
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 03 May 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1380451
This article is part of the Research Topic Community Series in Reducing Adverse Effects of Cancer Immunotherapy: Volume II View all 18 articles
 Mikalai Katsin1*
Mikalai Katsin1* Tatsiana Shman2
Tatsiana Shman2 Alexandr Migas2
Alexandr Migas2 Dzmitry Lutskovich2
Dzmitry Lutskovich2 Yuliya Serada1
Yuliya Serada1 Yauheniya Khalankova1
Yauheniya Khalankova1 Yuliya Kostina1
Yuliya Kostina1 Simon Dubovik3
Simon Dubovik3Corticosteroid therapy is the mainstay of immune effector cell-associated neurotoxicity syndrome (ICANS) management, although its use has been associated with worse overall survival (OS) and progression-free survival (PFS) after chimeric antigen receptor T-cell (CAR-T cell) therapy. Many options are being investigated for prophylaxis and management. Accumulating evidence supports the use of intrathecal (IT) chemotherapy for the management of high-grade ICANS. Here, we describe a case of a patient with stage IV Primary mediastinal B-cell lymphoma (PMBCL) successfully treated with IT methotrexate, cytarabine, and dexamethasone as first-line therapy for CD19 CAR-T cell-associated grade IV ICANS. The stable and rapid resolution of ICANS to grade 0 allowed us to discontinue systemic corticosteroid use, avoiding CAR-T cells ablation and ensuring preservation of CAR-T cell function. The described patient achieved a complete radiologic and clinical response to CD19 CAR-T cell therapy and remains disease-free after 9 months. This case demonstrates a promising example of how IT chemotherapy could be used as first-line treatment for the management of high-grade ICANS.
Chimeric antigen receptor T-cell (CAR-T cell) therapy has recently emerged as a novel treatment modality for the management of B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, and multiple myeloma, with high response rates and a potential for cure. To date, the Food and Drug Administration has approved six CAR-T cell products for different indications, and numerous CAR-T cell trials are being widely carried out. Despite great clinical success, complications such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) can be fatal and can pose obstacles in the clinical application of CAR-T cells. Anti-IL-6 receptor antibody (Tocilizumab) and corticosteroid therapy are the mainstream management strategies for CRS and ICANS, respectively (1). It was recently reported that a high cumulative corticosteroid dose, especially in high-grade ICANS, is associated with worse overall survival (OS) and progression-free survival (PFS) after CAR-T cell therapy due to the inhibition of CAR-T cell function and persistence (2). Various prophylaxis and preemptive strategies, such as early (3) and prophylactic (4) glucocorticosteroid administration, IL-1 receptor antagonist (Anakinra), anti-GM-CSF antibodies (Lenzilumab) and ibrutinib incorporation into the backbone of the CAR-T cell protocol (5), are being explored and have shown some evidence of success. Nevertheless, in cases of ICANS occurrence, strategies aimed at reducing the cumulative corticosteroid dose could impede systemic CAR-T cell depletion and on improve OS and PFS by controlling the pathological inflammatory response in the central nervous system (CNS). We report a case of relapsed/refractory (R/R) stage IV Primary Mediastinal B-Cell Lymphoma (PMBCL) developing late grade IV ICANS after CD19 CAR-T cell therapy. Intrathecal (IT) administration of methotrexate, cytarabine, and dexamethasone as first-line therapy successfully resolved ICANS IV within one day. The rapid response allowed us to discontinue intravenous (IV) dexamethasone and ensure CAR-T cell functional activity. The patient reached a sustained complete response according to positron emission tomography–computed tomography (PET-CT), which has lasted for over 9 months.
