- 1Department of Dermatology, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
- 2Department of Microbiology, Faculty of Biological Sciences, Quaid-I-Azam University, Islamabad, Pakistan
- 3Department of Dermatology, Changzheng Hospital, Second Military Medical University, Shanghai, China
- 4School of Pharmacy, Shandong University, Qingdao, Shandong, China
- 5Department of Dermatovenereology, Chengdu Second People’s Hospital, Chengdu, China
- 6Department of Dermatology, Tongren Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China
- 7Department of Dermatology, First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
- 8Department of Dermatology, Meizhou Dongshan Hospital, Meizhou, Guangdong, China
- 9Department of Dermatology, Meizhou People’s Hospital, Meizhou, Guangdong, China
- 10Department of Pharmacy, Shantou University School Medical College, Shantou, China
- 11Department of Clinical Laboratory, Skin and Venereal Diseases Prevention and Control Hospital of Shantou City, Shantou, Guangdong, China
- 12Department of Dermatology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
Interleukins (ILs) are vital in regulating the immune system, enabling to combat fungal diseases like candidiasis effectively. Their inhibition may cause enhanced susceptibility to infection. IL inhibitors have been employed to control autoimmune diseases and inhibitors of IL-17 and IL-23, for example, have been associated with an elevated risk of Candida infection. Thus, applying IL inhibitors might impact an individual’s susceptibility to Candida infections. Variations in the severity of Candida infections have been observed between individuals with different IL inhibitors, necessitating careful consideration of their specific risk profiles. IL-1 inhibitors (anakinra, canakinumab, and rilonacept), IL-2 inhibitors (daclizumab, and basiliximab), and IL-4 inhibitors (dupilumab) have rarely been associated with Candida infection. In contrast, tocilizumab, an inhibitor of IL-6, has demonstrated an elevated risk in the context of coronavirus disease 2019 (COVID-19) treatment, as evidenced by a 6.9% prevalence of candidemia among patients using the drug. Furthermore, the incidence of Candida infections appeared to be higher in patients exposed to IL-17 inhibitors than in those exposed to IL-23 inhibitors. Therefore, healthcare practitioners must maintain awareness of the risk of candidiasis associated with using of IL inhibitors before prescribing them. Future prospective studies need to exhaustively investigate candidiasis and its associated risk factors in patients receiving IL inhibitors. Implementing enduring surveillance methods is crucial to ensure IL inhibitors safe and efficient utilization of in clinical settings.
1 Introduction
Candidiasis is one of the most common fungal infections affecting humans, caused by Candida species (1). These opportunistic pathogens are typically present on the skin and mucous membranes of healthy individual’s without causing infections (2). However, the risk factors of candidiasis are heightened in patients with compromised immune systems, often due to conditions such as diabetes, renal failure or liver failure (1, 3).
The interaction of C. albicans Toll-like receptors (TLRs) or C-type lectin receptors is a multifaceted phenomenon due to the potential collaboration between TLRs and other pattern recognition receptors (PRRs). Additionally, the expression of fungal ligands on the surface is contingent upon the specific strain and morphotype, i.e., yeasts or hyphae, thereby influencing the resultant immune response (Th1/Th2/Th17; Figure 1) (4). Furthermore, a family of signaling proteins known as interleukins (ILs) plays a crucial role in the immune system. ILs are produced by various immune cells, including T cells, B cells, macrophages, and dendritic cells (DCs) (5). INF γ, TNFα, IL-2, IL-4, IL-5, IL-6, IL-8, L-10, IL-13, IL-10, TGFβ, IL-17, IL-21, IL-22 IL-23 and IL-26 are produced by Th1/Th2/Tregs/Th17 immune cells produced at the site of infections (Figure 1). ILs play a significant role in the host’s defense against bacterial and fungal pathogens (6–10) (Figure 1).
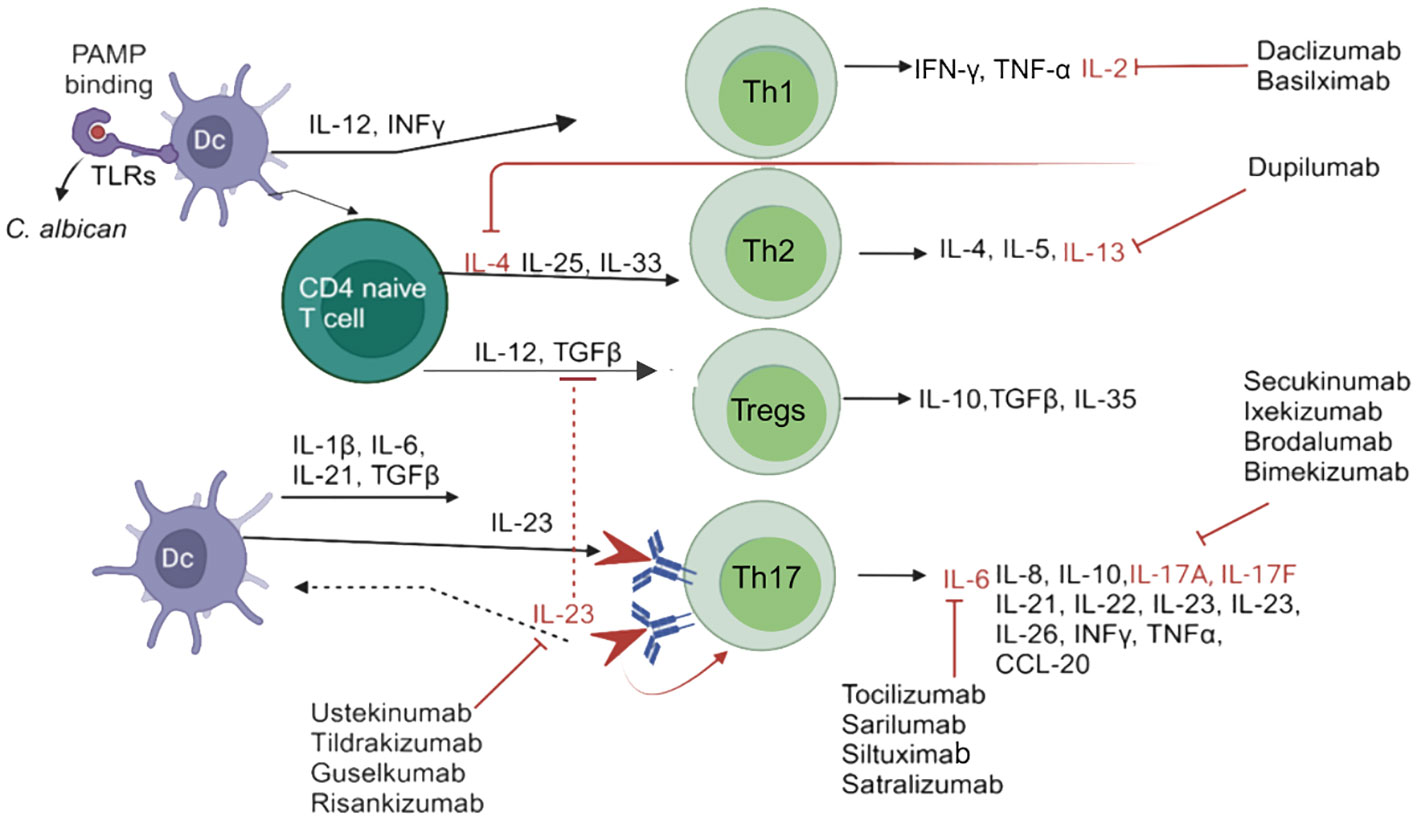
Figure 1 Schematic diagram of candidiasis and the immune system’s response for eliminating Candida species. In response to Candida species, DCs are activated, which promotes CD4+T cells or Th1, Th2, Tregs, and Th17 immune cells through various cytokines, in order to activate interleukins, and block by different interleukins inhibitors. IL-4, IL-13 inhibitors=Dupilumab; IL-6 inhibitors= Tocilizumab, Sarilumab, Siltuximab, Satralizumab; IL-17A, IL-17F inhibitors= Secukinumab, Ixekizumab, Brodalumab, Bimekizumab; IL-2 i inhibitors=Daclizumab, Basilximab; IL-23 inhibitors= Ustekinumab, Tildrakizumab, Guselkumab and Risankizumab. PAMP, pathogen-associated molecular patterns; IFN-γ, Interferon-gamma; IL, interleukin; Th, T helper cells; TGFβ, transforming growth factor beta; TLRs, Toll-like receptors. TNF-α, Tumor necrosis factor alpha.
