- Department of Respiratory and Critical Care Medicine, Chaohu Hospital Affiliated with Anhui Medical University, Chaohu, China
Tracheal small cell carcinoma (SCC) is a rare malignancy, for which the optimal treatment strategy has yet to be determined. Currently, treatment largely aligns with the therapeutic guidelines established for small cell lung cancer, although numerous unresolved issues remain. This paper details a case study of a patient with Stage IIIB primary tracheal SCC, who was treated with an immune-combined etoposide-platinum(EP) regimen. This treatment offers valuable insights into innovative approaches for managing such malignancies. Furthermore, the study includes a comprehensive literature review to better contextualize the findings. The patient, admitted on May 2, 2023, had been experiencing persistent symptoms of airway discomfort for 15 days. A bronchoscopy performed on May 4 revealed tracheal SCC, classified as T4N2M0, IIIB. Following the CAPSTONE-1 study’s methodology, the patient underwent six cycles of PD-L1(adebrelimab) combined with EP therapy, leading to significant relief of symptoms and the eventual disappearance of the tracheal mass.
Introduction
Primary tracheal cancer, a relatively uncommon malignant respiratory tumor, represents approximately 0.2% of such neoplasms (1). Its pathological subtypes include squamous cell carcinoma, adenoid cystic carcinoma, among others, with rarer forms like adenosquamous carcinoma, mucinous epidermoid carcinoma, undifferentiated glandular carcinoma, melanoma, and chondrosarcoma (2). In the early stages of the disease, symptoms often lack specificity (3), and some individuals may exhibit manifestations like cough, sputum production, chest tightness, and shortness of breath, often leading to misdiagnosis as other respiratory conditions like chronic obstructive pulmonary disease or bronchitis (4). Diagnosis is definitively established through imaging and histological examinations. As the tumor grows, obstruction of the tracheal lumen may occur, resulting in symptoms of respiratory distress. Involvement of blood vessels may lead to hemoptysis, and when lymph nodes are affected, symptoms such as lymphadenopathy and tenderness may manifest (2). Among these, SCC of the airway is particularly rare, with only a limited number of cases described. The clinical characteristics of tracheal SCC closely resemble those of squamous cell carcinoma of the airway, encompassing factors such as patient age, gender, ethnicity, disease severity, lymph node involvement, and treatment modalities, including surgery and radiation. Notably, patients with tracheal SCC are more likely to receive chemotherapy (5). Some scholars have posited that treatment regimens effective for small cell lung cancer might also be applicable to tracheal SCC (6). However, the optimal therapeutic approach for tracheal SCC continues to be a topic for further investigation. Addressing the need for a more comprehensive understanding of tracheal SCC, this paper reports on a case of airway SCC where significant relief was achieved through the adoption of immunotherapy combined with EP treatment, informed by the CAPSTONE-1 study methodology. methodology, this report includes a thorough review of relevant literature.
Case presentation
On May 2, 2023, an 83-year-old male was admitted, reporting a 15-day history of recurrent cough, sputum production, chest tightness, and wheezing. The patient described an exacerbation of these symptoms, characterized by white, viscous sputum, and noted that the chest tightness and wheezing intensified with physical activity. Despite self-medicating (details unspecified), the symptoms showed no significant improvement. Consequently, the patient sought medical care at our outpatient clinic. A pulmonary CT scan conducted on the same day revealed a lesion at the tracheal prominence indicative of malignant tumor (MT), enlarged mediastinal lymph nodes suggestive of lymphatic metastasis, a small nodule in the right lower lobe, and scattered inflammatory lesions in both lungs. The scan also showed signs of bronchiectasis and pulmonary emphysema (Figure 1A). The patient’s medical history was notably unremarkable for major illnesses or exposures, and there was no history of substance abuse or familial diseases. Comprehensive examinations conducted on May 3, 2023, including blood tests and scans, yielded normal results, apart from elevated tumor markers (CEA, NSE, and ProGRP).
