- Early Arthritis Clinic, National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland
Primary Sjögren’s syndrome (pSS) is an autoimmune disease, with B cell hyperactivation and autoantibody production as its immunological hallmarks. Although the distinction between immunoglobulin G4-related disease (IgG4-RD) and pSS, based on the presence or absence of certain autoantibodies, seems easy to make, possibility of elevated serum IgG4 concentration and often similar organ involvement may lead to a misdiagnosis. The increased serum concentration of IgG4 in IgG4-RD is not clearly linked to the pathogenesis of IgG-RD and it has been suggested that it may constitute just an epiphenomenon. The aim of this article is to discuss the presence of IgG4 in pSS and IgG4-RD and its potential significance for these two diseases.
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic autoimmune disease with a dominance of immunological features such as hypergammaglobulinemia, B and T cell activation, autoantibody production mainly against ribonucleoproteins (anti-SSA/Ro and anti-SSB/La antibodies), low levels of C3 and C4 components of the complement system, rheumatoid factor (RF) and cryoglobulin production. The main autoimmune and inflammatory process, with infiltrations of mononuclear cells, takes place in exocrine glands and internal organs. Due to the tropism of exocrine glands, of lacrimal and salivary glands in particular, the dysfunction of those glands is observed, causing dryness - one of the main clinical features of pSS (1).
The presence of anti-Ro/SSA and anti-La/SSB is typical for pSS (1). Less often, immunological tests may reveal anti-centromere B (CENP-B) and anti-citrullinated protein antibodies (ACPA) or antibodies more specific to other connective tissue diseases (2, 3). Rheumatoid factor may also be present in IgM, IgA, and IgG classes of immunoglobulins (4, 5). The association of pSS and primary biliary cholangitis with AMA-M2 autoantibodies or autoimmune thyroiditis with thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb) is also well established (6, 7).
Immunoglobulin G4-related disease (IgG4-RD) is another autoimmune disorder, which shares some of its clinical symptoms with pSS, including the presence of the inflammatory and autoimmune process in the salivary and thyroid glands and other similar organs. Its identification underlines the attention paid to immunoglobulin G4 in recent years, its role in the human immune system, and the connection of this antibody to various clinical states.
Interestingly, for years, Mikulicz’s disease (MD)—currently recognized as a clinical manifestation of IgG4-RD—was considered a form of pSS. It was described by a Polish surgeon, Jan Mikulicz-Radecki, in 1888 and its symptoms include sialadenitis and dacryoadenitis with salivary and lachrymal gland enlargement (8, 9). Another example of isolated submandibular gland involvement is chronic sclerosing sialadenitis, known as Kutner’s tumor (chronic sclerosing sialadenitis), which is also currently classified as a manifestation of IgG4-RD. It occurs without the involvement of other salivary glands, which is one of the elements that distinguishes it from pSS, where the characteristic feature is the involvement of the parotid and submandibular glands (8). The increased serum concentration of IgG4 is a recognized immunological determinant of IgG4-RD, while in the case of pSS, various relationships are presented - both a decrease and, in some studies, an increase in the concentration of this immunoglobulin. The still not fully recognized pathophysiological role of the IgG4, the research, that led to the distinguishing of IgG4-RD, as well as the similarities between this disease and pSS were the inspiration to write the present article.
Overview of the unique role of IgG4
In human serum, the IgG4 class of immunoglobulins is least abundant among other immunoglobulins G and constitutes approximately 5% of IgG (10). Serum IgG4 levels increase gradually from birth and by the age of approximately 10 years they usually reach adult levels, hindering the assessment of IgG4 levels in young children (11). Isolated IgG4 deficiency is very rare and more often occurs in combination with a deficiency of other immunoglobulins (IgG1, IgG2, or IgA). There are observations (mainly in children) indicating that in a case of IgG4 deficiency, recurrent respiratory infections, allergies, candidiasis, and chronic diarrhea occur (12, 13). IgG4 deficiency was also observed in patients with associated inflammatory bowel disease (14). Currently, a lot of attention is being paid to IgG4 hypergammaglobulinemia, which may occur in a healthy population (in ~5%), although increased serum concentration in a healthy population has not yet been proven to have clinical consequences (15).
IgG4 is attributed with an anti-inflammatory role (e.g., response to parasitic infections and allergies) and is also associated with a potentially pathogenic role in autoimmune diseases and with a response to biological treatment or cancer development (16).
Such varied perceptions of the role of IgG4 in immune processes are related to its specific features and abilities, different from other IgG molecules.
In 1997, the structure of an IgG4 Fc fragment was described (17, 18). The structure of human IgG4 is highly homologous (over 90%) to other IgG subclasses, but its unique properties result from specific amino acid differences in the heavy chain, especially in the hinge region, which allows for the phenomenon of fab arm exchange (FAE) (observed in vitro only for this subclass of immunoglobulin G) as well as the variability in CH3 domains (19, 20).
The unique property of IgG4 is FAE, which is possible due to relatively labile disulfide bonds between the heavy chains of IgG4 molecules, which allow for the phenomenon of recombination (exchange of half-molecules, each consisting of one heavy chain and one light chain). Such an exchange between two IgG4 molecules allows for the formation of a monovalent (for each antigen)/bispecific antibody, contrary to other IgG monomers, which are bivalent, but monospecific in nature (21, 22). The FAE phenomenon is also favored by a weaker CH3-CH3 interaction in IgG4, caused by the replacement of lysine with arginine (position 409) (19).
In a physiological state, IgG4 is an anti-inflammatory antibody and weakly activates the complement system. Under conditions of increased IgG4 concentration, the consumption of complement components was observed, which indicates such a possibility. It is suggested that special properties of IgG4, such as FAE and glycosylation of Fc fragments, affect complement activation (10, 19). It is also proposed that IgG4 blocks the binding of other immunoglobulin subclasses to the complement component 1q (C1q).
The glycosylation of the Fc IgG4 fragment is another process that plays a role in altering IgG4 effector functions (23). Glycosylation changes the affinity of IgG4 to the different FcγRs (24). Studies on the glycosylation of IgG and IgG4, including in the pathophysiology, (e.g., IgG4 in primary membranous nephropathy causes activation of the lectin pathway and induction of podocyte damage) indicated a pro-inflammatory role of this process and the possibility of the complement activation also by glycoforms of IgG4 (25, 26). Current research indicates that the process of glycosylation of IgG, including IgG4, may have a pro-inflammatory role and is associated with the pathogenesis of various inflammatory diseases. However, the assessment of this phenomenon requires further research.
In Oskam N et al. (27), the authors showed that complement activation by IgG4 is possible, but only at high antigen densities and high antibody concentrations. The researchers managed to demonstrate that such an activation occurred only through the classical pathway. It is possible that these potential pathways are enhanced by galactosylation, while the occurrence of FAE has the opposite effect. However, these findings do not explain the pathogenic role of IgG4 in IgG4-RD or, for example, in primary membranous nephropathy (pMN).
The antigen-specific activity of IgG4 under the influence of chronic antigen stimulation in allergic diseases and in the course of immunotherapy was studied more widely and earlier than the problem of IgG4-related disease (16).
In allergic diseases, as well as in IgG4-RD, T helper type 2 (Th2) cells play an important role associated with interleukin 4 (IL-4) and 13 (IL-13) production (Th2 cytokines). These cytokines stimulate B cells to switch to class IgG4 immunoglobulin production (28).
Because of this stimulation, B cells exposed to an allergen, in addition to producing allergenic IgE, produce IgG4. Immunoglobulin G4 competes with IgE to prevent the activation and the subsequent degranulation of mast cells and basophils. Subsequently, the IgE to IgG4 (IgE/IgG4) ratio decreases. This situation also occurs during allergen-specific immunotherapy. In such circumstances, the persistence of increased IgG4 concentration may be observed for a longer period of time, even up to 3 years (29). Because of the described observations in some diseases with IgE elevation, the protective role of IgG4 has been suggested.
A study on anti-drug antibodies in the biological treatment of RA revealed that adalimumab-treated patients developed antibodies against adalimumab mainly in the IgG4 isotype, rather than in the IgG1 isotype (30). Such observations were associated with longer (3 years in cited study) drug exposition. It was demonstrated that a longer exposition to an antigen (drug) can cause the development of more IgG4 anti-drug-antibodies—a finding which corresponds with other research on time of exposure to allergen/antigen and IgG4 antibodies production.
In summary, it is believed that in the case of allergic diseases and immunotherapy, IgG4 may have a potentially protective effect. A similar increase of both IgG4 and IgE levels is observed in helminth infections (31, 32). Furthermore, beekeepers and laboratory workers may exhibit high serum levels of the allergen-specific IgG4, which protects them from an anaphylactic reaction (20).
However, it is still not clearly established whether increased serum concentration of IgG4 plays an important role in diseases such as IgG4-RD or Crohn’s disease (CD) or whether it should be considered an epiphenomenon only (33).
IgG4 detection
In clinical practice, the detection of IgG4 currently is based on different tests, namely, radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and immunonephelometry, with two latter ones being most commonly used. In Su et al. (34), a comparison study between these tests proved good compatibility between ELISA and the nephelometric assays. The fact that the costs of performing an ELISA test are lower than immunonephelometry may also be important in planning research.
An antigen-binding radioimmunoassay (RIA) was used particularly in the oldest studies, in which it was used to measure liquid-phase IgG4 antibodies (35).
