- 1Internal Medicine Department, University Hospital of Nice, Cote d’Azur University, Nice, France
- 2Haematology Department , University Hospital of Nice, Cote d’Azur University, Nice, France
- 3Stroke Unit, UR2CA-URRIS Neurology, University Hospital Pasteur 2, Cote d’Azur University, Nice, France
- 4Mediterranean Centre for Molecular Medicine, Control of Gene Expression (COdEX), INSERM U1065, Nice, France
Introduction: Antiphospholipid syndrome (APS) is an autoimmune thrombotic disease with various systemic presentations. This study aimed to identify homogeneous groups of patients based on a non-supervised hierarchical cluster analysis and assess the rate of relapse associated with antinuclear antibodies (ANA).
Methods: This retrospective observational study enrolled patients, over a 90-month period, who had APS as defined by the 2006 Sydney classification criteria, and for whom ANA workup was performed. Agglomerative unsupervised hierarchical clustering was conducted to classify patients into subgroups using 24 variables reflecting a range of clinical and biological baseline features associated with APS.
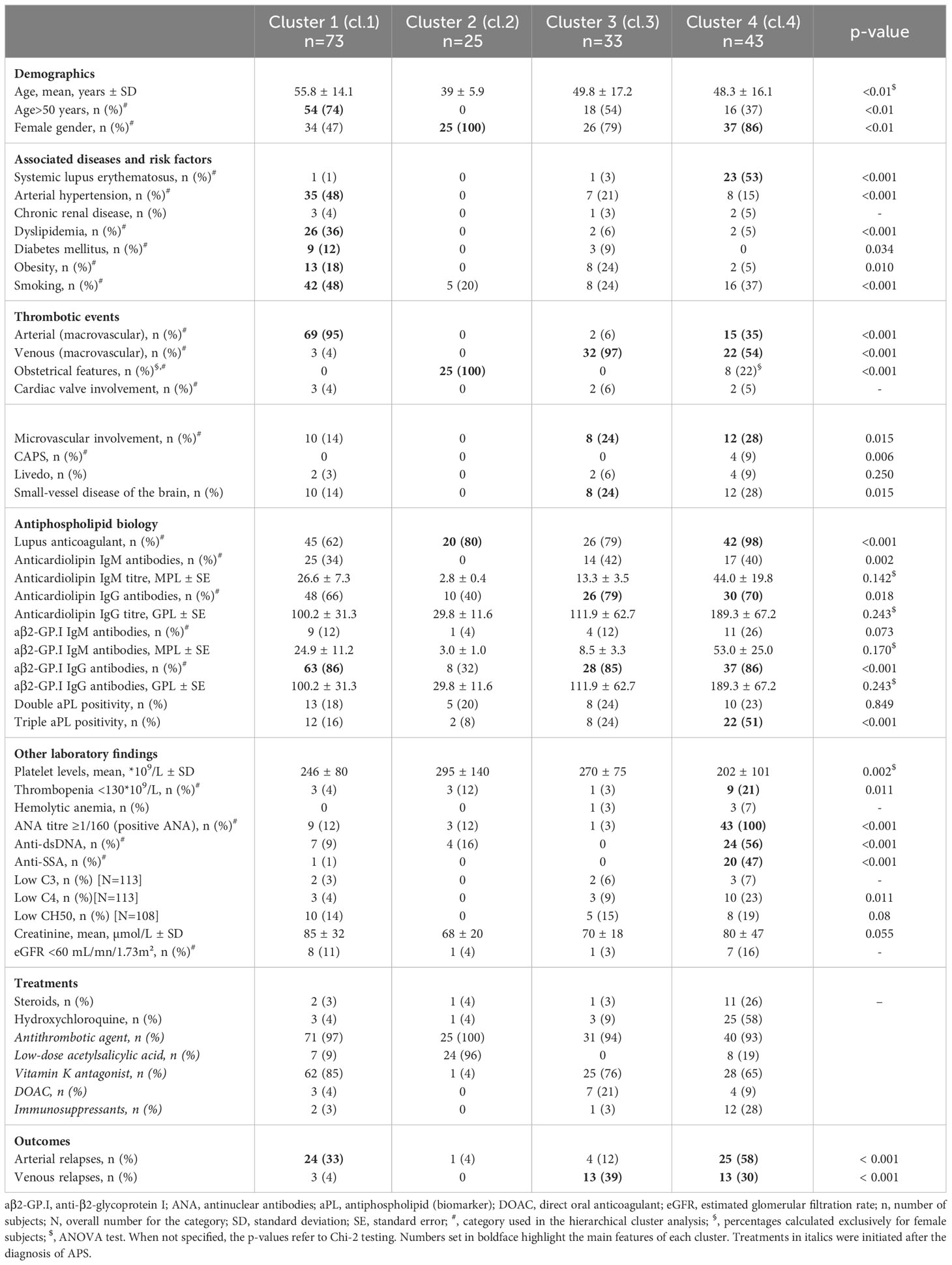
Results: Hundred and seventy-four patients were included and were categorized into four phenotypes. Cluster 1 (n=73) associated mostly middle-aged men with risk factors for cardiovascular disease. Obstetrical APS with low-risk thrombosis made up cluster 2 (n=25). Patients with venous thromboembolism (VTE), microvascular findings and double/triple positive APL antibodies (50%) were represented in cluster 3 (n=33). Whereas cluster 4 (n=43) characterized a predominantly female subpopulation with positive ANA and systemic lupus (n=23) that exhibited a high thrombotic risk and more frequent relapses (n=38) (p<0.001).
