- Division of Abdominal Cancer, Department of Medical Oncology, Cancer Center and Laboratory of Molecular Targeted Therapy in Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Objective: Programmed cell death protein-1 (PD-1) inhibitor-based therapy has demonstrated promising results in metastatic gastric cancer (MGC). However, the previous researches are mostly clinical trials and have reached various conclusions. Our objective is to investigate the efficacy of PD-1 inhibitor-based treatment as first-line therapy for MGC, utilizing real-world data from China, and further analyze predictive biomarkers for efficacy.
Methods: This retrospective study comprised 105 patients diagnosed with MGC who underwent various PD-1 inhibitor-based treatments as first-line therapy at West China Hospital of Sichuan University from January 2018 to December 2022. Patient characteristics, treatment regimens, and tumor responses were extracted. We also conducted univariate and multivariate analyses to assess the relationship between clinical features and treatment outcomes. Additionally, we evaluated the predictive efficacy of several commonly used biomarkers for PD-1 inhibitor treatments.
Results: Overall, after 28.0 months of follow-up among the 105 patients included in our study, the objective response rate (ORR) was 30.5%, and the disease control rate (DCR) was 89.5% post-treatment, with two individuals (1.9%) achieving complete response (CR). The median progression-free survival (mPFS) was 9.0 months, and the median overall survival (mOS) was 22.0 months. According to both univariate and multivariate analyses, favorable OS was associated with patients having Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1. Additionally, normal baseline levels of carcinoembryonic antigen (CEA), as well as the combination of PD-1 inhibitors with chemotherapy and trastuzumab in patients with human epidermal growth factor receptor 2 (HER2)-positive MGC, independently predicted longer PFS and OS. However, microsatellite instability/mismatch repair (MSI/MMR) status and Epstein-Barr virus (EBV) infection status were not significantly correlated with PFS or OS extension.
Conclusion: As the first-line treatment, PD-1 inhibitors, either as monotherapy or in combination therapy, are promising to prolong survival for patients with metastatic gastric cancer. Additionally, baseline level of CEA is a potential predictive biomarker for identifying patients mostly responsive to PD-1 inhibitors.
Introduction
Gastric cancer ranks among the most prevalent cancers globally, standing as the fourth most common cause of cancer-related deaths in 2020, with an estimated 769,000 fatalities (1). The incidence of gastric cancer exhibits regional variation, with a higher prevalence observed in East Asia, particularly in China. In 2022, it ranked as the fifth most common cancer in China, with estimated incidence and mortality rates of 10.5% and 12.4%, respectively (2). Regrettably, alongside the high incidence of this disease, the majority of patients are diagnosed at an advanced stage upon detection. This is often due to the early clinical symptoms of gastric cancer being mild and easily overlooked. In patients with advanced or metastatic gastric cancer (AGC/MGC), conventional systemic chemotherapy remains the predominant treatment option. Commonly used regimens typically revolve around fluorouracil-based and platinum-based treatments such as SOX (oxaliplatin + S-1), XELOX (oxaliplatin + capecitabine), FOLFOX (calcium folinate + fluorouracil + oxaliplatin) and so on. However, chemotherapy shows limited effect, with a median survival of 11–12 months (3). For human epidermal growth factor receptor 2 (HER2)-positive patients, the recommended first-line treatment option for advanced cancers is the combination of the anti-HER2 antibody trastuzumab with chemotherapy (4, 5). Nonetheless, patient benefit remains constrained, underscoring the imperative to investigate further treatment alternatives.
Immune checkpoint inhibitors (ICIs), including antibodies against programmed cell death protein-1 (PD-1) or its ligand (PD-L1), have been rapidly developed over the past few years and are now established treatments for chemotherapy-refractory gastric cancers (cancers that progress after two or more lines of chemotherapy) (6, 7). Previous clinical studies have confirmed that some gastric cancer patients may benefit from PD-1 inhibitor therapy. Regarding first-line treatments for AGC/MGC, the KEYNOTE-062 trial, the first global randomized phase III trial comparing the efficacy and safety of pembrolizumab, a humanized anti-PD-1 monoclonal antibody, with chemotherapeutic agents in AGC/MGC, demonstrated that pembrolizumab alone was not inferior to chemotherapy [median overall survival (mOS): 10.6 vs. 11.1 months, hazard ratio (HR): 0.91, 95% confidence interval (CI): 0.69–1.18]. Moreover, pembrolizumab even extended OS in patients with a combined positive score (CPS) of 10 or greater (mOS: 17.4 vs. 10.8 months, HR: 0.69, 95% CI: 0.49–0.97). However, pembrolizumab in combination with chemotherapy did not result in an improvement in OS or progression-free survival (PFS) compared to chemotherapy, either in patients with CPS ≥1 or CPS ≥10 (8). In another phase III trial, KEYNOTE-859, pembrolizumab in combination with chemotherapy prolonged patients’ OS and PFS compared to chemotherapy alone (9). For nivolumab, another clinically utilized PD-1 inhibitor, the combination of nivolumab with a chemotherapeutic agent notably enhanced PFS across all CPS subgroups in both the CheckMate 649 and ATTRACTION-4 trials. Additionally, in the CheckMate 649 trial, overall survival was prolonged. However, there was no significant difference between the two treatment modalities in the ATTRACTION-4 trial (10, 11). Regarding second- and third-line therapy, two trials conducted globally and in Asia showed favorable activity and a manageable safety profile for PD-1 inhibitors in patients with refractory advanced gastric cancer or gastroesophageal junction cancer (GEJC) who had received at least two prior therapies (12, 13). In contrast, another phase III trial, JAVELIN Gastric 300, which explored the efficacy of avelumab, a complete human IgG1 monoclonal antibody, as maintenance therapy in patients with AGC/GEJC, led to the conclusion that avelumab did not improve OS or PFS compared to chemotherapy as third-line treatment (14).
