- 1Department of Neurology, West China Hospital of Sichuan University, Chengdu, Sichuan, China
- 2Institute of Brain Science and Brain-inspired Technology of West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Neurology, Chengdu Shangjin Nanfu Hospital, Chengdu, Sichuan, China
Neuromyelitis optica spectrum disorder (NMOSD) is a rare demyelinating disease of the central nervous system primarily affecting the optic nerves, spinal cord, and brainstem. Viral infection may trigger NMOSD. Here, we report the case of a 34-year-old female presenting with a range of symptoms including nausea, vomiting, dysphagia, choking, and fatigue with unsteady gait, diplopia, hearing loss, left-sided facial paralysis, breathing difficulties, and hoarseness of voice. Her HBV DNA concentration, as determined by quantitative PCR analysis, exceeded 5×107 IU/ml in serum and 4.48×102 IU/ml in CSF. Next-generation sequencing of CSF revealed 1,528 HBV sequences in DNA analysis and 6 sequences in RNA analysis. Serum aquaporin-4 antibody (AQP4-Ab) titer was 1:10, and the CSF titer was 1:3.2. Brain magnetic resonance imaging showed high signal intensities in the brain stem, medulla oblongata, and left middle cerebellar peduncle with mild restricted-diffusion. The patient received antiviral and hepatoprotective medications before the high-dose methylprednisolone pulse therapy. However, the patient did not respond well to the first-line treatment. Subsequently, the patient received ofatumumab and inebilizumab. Throughout the follow-up period, there was a gradual improvement in her neurological symptoms, with no reactivation of hepatitis B or deterioration of liver function observed. Thereby, to the best of our knowledge, we report the first case of successful treatment with ofatumumab and inebilizumab in a patient with NMOSD concurrent with HBV infection.
1 Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare demyelinating disease of the central nervous system (CNS), primarily affecting the optic nerves, spinal cord, and brainstem (1). The current literature, including numerous studies and case reports, suggests an association between infections, vaccinations, and the onset of CNS demyelinating diseases, including NMOSD (2–6).
Chronic hepatitis B virus (HBV) infection is a significant global public health concern, with an estimated 296 million people infected in 2019 (7). The prevalence of HBV varies geographically; for example, China has approximately 70 million carriers of hepatitis B surface antigen (HBsAg), indicating a prevalence of 5%-6% (8, 9). Despite its global impact, NMOSD with active HBV replication remains rare. While many previous case reports have discussed the coexistence of NMOSD and HBV infection (3, 6), direct evidence of HBV presence in cerebrospinal fluid (CSF) is lacking, necessitating further research to establish a conclusive correlation between HBV and NMOSD.
In the context of immunosuppressive therapy for NMOSD, options such as high-dose intravenous methylprednisolone pulse therapy and other immunosuppressants like azathioprine (AZA), mycophenolate mofetil, ofatumumab, and inebilizumab are being used (10). It is crucial to acknowledge that immunosuppressive therapy may disrupt the immunologic control over HBV infection, potentially leading to HBV reactivation (HBVr) (11, 12). Therefore, selecting an appropriate treatment for NMOSD patients with coexisting HBV infection poses substantial challenges.
Despite its clinical importance, a consensus on the optimal approach to treating NMOSD patients with coexisting HBV infection is lacking. This report addresses this gap by presenting the first case of NMOSD with positive aquaporin-4 antibody (AQP4-Ab) and simultaneous detection of HBVr in both serum and CSF. The patient manifested symptoms consisted with area postrema syndrome (APS) and brainstem syndrome (BS). NMOSD remission was achieved through immunotherapy, including high-dose methylprednisolone pulse therapy, ofatumumab, and inebilizumab, without triggering HBVr. Further research is essential to refine the management strategies for NMOSD patients with concurrent HBV infection.
2 Case description
In May 2023, a 34-year-old Tibetan female was admitted to our hospital with a constellation of symptoms, including persistent nausea and vomiting for one month; dysphagia and choking for 25 days; and fatigue with unsteady gait for 20 days, which then followed by diplopia, hearing loss, left-sided facial paralysis, breathing difficulties, and voice hoarseness for 5 days. Before the onset of the illness, she experienced mild diarrhea that resolved within one day. The ongoing nausea and vomiting led to poor appetite, mental distress, and fatigue, necessitating intravenous nutritional support. Notably, she also had a past medical history of untreated chronic hepatitis B for 6 years. The patient denied any history of smoking or alcohol consumption, had no psychosocial background, and no family history of genetic diseases. Furthermore, there was no reported exposure to heavy metals.
Before seeking treatment at our hospital, the patient had previously consulted the gastroenterology department in another hospital for nausea and vomiting. Various investigations including routine blood test, amylase, lipase, electrolytes, and liver and kidney function tests were all performed with negative results. Abdominal CT and gastrointestinal endoscopy were also unremarkable. Symptomatic treatments, such as antiemetics and intravenous fluid therapy, were prescribed, but there was no improvement. Consequently, the patient was referred to our hospital for further evaluation.
