- 1West China School of Pharmacy, Sichuan University, Chengdu, China
- 2Department of Clinical Pharmacy, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 4West China Biomedical Big Data Center, Sichuan University, Chengdu, China
Objective: To estimate the cost-effectiveness of adding serplulimab to chemotherapy for metastatic squamous non-small cell lung cancer (NSCLC) patients in a first-line setting from a Chinese perspective.
Methods: A three-health state partitioned survival model was constructed to simulate disease development. The clinical data used in the model were derived from the ASTRUM-004 clinical trial. Only direct medical costs were included, and the utilities were derived from published literature. The quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratio (ICER) were employed to evaluate health outcomes. Additionally, a sensitivity analysis was performed to verify the robustness of the results.
Results: Compared with chemotherapy alone, the addition of serplulimab resulted in an increase of 0.63 QALYs with an incremental cost of $5,372.73, leading to an ICER of $8,528.14 per QALY. This ICER was significantly lower than 3 times China’s per capita GDP. The one-way sensitivity analysis suggested that the utility of PFS was the most sensitive factor on ICERs, followed by the price of serplulimab.
Conclusion: The combination of serplulimab and chemotherapy has been shown to be a cost-effective initial treatment option for patients with metastatic squamous NSCLC with the commonly accepted willingness-to-pay threshold of 3 times the GDP per capita per QALY in China.
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide (1). The associated economic burden makes it a serious public health concern, particularly in Asia, where it carries the heaviest economic burden compared to other cancer types (2, 3). Non-small cell lung cancer (NSCLC) is the most common subtype of lung cancer and accounts for approximately 80% of lung cancer cases (4). The survival of metastatic NSCLC patients has significantly improved due to the rapid progression of targeted drugs (5).
Nevertheless, EGFR mutations or ALK rearrangements are uncommon in squamous NSCLC, which accounts for approximately 25%-30% of all NSCLC cases, and is linked with a worse prognosis in comparison to lung adenocarcinoma (6). Immunotherapy presents a potential alternative to traditional treatment methods for patients lacking gene mutations or alterations. The substantial advancements achieved with immune checkpoint inhibitors have notably revolutionized the medical management of lung cancer, fostering a more effective and personalized approach (7). Pembrolizumab was the first programmed cell death protein-1(PD-1) inhibitor approved by the Food and Drug Administration (FDA) combined with chemotherapy for metastatic squamous NSCLC as a first-line treatment (8). Several domestic PD-1 inhibitors, such as sintilimab, tislelizumab, and camrelizumab, have received approval from the National Medical Products Administration (NMPA) for use in combination with chemotherapy for treating metastatic squamous NSCLC (9–11). However, the approval of these inhibitors was based on clinical studies that exclusively involved Chinese patients with metastatic squamous NSCLC. The Phase III IMpower131 trial reported no significant improvement in the overall survival (OS) of patients with metastatic squamous NSCLC when atezolizumab was added to platinum-based chemotherapy (12). Hence, there continues to be a global shortage of immune checkpoint inhibitors for patients with advanced squamous NSCLC.
In a recent study, the randomized, double-blind, phase III trial ASTRUM-004, conducted at 85 hospitals and academic research centers across 6 countries, assessed the efficacy of combining serplulimab with platinum-based chemotherapy in previously untreated patients with metastatic squamous NSCLC (13). Serplulimab significantly prolonged the median progression-free survival (PFS) by 2.6 months (8.3 months vs 5.7 months, hazard ratio (HR), 0.53; 95% confidence interval (CI), 0.42–0.67), and the median OS by 4.5 months (22.7 months vs. 18.2 months, HR, 0.73, IC, 0.58–0.93). Thus, serplulimab was approved in combination with chemotherapy for patients with metastatic squamous NSCLC by the NMPA in China.
Despite the considerable efficacy of serplulimab, its cost and occurrence of immune-related adverse events (AEs) may impose an additional financial burden on patients with metastatic squamous NSCLC. Hence, it has become essential to assess the economic implications of serplulimab. The aim of our analysis was to evaluate the cost-effectiveness of combining serplulimab with chemotherapy for metastatic squamous NSCLC patients without EGFR or ALK genomic tumor aberrations from the perspective of the Chinese healthcare payer.