IL inhibitors such as anakinra, canakinumab, basiliximab, daclizumab, and tocilizumab, have been used to treat IL-related autoimmune diseases. Based on current knowledge, specific IL inhibitors targeting IL-8, IL-10, IL-18, and IL-22 have not yet been reported. Cytokines also participate in host defense mechanisms against Candida species (11–13). Thus, certain IL inhibitors has been linked with an increased incidence of Candida infections (14). For instance, risankizumab, a selective anti-IL-23 antibody approved for the treatment of psoriasis, has been observed to induce mucocutaneous candidiasis in approximately 3.4% of cases (15). This interaction between candidiasis and IL inhibitors is a serious concern for patients undergoing treatment with such inhibitors (16).
Several studies have been compared the safety of the inhibitor medicines. For example, individuals with psoriasis receiving treatment in animal models, the inhibitor of tildrakizumab has shown a lower risk of candidiasis than Ustekinumab (17). In a clinical trial, a 2.3% incidence of candidiasis was observed in patients receiving Ustekinumab (17). However, information on risk factors associated with candidiasis is still scarce. This limitation may be attributed to the recency of that approval of IL inhibitors for treating autoimmune diseases, resulting in insufficient data (15).
Increasing awareness among physicians and researchers might help reduce the risk factors and occurrence of candidiasis. Therefore, this review aims to provide comprehensive insights into ILs and their role in the host immune response against candidiasis, and to discuss IL associated with candidiasis.
2 Candidiasis and the impact of interleukin inhibitors
2.1 Immune response to Candida infection
As specialized antigen-presenting cells (APCs), macrophages play a crucial role in the immune system’s defense against Candida. Upon activation by chemical signals from infected cells, they are recruited and phagocytize C. albicans, thereby eliminating and producing fungal protein antigens. DCs identify Candida species through PRRs interacting with pathogen-associated molecular patterns (PAMPs) on the fungal cell wall (18, 19). DCs facilitate the transfer of fungal antigens to lymph nodes, initiating an immune response and leading to T-cell development against Candida for long-term immunological defense (20, 21). DCs exhibit antigen-specific immune responses to C. albicans, with Langerhans cells driving Th17 responses. In contrast, cutaneous DCs without Langerhans cells stimulate Th1 and cytotoxic T-lymphocyte (CTL) responses, presenting antigens through the major histocompatibility complex class I (MHC I) route (10). T-lymphocytes recognize these antigens through receptors, releasing cytokines and differentiating into various Th cells. These dynamic interactions contribute collectively to the immune system’s ability to combat Candida infections (21, 22).
ILs modulate specific pathways during Candida infections. IL-1 and IL-6 mutually influence the production of IL-8, IL-17, and IL-22 through mitogen-activated protein kinase (MAPK) or nuclear factor-kappa B (NF-kB) specific pathways (23). IL-1 plays a role in recruiting immune cells and promoting inflammation in response to Candida infection. Furthermore, IL-12 regulates T cell proliferation and differentiation, with IL-2 enhancing interferon-gamma (INF-γ) production through the januskinase signal transducer and transcription factor (JAK-STAT) pathway during Candida infection (21, 23). IL-4 stimulates Th2 cell differentiation, antibody production, and IL-1, IL-6 and IL-8 production. IL-4 also plays a role in developing a Th2 immune response, inadvertently promoting fungal growth (24).
IL-10, inhibits inflammation and modulates the immune response, simultaneously blocking the production of IL-1, IL-6 and IL-8. IL-10 may contribute to Candida’s evasion strategy by reducing the inflammatory response, and increasing fungal persistence. Furthermore, IL-12 promotes the differentiation of Th1 cells and stimulates IFN-γ cells (21, 24). IL-17 comprises various subtypes, including IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25) and IL-17F. These ILs can activate the production of IL-6, IL-8, and IL-1β. IL-17 induces the production of IL-6 through the NF-kB pathway. During Candida infection, IL-17 play an important role in promoting inflammation and recruiting neutrophils (25). Conversely, IL-18 plays a pivotal role in enhancing the production of IFN-γ and modulating immune responses. It also promotes the production of IL-1 and IL-17. In the context of candidiasis, IL-18 stimulates cellular immunity and enhances IFN-γ production (26).
IL-22 plays a crucial role in promoting the function of epithelial cells and tissue repair, activating the production of IL-1, IL-6 and IL-8. During candidiasis, IL-22 enhances the immune cell response and facilitates tissue repair. IL-23 encourages Th17 cell development and induces the production of IL-17, serving as a critical factor in the host immunological response to Candida infection (12, 27). The primary homeostatic function of the IL-23/IL-17 pathway in the host is to defend against fungal and bacterial pathogens at mucosal barriers, concurrently overseeing barrier function (28). IL-23, a member of the IL-12-type cytokine family, comprises the p19 and p40 subunits, connected by a disulfide bond. Its interaction is mediated by two receptor chains, IL-12Rβ1 (shared with IL-12) and the IL-23-specific IL-23R (29)
The binding of IL-12 to the IL-12Rβ1 and IL-12Rβ2 receptors activates immune cells, including NK cells and T cells (30). Additionally, IL-12Rβ1 activation leads to the production of pro-inflammatory cytokines, such as IFN-γ and IL-2, promoting a Th1 response and enhancing the immune system’s ability to combat fungal pathogens (31, 32). Anomalous expression of IL-23 signaling can result in autoimmune diseases. In the context of candidiasis, IL-23 plays a crucial role in activating immune cells, including neutrophils and phagocytesthat enhance the immune response against fungal pathogens at the site of infection and facilitate tissue repair (10). The lack of IL-23 signaling pathways has been linked to increased susceptibility to candidiasis, underscoring the critical role of IL-23 in host defense against Candida (33). Immune cells also maintain a homeostasis of defense immunity to prevent excessive immune responses triggered by autoimmune diseases such as psoriasis, RA, and MS (34, 35). However, while IL inhibitors therapy has been used to treat autoimmune diseases, it comes with the risk of candidiasis, a significant concern during treatment.
2.2 Host mechanism by which interleukin inhibitors increase the risk of candidiasis
Candida species exhibit the capacity to infiltrate cells at the site of infection by binding to specific receptors. Initially, immune cells, such as macrophages and DCs, recognize molecules on the surface of Candida (10). The recognition of C. albicans manam by APS leads to the production of interleukins including IL-23 and chemokines (36). Th17 cells play a vital role by secreting various cytokines, notably IL-6, IL-17, IL-22, and IL-23, crucial for immune protection against Candida at mucosal sites throughout the body (25, 37). These cytokines also in attract and activate neutrophils, which engulf Candida species. Phagocytes, such as immune cells, respond by releasing antifungal proteins (e.g., Chemokine (C-C motif) ligand 2 (CCL2), antimicrobial peptides, and β-defensin) and pro-inflammatory chemokines (38, 39).
The described response mechanism effectively eliminates infectious molecules or kills Candida species within cells. However, neutralizing specific ILs, such as IL-17 RA (IL-17RA-/-) or IL-23p19 (IL-23p19-/-), has been found to increase susceptibility to oropharyngeal candidiasis and systemic candidiasis (25, 38). IL inhibitors are used to treat autoimmune diseases by inhibiting the recruitment and activation of immune cells such as DCs, macrophages, and neutrophils (40). Consequently, this inhibition hampers the production of chemokines and antimicrobial peptides increasing the risk of candidiasis due to the blockage of immune cell responses, allowing Candida to breach the epithelial barrier (41, 42). However, numerous studies have found that most candidiasis cases resulting from IL inhibitors are mild to moderate and do not lead to systemic infection (43).
3 IL inhibitors and Candida infection in immune mediated diseases
3.1 IL-1 inhibitors: anakinra, canakinumab, and rilonacept
IL-1 inhibitors, including anakinra, canakinumab, and rilonacept, are used to counteract the effects of IL-1, a cytokine that promotes inflammation and controls immune responses and inflammation. These inhibitors prevent the action of IL-1 and its receptors to reduce inflammation (44, 45). For instance, anakinra binds to IL-1 receptors, thereby blocking their binding with IL-1. This agent effectively manages joint pain and inflammation and is commonly used to treat rheumatoid arthritis (RA) (46).