Most of the above trials have demonstrated the effectiveness of PD-1 inhibitors in AGC/MGC, but the treatment response varies among the studies [objective response rate (ORR): 20%-65.1%] (8–10, 15). Findings on the benefits of OS, PFS, and ORR were also inconsistent. The clinical application of PD-1 inhibitor-based therapies is increasingly prevalent; however, there is limited real-world evidence regarding the use of PD-1 inhibitors as monotherapy or in combination treatments. This study endeavors to investigate the effectiveness of PD-1 inhibitor monotherapy or combination therapy in patients with metastatic gastric cancer through analysis of real-world clinical data. Furthermore, we conducted an additional analysis to explore the relationship between clinical characteristics and treatment efficacy, aiming to identify potential biomarkers for predicting the effectiveness of anti-PD-1 therapy.
Materials and methods
Study population
This retrospective, single-center study involved patients treated with PD-1 inhibitors monotherapy or a combination of chemotherapeutic agents and targeted agents, as documented by their medical history. We retrospectively collected information on MGC patients who received different PD-1 inhibitor-based treatments between January 2018 and December 2022 at West China Hospital of Sichuan University. The inclusion criteria were as follows: 1) pathologically confirmed gastric cancer; 2) no surgical options available after the initial diagnosis, or disease progression, metastasis, or recurrence after surgical cure; 3) receiving PD-1 inhibitor monotherapy or combination therapy in first line; 4) receiving at least 2 cycles of PD-1 inhibitor-based therapy; and 5) at least one measurable lesion; The major exclusion criteria included: 1) involvement of primary malignant tumors from other systems; and 2) loss to follow up for unknown reasons. We retrieved and integrated clinical information and basic characteristics from the records of the enrolled patients, including age, sex, HER2 expression status, Epstein-Barr virus (EBV) infection status, microsatellite instability/mismatch repair (MSI/MMR) status, carcinoembryonic antigen (CEA) baseline level, Eastern Cooperative Oncology Group performance status (ECOG PS), tumor stage, pathology type, metastatic organs, previous immunotherapy status, and treatment regimen. Patients were included regardless of their statuses of MMR proteins through immunohistochemical method or MSI through Next-Generation sequencing testing. The study was approved by the Ethics Committee of West China Hospital of Sichuan University, in accordance with the Declaration of Helsinki and the International Ethical Guidelines for Human Biomedical Research (Ethics Approval Number: 2023–2073).
Study design and treatment regimens
The chemotherapy regimens used SOX (oxaliplatin + S-1), TP (albumin paclitaxel + cisplatin), and XELOX (oxaliplatin + capecitabine), and the targeted drugs was the injectable trastuzumab (herceptin) for HER2-positive patients. The regimen of each PD-1 inhibitor for the concurrent use with chemotherapy drugs or targeted drugs is as follows: pembrolizumab (200mg), cedilimumab (200mg), tislelizumab (200mg) or toripalimab (240mg) for IV infusion every 3 weeks; or carrelizumab (200mg) or nivolumab (3mg/kg) for IV infusion every 2 weeks.
Assessment
The primary endpoint of this study was overall survival, defined as the time from the start of the patient’s PD-1 inhibitor-based therapy to the time of death from any cause, and the secondary endpoint was progression-free survival, defined as the time from the start of the patient’s PD-1 inhibitor-based therapy to the time of disease progression or death from any cause. Based on the Response Evaluation Criteria in Solid Tumors 1.1, the additional efficacy assessment metrics included: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), the objective response rate which is the sum of the proportions of complete response and partial response, as well as the disease control rate (DCR) which is defined as the proportion of cases achieving remission (CR+PR) and disease stability (SD) after treatment, representing the percentage of patients without disease progression.
Statistical analysis
Variables are presented as median (range) for continuous variables and numbers (%) for categorical variables. Relationships between categorical variables were examined by means of the Chi-square test. Univariate and multivariate analyses were performed to evaluate the prognostic impact on PFS and OS. The Kaplan–Meier method was applied to estimate survival probabilities and the log-rank test was carried out to assess heterogeneity within each prognostic factor. The HR was estimated using the Cox proportional hazard model. Cox proportional hazards regression analysis was carried out as a multivariate analysis to assess the prognostic value of the markers adjusted for the possible confounding effect of all the other factors included in the same model. All the statistical tests were two-sided, and differences for which p values were less than 0.05 were considered significant. All statistical analyses were performed using SPSS 27.0 software.
Results
Patients’ characteristics and treatment
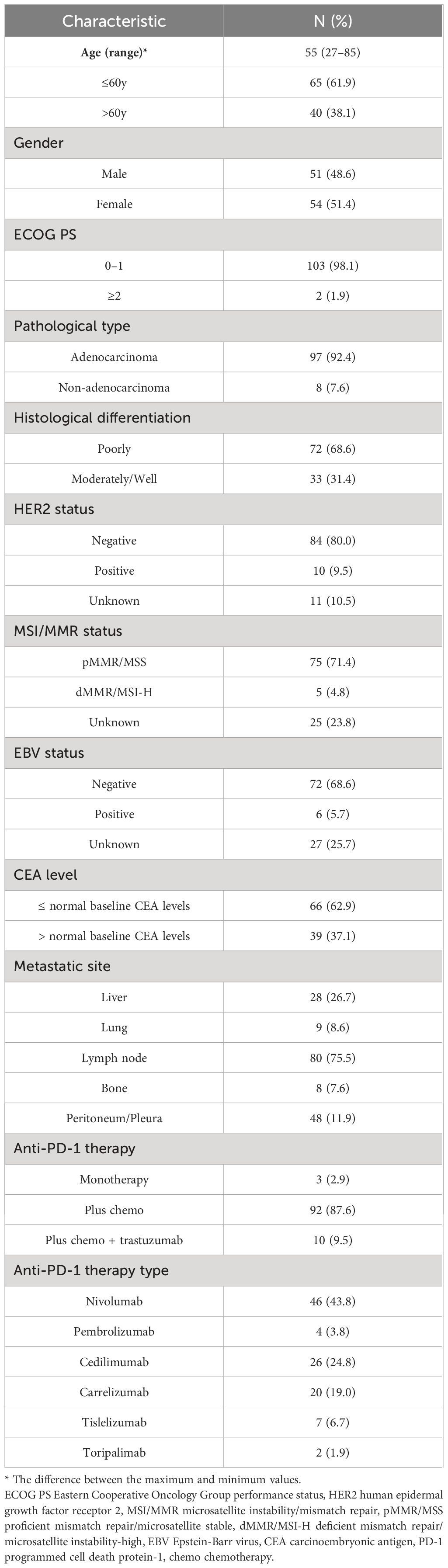
Our study included 105 patients whose median age was 55 years (range: 27–85). Among these patients, 51 (48.6%) were male, 103 (98.1%) had an ECOG PS score of 0–1, 97 (92.4%) had adenocarcinomas, 72 (68.6%) had poorly differentiated cancers. The numbers of tumors that metastasized to the liver, lung, lymph nodes, bone and peritoneum/pleura were 28 (26.7%), 9 (8.6%), 80 (75.5%), 8 (7.6%) and 48(11.9%), respectively. The PD-1 inhibitors used by patients included nivolumab (n=46, 43.8%), pembrolizumab (n=4, 3.8%), cedilimumab (n=26, 24.8%), carrelizumab (n=20, 19.0%), tislelizumab (n=7, 6.7%), toripalimab (n=2, 1.9%). A total of 3 (2.9%) individuals received treatment with PD-1 inhibitors alone, 92 (87.6%) received combined PD-1 inhibitor and chemotherapy, and 10 (9.5%) received combined PD-1 inhibitor, chemotherapy and targeted therapy. A total of 10 (9.5%) had positive HER2 status, 5 (4.8%) had deficient mismatch repair/microsatellite instability-high (dMMR/MSI-H) status, and 6 (5.7%) had positive EBV status, and 39 (37.1%) had CEA baseline level greater than normal baseline level. Table 1 shows more detailed information.