Methods
Clinical information
The criteria for inclusion in the ASTRUM-004 trial included patients diagnosed with stage IIIB-IV squamous NSCLC, aged 18 or older, lacking known EGFR mutations or ALK/ROS1 fusions, and without a history of systemic therapy for metastatic disease (13). In total, 809 patients were enrolled and randomized at a 2:1 ratio into two treatment groups. The treatment regimens for metastatic squamous NSCLC patients included the administration of carboplatin (at an area under the curve of 5-6 mg/mL per min on day 1) and albumin-bound paclitaxel (100 mg/m2 on days 1, 8, and 15) during a 3-week cycle for 4-6 cycles. Additionally, patients received either serplulimab (4.5 mg/kg on day 1) or a placebo for a maximum period of 2 years. The median follow-up duration was 31.1 months (range 0.2–41.6). Patients in the trial group received a median of 9.0 cycles of serplulimab. In the serplulimab plus chemotherapy group, patients received a median of 11.0 cycles of nab-paclitaxel, while in the placebo plus chemotherapy group, the median number of cycles was 10.0. Additionally, both groups of patients received a median of 4 cycles of carboplatin treatment.
Model overview and model transition probabilities
A partitioned survival model was constructed using TreeAge Pro 2020 software (TreeAge, Williamstown, MA, USA) to estimate the cost-effectiveness of adding serplulimab to chemotherapy regimen. Three exclusive states were included in the model: PFS, progressed disease (PD), and death (Figure 1). One month was set as a cycle in the model and the time horizon was 10 years. Quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs) were calculated as health outcomes.

Figure 1 The structure of the partitioned survival model for metastatic squamous NSCLC. PFS, progression-free. PD, progression.
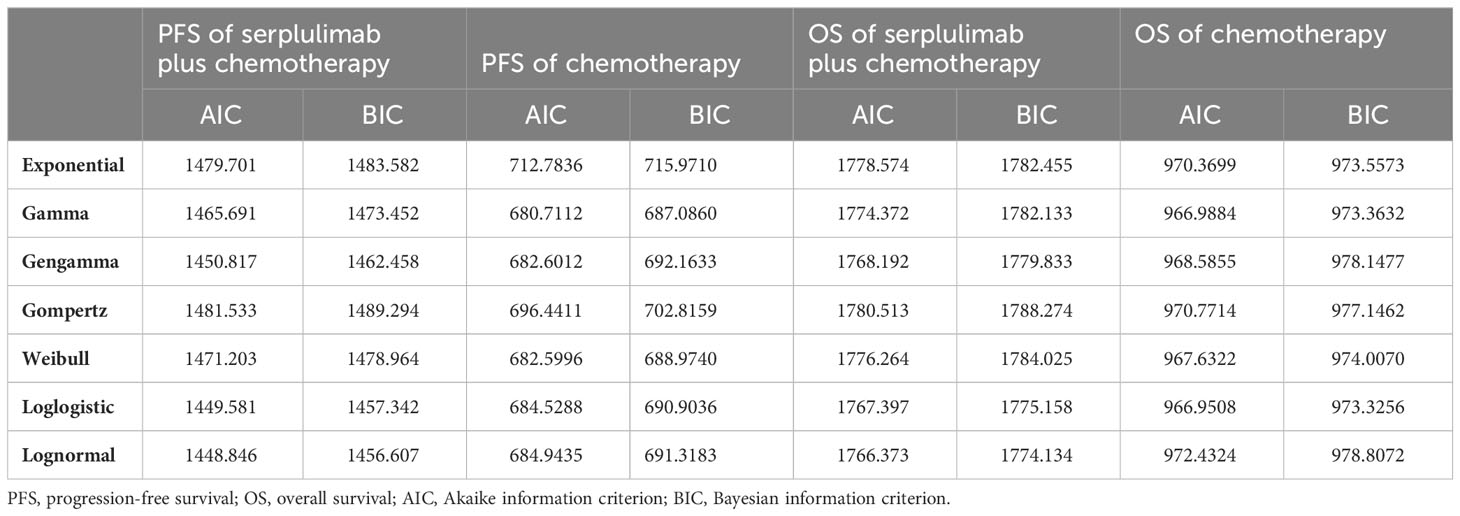
The Kaplan-Meier survival information for PFS and OS was derived from the ASTRUM-004 trial using the GetData graph digitizer (version 2.26, http://getdata-graph-digitizer.com/). Subsequently, individual patient data were reconstructed using the digitized R package (14). Survival probabilities were estimated using various distribution models, including Weibull, log-logistic, log-normal, gompertz, exponential, gengamma and gamma models. The optimal fitting distribution was evaluated through the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). The results of the survival curve simulation are shown in Figure 2 and Table 1. The best fitting distribution parameters for the PFS and OS data in the combination group were lognormal distributions. The most appropriate distribution parameters for the PFS and OS data in the chemotherapy alone group were the gamma and log-logistic distributions, respectively.