Conclusions: This study identified four homogenous groups of patients with APS listed as: i) cardiovascular and arterial risk, ii) obstetrical, iii) VTE and microvascular, and iv) ANA-positive APS. We found that ANA-positivity was associated with higher rates of relapse. Applying ANA status to classification criteria could constitute a novel approach to tailoring management for APS, based on phenotypic patterns and risk assessment.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disease with systemic features associated with arterial, venous, or microvascular thrombosis, pregnancy morbidity in patients with persistent antiphospholipid antibodies (aPL) (1). The latter refer to: lupus anticoagulant (LA), anticardiolipin (aCL) and anti-β2-glycoprotein I (aβ2-GP.I). Until recently, classification of APS relied on the Sapporo criteria (2) that were revised in 2006 (also known as the Sydney criteria) (1). Such criteria lacked specificity and did not account for clinical phenotypes outside of the obstetrical context (previously referred to as “non-criteria” features). Recently, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) published an updated approach for the classification of APS so as to provide “high specificity and a strong foundation for future research” (3). In reality, patients present with a wide range of manifestations with different risk profiles that do not necessarily reflect established clinical and/or biological domains. This may lead to dilemmas in decision-making, when classification criteria are used as diagnostic aids (4).
Previous studies have attempted to identify subgroups of patients with APS with common features and prognoses (5–12). Moreover, unsupervised statistical methods have been used to determine phenotypes in populations of individuals with APS (6–11). Hierarchical cluster analysis is one of such processes that can be used to assess the pertinence of clinical domains (from the newly established classification criteria) as well as relevant clinical and biological features commonly associated with APS. Among such features is the analysis of antinuclear antibodies (ANA), indiscriminately associated or not with well-defined systemic autoimmune diseases. The clinical value of ANA positivity therefore needs to be examined since it has not only been found to be associated with higher morbidity in the obstetrical context but also with a higher rate of thrombotic relapses in comparison to ANA-negative APS patients (13–15). ANA, however, do not appear in classification criteria domains (1–3).
We hypothesize that ANA-positivity is associated with a specific phenotype of APS. The aim of this study was to identify homogeneous groups of APS patients based on a hierarchical cluster analysis. We furthermore chose to assess differences in relapse-rates accordingly in patients with a complete ANA workup.
Materials and methods
Study population and definitions
This was a retrospective observational study that included patients treated at the University Hospital of Nice, France, over a period spanning from January 1st 2015 au June 30th 2022. Subjects aged 17 years and above were identified via a database search for antiphospholipid positivity, and those meeting the 2006 Sydney classification criteria for APS were selected for the study (2) (Supplementary Data, Supplementary Table S2). Patients with duplicate and/or missing data – as well as those that lacked an immunological work-up with ANA – were excluded from the study even if APS criteria were met.
Catastrophic antiphospholipid syndrome (CAPS) was defined according to Asherson et al. (16). Whereas, “microvascular involvement” referred to livedo racemosa, livedoid vasculopathy, aPL nephropathy and/or pulmonary hemorrhage based on the latest classification criteria (3). Diagnoses of systemic lupus erythematosus (SLE) were those made in the clinical setting and were retrospectively required to meet any of the classification criteria for the disease (17). ANA were considered positive for titres above 1/160 (expressed as “≥1/160” in the manuscript). Immunological markers with their ranges are presented in the Supplementary Data section (Supplementary Table S3).
Data were collected from patients’ digital medical files and included findings such as demographics, clinical characteristics (including comorbidities, initial symptoms, microvascular involvement and cardio- and cerebro-vascular risk factors) and laboratory workup. Focus was given to hematological and immunological features such as: LA, aCL and aβ2-GP.I with their IgM and IgG antibody isotypes, platelet counts, creatinine and kidney function, ANA and complement function tests. The estimated glomerular filtration rate (eGFR) was based on the Modification of Diet in Renal Disease Study Group (MDRD) method (18). Immuno-assays with their associated cut-off values are provided as Supplementary Data (Supplementary Table S3).
“Double-positive aPL” was defined as the positivity of any combination of two aPL biomarkers irrespective of immunoglobulin isotype for aCL and/or aβ2-GP.I, whereas “triple positivity” referred to the presence of all three aPL biomarkers (irrespective of immunoglobulin isotype).
Based on previous studies (6–11) (Supplementary Data, Supplementary Table S4), clinical relevance and the recent 2023 ACR/EULAR classification criteria for APS (3), we chose the following 24 categories for the hierarchical cluster analysis: age of >50 years, gender, arterial hypertension, diabetes mellitus, dyslipidemia, smoking, obesity, SLE, initial arterial and/or venous thrombotic event, cardiac valve involvement, microvascular involvement, CAPS, obstetric features, LA, IgM and IgG aCL, IgM and IgG aβ2-GP.I, thrombocytopenia <130*109/L, eGFR <60 mL/mn/1.73m², positive ANA, anti-SSA and anti-dsDNA antibody positivity.
Statistical analysis
Agglomerative unsupervised hierarchical clustering based on the Ward method (for linkage) was performed using the 24 previously stated variables. Euclidian distance was the used metric. The optimal number of clusters was estimated with silhouette scores based on the k-means algorithm for cluster analysis but was also visually assessed with the dendrogram representation of the fusion sequence. Principal coordinates analysis was subsequently used to visualize individual data in a 2-dimensional scatterplot.
Categorical variables were expressed as counts with percentages, and continuous variables as means with their standard deviation (SD). Categorical variables were compared using the Chi² test. ANOVA tests were used to evaluate the difference between multiple means. All analyses were two-tailed and p-values <0.05 were considered statistically significant for comparative studies.
Predictive modelling was performed using the Orange© data mining software (version 3.35.0) developed by the University of Ljubljana. The JASP© software (version 0.17.2.1) supported by the University of Amsterdam was used for descriptive and frequentist inference statistical analysis.
Data protection and ethics
Data were anonymized on collection and stored in our institutional electronic repository under the registration number 2023-512 as required by, and in compliance with, French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés) guidelines. In accordance with French law, due to its retrospective nature, this study did not require the validation of an Ethics Committee.
