
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 10 April 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1366489
This article is part of the Research Topic Response/Resistance to PD-1 Axis Inhibitors: Focus on the Tumor Microenvironment View all 12 articles
Cancer ranks among the foremost causes of mortality worldwide, posing a significant threat to human lives. The advent of tumor immunotherapy has substantially transformed the therapeutic landscape for numerous advanced malignancies, notably non-small cell lung cancer and melanoma. However, as immune checkpoint inhibitors (ICIs) are increasingly applied in clinical settings, a spectrum of undesired reactions, termed immune-related adverse events (irAEs), has emerged. These adverse reactions are associated with immunotherapy and can result in varying degrees of harm to the human body. Among these reactions, Immune checkpoint inhibitor-induced colitis (ICIIC) stands out as one of the most prevalent clinical adverse events. In contemporary times, traditional Chinese medicine (TCM) has demonstrated remarkable efficacy in addressing various maladies. Consequently, investigating the potential application and mechanisms of Chinese medicine in countering immune checkpoint inhibitor-induced colitis assumes significant importance in the treatment of this condition.
In recent years, the global cancer incidence has been steadily rising. Traditional cancer treatments include surgery, radiotherapy, chemotherapy, targeted therapy, and interventional therapy (1). Immunotherapy has become the fifth pillar of cancer management alongside surgery, chemotherapy, radiotherapy, and targeted therapy (2). Immune checkpoint inhibitors (ICIs) include nivolumab, pembrolizumab (PD-1 monoclonal antibodies), atezolizumab, avelumab, durvalumab (PD-L1 monoclonal antibodies), and ipilimumab (CTLA-4 inhibitor), among others (3). The side effects of immune checkpoint inhibitors have gained attention, affecting various systems like skin, gastrointestinal tract, heart, and more. Gastrointestinal immune-related adverse events (GI-irAEs)is a common issue (4). Traditional Chinese medicine (TCM), with a 2,000-year history, complements conventional cancer treatments effectively. TCM combined with antitumor therapy suppresses tumors, reduces drug resistance, and improves patient quality of life (1). Chinese herbal medicine is affordable, accessible, and minimally disruptive, making it widely accepted by patients. Investigating TCM’s potential in addressing immune checkpoint inhibitor-induced colitis offers new treatment approaches, enhancing patient quality of life and clinical care for this condition.
The primary symptoms of immune checkpoint inhibitor-induced colitis (ICIIC) include watery stool and non-bloody diarrhea, with abdominal pain, blood in the stool, nausea, vomiting, weight loss, and fever (1). Studies indicate that patients in the αCTLA-4 group have a higher incidence of GI-irAEs and more frequent diarrhea than patients in the αPD-1/PD-L1 group. Wang et al. reported colitis incidences of 9.1% with αCTLA-4 monotherapy, 1.3% with anti-PD-1/PD-L1 monotherapy, and 13.6% with combination therapy (2).
In a previous study (4), it was found that GI-irAEs typically occurred around 6-7 weeks after starting treatment with ipilimumab, an anti-CTLA-4 antibody. Another study showed that colitis began at 25.4 weeks in patients treated with anti-PD-1 monotherapy, compared with 7.2 weeks in patients treated with combination therapy (5). The incidence of diarrhea and colitis in patients treated with ipilimumab is approximately 30%-40% (6). Colitis typically affects the descending colon, but in certain instances, enteritis without colonic involvement occur, might leading to small bowel obstruction (7).
In most cases, mild GI-irAEs can resolve on their own or with close monitoring, and may not require discontinuation of ICIs. However, moderate to severe symptoms can lead to significant morbidity, affecting the patient’s nutritional and fluid balance status, and potentially necessitating hospitalization. This can also impact the patient’s eligibility for receiving further treatment (8). GI-irAEs can be classified into four grades of increasing severity in clinical practice, with corresponding diagnostic examinations and management strategies. Grades 1 and 2 involve mild to moderate diarrhea, while grades 3 and 4 are considered severe. Severe diarrhea can be accompanied by symptoms such as abdominal pain, rectal bleeding, and mucus in stool (9). At this critical stage, it can become life-threatening and potentially result in bowel perforation (7). Diagnostic evaluation for GI-irAEs includes stool microbiological culture and sensitivity testing, as well as colonoscopy if colitis is suspected or if diarrhea persists despite corticosteroid therapy (9). Endoscopy typically reveals inflammatory changes in the gastrointestinal tract, including erythema, inflammatory exudates, granularity, loss of vascularity, and ulcerations. Management usually involves the use of corticosteroids. In cases of steroid-refractory grades 3 and 4 diarrhea, second-line immunosuppressive therapies like budesonide, vedolizumab, or aminosalicylates have been reported as successful and safe options. Fecal microbiota transplantation (FMT) has also been used as a third-line therapy in some cases (10).
The exact pathogenic mechanism of systemic adverse reactions caused by ICIs is not fully understood, but it is believed that over-activated T cells contribute to the development of irAEs. The inhibition of CTLA-4 and PD-1 pathways, which act as negative regulators of T cell activation, can lead to irAEs. CTLA-4 functions to inhibit T cells at the initial stage of activation in the lymph nodes, whereas PD-1 regulates previously activated T cells in peripheral tissues or at tumor sites (11). The interaction of CTLA-4 on naive T cells to B7 on antigen-presenting cells (APCs) triggers an inhibitory signal upon T cell activation. Additionally, CTLA-4 can prompt transendocytosis of B7 molecules on APCs, further inhibiting T cell activation (12). PD-1 and its ligand PD-L1 interaction also leads to T cell inactivation. There may be a difference in the risk of colitis between PD-1/L1 inhibitors and CTLA-4 inhibitors, suggesting that CTLA-4 more thoroughly inhibits T cell activation (13). However, it is important to note that irAEs involve a complex interplay between various components of the immune system, including T cells, humoral immunity, and autoimmune diseases. Emerging evidence also suggests a possible association between the composition of the intestinal microbiota and the efficacy of immune checkpoint blockade (11, 14). The intricate relationship between the composition and ecology of the gut microbiota and the activation of specific T cells has been elucidated in prior research (15). Staphylococcal enterotoxin B has been demonstrated to elicit T cells activation, subsequently triggering the production of IFN-γ and IL-2 by these cells (16). A study has shown that human gut Actinobacterium Eggerthella lenta can induce intestinal Th17 activation by unblocking the Th17 transcription factor, exacerbating colitis in mice (17). In the therapeutic management of colitis induced by dextran sodium sulfate (DSS), FMT has shown promise in selectively reducing the representation of CD4+ and CD8+ T cells in the colon, thereby supporting intestinal homeostasis. This suggests a clear association between the gut microbiome and the modulation of T cell activity (18). Higher relative abundance of the Bacteroidetes phylum in the gut microbiota has been linked to a reduced rate of ipilimumab-induced colitis (19). FMT has been reported as a successful treatment for severe colitis associated with ICIs (20). These findings highlight the importance of regulating T cell activation, the intestinal microenvironment, and the gut microbiota in the management of irAEs.
The current clinical treatment for GI-irAEs mainly involves the use of steroid drugs. In refractory cases, infliximab and vedolizumab are commonly used. It has been observed that CTLA-4 inhibitors may require higher steroid doses compared to PD-1/PD-L1 inhibitors for the treatment of GI-irAEs (21). However, these drugs may not always be effective and can present challenges for physicians. The onset of GI-irAEs can vary widely, making it difficult to predict its development. The pathological features of GI-irAEs resemble those of chronic gastrointestinal inflammation, such as inflammatory bowel disease (IBD), ulcerative colitis (UC), and Crohn’s disease (CD) (22). ICIIC and IBD exhibit similarities in terms of endoscopy findings, histological evaluation, intestinal microbiota, and therapeutic approaches. However, the etiology of IBD is multifactorial, typically involving the intricate interplay of genetics, environmental factors, microbial factors, and immune-inflammatory responses. This complex interaction gives rise to a wide range of overlapping phenotypes, with each disease process displaying distinct clinical presentations (22). On the other hand, ICIIC commonly arises as a consequence of treatment with ICIs, and immunohistochemical analysis has revealed an elevated proportion of CD4+ cells and CD8+ cells in ICIIC (23, 24). It is crucial for physicians to quickly recognize and differentiate these conditions when managing immunosuppressive colitis to prevent worsening of the patient’s condition.