A 39-year-old male patient was admitted to our department with R/R stage IV PMBCL involving the mediastinum (170 mm), pleura, lungs, bone marrow, and lymph nodes from both sides of the diaphragm, along with dyspnea and B-symptoms. He was refractory to R-DA-EPOCH, R-MACOP-B, and Nivo+Bv. His blood CD3+ cell count was 1220.43 cells/μL, and leukapheresis was successfully performed. A lentiviral vector containing a second-generation CAR was constructed based on an expression cassette coding for a CD19-specific single-chain variable fragment (FMC63) fused to the IgG4 hinge, CD28 transmembrane domain, 4-1BB, and CD3z cytoplasmic signaling domains. Additionally, a truncated version of EGFR (EGFRt) was added after the P2A sequence as a surface marker for CAR-T cell tracking. IL-7 and IL-15 were used for CAR-T cells expansion. Following ex vivo lentiviral transduction and expansion, CAR-T cells totaling approximately 1.29 * 106 cells were obtained, with CD4+ CAR-T cells constituting 85.3% (Figure 1A). Restaging with PET-CT and subsequent lymphodepletion (decitabine, cyclophosphamide, and fludarabine) were performed. High-risk factors included a high intermediate age-adjusted International Prognostic Index -2, bulky disease, CRP 340 mg/L (6), and a CAR-HEMATOTOX score of 2 (7). After 7 days of CAR-T cell infusion, the patient’s symptoms improved, dyspnea resolved, inflammatory markers returned to normal values, and no signs of CRS or ICANS were registered in the first 10 days (see Figure 2). However, on day +14, the patient developed grade III ICANS with an Immune Effector Cell-Associated Encephalopathy (ICE) score of 2 points. No concomitant CRS has been observed. After the diagnosis of grade III ICANS, IV dexamethasone 20 mg was immediately initiated. A brain computed tomography (CT) scan did not reveal any intracranial abnormality, and an opportunity to perform an electroencephalography study was not available. Serum concentrations of inflammatory markers also significantly increased, and CAR-T cells reached Cmax at 56.3% (77.7 cells/μL) on day +14 (Figures 1C, 2).
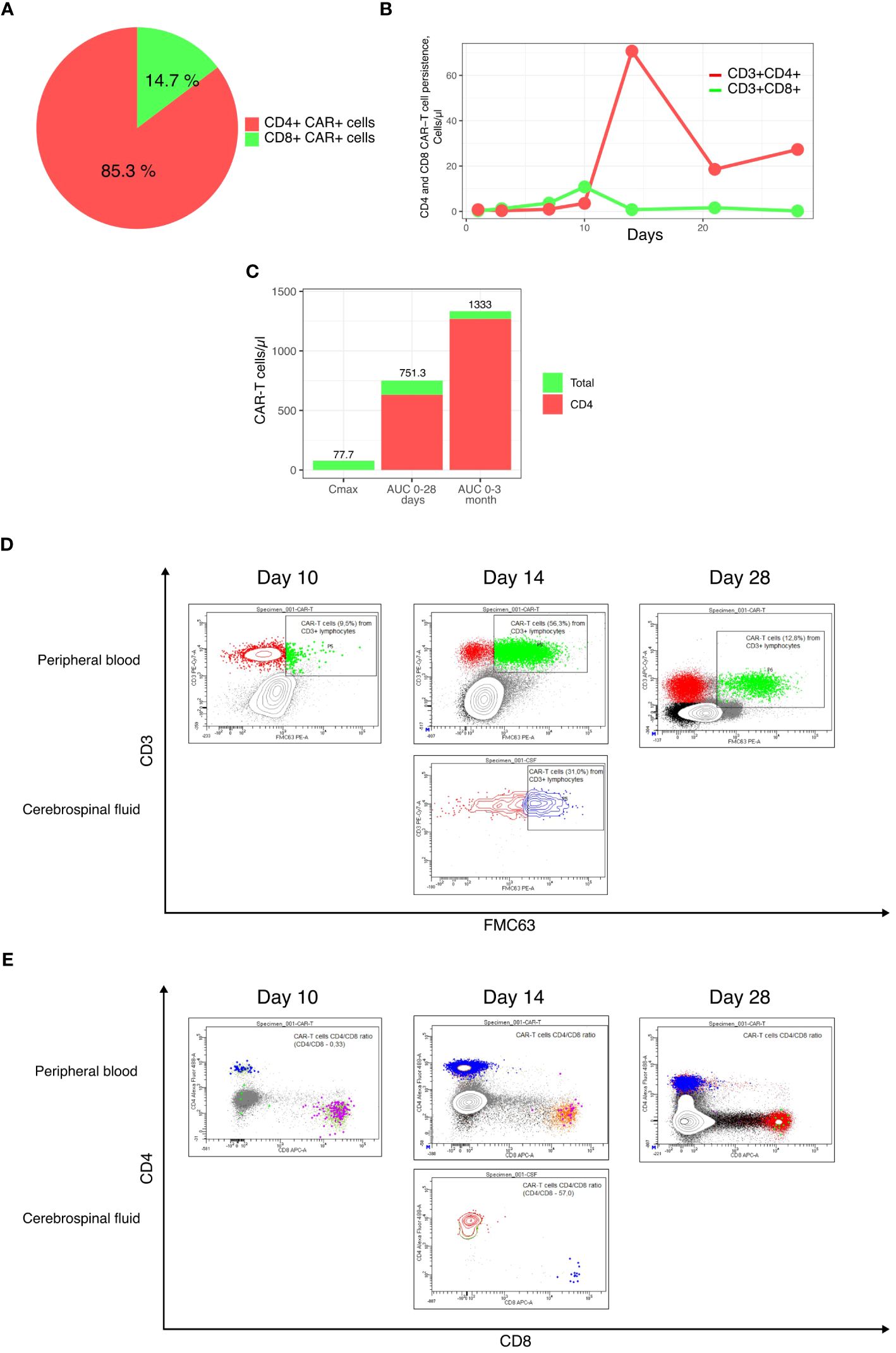