Results
Characteristics of the study population
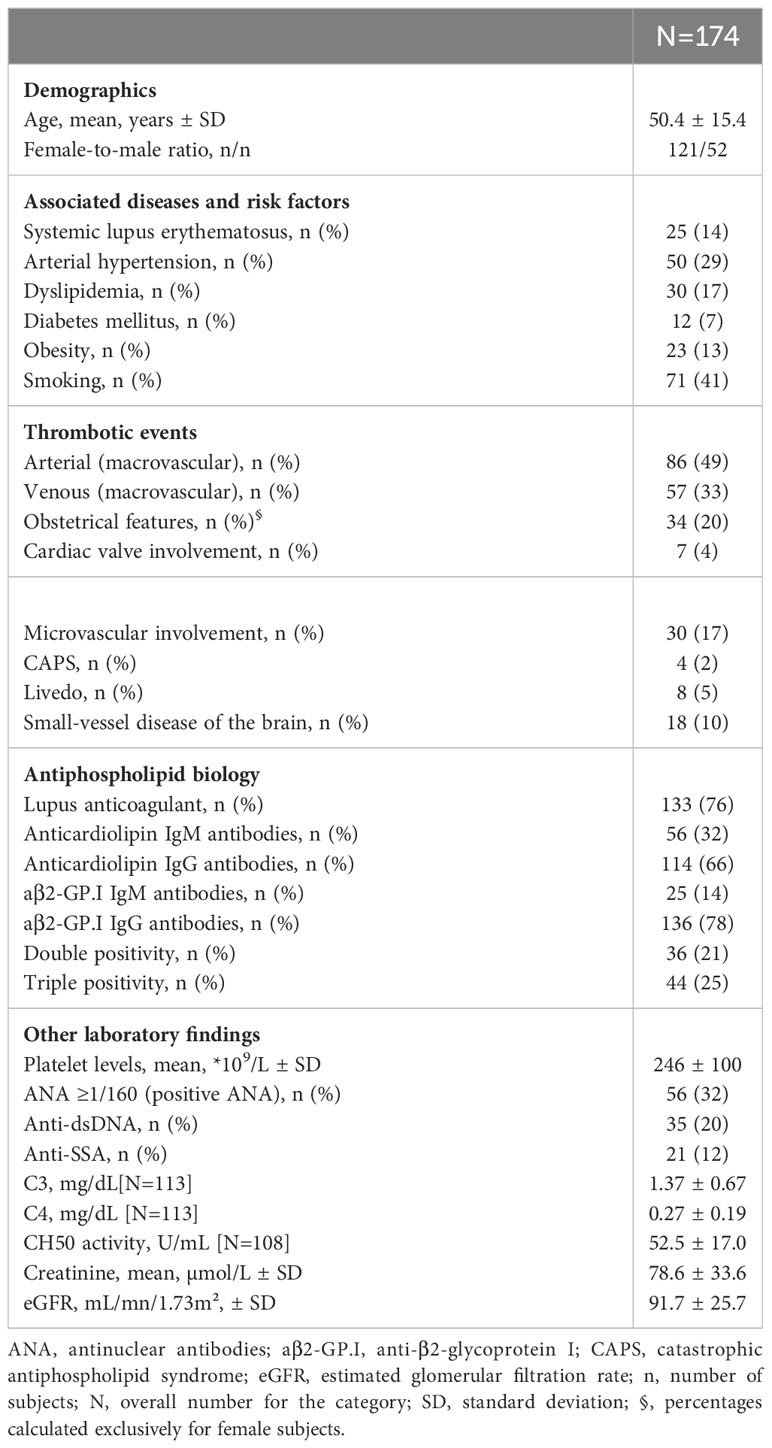
Over the 90-month study period, 174 patients – that were not only diagnosed with APS but for whom ANA were also performed – were included in the hierarchical analysis (Figure 1). Demographics, clinical and biological features at diagnosis are provided in Table 1.
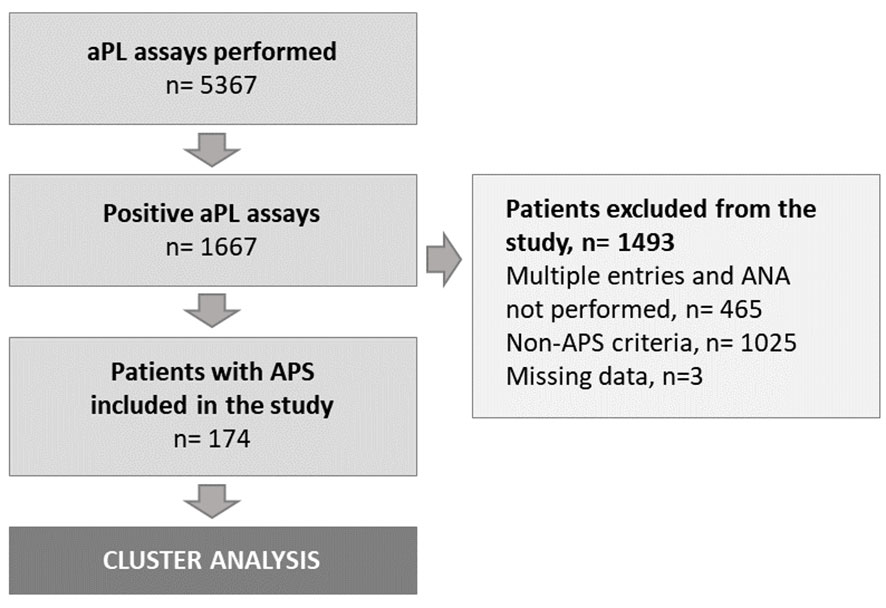
Figure 1 Study flow-chart. aPL, antiphospholipid; ANA, antinuclear antibodies; APS, antiphospholipid syndrome; n, number of subjects.
Antibody titres for aCL and/or aβ2-GP.I (at baseline) were not significantly different between the 4 clusters based on ANOVA testing.