Canakinumab is a monoclonal antibody specifically designed to target IL-1, inhibiting both its activity and downstream signaling. IL-1 activity is believed to play a role in the pathophysiology of diseases, such as cryopyrin-associated periodic syndromes (CAPS) and systemic juvenile idiopathic arthritis (SJIA). On the other hand, rilonacept, is a fusion protein consisting of an Fc region and the extracellular portion of the IL-1 receptor. It binds to both IL-1α and IL-1β, preventing their signaling by interacting with IL-1 receptors. Rilonacept is used to treat condition like CAPS and familial mediterranean fever (FMF) (47). In a randomized, double-blind trial phase with placebo followed by open-label extension for RA. 1346 patients with RA received anakinra subcutaneous injection for three years. Although, candidal esophagitis was reported in one patient approximately 2.5 years after initiating anakinra (100 mg/day) treatment (Table 1) (48).
3.2 IL-2-inhibitors: daclizumab, and basilximab
Daclizumab is a monoclonal antibody specifically designed to target the IL-2 receptor (IL-2R) for treating of multiple sclerosis (MS). It selectively binds to the CD25 component of IL-2R, expressed on activated T cells. By targeting and blocking IL-2R, daclizumab effectively modulates the immune response and reduces the activity of immune cells responsible for the inflammation and damage commonly observed in MS (69, 70).
Basiliximab, a monoclonal antibody, is an immunosuppressive organ transplantation agent designed to prevent rejection. By targeting CD25, basiliximab effectively decreases the risk of organ rejection by inhibiting the activation and proliferation of T cells pivotal in immuneresponses (71). In multicenter, randomized, double-blind studies investigating MS. 2236 patients received daclizumab 150 mg or 300 mg and the first incident of infection occurred at 162 days. Patients received daclizumab 150 mg the incidence of 12 oral candidiasis, and 12 vulvovaginal candidiasis occurred (49).
3.3 IL-4 and IL-13, inhibitor: dupilumab
The monoclonal antibody dupilumab has been developed to target the IL-4 receptor alpha (IL-4R), effectively blocking the signaling of both IL-4 and IL-13. Dupilumab is typically used to treat allergic conditions such as atopic dermatitis and asthma (72). Recently, in a case report, patient received dupilumab (600 mg) for the treatment of severe asthma and atopic dermatitis, after the 6 week the incident of candida infection occurred (72).
3.4 IL-6 inhibitors: tocilizumab, sarilumab, siltuximab, and satralizumab
The monoclonal antibody tocilizumab targets the IL-6 receptor (IL-6R) and is used to treat various autoimmune conditions, including giant cell arteritis, cytokine release SJIA, and RA. Tocilizumab works by inhibiting IL-6 signaling by binding to both the soluble and membrane-bound forms of IL-6R (73). By effectively blocking IL-6 signaling, tocilizumab serves to decrease inflammation and manages the signs and symptoms related of autoimmune disorders (74). In a report of cumulative safety data from five core phase III trials, two ongoing extension trials, and one clinical pharmacology. Overall, 22 opportunistic infections, of which six cases were candidiasis occurred in patients treated with tocilizumab 8mg/kg for RA (75). Sarilumab is a monoclonal antibody designed to target IL-6R and used to treat moderate-to-severe active RA. Through its binding to IL-6, sarilumab effectively inhibits IL-6 signaling. IL-6 is known for its role in promoting inflammation and is involved in the pathogenesis of RA. Sarilumab reduces inflammation and alleviates the symptoms associated with rheumatoid arthritis by suppressing IL-6 signaling (76). In a study called MOBILITY, double blind, randomized, placebo-controlled phase III trial, 200 mg of sarilumab was administered subcutaneously every two weeks in combination with methotrexate for the treatment of 45 patients with active RA, and one instance of candidal brochitis was reported (50).
IL-6, a pro-inflammatory cytokine with implications for immunological responses and inflammation, is the target of the monoclonal antibody siltuximab. This biologic agent is primarily treats multicentric castleman disease (MCD), a rare disorder characterized by abnormal lymph node enlargement and systemic inflammation. By selectively binding to IL-6 and inhibiting it signaling, siltuximab effectively reduces the inflammatory response associated with MCD. Siltuximab helps manage the symptoms and consequences of the illness by blocking IL-6 (77).
Satralizumab, a monoclonal antibody targeting IL-6R, is utilized in the treating of neuromyelitis optica spectrum disorder (NMOSD), a rare autoimmune disorder that primarily affecting the spinal cord and optic nerves. This therapeutic agent functions by binding to IL-6R, thereby preventing the binding of IL-6 to the receptor and its activation. By impeding IL-6 signaling, satralizumab helps reduce inflammation and prevents relapses in NMOSD (78).
3.5 IL-17 inhibitors: secukinumab, ixekizumab, brodalumab, and bimekizumab
The monoclonal antibody secukinumab is specifically designed to selectively target and suppress the pro-inflammatory cytokine IL-17A, which play a pivotal role in autoimmune diseases, such as psoriasis, ankylosing spondylitis, and psoriatic arthritis (57). To treat ankylosing spondylitis, a dosage of secukinumab has been selectively adjusted to a range of 150 to 300 mg. However, this adjustment has been associated with candidiasis in 11 (2.3%) cases for the lower dosage and 22 (4.7%) cases for the higher dosage (57).
The humanized monoclonal antibody ixekizumab is designed to target and suppress the production of IL-17A, a cytokine associated with autoimmune conditions such as psoriasis and psoriatic arthritis (59). Notably, pivotal phase III randomized, double-blind, placebo-controlled trials for psoriasis, known as UNCOVER-1, -2, and -3, that reported a total of 2,570 patients received ixekizumab, of which first dose was 160 mg followed by 80 mg every 2 or 4 weeks. Hence,16 incidences of candidiasis were reported and interestingly, an equal number instances were observed between weeks 0 and 60 and between weeks 0 and 12 (60–62).
Next, the human IgG2 monoclonal antibody known as brodalumab targets IL-17 receptor A (IL-17RA), a protein involved in the signaling cascade of IL-17 cytokines (79). Brodalumab reduces the inflammatory response associated with autoimmune diseases like psoriasis by inhibiting IL-17RA (80). In phase III clinical studies, namely AMAGINE -1, -2, and -3, 1338 adults with significant psoriasis were randomized to receive either brodalumab 210 mg or 140 mg. In all three studies involving brodalumab, 33 candidiasis cases were reported almost of them with a higher dosage of 210 mg (54–56).
The humanized IgG1 monoclonal antibody bimekizumab is designed to selectively target and suppress the pro-inflammatory cytokines IL-17A and IL-17F, both associated with autoimmune disorders (81). Bimekizumab is often administered to individual with psoriasis. Adult patients with moderate-to-severe psoriasis participated in three active-comparator-controlled, randomized phase III clinical trial: BE SURE, BE RADIANT, and BE VIVID. These studies utilized adalimumab, secukinumab, or Ustekinumab as active comparators, respectively. Candidiasis incidence was observed in the active-comparator group (0% to 5%), while in the bimekizumab group exhibited more candidiasis cases (9% to 21%) (63, 64, 82).