Figure 1 Final CAR-T cell product and CAR-T cell subsets persistence characteristics. (A) CD4+ and CD8+ subsets composition of the final CAR-T cell product; (B) Persistence of CD4+ and CD8+ CAR-T cells; (C) Cmax, AUC0-28 days and AUC0-3 months; (D, E) Flow cytometry plots of CAR-T cells in the peripheral blood and cerebrospinal fluid.
In our case, CAR-T cell expansion was primarily achieved through CD4+ CAR-T cells, resulting in a CD4+/CD8+ ratio of 89 (Figures 1B, 2). Within 1 hour after the initial dose of IV dexamethasone, the patient rapidly deteriorated, progressing to a comatose level of consciousness and non-responsiveness to tactile or auditory stimuli, consistent with ICANS grade IV. The patient did not require intubation or mechanical ventilation. A lumbar puncture was performed, revealing a normal opening pressure. Cerebrospinal fluid analysis ruled out viral and bacterial infections and indicated a white blood cell count of 2 cells/μl. CAR-T cells comprised 31.6% of leukocytes in the cerebrospinal fluid, with a CD4+/CD8+ CAR-T cell ratio of 54 (Figures 1D, E). In view of the low dose of CAR-T cells infused and the patient’s high-risk disease factors, first-line IT chemotherapy (methotrexate 15 mg, Ara-C 40 mg, dexamethasone 4 mg) was immediately implemented after the onset of grade IV ICANS to preserve CAR-T cell function. Within 6 hours of the IT chemotherapy, the patient showed a dramatic clinical improvement, with ICANS decreasing to grade II (ICE 6 points). In less than 32 hours after the IT chemotherapy, the patient’s ICANS had resolved to grade 0, inflammatory markers decreased, and steroid therapy was discontinued to ensure CAR-T cell function (Figure 2). The cumulative steroid dose was 96 mg of IV dexamethasone. CAR-T cells continued to be detected at significant levels in the peripheral blood (+28 days - 27.9 cells/μl, +3 months – 2.5 cells/μl, +6 months – 0.76 cells/μl), maintaining B-cells aplasia (Figures 1D, E, 2). PET/CT scans at +3 months (Figure 3) and CT scans at +6 and +9 months confirmed an ongoing complete remission (CR). The area under the curve (AUC) for 0-28 days of CAR-T cells amounted to 751.3 cells/μl, including CD4+CAR-T cells 632.2 cells/μl. The AUC for 0-3 months amounted to 1333 cells/μl, including CD4+ CAR-T cells 1269.4 cells/μl (Figure 1C).