For years, the problem of establishing a cut-off value for a significant IgG4 concentration, in a way as to maintain the greatest sensitivity and specificity of the tests, has been discussed. Especially since the identification of IgG4-RD, it has become important to determine what value of IgG4 concentration is most likely to be associated with this disease. The classification criteria for IgG4 – RD adopted a value of 1.35 (135 mg/dL) as the cut-off value (36) but in some studies, higher levels were noted as being more specific and sensitive (37, 38).
Carruthers et al. (39) noted that elevated serum IgG4 concentrations had only 60% specificity and a 34% positive predictive value (PPV). Therefore, the authors suggest that a doubled cut-off value may increase the specificity of the test.
In some studies, researchers highlighted that elevated serum concentrations of IgG4 or plasmablasts may be a hallmark of IgG4-RD (40).
This approach is determined by the difficulties in interpreting symptoms and diagnosing IgG4-RD in the presence of a normal/low serum IgG4 concentration. Although such a discussion is currently taking place mainly in relation to IgG4-RD, its outcome may have further consequences, leading to a re-evaluation of diagnostic criteria for other diseases, including pSS, related in various ways to IgG4.
The outline of pSS pathogenesis
The discussion of the potential role of IgG4 in pSS requires a basic understanding of pSS pathogenesis—a problem that still remains not yet fully understood. However, certain factors, such as a breach of immune tolerance, endothelial damage, release of autoantigens, and B cell activation with autoantibody production, were confirmed as immunological features of this chronic autoimmune disease, which is also known under the name of “autoimmune epithelitis” (41).
In recent years, the role of the activity of the interferon pathway in pSS, as well as other autoimmune rheumatic diseases, has been examined. The overexpression of type I IFN is called IFN-I signature (42). Other studies highlighted the significance of T lymphocytes as cells that influence the activation of B lymphocytes with the participation of stimulating factors (CTLA-4) and through the production of B cell stimulating factors such as BAFF/BlySS (43).
Sicca symptoms due to exocrine gland involvement remain the main clinical feature of pSS, although it does not take place in the early stages of pSS. Importantly, in different age groups of patients, we can expect different symptoms as they present with different levels of immune system activity and varied degrees of organ and exocrine gland damage. Several pSS phenotypes have been described and the type designation highlights sicca symptoms, systemic involvement, and organ damage as follows: sicca without organ damage, sicca with glandular involvement/mild systemic involvement, and sicca with severe systemic involvement (44).
The main immunological hallmarks of pSS are anti-ribonucleoprotein antibodies (anti Ro/SSA and anti-La/SSB). They belong to antibodies against extractable nuclear antigens (ENA) which are present in the cell’s cytoplasm. Anti-Ro/SSA ab was divided in 1981 by Lerner et al. (45) in two subclasses depending of a molecular weight: anti-Ro52 kDa and anti-Ro60 kDa.
Interestingly, Ro52 and Ro60 antigens are coded on different chromosomes. Ro60 is coded on chromosome 19 and forms complexes with small cytoplasmic RNA (hY-RNA complexes) which may be involved in binding misfolded mRNA and influence its degradation.
Ro52 is coded on chromosome 11, belongs to the tripartite motif protein (TRIM) family, and is involved in inflammation and apoptosis (46).
Some researchers suggest that anti-Ro60 abs are sufficient to diagnose pSS, while anti-La/SSB abs are heterogenous and may be present in other autoimmune diseases, such as systemic lupus erythematosus (SLE), neonatal lupus erythematosus (NLE), and inflammatory myositis as well as pSS. As has been concluded in recent studies, anti-Ro60 abs are considered a triggering factor for autoimmunity (glomerulonephritis, ultraviolet light radiation sensitivity). Anti-Ro52 abs are associated also with other autoimmune diseases, such as systemic sclerosis, rheumatoid arthritis, and primary cholangitis.
However, despite differences between anti-Ro/SSA subclasses, the measurement of anti-Ro/SS-A antibodies as a complex in an ENA panel is sufficient for the determination of their presence for diagnosis according to the current classification criteria of pSS (46).
In the Figure 1, the outline of the recognized pSS pathogenesis is presented.
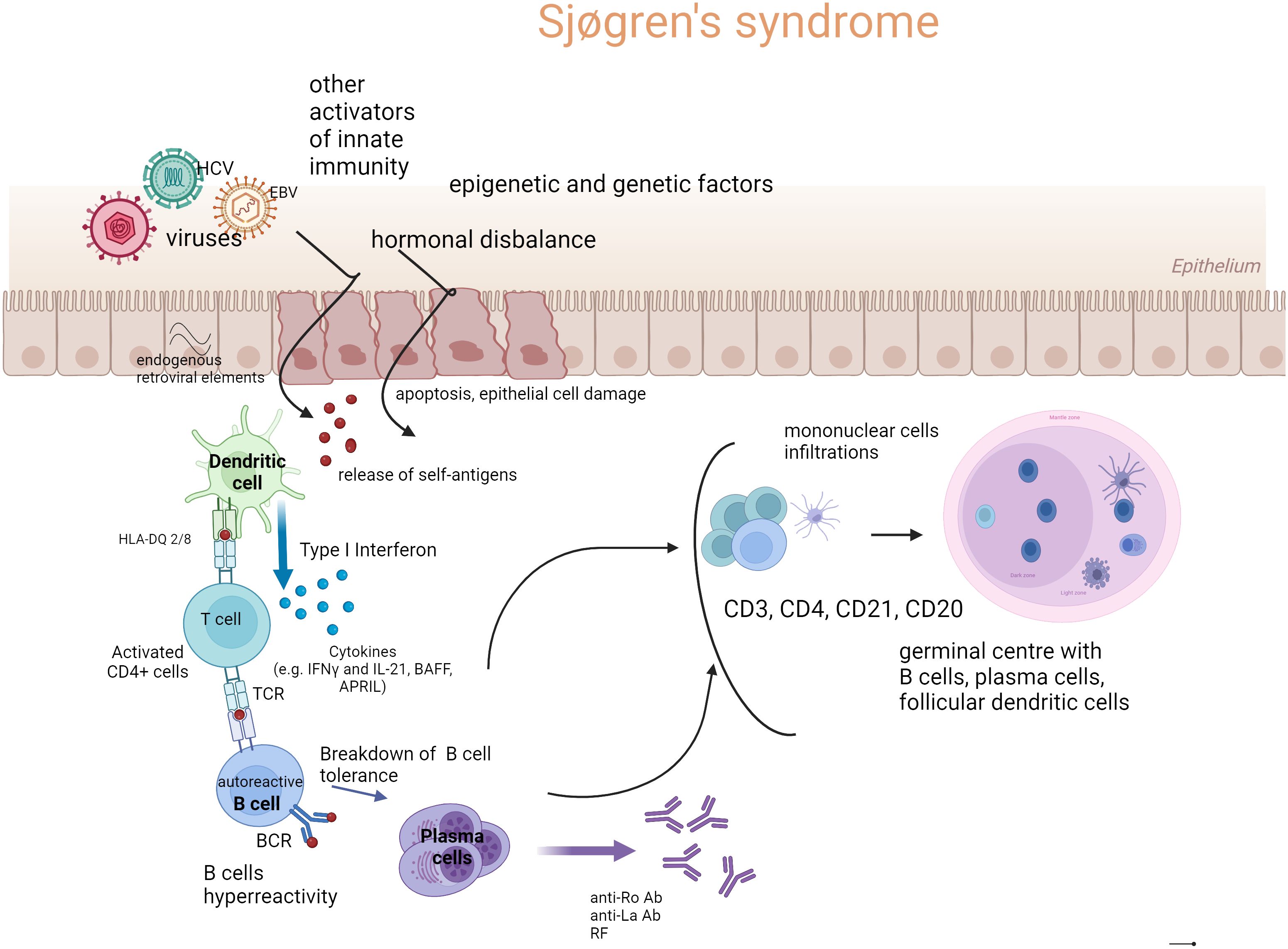
Figure 1. The outline of Sjögren’s syndrome pathogenesis (Created with BioRender.com). Genetic susceptibility, environmental factors such as mainly viral infections, but also bacterial infections, dysbiosis (microbiome disbalance), endogenous retroviral elements, hormonal disbalance (estrogen deficiency), and ultraviolet radiation (UV) are involved in epithelial cell damage and apoptosis. The endothelial cell damage and release of autoantigens stimulate innate and acquired immunity. The first-line immune response leads to the activation of dendritic cells (DC), especially of a type I signature of the IFN pathway, which releases interferons (IFNs). Subsequently, cross-talk between DC and T cells and their production of B cell stimulating factors (e.g., IFNγ, B cell activating factor (BAFF), a proliferation-inducing ligand (APRIL), and interleukin-21 (IL-21)) cause the activation of B cells and their hyperreactivity. Newly formed plasma cells produce autoantibodies, to ribonucleoproteins in particular, such as anti-Ro/anti-La antibodies and rheumatoid factors (RF) in various classes of immunoglobulins. In target organs (primarily salivary glands), cell infiltrates are formed, consisting of mononuclear cells such as dendritic cells (mainly CD21), T lymphocytes (CD4+, CD3+), CD20 B lymphocytes, and plasma cells. Under the influence of chronic and intense stimulation, tertiary lymphatic structures may be formed—germinal centers (GC), and activation of B cells beyond the control of T lymphocytes may occur. Such stimulation carries the risk of developing a lymphoproliferative process in pSS.
