- 1Department of General Surgery, The First Affiliated Hospital of Shandong First Medical University, Jinan, China
- 2Key Laboratory of Metabolism and Gastrointestinal Tumor, The First Affiliated Hospital of Shandong First Medical University, Jinan, China
- 3Department of Neurology, Shandong Province Third Hospital, Jinan, China
Background: The Prognostic Nutritional Index (PNI) has become an important predictive tool for assessing patients’ nutritional status and immune competence. It is widely used in prognostic evaluations for various cancer patients. However, the prognostic relevance of the Prognostic Nutritional Index (PNI) in gastric or gastro-esophageal junction cancer patients (GC/GEJC) undergoing immune checkpoint inhibitors (ICIs) treatment remains unclear. This meta-analysis aimed to determine the prognostic impact of PNI in this specific patient cohort.
Methods: We conducted a thorough literature search, covering prominent databases such as PubMed, Embase, Web of Science, SpringerLink, and the Cochrane Library. The search spanned from the inception of these databases up to December 5, 2023. Employing the 95% confidence interval and Hazard Ratio (HR), the study systematically evaluated the relationship between PNI and key prognostic indicators, including the objective remission rate (ORR), disease control rate (DCR), overall survival (OS) and progression-free survival (PFS) in GC/GEJC patients undergoing ICI treatment.
Results: Eight studies comprising 813 eligible patients were selected. With 7 studies consistently demonstrating superior Overall Survival (OS) in the high-Prognostic Nutritional Index (PNI) group compared to their low-PNI counterparts (HR 0.58, 95% CI: 0.47–0.71, P<0.001). Furthermore, the results derived from 6 studies pointed out that the significant correlation between he low-PNI and poorer progression-free survival (PFS) (HR 0.58, 95% CI: 0.47–0.71, P<0.001). Subgroup analyses were performed to validate the robustness of the results. In addition, we conducted a meta-analysis of three studies examining the correlation between PNI and objective response rate/disease control rate (ORR/DCR) and found that the ORR/DCR was significantly superior in the high PNI group (ORR: RR: 1.24, P=0.002; DCR: RR: 1.43, P=0.008).
Conclusion: This meta-analysis indicates that the low-PNI in GC/GEJC patients undergoing ICI treatment is significantly linked to worse OS and PFS. Therefore, PNI can serve as a prognostic indicator of post-treatment outcomes in patients with GC receiving ICIs. Further prospective studies are required to assess the reliability of these findings.
Systematic review registration: https://inplasy.com/, identifier INPLASY202450133.
1 Introduction
Gastric cancer is a malignant tumor that can aggressively invade the gastrointestinal tract and metastasize to distant sites. Globally, it ranks as the fifth most common cancer and the fourth leading cause of cancer-related mortality (1, 2). Although the incidence and mortality rates of gastric and gastroesophageal junction cancers have declined in recent years, these cancers still pose significant challenges and threats to human health (3). With changes in lifestyle habits, the incidence rate among younger populations is on the rise (4). Gastric and gastroesophageal junction cancers are characterized by insidious onset, rapid progression, high malignancy, and poor prognosis (5). The majority of patients diagnosed with gastric or gastroesophageal junction cancer are typically already in advanced stages of the disease at the time of diagnosis. As a result, the benefits of surgery are much lower compared to those for early-stage patients, and some may even lose the opportunity for curative surgery. In recent years, significant progress has been made in treating gastric and gastroesophageal junction cancers through combined chemotherapy and immunotherapy, particularly with the use of immune checkpoint inhibitors (ICIs) for advanced-stage cases (6). The combination of chemotherapy with immunotherapy and the integration of immune checkpoint inhibitors with other treatments have been continuously emerging (7). For example, The concurrent use of PD-1 and CTLA-4 inhibitors targets distinct stages of T cell activation., thereby producing a synergistic effect (8). Immune checkpoint inhibitors (ICIs) have become a mainstream treatment for various malignant tumors and have revolutionized traditional cancer therapy (9–13). ICIs have demonstrated significant advantages in improving the survival rates of patients with gastric or gastroesophageal junction cancer (14–17). In recent years, despite the improvement in prognosis for gastric or gastroesophageal junction cancer through the combination of chemotherapy and immune checkpoint inhibitors (ICIs), there has been an increasing incidence of immune-related adverse events (irAEs) associated with their use. Particularly, anti-CTLA-4 therapy appears to induce more severe irAEs compared to anti-PD-1/PD-L1 treatments, affecting multiple organ systems, and is more common in patients who respond well to treatment (18). As the approval of ICIs rapidly expands across various cancer types, knowledge regarding risk factors and biomarkers can help providers assess the risk of immune-related adverse events (irAEs) in patients. For instance, biomarkers such as cytokines (19), HLA (20), autoantibodies (21), gene expression profiles (22), among others, can aid in assessing the risk of irAEs. However, the above-mentioned biomarkers are costly and involve complex procedures. Additionally, tumor mutational burden (TMB) (23), tumor-infiltrating lymphocytes (24), microsatellite instability (MSI) (25), and other tumor biomarkers have been extensively studied as predictive biomarkers for PD-1/L1 inhibitor therapy. However, due to the relatively complex detection processes and a lack of consensus on numerical thresholds, their clinical application has been limited. Malnutrition accounts for 87% of gastric cancer patients, and the incidence of cachexia is as high as 65%-85%, exceeding all other tumors, and both malnutrition and cachexia incidence account for the first place of all tumors (26). The Prognostic Nutritional Index (PNI), initially proposed by the Buzby team (27), is used to assess overall nutritional and immune status. It quantifies serum albumin and peripheral blood lymphocyte count through a simple calculation. Clinicians can predict the risk of postoperative complications in surgical patients by assessing preoperative nutritional status (28). Evaluating the nutritional and immune status of the body holds significant clinical importance in predicting the prognosis of cancer patients (29–31). The mechanical obstruction and progression of gastric or gastroesophageal junction cancer deteriorate patients’ nutritional status, affecting serum albumin levels and impairing the host’s immune status (32). Additionally, lymphocytes, which are targeted by ICIs, inhibit tumors and play a crucial role in tumor immunity. Consequently, lymphocyte count is widely used as an indicator of immune competence (33). However, there is currently a lack of meta-analyses on the predictive significance of PNI in patients with gastric or gastroesophageal junction cancer undergoing ICI therapy. Therefore, we included relevant cohort studies to compare the prognosis and treatment response among different PNI groups of these patients after ICI treatment. This study aims to explore the prognostic value of PNI in this patient cohort.
2 Materials and methods
2.1 Search strategy
This meta-analysis strictly followed the guidelines specified in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (34). The PRISMA checklist ensures a comprehensive and transparent reporting of systematic reviews and meta-analyses, emphasizing methodological clarity and quality. The study protocol has been registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY)(Registration ID: INPLASY202450133). Thorough searches were conducted by two independent researchers, encompassing multiple databases, namely PubMed, Embase, Web of Science, SpringerLink, and the Cochrane Library. The search duration spanned from the establishment of these databases until December 13, 2023. It utilizes the following terms to investigate the predictive significance of PNI and ICIs in patients with gastric or gastro-esophageal junction cancer: “Prognostic Nutritional Index” or “PNI “ and “gastric” or “stomach” or “esophagogastric junction “or “esophageal” or “oesophageal” or “esophagus” or “esophageal” and “cancer” or “tumor “ or “carcinoma “ or “adenocarcinoma “ or “neoplasm” and “PD-L1 inhibitors” or “immune checkpoint inhibitors” or “programmed cell death ligand-1 inhibitors” or “immunotherapy” or “ICIs”. In addition to utilizing free search terms and Medical Subject Headings (MeSH) for searching within titles or abstracts, we screened the references of selected articles to ensure comprehensive retrieval.