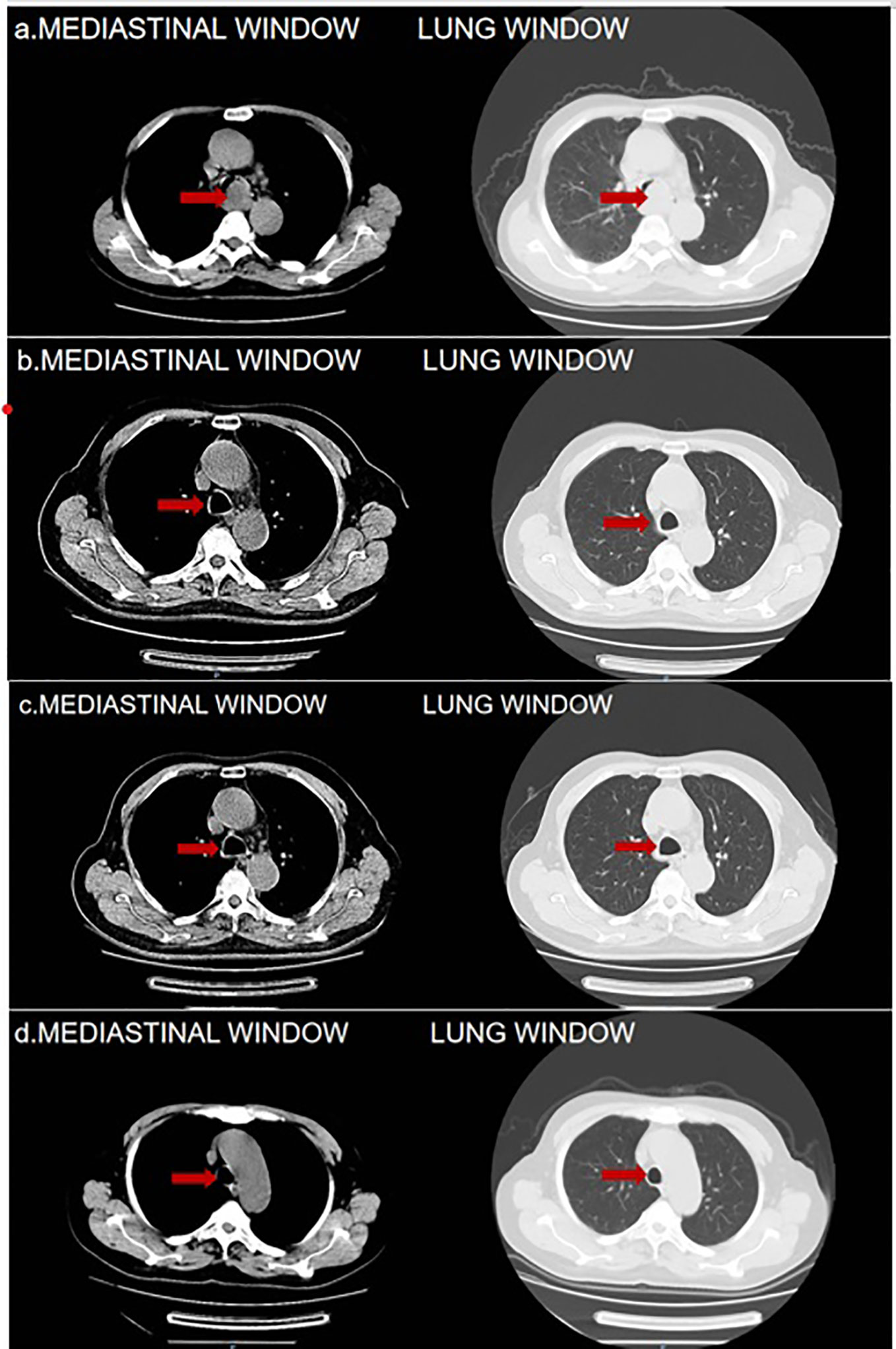
Figure 1 (A) 2023-5-2 Pulmonary CT Findings: Tracheal Prominence Occupancy. (B) 2023-6-28Pulmonary CT Findings: Significant Reduction in the Mass Below the Tracheal Prominence. (C, D) 2023-8-22 and 2023-11-1Pulmonary CT Findings: Absence of Mass Below the Tracheal Prominence in Both. The red arrows are used to indicate the location of tumor.
On May 5, 2023, a bronchoscopy identified an extrinsic compressive growth causing tracheal stenosis, with mucosal swelling and an irregular surface, approximately 13 cm below the glottis. The narrowed tracheal lumen measured about 10x5 mm, where a mucosal biopsy was obtained (Figure 2A). Histopathological examination and immunohistochemistry combined with diagnosis of tracheal SCC (Figure 3), with the tumor tissue expressing CD56+, TTF-1+, NapsinA-, P40-P63-, and LCA-Ki-67 approximately 90% positive. The patient, classified as stage IIIB (T4N2M0) with an ECOG performance status of 1, tolerating the treatment well and exhibiting no significant adverse reactions. Follow-up pulmonary CT on June 28 (Figure 1B), August 22 (Figure 1C), and November 1, 2023 (Figure 1D), demonstrated a marked reduction in the tracheal prominence An electron bronchoscopy on November 2, 2023, revealed slight scarring and some point-like projections at the lower end of the tracheal prominence, without tracheal obstruction (Figure 2B).