Diagnosis of primary Sjögren’s syndrome
To confirm pSS diagnosis, the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria should be met, and a differential diagnosis should also be performed to exclude other diseases (47). The elements of pSS classification criteria are presented in Table 1.
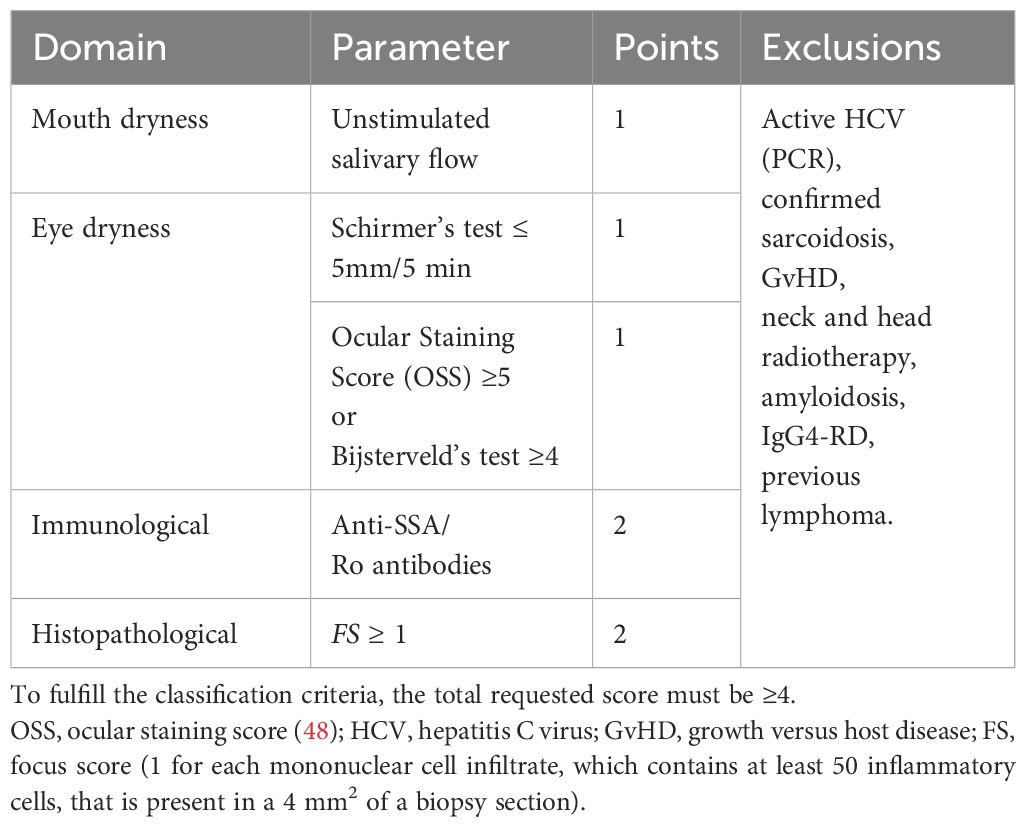
Table 1. EULAR/ACR classification criteria for primary Sjogren’s syndrome diagnosis (47).
The classification criteria are met if the final scoring reaches a minimum of four points and the exclusion criteria are not fulfilled.
A short introduction to IgG4-related disease
IgG4-related disease is a chronic autoinflammatory disease of an unknown etiology. The principal symptoms of IgG4-RD include the elevation of IgG4 serum concentration, characteristic infiltrations of mononuclear cells, fibrosis, and formation of pseudotumors. C-reactive protein elevation and fever are not characteristic of IgG4-RD as for other autoimmune-autoinflammatory diseases, e.g., Castleman’s disease, antineutrophil cytoplasmic antibody-associated vasculitis (especially eosinophilic granulomatosis with polyangiitis EGPA)—a phenomenon which should be considered in a differential diagnosis.
A histopathological examination is considered the gold standard of IgG-RD diagnosis, revealing storiform fibrosis, inflammation with infiltration by lymphocyte and plasmatic cells, eosinophilia, and obliterative phlebitis. The formation of germinal centers and lymphoid follicles is also observed.
A storiform pattern of fibrosis (similar to the woven fabric pattern in microscopy assessment) is considered typical for IgG4-RD, however, it can also be observed in neoplastic changes. In the late stage of IgG4-RD, acellular fibrosis dominates (49). Storiform fibrosis is accompanied by dense lymphoplasmacytic infiltrates, often partially eosinophilic infiltrates and obliterative phlebitis.
Obliterative vascular disease is a unique feature of IgG4-RD, not observed in other vasculitis, e.g., granulomatosis with polyangiitis (GPA), polyarteritis nodosa (PN), or microscopic polyangiitis (MPA). In IgG4-RD, vessel wall necrosis is not observed (49).
In infiltrations, IgG4 + plasma cells are dominant (greater than 10 IgG4 + plasma cells/HPF) with a ratio of IgG4 +/IgG + cells greater than 40%.
The current classification criteria for IgG4 - RD diagnosis were established and published in 2019 (36). The verification of whether a patient meets the classification criteria of IgG4-RD is divided into three steps.
The first step is based on the entry criteria (Table 2).

Table 2. Entry criteria for the evaluation for IgG4–RD according to ACR/EULAR classification criteria.
The second step is only possible after meeting the entry criteria. It requires a consideration of possible exclusions, which are listed in Table 3.

Table 3. List of exclusions (36).
In the final step, verification of the inclusion criteria is performed. Inclusion criteria are divided into eight domains. The findings in each domain are weighted, and cases with 20 points or more are classified as IgG4-RD. However, according to the ACR/EULAR IgG4 classification criteria, if any exclusion criterium is met, the patient cannot be further considered as having IgG4 -RD. As a rule, the highest weighted item in each domain is scored (Table 4).
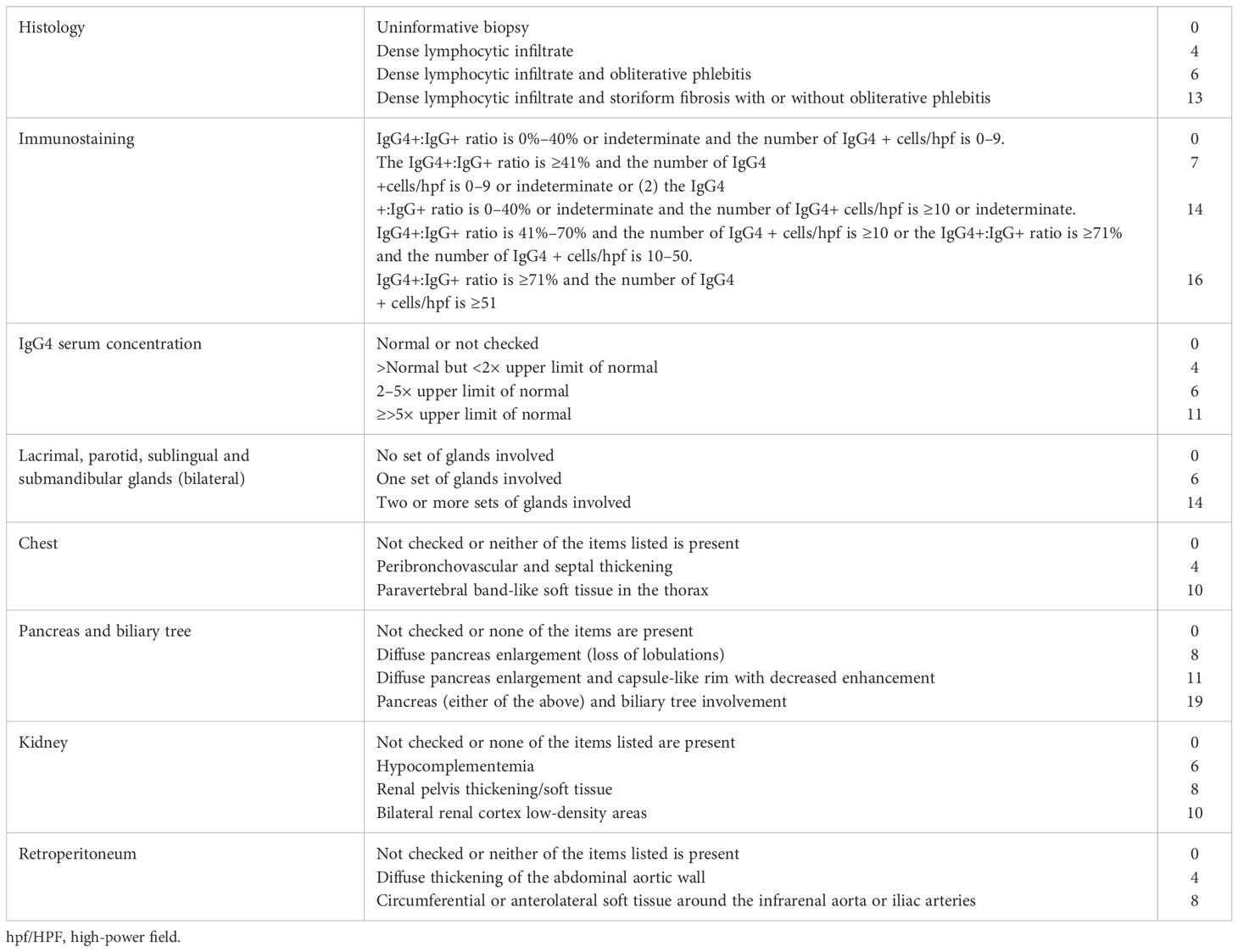
Table 4. Inclusion domains and items included in the IgG4 ACR/EULAR classification criteria (36).