ICANS is a potentially serious complication of CAR-T cell therapy, encompassing symptoms ranging from mild encephalopathy and disorientation to more severe and potentially fatal manifestations such as acute cerebral edema, paresis, plegia, seizures, and loss of consciousness. Approximately half of patients with diffuse large B-cell lymphoma experience ICANS, with 70% encountering any grade and 35% experiencing grade ≥3 ICANS following CD28-based anti-CD19 CAR-T cells (8). Conversely, 4-1bb-based anti-CD19 CAR-T cells are associated with a lower incidence and severity of ICANS, with 26% experiencing any grade and 10% experiencing grade ≥3 ICANS (9). In most cases, acute ICANS develops 4–6 days after CAR-T cell infusion and typically occurs after the onset of CRS or in the setting of improving or resolved CRS (10). Late-onset ICANS cases have also been reported (11, 12), lasting 5–13 days with complete symptom resolution in the majority of cases (13, 14). Clinical studies have identified IL-6, GM-CSF, IFN-γ, and IL-15 as major cytokines responsible for CNS inflammation after CAR-T cell therapy (15). While IL-1 hasn’t displayed a strong association with ICANS in clinical studies, anti-IL-1R antibodies have been shown to prevent ICANS in preclinical mouse models of CAR-T cell therapy (16). CAR-T cells produce IFN-γ, TNF-α, and GM-CSF at the tumor site to attract and activate macrophages/monocytes, which in turn produce large amounts of IL-1, IL-6, nitric oxide, and other cytokines associated with CRS presentation. The abundance of cytokines and soluble inflammatory mediators in the circulation leads to the activation of CNS endothelium and disruption of the blood-brain barrier (BBB), driven by CAR-T cells themselves and amplified by activated macrophages (17). Consequently, the severity of CRS is considered a predictor of neurotoxicity development in the clinical context (18). Subsequently, the diffusion of cytokines, migration of CAR-T cells and peripherally activated monocytes into the CNS, resident microglia activation, and neuronal cell injury occur. However, not all ICANS cases are preceded by CRS. Another mechanism of neuroinflammation and BBB disruption is the direct production of cytokines and mediators within the CNS (19). One additional pathophysiological mechanism of ICANS, particularly cerebral edema, could result from significant disruption of the BBB with a massive influx of cytokines, inflammatory mediators, and plasma leakage into the brain, even without any detection of CAR-T cells in the CNS (20).
The composition of CAR-T cell subpopulations in the apheresis material and the final CAR-T cell product may indeed impact the efficacy and toxicity of CAR-T cells. In the majority of cases, patients with B-cell malignancies tend to have a higher proportion of CD8+ T cells in the peripheral blood at the time of apheresis (21). CD4+ CAR-T cells have been shown to produce increased amounts of cytokines such as IFN-γ, TNF-α, and IL-2. Additionally, they demonstrate a robust proliferative capacity upon the recognition of tumor cells and tend to have a lesser degree of exhaustion, resulting in longer persistence (22–24). Baur et al. (25) investigated the contribution of CD4+ and CD8+ CAR-T cell expansion kinetics to the clinical response and the development of toxicities. They found a positive correlation between the AUC for CD4+ CAR-T cells at 0-28 days and 0-3 months and the development of CR at 1 and 3 months, respectively. The severity of CRS and ICANS also positively correlated with the AUC for CD4+ CAR-T cells at 0-28 days (25). This case is in concordance with the findings of the study, as the patient developed grade IV ICANS at the peak of CD4+ CAR-T cell expansion and eventually achieved a CR, largely due to the CD4+ CAR-T cell subset.
The majority of cases of ICANS are a consequence of hyperinflammation outside the CNS, making prophylaxis a key strategy to reduce its incidence and severity. Current prophylaxis options include steroids, anakinra, and JAK inhibitors (26). Clinical studies of the anti-GM-CSF antibody Lenzilumab are also underway, with results pending (27, 28). Anakinra can cross the BBB when given intravenously, although its large molecular size allows only 4% penetration into cerebrospinal fluid with standard dosing regimens (29). This suggests that Anakinra could be much more effective if prescribed earlier in the clinical scenario with the aim of preventing endothelial disruption and ICANS development. In a Phase II study of Anakinra prophylaxis, all-grade ICANS occurred in 19%, and severe ICANS occurred in 9.7% of patients (30). Another option for ICANS prophylaxis is the utilization of steroids. In the ZUMA-1 cohort study (NCT02348216), 40 patients received axicabtagene-ciloleucel (axi-cel) with CRS/ICANS prophylaxis, including once-daily oral dexamethasone 10 mg. This regimen resulted in 58% of patients experiencing any grade of ICANS and 13% experiencing grade 3 or higher ICANS (4).