Figure 2 (A)The exploration of survival curves for PFS. (B) The exploration of survival curves for OS. PFS, progression-free. OS, overall survival.
Cost and utility estimates
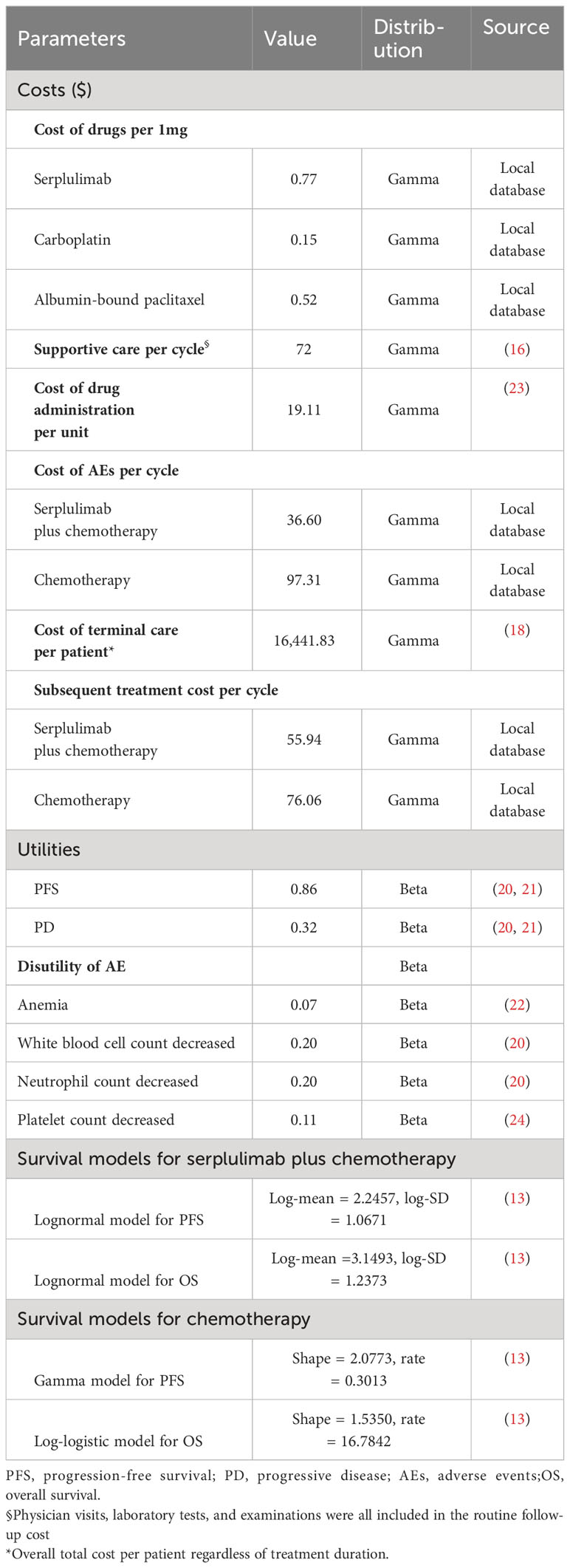
Direct costs, including the cost of medicine, cost of supportive care, cost of terminal care, and management cost of necessary AEs, were calculated from the Chinese payer perspective. In our model, we assumed that the average weight of the patients was 65 kg, with an average body surface area of 1.8m2 and a creatinine clearance rate (CCR) of 90 ml/min/1.73 m2 (15). The price of serplulimab and the prices of chemotherapy were sourced from the Chinese Drug Bidding Database (https://www.yaozh.com/). The costs associated with supportive care, drug administration, and terminal care were obtained from published literature (16–18). Metastatic squamous NSCLC patients in the placebo group were eligible to receive serplulimab monotherapy treatment after disease progression. Due to the limited information on second-line therapies, we assumed that the other patients had been treated with nivolumab, tislelizumab, or docetaxel according to clinical guidelines (19). All costs were converted to US dollars ($1 = ¥7.21), and the details of costs are presented in Table 2. The health utility values used in our study were all from the published literature. The utility value in the PFS state was 0.86, and the utility in the PD state was 0.32 (20, 21). Additionally, we determined the disutility values