2.2 Inclusion and exclusion criteria
Inclusion criteria: (1) The patient was diagnosed with GC/GEJC through a comprehensive evaluation, which included imaging studies, serum tumor marker tests, and a histopathological biopsy; (2) Received ICIs, either in combination with chemotherapy or as a stand-alone drug; (3) Provided survival data in the distant future such as overall survival (OS) or progression-free survival (PFS), and the existence of feedback of therapeutic data such as the objective remission rate (ORR) or the disease control rate (DCR); (4) Data such as HR and 95% CI can be obtained in the literature directly or indirectly;(5)The methods of study were either cohort study or randomized controlled study.
Exclusion criteria: (1) reviews, case reports, case series, conference abstracts, or commentaries; (2) data overlap or duplication; (3) the literature fails to provide complete raw data information.
2.3 Data extraction and quality assessment
Two researchers conducted independent literature searches, following predetermined criteria and specified strategies. This approach ensures a thorough and unbiased exploration of available literature, utilizing a systematic and structured methodology. Meticulous data extraction was performed, encompassing essential details such as the first author’s name, publication year, study country, design, sample size, gender distribution, treatment modalities, and survival analyses, including OS、PFS,、DCR、ORR. The quality of each study was meticulously evaluated using the Newcastle-Ottawa Scale (NOS) (35). Studies scoring above 6 points were considered high-quality indicators. This stringent evaluation ensures that only studies meeting robust methodological standards contribute to the overall analysis. Use the following equation to calculate the PNI value: 10 * serum albumin value + 0.005 * peripheral blood lymphocyte count. This standardized calculation method allows for consistent and comparable PNI values across the studies, enhancing the reliability and validity of the meta-analysis results.
2.4 Data statistics
Statistical analysis in this study was performed using Stata SE (version 12.0; StataCorp, College Station, Texas, USA). Heterogeneity among the studies was evaluated using Cochran’s Q-test and I2 statistics. In cases where heterogeneity was not significant (P≥0.10 or I²<50%), a fixed-effects model was applied; conversely, in the presence of significant heterogeneity (P<0.10 or I²≥50%), a random-effects model was employed for the meta-analysis. Effect sizes for dichotomous variables, such as ORR and DCR, were represented using RR along with their 95% CI. For survival data, including OS and PFS, Hazard Ratios (HR) and their 95% CI were utilized. The threshold for statistical significance was defined as P < 0.05. To evaluate publication bias, we examined the symmetry of the funnel plot and employed methods such as Egger’s linear regression and Begg’s regression, with a P-value < 0.05 indicating potential publication bias. A sensitivity analysis was conducted to assess the influence of individual studies on overall survival (OS) and progression-free survival (PFS).To explore the origins of heterogeneity, subgroup analyses were conducted, considering treatment modalities, sample sizes, cutoff values, and analytical models.
