- 1Medical Oncology Department, Paoli-Calmettes Institute, Marseille, France
- 2Neurology Department, Assitance Publique Hôpitaux de Marseille, Aix-Marseille University, Marseille, France
- 3Referral Centre for Neuromuscular Diseases and Amyotrophic Lateral Sclerosis (ALS), Hôpital La Timone, Marseille, France
- 4Radiology Department, Assitance Publique Hôpitaux de Marseille, Aix-Marseille University, Marseille, France
- 5Nuclear Medicine Department, Paoli-Calmettes Institut, Marseille, France
- 6Team Immunity and Cancer, Centre de Recherche en Canceírologie de Marseille (CRCM), Inserm, U1068, Centre national de la recherche scientifique (CNRS), Unité Mixte de Recherche 7258 (UMR7258), Paoli-Calmettes Institute, Aix-Marseille University, Marseille, France
Neurological immune-related adverse events (irAEs) due to immune checkpoint inhibitors (ICI) are rare complications of immunotherapy, particularly dreadful for patients and clinical teams. Indeed, neurological irAEs are potentially severe and their diagnosis require prompt recognition and treatment. Additionally, the spectrum of neurological irAEs is broad, affecting either neuromuscular junction, peripheral or central nervous system. Here, we described the case of a 55-year man with metastatic melanoma, facing a brutal right peripheral cerebral palsy after his third ipilimumab/nivolumab infusion. After the case presentation, we reviewed the literature about this rare complication of immunotherapy, and described its diagnosis work-up and clinical management.
Introduction
Immune checkpoint inhibitors (ICI) reinvigorate patient’s own immune system to recognize and destroy tumor cells (1). These treatments, notably the CTLA-4 inhibitor ipilimumab (Ipi) and the PD-1 inhibitor nivolumab (Nivo), revolutionized the treatment of advanced solid tumors (2). Indeed, in metastatic melanoma, a first-line combination of Ipi and Nivo lead to an impressive 5-year overall survival of 52% (3). However, these efficient treatments have numerous immune-related adverse events (irAEs), with a broad spectrum of severity, that potentially affect every organ of the patient (4). If the onset of irAES are frequently associated with increased efficacy of ICI (5), their management in routine clinical practice remain challenging, as each of them requires specific diagnostic procedure and sometimes specific treatments (6).