associated with AEs from previous literature (20, 22). The annual discount rate for both costs and utilities was set at 5%. As per the World Health Organization (WHO) recommendation, the willingness-to-pay (WTP) threshold was set at three times the gross domestic product (GDP) per capita in China ($38,052/QALY).
Sensitivity analysis
The robustness of the model was verified using one-way sensitivity analysis and probabilistic sensitivity analysis. In the one-way sensitivity analysis, model parameters were adjusted within a range of ±20% from their baseline values. For the probabilistic sensitivity analysis, 1,000 Monte Carlo simulations were conducted using parameters with specific probability distributions. A cost-effectiveness acceptability curve was generated to illustrate the ICERs across different WTP thresholds.
Results
Base case results
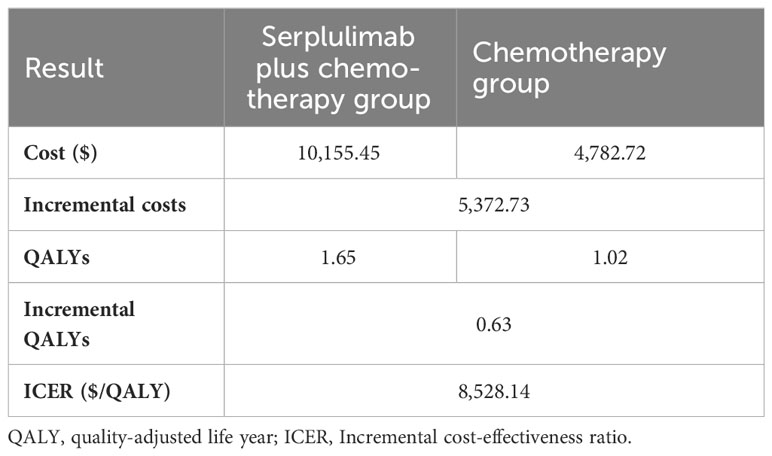
As shown in Figure 1, a partition survival model was built to assess the cost effectiveness of serplulimab plus chemotherapy and chemotherapy alone. The base case results of the two treatment regimens are presented in Table 3. Compared with chemotherapy alone, the addition of serplulimab resulted in an additional 0.63 QALYs (1.65 versus 1.02 QALYs), with costs increased by $5,372.73 ($10,155.45 versus $4,782.72), leading to an ICER of 8,528.14 per QALY. The ICER was lower than the WTP threshold, suggesting that the use of serplulimab for the first-line treatment of metastatic squamous NSCLC was cost-effective.