3 Results
3.1 Study selection and characteristics
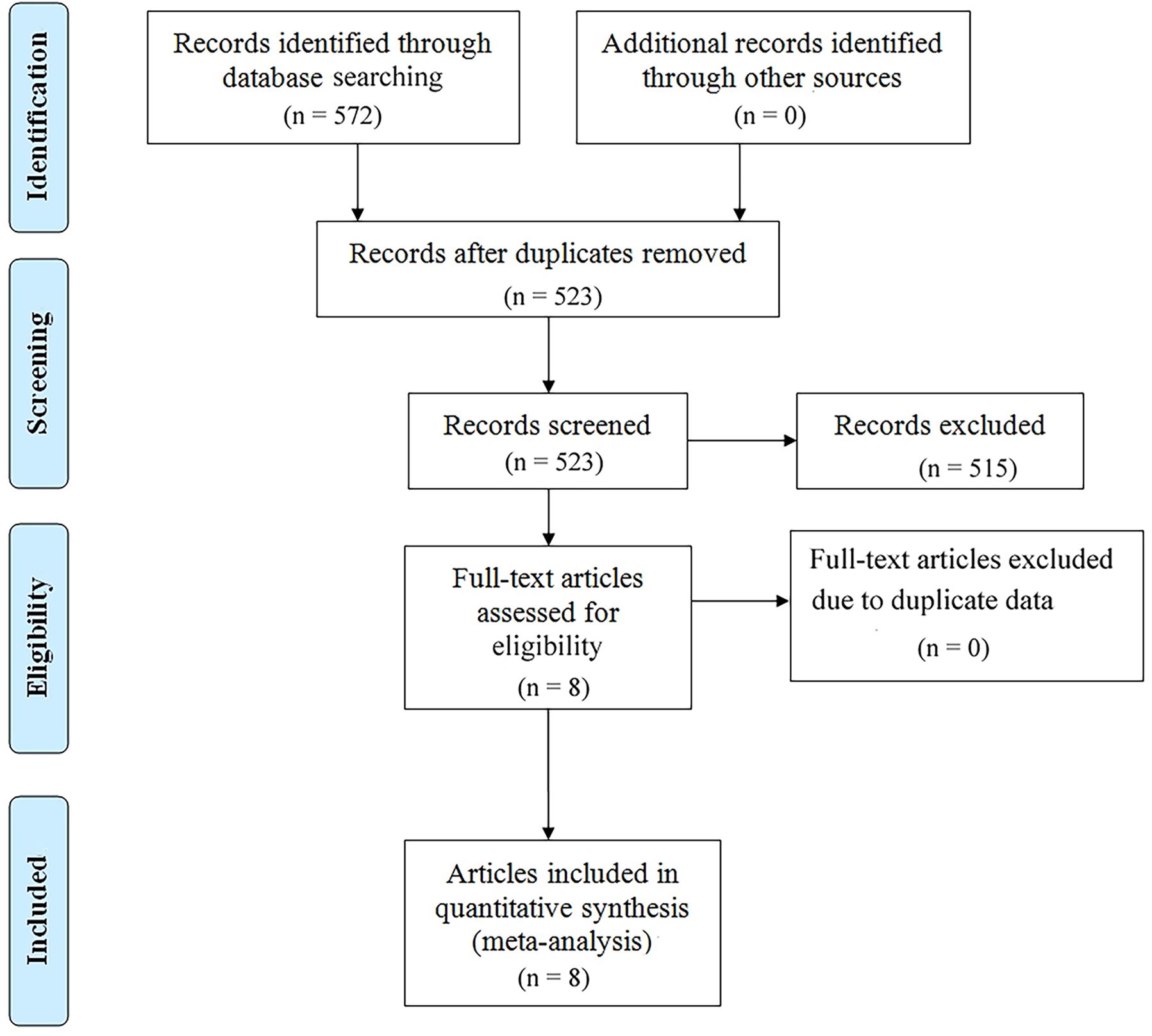
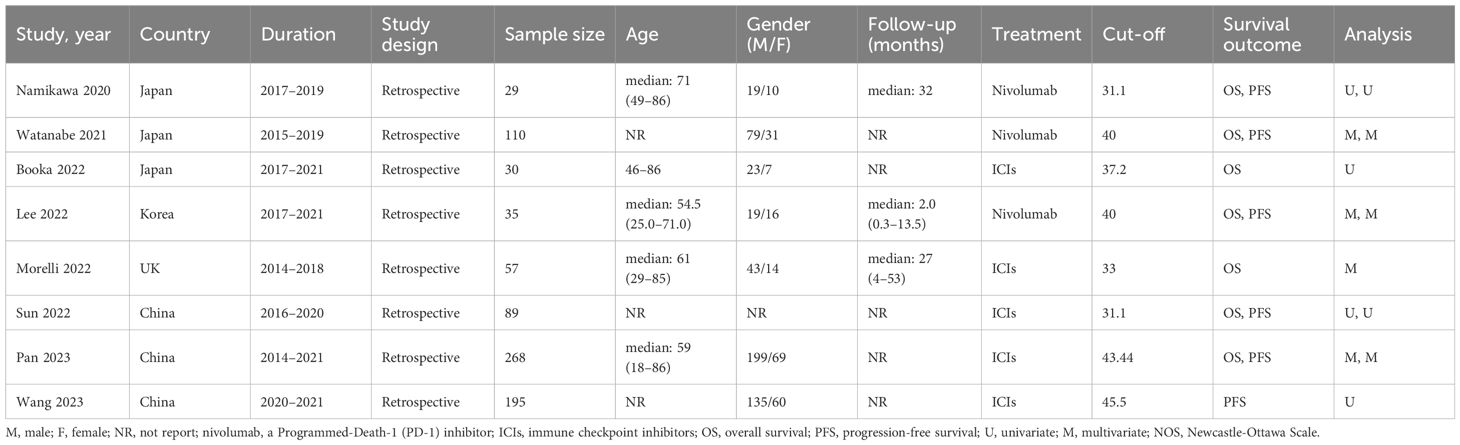
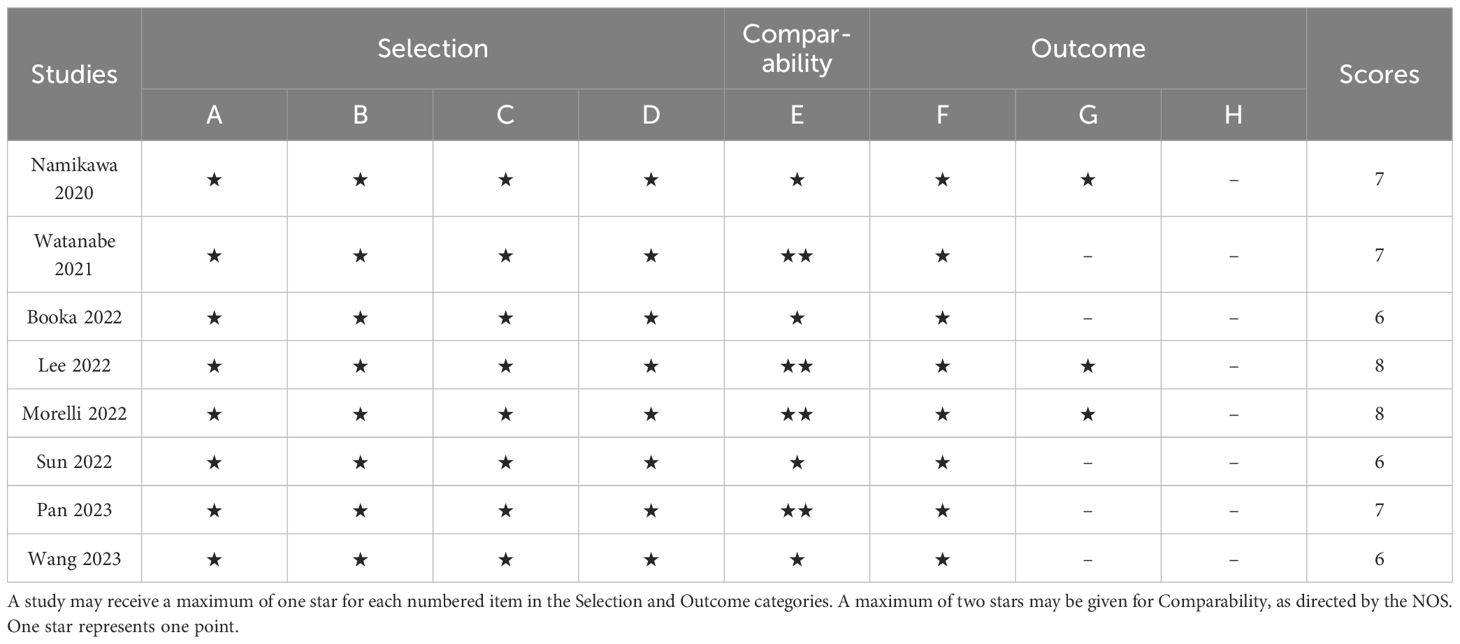
We searched PubMed, Embase, and Web of Science SpringerLink as well as Cochrane Library databases to find a total of 572 relevant papers, firstly, Based on the inclusion and exclusion criteria, 49 duplicates were removed from the dataset and then 515 papers were excluded by reviewing their abstracts, titles and other abbreviations, and finally, 8 articles were included after full-text review (36–43), These were retrospective studies with a total of 813 patients receiving ICIs. articles, These were retrospective studies, and a total of 813 patients with (GC/GEJC) treated with ICIs were included, and the flow chart is shown in Figure 1. Table 1 provides a summary of the characteristics of the included studies. All of these studies were published between 2020 and 2023, including three from China, three from Japan, one from the United States, and one from South Korea. All eight articles were retrospective studies. The sample size ranged from 29 to 268, totaling 813 patients. Five studies used ICIs alone and three studies used a combination of chemotherapy including ICIs. Two studies focused only on overall survival (OS), one study focused only on progression-free survival (PFS), and the remaining five studies documented both overall survival (OS) and PFS. According to the NOS rating sheet, all of the included studies scored between 6 and 8, indicating relatively high data quality. Table 2 presents the Newcastle-Ottawa Quality Assessment Scale (NOS) scores for all articles included in the analysis.
3.2 Prognostic impact of PNI on PFS and OS in GC/GEJC patients treated with ICIs
Seven studies with a total of 618 patients were analyzed for the correlation between PNI and OS, and the combined results showed that lower PNI was significantly correlated with poorer OS in patients (HR=0.58, 95%CI:0.47–0.71 p=0.001; Figure 2A). We also performed a correlation between PNI and PFS analysis, the combined results of 726 cases from 6 studies showed that high PNI before treatment was significantly correlated with better PFS in patients (HR=0.71 95% CI: 0.53–0.94, p=0.004; Figure 2B). In order to delve deeper into the prognostic influence of the prognostic nutritional index (PNI) on patients with gastric and gastroesophageal junction (GC/GEJC) cancers undergoing treatment with immune checkpoint inhibitors (ICIs) across various scenarios, and to identify potential sources of heterogeneity, subgroup analyses were conducted based on pre-established stratification criteria, and the results showed that, in terms of OS, both in terms of country (Japan, China, and other), sample size (>100, ≤100), cutoff value (>40, ≤40), and type of analysis (univariate group, multivariate group) Patients with low PNI had worse OS; while the results of subgroup analysis of PFS were not the same as those of OS, PNI did not accurately predict PFS in patients in (Japan, China) countries or with cutoff values <40 or sample sizes higher than 100 (Tables 3, 4).