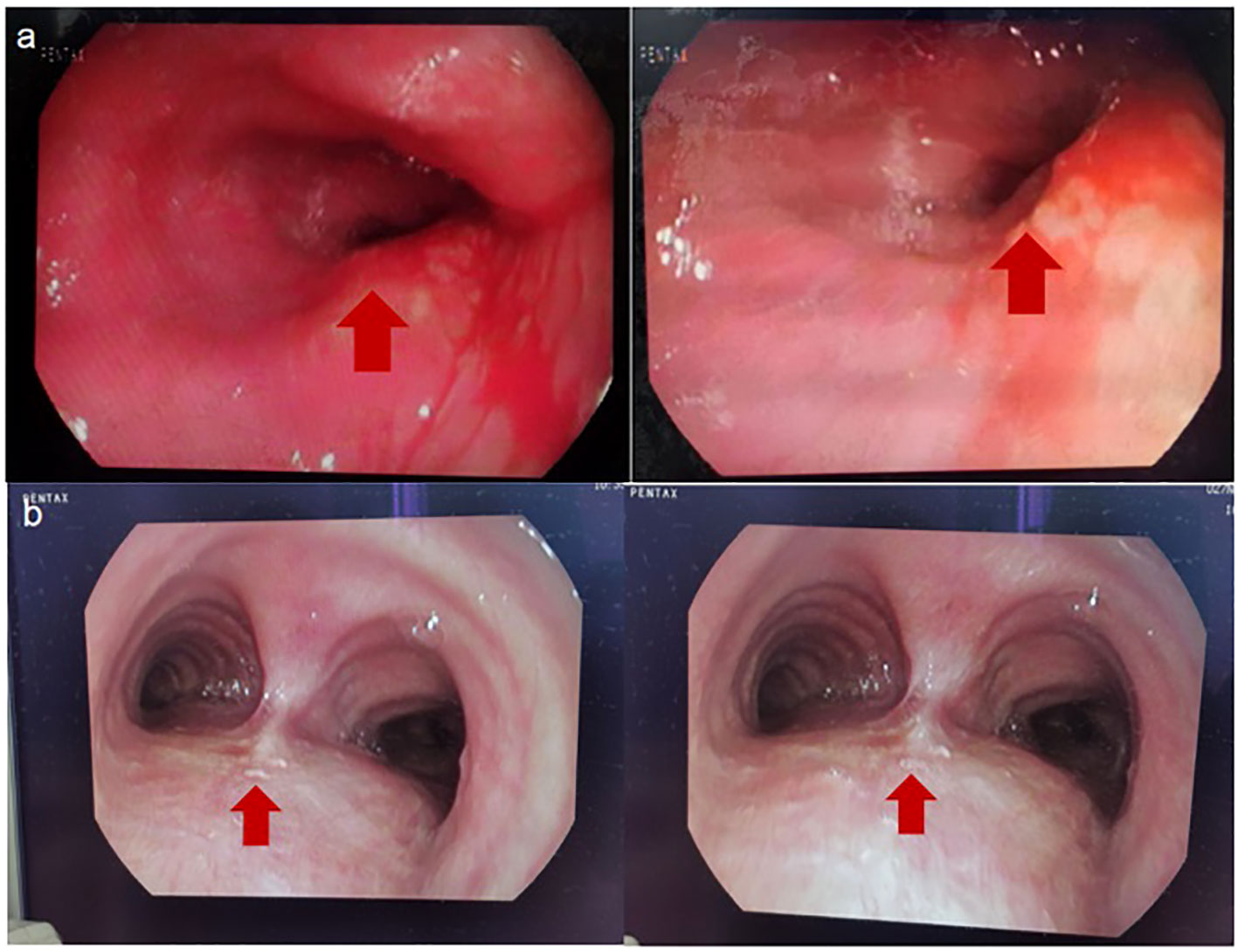
Figure 2 (A) 2023-5-5 Fibrobronchoscopy Reveals: Compression of the Tracheal Glottis, Narrowing of the Lumen, and Mucosal Swelling and Congestion. (B) 2023-11-2 Fibrobronchoscopy Reveals: The Tracheal Eminence Mass Has Disappeared, Localized with Slight Nodular Protrusions. The red arrows are used to indicate the location of tumor.
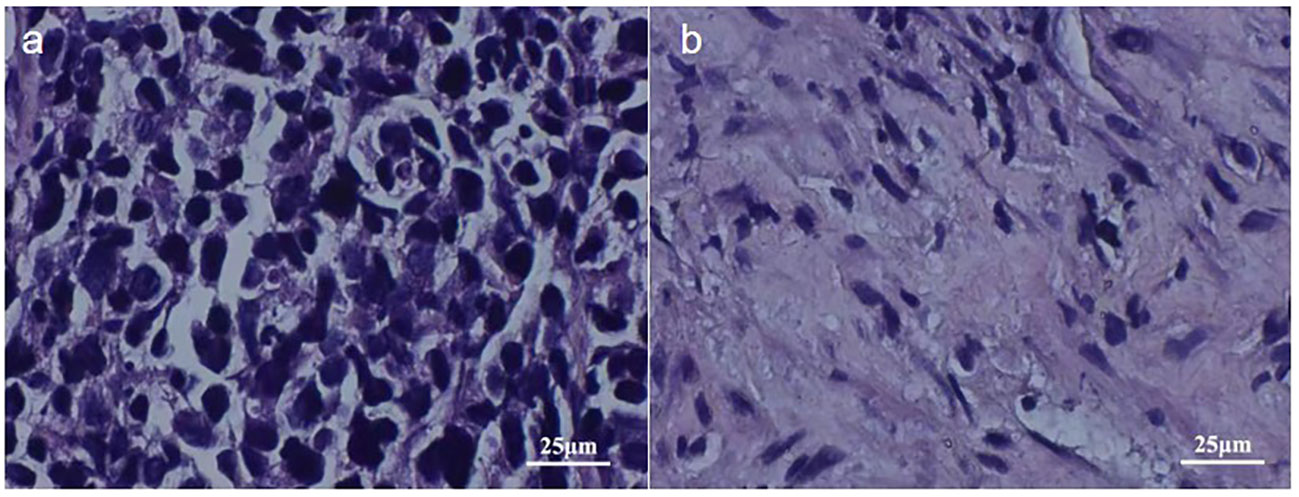
Figure 3 2023-5-5 Pathological Images: Image (A) Displays Tumor Tissue Cells Under 400x Magnification, Image (B) Presents Cells of Para-cancerous Tissue Under 400x Magnification.