Hierarchical cluster analysis
The k-means silhouette score algorithm was able to identify an optimal choice of 4 clusters. These 4 clusters were also visually individualized on the dendrogram (Figures 2, 3). Incomplete data were estimated to be less than 0.1%.
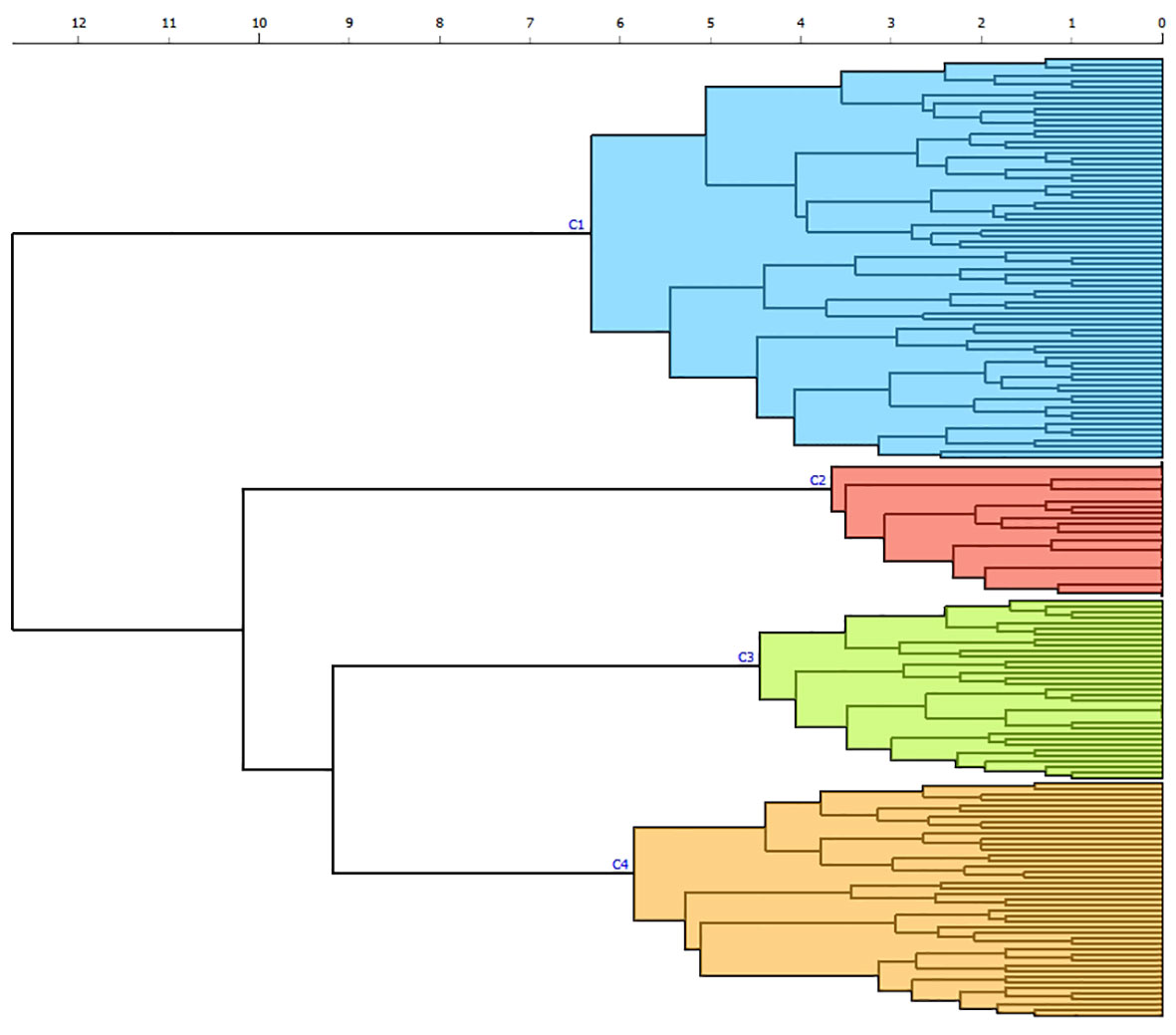
Figure 2 Dendrogram produced through hierarchical clustering of 24 clinical and biological categories in patients with APS. Euclidian distance is reported on the x-axis with horizontal branches reflecting degrees of dissimilarities between combined clusters. Each individual is analyzed on the y-axis, with vertical branches representing the combination of two clusters. Four different clusters (C1 to C4) are individualized by color according to the k-means algorithm: cluster 1 (blue), cluster 2 (red), cluster 3 (green), cluster 4 (orange).