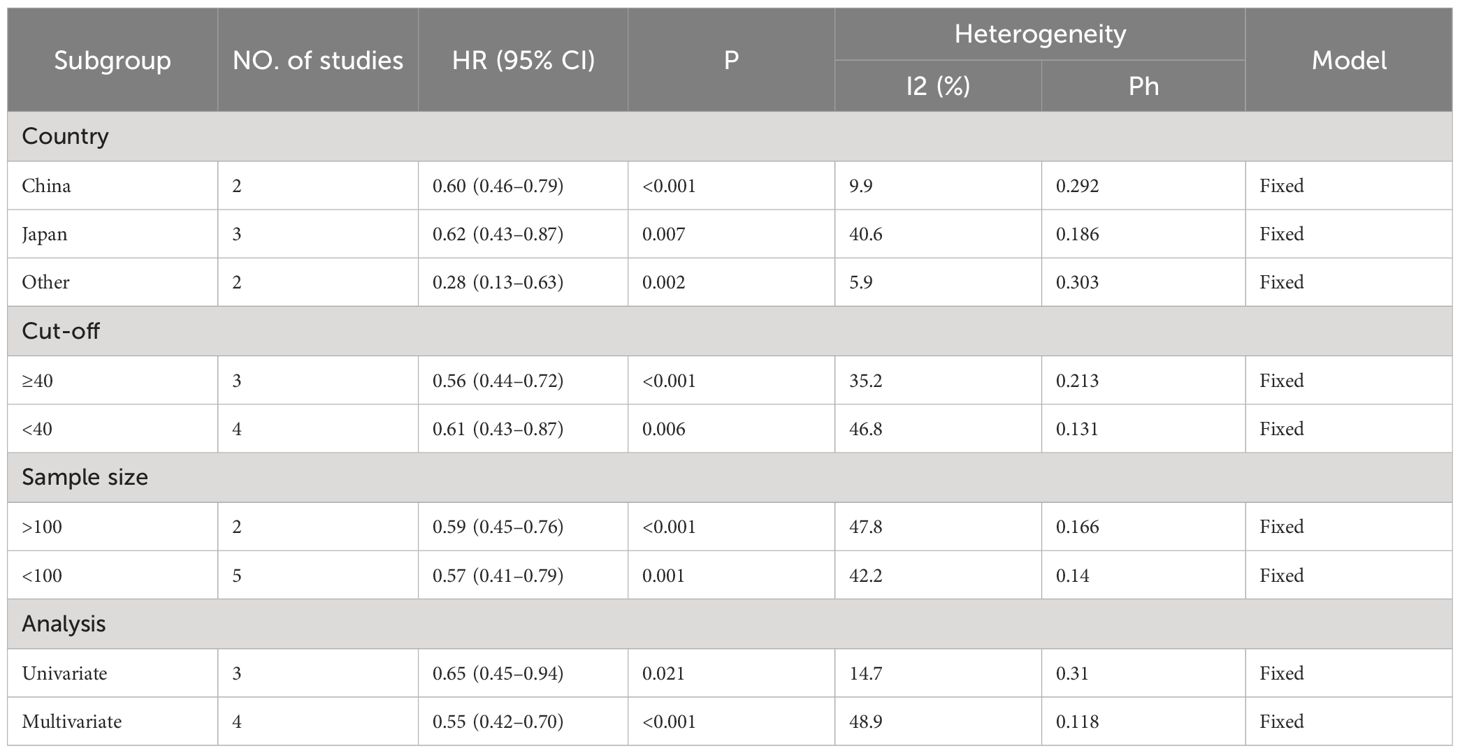
Table 3 Subgroup analysis evaluating the prognostic significance of PNI for OS in GC/GEJC patients treated with ICIs.
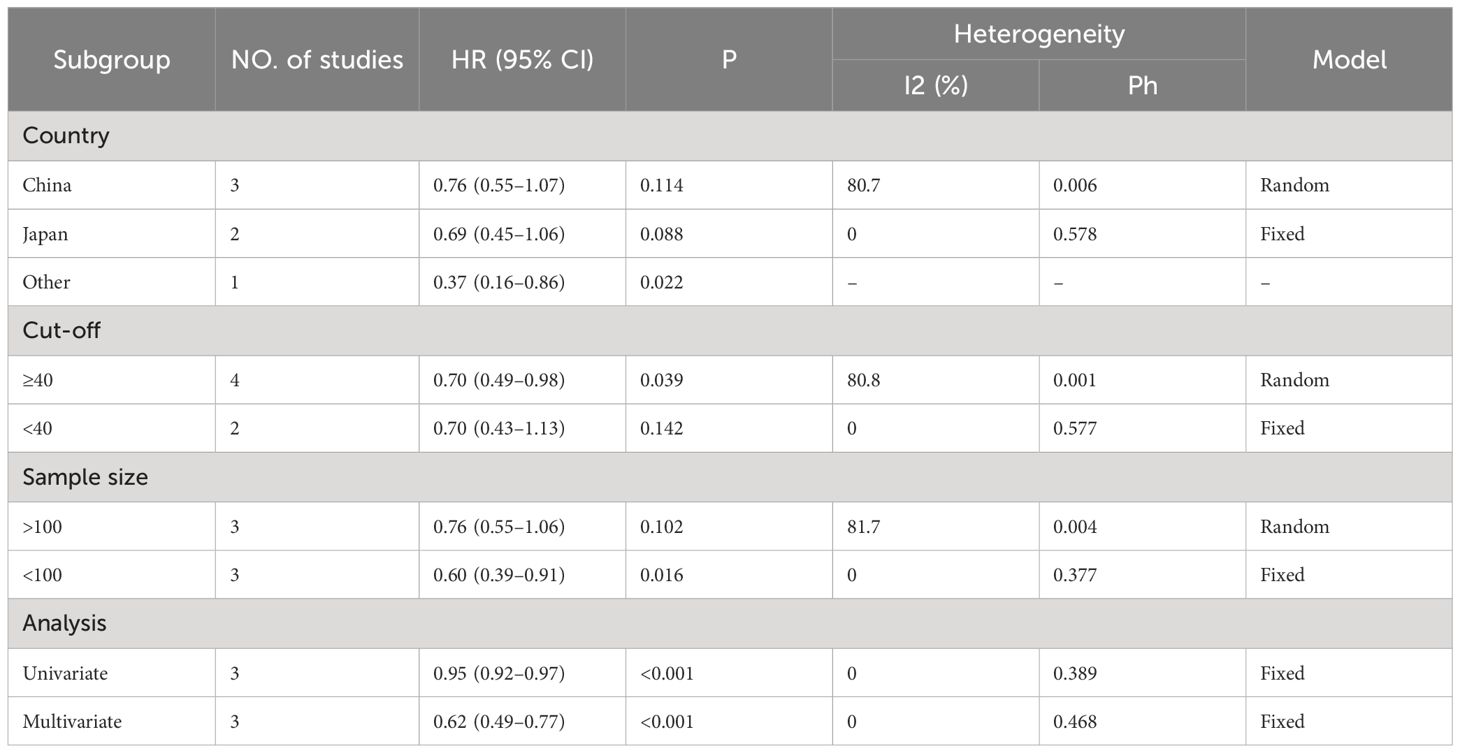
Table 4 Subgroup analysis evaluating the prognostic significance of PNI for PFS in GC/GEJC patients treated with ICIs.
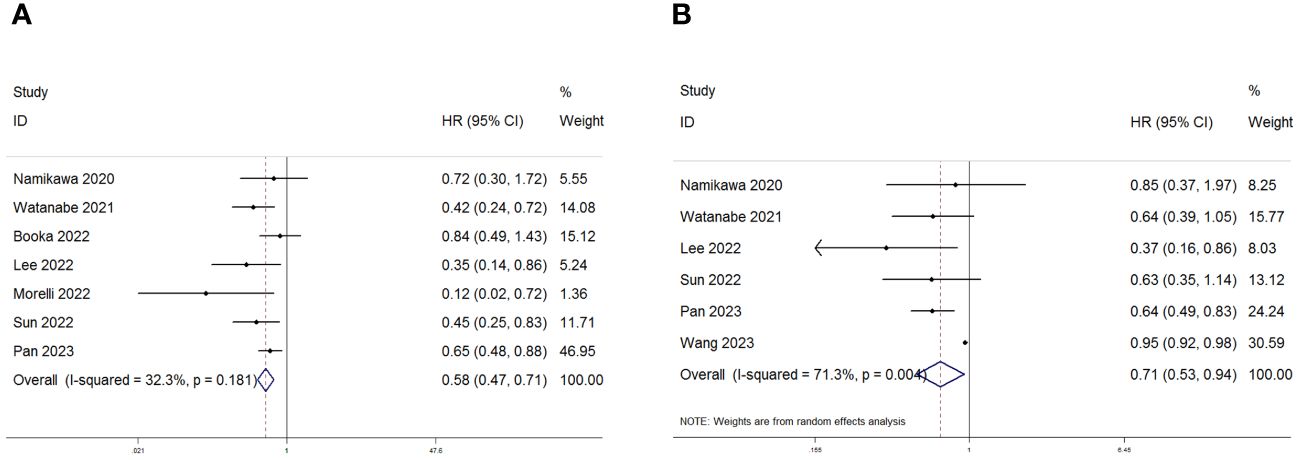
Figure 2 Forest plot for the association between PNI expression and (A) overall survival (OS) and (B) progression-free survival (PFS) in GC/GEJC patients receiving Immune Checkpoint Inhibitors (ICIs).