TCM has been used for centuries in China and East Asia and shows great potential in treating various complicated disease (25). Currently, there is limited research on the use of traditional Chinese medicine for the treatment of ICIIC. However, given the similarities between ICIIC and IBD in terms of endoscopic lesions, histopathological features, and treatment approaches (22), combined with TCM’s efficacy in treating IBD, it is plausible to consider the potential application of TCM in the treatment of ICIIC. Since the pathogenesis of ICIIC remains unclear, existing literature suggests that it may be associated with gastrointestinal immune inflammation and alterations in intestinal flora (20, 26–28). TCM can potentially target immune cells, the microenvironment, and intestinal flora effective treatment of ICIIC (Figure 1 and Table 1). The regulation of immune cells by TCM may occur through the following avenues: a) Reactivation of regulatory T cells (Tregs) to mediate immunosuppression. b) Inhibition of hyperactive macrophages. c) Restoration of the balance between T helper cells (Th1/Th2). d) Other mechanisms (66).
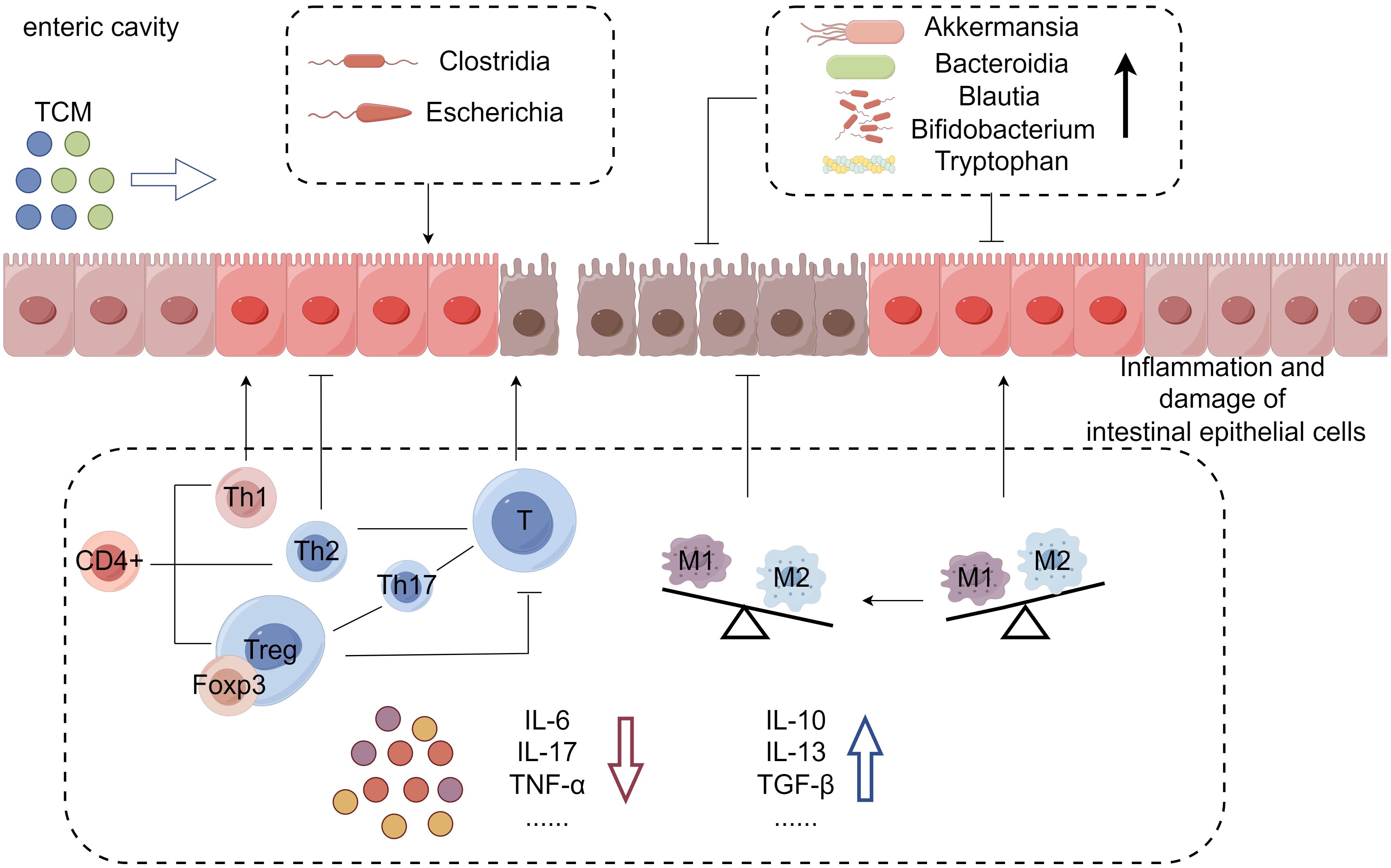
Figure 1 Mechanism of TCM in the treatment of immune checkpoint inhibitor-induced colitis: In this condition, overactivated T cells lead to intestinal epithelial inflammation, mucosal damage, barrier breakdown, and an imbalance of inflammatory factors. TCM can alleviate colitis symptoms by increasing anti-inflammatory factors, reducing pro-inflammatory factors, and altering intestinal flora and metabolites. This mechanism illustrates how TCM can effectively treat immune checkpoint inhibitor-induced colitis.
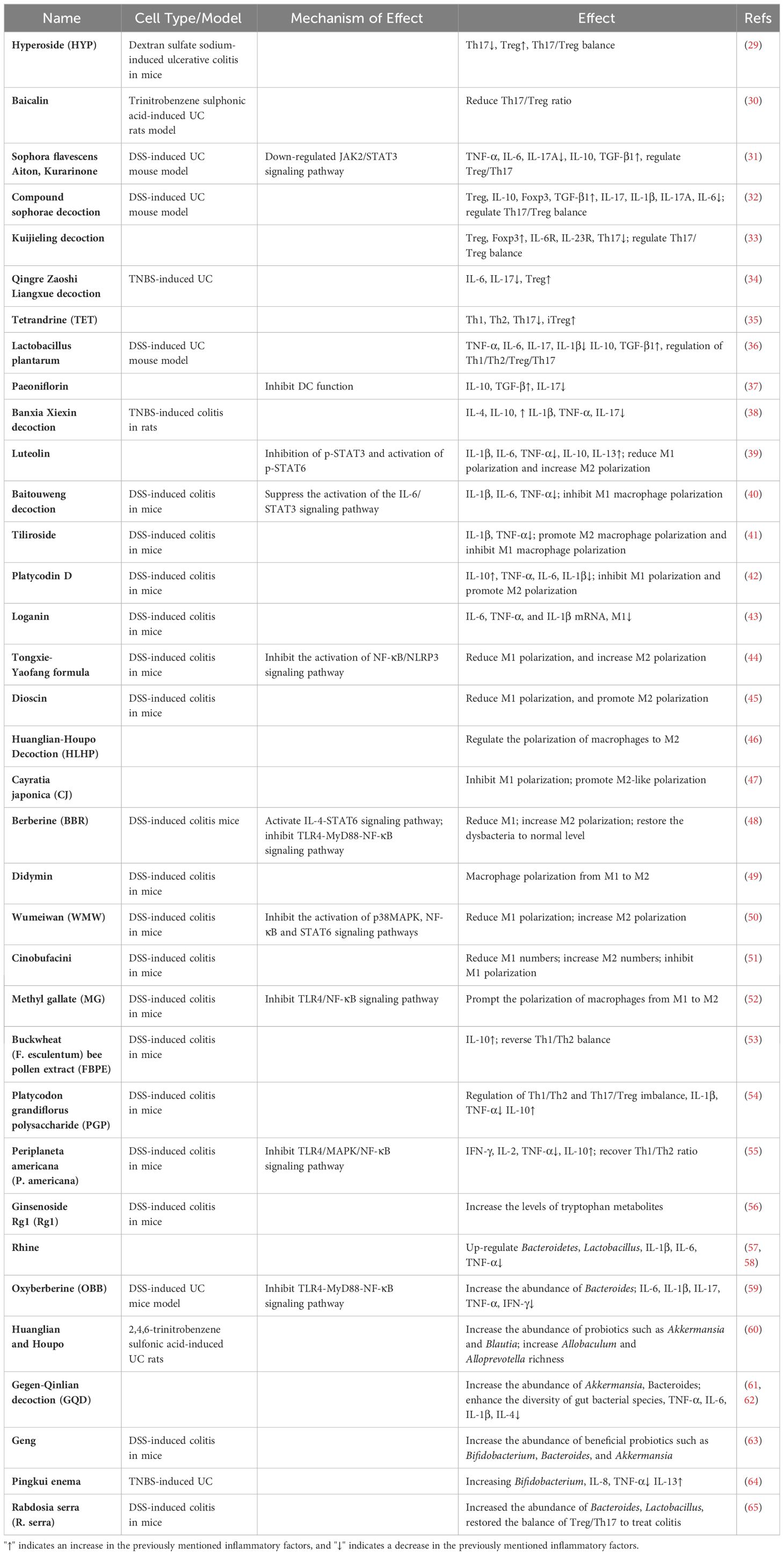
Table 1 Potential mechanism of Chinese medicine in treating immune checkpoint inhibitor-induced colitis.