Additional notes: a) in the immunostaining domain biopsies from lymph nodes, mucosal surfaces of the gastrointestinal tract and skin are not acceptable; b) ‘Indeterminate’ means that a pathologist is unable to clearly quantify the number of positively stained cells within an infiltrate, yet can still ascertain that the number of cells is at least 10/hpf.
The introduction of the 2019 ACR/EULAR IgG4-RD criteria is an attempt to exclude—with the optimal precision in the diagnosis of this multiorgan and multisymptomatic disease—all mimickers and maintain sufficient sensitivity and specificity (the first validation cohort had a specificity of 99.2% and sensitivity of 85.5%, and the second validation a specificity of 97.8% and sensitivity of 82.0%) (36).
Antibodies in the IgG4 subclass
In clinical practice, adverse reactions to food ingredients manifest with symptoms of intolerance as well as with the production of specific antibodies in various classes of immunoglobulins, especially in IgE, IgG, or IgG4. In food intolerance, IgG4 antibodies are associated with basophils and with mastocyte degranulation—elements similarly active in other allergic reactions. In food intolerance, total IgG and IgG4 are significantly increased. While an initial elevation of IgE is observed, a subsequent increase in IgG/IgG4 ratio constitutes a delayed persistent phase (from 24 hours to 5 days) of food intolerance (50). Therefore, for food allergy, diagnostic ELISA tests have been developed for specific IgG4 antibodies against food antigens (50).
In rheumatoid arthritis (RA), specific anti-citrullinated cyclic peptide antibodies (ACPAs) in the IgG4 class of immunoglobulins may occur. Carbone et al. (51) presented a metanalysis from three studies on 328 RA patients in total and concluded that elevated IgG4 ACPA was observed in 35.98% of patients. The Fab segments of RF can react with the Fc part of the IgG molecule and mainly generate IgM(RF)-IgG immune complexes. They can also recognize the Fc domains of IgG4 to form IgG4-RF immune complexes which may activate the complement system and cause synovial injury (52). In patients with RA, increased IgG4 serum concentration was also observed as was the presence of IgG4 in the inflammatory active synovium.
The focus on IgG4 led to the search for connections between this immunoglobulin and other autoimmune diseases. It seems obvious that, especially in IgG4-RD, various IgG4 associations were analyzed. Kiyama et al. (53) showed that in spite of the elevation of IgG4 in the sera of patients with IgG4-RD, the production of antinuclear antibodies (ANA) antibodies in the IgG4 subclass was not observed. Therefore, the authors suggested that the elevation of IgG4 levels is non-specific (epiphenomenon?) and not pathogenetic, concluding that IgG4-RD is not an autoimmune disease.
Demirci et al. (54) found a significant increase in serum levels of IgG4 in patients with celiac disease (CD) versus the control group and the authors concluded that IgG4 levels can constitute a biochemical marker for CD.
In an animal model in the study by Bi et al. (55), it was found that switching anti – ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) to the IgG4 subclass had a protective effect in a IgG4-mediated disease, thrombotic thrombocytopenic purpura (TTP), in contrast to IgG3 anti-ADAMTS13 antibodies. In pemphigus foliaceus (PF), autoantibodies to desmoglein 1 (anti-Dsg1) in the IgG4 subclass of immunoglobulins played an opposite role by reducing FcγR-binding affinity or ablating FcγRs, which enhanced their pathogenic function. In PF, the IgG1 subclass was revealed to be non-pathogenic (56).
An interesting topic, however beyond the scope of this article, is the existence of IgG4-nervous system dependent diseases including myasthenia with muscle specific tyrosine kinase (MuSK) antibodies or chronic inflammatory demyelinating polyneuropathy (CIDP) with neurofascin-155, contactin-1/CASPR-1 antibodies, and anti- LGI 1 as well as in CASPR2-associated limbic encephalitis, neuromyotonia, and Morvan syndrome (57, 58). The cases of anti-IgLON5 and anti-DPPX-spectrum CNS diseases related to IgG4 were also described (59).
Sjögren’s syndrome and antibodies in the immunoglobulin G4 class of immunoglobulins
Primary Sjögren’s syndrome is relatively common among systemic diseases of a connective tissue and is associated with the hyperactivity of B lymphocytes, which increases the possibility of the emergence of autoantibodies.
Antibodies to ribonucleoproteins are characteristic of pSS, but only anti-Ro/SS-A antibodies are included in the current classification criteria for this disease (47). However, antinuclear antibodies are present in the majority of pSS patients. Kiyjama et al.’s work (53) shows that ANA-IgG4 antibodies are rare in rheumatic diseases. Among the patients analyzed in the study only one, with pSS, had ANA-IgG4 antibodies. This patient did not meet the criteria for IgG4-RD. Although he had anti-Ro/Ss-A antibodies, the researchers did not indicate whether those included anti-Ro-IgG4. Antinuclear antibodies are usually present in IgG1-3 subclasses. They can also be present in low titers in IgG4-RD (a higher titer constitutes an exclusion criterion in IgG-RD diagnosis).
Wahren and colleagues (60), in 1994, investigated humoral response to Ro60kDa/SS-A and La/SS-B antigens and found that IgM and IgG (1-4) responses to these antigens coexist, while among IgG antibodies, IgG1 dominates.
In Sjögren’s syndrome, polyclonal hypergammaglobulinemia is one of the main laboratory findings and immunoglobulins usually belong to various classes. In pSS, hyperglobulinemia is associated with high serum levels of RF (IgM, IgA, and IgG). In rare cases, immunoglobulins of one class are responsible for the hyperglobulinemia. Liu et al. (61) studied immunoglobulin profiles in pSS and SLE patients. They showed that in pSS, IgG1, IgG2, and IgG3 were usually increased among the subclasses of immunoglobulin G, while there was a visible and significant reduced level of IgG4 in the sera of pSS patients and a decrease of the IgG4 to IgG ratio. Interestingly, in a cited work, the serum concentrations of IgG1-3 immunoglobulins were similar in pSS and SLE patients, however, a difference was observed between pSS and SLE in the IgG4 concentration. The authors did not find an explanation for this fact. In another work, a lower serum concentration of IgG4 in the pSS group (p=0.0435) and a significantly lower (p=0.0035) ratio of serum IgG4 to total IgG compared to healthy subjects, were confirmed. This study also showed a weak negative correlation (r= - 0.274) between C4 component complement levels and IgG4 (62).
There are also studies of pSS patients in which an increase of Ig-G4 serum concentration was shown, but it should be taken into account that such results concerned a small number of pSS patients, only 7.5% percent (n=10) of the pSS group (63).
The role of IgG4 in a context of the development of lymphomas and sialadenitis in primary Sjögren’s syndrome and IgG4-RD
Primary Sjögren’s syndrome increases the risk of lymphoma development due to lymphatic organ involvement, especially B cells. In pSS, all elements of the immune response leading to the stimulation of maturation and activation of B lymphocytes and the disruption of the controlling mechanisms (breaching the control of, among others, T lymphocytes) result in the hyperreactivity of B cells.
Mucosa-associated lymphoid tissue (MALT) lymphoma is the most often observed lymphoma emerging during a course of pSS. Its different types are distinguished depending on their localization: gut-associated lymphoid tissue (GALT), bronchus-associated lymphoid tissue (BALT), or nasal-associated lymphoid tissue (NALT) lymphoma. Salivary gland MALT lymphoma is the most frequent localization of the initial lymphoproliferation in pSS (64, 65).
The main risk factors for lymphoma development in pSS are presented in Table 5 (65).