3.3 Publication bias and sensitivity analysis
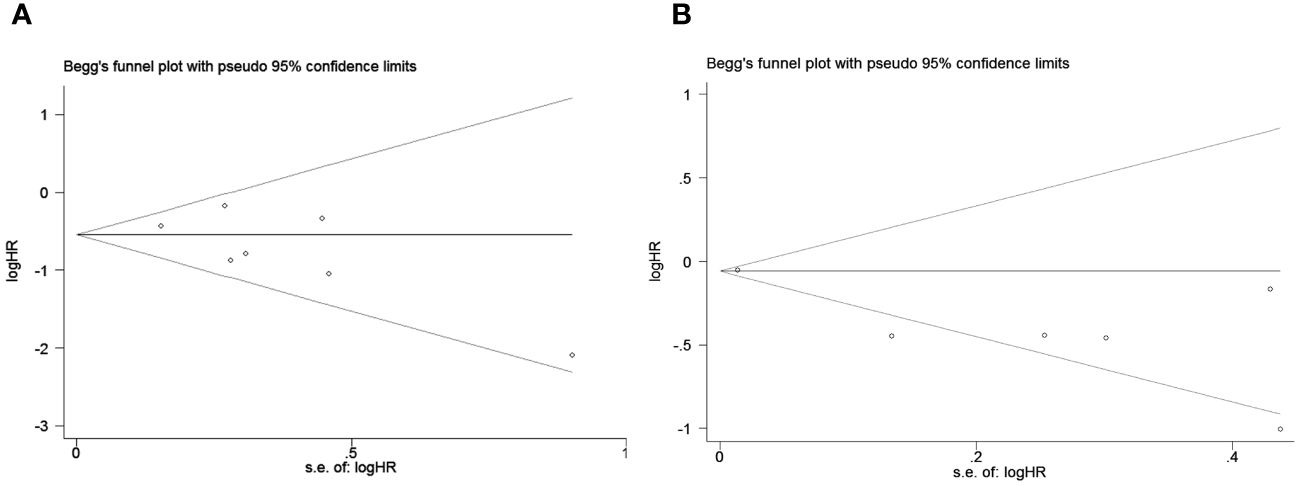
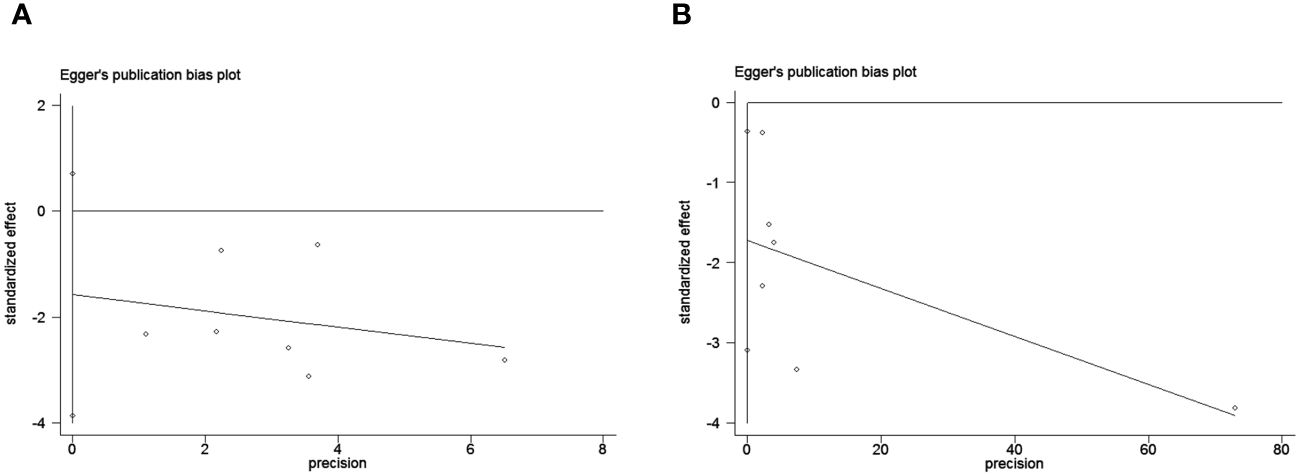
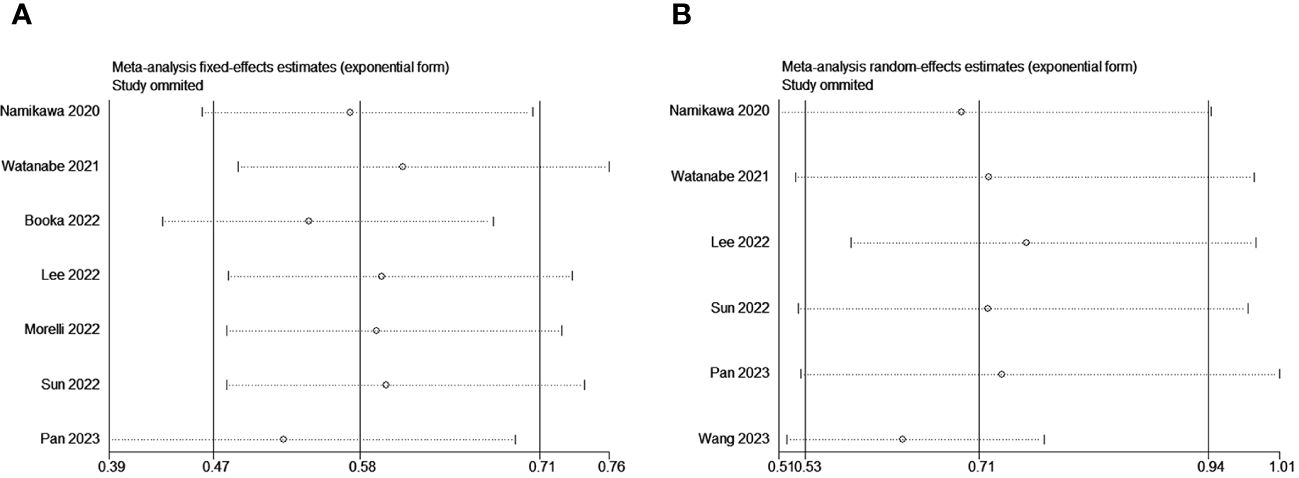
To assess potential publication bias, a combination of funnel plots, Begg test, and Egger’s test was employed. The funnel plots for OS exhibited a symmetrical pattern (Figure 3A). However, the symmetry of the funnel plot for progression-free survival (PFS) is not as effective as that for overall survival (OS) (Figure 3B). The Begg tests indicated no significant publication bias for OS or PFS (OS, p = 0.133; PFS, p = 1.000; Figure 4). The outcomes from the Egger (OS, p = 0.135; Figure 5A; PFS, p = 0.025; Figure 5B) tests revealed a probable publication bias inside the relevant PFS investigations. However, we then conducted a sensitivity analysis, that is, we eliminated each paper in turn and then conducted a summary analysis to see the impact on the final result. The results showed that no single study significantly affected the relationship between PNI and OS and patients’ progression-free survival (PFS)(Figure 6). This highlights the reliability of the observed correlation, as the results remain consistent and unaffected by individual studies.