Efficacy
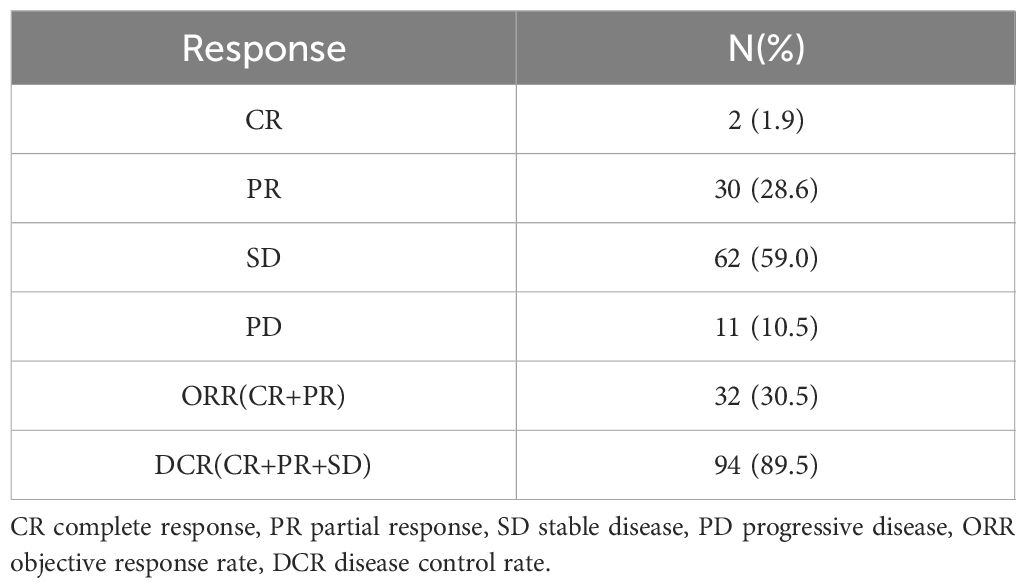
As of October 17, 2023, among the 105 individuals enrolled in the study, 2 (1.9%) patients achieved CR, and an ORR of 30.5% and a DCR of 89.5% were observed in the total population. Specific information can be found in Table 2. After 28.0 months (95% CI: 25.114–30.886) of follow-up, the median OS in the total population was 22.0 months (95% CI: 16.204–22.796; Figure 1A), and the median PFS was 9.0 months (95% CI: 7.184–10.816; Figure 1B). At the cut-off date, 40 patients were still alive.
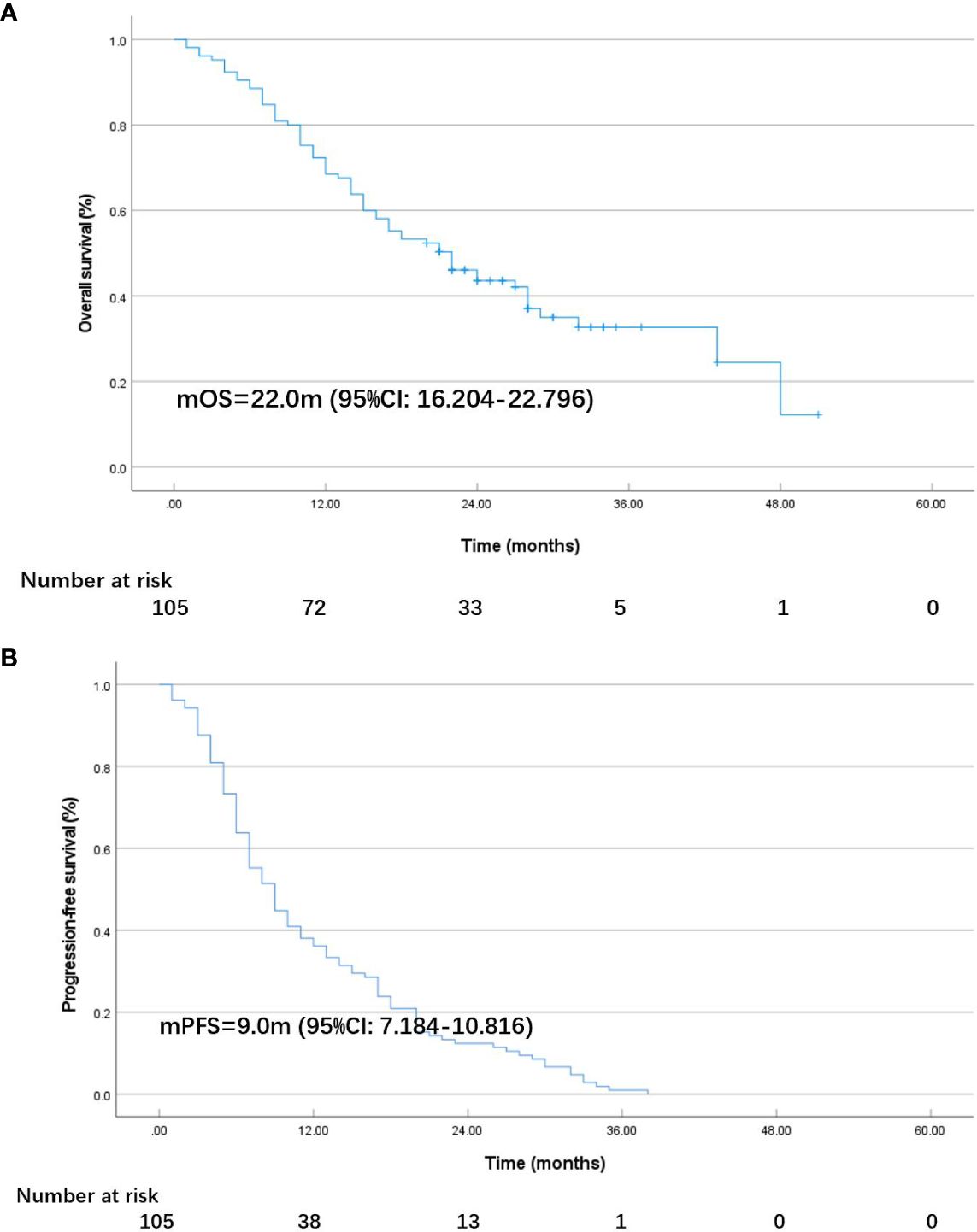
Figure 1 K-M plot of overall survival (OS) (A) and progression-free survival (PFS) (B) in all patients.
Univariate analysis and multivariate analysis
According to the results of the univariate analysis and multivariate analysis, favorable prognostic factors for OS included an ECOG PS score <2, CEA at the normal level, and the addition of targeted therapy for HER2-positive MGC patients (Tables 3, 4). The median OS for patients with