Additionally, from the initial admission until November 5, 2023, the patient was subjected to regular monitoring encompassing blood routine, liver and kidney function, myocardial enzyme spectrum, and thyroid function tests. During this monitoring period, mild fluctuations were observed in thyroid hormone levels, which ranged between 40-46 mg/L and a slight leukopenia, varying between 1-2 degrees, were noted. Other parameters, including liver and kidney function tests and myocardial enzyme spectrum, consistently indicated no significant abnormalities. Importantly, throughout this period, the patient did not develop any immune-related dermatomyositis.
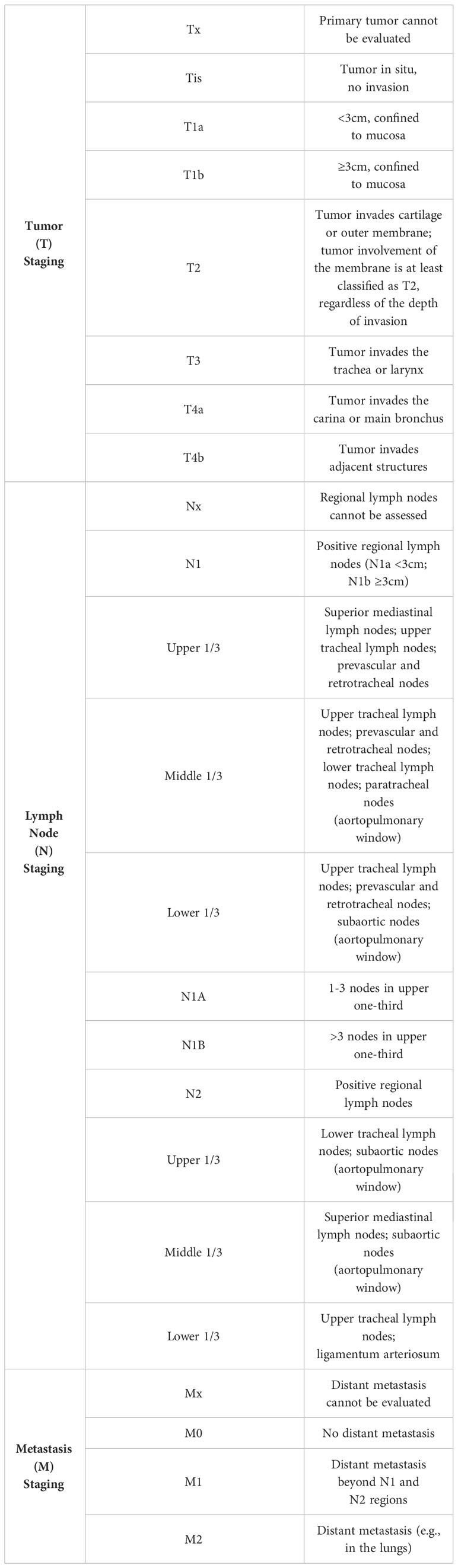
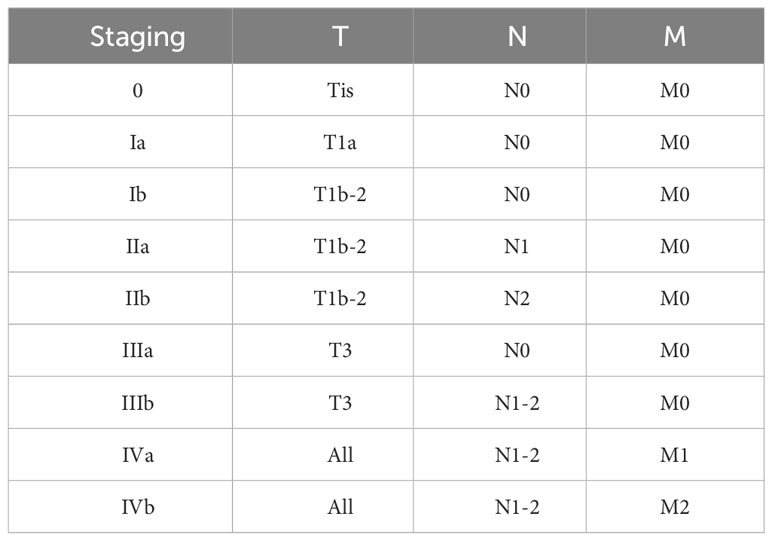
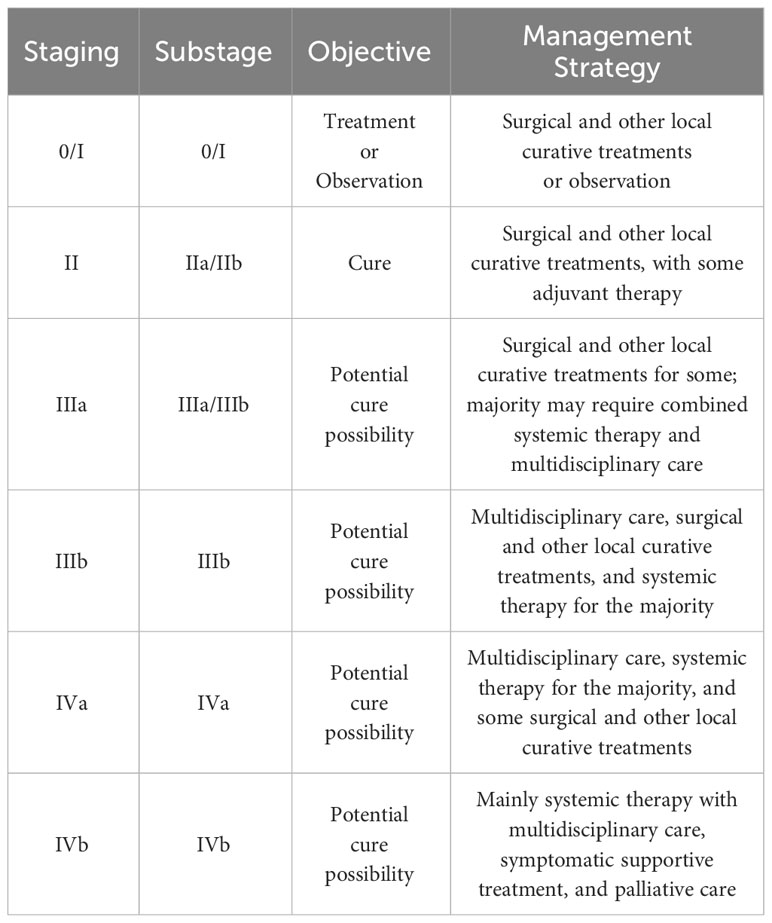
Discussion
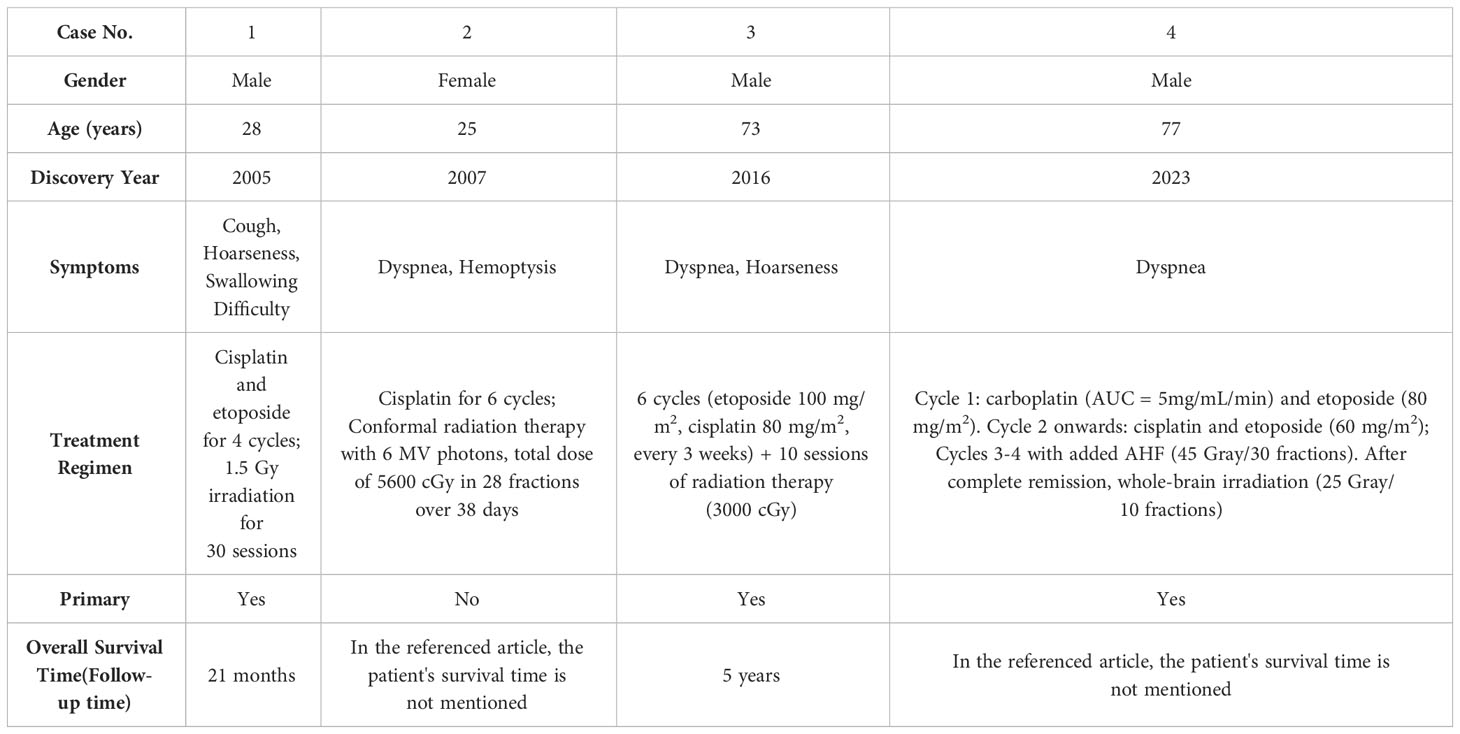
Primary tracheal tumors are considered rare, with primary SCC of the trachea being notably elusive, and its optimal treatment strategy remains insufficiently explored. Presently, clinical approaches often draw upon the treatment protocols established for small cell lung cancer. Nonetheless, significant inconsistencies are observed in the staging of tracheal malignant tumors compared to lung malignancies, along with variations in diagnostic and therapeutic procedures. To address this, a distinct staging and diagnostic-treatment workflow has been developed, employing the TNM staging system for classification (Table 1). This system stratifies cases into four stages based on diverse TNM manifestations (Table 2). Despite the availability of this