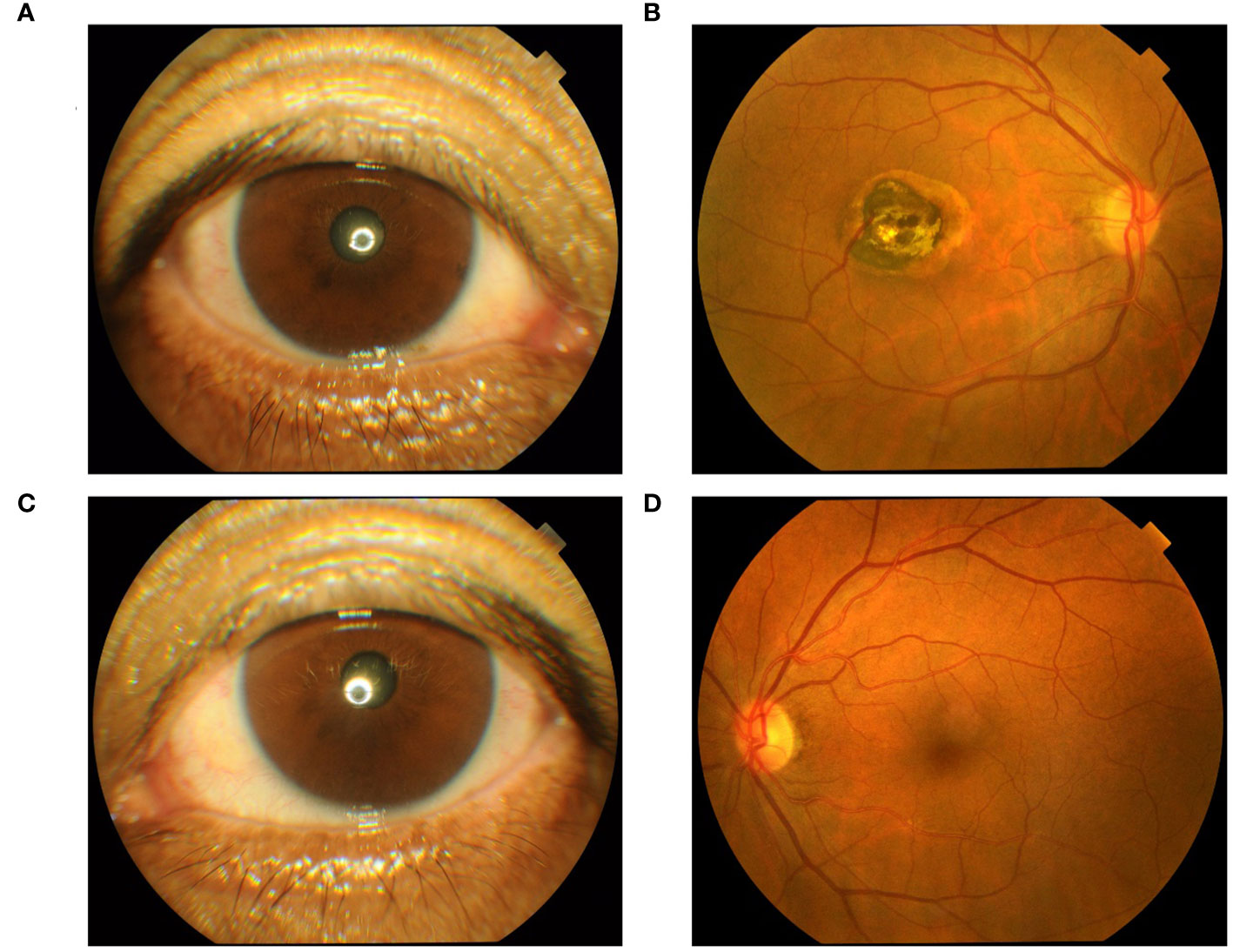
Figure 3 (A) Right anterior segment findings; (B) Right eye fundus screening results; (C) Left anterior segment findings; (D) Left eye fundus screening results.