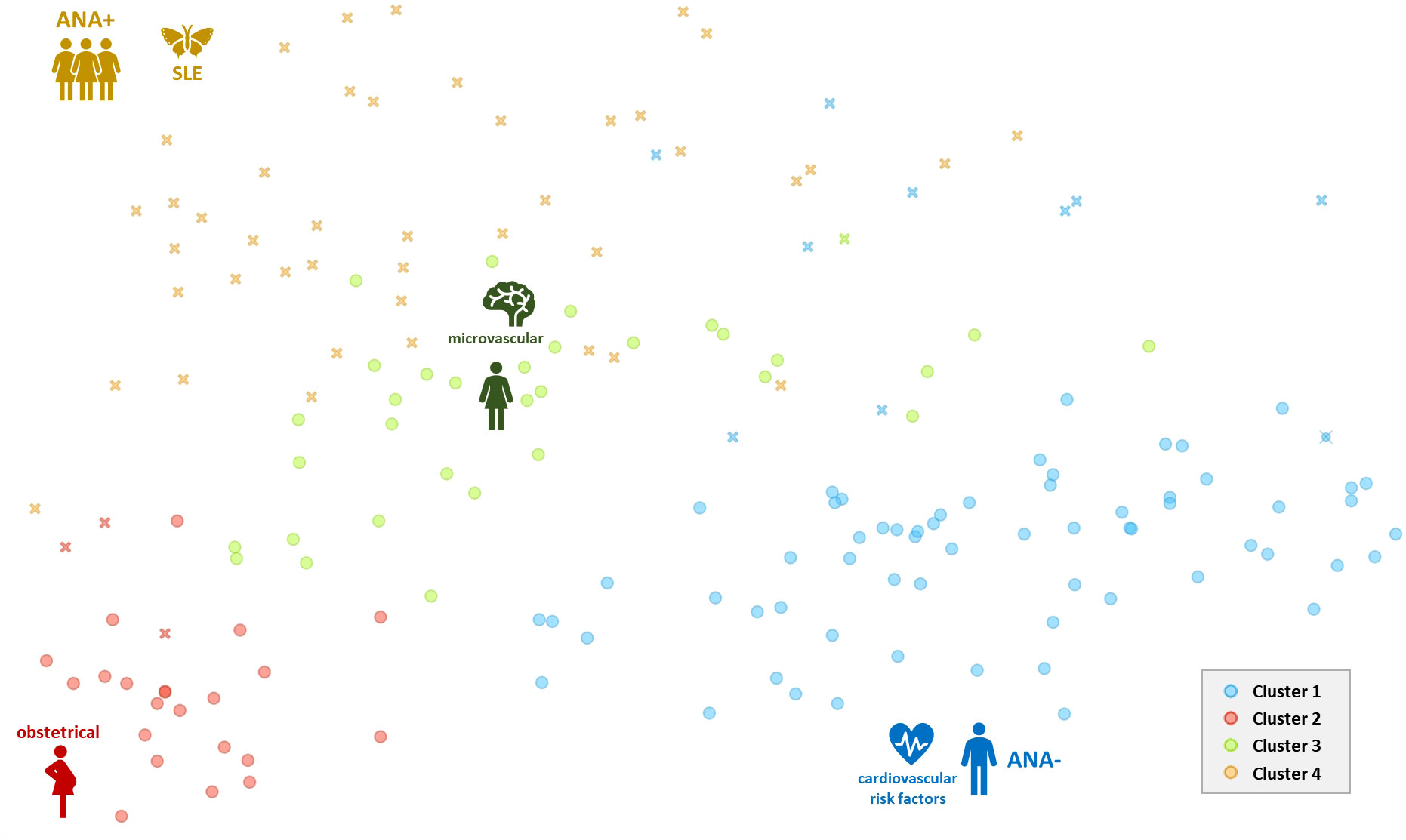
Figure 3 Two-dimensional scatterplot (biplot representation) of results from the principal coordinates analysis with the representation of 4 different clusters of individuals. Crosses (x) represent individuals with positive ANA. Each cluster is represented by a single color. ANA, antinuclear antibodies positivity (+) or negativity (-); SLE, systemic lupus erythematosus.
Cluster description
Cluster 1: cardiovascular and arterial risk
This group included 73 patients, with the highest proportion of middle-aged subjects (mean age of 55.8 years) and the lowest proportion of women (n=34). These patients presented with risk factors for cardiovascular disease such as arterial hypertension (48%), hyperlipidemia (36%), obesity (18%) and diabetes mellitus (12%); and 48% were smokers. 69/73 (95%) of patients had arterial thrombotic events at onset. aβ2-GP.I IgG antibodies were present in 86% cases. Arterial thrombotic relapses were observed in one in three patients despite ongoing and long-term anticoagulant therapy (Table 2). Most were on vitamin K antagonists (85%).
Cluster 2: obstetrical APS
This cluster (n=25) was numerically the smallest and included relatively young female patients (mean age of 39 ± 5.9 years) with obstetrical events, but without a prior history of venous or arterial thrombosis. One in five patients was a smoker but none had associated cardiovascular diseases nor a systemic autoimmune disorder. LA was found in 20 cases and ANA in only three cases. None had hypocomplementemia. Most received low-dose acetylsalicylic acid as long-term therapy with only one relapse (i.e. arterial thrombosis) whilst on this treatment.
Cluster 3: venous thromboembolism and microvascular involvement
Cluster 3 (n=33) included predominantly female patients (n=26) with very few cardiovascular risk factors. Most presented with venous thrombosis (97%) at onset. There were no obstetrical features. Small vessel disease of the brain was observed in 8/33 cases but only two were found to have some type of livedo. One in four patients had “double positive” aPL whilst another quarter were “triple positive” for aPL. Relapses were mostly venous (n=13) in a group of patients mostly on vitamin K antagonists (n=25). Of the seven patients on direct oral anticoagulants (DOAC), three relapsed with VTE.
Cluster 4: ANA-positive APS
Patients (n=43) in this cluster were mostly female (86%) and were all positive for ANA. More than half presented with SLE (n=23). The highest proportion of “triple positive” patients (51%) was found in this group with LA detected in 42/43 patients. Anti-dsDNA and anti-SSA antibodies were respectively found in 24 and 20 patients. Thrombocytopenia of less than 130*109/L was found in nine patients. More than half was on hydroxychloroquine at the time of APS diagnosis, and a quarter on steroids. Microvascular features were also associated with this group that included all four patients with CAPS.
Study of relapse rates in relation to ANA positivity
Taken independently, positive ANA were associated with relapse in the overall cohort of APS patients (p<0.01), with a likelihood ratio (LR) of 12.09. Anti-dsDNA and anti-SSA positivity were also found to be associated with relapse with, respectively, LR of 4.166 (p=0.041) and 3.892 (p=0.048). Patients from cluster 4 presented with the highest number of relapses, whether arterial or venous, compared to the three other groups (Table 2), regardless of anti-dsDNA and anti-SSA antibody-positivity (p=1 and p=0.740, respectively). Arterial thrombosis was more frequent in ANA-positive patients (Table 2).