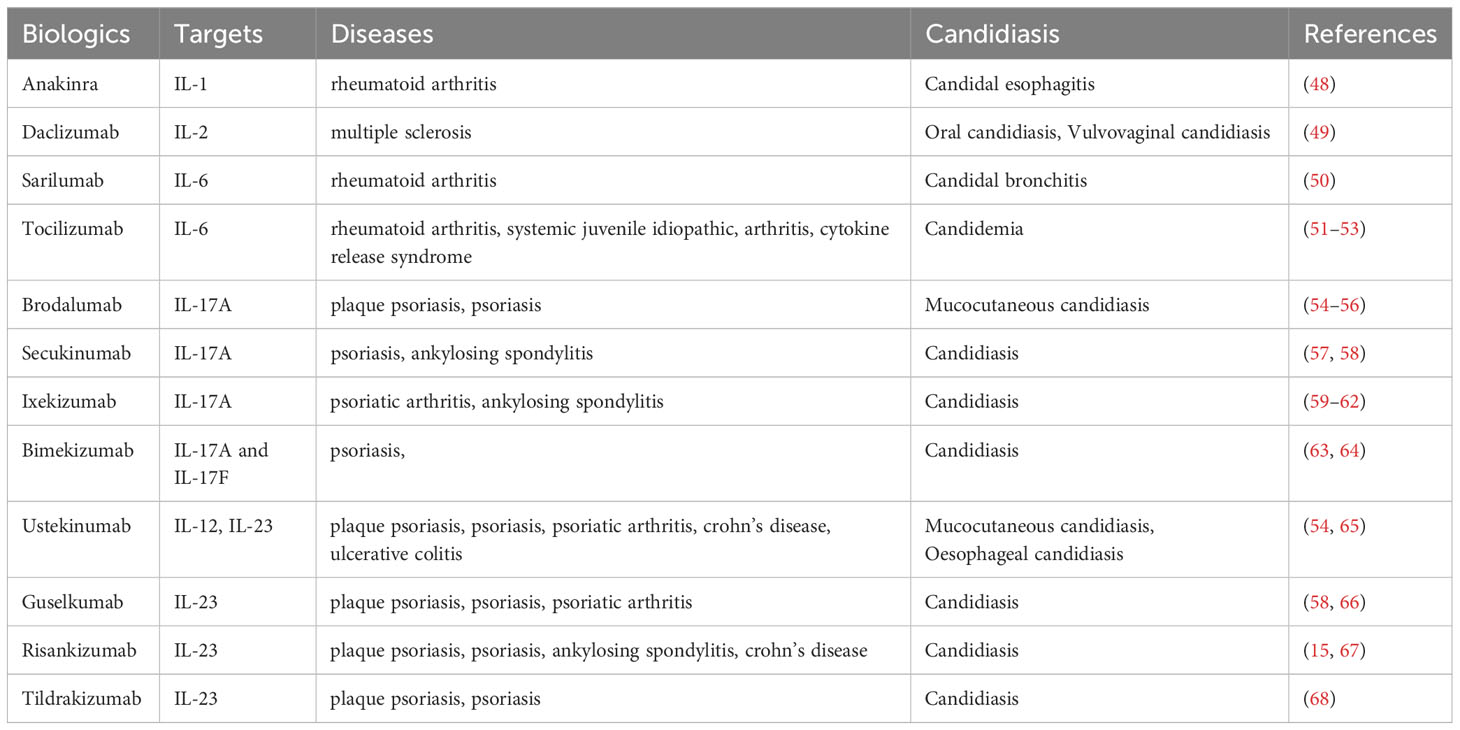
3.6 IL-23 inhibitors: ustekinumab, tildrakizumab, guselkumab and risankizumab
IL-23 inhibitors target and block IL-23, a cytokine that plays a crucial role upstream of Th17 lymphocyte activation. Four IL-23 inhibitors, namely ustekinumab, tildrakizumab, guselkumab, and risankizumab, have received approval for the treatment of psoriasis and psoriatic arthritis (83, 84). Ustekinumab, is a monoclonal antibody (IgG1 kappa) that binds to both the p19 and p40 subunits of the IL-23 protein. This biologic effectively suppresses IL-23 and IL-12 by interacting with the p40 subunit on IL-12 (85, 86). Ustekinumab is employed in treating moderate-to-severe plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis (87, 88).
The efficacy of ustekinumab and the occurrence of candidiasis infections have been studied in various clinical trials. In the UNITI trials, two cases of oesophageal candidiasis were reported: one in the placebo group and one in the group receiving 90 mg of ustekinumab every eight weeks (65). In another study encompassing two phases (AMAGINE-2 and AMAGINE-3) and in the CLEAR trials, brodalumab (210 mg or 140 mg) was compared with ustekinumab (45 mg or 90 mg) or placebo, a total of 3712 patients were examined and mucocutaneous Candida infections were reported in 1.3% of patients treated with ustekinumab at week 52 (54).
Tildrakizumab is a monoclonal antibody of the IgG1 class that specifically targets the p19 subunit of IL-23, a cytokine involved in the inflammatory responses of the immune system and implicated in conditions like psoriasis and psoriatic arthritis (Figure 2) (89, 90). Consequently, tildrakizumab effectively treats autoimmune diseases by inhibiting IL-23 and reducing inflammation (91). A comprehensive efficacy and safety trial, encompassed 391 patients with active psoriatic arthritis who received various doses of tildrakizumab (200 mg every four weeks, 100 mg every 12 weeks, or 20 mg every 12 weeks), alongside a placebo. In this trial, cases of candidiasis minimal (0.5% of all patients). Interestingly, both cases of candidiasis were observed in patients receiving tildrakizumab at a dosage of 200 mg every 4 weeks (68). Furthermore, guselkumab is an IgG1λ monoclonal antibody that inhibits IL-23 by targeting the p19 subunit (92). This biologic is used in the treatment of various conditions like pustular psoriasis, psoriatic arthritis, and chronic plaque psoriasis (93). In seven phase II/III studies (X-PLORE, VOYAGE 1, VOYAGE 2, NAVIGATE, ORION, ECLIPSE, Japan registration, randomized, double-blind, placebo-controlled trials for psoriasis, the 4252 patients who received guselkumab at dosage of 50-100 mg for weeks 0-16 weeks, three patients developed Candida infections, including two cases of vulvovaginal candidiasis and one of oral candidiasis. In the guselkumab (100 mg) treatment group, two patients developed candidiasis, comprising one case of vulvovaginal candidiasis and one of skin Candida (66). In the above seven phase II/III studies, 2891 patients were treated for psoriasis with guselkumab for up to 5 years. They suffered Candida infections, including vulvovaginal candidiasis (n=23), skin Candida (n=15), oral candidiasis (n=9), Candida infection (n=2), genital c andidiasis (n=2), and balanitis Candida (n=1) (66).
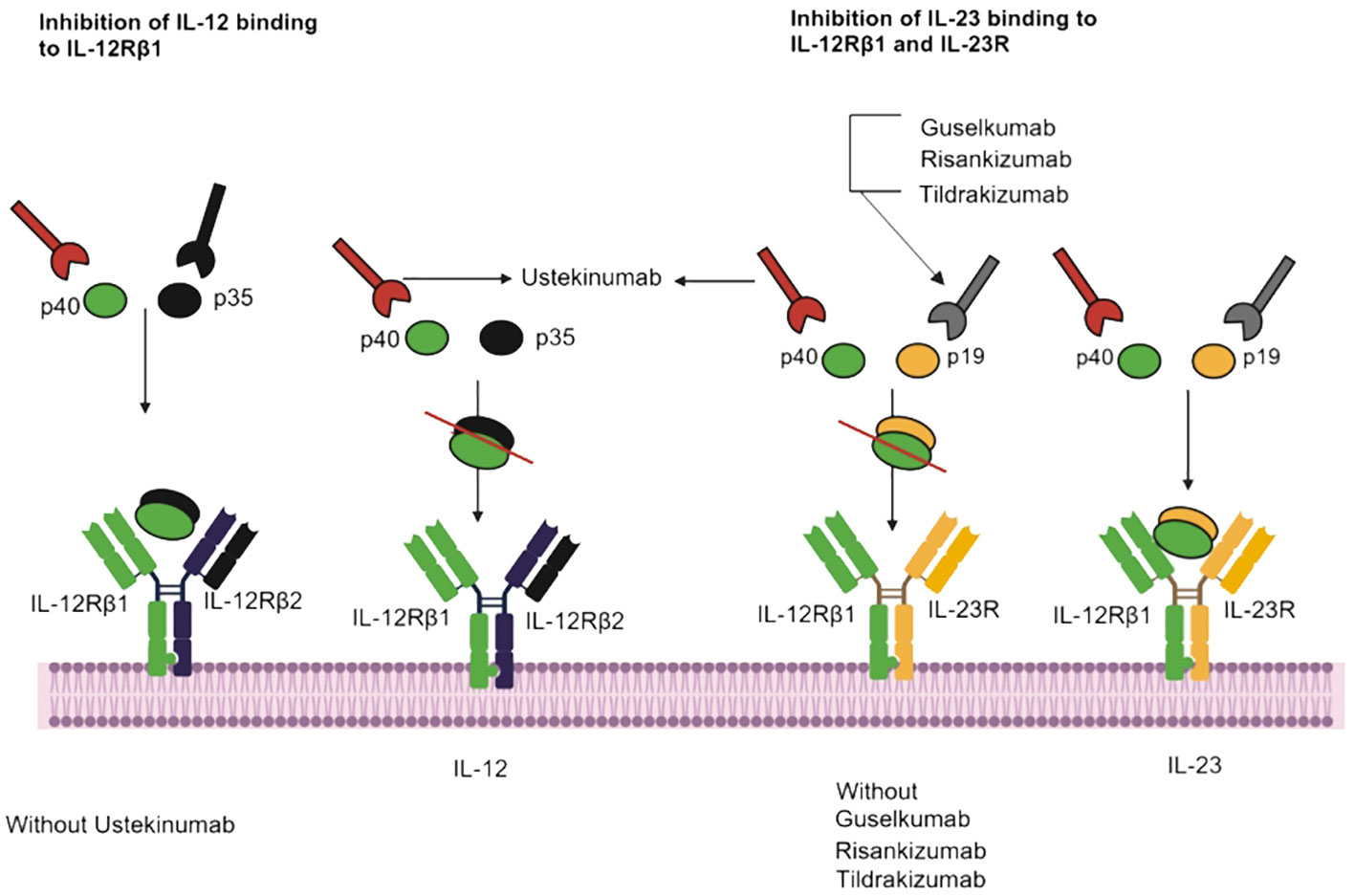
Figure 2 IL-23 inhibitors, binding sites, and mechanisms of action. Ustekinumab interacts with IL-12Rβ1, and Guselkumab, Risankizumab and Tildrakizumab inhibit IL-23 and IL-12Rβ1.