From the results of the ophthalmologic examination, our patient was not considered for the diagnosis of age-related macular degeneration (AMD), which is characterized by the accumulation of extracellular deposits and the progressive degeneration of photoreceptors and adjacent tissues (9–12). The prevalence increases gradually with age and usually affects vision for a short period of time, even leading to blindness (13, 14). In AMD, the damage is concentrated in the central portion of the retina, known as the macula. One of the features of AMD is the scattered or confluent areas of degeneration of RPE cells and overlying photoreceptors in the photoreceptors of the photoreceptors, which depend on the RPE for trophic support. Although our patient’s OCT results showed disturbed and uneven RPE reflection seen in the retina of the macular area of the right eye. However, there was no significant abnormality in the left eye OCT results. Another feature of AMD is the formation of choroidal neovascularization, in which immature blood vessels grow from the choroid below toward the outer retina. These immature blood vessels leak fluid below or inside the retina (10). In contrast, our patient’s funduscopic findings showed no vasculopathy. So we consider that this macular lesion is not so much related to aging as it is to the use of HCQ.
In addition, we performed a skin biopsy on the patient. HE staining (Figure 4A) showed excessive keratinization of the epidermal mesh basket, a significant increase in melanin in the basal layer, sparse lymphocyte and tissue cell infiltration around the blood vessels in the superficial dermis, and a few melanocytes and melanin granules were seen locally, considering drug-induced pigmentation. Therefore, it can be differentiated from skin pigmentation caused by exposure to ultraviolet light (15). Fontana-Masson staining (Figure 4B) showed a significant increase in melanin in the basal layer of the epidermis and a few melanin granules in the superficial dermis. Prussian blue staining and silver hexamine staining were negative (Figures 4C, D). Five items of direct immunofluorescence (Figure 5) C3, IgG, IgM, IgA and Fib were all negative.
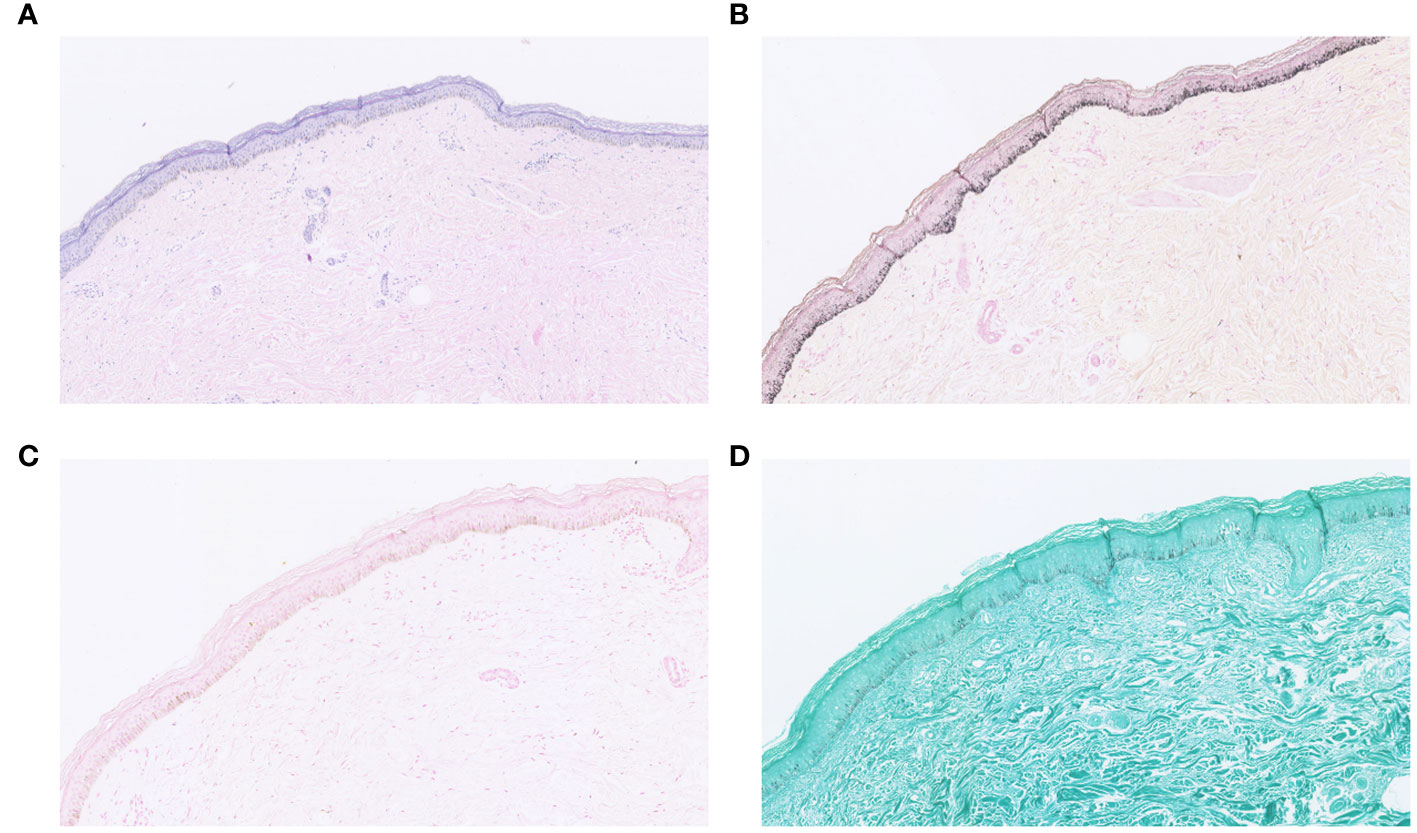
Figure 4 (A) HE staining; (B) Fontana-Masson staining; (C) Prussian blue staining; (D) silver hexamine staining.