Among every irAES, neurological irAES (N-irAES) are particularly difficult to manage, for several reasons: i) contrary to frequent irAES, like thyroid dysfunction or cutaneous toxicity, neurological complications might be life-threatening; ii) neurological complications are highly polymorphic as they might affect central nervous system (encephalitis, meningitis), peripheral nervous system (peripheral neuropathy, Guillain-Barre syndrome) or neuromuscular junction (myositis, myasthenic syndromes) (7); iii) the differential diagnoses are numerous (cancer progression, paraneoplastic syndrome, stroke…) and iv) the radiological and biological work-up necessary to diagnose N-irAES are frequently highly technologic (brain MRI, specific autoantibodies) or invasive (lumbar punction). Consequently, medical oncologists need clear knowledge to face neurological irAES and case descriptions are helpful in clinical practice.
Here, we report the case of a patient with metastatic melanoma experiencing a brutal facial palsy after three infusions of ipilimumab/nivolumab.
Case presentation
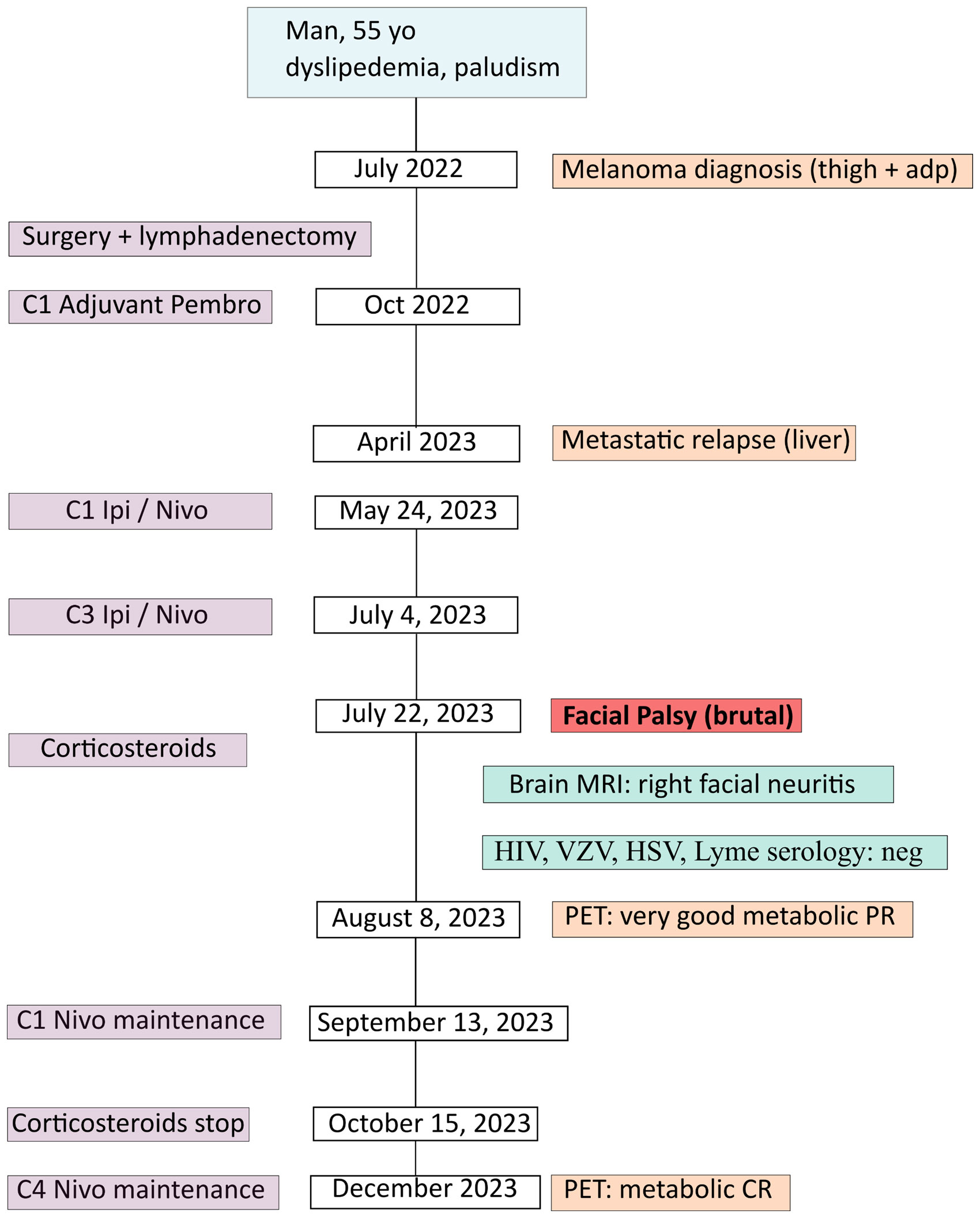
A 55 year-old man, ECOG-PS 0, with a medical history of dyslipidemia and non-severe malaria presented a right inguinal adenopathy, gradually increasing in volume, associated with a black skin lesion of the right thigh, surrounded with a perilesional vitiligo. In July 2022 (Figure 1: patient’s timeline), he consulted in our center (Paoli-Calmettes Institute, Cancer Center in Marseille, France) and beneficiated from a right inguinal lymph node biopsy (July 22, 2022). The histology revealed a malignant melanoma, PS100+, HMB 45+ and MelanA+. The PET-CT confirmed a hypermetabolic skin lesion with large right inguinal adenopathies, without distant metastasis and the cerebral MRI was normal. The next generation sequencing (in-house panel of 96 genes) revealed a BRAF mutation in exon 15, V600D (p.(Val600Asp), a punctual variation of TERT promotor (c.-146C>T) and focal homozygous deletions of genomic regions containing the loci CDKN2A and CDKN2B and TERT.
In August 2022, the patient had surgical excision of the skin lesion and a right inguinal lymph node dissection. As recommended in tumor board, the patient started adjuvant PD-1 inhibitor (pembrolizumab 200 mg/3 weeks), with C1D1 in October 2, 2022. Unfortunately, in April 2023, after only 6 months of adjuvant pembrolizumab the patient relapsed with multiple metastatic liver lesions and multiple sub-diaphragmatic lymph nodes, particularly in the right iliopelvic region (PET-CT of April 27, 2023: Figure 2A). The new tumor board recommended either the inclusion in a clinical trial (investigating Ipi/Nivo +/- anti IL-8) or the Ipi/Nivo combination in routine clinic that the patient preferred. The first three infusions of Ipi/Nivo were well tolerated [C1: May 24, 2023 (Day 1)/C2: June 13, 2023/C3: July 04, 2023], with only a grade 1 dermatitis with pruritus.