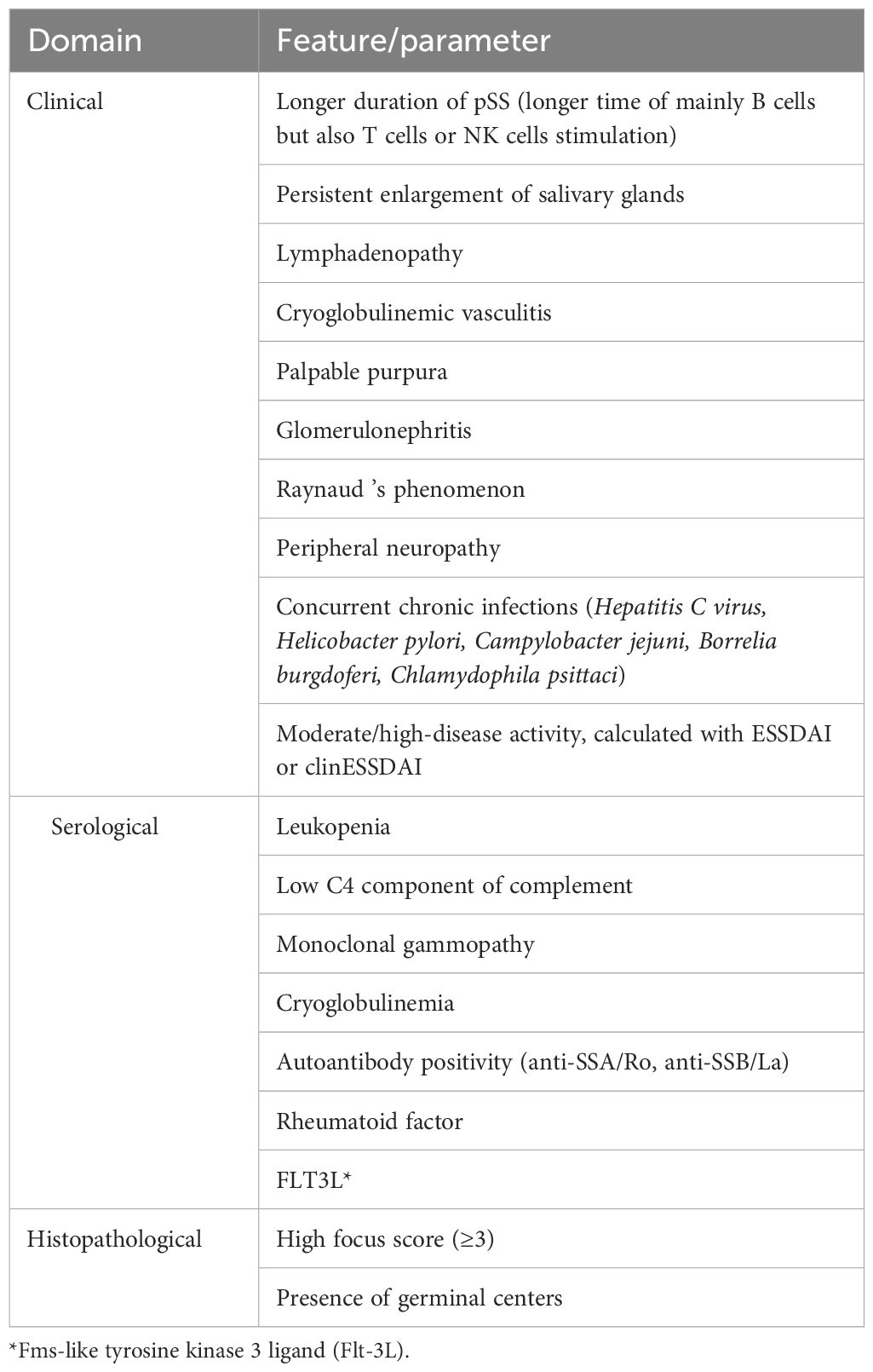
Table 5. The risk factors for lymphoma development in pSS. Risk factors confirmed by Fragkioudaki et al.’s study (65) are in blue.
Risk factors for lymphoma development include Fms-like tyrosine kinase 3 ligand (Flt-3L), a type I transmembrane protein activating Flt-3 which stimulates progenitor cells in bone marrow and blood. A study revealed that high levels of Flt-3L were associated with lymphoma (66). The Flt3/Flt3L cascade is responsible for the development and maintenance of DCs (67). Flt3L – IgG4 (modified Fc region) has been investigated as a drug in immunotherapy and adjuvant in vaccines and may potentially become a drug for autoimmune diseases (68).
Lymphomas such as MALT lymphoma and diffuse large B cell lymphoma (DLBCL) can also develop in IgG4-related sialadenitis and orbital IgG4-RD localization (69).
A picture of salivary glands in ultrasonography with a high SGUS score evaluation can be a diagnostic predictor of risk of lymphoma development, particularly when it coincides with a high minor salivary gland biopsy (MSGB) focus score (70). Although this imaging method is not included in pSS classification criteria, its results may suggest a need for further lymphoma diagnostics. Another useful method, which can be used as a second step to better establish a place suspected of tumor development, infiltration, and lymphoproliferation, is magnetic resonance imaging (MRI) (71).
A craniofacial MRI image from a patient with pSS is shown in Figure 2.
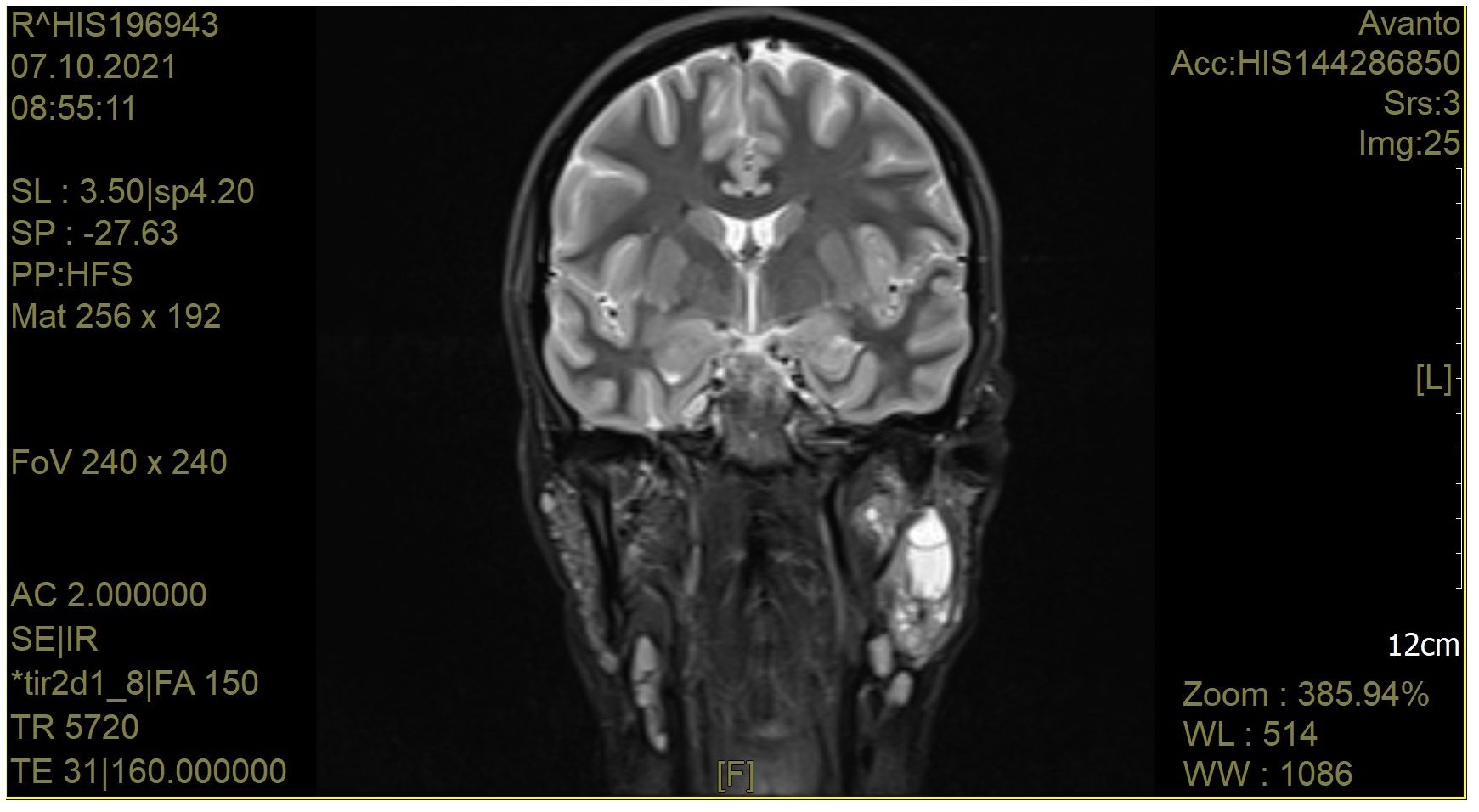
Figure 2. A craniofacial magnetic resonance image of a patient with pSS. The left parotid gland is bulging outwards. The right parotid gland is not enlarged. Altered vesicular structure of the parotid and submandibular glands. In the left parotid gland in the deep lobe, cystic lesions are visible. A group of cystic structures is visible in the central part of the left parotid gland. After i. v. contrast administration, no significant signal amplification was observed. Currently, the salivary ducts are not dilated. There are numerous lymphatic nodules in the parotid glands (approx. 6 mm). In the submandibular area, there are single lymph nodes up to 11 mm long. There are numerous lymph nodes in the neck, measuring up to 15 mm.
IgG4-related sialadenitis is quite often present in this disease and may suggest pSS, particularly in the presence of changes in the major salivary glands. In such instances, the IgG4 concentration in the serum should be determined and an analysis of cells from a biopsy of the minor salivary glands performed. An examination of the material from a biopsy of the major salivary glands is useful both for establishing a diagnosis of IgG4-RD and verifying whether lymphoproliferation occurred.
Pulmonary nodular lymphoid hyperplasia (PNLH, pulmonary pseudolymphoma) is characterized by an increase in IgG4+ plasma cells and a higher IgG4+/IgG+ plasma cell ratio compared to other pulmonary lymphoid proliferations. In contrast, in a low-grade B cell lymphoma and BALT lymphoma, which may be associated with pSS, such a phenomenon was not confirmed (72, 73).
The presence of pSS, as a preexisting autoimmune disorder, was also reported in cases of bronchial-associated lymphoid tissue lymphoma (a relatively rare disease) (74, 75).
There are also various reports of an increased risk of carcinogenesis in IgG4-RD. In Yu et al.’s (76) metanalysis of 10 studies, the overall standardized incidence ratio (SIR) estimated an increased risk of overall cancer in IgG4-RD patients (SIR 2.57 95% CI 1.72–3.84) compared with the general population. This risk was particularly high in cases of pancreatic cancer and lymphoma (SIR 4.07 95% CI 1.04–15.92, SIR 69.17 95% CI 3.91–1223.04, respectively) (76).