Sensitivity analysis
A one-way sensitivity analysis was conducted to explore the effects of parameter variations on the analysis results in this model. The findings are visualized in a tornado diagram (Figure 3), revealing that the utility of PFS and the cost of serplulimab had the most substantial impact on the ICER. Conversely, the costs associated with treating AEs and the expenses related to supportive care in both treatment regimens had a lesser impact on the ICER. Overall, the ICER of serpalimab plus chemotherapy compared with chemotherapy alone was less than $38,052 per QALY, despite fluctuations in individual parameters. The cost-effectiveness acceptability curve is shown in Figure 4, which illustrates the cost-effectiveness probability of serplulimab plus chemotherapy under different WTP thresholds. The cost-effectiveness acceptability curve indicated that at a threshold of $17,500, the probability of serplulimab plus chemotherapy being cost-effective was 99.9%.
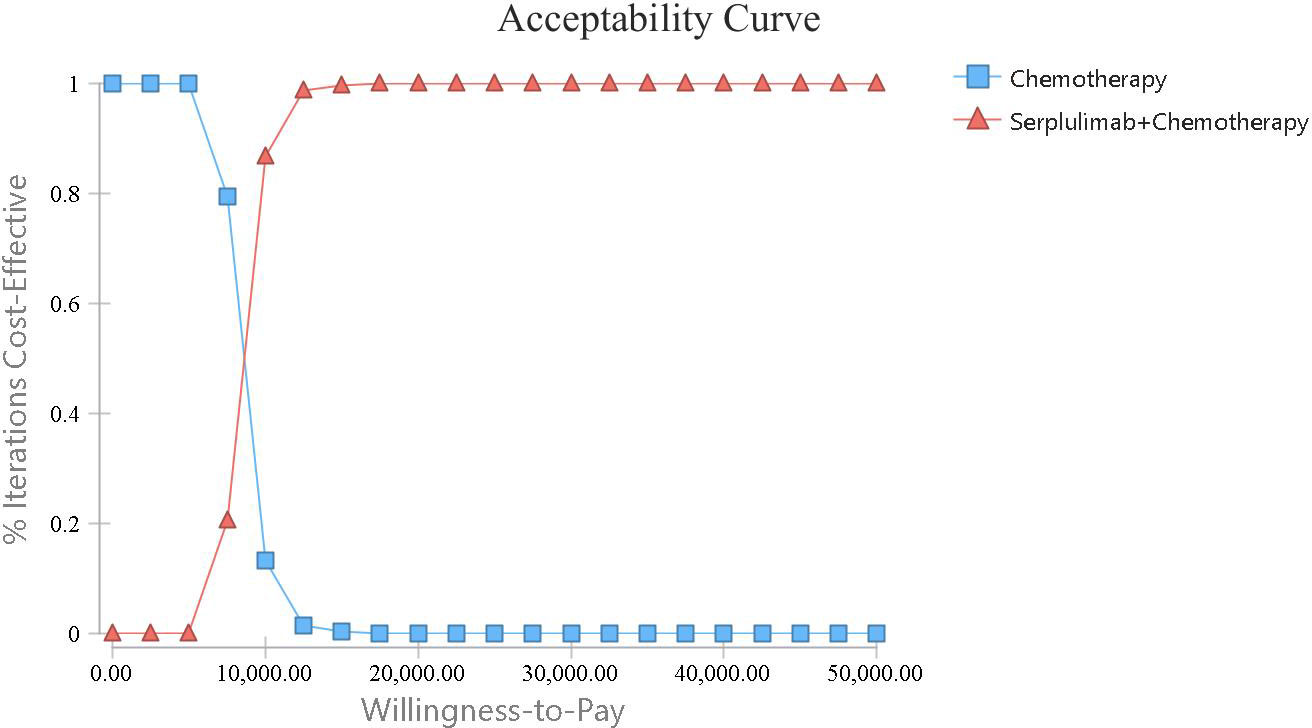
Figure 3 The cost-effectiveness acceptability curve comparing the addition of serplulimab to chemotherapy with chemotherapy alone.

Figure 4 The Tornado diagram.PFS, progression-free survival; PD, progressive disease; AEs, adverse events.