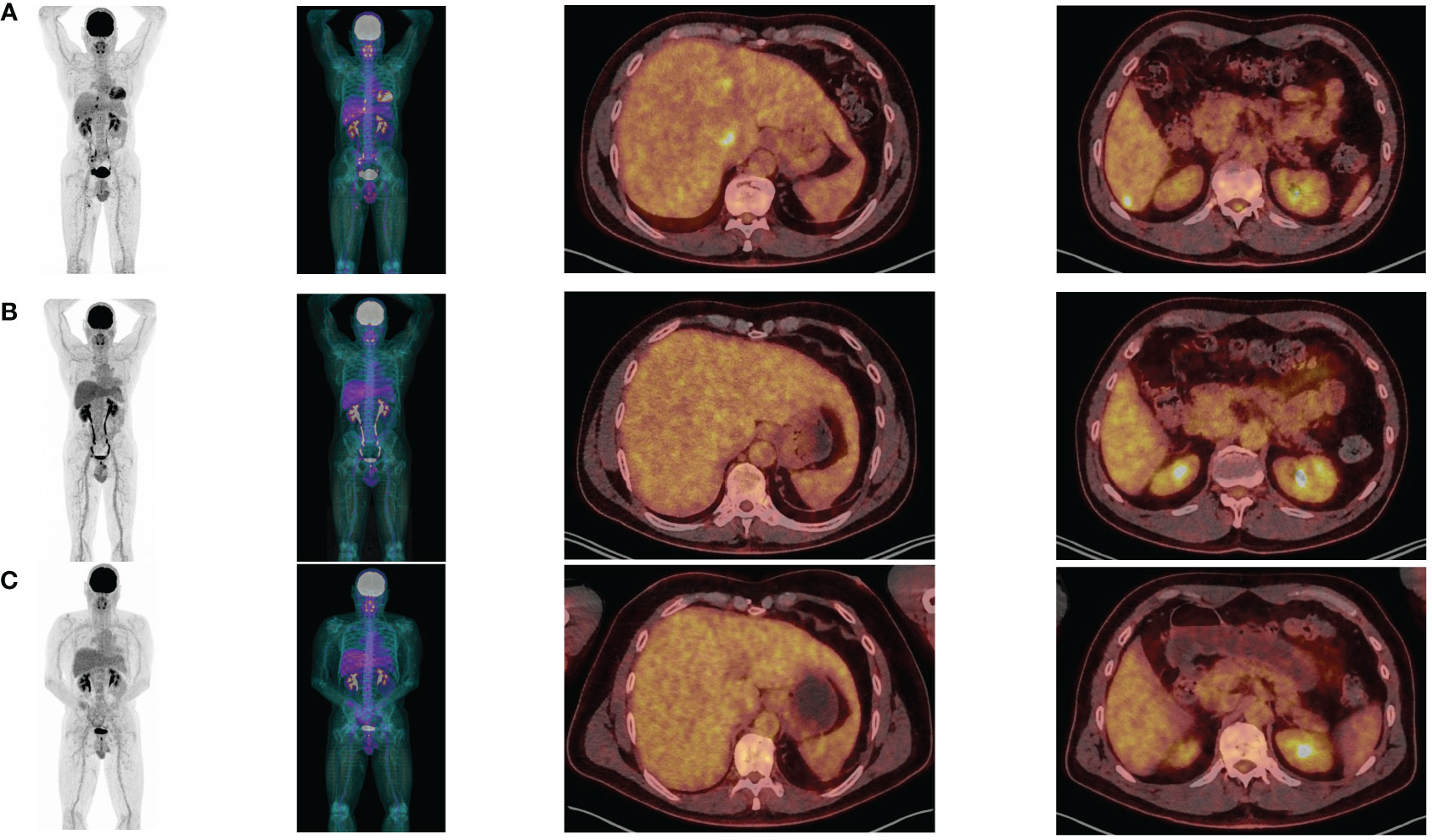
Figure 2 PET-CT at baseline (A), after 3 Ipilimumab/Nivolumab combinations (B) and after 3 Nivolumab maintenance (C).
However, on July 22, 2023 (Day 59), the patient presented with acute peripheral left facial palsy (grade 3 on CTCAE v5.0), with retro-auricular pain and numbness of the face. He consulted the emergency department in a local hospital. The clinical examination did not show any associated neurological signs (motor or sensitive deficit of the limb, cognitive impairment, additional cranial nerve injury) and the biological tests were normal. The cerebral CT scanner was normal. A diagnosis of Bell’s palsy was suspected: lumbar puncture was not performed and a treatment of oral prednisolone 1 mg/kg (80 mg) was started, together with ocular protection. The cerebral MRI in August 1, 2023 revealed no secondary localization, no signs suggestive of a stroke, but inflammation of the right facial nerve, suggestive of facial nerve neuritis (Figure 3). HIV, VZV, HSV and Lyme (Borrelia burgdorferi) serology were negative, glycemia and TSH were normal. Altogether, the concertation between oncologists and neurologists adopted the probable diagnosis of an immune-related facial palsy caused by ipilimumab/nivolumab combination. Consequently, we decided to stop ipilimumab/nivolumab combination.