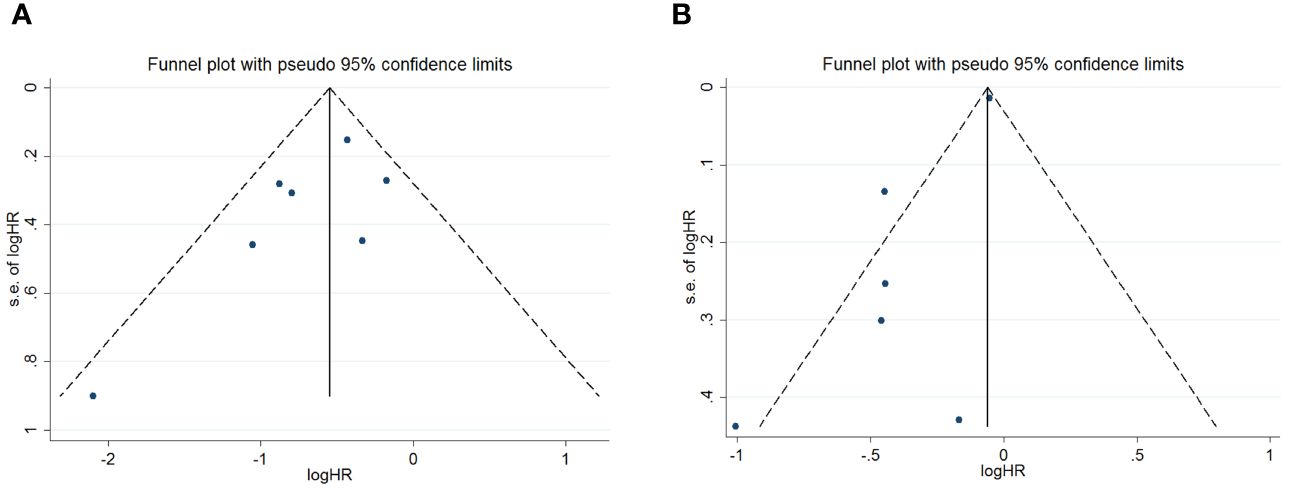
Figure 3 Funnel plots are utilized to assess the presence of publication bias in (A) overall survival (OS) and (B) progression-free survival (PFS).
3.4 PNI and ORR/DCR association
Among the eight studies examined, three specifically delved into the relationship between the PNI and the ORR and DCR. Consistently across these three studies, there was a unanimous reporting of a significant statistical correlation between PNI and both DCR and ORR. Subsequent meta-analysis further illuminated the elevated risk ratios (RR) for both ORR (RR=1.43, 95% CI: 1.10–1.87, P=0.008, I2 = 52.1%; Figure 7A) and DCR (RR=1.24, 95% CI: 1.08–1.41, P=0.002, I2 = 0%; Figure 7B). These results indicate a substantial increase in the likelihood of higher ORR and DCR associated with elevated PNI levels, as supported by the meta-analysis outcomes.
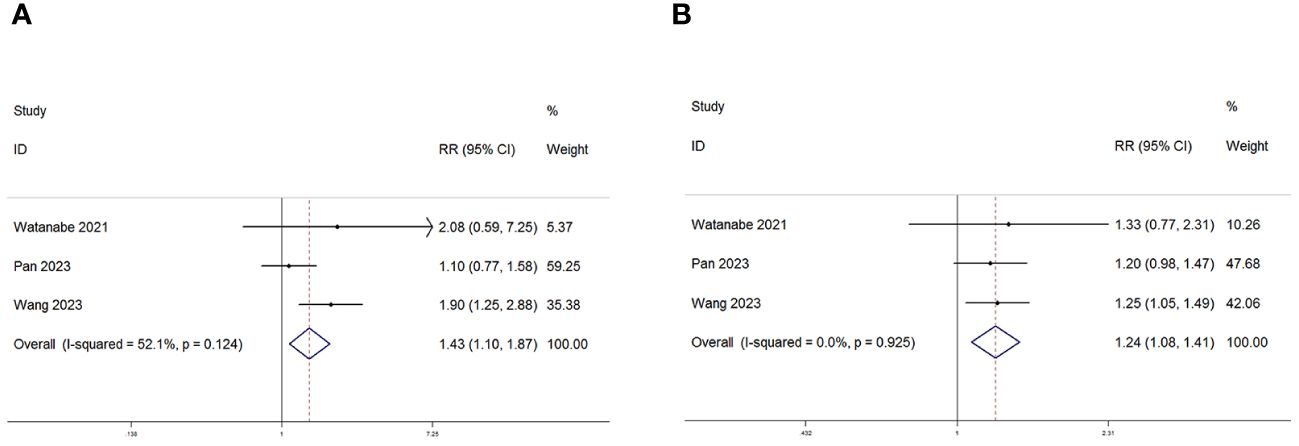
Figure 7 Forest plot for the association between PNI expression and (A) Objective response rate (ORR) and (B) Disease control rate (DCR) in GC/GEJC patients receiving Immune Checkpoint Inhibitors (ICIs).
4 Discussion
Gastric and gastroesophageal junction (GEJ) cancers are prevalent malignancies of the digestive tract. According to global cancer statistics, their incidence and mortality rates rank among the top five for all cancers (44–46). While the incidence and mortality rates of gastric cancer have been declining, the incidence of GEJ cancer has been gradually increasing (47). In recent years, immune checkpoint inhibitors (ICIs) have achieved significant advancements in cancer treatment, but their efficacy varies significantly among different patients (48). Additionally, there is limited ability to predict the survival outcomes of gastric and GEJ cancer patients treated with ICIs. Therefore, there is an urgent need to identify more effective, simplified, and precise indicators to assist clinicians in predicting post-treatment prognoses. Studies have demonstrated the prognostic significance of the prognostic nutritional index (PNI) as a biomarker in various cancer types (49–51). Nevertheless, the association between the prognostic nutritional index (PNI) and the prognosis of patients with gastric and GEJ cancer undergoing treatment with ICIs has not been conclusively documented, The aim of this meta-analysis is to explore the prognostic significance of PNI in gastric and GEJ cancer patients undergoing ICI therapy. According to our meta-analysis findings, a high prognostic nutritional index (PNI) serves as a favorable prognostic factor for cancer patients undergoing treatment with ICIs. Individuals with elevated PNIs tend to experience extended overall survival (OS) and progression-free survival (PFS) compared to those with lower PNIs. Additionally, they demonstrate increased objective response rates (ORR) and disease control rates (DCR).
As research into immunotherapy deepens, the immunotherapy strategies for gastric and gastroesophageal junction (GEJ) cancers are continually being refined. However, the factors influencing their efficacy remain unclear. Tumor mutational burden (TMB), tumor-infiltrating lymphocytes (TILs), microsatellite instability (MSI), and other tumor biomarkers have been extensively studied as predictive biomarkers for PD-1/L1 inhibitor therapy. However, their application in clinical settings is limited due to the relatively complex detection processes and the lack of consensus on numerical thresholds. The prognostic nutritional index (PNI) is a value derived from nutritional-related serological indicators, such as albumin and lymphocytes. The prognostic nutritional index (PNI) is computed using the formula: PNI = 10 * albumin value (g/dl) + 0.005 * total lymphocyte count in peripheral blood (per mm³).This index can reflect the prognosis of tumor patients (52). Low serum albumin is not only a marker of malnutrition but is also considered a biomarker of systemic inflammation, a view strongly supported by previous research (53). Studies have found that inflammation-related factors can hinder albumin synthesis, and oxidative stress can induce albumin denaturation. Both mechanisms play a crucial role in the rapid decline of serum albumin levels during inflammation (54, 55). In summary, the reduction of serum albumin reflects not only nutritional deficiencies but also a close association with systemic inflammation. Research indicates that serum albumin levels serve as an independent factor influencing the prognosis of patients with malignant tumors (56). Supported by previous research, the interference of inflammation-related factors with albumin synthesis and the denaturation of albumin induced by oxidative stress elucidate the mechanisms behind the rapid decline in serum albumin levels in individuals with inflammation. These insights highlight the multifaceted role of serum albumin as an important biomarker for nutritional status and systemic inflammation processes. Additionally, lymphocytes inhibit the occurrence and development of tumors through cytotoxicity in immune responses (57). Lee YJ’s team conducted a study showing that an elevation in peripheral blood lymphocyte counts before and after treatment correlated with improved progression-free survival (PFS) and overall survival (OS) in non-small cell lung cancer patients undergoing treatment with immune checkpoint inhibitors (ICIs) (58). Moreover, a meta-analysis investigating the relationship between pre-treatment neutrophil-to-lymphocyte ratio (NLR) and clinical outcomes of cancer immunotherapy revealed that a high pre-treatment NLR correlated with unfavorable outcomes of cancer immunotherapy (59).. Recent studies suggest that the activation or proliferation of lymphocytes at early stages can still contribute to improved responses to immunotherapy (60). In summary, it is not difficult to understand why the PNI can reflect the prognosis of tumor patients undergoing immunotherapy.