Discussion
Lung cancer contributes to approximately 25% of all cancer disability-adjusted life years (DALYs), with the greatest DALY burden in China (25). NSCLC, which includes squamous and nonsquamous cell carcinomas, is the most prevalent type of lung cancer, and each of these types of NSCLC has distinct biological characteristics (4). Squamous NSCLC is generally more aggressive than nonsquamous NSCLC (26). The difference in the immune microenvironment between squamous and nonsquamous NSCLC may impact immunotherapy outcomes in patients treated with PD-1/PD-L1 inhibitors (27). On a global scale, there is still a relative shortage of immunotherapeutic drugs available for treating metastatic squamous NSCLC. The ASTRUM-004 trial, an international multicenter phase III study, demonstrated the efficacy of serplulimab in metastatic squamous NSCLC, thereby presenting a novel treatment option for these patients. There is an urgent need to assess the cost-effectiveness of serplulimab in metastatic squamous NSCLC.
To the best of our knowledge, our study represents the first cost-effectiveness analysis of combining serplulimab with chemotherapy for metastatic squamous NSCLC patients in China. Our analysis indicated that the addition of serplulimab to chemotherapy was cost-effective, with an ICER of $8,528.14 per QALY, which was significantly lower than the WTP threshold. The model’s robustness was confirmed through one-way sensitivity and probabilistic sensitivity analyses. When the threshold of WTP was set as one time the GDP per capita, the probability of cost-effectiveness of serplulimab plus chemotherapy exceeded 99.0%. The one-way sensitivity analysis demonstrated that the utility of PFS was the most sensitive factor, followed by the cost of serplulimab. When utility of PFS ranged from 0.688 to 1, the ICERs ranged from $7,242.32 to $10,725.64, which was also below the WTP. Currently, serplulimab is not covered by health insurance in China, but there is a charitable drug donation program. If serplulimab is included in medical insurance through price negotiations, its price is expected to decrease in the future. Overall, serplulimab presents an affordable treatment option for Chinese metastatic squamous NSCLC patients.
The financial burden associated with treating lung cancer is substantial, often exceeding the annual household incomes of several families (28). In recent years, the emergence of immunotherapy drugs has significantly transformed cancer treatment. However, the use of these innovative drugs raises costs, further compounding the economic burden on families and society (29). To reduce the cost of cancer treatment, China has implemented a series of policies, including price negotiations. Following national price negotiations, the prices of innovative anti-cancer drugs have significantly decreased, thereby enhancing accessibility and affordability (30). By 2021, camrelizumab, sintilimab, and tislelizumab were included in the National Medical Insurance List in China (31). A decrease in the price of domestic PD-1 inhibitors plays a crucial role in diminishing national healthcare spending and easing the financial burden on patients. The addition of camrelizumab to chemotherapy was considered cost-effective from a healthcare perspective in China for metastatic NSCLC patients (32). Zhao et al. discovered that the combination of camrelizumab with paclitaxel-based chemotherapy, followed by docetaxel, within a selection of 11 treatment sequences for metastatic NSCLC patients, represented a cost-effective treatment option and tislelizumab was deemed the most effective and cost-effective second-line option (33). The combination of sintilimab and chemotherapy was proven to be more cost-effective than the combination of pembrolizumab and chemotherapy for patients with metastatic NSCLC in China, as the QALYs obtained in the sintilimab group were similar to those in the pembrolizumab group, with substantially lower costs (34).
Our research has the following limitations. First, as the utilities of patients treated with serplulimab have not been publicly reported, we obtained the utilities in our study from previous literature. The sensitivity analysis revealed that the PFS utility was the main influencing factor on the results, so it may have a certain impact on the model results. However, fluctuations in PFS within a range of ±20% did not affect the results of the study. Second, due to the lack of data on subsequent treatment in the ASTRUM-004 trial, we assumed that patients in this model received second-line treatment recommended by guidelines, which may not reflect clinical practice. Third, the use of parametric simulations to extrapolate PFS and OS for long-term survival introduced uncertainty. Nevertheless, the best fit parameter model was selected based on the AIC and BIC criteria. Forth, although sensitivity analyses demonstrated the robustness of the results, the differences in costs and health care in different countries may affect the validity and generalizability of our results. Finally, our analysis primarily depended on data from the ASTRUM-004 trial. In the real world, clinical outcomes may be influenced by a variety of factors, including organ function, medication adherence, and race. The effectiveness and economy of serplulimab in advanced squamous NSCLC patients still needs long-term follow-up and validation in clinical practice. Despite these limitations, our results have great significant of the selection of medicine for metastatic squamous NSCLC in China.