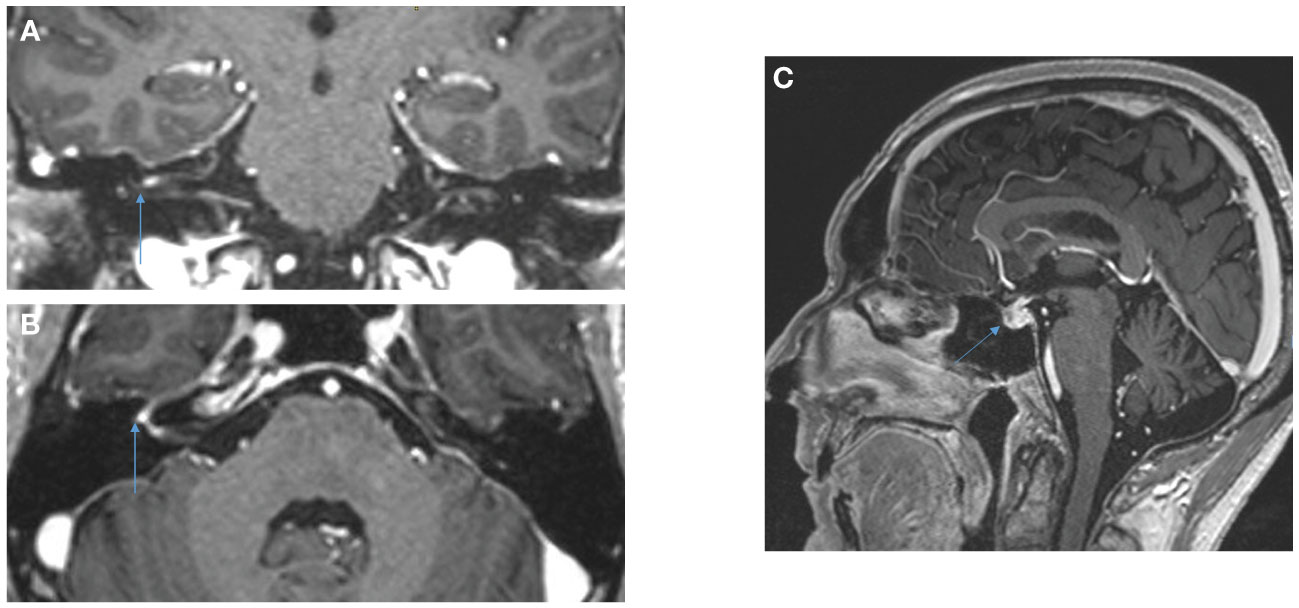
Figure 3 Cerebral MRI, Coronal (A) and axial (B) 3DT1MPRAGE sequences after gadolinium reconstruction showing contrast of the fundus of the internal auditory meatus and of the right geniculate ganglion attesting to right facial neuritis. Hypophisitis is shown in the sagittal sequence (C).
The clinical evolution was favorable: after 10 days of prednisolone, the facial palsy improved and the patients began to close his upper right eyelid. The prednisolone was decreased by 10 mg every week (until 10 mg, then 5 mg and stop) and in September 2023, the patient did not present any sign of cerebral palsy, nor ocular complication. Interestingly, two other irAEs were observed: i) an hypophysitis revealed by the brain MRI of August 1, 2023 (diffusely enlarged pituitary gland, not shown) and a corticotropic deficiency (8 a.m. blood level cortisol: 2 nmol/l; blood level ACTH: 0.7 pmol/l); ii) a vitiligo around 20% of body surface starting in September 2023.
Concerning efficacy, the PET-CT after three infusions of Ipi/Nivo (August 9, 2023) revealed a very good metabolic partial response (liver metastases of segment I and IV, right external iliac adenopathy) and a complete response on other liver metastases and other sub-diaphragmatic lymph nodes (Figure 2B). We canceled the fourth and last ipilimumab/nivolumab combination and started the maintenance therapy by nivolumab monotherapy 480 mg q4w in September 13, 2023. PET-CT after three nivolumab maintenance (December 20, 2023) revealed a metabolic complete response in liver and lymph nodes (Figure 2C). At the date of redaction of this case report, the patient was ECOG-PS 0, maintained an intense professional activity without any clinical sequelae of the metastastic melanoma or the former facial palsy.
Literature review and discussion
Here, we report the case of a patient presenting a facial palsy after 3 infusions of ipilimumab/nivolumab. As ICI-induced facial palsy might be challenging for oncologists, we will discuss the literature and the clinical management of this rare neurological irAE.
ICI-induced facial palsy: literature review
The first case of ICI-induced facial palsy was reported in 2015 by Altman et al. (8). The physiopathological mechanism of this neurological adverse event remains unknown to date, even if an immune tolerance breakdown is the most probable (9). Although rare, facial palsy has been reported in the literature with different ICIs agents and combinations: ipilimumab (10) pembrolizumab (11), atezolizumab (12), nivolumab (13), ipilimumab plus nivolumab (14), ipilimumab plus pembrolizumab (10), tremelimumab plus durvalumab (15). The Table 1 summarizes the main reported cases of facial palsy induced by ICIs reported in the literature. Interestingly, brain MRI was normal in approximately 50% of the cases and an additional irAE was observed in approximately 30% of the cases. In our patient, whereas ICI monotherapy by pembrolizumab was not associated with irAEs and let to tumor progression, Ipi/Nivo combination led to three irAEs (notably facial palsy) and a metabolic complete response at 7 months.