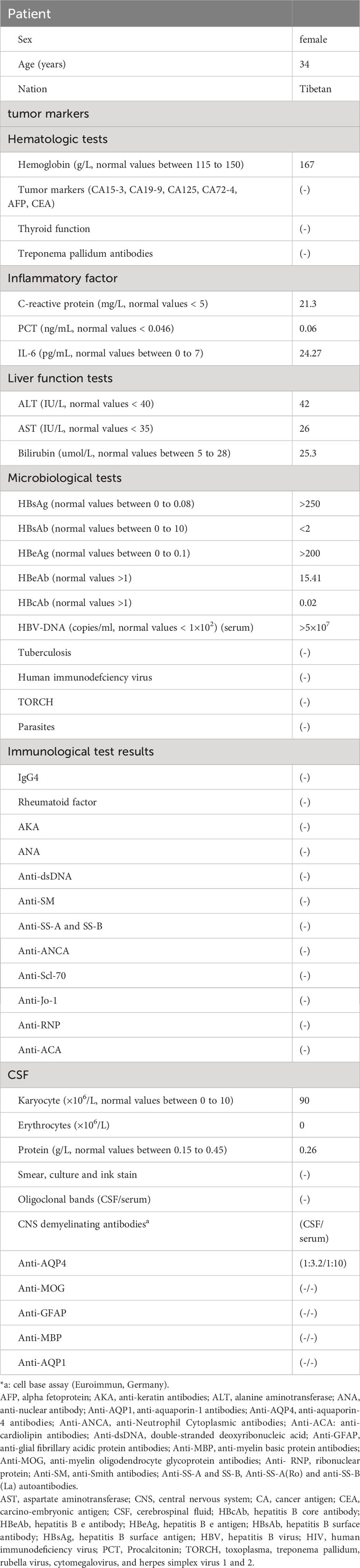
Upon admission, the physical examination revealed no abdominal tenderness or rebound pain and no signs of peritoneal irritation. The initial Expanded Disability Status Scale (EDSS) score was 6.5 points (Figure 1). The EDSS is a tool utilized to quantify neurological impairment in NMOSD, ranging from 0 to 10, with higher scores indicating more severe disability (13).
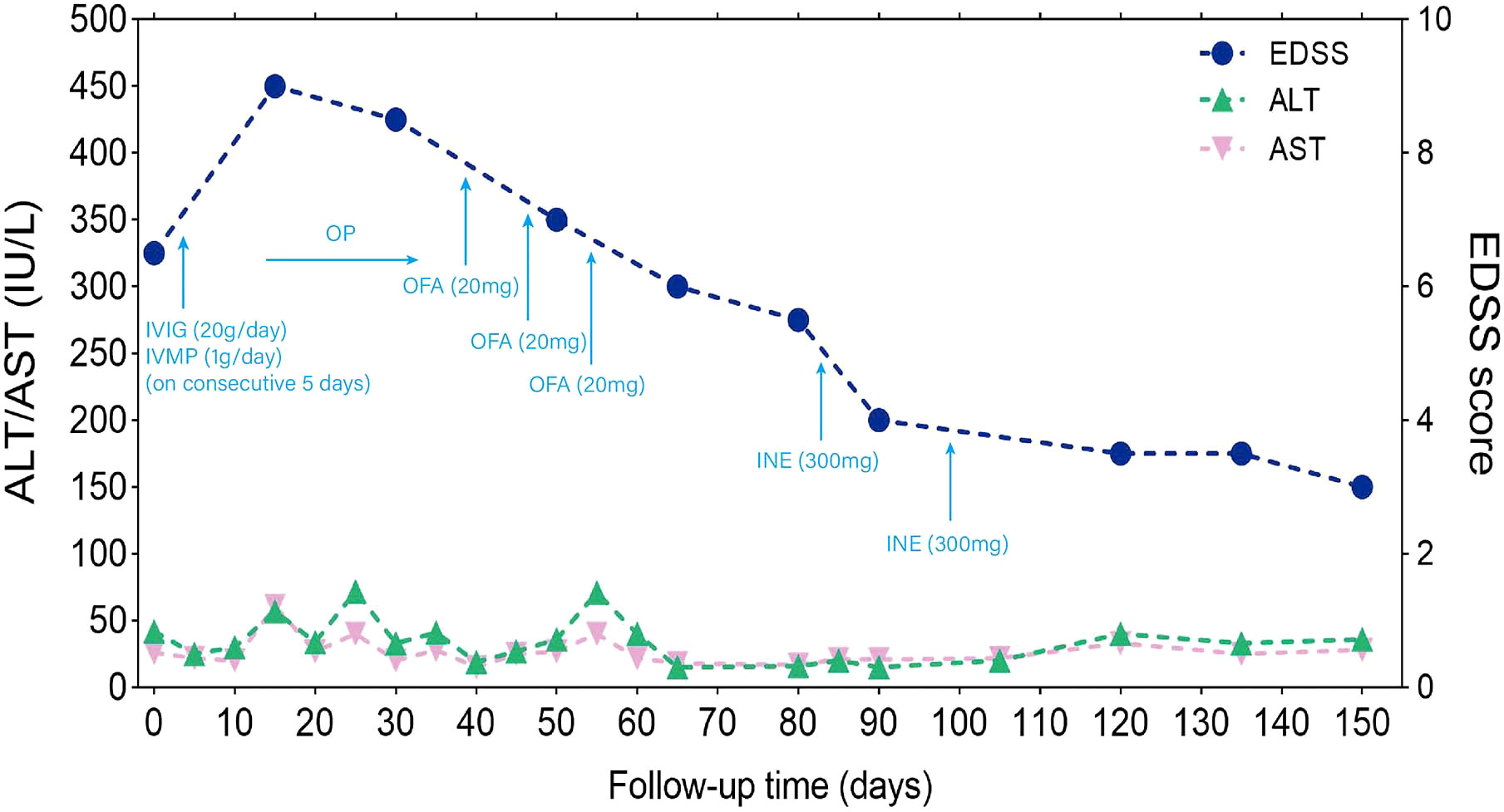
Figure 1 Timeline of disease disability course and different treatment regimes. The x-axis indicates the number of days after admission. The left y-axis indicates ALT (IU/L) and AST (IU/L). The right y-axis also indicated EDSS score. ALT: normal values < 40 IU/L ml; AST: normal values < 35 IU/L; EDSS is used to quantify the level of disability in NMOSD, ranging from 0 to 10, with higher scores representing increasing levels of disability; IVIG: 0.4 g/kg intravenous immunoglobulins daily for 5 days; IVMP, intravenous methylprednisolone 1000 mg for 5 consecutive days, followed by oral prednisone (1 mg/kg) with a weekly reduction of 5 mg. Throughout the entire immunotherapy process, ALT and AST showed some fluctuations without significant elevation. There was no clinical improvement after receiving IVMP and IVIG, and the EDSS score worsened to 9 on day 15 from admission. Ofatumumab was subcutaneously injection on days 39, 46, 53 (20 mg per dose) from admission; inebilizumab was intravenously infusion on days 83, 90 (300 mg per dose) from admission. After receiving ofatumumab and inebilizumab