Tregs, a subset of CD4+ T cells, play a crucial role in regulating autoimmune responses and maintaining immune balance. When activated, Tregs actively suppress the immune function of T lymphocytes. Foxp3 serves as a specific marker for Tregs and is essential in their development, peripheral expression, and functional maintenance, often referred to as the “primary regulator” of Tregs (67). In normal human biology, intestinal Tregs are essential for preserving immune stability in the gastrointestinal region by inhibiting abnormal immune reactions (68). Th17 cells, a type of T helper cells, are strongly linked to inflammation in the gut (66). Colitis typically presents with ulcer formation, infiltration by inflammatory cells, and an upregulation in pro-inflammatory cytokines such as IL-6, IL-12, IL-23. These pro-inflammatory factors contribute to intestinal inflammation and induce the conversion of naive T cells in the small intestine into Th17 cells, which release inflammatory cytokines like IL-17A and IL-17F. Notably, with the influence of TGF-β, intestinal DC can promote the induction of Tregs (68). Hyperoside (HYP) is found in a variety of plants such as Ligustrum phillideni, Hypericum, parsnips, and pecan. It has been shown to facilitate the differentiation of Tregs, suppress Th17 cells in cases of colitis, restore balance to the Th17/Treg axis, and ameliorate colitis (29). Baicalin, derived from Huangqin, a widely used Chinese herb in the treatment of ulcerative colitis, has been demonstrated to reduce the Th17/Treg ratio in TNBS-induced colitis, indicating its potential to alleviate colorectal inflammation (30). Triptolide (TPT), derived from the Chinese herb Tripterygium wilfordii, possesses strong immunosuppressive and anti-inflammatory characteristics. It efficiently inhibits the maturation of DCs. Additionally, TPT can increase the expression of DC-SIGN, promote the production of IL-10, and encourage DCs to adopt a tolerance-inducing phenotype. Consequently, TPT-treated DCs contribute to the expansion of Tregs (69). Various flavonoid compounds in the extract of Sophora flavescens (SFE) suppressed the productions of IL-6 and TNF-α in RAW 264.7 cells, with Kurarinone demonstrating significant potency. By downregulating the JAK2/STAT3 signaling pathway and controlling the differentiation of Th17 and Tregs, Kurarinone contributed to reestablishing the intestinal immune system’s balance in UC (31). The compound sophorae decoction, a Chinese herbal formula comprising six traditional Chinese herbs, demonstrated effective mitigation of colonic mucosal damage and reduction of IL-17A levels in colon tissue. In colitis-afflicted mice, it facilitated the development of Tregs, thereby modulating the Th17/Treg balance. The decoction upregulated the expression of IL-10, downregulated the concentration of IL-6, and increased TGF-β1 levels in colonic tissues, ultimately resulting in reduced colon inflammation in mice (32). The Kuijieling decoction, a TCM known for its impressive effectiveness in treating ulcerative colitis, functions by increasing the expression of Smad3 and Foxp3 to support Tregs differentiation. At the same time, it suppresses IL-6R and IL-23R genes expressions and the production of RORγt, resulting in the inhibition of Th17 differentiation and ultimately lowering the Th17/Treg cell ratio (33). The Qingre Zaoshi Liangxue decoction (QrLx), a Chinese herbal formula frequently employed for treating UC, has undergone extensive research. Studies have demonstrated that QrLx efficiently suppresses the expression of the inflammatory cytokine IL-6, as well as STAT3 and RORγt, which are associated with pro-inflammatory responses. Conversely, QrLx enhances the expression of Foxp3, a critical regulator of Tregs. These actions contribute to a considerable reduction in the ratio of Th17 cells and an increase in the ratio of Tregs (34). Tetrandrine (TET) is an anti-inflammatory bisbenzylisoquinoline alkaloid derived from Stephania tetrandra S. Moore, utilized in the management of autoimmune diseases, hypertension, and silicosis. In an experimental study, TET was found to strongly suppress the differentiation of pro-inflammatory Th1, Th2, and Th17 cells in vitro and in vivo, while leaving the differentiation of immunosuppressive iTreg cells unaffected (35). Lactobacillus plantarum was utilized to ferment Astragalus, a traditional Chinese medicine, and its potential therapeutic impact on DSS-induced colitis in mice was explored. The research uncovered that the fermented Astragalus (FA) reduced pro-inflammatory factors like TNF-α, IL-1β, IL-6, and IL-17, while enhancing anti-inflammatory factors like IL-10 and TGF-β. These observations imply that FA can adjust the inflammatory status by controlling the balance of cytokines associated with Th1, Th2, Th17, and Treg immune responses (36). In animal studies, paeoniflorin, derived from white paeony root, has demonstrated effectiveness in treating diseases resulting from immune overreactions by suppressing immune activation and response (70). A recent study revealed that paeoniflorin inhibits the maturation and immunostimulatory function of mouse bone marrow-derived DCs by enhancing the secretion of IL-10 and TGF-β, and decreasing IL-12 release and co-stimulatory molecules expression (37). Banxia Xiexin decoction has been shown to possess anti-inflammatory and antioxidant properties (71). The study indicated that the administration of Banxia Xiexin decoction decreased the disease activity index (DAI) score and colonic mass index, thereby alleviating histopathological damage in colitis-afflicted mice. Furthermore, it elevated the levels of anti-inflammatory factors such as IL-4 and IL-10, while decreasing the levels of pro-inflammatory factors such as IL-1β, TNF-α and IL-17 (38).
Macrophages are a multifaceted group of cells capable of performing a wide range of functions. In the intestines, macrophages play a vital role in maintaining immune balance and regulating inflammation. Overactive macrophages are involved in the body’s inflammatory response. Macrophages can be categorized into two phenotypes: M1 and M2. M1 macrophages primarily contribute to the initiation of inflammation and tissue damage, while M2 macrophages protect tissues from inflammation by regulating immune responses (72). Stimulation with lipopolysaccharide (LPS) and/or IFN-γ polarizes macrophages into M1 phenotype. These M1 macrophages produce inducible nitric oxide synthase (iNOS), IL-1, IL-6, and TNF-α. They also highly express CD86 and other proinflammatory cytokines, which aid in microbial clearance. On the other hand, stimulation with IL-4 or IL-13 polarizes macrophages into M2 phenotype. These M2 macrophages secrete anti-inflammatory factors such as arginase 1 (Arg-1) and IL-10. Additionally, they show increased expression of scavenger receptor CD163 or CD206, which helps alleviate inflammation and promote tissue regeneration. It is worth noting that macrophage polarization is regulated by various signaling pathways (39). Reducing the number of macrophages in the body or converting M1 macrophages to M2 macrophages might effectively alleviate the inflammatory response in ICIIC.