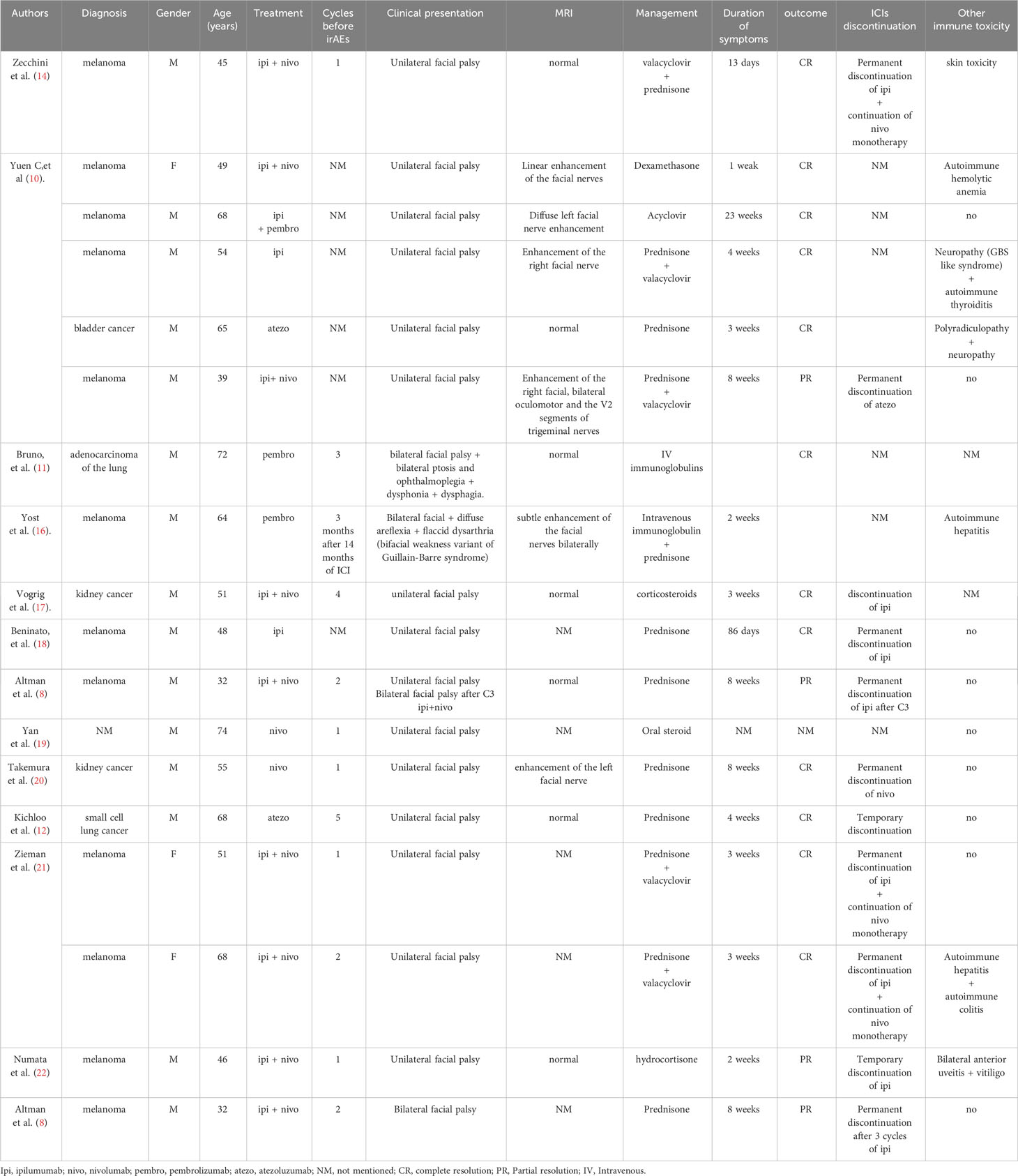
Table 1 Published cases of patients with checkpoint inhibitor-induced facial palsy (literature review).
A systematic analysis published in 2023 assessed the risk of facial palsy associated with ICIs based on data from 21 randomized trials including 10,779 patients treated with ICIs (15). Patients were treated for several primary cancers: melanoma, gastro-esophageal cancer, renal or urothelial cancer and malignant mesothelioma. An increased risk of facial palsy associated with ICIs was reported in 23 cases with OR 3.07 (95% CI: 1.4–6.5) compared to control treatment. Results of subgroup analysis indicated that OR of ICI-related FP did not vary significantly by tumor type, ICIs treatment schedule, case of events, study design, median PFS and publication status. In the same analysis, the FDA Adverse Event Reporting System database was retrospectively reviewed between 2011 and 2022. In total, 274 cases were identified. Most patients presenting facial palsy were treated with ICI only (82.1%) rather than with combination of ICI with other drug (17.9%). The median onset time of facial palsy was 5.5 weeks in this review and 15 weeks in another case series (18). In most of the cases, drug interruption was performed (78%) and clinical outcome was favorable (71.7%).