treatments, there was a gradual improvement in symptoms, and the EDSS score changed from 9 to 3. ALT, aspartate aminotransferase; AST, alanine aminotransferase; IVMP, intravenous methylprednisolone; IVIG, intravenous immunoglobulin; OP, oral prednisone; OFA, Ofatumumab; INE, inebilizumab; EDSS, Expanded Disability Status Scale.
3 Diagnostic assessment
The patient’s serum was positive for hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and hepatitis B core antigen (HBcAg). The concentration of HBV DNA in the serum, as determined by quantitative PCR analysis, exceeded 5×107 IU/ml. The HBV DNA concentration in CSF was also found to be greater than 4.48×102 IU/ml. On next-generation sequencing (NGS) of CSF, DNA analysis showed 1,528 HBV sequences, and RNA analysis showed 6 HBV sequences. The sequencing was performed using the Illumina NextSeq 550 sequencing platform (Illumina, San Diego, USA) and a SE75bp sequencing strategy (Figure 2). The test for anti-AQP4 antibodies was positive in both the serum (titer, 1:10) and CSF (titer, 1:3.2) with a commercial kit (Euroimmun, Germany) (Supplementary Figure 1). Other auxiliary laboratory examinations are listed in Table 1.
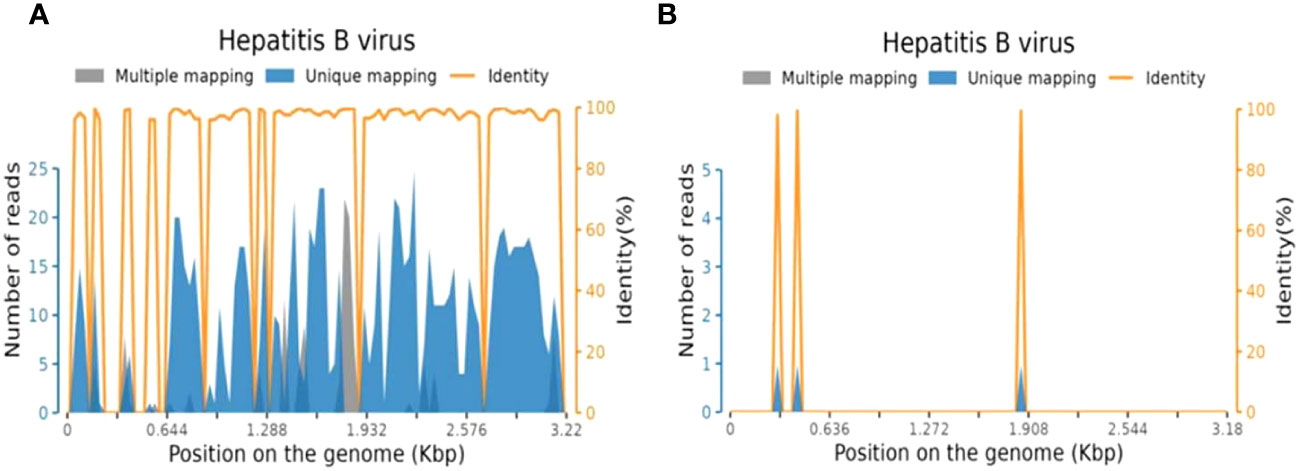
Figure 2 Sequence reads mapped to HBV in CSF from NGS results. (A) Sequence reads of HBV DNA in CSF with a total coverage of 69.18% and an average depth of 6.22 X; (B) Sequence reads of HBV RNA in CSF with a total coverage of 1.57% and an average depth of 1.00 X. HBV, hepatitis B virus; CSF, cerebrospinal fluid; NGS, next generation sequencing.