Despite prophylaxis, many patients still succumb to high-grade ICANS. The first-line therapy includes corticosteroids and supportive care. However, concerns are rising regarding the usage of steroids, as their cumulative dose negatively correlates with OS and PFS due to the loss of CAR-T cell function and persistence (2). Moreover, it elevates the risks of severe infections (31). Limited options are available for steroid-refractory ICANS, which include Anakinra, chemotherapy (15, 32), and more treatment modalities like dasatinib (33) are under investigation.
In one study, Anakinra was used in combination with steroids for the management of 6 patients with high-grade 3-4 ICANS after CD19 CAR-T cell therapy. Among the patients, 3 reached grade 0-I ICANS, 1 reached Grade II ICANS, and 2 did not respond. Among the responders, the mean cumulative dose of dexamethasone and the median time to ICANS resolution were 1446 mg and 28.5 days, respectively. Moreover, 2 of the initial responders succumbed to ICANS, disease progression, and hemophagocytic lymphohistiocytosis recurrence (34) (Table 1). In another study by Gazeau et al. (35), the utility of Anakinra implementation for the management of steroid-refractory ICANS after CD19 CAR-T cell therapy was explored. In this study, 26 patients with B-cell malignancy were recruited. Half of the patients received low-dose Anakinra (100-200 mg per day) and the other half received high-dose Anakinra (8 mg/kg/day) for managing steroid-refractory ICANS. The median ICANS resolution time was 15.5 days for all the patients. Improvement in ICANS was noted in 73% of patients, with higher response rates seen in the high-dose group compared to the low-dose group (100% versus 46%, respectively). The objective response rate and CR rates were 58% and 42% for the low-dose regimen and 77% and 53% for the high-dose regimen, respectively. The non-relapse mortality rate at day 30 was significantly lower in patients treated with high-dose Anakinra compared to low-dose Anakinra (0% versus 69%) (35) (Table 1). The drawback of these studies is the prolonged concurrent use of high dose steroids, which makes it difficult to evaluate the contribution of Anakinra to the clinical efficacy. Furthermore, the negative impact of steroids on CAR-T cell function, which could be observed later during the surveillance period, adds complexity to the evaluation. Some concerns regarding the lack of a clear and dramatic clinical effect on neurotoxicity relief by Anakinra, as well as the lack of corticosteroid tapering for managing ICANS, have been reported (39). Some recent studies indicate that the dose of Anakinra could be safely elevated up to 12 mg/kg/day with enhancement of its efficacy (40).
Due to the possibility that other cytokines, in addition to IL-1, could be implicated in the pathogenesis of ICANS, as well as the potential advantage of local control of CNS inflammation over systemic CAR-T cell depletion, treatment options such as IT administration of immunosuppressive drugs have been proposed. Clinical cases of IT therapy have been reported in the past (37, 41, 42), and recently, small clinical studies have shown favorable outcomes in steroid-refractory ICANS (37) (Table 1). Yucebay F. et al. reported a small clinical study investigating the IT administration of methotrexate 15mg, cytarabine 40mg, and hydrocortisone 50mg for the management of steroid-refractory or recurring high-grade ICANS following anti-CD19 CAR-T cell therapy. Overall, 5 out of 7 patients (71%) responded to IT chemotherapy, with total resolution of neurotoxicity occurring in a median of 11 days. The median time to IT chemotherapy from onset of neurotoxicity was 11 days, which could contribute to the late resolution of ICANS (37). A recent retrospective study has highlighted the superior efficacy of early IT hydrocortisone administration for steroid-refractory high-grade ICANS compared to late IT hydrocortisone or systemic corticosteroid therapy (36). In this study, the first group of 7 patients with steroid-refractory ICANS received IT hydrocortisone within the first 5 days after high-grade ICANS development. All patients recovered from ICANS, and the 1-year PFS and OS were 57.1% (Table 1). However, three of these patients who received additional treatment with anakinra died of infectious complications. The second group, consisting of 8 patients with high-grade ICANS, received late IT or conventional systemic corticosteroid therapy. For this group of patients, the estimated 1-year PFS and OS were 18.8% for all patients and 0% for patients with steroid-refractory ICANS (36). Solh M.M. et al. reported a clinical study that explored the safety and efficacy of IT administration of methotrexate and/or cytarabine for the treatment of ICANS. In this study, twelve patients received one or two doses of IT chemotherapy. All patients received systemic steroids, and 6 patients (50%) received Anakinra as part of their ICANS management. Eleven patients experienced resolution of their ICANS, with a median time to resolution of 2 days. Additionally, five patients had complete resolution within 24 hours of receiving IT chemotherapy. Among the five patients who did not respond to steroids, all experienced resolution of their ICANS symptoms after IT chemotherapy (38) (Table 1).