Neurological irAEs and ICI-induced facial palsy: epidemiology
Neurological irAEs (N-irAEs) are relatively rare with an estimated prevalence of 1-4% of ICI-treated patients (23, 24). Yet, with cardiological irAEs, neurological adverse events are among the most serious irAEs (25), with mortality rate of 28% for myasthenic syndrome and 21% for encephalitis. Thus, early detection, diagnosis and management of N-irAEs is a major issue. N-irAEs involving peripheral nervous system are twice more frequent than central nervous system (CNS) toxicity (7), and clinical as well as paraclinical characteristics of ICI-induced neuropathies differ from chemotherapy-induced neuropathies (26). Among N-irAEs, occurrence of facial palsy is particularly rare, with an incidence around 0.2% in the ICI-treated population (23 cases/10.779 patients) in the largest reported study (15). Furthermore, a recent study showed that combination of CTLA-4 and PD(L)-1 inhibitors is a risk factor of developing cranial neuropathies, including facial palsy (27).
Melanoma may have a potential risk of neurological irAE compared to other cancers. Indeed, in the literature described in Table 1, 12/18 patients presenting ICI-induced facial palsy are melanoma patients. This might be due to the early onset of ICI in melanoma patients since 2010 (which might increase the reported cases) and the high frequency of ICI combination, notably Ipi/Nivo which increase irAEs. However, a meta-analysis of 694 articles (7), melanoma was more frequent in patients with peripheral neuropathies (64/94, 68%; p = 0.003) and less common in encephalitis (19/56, 34%; p = 0.001).
ICI-induced facial palsy: diagnosis
The diagnosis of facial palsy is primarily clinical. Physicians need to eliminate central facial paralysis that would lead to look for CNS involvement with simple neurological examination. Then, associated neurological symptoms, notably for immune-related myasthenia gravis, must be carefully searched to adapt patient’s further exams and surveillance (28). It is mandatory to exclude differential diagnoses such as stroke or toxic, metabolic, infectious and endocrine neuropathies. Nevertheless, the main goal is to eliminate a carcinologic progression such as brain or leptomeningeal metastases (17, 28). Differentiating immune-related from a frigore facial palsy (Bell’s palsy) can be difficult. The delay between ICI introduction and the occurrence of firsts symptoms is estimated to 2 months (0.5-17.0 months) and might help ICI imputability (17).
Even if some of them are missing in our report, biological exams are important to eliminate other etiologies of reversible neuropathies: HbA1c, vitamin B12, TSH, vitamin B6, folate, serum protein electrophoresis, immunofixation and CPK. Other exams might be done depending on the context: ANA, ESR, CRP, ANCA, anti–smooth muscle, SSA/SSB, RNP, anti-dsDNA, ganglioside antibodies, anti-MAG, anti-Hu (ANNA-1 ab), thiamine, Lyme, hepatitis B or C, and HIV. Thus, to our knowledge, anti-onconeural antibodies has always been reported as negative when reported in isolated ICI-related facial palsy (7, 10).
Lumbar puncture aims to seek for carcinomatous cells. CSF analysis may show a pleiocytosis (10, 17), but may also be normal (11, 14). Nerve conduction study shows a reduced amplitude in the facial nerve and an altered blink reflex (11). Brain MRI may reveal an ipsilateral facial nerve enhancement after gadolinium infusion, or be normal (10, 17).
In the case of our patient, due to the presence of hypophysitis, sarcoidosis diagnosis should be considered, but the patient did not present symptoms in the organs classically touched in this pathology (lungs, lymph nodes, joints, skin, eyes, heart, kidneys, etc.). Additionally, even if our patient did not complain of other symptoms and tendinous reflexes where present, electromyography could have been performed in this patient to increase diagnostic sensitivity (29).
ICI-induced facial palsy: clinical management ant treatment
N-irAEs management first relies on ICI discontinuation. In some reported cases of combined ICI treatment, only one of them was discontinued (17). Oral corticosteroids (1-2 mg/kg/d) may be introduced, and slowly tapered within 4 to 6 weeks (27). Some authors have used intravenous immunoglobulin (IVIG) instead or with corticosteroids (17). It is mandatory to assess the disease course with early and repeated neurological examination to adapt patient’s management. In case of facial palsy being the inaugural symptom of a Guillain-Barre syndrome or a more diffuse neuropathy, further therapies might be considered such as plasma exchange (27).
The prognosis of isolated facial palsy is good with 70% of complete recovery, within a median of 41 days (18). However, recovery can be partial with persistent sequelae in approximately one patient out of four (17). In this context, rehabilitation strategies for facial nerve injuries is crucial and several techniques are available, including exercise, electrical stimulation, biofeedback, and facial neuromuscular retraining (30). In case of full recovery, reintroduction of ICI might be considered in case of absence of alternative therapy. Indeed, disease progression has been shown to be the main cause of death in patients with irAEs (24). To switch ICI subtype (from CTLA-4 to PD-1 inhibitors) in non-neurological irAEs has been associated to a lower risk of relapse (31), but evidence are lacking in N-irAEs. Yet, no clear guideline is currently available, and this attitude should be a case-by-case evaluation.