Risankizumab, a monoclonal antibody, has obtained approval for treating specific inflammatory conditions, such as moderate-to-severe psoriasis and psoriatic arthritis (94, 95). During placebo-controlled trials, open-label risankizumab clinical trials, 1306 patients were treated with 150 mg of risankizumab for primary psoriasis over a 16-week period, two cases of Candida infection occurred in this cohort (67). The safety and risk profiles of various IL inhibitors have been compared to assess their efficacy in addressing psoriasis. Notably, in patients treated with risankizumab, the reported rate of serious infection stood at 1.0 to 1.6 per 100 patient-years, compared to rates of 1.1 in the placebo group, 2.1 in patients treated with adalimumab and 5.3 in those treated with ustekinumab. Mucocutaneous candidiasis was noted in 3.4% of cases (15).
Additionally, in a phase 3 randomized controlled trial comparing guselkumab to secukinumab for the treatment of moderate-to-severe psoriasis, Candida infections occurred in 2% of the guselkumab-treated group and in 6% of those who received secukinumab (58). A recent trial investigating the new IL-17 inhibitor bimekizumab reported oropharyngeal candidiasis in 15% of patients, compared to 1% in those treated with ustekinumab (64). Patients receiving brodalumab were administered 210 mg or 140 mg maintenance doses every 2, 4, or 8 weeks between weeks 12 and 52. In the induction phase, patients in the placebo group received 210 mg of brodalumab every 2 weeks, while those in the ustekinumab group continued their treatment every 12 weeks. The incidence of mucocutaneous candidiasis was 4.8% among patients receiving brodalumab and 1.3% among those receiving ustekinumab at week 52 (54).
Overall, the risk of candidiasis associated with using IL12/23 inhibitors appear to be lower than that observed with IL17 inhibitors. Therefore, anti-IL-12/23 agents may be considered preferable for the treatment of autoimmune diseases. The variability in risk profile emphasized the important of selecting and tailoring treatment approaches, necessitating a thorough evaluation of the individual characteristics and factors associated with different groups of biological drugs. This approach ensures a comprehensive evaluation of individual patient factors, contributing to more inform and specialized medical treatment options.
3.7 Real world data on the risk of candidiasis during IL inhibitors
Several studies have examined the use of IL inhibitor therapy and its association with candida infection (Table 1). From June to November of 2021, 40 eligible patients received IL-13 and only had experienced candidiasis (96). Also, a single patient recently received dupilumab for the treatment of severe asthma and atopic dermatitis and an incident of Candida infection occurred. A total of 303 patients received IL-6 inhibitors and 12 had Candida infection including five candidemia with tocilizumab (400 mg) after two weeks (51, 52, 97, 98).
Furthermore, between 2017 and 2018, research was conducted where 391 patients received IL-23 inhibitors, with only two patients (0.5%) experiencing Candida infections both were on tildrakizumab 200 mg at four weeks (68). In 2021, a total of 955 patients received risankizumab at a dosage of 150 mg for up to 208 weeks, resulting in 20 reported cases of candidiasis. These cases manifested as seven cases of oral candidiasis, seven of genital candidiasis, four of esophageal candidiasis, one of intestinal candidiasis, and one of skin candidiasis (67). To further elucidate the study, a comprehensive analysis was conducted using aworld health organization (WHO) database containing 16,343,451 individual case safety reports. Among the reports, 50,353 cases pertained to candidiasis, of which 33,735 were categorized as proven or probable. Among the candidiasis cases, 427 reports (356, 1.05%, proven/probable candidiasis) were linked to anti-IL-17 drugs as the suspected cause, while 88 reports (63, 0.18%, proven/probable) were associated with anti-IL12/23 drugs (99). A population-based study examined the incidence of infections in patients with psoriasis with interleukin (IL)-23 inhibitors (IL-23i) and IL-17 inhibitors (IL-17i). A 5272 of patients received IL-23, IL-23i inhibitor, after which 104 cases of mucocutaneous candidiasis were noticed. In the same case, 15,160 patients received an IL-17 inhibitor, and mucocutaneous candidiasis was reported in 560 patients (100). In a retrospective study, 4866 adult patients with psoriasis were treated with an interleukin IL-17 or IL-23 inhibitor between 1 February 2015 and 31 October 2021. Due to the use of IL-17 inhibitor, 102 patients were infected with candida, while only 8 contracted the infection due to IL-23 inhibitor (101).
Moreover, a review compiled the clinical trial and real-world studies related to candidiasis incidences using IL-17 inhibitors. Here, the different IL-17 inhibitors and associated risk factors of candidiasis were compared. Additionally, guideline and safety assessment were provided in detail to healthcare professionals before prescribing IL-17 inhibitors (102). Moreover, a study examining biologics, including ustekinumab, and their potential for candidiasis risk, collected data from WHO VigiBase, the Population-based Drug Prescriptions Registry (PHARMO), and the Netherlands Psoriasis Cohort. This study revealed that of the 17,398 patients exposed to ustekinumab, 63 (0.36%) developed candidiasis. The manifestation included mucocutaneous (n=37), cutaneous (n=19), oropharyngeal (n=12), vulvovaginal (n=10), esophageal (n=6), onychomycosis (n=4), and candidemia (n=1) (99).
4 IL inhibitors and Candida infection in Covid-19
According to the World Health Organisation (WHO), an estimated15 million individuals died in the years of 2020- 2021 due to the COVID-19 pandemic (103). However, there were several interleukin inhibitors used for the treament of COVID-19 diseases (104). During phase 2a of a randomized, double-blind, placebo-controlled trial investigating treatment for COVID-19, 40 patients with Coronavirus disease received dupilumab given as two 300 mg subcutaneous injections at day 0 and with additional maintenance doses of 300 mg or placebo given on days 14 and 28. One patient who incident of oral candidiasis was reported during the whole study (96).
Recently, a study reported that out of 43 patients with severe COVID-19 pneumonia treated with tocilizumab, three (6.9%) developed candidemia (Table 1) (51). Another study compared tocilizumab use in patients with candidemia both with and without COVID-19 and found that it was 30 times more prevalent in COVID-19-associated candidemia (18.8% vs. 0.5% without COVID-19; p < 0.0001) (53). In a retrospective, observational cohort study, 179 patients with severe COVID-19 pneumonia patients were treated with tocilizumab (8 mg/kg body weight) twice a day, of which two had candidemia within 12 days (52). In another retrospective cohort study, tocilizumab (400 mg) was given to 65 patients for the treatment of severe COVID-19, and one patient had candidemia day 18 (97). In another example of an observational cohort study, 16 patient with COVID-19 were administered with tocilizumab (8 mg/dose) and five patient had candidemia (98). Furthermore, in an open-label therapy, a combination of tocilizumab (8 mg/kg up to 800 mg) and anakinra (100–300 mg) were given to thirty-one patients with severe COVID-19 pneumonia, in which one patient infected with candidal infection (105).