In our case, given that we infused a very small amount of only 1.29 * 106 CAR-T cells and the patient had high-risk disease features, we chose to manage the grade IV ICANS with the first-line IT chemotherapy (methotrexate, Ara-C, and dexamethasone) to avoid high cumulative doses of systemic steroids. The patient responded very rapidly, with complete resolution of ICANS in less than 32 hours after the onset, and the cumulative dose of steroids was only 96 mg. This more targeted approach of ICANS management helped to prolong the persistence of CAR-T cells, resulting in a durable complete response. Although our patient received a significantly lower cumulative dose of steroids compared to those reported in clinical trials (Table 1), it is possible that high-dose systemic steroids could also have contributed to his rapid improvement. However, the rapidity of his response suggests that our early intervention played a role in reversing his ICANS. The potential benefits of IT chemotherapy include direct delivery of immunosuppressive drugs into the CNS, leading to rapid biodistribution in the brain, rapid resolution of neurotoxicity, and dose reduction of systemic steroids. However, it’s important to note that there could be contraindications to the procedure, such as coagulopathy, thrombocytopenia, etc., which may make it difficult to perform an IT chemotherapy in every patient.
CD4+ CAR-T cells may play a role in both the toxicity and efficacy of CAR-T cell therapy even when infused at very low levels. In cases of high-grade ICANS, early IT therapy with methotrexate, Ara-C, and dexamethasone is a viable option to accelerate the resolution of ICANS and reduce the cumulative dose of systemic corticosteroids. Further clinical trials are needed to evaluate the safety and efficacy of this approach, as it could potentially provide a more targeted and effective management strategy for ICANS associated with CAR-T cell therapy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Independent ethical committee of the health care institution “Vitebsk Regional Clinical Oncology Dispensary”. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MK: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Supervision, Validation. TS: Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. DL: Writing – original draft, Writing – review & editing. YS: Writing – original draft, Writing – review & editing. YKh: Writing – original draft, Writing – review & editing. YKo: Writing – original draft, Writing – review & editing. SD: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gu T, Hu K, Si X, Hu Y, Huang H. Mechanisms of immune effector cell-associated neurotoxicity syndrome after CAR-T treatment. WIREs Mech Dis. (2022) 14(6):e1576. doi: 10.1002/wsbm.1576
2. Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Iyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. (2021) 137:3272–6. doi: 10.1182/blood.2020008865
3. Topp MS, van Meerten T, Houot R, Minnema MC, Bouabdallah K, Lugtenburg PJ, et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. (2021) 195:388–98. doi: 10.1111/bjh.17673
4. Oluwole OO, Bouabdallah K, Muñoz J, De Guibert S, Vose JM, Bartlett NL, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. (2021) 194:690–700. doi: 10.1111/bjh.17527
5. Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. (2020) 135:1650–60. doi: 10.1182/blood.2019002936
6. Garcia-Recio M, Wudhikarn K, Pennisi M, Alonso-Trillo R, Flynn J, Shouval R, et al. The international prognostic index is associated with outcomes in diffuse large B cell lymphoma after chimeric antigen receptor T cell therapy. Transplant Cell Ther. (2021) 27:233–40. doi: 10.1016/j.jtct.2020.10.022
7. Rejeski K, Perez A, Iacoboni G, Penack O, Bücklein V, Jentzsch L, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. (2022) 10:1–14. doi: 10.1136/jitc-2021-004475
8. Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. (2020) 38:3095–106. doi: 10.1200/JCO.19.02103
9. Riedell PA, Grady C, Nastoupil LJ, Luna A, Ahmed N, Maziarz RT, et al. Lisocabtagene maraleucel in relapsed/refractory large B-cell lymphoma: real world analysis from the cell therapy consortium. Blood. (2023) 142:617–7. doi: 10.1182/blood-2023-184862
10. Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. (2018) 32:1091–101. doi: 10.1007/s40263-018-0582-9
11. Jung S, Greiner J, Von Harsdorf S, Popovic P, Moll R, Schittenhelm J, et al. Fatal late-onset CAR T-cell-mediated encephalitis after axicabtagene-ciloleucel in a patient with large B-cell lymphoma. Available online at: http://ashpublications.org/bloodadvances/article-pdf/5/19/3789/1824771/advancesadv2021004889.pdf.