In IgG4-RD the main risk factors for malignancy development are currently considered to be (77):
- autoimmune pancreatitis
- eosinophilia
However, the data about eosinophilia are conflicting. In some reports, it is presented as a protective factor (78) while in others, as a risk for cancer development (77).
IgG4-RD lymphadenopathy in some reports was misdiagnosed as Hodgkin’s lymphoma (79) but most reported cases indicate that MALT lymphomas more frequently occur in orbital IgG4-RD. The majority of these reports are derived from Asia (76). Further studies and meta-analyses should be performed to reliably assess the actual situation.
Taking into account that the possibility of lymphoma development exists both in pSS and IgG4-RD, but is higher in pSS, the differentiation between these two diseases may, in some especially serologically unclear cases, be crucial for diagnosis, proper treatment, and estimating the risk of lymphoma.
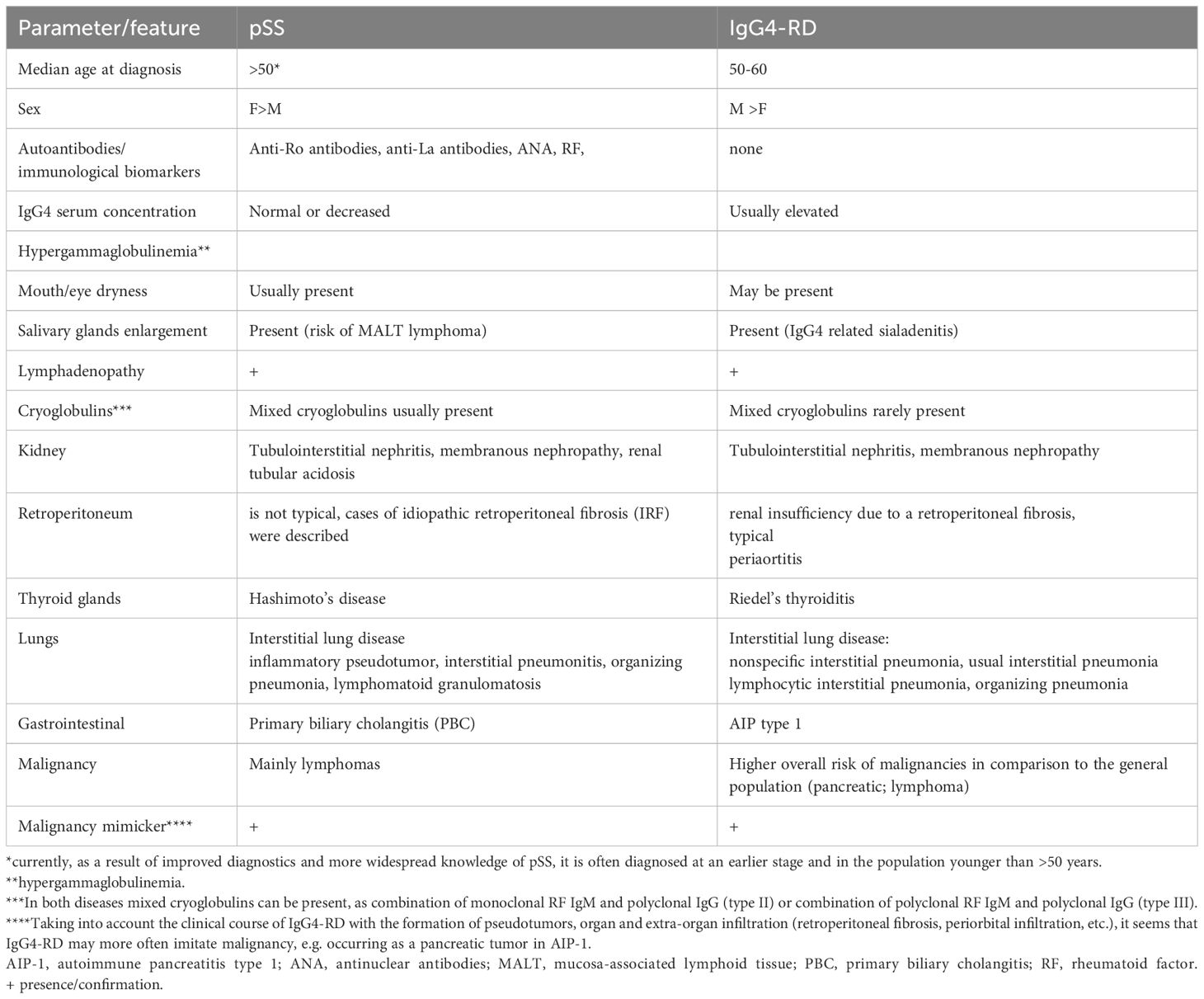
A comparison of the main clinical features of primary Sjogren’s syndrome and IgG4-related disease
A summary of the main features of pSS and IgG4-RD is presented in Table 6.
When analyzing organ involvement in pSS compared to IgG4-RD, similarities and tropism of certain organs, e.g., salivary glands, in both diseases should be taken into account. Certain locations and types of lesions may be more indicative of one of these diseases. Thus, changes only in submandibular glands (e.g., Kuttner’s tumor, defined as a chronic sclerosing sialadenitis) without parotid gland involvement is a common feature of IgG4-RD and is rarely seen in pSS (8, 80).
Based on the studies indicating a reduction in IgG4 in the serum of patients with pSS (60), in the absence of specific autoantibodies, the assessment of IgG4 concentration may prove vital for the differentiation between pSS and IgG4-RD, with an increased concentration pointing to IgG4-RD and a decreased one indicating pSS.
Despite adopting the presence of several diseases as an exclusion criterium for the IgG4-RD diagnosis, there is an ongoing discussion on the potential possibility of IgG4-RD overlapping or co-existing with other diseases (81, 82). Addressing this problem requires further research and data gathering, which exceed the scope of this work.
Conclusions
The main difference between IgG4-RD and pSS in the context of the presence of IgG4 is that while the serum concentration of IgG4 in IgG4-RD is typically elevated, the same phenomenon does not occur in pSS and some studies indicate a decrease in IgG4 serum level in pSS below its normal range.
This phenomenon may have a diagnostic value—in particular in the context of similar clinical features and affected organs in pSS and IgG4-RD—but its significance is not clear and this issue requires more research.
The role of IgG4 in pSS, as well as in IgG4-RD, is not fully understood and should be a subject of a further research. Currently, the still incomplete understanding of the dual role IgG4 plays in the immune response undermines the proper interpretation of the obtained test results.