5 Discussion and conclusion
This comprehensive review highlights the intricate relationships between IL inhibitors, immunological responses, and the associated risks of opportunistic infections, such as candidiasis. Clinicians must possess a profound understanding of the mechanisms of these inhibitors to make informed clinical decisions and strike an appropriate balance between suppressing the immune system and preventing potential adverse effects. The positive impact of targeted therapy is most evident with IL-1 inhibitors, which reduce inflammation by blocking IL-1 signaling (106). IL-2 inhibitors provide valuable insight into precise immune regulation by targeting activated T cells (107). Most importantly, instances of Candida infections associated with IL-1 and IL-2 inhibitors are rarely reported. Furthermore, IL-4 and IL-13 inhibitors are promising for allergy treatment, albeit with candidiasis concerns, and IL-6 inhibitors curb autoimmune responses by interrupting IL-6 signaling interleukin (108). Notably, IL-17 inhibitors, used intreating complex autoimmune diseases, represent significant therapeutic options. Furthermore, IL-23 inhibitors block IL-23, a cytokine upstream of and crucial for the activation of Th17 lymphocytes consequently reducing the production of IL-17, IL-12, and IL-22 (109). However, limitations to the therapeutic potential of IL inhibitors do exist. This review underscores the risks of candidiasis associated with IL inhibitors several classes. Professionals must navigate these complex scenarios as the field evolves, weighing the benefits of immune system regulation against potential infection risks. In this dynamic setting, it is crucial for healthcare professional from various fields including, rheumatologists, immunologists, infectious disease experts, and other medical specialists, to collaborate toward the effective use of IL inhibitors.
In conclusion, this review comprehensive describes the intricate interplay between IL inhibitors, immunological responses, and the looming threat of candidiasis. Candida infections commonly occur hospitalized patients, particularly in immunocompromised. Hence, the use of IL inhibitor in the treatment of autoimmune diseases or COVID-19 poses a serious concern, as they inhibits immune response, and increasing the risk of candidiasis. It is crucial for clinicians to carefully balance therapeutic advantages with the risk of opportunistic infections as biological treatments reshape the management of autoimmune diseases. The advent of precision medicine heralds a new era of focused treatments, where a deep understanding of the complex functions of the immune system will inform individual choices.
Author contributions
SK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HB: Formal analysis, Methodology, Writing – review & editing. MK: Formal analysis, Investigation, Writing – review & editing. WF: Validation, Visualization, Writing – review & editing. WC: Formal analysis, Writing – review & editing. BY: Formal analysis, Writing – review & editing. N-JS: Formal analysis, Writing – review & editing. ZL: Formal analysis, Writing – review & editing. DZ: Methodology, Writing – review & editing. FY: Writing – review & editing. XW: Investigation, Writing – review & editing. QW: Investigation, Writing – review & editing. LC: Project administration, Resources, Writing – review & editing. BH: Validation, Visualization, Writing – review & editing. JW: Data curation, Writing – review & editing. CM: Validation, Writing – review & editing. LL: Data curation, Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. ZY: Investigation, Methodology, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is funded by the Second Affiliated Hospital of Shantou University Medical College to SK and his mentor YZ.
Acknowledgments
All the Authors are thankful to the Second Affiliated Hospital of Shantou University Medical College for supporting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hani U, Shivakumar HG, Vaghela R, Osmani RA, Shrivastava A. Candidiasis: a fungal infection–current challenges and progress in prevention and treatment. Infect Disord Drug Targets. (2015) 15:42–52. doi: 10.2174/1871526515666150320162036
2. Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. (2014) 10:95–105. doi: 10.2147/TCRM
3. Bilal H, Shafiq M, Hou B, Islam R, Khan MN, Khan RU, et al. Distribution and antifungal susceptibility pattern of Candida species from mainland China: A systematic analysis. Virulence. (2022) 13:1573–89. doi: 10.1080/21505594.2022.2123325
4. Luisa Gil M, Murciano C, Yáñez A, Gozalbo D. Role of Toll-like receptors in systemic Candida albicans infections. Front bioscience (Landmark edition). (2016) 21:278–302. doi: 10.2741/3263
5. Fietta P, Costa E, Delsante G. Interleukins (ILs), a fascinating family of cytokines. Part I: ILs from IL-1 to IL-19. Theor Biol Forum. (2014) 107:13–45.
6. Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol. (2012) 51:389–95. doi: 10.1111/j.1365-4632.2011.05154.x
7. Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. (2015) 21:719–29. doi: 10.1038/nm.3895
8. McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. (2006) 27:17–23. doi: 10.1016/j.it.2005.10.003
9. Xu M, Mizoguchi I, Morishima N, Chiba Y, Mizuguchi J, Yoshimoto T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin Dev Immunol 2010. (2010). doi: 10.1155/2010/832454
10. Richardson JP, Moyes DL. Adaptive immune responses to Candida albicans infection. Virulence. (2015) 6:327–37. doi: 10.1080/21505594.2015.1004977
11. De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. (2010) 3:361–73. doi: 10.1038/mi.2010.22
12. Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. (2010) 185:5453–62. doi: 10.4049/jimmunol.1001153
13. Kisand K, Bøe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. (2010) 207:299–308. doi: 10.1084/jem.20091669
14. Lee MP, Wu KK, Lee EB, Wu JJ. Risk for deep fungal infections during IL-17 and IL-23 inhibitor therapy for psoriasis. Cutis. (2020) 106:199–205. doi: 10.12788/cutis
15. Shoor S. Risk of serious infection associated with agents that target T-cell activation and interleukin-17 and interleukin-23 cytokines. Infect Dis Clin North Am. (2020) 34:179–89. doi: 10.1016/j.idc.2020.02.001
16. Feng Y, Zhou B, Wang Z, Xu G, Wang L, Zhang T, et al. Risk of candida infection and serious infections in patients with moderate-to-severe psoriasis receiving biologics: A systematic review and meta-analysis of randomized controlled trials. Int J Clin Pract. (2022) 2022:2442603. doi: 10.1155/2022/2442603
17. Frieder J, Kivelevitch D, Haugh I, Watson I, Menter A. Anti-IL-23 and anti-IL-17 biologic agents for the treatment of immune-mediated inflammatory conditions. Clin Pharmacol Ther. (2018) 103:88–101. doi: 10.1002/cpt.893
18. Kaisho T, Akira S. Regulation of dendritic cell function through toll-like receptors. Curr Mol Med. (2003) 3:759–71. doi: 10.2174/1566524033479366
19. Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. (2013) 34:521–30. doi: 10.1016/j.it.2013.07.006
20. Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. (2002) 20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828
21. Pathakumari B, Liang G, Liu W. Immune defence to invasive fungal infections: A comprehensive review. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2020) 130:110550. doi: 10.1016/j.biopha.2020.110550
22. Gong J, Zeng Q, Yu D, Duan YG, Lymphocytes T. and testicular immunity: A new insight into immune regulation in testes. Int J Mol Sci. (2020) 22(1):57. doi: 10.3390/ijms22010057
23. Gao Y, Liang G, Wang Q, She X, Shi D, Shen Y, et al. Different host immunological response to C. albicans by human oral and vaginal epithelial cells. Mycopathologia. (2019) 184:1–12. doi: 10.1007/s11046-018-0301-6