12. Beck K, Hasan S, Mehta S, Sanchez CV. Delayed onset ICANS leading to bilateral cranial neuropathies: two case reports (P12-13.005). Neurology. (2023) 100:1915. doi: 10.1212/WNL.0000000000202192
13. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. New Engl J Med. (2017) 377:2545–54. doi: 10.1056/NEJMoa1708566
14. Brown BD, Tambaro FP, Kohorst M, Chi L, Mahadeo KM, Tewari P, et al. Immune effector cell associated neurotoxicity (ICANS) in pediatric and young adult patients following chimeric antigen receptor (CAR) T-cell therapy: can we optimize early diagnosis? Front Oncol. (2021) 11:1–9. doi: 10.3389/fonc.2021.634445
15. Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T cell-associated neurotoxicity. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.577027
16. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. (2018) 24:739–48. doi: 10.1038/s41591-018-0036-4
17. Rosen RS, Yang JH, Peña JS, Schloss R, Yarmush ML. An in vitro model of the macrophage-endothelial interface to characterize CAR T-cell induced cytokine storm. Sci Rep. (2023) 13:1–14. doi: 10.1038/s41598-023-46114-y
18. Chou CK, Turtle CJ. Insight into mechanisms associated with cytokine release syndrome and neurotoxicity after CD19 CAR-T cell immunotherapy. Bone Marrow Transplant. (2019) 54:780–4. doi: 10.1038/s41409-019-0602-5
19. Torre M, Solomon IH, Sutherland CL, Nikiforow S, DeAngelo DJ, Stone RM, et al. Neuropathology of a case with fatal CAR T-cell-associated cerebral edema. J Neuropathol Exp Neurol. (2018) 77:877–82. doi: 10.1093/jnen/nly064
20. Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. (2022) 22:85–96. doi: 10.1038/s41577-021-00547-6
21. Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. (2016) 30:492–500. doi: 10.1038/leu.2015.247
22. Galli E, Bellesi S, Pansini I, Di Cesare G, Iacovelli C, Malafronte R, et al. The CD4/CD8 ratio of infused CD19-CAR-T is a prognostic factor for efficacy and toxicity. Br J Haematol. (2023) :564–70. doi: 10.1111/bjh.19117
23. Wang D, Aguilar B, Starr R, Alizadeh D, Brito A, Sarkissian A, et al. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight. (2018) 3:3-4. doi: 10.1172/jci.insight.99048
24. Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nat. (2022) 602:503–9. doi: 10.1038/s41586-021-04390-6
25. Baur K, Buser A, Jeker LT, Khanna N, Läubli H, Heim D, et al. CD4+ CAR T-cell expansion is associated with response and therapy related toxicities in patients with B-cell lymphomas. Bone Marrow Transplant. (2023) 58:1048–50. doi: 10.1038/s41409-023-02016-1
26. Frigault MJ, Maziarz RT, Park JH, Lazaryan A, Shah NN, Svoboda J, et al. Itacitinib for the prevention of immune effector cell therapy-associated cytokine release syndrome: results from the phase 2 Incb 39110-211 placebo-controlled randomized cohort. Blood. (2023) 142:356–6. doi: 10.1182/blood-2023-180205
27. Oluwole OO, Kenderian SS, Shiraz P, Karmali R, Reshef R, McCarthy PL, et al. ZUMA-19: A phase 1/2 study of axicabtagene ciloleucel plus lenzilumab in patients with relapsed or refractory large B-cell lymphoma. Blood. (2022) 140:10318–20. doi: 10.1182/blood-2022-167688
28. Kenderian SS, Durrant C, Chappell D, Ahmed O, Kilcoyne A. A phase 2/3 randomized, placebo-controlled, open-label, multi-center trial of lenzilumab to improve the safety and efficacy of CAR-T cell therapy in adults with relapsed or refractory large B-cell lymphoma (The SHIELD study). Blood. (2021) 138:1758–8. doi: 10.1182/blood-2021-153987