Luteolin, a natural flavonoid compound found in vegetables, fruits, and herbs, exhibits strong anti-inflammatory effects and possesses various beneficial biological properties, including anti-tumor, antioxidant, anti-infective, immunomodulatory, and cardioprotective activities. Studies have shown that luteolin not only reduces M1 polarization of macrophages and increases M2 polarization of macrophages, but also reduces pro-inflammatory IL-6 and TNF-α, and increases anti-inflammatory IL-10, thereby alleviating intestinal immune inflammation (39). In a study, it was demonstrated that Baitouweng decoction improved colon shortening, reversed body weight loss, and decreased DAI scores. Additionally, it was found to lower the levels of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. This decoction also ameliorated colonic pathological damage and inhibited M1 macrophage polarization. These effects were mediated by suppressing the activation of the IL-6/STAT3 signaling pathway in a DSS-induced colitis model (40). Tiliroside has been proven to promote M2 macrophage polarization while inhibiting M1 macrophage polarization. It achieves this by regulating macrophage polarization through the blocking of the glycolysis pathway. As a result, tiliroside possesses the ability to ameliorate pathological changes in the colon, increase survival rates, decrease DAI scores, and promote longer colon length (41). Platycodin D inhibits M1 macrophage polarization and promotes M2 macrophage polarization. It also protects intestinal barrier function and improves intestinal inflammation. Studies have shown that platycodin D is capable of suppressing peripheral and colonic tissue inflammation, reducing levels of IL-1β, IL-6, IL-10, and TNF-α, and alleviating colonic pathological damage (42). Loganin, an extract of Cornus officinalis, can significantly reduce the polarization of M1 macrophages and the expression of M1 macrophage-associated pro-inflammatory chemokines and cytokines in UC mice, which is beneficial for the treatment of UC (43). The Tongxie-Yaofang formula reduces M1 polarization of macrophages by inhibiting the activation of the NF-κB/NLRP3 signaling pathway and significantly increases M2 polarization, leading to a significant improvement effect on acute colitis (44). Dioscin, a traditional Chinese medicine compound isolated from Dioscorea, has powerful anti-inflammatory and immunomodulatory effects. In experiments using Dioscin to interfere with DSS-induced colitis in mice, it was found that Dioscin can improve colitis in mice, reduce the polarization of M1 macrophages, and significantly promote the polarization of M2 macrophages (45). The Huanglian-Houpo Decoction (HLHP) is a typical prescription for treating gastrointestinal diseases in ancient Chinese medicine. A study has shown that HL and HP (EHLHP) can regulate the polarization to M2 macrophages, reduce the level of inflammation, repair the intestinal mucosal barrier, and improve UC symptoms in experimental animals (46). Cayratia japonica (CJ) is a widely used folk medicine whose two main ingredients, rutin and quercetin, have been demonstrated to be effective in the treatment of inflammatory bowel disease (73). One study revealed that CJ can inhibit the TLR4/MAPK/NF-κB signaling pathway and macrophage M1 polarization. This promotes M2-like polarization, thereby reducing DSS-induced immunoinflammation in UC models and lessening colon damage (47). Berberine (BBR) is a common treatment for gastrointestinal disorders. It modulates inflammatory factors, decreasing the presence of M1 macrophages, and enhances STAT6 phosphorylation by activating downstream elements of the IL-4-STAT6 signaling pathway. This significantly promotes M2 polarization and effectively alleviates symptoms in UC mouse models (48). Didymin has the ability to convert the proinflammatory M1-like macrophage phenotype into an anti-inflammatory M2-like macrophage phenotype in an inflamed environment. However, it does not alter the polarization of M2-like macrophages (49). Wumeiwan (WMW) is commonly used to treat diarrhea resulting from colitis. A study has demonstrated that WMW inhibits intestinal inflammation and repairs damaged intestinal mucosa by suppressing the activation of p38MAPK, NF-κB, and STAT6 signaling pathways. It also inhibits M1 polarization, promotes M2 polarization, and regulates the levels of inflammatory factors (50). Cinobufacini, a compound with anti-inflammatory and anti-tumor properties, has demonstrated the ability to decrease M1 macrophage counts, significantly increase M2 macrophage numbers, and profoundly inhibit M1 polarization, thereby improving colitis (51). Sanguisorba officinalis L. (SOL), known for its potent anti-inflammatory effects, contains a component called Methyl gallate (MG) that exhibits efficient anti-UC efficacy. A study has revealed that MG can effectively prompt the polarization of macrophages from M1 to M2 and inhibit the release of inflammatory cytokines (52).
Colitis is classified as an inflammatory bowel disease characterized by an imbalance in the immune response, involving both pro-inflammatory (Th1) and anti-inflammatory (Th2) cytokines (74). T helper cells are critical in facilitating adaptive immune responses and inflammatory reactions, including autoimmunity, asthma, and allergies, by reacting to specific pathogens or autoantigens. CD4+ T cells are divided into Th1 cells and Th2 cells based on their cytokine secretion profile. Under normal conditions, there is a dynamic balance between Th1 and Th2 in the body. However, when tissue inflammation occurs, this balance is disrupted, and an excess of Th1 and Th2 can contribute to the development of autoimmune diseases (75).
Buckwheat (F. esculentum) bee pollen extract (FBPE) is abundant in nutritional components, including luteolin, resveratrol, kaempferol, and other active ingredients. Research has demonstrated that it can regulate immune function, restore the Th1/Th2 balance, thereby alleviating clinical symptoms and tissue damage in colitis-induced mice. Furthermore, FBPE enhances intestinal epithelial barrier function and exhibits a protective effect on colitis (53). Platycodon grandiflorus polysaccharide (PGP), a main component of P. grandiflorus, has been shown to effectively reduce the levels of Th1 or Th17 related cytokines and transcription factors, while increase the levels of Th2 or Treg related counterparts, and treat UC by regulating the immune balance between Th1/Th2 and Th17/Treg cells (54). One study applied Periplaneta americana (P. americana) to acute colitis mice model, and the results showed that P. americana can regulate cytokines. The significantly elevated Th1/Th2 ratio in a mouse model of acute colitis was restored to normal, and DSS induced inflammation was suppressed through immunomodulatory effects (55).
In cases of gastrointestinal inflammation, specific amino acids and metabolites are involved in the inflammatory process. The intervention of ginsenoside Rg1 (Rg1) can affect various metabolic pathways in the gut microbiota, including the regulation of valine, leucine, and vitamin B6 metabolism. The most notable impact is seen in the regulation of tryptophan metabolism. Rg1 has the ability to elevate the levels of tryptophan metabolites, which helps protect the intestinal barrier and decrease colon inflammation in mice with UC (56).
The role of gut microbiota dysbiosis in the development and progression of various diseases has been increasingly supported by evidence (25). Given the similarities between IBD and ICIIC with other chronic intestinal inflammations, regulating the intestinal flora becomes crucial in the treatment of intestinal disorders. TCM follows the principle of “treating different diseases using the same method,” which is a fundamental concept in TCM theory. When multiple diseases share a common underlying cause, TCM practitioners often prescribe similar or identical treatments. Network pharmacology research has also identified shared pathogenic mechanisms across different diseases, allowing TCM to address multiple conditions by targeting these core factors (25). Numerous studies have shown that herbal medicine’s efficacy is closely related to improving gut microbiota.