independent staging protocol, ta gap persists due to the lack of large-scale clinical research focused on optimal treatment strategies. As a result, treatment procedures are predominantly based on clinical staging (Table 3) (7, 8). The primary treatment approach for these cases typically involves a combination of radiation therapy and chemotherapy; however, the efficacy of various regimen remains to be definitively established. Furthermore, the management of tracheal SCC is characterized by its diversity, potentially influencing the overall treatment effectiveness. Building upon the foundation of TNM staging, this study entails a retrospective analysis of global tracheal SCC cases reported since 2004. The subsequent table (Table 4) delineates the current challenges in treatment (4, 6, 9, 10):
In the four cases under consideration, the predominant symptoms included respiratory distress and hoarseness, with some patients also presenting with cough and swallowing difficulties, indicating possible esophageal involvement. Of these cases, three were identified as primary, while one was classified as secondary. All cases were treated with a combination of chemotherapy and radiotherapy. The maximum recorded survival period was 5 years, though the total survival durationfor some cases has not been comprehensively documented. Notably, tracheal SCC is characterized by a wide age range at onset, prolonged overall case duration, scarce reported instances, extremely low incidence rates, and no apparent gender predilection. These cases highlight that the optimal treatment modalities for tracheal SCC necessitate further in-depth investigation. Typically, tracheal SCC presents as an intrabronchial mass, and currently, there are no well-established treatment guidelines. The primary therapeutic strategies are derived from historical treatment experiences and include surgical intervention alone, combined surgery and chemotherapy, or chemotherapy alone (6). Over the past few decades, EP chemotherapy has remained the standard frontline treatment for extensive-stage small cell lung cancer (ES-SCLC), with no significantly superior alternatives yet identified. This regimen typically involves cisplatin and etoposide, administered with or without concurrent radiotherapy. Despite the initial sensitivity of ES-SCLC to EP chemotherapy, resistance development is nearly inevitable, leading to tumor recurrence within six months and an objective response rate of approximately 50-60%. Notably, over the past two decades, no significant breakthrough in medical interventions have been achieved, and patient outcomes have not demonstrated marked improvement (11). In contrast, monotherapy with chemotherapy tends to yields less favorable results, and the addition of radiotherapy can introduce unpredictable risks (12). The optimal therapeutic approach and overall efficacy for tracheal SCC remain elusive, presenting significant challenges in its clinical management. Consequently, there is a pressing need for further research and comprehensive clinical studies to establish a more effective andpersonalize treatment strategy for this rare malignancy.