According to the patient ‘s medical history and examination results, we believe that the patient ‘s long-term use of HCQ in the treatment of RA, resulting in hyperpigmentation of the skin and bull’s-eye maculopathy. Therefore, we decided to discontinue HCQ and replace it with iguratimod tablets (50mg/d) and prednisone acetate tablets (5mg/d) to control the disease. It is important to note that iguratimod is only used in China and Japan for the treatment of RA and has been shown to be effective (16–18). Since then, we followed up the patient for three months. After discontinuation of HCQ and treatment with iguratimod tablets, the hyperpigmentation of the patient ‘s skin was gradually reduced, and the symptoms of blurred vision were not significantly improved.
Based on the patient’s clinical presentation and ophthalmologic findings, we reviewed similar cases reported in PubMed, Embase, Web of Science and other databases from the establishment of the database to December 2023. We used “hydroxychloroquine”, “plaquenil”, “case study”, “case report”, “bull’s-eye maculopathy” and “hyperpigmentation” as the search terms to filter out the eligible literature and extract the relevant information of the cases. From the results of our literature search, a total of 181 articles were retrieved from the literature related to hyperpigmentation of the skin. After screening, 18 articles were obtained, the total number of cases was 20, female patients were more than male patients, distributed in different countries or regions, the primary disease was mainly RA or SLE, the color of skin pigmentation was mainly blue/gray, and the site of skin pigmentation was mainly on the face, the upper limbs or the lower limbs, and the daily dosage of HCQ was mainly 400mg, and the skin pigmentation of the over-pigmented skin of the majority of the patients could be gradually subsided after stopping the HCQ. Table 1 shows the detailed clinical features of these cases. A total of 46 articles were retrieved from the literature related to macular degeneration of the bull’s eye. After screening 11 valid literature were obtained, the total number of cases was 13, all were female patients, the daily dose of HCQ was 200-1200mg and the approximate cumulative dose was 438-2920g, and most of the patients’ solution was to stop HCQ therapy. Table 2 shows the detailed clinical features of these cases.