The objective of this study is to determine whether the prognostic nutritional index (PNI) can serve as a predictor of survival outcomes in patients diagnosed with gastric and gastroesophageal junction (GEJ) cancers undergoing treatment with immune checkpoint inhibitors (ICIs). We performed a comprehensive meta-analysis using data from eight pertinent trials, encompassing 813 patients from four different countries. Our analysis found that a high PNI is significantly associated with better survival rates, indicating that elevated PNI is significantly correlated with improved overall survival (OS) and progression-free survival (PFS) (OS: HR=0.58, 95% CI: 0.47–0.71, p=0.001; PFS: HR=0.71, 95% CI: 0.53–0.94, p=0.004). These results are consistent with most studies, including those by Lee (36) and Pan (43). However, they contradict Booka’s findings, where a retrospective analysis of 30 GEJ cancer patients treated with pembrolizumab or nivolumab showed no significant impact of PNI on OS (P=0.111) and PFS (P=0.381) (38). The discrepancy may be due to the small sample size in Booka’s study, which could introduce bias. Additionally, our subgroup analysis indicated that higher PNI values consistently correlate with better OS regardless of country (China, Japan, Other), sample size (≥100 or <100), cut-off value (>40 or ≤40), and analysis type (multivariate or univariate). Notably, the heterogeneity in PFS might be attributed to differences in country, a cut-off value less than 40, or a sample size greater than 100.To assess potential publication bias, we employed several methods, including funnel plot analysis, Begg’s test, and Egger’s test. Sensitivity analysis and assessment of publication bias further corroborated the steadfastness of the conclusions drawn in this meta-analysis. According to the meta-analysis results, elevated PNI levels significantly increase the likelihood of higher objective response rates (ORR) and disease control rates (DCR). Overall, this meta-analysis has clinical significance, emphasizing the crucial role of optimizing nutritional and immune levels in patients undergoing ICI treatment. The observed associations suggest that by enhancing these factors, the burden of tumors on the body can be alleviated, ultimately improving patient outcomes. Some studies indicate that providing immune-enhancing enteral nutrition is beneficial for improving clinical outcomes and reducing complications in gastric cancer (61, 62). These insights provide a valuable perspective for developing tailored strategies for managing gastric and gastroesophageal junction cancers (GC/GEJC) and integrating ICI treatments. It is important to acknowledge certain limitations when interpreting our findings. Firstly, The sample size in this analysis is relatively limited, with most data originating from Asian countries. Therefore, the value of PNI in European and other populations needs further exploration to determine its applicability across different demographics. Secondly, all included studies in this analysis were retrospective, with inevitable selection biases. Consequently, more high-quality, large-sample, prospective studies are needed in the future to confirm and refine our findings.
5 Conclusions
Lower pre-treatment PNI values in patients with gastric and gastroesophageal combined tumors are closely associated with poorer prognosis after patients are treated with ICIs, but further multicenter, prospective, large-data studies are needed to support the study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SH: Conceptualization, Writing – original draft, Writing – review & editing. DS: Investigation, Project administration, Writing – review & editing. RH: Conceptualization, Writing – original draft, Writing – review & editing. LL: Data curation, Formal analysis, Writing – review & editing. YZ: Data curation, Formal analysis, Writing – review & editing. JZ: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Youth Science Foundation Funding Program of Shandong First Medical University (Project No. 2022002) and the 2023 Youth Talent Promotion Project of Shandong Medical Association (Project No. 2023_LC_0228).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Sexton RE, Al HM, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. (2020) 39:1179–203. doi: 10.1007/s10555-020-09925-3
4. Jin G, Lv J, Yang M, Wang M, Zhu M, Wang T, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. (2020) 21:1378–86. doi: 10.1016/S1470-2045(20)30460-5
5. Lopez MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. (2023) 181:103841. doi: 10.1016/j.critrevonc.2022.103841
6. Pavlakis N, Tincknell G, Lim LE, Muro K, Obermannova R, Lorenzen S, et al. European-Australasian consensus on the management of advanced gastric and gastro-oesophageal junction cancer: current practice and new directions. Ther Adv Med Oncol. (2022) 14:7436390. doi: 10.1177/17588359221118874
7. Downs-Canner S, Mittendorf EA. Preoperative immunotherapy combined with chemotherapy for triple-negative breast cancer: perspective on the KEYNOTE-522 study. Ann Surg Oncol. (2023) 30:3166–69. doi: 10.1245/s10434-023-13267-z
8. Huang S, Zheng G, Yang K. Neoadjuvant PD-1/PD-L1 combined with CTLA-4 inhibitors for solid Malignancies: a systematic review and meta-analysis. World J Surg Oncol. (2023) 21:349. doi: 10.1186/s12957-023-03212-5
9. Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. (2020) 37:443–55. doi: 10.1016/j.ccell.2020.03.017
10. Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. (2020) 18:479–89. doi: 10.6004/jnccn.2020.7554
11. Reda M, Ngamcherdtrakul W, Nelson MA, Siriwon N, Wang R, Zaidan HY, et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat Commun. (2022) 13:4261. doi: 10.1038/s41467-022-31926-9
12. Fennell DA, Dulloo S, Harber J. Immunotherapy approaches for Malignant pleural mesothelioma. Nat Rev Clin Oncol. (2022) 19:573–84. doi: 10.1038/s41571-022-00649-7
13. Boukouris AE, Theochari M, Stefanou D, Papalambros A, Felekouras E, Gogas H, et al. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit Rev Oncol Hematol. (2022) 173:103663. doi: 10.1016/j.critrevonc.2022.103663
14. Zhao JJ, Yap D, Chan YH, Tan B, Teo CB, Syn NL, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. (2022) 40:392–402. doi: 10.1200/JCO.21.01862
15. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
16. Chen Y, Bai B, Ying K, Pan H, Xie B. Anti-PD-1 combined with targeted therapy: Theory and practice in gastric and colorectal cancer. Biochim Biophys Acta Rev Cancer. (2022) 1877:188775. doi: 10.1016/j.bbcan.2022.188775
17. Vafaei S, Zekiy AO, Khanamir RA, Zaman BA, Ghayourvahdat A, Azimizonuzi H, et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. (2022) 22:2. doi: 10.1186/s12935-021-02407-8
18. Geukes FM, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. Esmo Open. (2018) 3:e278. doi: 10.1136/esmoopen-2017-000278
19. Limagne E, Nuttin L, Thibaudin M, Jacquin E, Aucagne R, Bon M, et al. MEK inhibition overcomes chemoimmunotherapy resistance by inducing CXCL10 in cancer cells. Cancer Cell. (2022) 40:136–52. doi: 10.1016/j.ccell.2021.12.009
20. Naranbhai V, Viard M, Dean M, Groha S, Braun DA, Labaki C, et al. HLA-A*03 and response to immune checkpoint blockade in cancer: an epidemiological biomarker study. Lancet Oncol. (2022) 23:172–84. doi: 10.1016/S1470-2045(21)00582-9
21. Les I, Martinez M, Perez-Francisco I, Cabero M, Teijeira L, Arrazubi V, et al. Predictive biomarkers for checkpoint inhibitor immune-related adverse events. Cancers (Basel). (2023) 15:1629. doi: 10.3390/cancers15051629
22. Shi Y, Lei Y, Liu L, Zhang S, Wang W, Zhao J, et al. Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer. Cancer Med. (2021) 10:2216–31. doi: 10.1002/cam4.3649
23. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. (2018) 362:eaar3593. doi: 10.1126/science.aar3593
24. Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, et al. Impact of tumor-infiltrating T cells on survival in patients with Malignant pleural mesothelioma. J Thorac Cardiovasc Surg. (2008) 135:823–29. doi: 10.1016/j.jtcvs.2007.10.026
25. Luchini C, Bibeau F, Ligtenberg M, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. (2019) 30:1232–43. doi: 10.1093/annonc/mdz116
26. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–05.
27. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) 139:160–67. doi: 10.1016/0002-9610(80)90246-9
28. Liu X, Qiu H, Kong P, Zhou Z, Sun X. Gastric cancer, nutritional status, and outcome. Onco Targets Ther. (2017) 10:2107–14. doi: 10.2147/OTT.S132432
29. Liu X, Wu Z, Lin E, Li W, Chen Y, Sun X, et al. Systemic prognostic score and nomogram based on inflammatory, nutritional and tumor markers predict cancer-specific survival in stage II-III gastric cancer patients with adjuvant chemotherapy. Clin Nutr. (2019) 38:1853–60. doi: 10.1016/j.clnu.2018.07.015
30. Oh SE, Choi MG, Seo JM, An JY, Lee JH, Sohn TS, et al. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr. (2019) 38:870–76. doi: 10.1016/j.clnu.2018.02.015
31. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. (2011) 98:268–74. doi: 10.1002/bjs.7305
32. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
33. Huber C, Bobek N, Kuball J, Thaler S, Hoffarth S, Huber C, et al. Inhibitors of apoptosis confer resistance to tumour suppression by adoptively transplanted cytotoxic T-lymphocytes in vitro and in vivo. Cell Death Differ. (2005) 12:317–25. doi: 10.1038/sj.cdd.4401563
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
35. Moskalewicz A, Oremus M. No clear choice between Newcastle-Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. (2020) 120:94–103. doi: 10.1016/j.jclinepi.2019.12.013
36. Lee J, Choi SH, Baek JH, Baek DW, Kim JG, Kang BW. Clinical impact of prognostic nutrition index for advanced gastric cancer patients with peritoneal metastases treated nivolumab monotherapy. Chonnam Med J. (2022) 58:24–8. doi: 10.4068/cmj.2022.58.1.24
37. Watanabe H, Yamada T, Komori K, Hara K, Kano K, Takahashi K, et al. Effect of prognostic nutrition index in gastric or gastro-oesophageal junction cancer patients undergoing nivolumab monotherapy. In Vivo. (2021) 35:563–69. doi: 10.21873/invivo.12292
38. Booka E, Kikuchi H, Haneda R, Soneda W, Kawata S, Murakami T, et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Res. (2022) 42:2977–87. doi: 10.21873/anticanres.15781
39. Morelli C, Formica V, Patrikidou A, Rofei M, Shiu KK, Riondino S, et al. Nutritional index for immune-checkpoint inhibitor in patients with metastatic gastro-esophageal junction/gastric cancer. J Gastrointest Oncol. (2022) 13:2072–81. doi: 10.21037/jgo-22-217
40. Wang X, Liu X, Dai H, Jia J. Peripheral blood nutrient indices as biomarkers for anti−PD−1 therapy efficacy and prognosis in patients with advanced gastric cancer. Oncol Lett. (2023) 26:397. doi: 10.3892/ol.2023.13983
41. Namikawa T, Yokota K, Tanioka N, Fukudome I, Iwabu J, Munekage M, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today. (2020) 50:1486–95. doi: 10.1007/s00595-020-02048-w
42. Pan Y, Ma Y, Dai G. The prognostic value of the prognostic nutritional index in patients with advanced or metastatic gastric cancer treated with immunotherapy. Nutrients. (2023) 15:4290. doi: 10.3390/nu15194290
43. Sun H, Chen L, Huang R, Pan H, Zuo Y, Zhao R, et al. Prognostic nutritional index for predicting the clinical outcomes of patients with gastric cancer who received immune checkpoint inhibitors. Front Nutr. (2022) 9:1038118. doi: 10.3389/fnut.2022.1038118
44. Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. (2023) 401:1655–68. doi: 10.1016/S0140-6736(23)00620-7
45. Hess T, Maj C, Gehlen J, Borisov O, Haas SL, Gockel I, et al. Dissecting the genetic heterogeneity of gastric cancer. Ebiomedicine. (2023) 92:104616. doi: 10.1016/j.ebiom.2023.104616
46. Formica V, Morelli C, Patrikidou A, Shiu KK, Nardecchia A, Lucchetti J, et al. A systematic review and meta-analysis of PD-1/PD-L1 inhibitors in specific patient subgroups with advanced gastro-oesophageal junction and gastric adenocarcinoma. Crit Rev Oncol Hematol. (2021) 157:103173. doi: 10.1016/j.critrevonc.2020.103173
47. Wang C, Zhang J, Cai M, Zhu Z, Gu W, Yu Y, et al. DBGC: A database of human gastric cancer. PloS One. (2015) 10:e142591. doi: 10.1371/journal.pone.0142591
48. Jain P, Jain C, Velcheti V. Role of immune-checkpoint inhibitors in lung cancer. Ther Adv Respir Dis. (2018) 12:1825482181. doi: 10.1177/1753465817750075
49. Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: Review and meta-analysis. Clin Chim Acta. (2018) 486:303–10. doi: 10.1016/j.cca.2018.08.030
50. Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. (2016) 42:1176–82. doi: 10.1016/j.ejso.2016.05.029
51. Jiao H, Wang L, Zhou X, Wu J, Li T. Prognostic ability of nutritional indices for outcomes of bladder cancer: A systematic review and meta-analysis. Urol Int. (2023) 107:886–94. doi: 10.1159/000531884
52. Nakatani M, Migita K, Matsumoto S, Wakatsuki K, Ito M, Nakade H, et al. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus. (2017) 30:1–07. doi: 10.1093/dote/dox020
53. Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. (2021) 36:22–8. doi: 10.1002/ncp.10588
54. Coffelt SB, de Visser KE. Cancer: Inflammation lights the way to metastasis. Nature. (2014) 507:48–9. doi: 10.1038/nature13062
55. Bito R, Hino S, Baba A, Tanaka M, Watabe H, Kawabata H. Degradation of oxidative stress-induced denatured albumin in rat liver endothelial cells. Am J Physiol Cell Physiol. (2005) 289:C531–42. doi: 10.1152/ajpcell.00431.2004
56. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr Cancer. (2017) 69:1151–76. doi: 10.1080/01635581.2017.1367947
57. Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. (2018) 18:285. doi: 10.1186/s12885-018-4201-4
58. Lee YJ, Park YS, Lee HW, Park TY, Lee JK, Heo EY. Peripheral lymphocyte count as a surrogate marker of immune checkpoint inhibitor therapy outcomes in patients with non-small-cell lung cancer. Sci Rep. (2022) 12:626. doi: 10.1038/s41598-021-04630-9
59. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. (2020) 18:360. doi: 10.1186/s12916-020-01817-1
60. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. (2021) 21:345–59. doi: 10.1038/s41568-021-00347-z
61. Choi WJ, Kim J. Nutritional care of gastric cancer patients with clinical outcomes and complications: A review. Clin Nutr Res. (2016) 5:65–78. doi: 10.7762/cnr.2016.5.2.65
Keywords: gastric cancer, immune checkpoint inhibitors, gastro-esophageal junction cancer, prognostic nutritional index, meta – analysis
Citation: Hou S, Song D, Hao R, Li L, Zhang Y and Zhu J (2024) Prognostic relevance of prognostic nutritional indices in gastric or gastro-esophageal junction cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Immunol. 15:1382417. doi: 10.3389/fimmu.2024.1382417
Received: 05 February 2024; Accepted: 06 June 2024;
Published: 20 June 2024.
Edited by:
Paulo Rodrigues-Santos, University of Coimbra, PortugalReviewed by:
Fernando Mendes, Polytechnical Institute of Coimbra, PortugalSina Azadnajafabad, University of Leeds, United Kingdom
Copyright © 2024 Hou, Song, Hao, Li, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiankang Zhu, MjQ5MjE3NTIzQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shufu Hou
Shufu Hou Dandan Song
Dandan Song Ruiqi Hao
Ruiqi Hao Linchuan Li1,2
Linchuan Li1,2