ECOG PS score of 0–1 was 22.0 months, while for those with a score ≥2, it was 1.0 months (HR: 6.327, 95% CI: 1.502–26.648; p=0.012, Figure 2A). In the subgroups, patients with elevated CEA levels at baseline exhibited poorer immunotherapy responsiveness than those with normal CEA level: mOS (15.0 vs. 27.0 months, HR: 1.754, 95% CI: 1.031–2.985; p=0.038, Figure 2B) and mPFS (6.0 vs. 9.0 months, HR: 1.533, 95% CI: 0.992–2.369; p=0.054). Additionally, the combination of PD-1 inhibitors with chemotherapy and trastuzumab, compared to PD-1 inhibitors monotherapy or combined with chemotherapy alone, may result in a better mOS (>28.0 vs. 21.0 months, HR: 0.299, 95% CI: 0.093–0.962; p=0.043, Figure 2C, Table 3) and mPFS (12.0 vs. 8.0 months, HR: 0.597, 95% CI: 0.308–1.158; p=0.127, Table 4), however, PFS were not significantly different between the two subgroups.
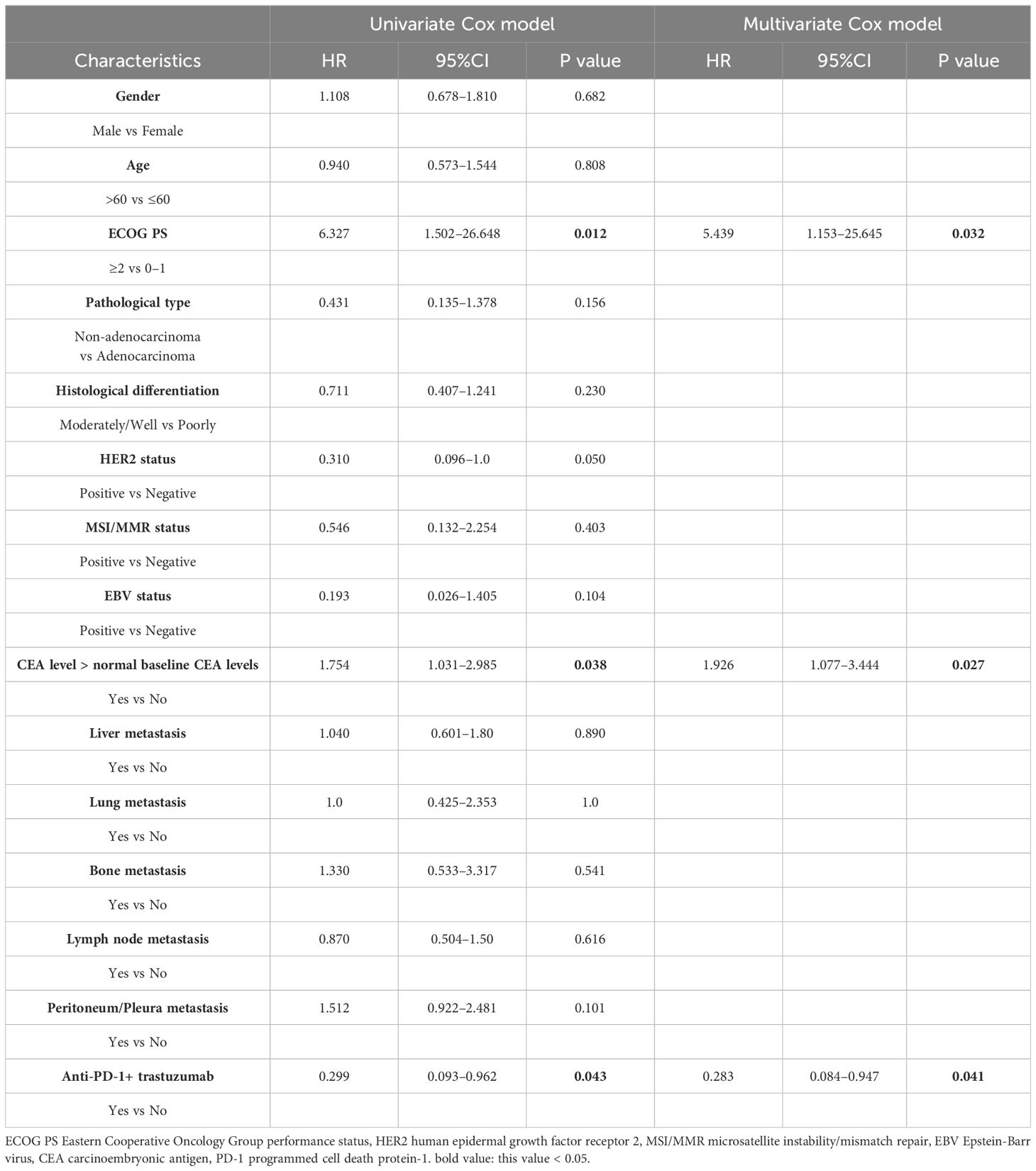
Table 3 Univariate analysis and multivariate analysis of clinical variables for the prediction of overall survival.