Conclusion
In conclusion, the addition of serplulimab to chemotherapy, compared with chemotherapy alone, is cost-effective for first-line treatment of metastatic squamous NSCLC from the perspective of the Chinese health system. The study’s conclusions are beneficial for guiding clinical decision-making and allocating health resources.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HZ: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft. YZ: Data curation, Visualization, Writing – review & editing. FW: Formal analysis, Project administration, Writing – review & editing. MH: Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Med-X Center for Informatics funding project (YGJC009).
Acknowledgments
This research was supported by National Key Clinical Specialties Construction Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chen SM, Cao Z, Prettner K, Kuhn M, Yang JT, Jiao LR, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. (2023) 9:465–72. doi: 10.1001/jamaoncol.2022.7826
3. Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. (2023) 20:624–39. doi: 10.1038/s41571-023-00798-3
4. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature.(2018) 553:446–54. doi: 10.1038/nature25183
5. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. (2020) 383:640–9. doi: 10.1056/NEJMoa1916623
6. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn PA Jr., et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. (2018) 13:165–83. doi: 10.1016/j.jtho.2017.11.111
7. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. (2020) 198:897–907. doi: 10.1007/s00408-020-00407-5
8. Yuan H, Liu J, Zhang J. The current landscape of immune checkpoint blockade in metastatic lung squamous cell carcinoma. Molecules. (2021) 26:1392. doi: 10.3390/molecules26051392
9. Ren SX, Chen JH, Xu XX, Jiang T, Cheng Y, Chen GY, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol. (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
10. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol. (2021) 16:1501–11. doi: 10.1016/j.jtho.2021.04.011
11. Wang J, Lu S, Yu XM, Hu YP, Sun YP, Wang ZJ, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
12. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. (2020) 15:1351–60. doi: 10.1016/j.jtho.2020.03.028
13. Zhou C, Hu Y, Arkania E, Kilickap S, Ying K, Xu F, et al. A global phase 3 study of serplulimab plus chemotherapy as first-line treatment for advanced squamous non-small-cell lung cancer (ASTRUM-004). Cancer Cell. (2023) 42:198–208.e3. doi: 10.1016/j.ccell.2023.12.004
14. Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodology. (2012) 12:9. doi: 10.1186/1471-2288-12-9
15. Zhu C, Xing XX, Wu B, Liang G, Han G, Lin CX, et al. Cost-effectiveness analysis of camrelizumab plus chemotherapy vs. Chemotherapy alone as the first-line treatment in patients with IIIB-IV non-squamous non-small cell lung cancer (NSCLC) without EGFR and ALK alteration from a perspective of health - care system in China. Front Pharmacol. (2021) 12:735536. doi: 10.3389/fphar.2021.735536
16. Gu XH, Zhang Q, Chu YB, Zhao YY, Zhang YJ, Kuo D, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. (2019) 127:84–9. doi: 10.1016/j.lungcan.2018.11.029
17. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. (2018) 6:124. doi: 10.1186/s40425-018-0440-9
18. Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Ejzykowicz F, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr Med Res Opin. (2019) 35:1241–56. doi: 10.1080/03007995.2019.1571297
19. Oncology Society of Chinese Medical A, Chinese Medical Association Publishing H. Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer. 2023 edition. Zhonghua Zhong Liu Za Zhi (2023) 45:539–74. doi: 10.3760/cma.j.cn112152-20230510-00200
20. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: An international study. Asia-Pac J Clin Onco. (2017) 13:E195–203. doi: 10.1111/ajco.12477
21. Shen YJ, Wu B, Wang XH, Zhu J. Health state utilities in patients with advanced non-small-cell lung cancer in China. J Comp Effect Res. (2018) 7:443–52. doi: 10.2217/cer-2017-0069
22. Wan XM, Luo X, Tan CQ, Zeng XH, Zhang YC, Peng LB. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: A United States-based cost-effectiveness analysis. Cancer-Am Cancer Soc. (2019) 125:3526–34. doi: 10.1002/cncr.32368
23. Wu B, Lu S. The effect of PD-L1 categories-directed pembrolizumab plus chemotherapy for newly diagnosed metastatic non-small-cell lung cancer: a cost-effectiveness analysis. Transl Lung Cancer Res. (2020) 9:1770–84. doi: 10.21037/tlcr
24. Konidaris G, Paul E, Kuznik A, Keeping S, Chen CI, Sasane M, et al. Assessing the value of cemiplimab for adults with advanced cutaneous squamous cell carcinoma: A cost-effectiveness analysis. Value Health. (2021) 24:377–87. doi: 10.1016/j.jval.2020.09.014
25. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). (2021) 41:1037–48. doi: 10.1002/cac2.12197
26. Wang W, Liu H, Li G. What's the difference between lung adenocarcinoma and lung squamous cell carcinoma? Evidence from a retrospective analysis in a cohort of Chinese patients. Front Endocrinol (Lausanne). (2022) 13:947443. doi: 10.3389/fendo.2022.947443
27. Meng X, Gao Y, Yang L, Jing H, Teng F, Huang Z, et al. Immune microenvironment differences between squamous and non-squamous non-small-cell lung cancer and their influence on the prognosis. Clin Lung Cancer. (2019) 20:48–58. doi: 10.1016/j.cllc.2018.09.012
28. Zhang X, Liu S, Liu Y, Du J, Fu W, Zhao X, et al. Economic burden for lung cancer survivors in urban China. Int J Environ Res Public Health. (2017) 14:308. doi: 10.3390/ijerph14030308
29. Ocran Mattila P, Ahmad R, Hasan SS, Babar ZU. Availability, affordability, access, and pricing of anti-cancer medicines in low- and middle-income countries: A systematic review of literature. Front Public Health. (2021) 9:628744 doi: 10.3389/fpubh.2021.628744
30. Zhou J, Lan T, Lu H, Pan J. Price negotiation and pricing of anticancer drugs in China: An observational study. PloS Med. (2024) 21:e1004332. doi: 10.1371/journal.pmed.1004332
31. Liu GG, Wu J, He X, Jiang Y. Policy updates on access to and affordability of innovative medicines in China. Value Health Reg Issues. (2022) 30:59–66. doi: 10.1016/j.vhri.2021.12.003
32. Liang XY, Chen XY, Li HJ, Li Y. Cost-effectiveness of camrelizumab plus chemotherapy in advanced squamous non-small-cell lung cancer. Immunotherapy-Uk. (2023) 15:1133–42. doi: 10.2217/imt-2023-0008
33. Zhao M, Shao T, Chi Z, Tang W. Effectiveness and cost-effectiveness analysis of 11 treatment paths, seven first-line and three second-line treatments for Chinese patients with advanced wild-type squamous non-small cell lung cancer: A sequential model. Front Public Health. (2023) 11:1051484doi: 10.3389/fpubh.2023.1051484
Keywords: cost-effectiveness, serplulimab, chemotherapy, squamous non-small cell lung cancer, partitioned survival model
Citation: Zheng H, Zeng Y, Wen F and Hu M (2024) Cost-effectiveness of additional serplulimab to chemotherapy in metastatic squamous non-small cell lung cancer patients. Front. Immunol. 15:1382088. doi: 10.3389/fimmu.2024.1382088
Received: 05 February 2024; Accepted: 08 April 2024;
Published: 22 April 2024.
Edited by:
Ying Han, Central South University, ChinaReviewed by:
Jianjun Zhang, University of Texas MD Anderson Cancer Center, United StatesAlberto Pavan, Azienda ULSS 3 Serenissima, Italy
Copyright © 2024 Zheng, Zeng, Wen and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Hu, aHVtaW5nQHNjdS5lZHUuY24=
 Hanrui Zheng
Hanrui Zheng Ya Zeng
Ya Zeng Feng Wen3,4
Feng Wen3,4 Ming Hu
Ming Hu