24. Shoham S, Levitz SM. The immune response to fungal infections. Br J haematology. (2005) 129:569–82. doi: 10.1111/j.1365-2141.2005.05397.x
25. Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. (2009) 206:299–311. doi: 10.1084/jem.20081463
26. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. (2018) 4:18026. doi: 10.1038/nrdp.2018.26
27. Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. (2008) 128:2640–5. doi: 10.1038/jid.2008.139
28. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. (2014) 14:585–600. doi: 10.1038/nri3707
29. Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. (2012) 13:722–8. doi: 10.1038/ni.2366
30. Floss DM, Schröder J, Franke M, Scheller J. Insights into IL-23 biology: From structure to function. Cytokine Growth Factor Rev. (2015) 26:569–78. doi: 10.1016/j.cytogfr.2015.07.005
31. Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. (2000) 407:916–20. doi: 10.1038/35038103
32. Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. (2007) 19:362–71. doi: 10.1016/j.smim.2007.10.007
33. Nur S, Sparber F, Lemberg C, Guiducci E, Schweizer TA, Zwicky P, et al. IL-23 supports host defense against systemic Candida albicans infection by ensuring myeloid cell survival. PLoS Pathog. (2019) 15:e1008115. doi: 10.1371/journal.ppat.1008115
34. Mohammadi H, Sharafkandi N, Hemmatzadeh M, Azizi G, Karimi M, Jadidi-Niaragh F, et al. The role of innate lymphoid cells in health and disease. J Cell Physiol. (2018) 233:4512–29. doi: 10.1002/jcp.26250
35. Saferding V, Blüml S. Innate immunity as the trigger of systemic autoimmune diseases. J Autoimmun. (2020) 110:102382. doi: 10.1016/j.jaut.2019.102382
36. Smeekens SP, van de Veerdonk FL, van der Meer JW, Kullberg BJ, Joosten LA, Netea MG. The Candida Th17 response is dependent on mannan- and beta-glucan-induced prostaglandin E2. . Int Immunol. (2010) 22:889–95. doi: 10.1093/intimm/dxq442
37. McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev. (2014) 260:129–44. doi: 10.1111/imr.12183
38. Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. (2004) 190:624–31. doi: 10.1086/422329
39. Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. (2013) 252:116–32. doi: 10.1111/imr.12027
40. Navegantes KC, de Souza Gomes R, Pereira PAT, Czaikoski PG, Azevedo CHM, Monteiro MC. Immune modulation of some autoimmune diseases: the critical role of macrophages and neutrophils in the innate and adaptive immunity. J Transl Med. (2017) 15:36. doi: 10.1186/s12967-017-1141-8
41. Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. (2010) 1:440–64. doi: 10.4161/viru.1.5.12983
42. Moyes DL, Naglik JR. Mucosal immunity and Candida albicans infection. Clin Dev Immunol. (2011) 2011:346307. doi: 10.1155/2011/346307
43. Blauvelt A, Papp KA, Lebwohl MG, Green LJ, Hsu S, Bhatt V, et al. Rapid onset of action in patients with moderate-to-severe psoriasis treated with brodalumab: A pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). J Am Acad Dermatol. (2017) 77:372–4. doi: 10.1016/j.jaad.2017.03.026
44. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discovery. (2012) 11:633–52. doi: 10.1038/nrd3800
45. Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. (2013) 25:469–84. doi: 10.1016/j.smim.2013.10.008
46. Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatol (Oxford England). (2002) 41:972–80. doi: 10.1093/rheumatology/41.9.972
47. Hausmann JS. Targeting cytokines to treat autoinflammatory diseases. Clin Immunol (Orlando Fla.). (2019) 206:23–32. doi: 10.1016/j.clim.2018.10.016
48. Fleischmann RM, Tesser J, Schiff MH, Schechtman J, Burmester GR, Bennett R, et al. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann rheumatic Dis. (2006) 65:1006–12. doi: 10.1136/ard.2005.048371
49. Giovannoni G, Kappos L, Gold R, Khatri BO, Selmaj K, Umans K, et al. Safety and tolerability profile of daclizumab in patients with relapsing-remitting multiple sclerosis: An integrated analysis of clinical studies. Multiple sclerosis related Disord. (2016) 9:36–46. doi: 10.1016/j.msard.2016.05.010
50. Genovese MC, Fleischmann R, Kivitz AJ, Rell-Bakalarska M, Martincova R, Fiore S, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: Results of a phase III study. Arthritis Rheumatol (Hoboken N.J.). (2015) 67:1424–37. doi: 10.1002/art.39093
51. Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev. (2020) 19:102564. doi: 10.1016/j.autrev.2020.102564
52. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. (2020) 2:e474–84. doi: 10.1016/S2665-9913(20)30173-9
53. Seagle EE, Jackson BR, Lockhart SR, Georgacopoulos O, Nunnally NS, Roland J, et al. The landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis an Off Publ Infect Dis Soc America. (2022) 74:802–11. doi: 10.1093/cid/ciab562
54. Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New Engl J Med. (2015) 373:1318–28. doi: 10.1056/NEJMoa1503824
55. Papp K, Menter A, Leonardi C, Soung J, Weiss S, Pillai R, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. (2020) 183:1037–48. doi: 10.1111/bjd.19132
56. Puig L, Lebwohl M, Bachelez H, Sobell J, Jacobson AA. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. (2020) 82:352–9. doi: 10.1016/j.jaad.2019.05.095
57. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. New Engl J Med. (2014) 371:326–38. doi: 10.1056/NEJMoa1314258
58. Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet (London England). (2019) 394:831–9. doi: 10.1016/S0140-6736(19)31773-8
59. Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann rheumatic Dis 72 Suppl 2. (2013), ii116–23. doi: 10.1136/annrheumdis-2012-202371
60. Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet (London England). (2015) 386:541–51. doi: 10.1016/S0140-6736(15)60125-8
61. Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New Engl J Med. (2016) 375:2102. doi: 10.1056/NEJMc1610828
62. Blauvelt A, Lebwohl MG, Mabuchi T, Leung A, Garrelts A, Crane H, et al. Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. (2021) 85:360–8. doi: 10.1016/j.jaad.2020.11.022
63. Griffith SK, Ahn GS, Wu JJ. Bimekizumab versus adalimumab in plaque psoriasis. New Engl J Med. (2021) 385:1149–50. doi: 10.1056/NEJMc2113092
64. Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet (London England). (2021) 397:487–98. doi: 10.1016/S0140-6736(21)00125-2
65. Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for crohn's disease. New Engl J Med. (2016) 375:1946–60. doi: 10.1056/NEJMoa1602773
66. Lebwohl MG, Merola JF, Rowland K, Miller M, Yang YW, Yu J, et al. Safety of guselkumab treatment for up to 5 years in patients with moderate-to-severe psoriasis: Pooled analyses across seven clinical trials with greater than 8600 patient-years of exposure. Br J Dermatol. (2023) 189(1):42–52. doi: 10.1093/bjd/ljad115
67. Papp KA, Lebwohl MG, Puig L, Ohtsuki M, Beissert S, Zeng J, et al. Long-term efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial beyond 3 years of follow-up. Br J Dermatol. (2021) 185:1135–45. doi: 10.1111/bjd.20595
68. Mease PJ, Chohan S, Fructuoso FJG, Luggen ME, Rahman P, Raychaudhuri SP, et al. Efficacy and safety of tildrakizumab in patients with active psoriatic arthritis: results of a randomised, double-blind, placebo-controlled, multiple-dose, 52-week phase IIb study. Ann rheumatic Dis. (2021) 80:1147–57. doi: 10.1136/annrheumdis-2020-219014
69. Bielekova B. Daclizumab therapy for multiple sclerosis. Neurother J Am Soc Exp Neurother. (2013) 10:55–67. doi: 10.1007/s13311-012-0147-4
70. Pfender N, Martin R. Daclizumab (anti-CD25) in multiple sclerosis. Exp Neurol. (2014) 262:44–51. doi: 10.1016/j.expneurol.2014.04.015
71. Church AC. Clinical advances in therapies targeting the interleukin-2 receptor. QJM monthly J Assoc Physicians. (2003) 96:91–102. doi: 10.1093/qjmed/hcg014
72. Ricciardolo FLM, Bertolini F, Carriero V. The role of dupilumab in severe asthma. Biomedicines. (2021) 9(9):1096. doi: 10.3390/biomedicines9091096