Author contributions
MM: Conceptualization, Formal analysis, Supervision, Visualization, Writing – original draft, Writing – review & editing, Data curation, Methodology, Resources. KK: Data curation, Formal analysis, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article
Acknowledgments
We would like to thank Krzysztof Maśliński for the linguistic correction of the text.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jonsson R, Vogelsang P, Volchenkov R, Espinosa A, Wahren-Herlenius M, Appel S. The complexity of Sjögren's syndrome: novel aspects on pathogenesis. Immunol Lett. (2011) 141:1–9. doi: 10.1016/j.imlet.2011.06.007
2. Park Y, Lee J, Koh JH, Choe JY, Sung YK, Lee SS, et al. Clinical influences of anticentromere antibody on primary Sjögren's syndrome in a prospective Korean cohort. Korean J Intern Med. (2021) 36:1492–503. doi: 10.3904/kjim.2020.146
3. Gottenberg JE, Mignot S, Nicaise-Rolland P, Cohen-Solal J, Aucouturier F, Goetz J, et al. Prevalence of anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with primary Sjögren's syndrome. Ann Rheum Dis. (2005) 64:114–7. doi: 10.1136/ard.2003.019794
4. Maślińska M, Mańczak M, Kwiatkowska B, Ramsperger V, Shen L, Suresh L. IgA immunoglobulin isotype of rheumatoid factor in primary Sjögren's syndrome. Rheumatol Int. (2021) 41:643–9. doi: 10.1007/s00296-020-04782-3
5. Meek B, Kelder JC, Claessen AME, van Houte AJ, Ter Borg EJ. Rheumatoid factor isotype and Ro epitope distribution in primary Sjögren syndrome and rheumatoid arthritis with keratoconjunctivitis sicca. RheumatolInt. (2018) 38:1487–93. doi: 10.1007/s00296-018-4090-5
6. Deng X, Li J, Hou S, Ci B, Liu B, Xu K. Prevalence and impact of Sjögren's syndrome in primary biliary cholangitis: a systematic review and meta-analysis. Ann Hepatol. (2022) 27:100746. doi: 10.1016/j.aohep.2022.100746
7. Baldini C, Ferro F, Mosca M, Fallahi P, Antonelli A. The association of Sjögren syndrome and autoimmune thyroid disorders. Front Endocrinol (Lausanne). (2018) 9:121. doi: 10.3389/fendo.2018.00121
8. Kamiński B, Błochowiak K. Mikulicz's disease and Küttner's tumor as manifestations of IgG4-related diseases: a review of the literature. Reumatologia. (2020) 58:243–50. doi: 10.5114/reum.2020.98437
9. Yamamoto M, Harada S, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, et al. Clinical and pathological differences between Mikulicz's disease and Sjögren's syndrome. Rheumatology. (2005) 44:227–34. doi: 10.1093/rheumatology/keh447
10. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: From structure to effector functions. Front Immunol. (2014) 5:520. doi: 10.3389/fimmu.2014.00520
11. Schauer U, Stemberg F, Rieger CH, Borte M, Schubert S, Riedel F, et al. IgG. IgG subclass concentrations in certified reference material 470 and reference values for children and adults determined with the binding site reagents. Clin Chem. (2003) 49:1924–9. doi: 10.1373/clinchem.2003.022350
12. Parker AR, Skold M, Ramsden DB, Ocejo-Vinyals JG, López-Hoyos M, Harding S. The clinical utility of measuring IgG subclass immunoglobulins during immunological investigation for suspected primary antibody deficiencies. Lab Med. (2017) 48:314–25. doi: 10.1093/labmed/lmx058
13. Moss RB, Carmack MA, Esrig S. Deficiency of IgG4 in children: association of isolated IgG4 deficiency with recurrent respiratory tract infection. J Pediatr. (1992) 120:16–21. doi: 10.1016/S0022-3476(05)80590-6
14. Rawat A, Suri D, Gupta A, Saikia B, Minz RW, Singh S. Isolated immunoglobulin G4 subclass deficiency in a child with bronchiectasis. Indian J Pediatr. (2014) 81:932–3. doi: 10.1007/s12098-013-1247-9
15. Koutroumpakis F, Evans Phillips A, Yadav D, Machicado JD, Ahsan M, Ramos Rivers A, et al. Serum IgG4 subclass deficiency defines a distinct, commonly encountered, severe inflammatory bowel disease subtype. Inflammation Bowel Dis. (2021) 27:855–63. doi: 10.1093/ibd/izaa230
16. Rispens T, Huijbers MG. The unique properties of IgG4 and its roles in health and disease. Nat Rev Immunol. (2023) 23:763–78. doi: 10.1038/s41577-023-00871-z
17. Sohi MK, Corper AL, Wan T, Steinitz M, Jefferis R, Beale D, et al. Crystallization of a complex between the Fab fragment of a human immunoglobulin M (IgM) rheumatoid factor (RF-AN) and the Fc fragment of human IgG4. Immunology. (1996) 88:636–41. doi: 10.1046/j.1365-2567.1996.d01-692.x
18. Corper AL, Sohi MK, Bonagura VR, Steinitz M, Jefferis R, Feinstein A, et al. Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody-antigen interaction. Nat Struct Biol. (1997) 4:374–81. doi: 10.1038/nsb0597-374
19. Lighaam L, Rispens T. The immunobiology of immunoglobulin G4. Semin Liver Disease. (2016) 36:200–15. doi: 10.1055/s-0036-1584322
20. Trampert DC, Hubers LM, van de Graaf SFJ, Beuers U. On the role of IgG4 in inflammatory conditions: lessons for IgG4-related disease. Biochim Biophys Acta (BBA) - Mol Basis Dis. (2018) 1864:1401–9. doi: 10.1016/j.bbadis.2017.07.038
21. Schuurman J, Labrijn AF, Parren PW. Fab-arm exchange: what's in a name? MAbs. (2012) 4:636. doi: 10.4161/mabs.22075
22. Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, et al. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol. (2014) 426:630–44. doi: 10.1016/j.jmb.2013.10.039
23. Kozłowska K, Rydlewska M, Ząbczyńska M, Pocheć E. Glikozylacja IgG w chorobach autoimmunizacyjnych. Postępy Higieny i Medycyny Doświadczalnej. (2018) 72:975–90. doi: 10.5604/01.3001.0012.7351
24. Wang TT. IgG fc glycosylation in human immunity. Curr Top Microbiol Immunol. (2019) 423:63–75. doi: 10.1007/82_2019_152
25. Konno N, Sugimoto M, Takagi T, Furuya M, Asano T, Sato S, et al. Changes in N-glycans of IgG4 and its relationship with the existence of hypocomplementemia and individual organ involvement in patients with IgG4-related disease. PLoS One. (2018) 13:e0196163. doi: 10.1371/journal.pone.0196163
26. Haddad G, Lorenzen JM, Ma H, de Haan N, Seeger H, Zaghrini C, et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J Clin Invest. (2021) 131:e140453. doi: 10.1172/JCI140453
27. Oskam N, Damelang T, Streutker M, Ooijevaar-de Heer P, Nouta J, Koeleman C, et al. Factors affecting IgG4-mediated complement activation. Front Immunol. (2023) 14:1087532. doi: 10.3389/fimmu.2023.1087532
28. Moriyama M, Nakamura S. Th1/Th2 immune balance and other T helper subsets in IgG4-related disease. Curr Top Microbiol Immunol. (2017) 401:75–83. doi: 10.1007/82_2016_40
29. Pavón-Romero GF, Parra-Vargas MI, Ramírez-Jiménez F, Melgoza-Ruiz E, Serrano-Pérez NH, Teran LM. Allergen immunotherapy: current and future trends. Cells. (2022) 11:212. doi: 10.3390/cells11020212
30. Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. (2011) 305:1460–8. doi: 10.1001/jama.2011.406
31. Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, et al. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. (2005) 7:990–6. doi: 10.1016/j.micinf.2005.03.036
32. Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol. (2010) 104:455–64. doi: 10.1179/136485910X12786389891407
33. Bushara O, Escobar DJ, Weinberg SE, Sun L, Liao J, Yang GY. The possible pathogenic role of IgG4-producing plasmablasts in stricturing Crohn's disease. Pathobiology. (2022) 89:187–97. doi: 10.1159/000521259
34. Xu Wl., Ling Yc., Wang Zk., Deng F. Diagnostic performance of serum IgG4 level for IgG4-related disease: a meta-analysis. Sci Rep. (2016) 6:32035. doi: 10.1038/srep32035
35. Rowntree S, Platts-Mills TA, Cogswell JJ, Mitchell EB. A subclass IgG4-specific antigen-binding radioimmunoassay (RIA): comparison between IgG and IgG4 antibodies to food and inhaled antigens in adult atopic dermatitis after desensitization treatment and during development of antibody responses in children. J Allergy Clin Immunol. (1987) 80:622–30. doi: 10.1016/0091-6749(87)90017-0
36. Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, et al. Members of the ACR/EULAR IgG4-RD classification criteria working group. The 2019 American college of rheumatology/European league against rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. (2020) 79:77–87. doi: 10.1136/annrheumdis-2019-216561
37. Yu KH, Chan TM, Tsai PH, Chen CH, Chang PY. Diagnostic performance of serum IgG4 levels in patients with IgG4-related disease. Med (Baltimore). (2015) 94:e1707. doi: 10.1097/MD.0000000000001707
38. Ebbo M, Grados A, Bernit E, Vély F, Boucraut J, Harlé JR, et al. Pathologies associated with serum IgG4 elevation. Int J Rheumatol. (2012) 2012:602809. doi: 10.1155/2012/602809
39. Carruthers M, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. (2015) 74:14–8. doi: 10.1136/annrheumdis-2013-204907
40. Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della-Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. (2015) 74:190–5. doi: 10.1136/annrheumdis-2014-205233
41. Ogawa Y, Takeuchi T, Tsubota K. Autoimmune epithelitis and chronic inflammation in Sjögren's syndrome-related dry eye disease. Int J Mol Sci. (2021) 22:11820. doi: 10.3390/ijms222111820
42. Del Papa N, Minniti A, Lorini M, Carbonelli V, Maglione W, Pignataro F, et al. The role of interferons in the pathogenesis of Sjögren's syndrome and future therapeutic perspectives. Biomolecules. (2021) 11:251. doi: 10.3390/biom11020251