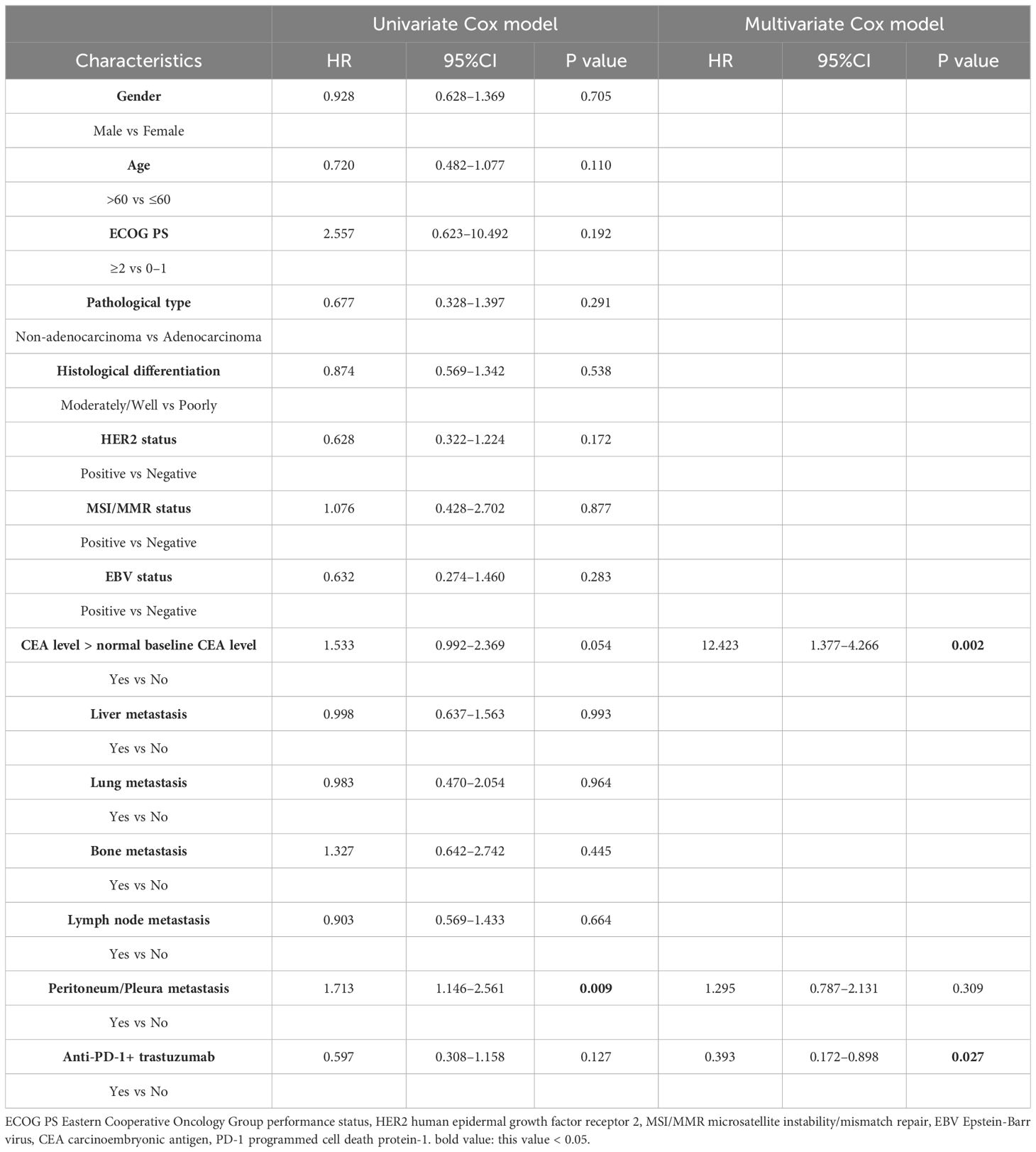
Table 4 Univariate analysis and multivariate analysis of clinical variables for the prediction of progression free survival.