Currently, studies have shown a relevant connection between the intestinal flora and irAEs. Manipulating the gut microbiota has demonstrated significant and rapid improvement in irAEs associated with ICIs, as observed in both preclinical and clinical trials. A case series reported successful treatment of ICIIC through fecal microbiota transplantation, resulting in the reconstruction of the intestinal microbiome and an increased proportion of regulatory T cells in the colon mucosa, highlighting the potential of modulating the intestinal microbiome to mitigate ICIIC (20). The study showed that colitis in ICIIC patients predominated with Clostridia and Escherichia, the latter of which is associated with an intestinal disorder. After treatment, the increase of Akkermansia, Bacteroidia, Blautia and Bifidobacterium was associated with the improvement of colitis (20). Lactobacillus reuteri has demonstrated alleviation of ICIIC by downregulating group 3 innate lymphocytes (ILC3s) (76). Wang et al. (77) proved that the combinations of anti-CTLA-4 and anti-PD-1 antibody treatments induced colitis, in the mouse model, fecal microbial sequencing showed that the abundance of Lactobacillus in the intestinal flora of mice with immune checkpoint associated colitis was significantly reduced. Many studies have shown that gut microbiota is also involved in Th17/Treg balance (78, 79), such as Lactobacillus and Bifidobacterium are involved in Treg development and T cell response (80). In addition, a prospective study revealed that ipilimumab treatment did not change the microbiome composition, while the occurrence of ICIIC was associated with a decrease in microbial diversity, such as several genera in Firmicutes were significantly reduced (11). Improving intestinal flora richness to improve intestinal flora disturbance is one of the functions of TCM. Rhine up-regulates Bacteroidetes, especially Rikenellaceae, a kind of Bacteroidetes, which can enhance the barrier function of intestinal epithelial cells, play a beneficial role in anti-inflammation and UC, and is negatively correlated with pro-inflammatory factors, such as IL-1β, IL-6 and TNF-α. It can effectively inhibit the production of proinflammatory cytokines (57). Another study showed that Rhine can also increase lactobacillus levels, protect the intestinal barrier, and reduce colitis (58). Berberine (BBR), an isoquinoline alkaloid derived from various Chinese herbal medicines, has been extensively utilized in the treatment of dysentery and colitis. In a study, it was found that the gut microbiota can metabolize BBR into Oxyberberine (OBB) through an oxidation reaction. Oxyberberine (OBB) possesses a spectrum of pharmacological benefits, encompassing anti-inflammatory, anti-fungal, anti-tumor, and anti-arrhythmic properties, with OBB outperforming BBR in terms of anti-inflammatory, anti-fungal, and anti-arrhythmic efficacy. This study showed that both OBB and BBR can increase the abundance of Bacteroides, and OBB has superior anti-colitis activities, including improving the intestinal mucosal barrier, inhibiting colitis tissue damage, and reducing pro-inflammatory factors such as IL-6, IL-1β, IL-17, TNF-α and IFN-γ (59). Coptis chinensis Franch (referred to as Huanglian in Chinese, HL) and Magnoliae officinalis (known as Houpo, HP) have been traditionally used in folk medicine for hundreds of years to treat gastrointestinal disorders, including ulcers and inflammation. A study demonstrated the therapeutic effects of HL and HP on 2,4, 6-trinitrobenzene-sulfonic acid-induced UC rats and their effects on intestinal flora in UC rats. The results showed that HL+HP supplementation augmented the abundance of beneficial bacteria such as Akkermansia and Blautia, significantly reduced the harmful flora, improved colitis, and demonstrated the ability to suppress inflammatory responses (60). Gegen-Qinlian decoction (GQD) stands as a classic formulation in TCM, comprising four medicinal herbs: Puerariae Lobatae Radix, Scutellariae Radix, Coptidis Rhizoma, and Glycyrrhizae Radix et Rhizoma Praeparata cum Melle. GQD demonstrates efficacy in treating inflammatory intestinal diseases, encompassing diarrhea, ulcerative colitis, and intestinal adverse reactions induced by chemotherapy drugs. Studies have revealed that GQD treatment enhances the diversity of gut bacterial species and reshapes the composition of gut microbial communities. Specifically, GQD treatment increased the relative abundance of gut bacteria, including Akkermansia, Bacteroides and others (61). Another study showed that GQD treatment of colitis also significantly reduced TNF-α, IL-6, IL-1β, and IL-4 (62). Geng, known as the “king” of TCM, exhibits notable therapeutic effects on various diseases. Treatment with ginseng has been shown to increase the abundance of beneficial probiotics such as Bifidobacterium, Bacteroides, and Akkermansia, and improve colitis and diarrhea effectively by increasing Bacteroidetes and Lactobacillus. Furthermore, certain bacteria, including Bacteroides, Eubacterium, and Bifidobacterium, have been found to transform ginsenosides, improving the absorption rate of ginseng to improve colitis (63). Pingkui enema, a herbal compound used to treat intestinal diseases, has been shown to have a significant therapeutic effect on TNBS-induced UC by increasing the content of Bifidobacterium and adhesin receptors of bifidobacterium, reducing the concentration of IL-8 and TNF-α, and increasing the concentration of IL-13 (64). Rabdosia serra (R. serra) can increase the abundance of Bacteroides, Lactobacillus and other beneficial bacteria, regulate immune factors, and restore the balance of Treg/Th17 to treat colitis (65).
In traditional Chinese medicine theory, long-term improper diet and emotional stimulation can impact visceral function and impair the body’s healthy qi. A deficiency in healthy qi can weaken the body’s ability to resist external pathogens and clear abnormal cells. Additionally, prolonged visceral dysfunction, abnormal transport, phlegm stagnation, blood stasis, and the accumulation of turbid phlegm can contribute to cancer toxin buildup, ultimately leading to tumor formation. Therefore, the occurrence of tumors is believed to result from the struggle between healthy and pathogenic qi within the human body.
ICIs are a novel category of immunotherapy drugs used for anti-tumor treatment by activating the immune system to eliminate cancer cells through immune checkpoint blockade. Although PD-1/PD-L1 immunosuppressants have demonstrated significant effectiveness in this regard, their mechanism of action can disrupt the balance of the body’s immune environment, leading to irAEs. Colitis, a common side effect associated with immune checkpoint inhibitors, is categorized in TCM as “diarrhea” or “dysentery,” with diarrhea being the primary clinical symptom. According to TCM, the key pathogenesis of this condition is spleen deficiency with dampness. Spleen deficiency is characterized by digestive issues and imbalanced enteric microflora. Dampness accumulation transforms into heat, leading to the build-up of heat toxicity internally. Clinical indicators include sticky, sluggish stools, and tenesmus. Elevated levels of pro-inflammatory cytokines can result in heightened systemic inflammatory responses, increased vascular permeability, and damage to visceral tissues. Therefore, the guiding principle for treating this condition involves fortifying the body’s resistance to eradicate the pathogenic factors. The treatment method focuses on clearing heat and detoxification, as well as strengthening the spleen and dispelling dampness (Figure 2).
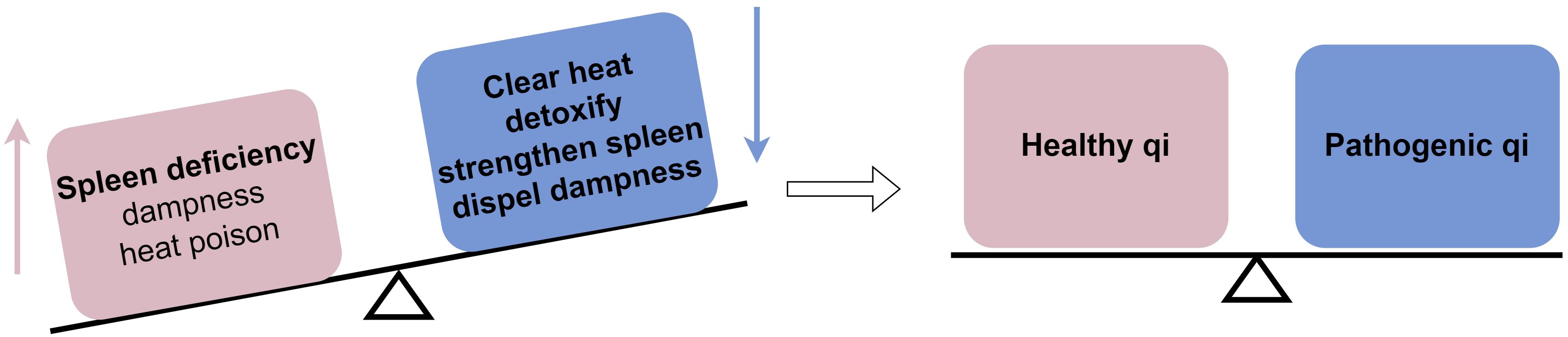
Figure 2 Mechanism by which TCM affects immune checkpoint inhibitor-induced colitis from a TCM standpoint: TCM helps the body achieve a balance between healthy qi and pathogenic qi by eliminating pathogenic factors.