Additionally, comprehensive investigations, including routine stool analysis, abdominal ultrasound, enhanced CT scans of the upper and lower abdomen, gastroscopy, upper abdominal MRI, and tests for pancreatic enzymes, lipase, and amylase all revealed no abnormalities, thereby ruling out digestive system abnormalities. EEG result was within normal limits. Brain MRI using T2-weighted sequences showed high signals in the brain stem, medulla oblongata, and left middle cerebellar peduncle with mild restricted-diffusion, but no significant enhancement (Figures 3A–E).
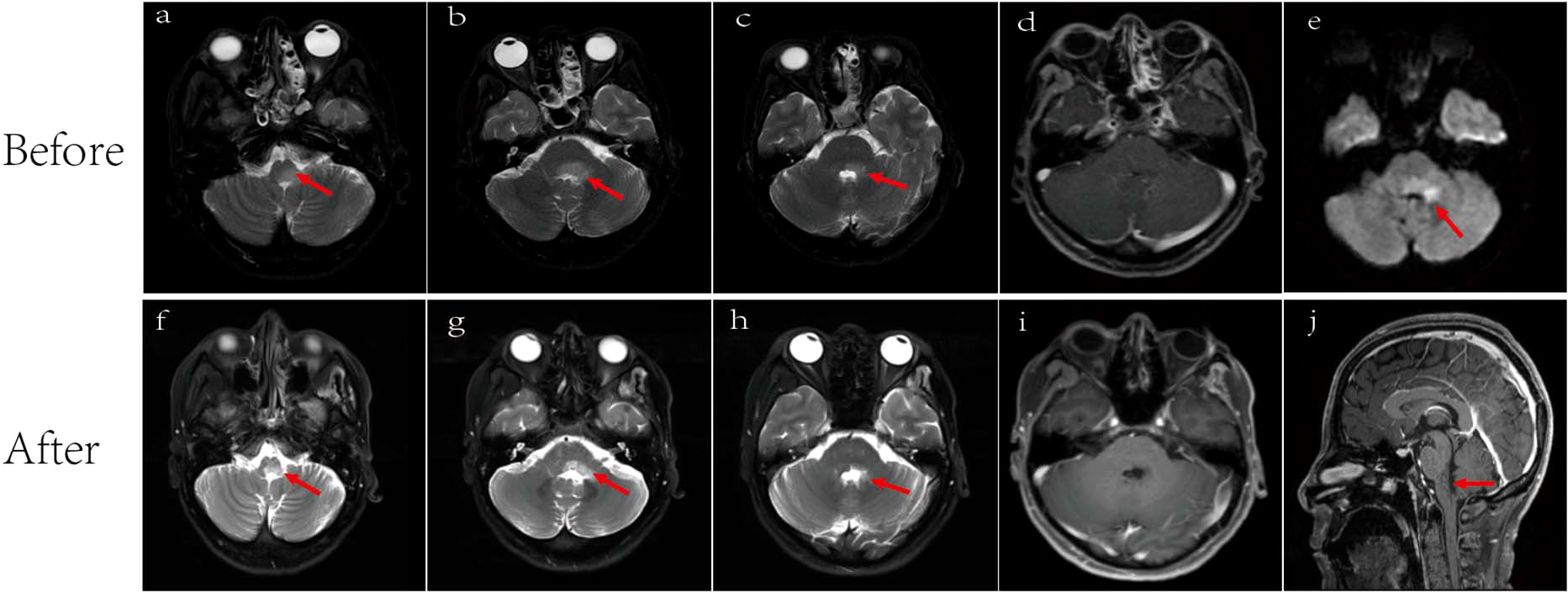
Figure 3 Brain MRI. Lesions (arrowed) on brain MRI images before (A–E) and after (F-J) antiviral and immunotherapy showing obviously manifested shrinkage after treatment. (A) T2-weighted showed medulla oblongata lesion. Lesion area: 2.4 cm2; (B) T2-weighted showed dorsal aspect of the brainstem and left cerebellar peduncle lesions. Lesion area: 3.3 cm2; (C) T2-weighted showed dorsal aspect of the brainstem and left cerebellar peduncle lesions. Lesion area: 1.8cm2; (D) No significant enhancement before treatment; (E) Diffusion-weighted MRI (DWI) showed mild limited diffusion. Lesion area: 1.7 cm2; (F) T2-weighted showed a reduction in the area of medullary lesions, with an area of 1.0 cm²; (G) T2-weighted showed a reduction in the area of brainstem and left cerebellar peduncle lesions, with an area of 2.0 cm2; (H) T2-weighted showed a reduction in the area of brainstem and left cerebellar peduncle lesions, with an area of 0.7 cm2; (I) No significant enhancement after treatment; (J) Sagittal view showed medulla oblongata lesion.