73. Tanaka T, Narazaki M, Kishimoto T. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS Lett. (2011) 585:3699–709. doi: 10.1016/j.febslet.2011.03.023
74. Mano Y, Shibata K, Sumigama S, Hayakawa H, Ino K, Yamamoto E, et al. Tocilizumab inhibits interleukin-6-mediated matrix metalloproteinase-2 and -9 secretions from human amnion cells in preterm premature rupture of membranes. Gynecologic obstetric Invest. (2009) 68:145–53. doi: 10.1159/000229021
75. Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. (2011) 13:R141. doi: 10.1186/ar3455
76. Lamb YN, Deeks ED. Sarilumab: A review in moderate to severe rheumatoid arthritis. Drugs. (2018) 78:929–40. doi: 10.1007/s40265-018-0929-z
77. van Rhee F, Fayad L, Voorhees P, Furman R, Lonial S, Borghaei H, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman's disease. J Clin Oncol Off J Am Soc Clin Oncol. (2010) 28:3701–8. doi: 10.1200/JCO.2009.27.2377
78. Fujihara K, Bennett JL, de Seze J, Haramura M, Kleiter I, Weinshenker BG, et al. Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurology(R) neuroimmunology Neuroinflamm. (2020) 7(5):e841. doi: 10.1212/NXI.0000000000000841
79. Bethesda M. Drugs and lactation database (LactMed). Natl Library Med (US): Bethesda MD USA. (2006). doi: 10.1080/15424065.2012.735134
80. Vidal S, Puig L, Carrascosa-Carrillo JM, González-Cantero Á, Ruiz-Carrascosa JC, Velasco-Pastor AM. From messengers to receptors in psoriasis: The role of IL-17RA in disease and treatment. Int J Mol Sci. (2021) 22(13):6740. doi: 10.3390/ijms22136740
81. Adams R, Maroof A, Baker T, Lawson ADG, Oliver R, Paveley R, et al. Bimekizumab, a novel humanized igG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. (2020) 11:1894. doi: 10.3389/fimmu.2020.01894
82. Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus secukinumab in plaque psoriasis. New Engl J Med. (2021) 385:142–52. doi: 10.1056/NEJMoa2102383
83. Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: The clinical importance of its divergence in skin and joints. Int J Mol Sci. (2018) 19(2):530. doi: 10.3390/ijms19020530
84. Fragoulis GE, Siebert S. The role of IL-23 and the use of IL-23 inhibitors in psoriatic arthritis. Musculoskeletal Care. (2022) 20 Suppl 1:S12–s21. doi: 10.1002/msc.1694
85. Luo J, Wu SJ, Lacy ER, Orlovsky Y, Baker A, Teplyakov A, et al. Structural basis for the dual recognition of IL-12 and IL-23 by ustekinumab. J Mol Biol. (2010) 402:797–812. doi: 10.1016/j.jmb.2010.07.046
86. Benson JM, Sachs CW, Treacy G, Zhou H, Pendley CE, Brodmerkel CM, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. (2011) 29:615–24. doi: 10.1038/nbt.1903
87. Jeon C, Sekhon S, Yan D, Afifi L, Nakamura M, Bhutani T. Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Hum Vaccin Immunother. (2017) 13:2247–59. doi: 10.1080/21645515.2017.1356498
89. Mease PJ. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr Opin Rheumatol. (2015) 27:127–33. doi: 10.1097/BOR.0000000000000147
90. Papp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR). J Drugs Dermatol. (2015) 14:706–14.
91. Puig L. The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol. (2017) 13:525–34. doi: 10.1080/1744666X.2017.1292137
92. Nawas Z, Hatch M, Ramos E, Liu M, Tong Y, Peranteau A, et al. A review of guselkumab, an IL-23 inhibitor, for moderate-to-severe plaque psoriasis. Skin Ther Lett. (2017) 22:8–10.
93. Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. (2017) 76:405–17. doi: 10.1016/j.jaad.2016.11.041
94. Yang K, Oak ASW, Elewski BE. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: A comprehensive review. Am J Clin Dermatol. (2021) 22:173–92. doi: 10.1007/s40257-020-00578-0
95. Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. New Engl J Med. (2017) 376:1551–60. doi: 10.1056/NEJMoa1607017
96. Sasson J, Donlan AN, Ma JZ, Haughey HM, Coleman R, Nayak U, et al. Safety and efficacy of dupilumab for the treatment of hospitalized patients with moderate to severe coronavirus disease 2019: A phase 2a trial. Open Forum Infect Dis. (2022) 9:ofac343. doi: 10.1093/ofid/ofac343
97. Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Internal Med. (2020) 76:43–9. doi: 10.1016/j.ejim.2020.05.021
98. Burger BJ, Epps SM, Cardenas VM, Jagana R, Meena NK, Atchley WT. Tocilizumab is associated with increased risk of fungal infections among critically ill patients with COVID-19 and acute renal failure: An observational cohort study. Life (Basel Switzerland). (2023) 13(8):1752. doi: 10.3390/life13081752
99. Davidson L, van den Reek J, Bruno M, Hunsel Fv, Herings RMC, Matzaraki V, et al. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources. Lancet Reg Health Eur. (2022) 13:100266. doi: 10.1016/j.lanepe.2021.100266
100. Kridin K, Zirpel H, Mruwat N, Ludwig RJ, Thaci D. Evaluating the risk of infections under interleukin 23 and interleukin 17 inhibitors relative to tumour necrosis factor inhibitors - A population-based study. J Eur Acad Dermatol Venereology JEADV. (2023) 37:2319–26. doi: 10.1111/jdv.19328
101. Torres T, Puig L, Vender R, Yeung J, Carrascosa JM, Piaserico S, et al. Drug survival of interleukin (IL)−17 and IL−23 inhibitors for the treatment of psoriasis: A retrospective multi−country, multicentric cohort study. Am J Clin Dermatol. (2022) 23:891–904. doi: 10.1007/s40257-022-00722-y
102. Bilal H, Khan MN, Khan S, Fang W, Chang W, Yin B, et al. Risk of candidiasis associated with interleukin-17 inhibitors: implications and management. Mycology. (2023), 1–15. doi: 10.1080/21501203.2023.2265664
103. Adam D. 15 million people have died in the pandemic, WHO says. Nature. (2022) 605:206. doi: 10.1038/d41586-022-01245-6
104. Convertino I, Tuccori M, Ferraro S, Valdiserra G, Cappello E, Focosi D, et al. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit Care (London England). (2020) 24:331. doi: 10.1186/s13054-020-03020-3
105. Haibel H, Poddubnyy D, Angermair S, Allers K, Vahldiek JL, Schumann M, et al. Successful treatment of severe COVID-19 pneumonia, a case series with simultaneous interleukin-1 and interleukin-6 blockade with 1-month follow-up. Ther Adv musculoskeletal Dis. (2022) 4:14. doi: 10.1177/1759720X221116405
106. Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol. (2006) 20:879–96. doi: 10.1016/j.berh.2006.06.004
107. Orozco Valencia A, Camargo Knirsch M, Suavinho Ferro E, Antonio Stephano M. Interleukin-2 as immunotherapeutic in the autoimmune diseases. Int Immunopharmacol. (2020) 81:106296. doi: 10.1016/j.intimp.2020.106296
108. Allocca M, Jovani M, Fiorino G, Schreiber S, Danese S. Anti-IL-6 treatment for inflammatory bowel diseases: next cytokine, next target. Curr Drug Targets. (2013) 14:1508–21. doi: 10.2174/13894501113146660224
Keywords: interleukins (ILs), immune system, signaling pathways, interleukin inhibitors, candidiasis
Citation: Khan S, Bilal H, Khan MN, Fang W, Chang W, Yin B, Song N-j, Liu Z, Zhang D, Yao F, Wang X, Wang Q, Cai L, Hou B, Wang J, Mao C, Liu L and Zeng Y (2024) Interleukin inhibitors and the associated risk of candidiasis. Front. Immunol. 15:1372693. doi: 10.3389/fimmu.2024.1372693
Received: 18 January 2024; Accepted: 18 March 2024;
Published: 28 March 2024.
Edited by:
Scott Kenneth Durum, National Cancer Institute (NIH), United StatesReviewed by:
Debora Decote-Ricardo, Federal Rural University of Rio de Janeiro, BrazilIrma Convertino, University of Pisa, Italy
Celio Geraldo Freire-de-Lima, Federal University of Rio de Janeiro, Brazil
Copyright © 2024 Khan, Bilal, Khan, Fang, Chang, Yin, Song, Liu, Zhang, Yao, Wang, Wang, Cai, Hou, Wang, Mao, Liu and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuebin Zeng, emVuZ195YkAxNjMuY29t
 Sabir Khan
Sabir Khan Hazrat Bilal
Hazrat Bilal Muhammad Nadeem Khan
Muhammad Nadeem Khan Wenjie Fang
Wenjie Fang Wenqiang Chang
Wenqiang Chang Bin Yin5
Bin Yin5 Fen Yao
Fen Yao Qian Wang
Qian Wang Chunyan Mao
Chunyan Mao Lingxi Liu
Lingxi Liu Yuebin Zeng
Yuebin Zeng