Hyperpigmentation of the skin caused by antimalarial treatment has been reported since the Second World War (48). However, the associated skin hyperpigmentation due to HCQ seems to be uncommon compared to other antimalarials such as chloroquine (28, 49). One study showed that the onset of HCQ-associated skin hyperpigmentation ranged from 3 months to 22 years after the start of treatment, with a median of 6.1 years (50). In our reported case, the patient developed hyperpigmentation of the skin about 3 years after the start of treatment, and the treatment was to discontinue HCQs and to control the RA with iguratimod tablets, which led to a gradual reduction of the patient’s skin hyperpigmentation over several months (22, 25). Although there is evidence that both melanin and iron deposition can be present in the dermis in HCQ-induced hyperpigmented skin lesions (50), the exact mechanism is unknown (30). Our patients completed the relevant pathological examination. Fontana-Masson staining (Figure 4B) showed a significant increase in melanin in the basal layer of the epidermis and a few melanin granules in the superficial layer of the dermis. Prussian blue staining was negative (Figure 4C). Therefore, we describe the pathological features of some cases (Table 3). In terms of treatment, most of the investigators took the approach of discontinuing HCQ treatment to prevent further exacerbation of HCQ-induced hyperpigmentation of the skin. Most patients were able to reduce their hyperpigmentation after discontinuing HCQ treatment.
Long-term treatment with HCQ can lead to retinal toxicity (51). This retinal change is typically characterized by a thinning of the photoreceptor layer, starting with a paracentral sulcus ring and progressing over time to a “bull’s-eye” maculopathy, caused by pericentral atrophy and retention of the central sulcus (52). Some researchers have suggested that HCQ may be associated with bilateral retinopathy involving not only the macula but also the peripheral retina (39). The relationship between HCQ-related skin pigmentation and ocular toxicity is currently unknown (28); However, there is a need for regular ophthalmological follow-ups so that the patient’s eye condition can be understood (30). Our patient developed vision loss after 36 months of HCQ treatment, with a cumulative dose of about 438 g. Ophthalmological examination revealed macular lesions in the right eye. Table 2 describes some of the published information on similar cases, and the case we reported is similar to those reported in the literature. In terms of treatment, most of the investigators took to discontinuing HCQ treatment to prevent further worsening of the ocular adverse effects caused by HCQ. However, patients who have developed bull’s-eye macular degeneration are difficult to rehabilitate (41). In terms of ophthalmic examination, Fung et al. (40) suggest that OCT may be a useful, non-invasive clinical assessment if a patient presents with new visual changes associated with HCQ. In addition, OCT findings of diffuse retinal atrophy or increased reflectivity around the macular central pucker can support the port suspected diagnosis of HCQ-associated retinal toxicity.
In conclusion, HCQ can cause adverse reactions such as hyperpigmentation of the skin and macular degeneration of the bull’s eye, clinicians should be aware of early cutaneous symptoms and HCQ-associated ophthalmotoxicity in patients with rheumatic diseases on HCQ sulphate and should actively monitor patients, have them undergo regular ophthalmological examinations and give appropriate treatment to prevent exacerbation of symptoms.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
J-PP: Writing – original draft, Writing – review & editing. X-YY: Writing – original draft. FL: Writing – original draft. X-MY: Writing – original draft. HX: Writing – original draft. W-KM: Writing – original draft. X-MY: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by the National Natural Science Foundation of China, 82160869, 82374494; Guizhou Provincial Key Technology R&D Program, Qiankehe Support [2021] General 006; Guizhou Provincial Basic Research Program(Natural Science) Qiankehe Basic-ZK[2023] General 412; Guizhou Kehe Academic New Seedling[2023-36]; Guizhou University of Traditional Chinese Medicine National and Provincial Scientific and Technological Innovation Talent Teams Cultivation Project, Guizhou University of Traditional Chinese Medicine TD He Zi [2022]004.