According to the 2015 International Diagnostic Consensus for NMOSD (1), the patient’s clinical symptoms and MRI findings align with two (APS and BS) of the six core clinical syndromes of NMOSD. The comprehensive gastrointestinal examination ruled out digestive system diseases. CSF analysis and the results of other antibodies targeting specific CNS demyelinating diseases, such as anti-MOG and anti-GFAP antibodies, also ruled out other CNS infections and demyelinating diseases. Whereas the presence of positive APQ4-Ab in both serum and CSF confirms the diagnosis of NMOSD.
4 Therapeutic process and follow-up
During the 150-day follow-up period, the timeline of disease disability, different treatment regimens, and changes in liver function were depicted in Figure 1. Upon admission, an aggressive treatment approach was initiated, including intravenous high-dose methylprednisolone (1g/day) and immunoglobulins (0.4mg/kg) for five days, followed by a tapered oral prednisone regimen (1mg/kg) with a weekly reduction of 5mg. To inhibit HBV replication, entecavir (0.5mg/day) was prescribed, alongside propofol tenofovir fumarate tablets and hepatoprotective drugs. It is noteworthy that although plasmapheresis (PE) is also considered a first-line treatment for NMOSD, we opted not to go for PE in this patient because he already had received IVIG, and the use of PE may attenuate the effect of IVIG.
On day 15, the patient developed steroid-related complications including gastrointestinal bleeding and severe pulmonary infection accompanied by a decrease in oxygen saturation. The EDSS score worsened to 9. Therefore, immediate interventions including non-invasive ventilator support, antimicrobial therapy, gastric acid suppression, and intravenous nutritional support, among other symptomatic treatments were implemented. After implementing these interventions along with a gradual reduction in oral prednisone, a repeated chest CT on day 38 showed a decrease in pulmonary inflammation as compared with the previous scan. The inflammatory markers also significantly decreased and her temperature returned to the baseline. ALT and AST levels were relatively stable. Hemoglobin and stool occult blood were normal, with no signs of gastrointestinal bleeding. At this point, immunotherapy was introduced, with subcutaneous ofatumumab injections on days 39, 46, and 53 (20 mg per dose) from admission.
After the administration of ofatumumab, the patient’s symptoms gradually improved (Figure 1), and no HBVr was observed. We then considered switching to inebilizumab, a humanized monoclonal antibody targeting CD19-positive B cells, that was approved by the Food and Drug Administration (FDA) in 2020 for relapse prevention in adult patients with AQP4-Ab seropositive NMOSD. Therefore, the patients received inebilizumab infusion (300 mg) on days 83 and 98.
The patient demonstrated good compliance with the recommended intervention and actively participated in follow-up assessments, which included liver and kidney function tests, complete blood count, electrolyte levels, and CSF examination. All test results were within normal limits. By day 90, significant progress was evident, with brain MRI revealing reduced lesions compared to the previous scan (Figures 3F–J) and the HBV DNA in serum dropping to 1.32×105 IU/mL. The patient’s symptoms also improved, reflected in the EDSS score, which decreased from 9 to 3 (Figure 1). Interestingly, the treatment was well-tolerated, with no hepatitis outbreaks, deterioration of liver function, or any adverse reactions reported. Additionally, the count of CD20-positive B cells decreased from 238 cells/ul to 5 cells/ul, indicating a positive response to therapy.
5 Discussion
This is the first report of detectable HBV in the CSF of a patient with NMOSD who was successfully treated with ofatumumab and inebilizumab. NMOSD is a rare autoimmune disorder characterized by recurrent optic neuritis (ON) and transverse myelitis (TM). While the exact etiology remains elusive, substantial evidence implicates infectious agents, primarily viral infections (3, 6, 14). The 2015 international diagnostic consensus for NMOSD recognizes six major clinical characteristics including APS and BS (1). Notably, the area postrema, a vomiting center in the caudal and dorsal brainstem, lacks a blood-brain barrier and exhibits heightened expression of AQP4-Ab. The involvement of this area potentially leads to intractable nausea, vomiting, or hiccups (15). Brainstem syndromes, overlapping with area postrema syndrome, may also manifest with oculomotor dysfunction (e.g., diplopia and nystagmus) or other cranial nerve palsies (16). In this report, our patient presented with APS and BS. In the previously reported case series, ON and TM were the most frequently observed clinical characteristics among NMOSD patients with concurrent HBV infection; but one case presented with BS (3, 6, 14). The current literature suggests that ON tends to be more aggressive among people with chronic HBV infection compared to those without HBV (17). Similarly, our patient exhibited significant disability at disease onset. Further research is warranted to explore the relationship between HBV load in the CSF and the severity of NMOSD.