43. Smulski CR, Eibel H. BAFF and BAFF-receptor in B cell selection and survival. Front Immunol. (2018) 9:2285. doi: 10.3389/fimmu.2018.02285
44. Goules AV, Tzioufas AG. Primary Sjögren’s syndrome: clinical phenotypes, outcome and the development of biomarkers. Immunol Res. (2017) 65:331–44. doi: 10.1007/s12026-016-8844-4
45. Lerner MR, Boyle JA, Hardin JA, Steitz JA. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. (1981) 211:400–2. doi: 10.1126/science.6164096
46. Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun. (2012) 39:77–82. doi: 10.1016/j.jaut.2012.01.014
47. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American college of rheumatology/European league against rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. (2017) 69:35–45. doi: 10.1002/art.39859
48. Whitcher JP, Shiboski CH, Shiboski SC, Heidenreich AM, Kitagawa K, Zhang S, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren's Syndrome International Registry. Am J Ophthalmol. (2010) 149:405–15. doi: 10.1016/j.ajo.2009.09.013
49. Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. (2014) 9:315–47. doi: 10.1146/annurev-pathol-012513-104708
50. Platts-Mills TAE, Keshavarz B, Wilson JM, Li RC, Heymann PW, Gold DR, et al. An overview of the relevance of IgG4 antibodies in allergic disease with a focus on food allergens. Children (Basel). (2021) 8:418. doi: 10.3390/children8050418
51. Carbone G, Wilson A, Diehl SA, Bunn J, Cooper SM, Rincon M. Interleukin-6 receptor blockade selectively reduces IL-21 production by CD4 T cells and IgG4 autoantibodies in rheumatoid arthritis. Int J Biol Sci. (2013) 9:279–88. doi: 10.7150/ijbs.5996
52. Sakthiswary R, Shaharir SS, Wahab AA. Frequency and clinical significance of elevated IgG4 in rheumatoid arthritis: A systematic review. Biomedicines. (2022) 10:558. doi: 10.3390/biomedicines10030558
53. Kiyama K, Yoshifuji H, Kandou T, Hosono Y, Kitagori K, Nakashima R, et al. Screening for IgG4-type anti-nuclear antibodies in IgG4-related disease. BMC Musculoskelet Disord. (2015) 16:129. doi: 10.1186/s12891-015-0584-4
54. Demirci H, Polat Z, Ozturk K, Kekilli M, Kantarcioglu M, Sahiner F, et al. The degree of mucosal damage to the small intestine and serum immunoglobulin G4 levels correlate with celiac disease. Eur J Gastroenterol Hepatol. (2015) 27:781–4. doi: 10.1097/MEG.0000000000000362
55. Bi Y, Su J, Zhou S, Zhao Y, Zhang Y, Zhang H, et al. Distinct impact of IgG subclass on autoantibody pathogenicity in different IgG4-mediated diseases. Elife. (2022) 11:e76223. doi: 10.7554/eLife.76223
56. Koneczny I. Update on IgG4-mediated autoimmune diseases: New insights and new family members. Autoimmun Rev. (2020) 19(10):102646. doi: 10.1016/j.autrev.2020.102646
57. Huijbers MG, Querol LA, Niks EH, Plomp JJ, van der Maarel SM, Graus F, et al. The expanding field of IgG4-mediated neurological autoimmune disorders. Eur J Neurol. (2015) 22:1151–61. doi: 10.1111/ene.12758
58. Dalakas MC. Autoimmune neurological disorders with IgG4 antibodies: a distinct disease spectrum with unique IgG4 functions responding to anti-B cell therapies. Neurotherapeutics. (2022) 19:741–52. doi: 10.1007/s13311-022-01210-1
59. Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjögren's syndrome. Rheum Dis Clin North Am. (2016) 42:419–34. doi: 10.1016/j.rdc.2016.03.002
60. Wahren M, Ringertz NR, Pettersson I. IgM and IgG subclass distribution of human anti-Ro/SSA 60 kDa autoantibodies. Scandinavian J Immunol. (1994) 39:179–83. doi: 10.1111/j.1365-3083.1994.tb03357.x
61. Liu Y, Li J. Preferentially immunoglobulin (IgG) subclasses production in primary Sjögren’s syndrome patients. Clin Chem Lab Med (CCLM). (2012) 50(2):345–9. doi: 10.1515/cclm.2011.771
62. Maślińska M, Wojciechowska B, Mańczak M, Kwiatkowska B. Serum immunoglobulin G4 in Sjögren’s syndrome: a pilot study. Rheumatol Int. (2020) 40:555–61. doi: 10.1007/s00296-020-04529-0
63. Mavragani CP, Fragoulis GE, Rontogianni D, Kanariou M, Moutsopoulos HM. Elevated IgG4 serum levels among primary Sjögren's syndrome patients: do they unmask underlying IgG4-related disease? Arthritis Care Res (Hoboken). (2014) 5:773–7. doi: 10.1002/acr.22216
64. Voulgarelis M, Ziakas PD, Papageorgiou A, Baimpa E, Tzioufas AG, Moutsopoulos HM. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjogren syndrome. Medicine. (2012) 91:1–9. doi: 10.1097/MD.0b013e31824125e4
65. Fragkioudaki S, Mavragani CP, Moutsopoulos HM. Predicting the risk for lymphoma development in Sjogren syndrome: An easy tool for clinical use. Med (Baltimore). (2016) 95:25. doi: 10.1097/MD.0000000000003766
66. Tobón GJ, Renaudineau Y, Hillion S, Cornec D, Devauchelle-Pensec V, Youinou P, et al. The Fms-like tyrosine kinase 3 ligand, a mediator of B cell survival, is also a marker of lymphoma in primary Sjögren's syndrome. Arthritis Rheumatol. (2010) 1:3447–56. doi: 10.1002/art.27611
67. Wilson KR, Villadangos JA, Mintern JD. Dendritic cell Flt3 - regulation, roles and repercussions for immunotherapy. Immunol Cell Biol. (2021) 99:962–71. doi: 10.1111/imcb.12484
68. Ramos MI, Tak PP, Lebre MC. Fms-like tyrosine kinase 3 ligand-dependent dendritic cells in autoimmune inflammation. Autoimmun Rev. (2014) 2:117–24. doi: 10.1016/j.autrev.2013.09.010
69. Bledsoe JR, Wallace ZS, Stone JH, Deshpande V, Ferry JA. Lymphomas in IgG4-related disease: clinicopathologic features in a Western population. Virchows Arch. (2018) 5:839–52. doi: 10.1007/s00428-017-2286-9
70. Chatzis L, Goules AV, Pezoulas V, Baldini C, Gandolfo S, Skopouli FN, et al. A biomarker for lymphoma development in Sjogren's syndrome: Salivary gland focus score. J Autoimmun. (2021) 121:102648. doi: 10.1016/j.jaut.2021.102648
71. Alunno A, Leone MC, Giacomelli R, Gerli R, Carubbi F. Lymphoma and lymphomagenesis in primary Sjögren's syndrome. Front Med (Lausanne). (2018) 5:102. doi: 10.3389/fmed.2018.00102
72. Guinee DG Jr., Franks TJ, Gerbino AJ, Murakami SS, Acree SC, Kross MI. Pulmonary nodular lymphoid hyperplasia (pulmonary pseudolymphoma): the significance of increased numbers of IgG4-positive plasma cells. Am J Surg Pathol. (2010) 34:1812–9. doi: 10.1097/PAS.0b013e318282d0fa
73. Nishimura Y, Wien EA, Nishimura MF, Nishikori A, Sato Y, Otsuka F. Clinical characteristics and outcomes of IgG4-positive marginal zone lymphoma: Systematic scoping review. Pathol Int. (2022) 72:361–70. doi: 10.1111/pin.13251
74. Yachoui R, Leon C, Sitwala K, Kreidy M. Pulmonary MALT lymphoma in patients with Sjögren's syndrome. Clin Med Res. (2017) 15:6–12. doi: 10.3121/cmr.2017.1341e3766
75. Mole EN, Papadakos VT, Sfontouris CI. Bronchus-associated lymphoid tissue lymphoma (BALT) in a patient with primary Sjögren's syndrome. Mediterr J Rheumatol. (2017) 28:52–6. doi: 10.31138/mjr.28.1.52
76. Yu T, Wu Y, Liu J, Zhuang Y, Jin X, Wang L. The risk of Malignancy in patients with IgG4-related disease: a systematic review and meta-analysis. Arthritis Res Ther. (2022) 24:14. doi: 10.1186/s13075-021-02652-2
77. Liu Y, Fu J, Ning X, Li H, Ma X, Wang K, et al. Malignancy risk of immunoglobin G4-related disease: evidence from a large cohort multicenter retrospective study. Rheumatol Ther. (2021) 8:1207–21. doi: 10.1007/s40744-021-00326-8
78. Tang H, Yang H, Zhang P, Wu D, Zhang S, Zho J, et al. Malignancy and IgG4-related disease: the incidence, related factors and prognosis from a prospective cohort study in China. Sci Rep. (2020) 10:4910. doi: 10.1038/s41598-020-61585-z
79. Nakayama R, Matsumoto Y, Horiike S, Kobayashi S, Nakao R, Nagoshi H, et al. Close pathogenetic relationship between ocular immunoglobulin G4-related disease (IgG4-RD) and ocular adnexal mucosa-associated lymphoid tissue (MALT) lymphoma. Leuk Lymphoma. (2014) 55:1198–202. doi: 10.3109/10428194.2013.823494
80. Putra J, Ornstein DL. Küttner tumor: IgG4-related disease of the submandibular gland. Head Neck Pathol. (2016) 10:530–2. doi: 10.1007/s12105-016-0729-2
81. Baisya R, Yerram KV, Baby A, Devarasetti PK, Rajasekhar L. Immunoglobulin G4-related lesions in autoimmune diseases: unusual presentations at atypical sites-A tale of 2 cases with literature review. Eur J Rheumatol. (2023) 10:169–75. doi: 10.5152/eurjrheum.2023.23052
Keywords: immunoglobulin G4, Sjögren’s syndrome, IgG4-related disease, autoimmunity, lymphomas
Citation: Maslinska M and Kostyra-Grabczak K (2024) Immunoglobulin G4 in primary Sjögren’s syndrome and IgG4-related disease - connections and dissimilarities. Front. Immunol. 15:1376723. doi: 10.3389/fimmu.2024.1376723
Received: 26 January 2024; Accepted: 26 July 2024;
Published: 19 September 2024.
Edited by:
Inga Koneczny, Medical University of Vienna, AustriaReviewed by:
Kazunori Yamada, Kanazawa Medical University, JapanFederico Pratesi, University of Pisa, Italy
Hani S. Mousa, University of Cambridge, United Kingdom
Copyright © 2024 Maslinska and Kostyra-Grabczak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Maslinska, bWFzbGluc2thbUBnbWFpbC5jb20=
 Maria Maslinska
Maria Maslinska Kinga Kostyra-Grabczak
Kinga Kostyra-Grabczak