We appreciate Yuzheng Yang (Organization: Guizhou University of Traditional Chinese Medicine, Guiyang, China) for organizing the data, Yuheng Shi and Shasha Du (Organization: Ophthalmology Department, Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, China) for performing the ophthalmologic examinations, and all authors for their cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. (2015) 70:1608–21. doi: 10.1093/jac/dkv018
2. Bai L, Li H, Li J, Song J, Zhou Y, Liu B, et al. Immunosuppressive effect of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via regulating the differentiation of CD4+ T cell subsets in rats. Int Immunopharmacol. (2019) 70:313–23. doi: 10.1016/j.intimp.2019.02.056
3. Chandler LC, Yusuf IH, McClements ME, Barnard AR, MacLaren RE, Xue K. Immunomodulatory effects of hydroxychloroquine and chloroquine in viral infections and their potential application in retinal gene therapy. Int J Mol Sci. (2020) 21:1. doi: 10.3390/ijms21144972
4. Richard SA, Kampo S, Hechavarria ME, Sackey M, Buunaaim ADB, Kuugbee ED, et al. Elucidating the pivotal immunomodulatory and anti-inflammatory potentials of chloroquine and hydroxychloroquine. J Immunol Res. (2020) 2020:4582612. doi: 10.1155/2020/4582612
5. Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. (2020) 12:e12476. doi: 10.15252/emmm.202012476
6. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. (2020) 16:155–66. doi: 10.1038/s41584-020-0372-x
7. Alveyn E, Galloway J. When should we start screening for hydroxychloroquine retinopathy? Rheumatol (Oxford England). (2022) 61:3097–8. doi: 10.1093/rheumatology/keac043
8. Proano C, Kimball GP. Hydroxychloroquine retinal toxicity. New Engl J Med. (2019) 380:e27. doi: 10.1056/NEJMicm1304542
9. Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, et al. Age-related macular degeneration. Nat Rev Dis Primers. (2021) 7:31. doi: 10.1038/s41572-021-00265-2
10. Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. (2012) 75:26–39. doi: 10.1016/j.neuron.2012.06.018
11. Chirco KR, Potempa LA. C-reactive protein as a mediator of complement activation and inflammatory signaling in age-related macular degeneration. Front Immunol. (2018) 9:539. doi: 10.3389/fimmu.2018.00539
12. Clark SJ, McHarg S, Tilakaratna V, Brace N, Bishop PN. Bruch's membrane compartmentalizes complement regulation in the eye with implications for therapeutic design in age-related macular degeneration. Front Immunol. (2017) 8:1778. doi: 10.3389/fimmu.2017.01778
13. Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol (Chicago Ill 1960). (2004) 122:564–72. doi: 10.1001/archopht.122.4.564
14. Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. (2008) 115:116–26. doi: 10.1016/j.ophtha.2007.03.008
15. D'Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. (2013) 14:12222–48. doi: 10.3390/ijms140612222
16. Hu CJ, Zhang L, Zhou S, Jiang N, Zhao JL, Wang Q, et al. Effectiveness of iguratimod as monotherapy or combined therapy in patients with rheumatoid arthritis: a systematic review and meta-analysis of RCTs. J orthopaedic Surg Res. (2021) 16:457. doi: 10.1186/s13018-021-02603-2
17. Tanaka K, Yamaguchi T, Hara M. Iguratimod for the treatment of rheumatoid arthritis in Japan. Expert Rev Clin Immunol. (2015) 11:565–73. doi: 10.1586/1744666x.2015.1027151
18. Xie S, Li S, Tian J, Li F. Iguratimod as a new drug for rheumatoid arthritis: current landscape. Front Pharmacol. (2020) 11:73. doi: 10.3389/fphar.2020.00073