Discussion
This study identified four different clusters of patients from a cohort of patients with APS and a complete ANA workup based on an unsupervised clustering method. These phenotypic groups are concisely defined as follows: i) cardiovascular and arterial risk, ii) obstetrical, iii) VTE and microvascular, and iv) ANA-positive APS.
Findings from our study promote the need to include ANA-positivity in the multimodal approach for risk assessment in APS. Most cluster analysis studies on this topic focus on new ways to redefine APS classifications, its diagnosis or to understand disease mechanisms (6–10, 19) (Supplementary Data, Supplementary Table S4). Few studies, other than ours, have sought to focus on ANA-positivity as a marker of “high risk” APS, outside of SLE (11, 15).
We found that ANA-positivity is associated with a specific phenotype of APS and with higher rates of relapse. To a certain extent, our findings reflect the paradigm shift promoted by the recent 2023 ACR/EULAR APS classification criteria (3). Therefore, we believe that combining the latter with knowledge of ANA status could constitute a novel approach to risk management in APS based on phenotypic patterns.
Cardiovascular and arterial risk
Patients with cardiovascular disease and aPL positivity are at a high risk of thrombosis as they are likely to have pro-inflammatory endothelial damage in addition to atherosclerosis (5, 20). Such patients also constitute the largest subpopulation amongst subjects with APS as reflected in previous studies (6–11, 19) (Supplementary Data, Supplementary Table S4). It is therefore hardly surprising that our analysis identified this “cardiovascular risk” group that included mostly middle-aged men with arterial thrombosis. Hypertension and medium/high titres of IgG aCL were also identified as risk factors for initial thrombotic events and echoes previous findings (21). However, one should note that risk-assessment of cardiovascular disease, according to the recent classification criteria, might redefine disease status in previously APS-classified patients (3). This implies that the implementation of anticoagulation in patients with a high-risk profile would depend on future relapses and/or criterion from other clinical domains – if such an approach were to be taken literally. Recurrent thrombosis in APS is difficult to assess with rates ranging from 20% to 30% within the first ten years from disease-onset (5, 22, 23). Studies have suggested that thrombotic patterns do not change during the course of the disease (i.e. relapses are either arterial or venous) and thus highlight the importance of an early characterisation of APS phenotypes (6, 12, 24).
Obstetrical APS
Our findings also matched those from previous studies relating to obstetrical events (6, 7, 11). Other authors were not able to individualize such a group due to the variables chosen and/or profile of patients included in the analysis (8, 10). This was particularly the case in the study by Nguyen et al. whose study-population was biased by a high proportion of patients with CAPS (10). Based on our findings, one might argue that the “obstetrical” phenotype is associated with a lesser risk of relapse and, in the absence of macrothrombotic events, antiplatelet aggregating agents may suffice. This observation seems to reflect 2019 EULAR guidelines that recommend low-dose acetylsalicylic acid in non-pregnant women with a history of obstetric APS (after risk/benefit evaluation) as well as in pregnant women with a high-risk aPL profile without a prior history of thrombosis nor pregnancy complications (25). Use of low-dose acetylsalicylic acid could also be considered in patients with clinical “non-criteria” obstetric APS (a grade D recommendation) (25). According to EUROAPS data, thrombotic events occur mostly during pregnancy or the puerperium with only a small subset of patients developing SLE over time (i.e. less than 6%) (26).
VTE and microvascular APS
Our analysis identified a cluster of patients, mostly female, with VTE and venous relapses (cluster 3). Subjects presented with double or triple positive aPL defining a high-risk profile for two thirds of this subpopulation. This group shared similarities with a frequently described phenotype of patients that have triple-positive aPL and VTE (7–10). Caution should be exercised for treating such patients with DOAC in the absence of phenotype-based clinical trials (27, 28).
Cluster 4 was interesting to analyze since it identified ANA-positive patients with high-risk APS. This particular phenotype has been described in previous studies in groups associating either/or: “non-criteria” features, SLE, cytopenias, microthrombotic events and more frequent arterial thrombosis in mostly female patients (7–11) (Supplementary Data, Supplementary Table S4). ANA-positive APS has been shown to have more “non-criteria” manifestations compared to other forms, an increased frequency of triple-positive aPL and higher rates of relapse (5, 15, 25). In our cohort, thrombosis had the highest rate in this group and occurred despite immunosuppressant drugs, antithrombotic agents and/or hydroxychloroquine intake.
ANA-positive APS
A prior study found that, of the 43% of patients with SLE with positive aPL, only a third were found to have APS (29). The soon-to-be updated EULAR recommendations regarding the management of SLE-associated APS do not, in these aspects, differ from the 2019 guidelines that state that low-dose acetylsalicylic acid can be prescribed in asymptomatic patients with a high-risk aPL profile (30). In light of findings from previous studies and ours, we believe that ANA-positivity should be acknowledged as a risk factor for potential relapses irrespective of a clinical diagnosis of connective tissue disease. Of note, within this cluster, one out of two patients did not present with SLE. This does not imply that anticoagulation is to be started in asymptomatic aPL-positive patients with ANA, but that clinical and biological work-up should focus on microvascular features and/or cytopenias by, for example, assessing for silent APS nephropathy just as one would for lupus nephritis (31). Therefore close monitoring is warranted, especially considering a propensity for CAPS that is associated with a higher mortality rate primarily due to severe cardiac and cerebral involvement (32). Overlapping and associated pro-thrombotic features (including medication) also need to be addressed (19). Furthermore, patients with ANA-positive APS require optimal anticoagulation with vitamin K antagonists although treatment of isolated VTE with DOAC, in this setting, is still a matter of debate (27, 33).