Prior to the introduction of immune checkpoint inhibitors (ICIs), this innovative class of drugs has revolutionized immunotherapeutic options for patients with ES-SCLC, significantly improving survival rates. The combination of ICI with the EP treatment regimen has become a cornerstone in the therapeutic strategy for patients with ES-SCLC (11, 13). Notably, the IMpower 133 regimen, which incorporates the PD-L1 antibody atezolizumab into platinum-based chemotherapy, has demonstrated enhanced overall survival (OS) compared to chemotherapy alone. In a similar vein, the CASPIAN regimen, which integrates the PD-L1 antibody durvalumab with chemotherapy, has also yielded improvements in overall survival (OS) (14). Consequently, adebrelimab is being considered as a potent therapeutic agent for tracheal SCC. Adebrelimab is a recombinant fully humanized IgG4 monoclonal antibody, exhibits high affinity and specificity for PD-L1. It was officially approved for cancer therapy by the China Drug Evaluation Center (CDE) in 2022 (15). The results of the multicenter phase III clinical trial of adebrelimab, employing the CAPSTONE-1 study design, have now solidified its role as a first-line treatment regimen for ES-SCLC (16). Specifically, this study enrolled a total of 462 patients with ES-SCLC, dividing them equally into two groups: the adebrelimab group, comprising 230 patients (50%) received adebrelimab in combination with chemotherapy, while the placebo group, also consisting of 232 patients (50%), received a placebo alongside chemotherapy. Patients in the adebrelimab group were treated with 4-6 cycles of carboplatin (area under the curve 5 mg/mL per minute, day 1) and etoposide (body surface area 100 mg/m2, days 1-3), augmented with adebrelimab (20 mg/kg, day 1). In contrast, the placebo group underwent 4-6 cycles of the same chemotherapy regimen, but with a placebo, over a treatment cycle of 21 days. Notably, the median overall survival in the adebrelimab group exhibited significant improvement compared to the placebo group. The most common grade 3 or 4 treatment-related adverse events were decreased neutrophil count, white blood cell count, platelet count, and hemoglobin level. The incidence of severe treatment-related adverse events was notably lower in the adebrelimab group. Importantly, there were two cases of treatment-related deaths reported in both the adebrelimab and placebo group among the four cases documented (17). Consequently, the addition of adebrelimab is observed to significantly enhance overall survival rate in patients with ES-SCLC, while maintaining an acceptable safety profile. These findings advocate for the implementation of this combination therapy as a novel first-line treatment option for patients with ES-SCLC.
This article presents a case of primary tracheal SCC in an 83-year-old male patient. The lung CT scan showed bronchiectasis and pulmonary emphysema, along with a lesion occupying the tracheal carina. Bronchoscopy revealed a neoplasm obstructing the lower part of the glottis, situated approximately 13 cm below the vocal cords. The histopathological analysis of the bronchial biopsy specimen, supplemented with immunohistochemical studies, confirmed the diagnosis of trachealSCC. This diagnosis was established based on the clinical data, morphological characteristics, and immunohistochemistry findings, indicating primary tracheal SCC without metastatic involvement. Tracheal SCC typically manifests as an intrabronchial mass. In this case, involving an 83-year-old patient with compromised baseline health and diagnosed with primary tracheal SCC(not secondary to other malignancies), we adhered to the treatment protocol from the CAPSTONE-1 study. Given the proven efficacy of immunotherapy combined with chemotherapy in treating ES-SCLC, we opted for a similar therapeutic regimen. Due to the patient’s advanced age and potential for chemotherapy resistance, a reduced dose of carboplatin was administered, alongside etoposide at 0.1g on days 1-5 and carboplatin 200mg on day 5, combined with adebrelimab 1.2g. Remarkably, after just two cycles of chemotherapy and immunotherapy, there was a significant reduction in the tracheal mass below the carina, and following six cycles, the mass completely disappeared without any severe adverse reactions. This combined approach of chemotherapy and immunotherapy demonstrated superior efficacy compared to chemotherapy plus radiotherapy, offering the patient a less distressing treatment course, maintaining overall well-being, and suggesting a novel therapeutic strategy for similar cases.
Bronchial carcinomas are relatively rare with small cell bronchial carcinoma being even more uncommon. Currently, the optimal treatment approach for such rare diseases remains unclear, The standard regimen typically involves platinum-based chemotherapy, commonly combining cisplatin and etoposide. This particular case of SCC offers a new perspective on potential treatment strategies. Taking into account the patient’s physical condition, the integration of immunotherapy with EP regimen can effectively enhance survival rates for patients with tracheal SCC, thereby presenting a novel therapeutic avenue.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
We asked the ethics committee and they said this type doesn’t require approval. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YC: Writing – original draft. HZ: Writing – review & editing. DW: Resources, Writing – review & editing. YY: Resources, Writing – review & editing. JG: Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1356268/full#supplementary-material
References
1. Junker K. Pathology of tracheal tumors. Thorac Surg Clin (2014) 24(1):7–11. doi: 10.1016/j.thorsurg.2013.09.008
2. Rea F, Zuin A. Tracheal resection and reconstruction for Malignant disease. J Thorac Dis (2016) 8(Suppl 2):S148–52. doi: 10.3978/j.issn.2072-1439.2016.02.04
3. Moores D, Mane P. Pathology of primary tracheobronchial Malignancies other than adenoid cystic carcinomas. Thorac Surg Clin (2018) 28(2):149–54. doi: 10.1016/j.thorsurg.2018.01.003
4. Ahn JH, Chung JH, Lee KH, Shin KC, Choi EY, Jin HJ. Primary tracheal small cell carcinoma treated by concurrent chemoradiotherapy. Soonchunhyang Med Sci (2016) 22(2):132–5. doi: 10.15746/sms.16.030
5. Chen K, Yang Z, Zhang X, Zhao T, Zhang X, Li W, et al. Clinical features and prognosis of primary tracheal small cell carcinoma: a population-based analysis. Transl Cancer Res (2020) 9(2):882–90. doi: 10.21037/tcr.2019.12.29
6. Sugimoto C, Teranishi S, Sawazumi T, Nagaoka S, Nagayama H, Segawa W, et al. Primary tracheal small-cell carcinoma detected 11 months after surgery for pulmonary large-cell neuroendocrine carcinoma: A case report. Thorac Cancer (2023) 14(13):1212–6. doi: 10.1111/1759-7714.14860
7. Wu Y, Cheng Y, Zhou Q, Lin D, Wang J, Wang C, et al. Chinese society of clinical oncology guidelines working committee. Chinese Society of Clinical Oncology (CSCO) Primary lung Cancer Diagnosis and treatment guidelines 2017.V1 (2017) 5(2):71–81.
8. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Bharat A, et al. NCCN clinical practice guidelines in oncology. Non Small Cell Lung Cancer. Version 1.2019 (2019) 5-6, NSCL1–NSCL17, ST1-ST3.
9. Jain S, Agarwal JP, Gupta T, Parikh PM, Mistry RC, Menon H, et al. Case report: Second primary small cell carcinoma of the trachea in a breast cancer survivor: A case report and literature review. Br J Radiol (2008) 81(964):e120–2. doi: 10.1259/bjr/97077007
10. Takano S, Kusumoto M, Tateishi U, Matsuno Y, Oe Y, Asamura H. An example of a juvenile tracheal primary small cell carcinoma. Lung Cancer (2005) 45(2):133–7. doi: 10.2482/haigan.45.133
11. Wang T, Li Y, Zheng X. Cost-effectiveness of the combination of immunotherapy and chemotherapy for extensive-stage small-cell lung cancer: A systematic review. BMC Health Serv Res (2023) 23(1):691. doi: 10.1186/s12913-023-09727-7
12. Huang W, Tan P, Xu Z. [Secondary or second primary endotracheal cancer after surgery for primary lung cancer: Case series and review of the literature]. Zhongguo Fei Ai Za Zhi (2023) 26(7):545–52. Chinese. doi: 10.3779/j.issn.1009-3419.2023.101.20
13. Al-Salama ZT. Durvalumab: A review in extensive-stage SCLC. Target Oncol (2021) 16(6):857–64. doi: 10.1007/s11523-021-00843-0
14. Mathieu L, Shah S, Pai-Scherf L, Larkins E, Vallejo J, Li X, et al. FDA approval summary: Adebrelimab and durvalumab in combination with platinum-based chemotherapy in extensive stage small cell lung cancer. Oncologist (2021) 26(5):433–8. doi: 10.1002/onco.13752
15. Gan Y, Shi F, Zhu H, Han S, Li D. Adebrelimab plus chemotherapy vs. chemotherapy for treatment of extensive-stage small-cell lung cancer from the US and Chinese healthcare sector perspectives: A cost-effectiveness analysis to inform drug pricing. Front Pharmacol (2023) 14:1241130. doi: 10.3389/fphar.2023.1241130
16. Li H, Han H, Li C, Wu R, Wang Z, Wang Y, et al. Efficacy and safety of first-line PD-1/PD-L1 inhibitor combinations for extensive-stage small-cell lung cancer: A Bayesian network meta-analysis. Ther Adv Med Oncol (2023) 15:17588359231189430. doi: 10.1177/17588359231189430
17. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(6):739–47. doi: 10.1016/S1470-2045(22)00224-8
Keywords: tracheal small cell carcinoma, immune-combined therapy, etoposide-platinum regimen, adebrelimab, optimal treatment strategy
Citation: Chen Y, Zhu H, Wang D, Ye Y and Gao J (2024) Case report: A novel perspective on the treatment of primary tracheal small cell carcinoma: a patient’s experience with immuno-combined EP therapy and literature review. Front. Immunol. 15:1356268. doi: 10.3389/fimmu.2024.1356268
Received: 15 December 2023; Accepted: 08 January 2024;
Published: 29 January 2024.
Edited by:
Yanqing Liu, Columbia University, United StatesReviewed by:
Weiping Li, Columbia University, United StatesXinlei Sun, The University of Texas MD Anderson Cancer Center, United States
Mengdie Feng, Baylor College of Medicine, United States
Copyright © 2024 Chen, Zhu, Wang, Ye and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbin Zhu, emh1aG9uZ2Jpbjc4OEAxNjMuY29t
 Yu Chen
Yu Chen Hongbin Zhu
Hongbin Zhu