19. True DG, Bryant LR, Harris MD, Bernert RA. Clinical images: Hydroxychloroquine-associated mucocutaneous hyperpigmentation. Arthritis rheumatism. (2002) 46:1698. doi: 10.1002/art.10278
20. Millard TP, Kirk A, Ratnavel R. Cutaneous hyperpigmentation during therapy with hydroxychloroquine. Clin Exp Dermatol. (2004) 29:92–3. doi: 10.1111/j.1365-2230.2004.01412.x
21. Reynaert S, Setterfield J, Black MM. Hydroxychloroquine-induced pigmentation in two patients with systemic lupus erythematosus. J Eur Acad Dermatol Venereology. (2006) 20:487–8. doi: 10.1111/j.1468-3083.2006.01494.x
22. Amichai B, Gat A, Grunwald MH. Cutaneous hyperpigmentation during therapy with hydroxychloroquine. J Clin Rheumatol. (2007) 13:113. doi: 10.1097/01.rhu.0000260649.36417.09
23. Melikoglu MA, Melikoglu M, Gurbuz U, Budak BS, Kacar C. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. (2008) 33:699–701. doi: 10.1111/jcp.2008.33.issue-6
24. Puri PK, Lountzis NI, Tyler W, Ferringer T. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J cutaneous Pathol. (2008) 35:1134–7. doi: 10.1111/j.1600-0560.2008.01004.x
25. Rood MJ, Vermeer MH, Huizinga TW. Hyperpigmentation of the skin due to hydroxychloroquine. Scandinavian J Rheumatol. (2008) 37:158. doi: 10.1080/03009740701769735
26. Morrison LK, Nordlund JJ, Heffernan MP. Persistent cutaneous hyperpigmentation due to hydroxychloroquinone one year after therapy discontinuation. Dermatol Online J. (2009) 15:15. doi: 10.5070/D32T07Q0NW
27. Cho EB, Kim BC, Park EJ, Kwon IH, Cho HJ, Kim KH, et al. Hydroxychloroquine-induced hyperpigmentation. J Dermatol. (2012) 39:859–60. doi: 10.1111/j.1346-8138.2012.01591.x
28. Mir A, Boyd KP, Meehan SA, McLellan B. Hydroxycholoroquine-induced hyperpigmentation. Dermatol Online J. (2013) 19:20723. doi: 10.5070/D31912020723
29. Cohen PR. Hydroxychloroquine-associated hyperpigmentation mimicking elder abuse. Dermatol Ther. (2013) 3:203–10. doi: 10.1007/s13555-013-0032-z
30. Tracy CL, Blakey B, Parker G, Roebuck J. Hydroxychloroquine-induced hyperpigmentation. J Clin Rheumatol. (2013) 19:292. doi: 10.1097/RHU.0b013e31829d547b
31. Sawalha AH. Hydroxychloroquine-induced hyperpigmentation of the skin. J Rheumatol. (2015) 42:135–6. doi: 10.3899/jrheum.140995
32. Coulombe J, Boccara O. Hydroxychloroquine-related skin discoloration. CMAJ Can Med Assoc J. (2017) 189:E212. doi: 10.1503/cmaj.150622
33. Ivo R, Lopes CA, Reis R. Woman in grey: hydroxychloroquine-induced hyperpigmentation. BMJ Case Rep. (2018) 11:1. doi: 10.1136/bcr-2018-227305
34. Thakur V, Dalla A, Kumar S, Kumaran MS, Aggarwal D, Radotra BD. Hydroxychloroquine induced cutaneous pigmentation: a unique pattern. Postgraduate Med J. (2019) 95:169–70. doi: 10.1136/postgradmedj-2018-136377
35. Tosios KI, Kalogirou EM, Sklavounou A. Drug-associated hyperpigmentation of the oral mucosa: report of four cases. Oral surgery Oral medicine Oral Pathol Oral Radiol. (2018) 125:e54–66. doi: 10.1016/j.oooo.2017.10.006
36. Tekgöz E, Akıncıoğlu E, Çınar M, Yılmaz S. A case of exogenous ochronosis associated with hydroxychloroquine. Eur J Rheumatol. (2018) 5:206–8. doi: 10.5152/eurjrheum.2018.17190
37. Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (plaquenil) therapy. Am J Ophthalmol. (1967) 64:245–52. doi: 10.1016/0002-9394(67)92518-4
38. Johnson MW, Vine AK. Hydroxychloroquine therapy in massive total doses without retinal toxicity. Am J Ophthalmol. (1987) 104:139–44. doi: 10.1016/0002-9394(87)90005-5