Oncological perspective: quick and multi-disciplinary management
In conclusion, ICI-induced facial palsy is a rare complication that a medical oncologist rarely sees in his routine practice. The key in the management of these rare N-irAEs is the multi-disciplinary network between oncologists and neurologists or internists (32). This essential collaboration can take various forms, such as external consultants, immuno-toxicity meetings, mailing lists, messaging applications (33). Quick clinical evaluation, quick imaging and lab tests are essential to eliminate differential diagnoses and start early corticosteroid treatment, effective in 70-80% of cases (15, 34). Building a strong network to deal with rare and life-threatening irAEs is one of the new challenge of medical oncology, a medical specialty where multi-disciplinary care has always been highly estimated.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institut Paoli Calmettes institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EM: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. GC: Writing – review & editing, Writing – original draft. RM: Writing – review & editing, Investigation. SC: Writing – review & editing, Visualization. NC: Writing – review & editing, Visualization. BC: Writing – review & editing, Supervision. AC: Writing – review & editing, Supervision, Conceptualization. PR: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Our work is supported by Paoli-Calmettes Institute and Aix-Marseille University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AE, Adverse Events; CNS, Central Nervous System ; CTLA-4, Cytotoxic T-lymphocyte-associated protein 4; ECOG-PS, Eastern Cooperative Oncology Group - Performance Status; IC, Immune Checkpoint Inhibitors; irAEs, Immune-Related Adverse Events; Ipi, Ipilimumab; MRI, Magnetic Resonance Imaging; Nivo, Nivolumab; N-irAEs, Neurological irAEs; PD-1/PD-L1, Programmed cell Death protein 1/Programmed cell Death-Ligand 1; PET-CT, Positron Emission Tomography Computed Tomodensitometry Scanner.
References
1. Couzin-Frankel J. Breakthrough of the year 2013. Cancer Immunother Sci. (2013) 342:1432–3. doi: 10.1126/science.342.6165.1432
2. Wolchok J. Putting the immunologic brakes on cancer. Cell. (2018) 175:1452–4. doi: 10.1016/j.cell.2018.11.006
3. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. (2021) 40:127–37. doi: 10.1200/JCO.21.02229
4. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol Off J Eur Soc Med Oncol. (2016) 27:559–74. doi: 10.1093/annonc/mdv623
5. Beaufils M, Amodru V, Tejeda M, Boher JM, Zemmour C, Chanez B, et al. Dysthyroidism during immune checkpoint inhibitors is associated with improved overall survival in adult cancers: data mining of 1385 electronic patient records. J Immunother Cancer. (2023) 11:e006786. doi: 10.1136/jitc-2023-006786
6. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001
7. Marini A, Bernardini A, Gigli GL, Valente M, Muñiz-Castrillo S, Honnorat J, et al. Neurologic adverse events of immune checkpoint inhibitors: A systematic review. Neurology. (2021) 96:754–66. doi: 10.1212/WNL.0000000000011795
8. Altman AL, Golub JS, Pensak ML, Samy RN. Bilateral facial palsy following ipilimumab infusion for melanoma. Otolaryngol Neck Surg. (2015) 153:894–5. doi: 10.1177/0194599815606701
9. Vogrig A, Muñiz-Castrillo S, Desestret V, Joubert B, Honnorat J. Pathophysiology of paraneoplastic and autoimmune encephalitis: genes, infections, and checkpoint inhibitors. Ther Adv Neurol Disord. (2020) 13:1756286420932797. doi: 10.1177/1756286420932797
10. Yuen C, Reid P, Zhang Z, Soliven B, Luke JJ, Rezania K. Facial palsy induced by checkpoint blockade: A single center retrospective study. J Immunother Hagerstown Md 1997. (2019) 42:94–6. doi: 10.1097/CJI.0000000000000254
11. Bruno F, Palmiero RA, Ferrero B, Franchino F, Pellerino A, Milanesi E, et al. Pembrolizumab-induced isolated cranial neuropathy: A rare case report and review of literature. Front Neurol. (2021) 12:669493. doi: 10.3389/fneur.2021.669493
12. Kichloo A, Albosta MS, Jamal SM, Aljadah M, Wani F, Selene I, et al. Atezolizumab-induced bell’s palsy in a patient with small cell lung cancer. J Investig Med High Impact Case Rep. (2020) 8:2324709620965010. doi: 10.1177/2324709620965010
13. Sakoh T, Kanzaki M, Miyamoto A, Mochizuki S, Kakumoto T, Sato K, et al. Ramsay-Hunt syndrome and subsequent sensory neuropathy as potential immune-related adverse events of nivolumab: a case report. BMC Cancer. (2019) 19:1220. doi: 10.1186/s12885-019-6444-0
14. Zecchini JM, Kim S, Yum K, Friedlander P. Development of bell’s palsy after treatment with ipilimumab and nivolumab for metastatic melanoma: A case report. J Immunother. (2018) 41:39. doi: 10.1097/CJI.0000000000000184