Limitations and biases
Our study has limitations – the most important of which, being its retrospective nature that may have introduced a selection bias. The latter may have been increased by the deliberate choice of requiring an immunological work-up with ANA and/or the speciality of the physician ordering the analysis. However, this also appears to be one of its strengths since missing data were extremely limited (i.e. less than 0.1%). Another limit of our study relates to the cluster methodology itself, since clinical and biological changes overtime cannot be assessed. Therefore, one cannot exclude the influence of clinical events and treatments on the “re-categorisation” of patients at a given time. However, based on evidence from long-term registry follow-up, changes in disease course do seem exceptional (12, 24). The quality of the clustering process reflects the very stringent inclusion criteria but it does not exempt us from recognizing an unintentional overlap with unreported prothrombotic factors. Our choice of variables for the cluster analysis was established based on previous studies with an emphasis on clinical categories from the recent APS classification criteria (3, 7–11). Our findings are therefore not only in line with previous initiatives but also refine the categorisation of patients with APS; though they may not be extrapolated to different ethnic groups. Our approach chose to focus on clinically relevant events and therefore “asymptomatic patients” were not considered.
Clinical significance and perspectives
The clusters that were identified reflect different clinical presentations and risk profiles. From the clinician’s perspective, patient management could be decided according to the predominant phenotype. For instance, obstetrical APS may only require low-dose acetylsalicylic acid as such individuals qualify as part of a “low risk” group (25). However, attitudes would be different if individuals were to present with: “triple positive” aPL, arterial thrombosis, microvascular involvement, cardiac valve thickening/vegetations (28). From our study, one might add ANA-positivity to these criteria. In such “high risk” cases, full-dose anticoagulation with vitamin K antagonists and/or heparin constitute the corner stone of therapy, in keeping with international guidelines (25). In the case of CAPS, triple therapy associating steroids and plasma exchange to anticoagulation, with rituximab as a first-line immunosuppressor with/without follow-up intravenous immunoglobulins whilst preferring cyclophosphamide in cases with positive ANA (34). We would reserve eculizumab for refractory forms of CAPS, especially in documented complement-mediated thrombotic microangiopathy (35). Similarly, future studies studying anticoagulant and immunosuppressive strategies would require adopting a phenotype-based approach.
Conclusion
This study successfully identified four distinct clusters within APS: i) cardiovascular and arterial risk, ii) obstetrical, iii) VTE and microvascular, and iv) ANA-positive APS. It underscores the importance of microvascular involvement and ANA-positivity in high-risk forms, and categorises a low-risk obstetrical phenotype. It further brings awareness to risk factors for cardiovascular disease that may be confounders for APS in aPL-positive patients but may also constitute a specific phenotype. Finally, our findings promote a novel phenotype-based approach to risk assessment in APS that could hopefully lead to better tailored treatment strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, on request.
Ethics statement
Ethical approval was not required for the studies involving humans because this study belongs to the “category 3” of medical research in human participants as defined by the 2016 French law (décret n° 2016-1537 - loi Jardé). The latter states that approval from an ethics committee is not required for retrospective studies (that do not involve any risks). Such is the case of our submission. This information appears in the manuscript. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MO: Data curation, Investigation, Writing – original draft, Writing – review & editing. PT: Supervision, Validation, Writing – original draft, Writing – review & editing. BC: Supervision, Validation, Writing – original draft, Writing – review & editing. NM: Conceptualization, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to all clinicians who gave their input and/or helped in the recruitment of patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1361062/full#supplementary-material
References
1. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost JTH (2006) 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheumatol (1999) 42:1309–11. doi: 10.1002/(ISSN)1529-0131
3. Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo M-C, et al. ACR/EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol Hoboken NJ (2023). doi: 10.1002/art.42624
4. Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (2015) 67:891–7. doi: 10.1002/acr.22583
5. Cervera R, Piette J-C, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheumatol (2002) 46:1019–27. doi: 10.1002/art.10187
6. Krause I, Leibovici L, Blank M, Shoenfeld Y. Clusters of disease manifestations in patients with antiphospholipid syndrome demonstrated by factor analysis. Lupus (2007) 16:176–80. doi: 10.1177/0961203306075977
7. Sciascia S, Radin M, Cecchi I, Bertolaccini ML, Bertero MT, Rubini E, et al. Identifying phenotypes of patients with antiphospholipid antibodies: results from a cluster analysis in a large cohort of patients. Rheumatology (2021) 60:1106–13. doi: 10.1093/rheumatology/kez596
8. Zuily S, Clerc-Urmès I, Bauman C, Andrade D, Sciascia S, Pengo V, et al. Cluster analysis for the identification of clinical phenotypes among antiphospholipid antibody-positive patients from the APS ACTION Registry. Lupus (2020) 29:1353–63. doi: 10.1177/0961203320940776
9. Ogata Y, Fujieda Y, Sugawara M, Sato T, Ohnishi N, Kono M, et al. Morbidity and mortality in antiphospholipid syndrome based on cluster analysis: a 10-year longitudinal cohort study. Rheumatol Oxf Engl (2021) 60:1331–7. doi: 10.1093/rheumatology/keaa542