In TCM, Codonopsis is known for its spleen-tonifying properties. Codonopsis polysaccharide, a compound derived from the herb Codonopsis, has demonstrated immunomodulatory effects, particularly in maintaining immune balance. Thus, it may help address the immune imbalance caused by ICIs (81). Gegen Qinlian decoction (GGQLD) and Huanglian Jiedu decoction (HLJDD) are renowned for their heat-clearing properties and have been found to possess significant anti-inflammatory effects. This makes them suitable for the treatment of acute or chronic inflammatory diseases. Huanglian Decoction (HLD) has been shown to alleviate the symptoms of DSS-induced colitis, reduce histological damage, downregulate the level of pro-inflammatory cytokines, and improve dysfunctional intestinal flora (82). Baitouweng decoction and Huangqin decoction (HQD) are known for their ability to “clear heat and stop diarrhea” and are commonly utilized in the treatment of damp-heat type ulcerative colitis. HQD has been demonstrated to reduce pro-inflammatory factors, regulate intestinal flora, effectively alleviate symptoms in mice with ulcerative colitis, and improve their mental state (83). Shenling Baizhu powder has been demonstrated to enhance human immune function, effectively alleviating diarrhea symptoms, regulating immune factors, and improving intestinal flora (84, 85). Shaoyao decoction (SYD) is a TCM prescription with a history dating back to the Jin-Yuan Dynasty. It is commonly employed for the treatment of various inflammatory bowel diseases and intestinal dampness-heat syndrome. Research has indicated that SYD can significantly inhibit the production of pro-inflammatory cytokines and chemokines, especially IL-1β, IL-6, and IFN-γ. Moreover, it has been demonstrated to markedly reduce intestinal mucosal damage in DSS-induced UC mouse models and protect the intestinal barrier (86). Sanhuangshu’ai decoction (SH) is a combination of four commonly used Chinese herbal medicines: Coptidis Rhizoma, Scutellariae Radix, Phellodendri Chinensis Cortex, and Artemisiae Argyi Folium. Research has indicated that this decoction can effectively improve colitis in mice induced by DSS (87).
Tumors are severe diseases that pose a significant threat to human health and diminish overall quality of life. Although tumor immunotherapy offers new hope for countless cancer patients, frequent adverse reactions and serious side effects often expose patients to additional risks. Traditional Chinese medicine has been widely used in clinical practice in China, particularly for managing side effects caused by conventional cancer treatments. The principle of “same treatment for different diseases” in TCM suggests its wide applicability in treating various inflammatory intestinal diseases, an effectiveness that has been supported by experts and scholars. Exploring the potential of using TCM for immune checkpoint inhibitor-induced colitis could offer a novel therapeutic approach and method for clinical treatment, bringing much-needed relief to many patients and significantly improving their quality of life. In future, there is promise in discovering and implementing additional TCM approaches that can prevent and alleviate immune-related adverse events in clinical settings. However, TCM research on irAEs is still in its early stages, with limited available literature. A wealth of clinical and practical research evidence is needed to establish the efficacy of TCM and provide better clinical guidance for the prevention and treatment of irAEs using TCM.
JW: Writing – original draft. ZG: Writing – original draft. MS: Writing – review & editing. QX: Supervision, Writing – review & editing. HX: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Shandong Province [grant numbers ZR2022MH207, ZR2021LZY019]; the Shandong Provincial Key Discipline Construction Project of Traditional Chinese Medicine (Clinical Discipline of Integrated Traditional Chinese and Western Medicine); National Natural Science Foundation of China [grant numbers 82104723]; and Shandong Provincial Qianfoshan Hospital [grant numbers QYPY2020NSFC1015].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li Y, Kang X, Wang H, Guo X, Zhou J, Duan L. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated adverse events in the digestive system. Thorac Cancer. (2020) 11:829–34. doi: 10.1111/1759-7714.13338
2. Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. (2017) 6(6):e1344805. doi: 10.1080/2162402X.2017.1344805
3. Bishay K, Tandon P, Bourassa-Blanchette S, Laurie SA, McCurdy JD. The risk of diarrhea and colitis in patients with lung cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Curr Oncol. (2020) 27:e486–e94. doi: 10.3747/co.27.6251
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. (2012) 30:2691–7. doi: 10.1200/JCO.2012.41.6750
5. Wang DY, Mooradian MJ, Kim D, Shah NJ, Fenton SE, Conry RM, et al. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology. (2019) 8:e1524695. doi: 10.1080/2162402X.2018.1524695
6. Hodi S, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–723. doi: 10.1056/NEJMoa1003466
7. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. (2017) 8:49. doi: 10.3389/fphar.2017.00049
8. Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. (2018) 10:1758835918764628. doi: 10.1177/1758835918764628
9. Lomax AJ, McNeil C. Acute management of autoimmune toxicity in cancer patients on immunotherapy: Common toxicities and the approach for the emergency physician. Emerg Med Australas. (2017) 29:245–51. doi: 10.1111/1742-6723.12718
10. Rajha E, Chaftari P, Kamal M, Maamari J, Chaftari C, Yeung SJ. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol Rep (Oxf). (2020) 8:25–30. doi: 10.1093/gastro/goz065
11. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol.. (2017) 28:1368–79. doi: 10.1093/annonc/mdx108
12. Liu X, Shi Y, Zhang D, Zhou Q, Liu J, Chen M, et al. Risk factors for immune-related adverse events: what have we learned and what lies ahead? Biomark Res. (2021) 9:79. doi: 10.1186/s40364-021-00314-8
13. Yao J, Li M, Zhang H, Ge Y, Weygant N, An G. Differential risks of immune-related colitis among various immune checkpoint inhibitor regimens. Int Immunopharmacol. (2020) 87:106770. doi: 10.1016/j.intimp.2020.106770
14. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
15. Chiaranunt P, Tometich JT, Ji J, Hand TW. T cell proliferation and colitis are initiated by defined intestinal microbes. J Immunol. (2018) 201:243–50. doi: 10.4049/jimmunol.1800236
16. Ito K, Takaishi H, Jin Y, Song F, Denning TL, Ernst PB. Staphylococcal enterotoxin B stimulates expansion of autoreactive T cells that induce apoptosis in intestinal epithelial cells: regulation of autoreactive responses by IL-10. J Immunol. (2000) 164:2994–3001. doi: 10.4049/jimmunol.164.6.2994
17. Alexander M, Ang QY, Nayak RR, Bustion AE, Sandy M, Zhang B, et al. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe. (2022) 30:17–30.e9. doi: 10.1016/j.chom.2021.11.001
18. Wen X, Wang HG, Zhang MN, Zhang MH, Wang H, Yang XZ. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J Gastroenterol. (2021) 27:2834–49. doi: 10.3748/wjg.v27.i21.2834
19. Segui E, Zamora-Martinez C, Barreto TD, Padrosa J, Viladot M, Marco-Hernandez J. Severe immune-related adverse events: A case series of patients needing hospital admission in a Spanish oncology referral center and review of the literature. Diagnostics (Basel). (2022) 12:2116. doi: 10.3390/diagnostics12092116
20. Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. (2018) 24:1804–8. doi: 10.1038/s41591-018-0238-9
21. Yamada K, Sawada T, Nakamura M, Yamamura T, Maeda K, Ishikawa E, et al. Clinical characteristics of gastrointestinal immune-related adverse events of immune checkpoint inhibitors and their association with survival. World J Gastroenterol. (2021) 27:7190–206. doi: 10.3748/wjg.v27.i41.7190