39. Weiner A, Sandberg MA, Gaudio AR, Kini MM, Berson EL. Hydroxychloroquine retinopathy. Am J Ophthalmol. (1991) 112:528–34. doi: 10.1016/s0002-9394(14)76853-9
40. Fung AE, Samy CN, Rosenfeld PJ. Optical coherence tomography findings in hydroxychloroquine and chloroquine-associated maculopathy. Retinal cases Brief Rep. (2007) 1:128–30. doi: 10.1097/01.iae.0000226540.61840.d7
41. Salu P, Uvijls A, van den Brande P, Leroy BP. Normalization of generalized retinal function and progression of maculopathy after cessation of therapy in a case of severe hydroxychloroquine retinopathy with 19 years follow-up. Documenta ophthalmologica Adv Ophthalmol. (2010) 120:251–64. doi: 10.1007/s10633-010-9220-7
42. Phillips BN, Chun DW. Hydroxychloroquine retinopathy after short-term therapy. Retinal Cases Brief Rep. (2014) 8:67–9. doi: 10.1097/icb.0000000000000006
43. Wong KL, Pautler SE, Browning DJ. Near-infrared reflectance bull's eye maculopathy as an early indication of hydroxychloroquine toxicity. Clin Ophthalmol (Auckland NZ). (2015) 9:521–5. doi: 10.2147/opth.S76963
44. Brandao LM, Palmowski-Wolfe AM. A possible early sign of hydroxychloroquine macular toxicity. Documenta Ophthalmologica Adv Ophthalmol. (2016) 132:75–81. doi: 10.1007/s10633-015-9521-y
45. Modi YS, Singh RP. Bull's-eye maculopathy associated with hydroxychloroquine. New Engl J Med. (2019) 380:1656. doi: 10.1056/NEJMicm1412167
46. Pellerano F, Chahín G, Niurka Leonor M, Stern H. Hydroxychloroquine-induced bull's eye maculopathy. Lancet Rheumatol. (2020) 2:e120. doi: 10.1016/s2665-9913(19)30040-2
47. Ameen Ismail A, Sadek SH, Hatata RM. Early onset monocular hydroxychloroquine maculopathy in a systemic lupus erythematosus patient with history of central retinal artery occlusion: a case report. BMC Ophthalmol. (2022) 22:434. doi: 10.1186/s12886-022-02657-8
48. Lippard VW, Kauer GL Jr. Pigmentation of the palate and subungual tissues associated with suppressive quinacrine hydrochloride therapy. Am J Trop Med hygiene. (1945) 25:469–71. doi: 10.4269/ajtmh.1945.s1-25.469
49. Skare T, Ribeiro CF, Souza FH, Haendchen L, Jordão JM. Antimalarial cutaneous side effects: a study in 209 users. Cutaneous Ocular Toxicol. (2011) 30:45–9. doi: 10.3109/15569527.2010.521225
50. Jallouli M, Francès C, Piette JC, Huong du LT, Moguelet P, Factor C, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. (2013) 149:935–40. doi: 10.1001/jamadermatol.2013.709
51. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. (2014) 132:1453–60. doi: 10.1001/jamaophthalmol.2014.3459
Keywords: hydroxychloroquine, adverse drug reaction, hyperpigmentation of the skin, bull’s-eye maculopathy, literature review, autoimmune disease
Citation: Peng J-p, Yang X-y, Luo F, Yuan X-m, Xiong H, Ma W-k and Yao X-m (2024) Hydroxychloroquine-induced hyperpigmentation of the skin and bull’s-eye maculopathy in rheumatic patients: a case report and literature review. Front. Immunol. 15:1383343. doi: 10.3389/fimmu.2024.1383343
Received: 07 February 2024; Accepted: 27 March 2024;
Published: 10 April 2024.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Seyed Parsa Eftekhar, Babol University of Medical Sciences, IranCopyright © 2024 Peng, Yang, Luo, Yuan, Xiong, Ma and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-ming Yao, eXhtaW5nMTlAZm94bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.