15. Zhu J, Li J, Zheng Y, Gao S, He Z, Qiu K, et al. Facial palsy induced by immune checkpoint blockade: A systematic analysis of clinical trials and a pharmacovigilance study of postmarketing data. Int Immunopharmacol. (2023) 125:111184. doi: 10.1016/j.intimp.2023.111184
16. Yost MD, Chou CZ, Botha H, Block MS, Liewluck T. Facial diplegia after pembrolizumab treatment. Muscle Nerve. (2017) 56:E20–1. doi: 10.1002/mus.25663
17. Vogrig A, Muñiz-Castrillo S, Joubert B, Picard G, Rogemond V, Skowron F, et al. Cranial nerve disorders associated with immune checkpoint inhibitors. Neurology. (2021) 96:e866–75. doi: 10.1212/WNL.0000000000011340
18. Beninato T, Fucà G, Di Guardo L, Vetrano I, Valeri B, Nesa F, et al. Immune-related Bell’s palsy in melanoma patients treated with immune checkpoint inhibitors. Melanoma Res. (2021) 31:178–80. doi: 10.1097/CMR.0000000000000715
19. Yan C, Huang M, Swetlik C, Toljan K, Mahadeen AZ, Bena J, et al. Predictors for the development of neurological immune-related adverse events of immune checkpoint inhibitors and impact on mortality. Eur J Neurol. (2023) 30:3221–7. doi: 10.1111/ene.15942
20. Takemura K, Yamanaka T, Hayashida M, Kizawa R, Yamaguchi T, Tanabe Y, et al. Bell’s palsy during rechallenge of immune checkpoint inhibitor. IJU Case Rep. (2023) 6:144–6. doi: 10.1002/iju5.12572
21. Zieman D, Frankel AE. Autoimmune bell’s palsy following immunotherapy for metastatic melanoma: A report of 2 cases. J Immunother. (2019) 42:318. doi: 10.1097/CJI.0000000000000291
22. Numata S, Iwata Y, Okumura R, Arima M, Kobayashi T, Watanabe S, et al. Bilateral anterior uveitis and unilateral facial palsy due to ipilimumab for metastatic melanoma in an individual with human leukocyte antigen DR4: A case report. J Dermatol. (2018) 45:113–4. doi: 10.1111/1346-8138.13779
23. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer Oxf Engl 1990. (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001
24. Sechi E, Markovic SN, McKeon A, Dubey D, Liewluck T, Lennon VA, et al. Neurologic autoimmunity and immune checkpoint inhibitors. Neurology. (2020) 95:e2442–52. doi: 10.1212/WNL.0000000000010632
25. Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
26. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
27. Farina A, Villagrán-García M, Honnorat J. Neurological adverse events of immune checkpoint inhibitors: An update of clinical presentations, diagnosis, and management. Rev Neurol (Paris). (2023) 179:506–15. doi: 10.1016/j.neurol.2023.03.003
28. Psimaras D, Velasco R, Birzu C, Tamburin S, Lustberg M, Bruna J, et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: From pathogenesis to treatment. J Peripher Nerv Syst. (2019) 24:S74–85. doi: 10.1111/jns.12339
29. Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Rev Neurol. (2019) 15:671–83. doi: 10.1038/s41582-019-0250-9
30. Novak CB. Rehabilitation strategies for facial nerve injuries. Semin Plast Surg. (2004) 18:47–52. doi: 10.1055/s-2004-823123
31. Villagrán-García M, Velasco R. Neurotoxicity and safety of the rechallenge of immune checkpoint inhibitors: a growing issue in neuro-oncology practice. Neurol Sci. (2022) 43:2339–61. doi: 10.1007/s10072-022-05920-4
32. Pensato U, Guarino M, Muccioli L. The role of neurologists in the era of cancer immunotherapy: Focus on CAR T-cell therapy and immune checkpoint inhibitors. Front Neurol. (2022) 13:936141. doi: 10.3389/fneur.2022.936141
33. Ruste V, Goldschmidt V, Laparra A, Messayke S, Danlos F-X, Romano-Martin P, et al. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: A prospective study of the French REISAMIC registry. Eur J Cancer. (2021) 158:217–24. doi: 10.1016/j.ejca.2021.08.048
Keywords: cerebral palsy, immune checkpoint inhibitors, immune toxicity, neurological toxicity, nivolumab, ipilimumab
Citation: Mezni E, Corazza G, Mari R, Coze S, Charrier N, Chanez B, Chretien AS and Rochigneux P (2024) Facial palsy after administration of immune checkpoint inhibitors: case report, literature review and clinical care management. Front. Immunol. 15:1375497. doi: 10.3389/fimmu.2024.1375497
Received: 23 January 2024; Accepted: 11 March 2024;
Published: 22 March 2024.
Edited by:
Jonathan Pol, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2024 Mezni, Corazza, Mari, Coze, Charrier, Chanez, Chretien and Rochigneux. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philippe Rochigneux, cm9jaGlnbmV1eHBAaXBjLnVuaWNhbmNlci5mcg==
 Essia Mezni
Essia Mezni Giovanni Corazza
Giovanni Corazza Roxane Mari
Roxane Mari Stephanie Coze4
Stephanie Coze4 Brice Chanez
Brice Chanez Anne Sophie Chretien
Anne Sophie Chretien Philippe Rochigneux
Philippe Rochigneux