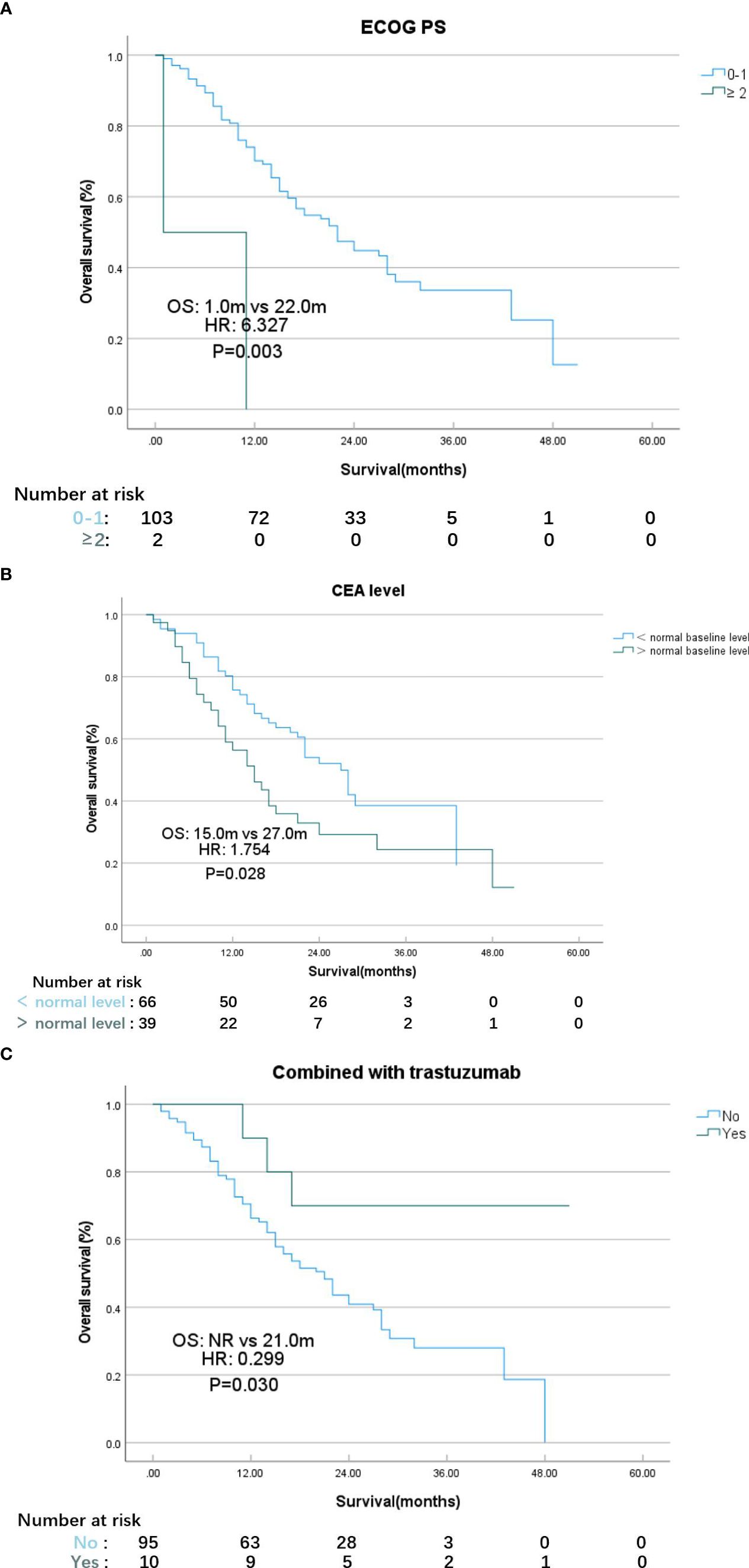
Figure 2 K-M plot of overall survival for patients with different ECOG PS scores (A), CEA level (B), therapy strategy (C). (A) ECOG PS Eastern Cooperative Oncology Group performance status. (B) CEA carcinoembryonic antigen. (C) NR not reached.
In the univariate analysis, considering that true effect of these factors may be masked by the effects of other confounding factors in single-factor analysis, we selected several indicators that were meaningful in the univariate analysis of OS for multivariate analysis, with favorable prognostic factors including CEA at the normal level (HR: 12.423, 95% CI: 1.377–4.266; p=0.002) and the addition of targeted therapy, trastuzumab, to immunotherapy (HR: 0.393, 95% CI: 0.172–0.898; p=0.027).
Molecular features associated with OS and PFS
MSI/MMR status and EBV infection status are commonly used molecular biomarkers for immunotherapy. However, this study found no significant correlation between different MSI/MMR status (positive vs. negative) and EBV infection status (positive vs. negative) with changes in mOS (MSI/MMR: 24.0 vs. 22.0 months, p=0.403; EBV: >28.0 vs. 22.0 months, p=0.104) and mPFS (MSI/MMR: 6.0 vs. 9.0 months, p=0.877; EBV: 17.0 vs. 9.0 months, p=0.283). Therefore, these two biomarkers cannot currently be deemed predictive of responsiveness to immunotherapy.
Discussion
Progressive gastric cancer has a poor prognosis and current treatment options are limited. With the rise of immunotherapy in recent years, PD-1 inhibitors such as nivolumab and pembrolizumab in combination with chemotherapy also have been recommended for treatment of metastatic gastric cancer (7, 16, 17). However, most previous conclusions were drawn from clinical trials, and there is considerable variation in patient outcomes, with ORR ranging from 20% to 65.1% (9, 10, 15, 18). Hence, it is imperative to investigate the real-world benefits that gastric cancer patients derive from first-line treatment with immune checkpoint inhibitors. To the best of our knowledge, this study represents a largest single-center real-world analysis among Chinese patients with MGC undergoing first-line treatment with anti-PD-1 therapy. Our findings suggest that first-line treatment with PD-1 inhibitors yields significant efficacy, particularly show that the median OS was 22.0 months (95% CI: 16.204–22.796), and the median PFS was 9.0 months (95% CI: 7.184–10.816) with an ORR of 30.5% and a DCR of 89.5%. Furthermore, compared to chemotherapy regimens alone in previous studies (OS:6.2–14 months, PFS: 4.3–12.1 months), the efficacy of chemotherapy combined with PD-1 inhibitors was indeed superior (3). Our patients obtained a longer OS.
It appears that patients may potentially benefit more from combination therapy with chemotherapy plus trastuzumab compared to PD-1 monotherapy or combination with chemotherapy alone. After a follow-up period of 28.0 months in our study, patients treated with PD-1 inhibitors exhibit a mOS of 21 months, while those receiving the combination of PD-1 inhibitors and trastuzumab remain alive, and thus have not yet reached their mOS. For HER2-positive patients, the current recommended standard treatment entails combining chemotherapy with trastuzumab. Recent studies have shown that adding PD-1 inhibitors to the combination of chemotherapy and trastuzumab can enhance efficacy in HER2-positive patients (19–21). Our study further supports this evidence. Nevertheless, due to the limited number of HER2-positive cases, further analysis and expansion of this patient subgroup are warranted.