29. Mehta P, Cron RQ, Hartwell J, Manson JJ, Tattersall RS. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. (2020) 2:e358–67. doi: 10.1016/S2665-9913(20)30096-5
30. Park JH, Nath K, Devlin SM, Sauter CS, Palomba ML, Shah G, et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat Med. (2023) 29:1710–7. doi: 10.1038/s41591-023-02404-6
31. Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. (2023) 141:2430–42. doi: 10.1182/blood.2022017414
32. Genoud V, Migliorini D. Novel pathophysiological insights into CAR-T cell associated neurotoxicity. Front Neurol. (2023) 14:1108297. doi: 10.3389/fneur.2023.1108297
33. Baur K, Heim D, Beerlage A, Poerings AS, Kopp B, Medinger M, et al. Dasatinib for treatment of CAR T-cell therapy-related complications. J Immunother Cancer. (2022) 10:1. doi: 10.1136/jitc-2022-005956
34. Strati P, Ahmed S, Kebriaei P, Nastoupil LJ, Claussen CM, Watson G, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. (2020) 4:3123–7. doi: 10.1182/bloodadvances.2020002328
35. Gazeau N, Barba P, Iacoboni G, Kwon M, Bailen R, Reguera JL, et al. Safety and efficacy of two anakinra dose regimens for refractory CRS or Icans after CAR T-cell therapy. Blood. (2021) 138:2816–6. doi: 10.1182/blood-2021-147454
36. Zurko JC, Johnson BD, Aschenbrenner E, Fenske TS, Hamadani M, Hari P, et al. Use of early intrathecal therapy to manage high-grade immune effector cell-associated neurotoxicity syndrome. JAMA Oncol. (2022) 8:773–5. doi: 10.1001/jamaoncol.2022.0070
37. Yucebay F, Maakaron J, Grana A, Jaglowski S, Roddy J. Intrathecal chemotherapy: an alternative treatment strategy to prolonged corticosteroids for severe CAR T associated neurotoxicity. Biol Blood Marrow Transplant. (2020) 26:S312. doi: 10.1016/j.bbmt.2019.12.390
38. Solh M. Intrathecal chemotherapy as treatment for chimeric antigen receptor T cell (CAR T) therapy associated neurotoxicity. ASH. (2023) 42(Supplement 1):2138. doi: 10.1182/blood-2023-186585
39. Wehrli M, Gallagher K, Chen YB, Leick MB, McAfee SL, El-Jawahri AR, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J Immunother Cancer. (2022) 10:4. doi: 10.1136/jitc-2021-003847
40. Gazeau N, Liang EC, Wu Q, Voutsinas JM, Barba P, Iacoboni G, et al. Anakinra for refractory cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T cell therapy. Transplant Cell Ther. (2023) 29:430–7. doi: 10.1016/j.jtct.2023.04.001
41. Shah NN, Johnson BD, Fenske TS, Raj RV, Hari P. Intrathecal chemotherapy for management of steroid-refractory CAR T-cell–associated neurotoxicity syndrome. Blood Adv. (2020) 4:2119–22. doi: 10.1182/bloodadvances.2020001626
Keywords: ICANS, intrathecal chemotherapy, CD19 CAR-T cells, anakinra, corticosteroids
Citation: Katsin M, Shman T, Migas A, Lutskovich D, Serada Y, Khalankova Y, Kostina Y and Dubovik S (2024) Case report: Rapid resolution of grade IV ICANS after first line intrathecal chemotherapy with methotrexate, cytarabine and dexamethasone. Front. Immunol. 15:1380451. doi: 10.3389/fimmu.2024.1380451
Received: 01 February 2024; Accepted: 22 April 2024;
Published: 03 May 2024.
Edited by:
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, BrazilReviewed by:
Reuben Benjamin, King’s College London, United KingdomCopyright © 2024 Katsin, Shman, Migas, Lutskovich, Serada, Khalankova, Kostina and Dubovik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikalai Katsin, b25jb2dlbXZvY29kQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.