Numerous studies have noticed a relationship between viral infections and the development of NMOSD (2–6), with some having proposed a role for Helicobacter pylori and Clostridium perfringens (18, 19). By using NGS testing, we detected HBV DNA and HBV RNA in the CSF and ruled out other infections. Despite the decrease in HBV replication activity with the administration of anti-HBV drugs and hepatoprotective agents, HBV DNA was still detectable in the CSF even after treatment. A recent study suggested that HBV DNA can persist in the CSF even after antiviral therapy, this is possibly due to the limited penetration of most nucleos(t)ide analogs through the blood-brain barrier, which results in insufficient drug concentration to clear HBV (20). The presence of HBV in the CSF may increase the risk of CNS infection. Some theories proposed that molecular mimicry and immunological cross-reactivity between HBsAg and myelin antigens contribute to the development of demyelinating diseases in the CNS (21). Several hypotheses have also been proposed on how peripheral blood HBV can lead to CNS infection, including the disruption of the blood-brain barrier, production of hepatitis B immune complexes, and unequal transfer of virions and/or subviral particles across the blood-brain barrier (22–25). Though many previous studies have reported the detection of HBV DNA in the CSF of HBV-infected patients (20, 26–28), detecting HBV in the CSF of patients with NMOSD with concurrent HBV infection is rare, and the exact pathophysiological mechanisms are not clear (3, 6, 14). Our case suggests that HBV may trigger or contribute to the underlying mechanisms of NMOSD.
Limited studies have reported the replication of HBV in the CNS (20, 29–31). It remains uncertain whether HBV exclusively resides in the CNS following blood transmission or if it can actively replicate within the CNS of HBV-infected individuals. In our case, the high level of HBV replication might have resulted from blood contamination during the lumbar puncture procedure, while the absence of red blood cells in the CSF suggests that significant contamination is improbable. Moreover, Ene et al. isolated HBV from the CSF of patients co-infected with HIV and HBV and noticed that the replication potential of HBV in these patients could contribute to its ability to replicate in the CNS and trigger autoimmune inflammation (29).
Corticosteroids, a common first-line treatment for NMOSD, have the potential to reactivate HBV by suppressing T cell-mediated immunity and activating a glucocorticoid-responsive transcriptional element in the HBV genome (32). Antiviral treatment can mitigate both HBV integration and hepatocyte clonal expansion (33). Consistent with previous studies (3, 6, 14), our patient received high-dose methylprednisolone pulse therapy along with antiviral and hepatoprotective medications. Although some fluctuations in the results of liver function tests was noted, there was no evident elevation or hepatitis B outbreak. Researchers recommend prophylactic antiviral treatment for patients receiving high-dose methylprednisolone therapy (12, 34). Accordingly, our patient also received antiviral and hepatoprotective medications before initiating high-dose methylprednisolone pulse therapy.
In the remission phase, immunosuppressive agents are crucial to prevent relapse. It’s noteworthy that spontaneous HBVr may occur, but it is more commonly triggered by immunosuppressive therapy (12, 31). Currently, Eculizumab, inebilizumab, satralizumab, and ravulizumab are approved by the US FDA and the European Medicines Agency (EMA) for the treatment of AQP4-Ab positive NMOSD (35, 36). In China, ravulizumab is not approved for the treatment of NMOSD, whereas eculizumab and satralizumab are relatively expensive and not covered by medical insurance. Inebilizumab is a humanized anti-CD19 monoclonal antibody, while ofatumumab is a fully human anti-CD20 monoclonal antibody (36). Considering that CD19 is more widely expressed in B-lineage cells than CD20, we chose to use ofatumumab initially, which we thought might be safer (37). Although ofatumumab is an off-label drug, its mechanism of action suggests its potential use in the treatment of NMOSD. The efficiency of ofatumumab in patients with NMOSD has been reported in numerous studies (38–41). An observational study also found that patients with chronic lymphocytic leukemia combined with HBV did not experience HBVr after receiving ofatumumab treatment (42). Similarly, after ofatumumab treatment, not only did our patient’s symptoms significantly improve, but also HBVr did not occur. However, we later decided to switch the patient to inebilizumb, as it is the only medication covered by the medical insurance system for NMOSD in China. A recent report by Sadowsky, D. et al. described a case of an NMOSD patient with concurrent HBV and tuberculosis who switched from AZA to inebilizumab; however, neither the efficacy nor the tolerance of inebilizumab was reported (14). In our case, no HBV outbreaks, worsening liver function, or adverse events were observed at the last follow-up. We demonstrated the safety and efficacy of both ofatumumab and inebilizumab in NMOSD patients with HBV infection following the administration of anti-HBV agents and hepatoprotective drugs. However, this case report has certain limitations. Due to the insufficient evidence regarding the treatment of NMOSD patients with concurrent HBV infection and the use of ofatumumab and inebilizumab in such patients, the therapeutic regimen we adopted needs further research and observation. It remains unclear whether the patient’s condition will recur in the future and whether there will be adverse reactions.
In summary, our case suggests that ofatumumab and inebilizumab might serve as an effective and safe alternative for NMOSD patients with concurrent HBV infection. Large-scale studies are needed for further verification.