22. Shirwaikar Thomas A, Hanauer S, Wang Y. Immune checkpoint inhibitor enterocolitis vs idiopathic inflammatory bowel disease. Clin Gastroenterol Hepatol. (2023) 21:878–90. doi: 10.1016/j.cgh.2022.10.004
23. Bamias G, Delladetsima I, Perdiki M, Siakavellas SI, Goukos D, Papatheodoridis GV, et al. Immunological characteristics of colitis associated with anti-CTLA-4 antibody therapy. Cancer Invest. (2017) 35:443–55. doi: 10.1080/07357907.2017.1324032
24. Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. (2018) 3:e000278. doi: 10.1136/esmoopen-2017-000278
25. Zhang B, Liu K, Yang H, Jin Z, Ding Q, Zhao L. Gut microbiota: the potential key target of TCM's therapeutic effect of treating different diseases using the same method-UC and T2DM as examples. Front Cell Infect Microbiol. (2022) 12:855075. doi: 10.3389/fcimb.2022.855075
26. Rocha M, Correia de Sousa J, Salgado M, Araujo A, Pedroto I. Management of gastrointestinal toxicity from immune checkpoint inhibitor. GE Port J Gastroenterol. (2019) 26:268–74. doi: 10.1159/000494569
27. Assarzadegan N, Montgomery E, Anders RA. Immune checkpoint inhibitor colitis: the flip side of the wonder drugs. Virchows Arch. (2018) 472:125–33. doi: 10.1007/s00428-017-2267-z
28. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58
29. Cheng C, Zhang W, Zhang C, Ji P, Wu X, Sha Z, et al. Hyperoside ameliorates DSS-induced colitis through MKRN1-mediated regulation of PPARgamma signaling and th17/treg balance. J Agric Food Chem. (2021) 69:15240–51. doi: 10.1021/acs.jafc.1c06292
30. Zhu L, Xu LZ, Zhao S, Shen ZF, Shen H, Zhan LB. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl Microbiol Biotechnol. (2020) 104:5449–60. doi: 10.1007/s00253-020-10527-w
31. Li Z, Lin M, Li Y, Shao J, Huang R, Qiu Y, et al. Total flavonoids of Sophora flavescens and kurarinone ameliorated ulcerative colitis by regulating Th17/Treg cell homeostasis. J Ethnopharmacol. (2022) 297:115500. doi: 10.1016/j.jep.2022.115500
32. Xu M, Duan XY, Chen QY, Fan H, Hong ZC, Deng SJ, et al. Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance. BioMed Pharmacother. (2019) 109:2396–408. doi: 10.1016/j.biopha.2018.11.087
33. Xiao S, Yan Y, Shao M, Zhou X, Niu Z, Wu Y, et al. Kuijieling decoction regulates the Treg/Th17 cell balance in ulcerative colitis through the RA/RARalpha signaling pathway. J Ethnopharmacol. (2024) 318:116909. doi: 10.1016/j.jep.2023.116909
34. Zhang M, Fan H, Tan S, Tang Q, Liu X, Zuo D, et al. The Chinese medicinal herb decoction QRZSLXF enhances anti-inflammatory effect in TNBS-induced colitis via balancing Th17/Tregs differentiation. J Ethnopharmacol. (2020) 251:112549. doi: 10.1016/j.jep.2020.112549
35. Zou H, He T, Chen X. Tetrandrine inhibits differentiation of proinflammatory subsets of T helper cells but spares de novo differentiation of iTreg cells. Int Immunopharmacol. (2019) 69:307–12. doi: 10.1016/j.intimp.2019.01.040
36. Li J, Ma Y, Li X, Wang Y, Huo Z, Lin Y, et al. Fermented Astragalus and its metabolites regulate inflammatory status and gut microbiota to repair intestinal barrier damage in dextran sulfate sodium-induced ulcerative colitis. Front Nutr. (2022) 9:1035912. doi: 10.3389/fnut.2022.1035912
37. Shi D, Ma A, Zheng H, Huo G, Yan H, Fu H, et al. Paeoniflorin inhibits the maturation and immunostimulatory function of allergen-induced murine dendritic cells. Int Immunopharmacol. (2014) 19:221–32. doi: 10.1016/j.intimp.2014.02.001
38. Wang W, Xu C, Li X, Wang Z, Yang J, Shen Y, et al. Exploration of the potential mechanism of Banxia Xiexin Decoction for the effects on TNBS-induced ulcerative colitis rats with the assistance of network pharmacology analysis. J Ethnopharmacol. (2021) 277:114197. doi: 10.1016/j.jep.2021.114197
39. Wang S, Cao M, Xu S, Shi J, Mao X, Yao X, et al. Luteolin alters macrophage polarization to inhibit inflammation. Inflammation. (2020) 43:95–108. doi: 10.1007/s10753-019-01099-7
40. Xuan-Qing C, Xiang-Yu LV, Shi-Jia LIU. Baitouweng decoction alleviates dextran sulfate sodium-induced ulcerative colitis by regulating intestinal microbiota and the IL-6/STAT3 signaling pathway. J Ethnopharmacol. (2021) 265:113357. doi: 10.1016/j.jep.2020.113357
41. Zhuang H, Lv Q, Zhong C, Cui Y, He L, Zhang C, et al. Tiliroside ameliorates ulcerative colitis by restoring the M1/M2 macrophage balance via the HIF-1alpha/glycolysis pathway. Front Immunol. (2021) 12:649463. doi: 10.3389/fimmu.2021.649463
42. Guo R, Meng Q, Wang B, Li F. Anti-inflammatory effects of Platycodin D on dextran sulfate sodium (DSS) induced colitis and E. coli Lipopolysaccharide (LPS) induced inflammation. Int Immunopharmacol. (2021) 94:107474. doi: 10.1016/j.intimp.2021.107474
43. Liu S, Shen H, Li J, Gong Y, Bao H, Zhang J, et al. Loganin inhibits macrophage M1 polarization and modulates sirt1/NF-kappaB signaling pathway to attenuate ulcerative colitis. Bioengineered. (2020) 11:628–39. doi: 10.1080/21655979.2020.1774992
44. Zhang HY, Zeng HR, Wei HZ, Chu XY, Zhu HT, Zhao B, et al. Tongxie-Yaofang formula regulated macrophage polarization to ameliorate DSS-induced colitis via NF-kappaB/NLRP3 signaling pathway. Phytomedicine. (2022) 107:154455. doi: 10.1016/j.phymed.2022.154455
45. Wu MM, Wang QM, Huang BY, Mai CT, Wang CL, Wang TT, et al. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol Res. (2021) 172:105796. doi: 10.1016/j.phrs.2021.105796
46. Cheng W, Wang X, Wu Y, Li W, Fu C, Zou L, et al. Huanglian-Houpo extract attenuates DSS-induced UC mice by protecting intestinal mucosal barrier and regulating macrophage polarization. J Ethnopharmacol. (2023) 307:116181. doi: 10.1016/j.jep.2023.116181
47. Sun J, Zhao P, Ding X, Li F, Jiang J, Huang H, et al. Cayratia japonica Prevents Ulcerative Colitis by Promoting M2 Macrophage Polarization through Blocking the TLR4/MAPK/NF-kappaB Pathway. Mediators Inflam. (2022) 2022:1108569. doi: 10.1155/2022/1108569
48. Xiong K, Deng J, Yue T, Hu W, Zeng X, Yang T, et al. Berberine promotes M2 macrophage polarisation through the IL-4-STAT6 signalling pathway in ulcerative colitis treatment. Heliyon. (2023) 9:e14176. doi: 10.1016/j.heliyon.2023.e14176
49. Lv Q, Xing Y, Liu Y, Chen Q, Xu J, Hu L, et al. Didymin switches M1-like toward M2-like macrophage to ameliorate ulcerative colitis via fatty acid oxidation. Pharmacol Res. (2021) 169:105613. doi: 10.1016/j.phrs.2021.105613
50. Yan S, Wei H, Jia R, Zhen M, Bao S, Wang W, et al. Wu-mei-wan ameliorates murine ulcerative colitis by regulating macrophage polarization. Front Pharmacol. (2022) 13:859167. doi: 10.3389/fphar.2022.859167
51. Wang SW, Bai YF, Weng YY, Fan XY, Huang H, Zheng F, et al. Cinobufacini ameliorates dextran sulfate sodium-induced colitis in mice through inhibiting M1 macrophage polarization. J Pharmacol Exp Ther. (2019) 368:391–400. doi: 10.1124/jpet.118.254516
52. Zhou P, Lai J, Li Y, Deng J, Zhao C, Huang Q, et al. Methyl gallate alleviates acute ulcerative colitis by modulating gut microbiota and inhibiting TLR4/NF-kappaB pathway. Int J Mol Sci. (2022) 23:14024. doi: 10.3390/ijms232214024
53. Chen S, Xu Y, Cheng N, Li F, Zhao H, Bai N, et al. Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum esculentum Moench) Bee Pollen Extract. Foods. (2022) 11:1293. doi: 10.3390/foods11091293
54. Liu Y, Dong Y, Shen W, Du J, Sun Q, Yang Y, et al. Platycodon grandiflorus polysaccharide regulates colonic immunity through mesenteric lymphatic circulation to attenuate ulcerative colitis. Chin J Nat Med. (2023) 21:263–78. doi: 10.1016/S1875-5364(23)60435-2