Although anti-PD-1 treatment has improved patient prognosis compared to early studies, a considerable portion of patients still do not benefit from it, and there is also an increase of the risk of severe immune-related adverse effects. Therefore, researchers continue to explore and identify biomarkers to select the most suitable candidates for immunotherapy. Previous experiments have explored various biomarkers for predicting the efficacy of immunotherapy, including two gastric cancer genomic subtypes identified by The Cancer Genome Atlas (TCGA) studies: EBV infection status and MSI status (22). EBV infection can upregulate PD-L1 expression (23, 24), while DNA replication defects caused by dMMR/MSI-H result in the accumulation of mutations and the expression of new antigens, which may serve as potential targets for immune cells (25). Clinical trial findings suggest that patients with EBV infection or dMMR/MSI-H tumors may be more sensitive to immunotherapy (26–29). However, some experiments also demonstrate that these two biomarkers may not predict the response to immunotherapy in gastric cancer effectively (30–32). In this study, the ORR for two biomarkers EBV-positive and dMMR/MSI-H were 66.7% and 40%, respectively, which align with previous research findings (28, 29, 33). However, there were no significant statistical differences in OS and PFS between the subgroups. This lack of significance may be attributed to the low proportion of patients testing positive for these indicators. Consequently, the small sample size of these patients may have limited statistical power, underscoring the necessity to expand the sample size.
Currently, these biomarkers have limited clinical utility due to their uncertain predictive value, low positivity rates, and high cost. Therefore, we are striving to identify a more effective and convenient biomarker to predict the efficacy of immunotherapy. CEA is a widely used tumor marker in gastric cancer, playing a significant role in disease diagnosis and prognosis (34, 35). Additionally, research has demonstrated ability of CEA to predict the efficacy of PD-1 inhibitors, in non-small cell lung cancer. CEA values have been identified as predictors of patient responsiveness to immune checkpoint inhibitors (36, 37). In the current study, patients with CEA levels above the normal level had a mOS of 15.0 months, while those with normal CEA levels had a mOS of 27.0 months. Similarly, the mPFS was 6.0 months for patients with CEA levels above the standard level and 9.0 months for those with normal level, with statistically significant differences observed. It can be inferred that patients with CEA baseline levels below the standard threshold may derive greater benefits from immunotherapy. In the future, CEA levels might be utilized to predict patients’ response to anti-PD-1 therapy.
This study also has some limitations. Firstly, our study is a retrospective analysis, which inevitably introduces biases. Secondly, despite being the largest single-center, retrospective study to date, the sample size remains insufficient, for instance, efficacy comparisons among various PD-1 inhibitors were not feasible. Thirdly, due to the retrospective nature of the study, certain molecular markers in patients were not assessed, such as PD-L1 expression and tumor mutation burden (TMB), thus preventing analysis of these markers’ predictive value for treatment efficacy.
Conclusion
In conclusion, our study illustrates the effectiveness of PD-1 inhibitor-based therapy for patients with metastatic gastric cancer. Additionally, we found that carcinoembryonic antigen shows promise as a predictor of immunotherapy efficacy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Research Ethics Committee of West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because studies utilizing medical records were obtained from previous clinical consultations.
Author contributions
YD: Writing – original draft, Writing – review & editing. JL: Writing – review & editing. SZ: Writing – review & editing. FB: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to my colleagues who contributed to this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
3. Wagner AD, Syn NLX, Moehler M, Grothe W, Yong WP, Tai B-C, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Systematic Rev. (2017) 2017. doi: 10.1002/14651858.CD004064.pub4
4. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of her2-positive advanced gastric or gastro-oesophageal junction cancer (Toga): A phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
5. Qin S, Ji J, Xu R-H, Wang W, Tang Y, Bi F, et al. Treatment patterns and outcomes in chinese patients with gastric cancer by her2 status: A noninterventional registry study (Evidence). Oncologist. (2021) 26:e1567–e80. doi: 10.1002/onco.13826
6. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140–6736(20)31288–5
7. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1005–20. doi: 10.1016/j.annonc.2022.07.004
8. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the keynote-062 phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:1571–80. doi: 10.1001/jamaoncol.2020.3370
9. Rha SY, Wyrwicz LS, Weber PEY, Bai Y, Ryu MH, Lee J, et al. Vp1–2023: pembrolizumab (Pembro) plus chemotherapy (Chemo) as first-line therapy for advanced her2-negative gastric or gastroesophageal junction (G/gej) cancer: phase iii keynote-859 study. Ann Oncol. (2023) 34:319–20. doi: 10.1016/j.annonc.2023.01.006
10. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140–6736(21)00797–2
11. Kang Y-K, Chen L-T, Ryu M-H, Oh D-Y, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with her2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (Attraction-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:234–47. doi: 10.1016/s1470–2045(21)00692–6
12. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, MaChado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical keynote-059 trial. JAMA Oncol. (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
13. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in Patients with Advanced Gastric or Gastro-Oesophageal Junction Cancer Refractory to, or Intolerant of, at Least Two Previous Chemotherapy Regimens (Ono-4538–12, Attraction-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet. (2017) 390:2461–71. doi: 10.1016/S0140–6736(17)31827–5
14. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase iii, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of javelin gastric 300. Ann Oncol. (2018) 29:2052–60. doi: 10.1093/annonc/mdy264
15. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Lba53 sintilimab plus chemotherapy (Chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/gej) adenocarcinoma (Orient-16): first results of a randomized, double-blind, phase iii study. Ann Oncol. (2021) 32:S1331. doi: 10.1016/j.annonc.2021.08.2133
16. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The chinese society of clinical oncology (Csco): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. (2021) 41:747–95. doi: 10.1002/cac2.12193
17. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
18. Li T, Liu T, Zhao L, Liu L, Zheng X, Wang J, et al. Effectiveness and safety of anti-pd-1 monotherapy or combination therapy in chinese advanced gastric cancer: A real-world study. Front Oncol. (2023) 12:976078. doi: 10.3389/fonc.2022.976078
19. Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The keynote-811 trial of dual pd-1 and her2 blockade in her2-positive gastric cancer. Nature. (2021) 600:727–30. doi: 10.1038/s41586–021-04161–3
20. Rha SY, Lee C-K, Kim HS, Kang B, Jung M, Bae WK, et al. Targeting her2 in combination with anti-pd-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase ib/ii trial of first-line triplet regimen (Pembrolizumab, trastuzumab, chemotherapy) for her2-positive advanced gastric cancer (Agc). J Clin Oncol. (2020) 38:3081. doi: 10.1200/JCO.2020.38.15_suppl.3081
21. Triulzi T, Forte L, Regondi V, Di Modica M, Ghirelli C, Carcangiu ML, et al. Her2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology. (2019) 8:e1512942. doi: 10.1080/2162402X.2018.1512942
22. Kim ST, Cristescu R, Bass AJ, Kim K-M, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to pd-1 inhibition in metastatic gastric cancer. Nat Med. (2018) 24:1449–58. doi: 10.1038/s41591-018-0101-z
23. Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, et al. Abundant pd-L1 expression in epstein-barr virus-infected gastric cancers. Oncotarget. (2016) 7:32925–32. doi: 10.18632/oncotarget.9076
24. Kim SY, Park C, Kim H-J, Park J, Hwang J, Kim J-I, et al. Deregulation of immune response genes in patients with epstein-barr virus-associated gastric cancer and outcomes. Gastroenterology. (2015) 148:137–47.e9. doi: 10.1053/j.gastro.2014.09.020
25. Lee V, Murphy A, Le DT, Diaz LA. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist. (2016) 21:1200–11. doi: 10.1634/theoncologist.2016–0046
26. Xie T, Liu Y, Zhang Z, Zhang X, Gong J, Qi C, et al. Positive status of epstein-barr virus as a biomarker for gastric cancer immunotherapy: A prospective observational study. J Immunother. (2020) 43:139–44. doi: 10.1097/CJI.0000000000000316
27. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. Pd-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
28. Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the keynote-059, keynote-061, and keynote-062 clinical trials. JAMA Oncol. (2021) 7:895–902. doi: 10.1001/jamaoncol.2021.0275
29. Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J ImmunoTher Cancer. (2019) 7(1):24. doi: 10.1186/s40425–019-0514–3
30. Sun J-Y, Zhang D, Wu S, Xu M, Zhou X, Lu X-J, et al. Resistance to pd-1/pd-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. biomark Res. (2020) 8(1):35. doi: 10.1186/s40364–020-00212–5
31. Sun Y-T, Guan W-L, Zhao Q, Wang D-S, Lu S-X, He C-Y, et al. Pd-1 antibody camrelizumab for epstein-barr virus-positive metastatic gastric cancer: A single-arm, open-label, phase 2 trial. Am J Cancer Res. (2021) 11:5006–15.
32. Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a pd-1 antibody in phase ib/ii clinical trial nct02915432. Ann Oncol. (2019) 30:1479–86. doi: 10.1093/annonc/mdz197
33. Cai H, Li M, Deng R, Wang M, Shi Y. Advances in molecular biomarkers research and clinical application progress for gastric cancer immunotherapy. biomark Res. (2022) 10(1):67. doi: 10.1186/s40364–022-00413–0
34. Ramphal W, Boeding JRE, van Iwaarden M, Schreinemakers JMJ, Rutten HJT, Crolla RMPH, et al. Serum carcinoembryonic antigen to predict recurrence in the follow-up of patients with colorectal cancer. Int J Biol Markers. (2019) 34:60–8. doi: 10.1177/1724600818820679
35. Lee JH, Kim DY, Kim SH, Cho HM, Shim BY, Kim TH, et al. Carcinoembryonic antigen has prognostic value for tumor downstaging and recurrence in rectal cancer after preoperative chemoradiotherapy and curative surgery: A multi-institutional and case-matched control study of krog 14–12. Radiother Oncol. (2015) 116:202–8. doi: 10.1016/j.radonc.2015.07.049
36. Dall’Olio FG, Abbati F, Facchinetti F, Massucci M, Melotti B, Squadrilli A, et al. Cea and cyfra 21–1 as prognostic biomarker and as a tool for treatment monitoring in advanced nsclc treated with immune checkpoint inhibitors. Ther Adv Med Oncol. (2020) 12:1758835920952994. doi: 10.1177/1758835920952994
37. Wen S, Du X, Chen Y, Xia J, Wang R, Zhu M, et al. Association between changes in thioredoxin reductase and other peripheral blood biomarkers with response to pd-1 inhibitor-based combination immunotherapy in non-small cell lung cancer: A retrospective study. Trans Lung Cancer Res. (2022) 11:757–75. doi: 10.21037/tlcr-22–300
Keywords: PD-1 inhibitors, gastric cancer, immunotherapy, real-word study, drug response biomarkers
Citation: Duan Y, Li J, Zhou S and Bi F (2024) Effectiveness of PD-1 inhibitor-based first-line therapy in Chinese patients with metastatic gastric cancer: a retrospective real-world study. Front. Immunol. 15:1370860. doi: 10.3389/fimmu.2024.1370860
Received: 15 January 2024; Accepted: 21 May 2024;
Published: 12 June 2024.
Edited by:
Yih-Horng Shiao, United States Patent and Trademark Office, United StatesReviewed by:
Zhanjun Guo, Fourth Hospital of Hebei Medical University, ChinaKeren Jia, Peking University, China
Copyright © 2024 Duan, Li, Zhou and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Bi, YmlmZW5nQHNjdS5lZHUuY24=
†These authors have contributed equally to this work
 Yichun Duan
Yichun Duan Jielang Li
Jielang Li Shuang Zhou
Shuang Zhou Feng Bi
Feng Bi