10. Nguyen Y, Yelnik CM, Morel N, Paule R, Stammler R, Plaçais L, et al. Determination of four homogeneous subgroups of patients with antiphospholipid syndrome: a cluster analysis based on 509 cases. Rheumatol Oxf Engl (2022) 62(8):2813–9. doi: 10.1093/rheumatology/keac548
11. Guedon AF, Ricard L, Laurent C, De Moreuil C, Urbanski G, Deriaz S, et al. Identifying high-risk profile in primary antiphospholipid syndrome through cluster analysis: French multicentric cohort study. RMD Open (2023) 9:e002881. doi: 10.1136/rmdopen-2022-002881
12. Niznik S, Rapoport MJ, Avnery O, Lubetsky A, Haj Yahia S, Ellis MH, et al. Patterns of recurrent thrombosis in primary antiphospholipid syndrome-multicenter, real-life long-term follow-up. Front Immunol (2022) 13:843718. doi: 10.3389/fimmu.2022.843718
13. Li X, Deng X, Duan H, Zeng L, Zhou J, Liu C, et al. Clinical features associated with pregnancy outcomes in women with positive antiphospholipid antibodies and previous adverse pregnancy outcomes: a real-world prospective study. Clin Rheumatol (2021) 40:193–204. doi: 10.1007/s10067-020-05203-3
14. Natorska J, Celińska-Löwenhoff M, Undas AI. High prevalence of antinuclear antibodies in patients following venous thromboembolism. Adv Clin Exp Med (2018) 27:827–32. doi: 10.17219/acem/78361
15. Ricard L, Laurent C, Papo M, Deriaz S, Catano J, Alamowitch S, et al. Clinical and prognostic significance of antinuclear antibodies in primary antiphospholipid syndrome: A multicenter retrospective study. Joint Bone Spine (2022) 89:105297. doi: 10.1016/j.jbspin.2021.105297
16. Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus (2003) 12:530–4. doi: 10.1191/0961203303lu394oa
17. Aringer M, Costenbader KH, Daikh DI, Brinks R, Mosca M, Ramsey-Goldman R, et al. EULAR/ACR classification criteria for systemic lupus erythematosus. Arthritis Rheumatol Hoboken NJ (2019) 71:1400–12. doi: 10.1002/art.40930
18. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med (1999) 130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002
19. Pessach I, Kyriakou E, Kalampokas E, Kalampokas T, Bitsani A, Kotsianidis I. Antiphospholipid syndrome in cardiovascular disease and cancer. Eur J Haematol (2023). doi: 10.1111/ejh.14096
20. Belizna CC, Richard V, Thuillez C, Lévesque H, Shoenfeld Y. Insights into atherosclerosis therapy in antiphospholipid syndrome. Autoimmun Rev (2007) 7:46–51. doi: 10.1016/j.autrev.2007.06.002
21. Ruffatti A, Del Ross T, Ciprian M, Nuzzo M, Rampudda M, Bertero MT, et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers. A multicentre, retrospective follow-up study. Ann Rheum Dis (2009) 68:397–9. doi: 10.1136/ard.2008.096669
22. Erkan D, Yazici Y, Sobel R. Primary antiphospholipid syndrome: functional outcome after 10 years. J Rheumatol (2000) 27:2817–21.
23. Jackson WG, Oromendia C, Unlu O, Erkan D, DeSancho MT. Recurrent thrombosis in patients with antiphospholipid antibodies and arterial thrombosis on antithrombotic therapy. Blood Adv (2017) 1:2320–4. doi: 10.1182/bloodadvances.2017008185
24. Finazzi G, Brancaccio V, Moia M, De Stefano V, Berrettini M, Barbui T, et al. Natural history and risk factors for thrombosis in 360 patients with antiphospholipid antibodies: a four-year prospective study from the Italian Registry. Am J Med (1996) 100:530–6. doi: 10.1016/S0002-9343(96)00060-5
25. Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis (2019) 78:1296–304. doi: 10.1136/annrheumdis-2019-215213
26. Alijotas-Reig J, Ferrer-Oliveras R, Ruffatti A, Tincani A, Lefkou E, Bertero MT, et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): A survey of 247 consecutive cases. Autoimmun Rev (2015) 14:387–95. doi: 10.1016/j.autrev.2014.12.010
27. Khairani CD, Bejjani A, Piazza G, Jimenez D, Monreal M, Chatterjee S, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol (2023) 81:16–30. doi: 10.1016/j.jacc.2022.10.008
28. Zuily S, Cohen H, Isenberg D, Woller SC, Crowther M, Dufrost V, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost JTH (2020) 18:2126–37. doi: 10.1111/jth.14935.
29. Ruiz-Irastorza G, Egurbide M-V, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med (2004) 164:77–82. doi: 10.1001/archinte.164.1.77
30. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis (2019) 78:736–45. doi: 10.1136/annrheumdis-2019-215089
31. Martis N, Jamme M, Bagnis-Isnard C, Pouteil-Noble C, Presne C, Vigneau C, et al. Systemic autoimmune disorders associated with thrombotic microangiopathy: A cross-sectional analysis from the French National TMA registry: Systemic autoimmune disease-associated TMA. Eur J Intern Med (2021) 93:78–86. doi: 10.1016/j.ejim.2021.05.040
32. Rodríguez-Pintó I, Moitinho M, Santacreu I, Shoenfeld Y, Erkan D, Espinosa G, et al. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun Rev (2016) 15:1120–4. doi: 10.1016/j.autrev.2016.09.010
33. Cohen H, Hunt BJ, Efthymiou M, Arachchillage DRJ, Mackie IJ, Clawson S, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol (2016) 3:e426–436. doi: 10.1016/S2352-3026(16)30079-5
34. Cervera R, Rodríguez-Pintó I, Espinosa G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: A comprehensive review. J Autoimmun (2018) 92:1–11. doi: 10.1016/j.jaut.2018.05.007
Keywords: antibodies, antiphospholipid, antiphospholipid syndrome, lupus erythematosus, systemic, heart disease risk factors, connective tissue diseases
Citation: Ottavi M, Toulon P, Casolla B and Martis N (2024) Four clinical and biological phenotypes in antiphospholipid syndrome: a cluster analysis of 174 patients with antinuclear antibody tests. Front. Immunol. 15:1361062. doi: 10.3389/fimmu.2024.1361062
Received: 24 December 2023; Accepted: 05 February 2024;
Published: 19 February 2024.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Luis Del Carpio-Orantes, Mexican Social Security Institute (IMSS), MexicoKhalil Hajiasgharzadeh, Tabriz University of Medical Sciences, Iran
Copyright © 2024 Ottavi, Toulon, Casolla and Martis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nihal Martis, bWFydGlzLm5AY2h1LW5pY2UuZnI=
 Marie Ottavi
Marie Ottavi Pierre Toulon
Pierre Toulon Barbara Casolla3
Barbara Casolla3 Nihal Martis
Nihal Martis