55. Lu K, Zhou J, Deng J, Li Y, Wu C, Bao J. Periplaneta americana Oligosaccharides Exert Anti-Inflammatory Activity through Immunoregulation and Modulation of Gut Microbiota in Acute Colitis Mice Model. Molecules. (2021) 26:1718. doi: 10.3390/molecules26061718
56. Cheng H, Liu J, Zhang D, Wang J, Tan Y, Feng W, et al. Ginsenoside rg1 alleviates acute ulcerative colitis by modulating gut microbiota and microbial tryptophan metabolism. Front Immunol. (2022) 13:817600. doi: 10.3389/fimmu.2022.817600
57. Dong L, Du H, Zhang M, Xu H, Pu X, Chen Q, et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother Res. (2022) 36:2081–94. doi: 10.1002/ptr.7429
58. Wu J, Wei Z, Cheng P, Qian C, Xu F, Yang Y, et al. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. (2020) 10:10665–79. doi: 10.7150/thno.43528
59. Li C, Ai G, Wang Y, Lu Q, Luo C, Tan L, et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kappaB pathway. Pharmacol Res. (2020) 152:104603. doi: 10.1016/j.phrs.2019.104603
60. Xie Q, Li H, Ma R, Ren M, Li Y, Li J, et al. Effect of Coptis chinensis franch and Magnolia officinalis on intestinal flora and intestinal barrier in a TNBS-induced ulcerative colitis rats model. Phytomedicine. (2022) 97:153927. doi: 10.1016/j.phymed.2022.153927
61. Liu CS, Liang X, Wei XH, Jin Z, Chen FL, Tang QF, et al. Gegen qinlian decoction treats diarrhea in piglets by modulating gut microbiota and short-chain fatty acids. Front Microbiol. (2019) 10:825. doi: 10.3389/fmicb.2019.00825
62. Li R, Chen Y, Shi M, Xu X, Zhao Y, Wu X, et al. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-kappaB signaling and enhancing antioxidant effect. Phytomedicine. (2016) 23:1012–20. doi: 10.1016/j.phymed.2016.06.010
63. Chen Z, Zhang Z, Liu J, Qi H, Li J, Chen J, et al. Gut microbiota: therapeutic targets of ginseng against multiple disorders and ginsenoside transformation. Front Cell Infect Microbiol. (2022) 12:853981. doi: 10.3389/fcimb.2022.853981
64. Yun HF, Liu R, Han D, Zhao X, Guo JW, Yan FJ, et al. Pingkui enema alleviates TNBS-induced ulcerative colitis by regulation of inflammatory factors, gut bifidobacterium, and intestinal mucosal barrier in rats. Evid Based Complement Alternat Med. (2020) 2020:3896948. doi: 10.1155/2020/3896948
65. Li H, Wang Y, Shao S, Yu H, Wang D, Li C, et al. Rabdosia serra alleviates dextran sulfate sodium salt-induced colitis in mice through anti-inflammation, regulating Th17/Treg balance, maintaining intestinal barrier integrity, and modulating gut microbiota. J Pharm Anal. (2022) 12:824–38. doi: 10.1016/j.jpha.2022.08.001
66. Wang CZ, Wan C, Luo Y, Zhang CF, Zhang QH, Chen L, et al. Effects of dihydroartemisinin, a metabolite of artemisinin, on colon cancer chemoprevention and adaptive immune regulation. Mol Biol Rep. (2022) 49:2695–709. doi: 10.1007/s11033-021-07079-1
67. Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. BioMed Pharmacother. (2020) 121:109570. doi: 10.1016/j.biopha.2019.109570
68. McNamee EN, Masterson JC, Veny M, Collins CB, Jedlicka P, Byrne FR, et al. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn's-like murine ileitis. J Leukoc Biol. (2015) 97:1011–22. doi: 10.1189/jlb.3HI0614-303R
69. Liu Y, Chen Y, Lamb JR, Tam PK. Triptolide, a component of Chinese herbal medicine, modulates the functional phenotype of dendritic cells. Transplantation. (2007) 84:1517–26. doi: 10.1097/01.tp.0000289990.55668.0d
70. Chen D, Li Y, Wang X, Li K, Jing Y, He J, et al. Generation of regulatory dendritic cells after treatment with paeoniflorin. Immunol Res. (2016) 64:988–1000. doi: 10.1007/s12026-015-8773-7
71. Chen G, Yang Y, Liu M, Teng Z, Ye J, Xu Y, et al. Banxia xiexin decoction protects against dextran sulfate sodium-induced chronic ulcerative colitis in mice. J Ethnopharmacol. (2015) 166:149–56. doi: 10.1016/j.jep.2015.03.027
72. Jiandong L, Yang Y, Peng J, Xiang M, Wang D, Xiong G, et al. Trichosanthes kirilowii lectin ameliorates streptozocin-induced kidney injury via modulation of the balance between M1/M2 phenotype macrophage. BioMed Pharmacother. (2019) 109:93–102. doi: 10.1016/j.biopha.2018.10.060
73. Habtemariam S, Belai A. Natural therapies of the inflammatory bowel disease: the case of rutin and its aglycone, quercetin. Mini Rev Med Chem. (2018) 18:234–43. doi: 10.2174/1389557517666170120152417
74. Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. (2014) 20(1):6–21. doi: 10.3748/wjg.v20.i1.6
75. Bing X, Xuelei L, Wanwei D, Linlang L, Keyan C. EGCG maintains th1/th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/myD88/NF-kappaB signaling pathway in rats. Can J Gastroenterol Hepatol. (2017) 2017:3057268. doi: 10.1155/2017/3057268
76. Huang J, Jiang Z, Wang Y, Fan X, Cai J, Yao X, et al. Modulation of gut microbiota to overcome resistance to immune checkpoint blockade in cancer immunotherapy. Curr Opin Pharmacol. (2020) 54:1–10. doi: 10.1016/j.coph.2020.06.004
77. Wang T, Zheng N, Luo Q, Jiang L, He B, Yuan X, et al. Probiotics lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front Immunol. (2019) 10:1235. doi: 10.3389/fimmu.2019.01235
78. Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD Treat Targeting Gut Microbiome. Pathog. (2019) 8(3). doi: 10.3390/pathogens8030126
79. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. (2009) 9:313–23. doi: 10.1038/nri2515
80. Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. (2010) 28:623–67. doi: 10.1146/annurev-immunol-030409-101330
81. Deng X, Fu Y, Luo S, Luo X, Wang Q, Hu M, et al. Polysaccharide from Radix Codonopsis has beneficial effects on the maintenance of T-cell balance in mice. BioMed Pharmacother. (2019) 112:108682. doi: 10.1016/j.biopha.2019.108682
82. Hao W, Chen Z, Wang L, Yuan Q, Gao C, Ma M, et al. Classical prescription Huanglian Decoction relieves ulcerative colitis via maintaining intestinal barrier integrity and modulating gut microbiota. Phytomedicine. (2022) 107:154468. doi: 10.1016/j.phymed.2022.154468
83. Zheng Y, Liang C, Li Z, Chen J, Chen Z, Jiang Y, et al. Study on the mechanism of Huangqin Decoction on rats with ulcerative colitis of damp-heat type base on mtDNA, TLR4, p-PI3K, p-Akt protein expression and microbiota. J Ethnopharmacol. (2022) 295:115356. doi: 10.1016/j.jep.2022.115356
84. Rao K, Qin S, Yang Y, Zhan K, Wu H, Zheng H, et al. Shenling baizhu powder alleviates TNBS-induced colitis in rats by improving intestinal epithelial permeability and inhibiting inflammation through the TLR5/myD88/NF-kappaB pathway. Front Pharmacol. (2022) 13:883918. doi: 10.3389/fphar.2022.883918
85. Chen Q, Xiao Z, He QY, Zhang RR, Chen SX, Dong JW, et al. Effect of Shenling Baizhu powder on immunity to diarrheal disease: A systematic review and meta-analysis. Front Pharmacol. (2022) 13:938932. doi: 10.3389/fphar.2022.938932
86. Wei YY, Fan YM, Ga Y, Zhang YN, Han JC, Hao ZH. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-kappaB pathway. Phytomedicine. (2021) 92:153743. doi: 10.1016/j.phymed.2021.153743
Keywords: traditional Chinese medicine, immune checkpoint inhibitor-induced colitis, potential application, mechanisms, immune
Citation: Wang J, Guo Z, Shen M, Xie Q and Xiang H (2024) Potential application mechanism of traditional Chinese medicine in treating immune checkpoint inhibitor-induced colitis. Front. Immunol. 15:1366489. doi: 10.3389/fimmu.2024.1366489
Received: 06 January 2024; Accepted: 08 March 2024;
Published: 10 April 2024.
Edited by:
Ioannis Vathiotis, National and Kapodistrian University of Athens, GreeceReviewed by:
Hongliang Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2024 Wang, Guo, Shen, Xie and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Xie, eGllcWlAc2RmbXUuZWR1LmNu; Hongjie Xiang, eGhqbHloQDEyNi5jb20=
†These authors have contributed equally to this work
‡ORCID: Qi Xie, orcid.org/0000-0002-3152-9834
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.