Patient perspective
The patient conveyed that the entire course has been exceptionally challenging. Due to the initially presenting symptoms of nausea and vomiting and not receiving proper examination and medical consultation, she experienced the consequences of delayed diagnosis and management. Following diagnosis and initial treatment, her condition worsened, leading to frustration and emotional distress. Speech impairment, swallowing difficulties, choking on liquids, and limb weakness intensified her anxiety. However, after starting immunosuppressive therapy, she noticed a gradual improvement in her symptoms. The improvement in symptoms greatly encouraged her. Following the improvement in her breathing ability, she no longer required non-invasive ventilation, this also alleviated some of the financial burdens associated with medical care. The patient was highly satisfied with the treatment efficacy, drug safety, and the care received during hospitalization.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of the Medical School of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LC: Conceptualization, Formal analysis, Methodology, Software, Supervision, Writing – original draft. XL: Conceptualization, Writing – review & editing. HZ: Conceptualization, Writing – review & editing. JL: Conceptualization, Writing – review & editing. DZ: Conceptualization, Supervision, Writing – review & editing. ZH: Conceptualization, Formal analysis, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Open access funded by Helsinki University Library. This work was supported by the National Key Research and Development Program of China (Grant No. 2022YFC2503800), and Clinical Research Incubation Project of West China Hospital of Sichuan University (22HXFH022).
Acknowledgments
We thank the patient and her spouse for agreeing the paper published. We would like to express our sincere gratitude to Ammar T. Abdulaziz for his invaluable contribution to the English editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1351782/full#supplementary-material
Supplementary Figure 1 | The result of anti-aquaporin 4 antibody (Anti-AQP4) and anti-aquaporin 1 antibody (Anti-AQP1) detection in the serum and cerebrospinal fluid (CSF). Anti-AQP4 and Anti-AQP1 antibodies in the serum and CSF of the patient were positive, as tested by the cell-based assay (CBA) method. (A) Anti-AQP4 in serum; (B) Anti-AQP1 in serum; (C) Anti-AQP4 in CSF; (D) Anti-AQP1 in CSF.
Abbreviations
AQP4-Ab, Aquaporin-4 antibody; APS, Area postrema syndrome; AZA, Azathioprine; BS, Brainstem syndrome; CNS, Central nervous system; CSF, Cerebrospinal fluid; EDSS, Expanded Disability Status Scale; FDA, Food and Drug Administration; HBsAg, Hepatitis B surface antigen; HBV, Hepatitis B virus; HBVr, HBV reactivation; NGS, Next-generation sequencing; NMOSD, Neuromyelitis optica spectrum disorder; ON, Optic neuritis; PE, plasmapheresis; TM, Transverse myelitis.
References
1. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology (2015) 85(2):177–89. doi: 10.1212/WNL.0000000000001729
2. Fan M, Qiu W, Bu B, Xu Y, Yang H, Huang D, et al. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm (2020) 7(5):e787. doi: 10.1212/NXI.0000000000000787
3. Liu J, Xu L, Chen ZL, Li M, Yi H, Peng FH. Comprehensive analysis of patients with neuromyelitis optica spectrum disorder (NMOSD) combined with chronic hepatitis B (CHB) infection and seropositive for anti-aquaporin-4 antibody. Bosn J Basic Med Sci (2018) 18(1):35–42. doi: 10.17305/bjbms.2017.2255
4. Anamnart C, Tisavipat N, Owattanapanich W, Apiwattanakul M, Savangned P, Prayoonwiwat N, et al. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: case report and systematic review. Mult Scler Relat Disord (2022) 58:103414. doi: 10.1016/j.msard.2021.103414
5. Francis AG, Elhadd K, Camera V, Ferreira Dos Santos M, Rocchi C, Adib-Samii P, et al. Acute inflammatory diseases of the central nervous system after SARS-CoV-2 vaccination. Neurol Neuroimmunol Neuroinflamm (2023) 10(1):e200063. doi: 10.1212/NXI.0000000000200063
6. Lei J, Wang H. Neuromyelitis optica spectrum disorder with active replication of hepatitis B virus and seropositive anti-aquaporin-4 antibody: A case report. Med (Baltimore) (2021) 100(38):e27207. doi: 10.1097/MD.0000000000027207
7. World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections (2021). Available at: https://www.who.int/publications/i/item/9789240027077 (Accessed 28 December 2021).
8. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ (2019) 97(3):230–8. doi: 10.2471/BLT.18.219469
9. Liu L, Wang L, Zhang H, Ou W, Li D, Feng Y, et al. Changing epidemiology of hepatitis B virus and hepatitis C virus coinfection in a human immunodeficiency virus-positive population in China: results from the third and fourth nationwide molecular epidemiologic surveys. Clin Infect Dis (2021) 73(4):642–9. doi: 10.1093/cid/ciab058
10. Holmøy T, Høglund RA, Illes Z, Myhr KM, Torkildsen Ø Recent progress in maintenance treatment of neuromyelitis optica spectrum disorder. J Neurol (2021) 268(12):4522–36 doi: 10.1007/s00415-020-10235-5
11. Kempinska A, Kwak EJ, Angel JB. Reactivation of hepatitis B infection following allogeneic bone marrow transplantation in a hepatitis B–immune patient: case report and review of the literature. Clin Infect Dis (2005) 41(9):1277–82. doi: 10.1086/496924
12. Gonzalez SA, Perrillo RP. Hepatitis B virus reactivation in the setting of cancer chemotherapy and other immunosuppressive drug therapy. Clin Infect Dis (2016) 62(Suppl 4):S306–13. doi: 10.1093/cid/ciw043
13. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology (1983) 33(11):1444–52. doi: 10.1212/wnl.33.11.1444
14. Sadowsky D, Delijani K, Davis W, Safadi A, Brayo P, Osborne B. Neuromyelitis optica spectrum disorder management in the setting of chronic hepatitis B and latent tuberculosis: A case report. Neurohospitalist (2023) 13(4):361–3. doi: 10.1177/19418744231171464
15. Wingerchuk DM, Lucchinetti CF. Neuromyelitis optica spectrum disorder. N Engl J Med (2022) 387(7):631–9. doi: 10.1056/NEJMra1904655
16. Huda S, Whittam D, Bhojak M, Chamberlain J, Noonan C, Jacob A. Neuromyelitis optica spectrum disorders. Clin Med (Lond) (2019) 19(2):169–76. doi: 10.7861/clinmedicine.19-2-169
17. Zhao S, Chen T, Peng C, Zhou H, Li H, Huang D, et al. The putative acceleration of optic neuritis when combined with chronic hepatitis B. J Neurol Sci (2015) 358(1-2):207–12. doi: 10.1016/j.jns.2015.08.1538
18. Malli C, Pandit L, D’Cunha A, Sudhir A. Helicobacter pylori infection may influence prevalence and disease course in myelin oligodendrocyte glycoprotein antibody associated disorder (MOGAD) similar to MS but not AQP4-IgG associated NMOSD. Front Immunol (2023) 14:1162248. doi: 10.3389/fimmu.2023.1162248
19. Dunalska A, Saramak K, Szejko N. The role of gut microbiome in the pathogenesis of multiple sclerosis and related disorders. Cells (2023) 12(13):1760. doi: 10.3390/cells12131760
20. Xu L, Zhou M, Peng X, Xu Y, Huang F, Wang L, et al. The central nervous system is a potential reservoir and possible origin of drug resistance in hepatitis B infection. J Virus Erad (2023) 9(3):100348. doi: 10.1016/j.jve.2023.100348
21. Bogdanos DP, Smith H, Ma Y, Baum H, Mieli-Vergani G, Vergani D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol (2005) 12(3):217–24. doi: 10.1080/17402520500285247
22. Yimam KK, Merriman RB, Frederick RT. A rare case of acute hepatitis B virus infection causing guillain-barré Syndrome. Gastroenterol Hepatol (2013) 9(2):121–3.
23. Pronier C, Guyader D, Jezequel C, Tattevin P, Thibault V. Contribution of quantitative viral markers to document hepatitis B virus compartmentalization in cerebrospinal fluid during hepatitis B with neuropathies. J Neurovirol (2018) 24(6):769–72. doi: 10.1007/s13365-018-0662-0
24. Stübgen J-P. Neuromuscular disorders associated with hepatitis B virus infection. J Clin Neuromuscul Dis (2011) 13(1):26–37. doi: 10.1097/CND.0b013e3181df2b2b
25. Tsukada N, Koh CS, Inoue A, Yanagisawa N. Demyelinating neuropathy associated with hepatitis B virus infection. Detection of immune complexes composed of hepatitis B virus surface antigen. J Neurol Sci (1987) 77(2-3):203–16. doi: 10.1016/0022-510x(87)90123-7
26. Pao CC, Wu SY, Hung IJ, Ng KT, Liaw YF, Lo SJ. Intra blood-cerebrospinal fluid-barrier detection of hepatitis B virus. Biochem Biophys Res Commun (1987) 146(2):452–5. doi: 10.1016/0006-291x(87)90550-x
27. Inoue J, Ueno Y, Kogure T, Nagasaki F, Kimura O, Obara N, et al. Analysis of the full-length genome of hepatitis B virus in the serum and cerebrospinal fluid of a patient with acute hepatitis B and transverse myelitis. J Clin Virol (2008) 41(4):301–4. doi: 10.1016/j.jcv.2008.01.002
28. Anlar B, Pinar A, Yaşar Anlar F, Engin D, Ustaçelebi S, Kocagöz T, et al. Viral studies in the cerebrospinal fluid in subacute sclerosing panencephalitis. J Infect (2002) 44(3):176–80. doi: 10.1053/jinf.2002.0974
29. Ene L, Duiculescu D, Tardei G, Ruta S, Smith DM, Mehta S, et al. Hepatitis B virus compartmentalization in the cerebrospinal fluid of HIV-infected patients. Clin Microbiol Infect (2015) 21(4):387 e5–8. doi: 10.1016/j.cmi.2014.11.012
30. Mastroeni D, Nolz J, Sekar S, Delvaux E, Serrano G, Cuyugan L, et al. Laser-captured microglia in the Alzheimer’s and Parkinson’s brain reveal unique regional expression profiles and suggest a potential role for hepatitis B in the Alzheimer’s brain. Neurobiol Aging (2018) 63:12–21. doi: 10.1016/j.neurobiolaging.2017.10.019
31. Kim MG, Park SY, Kim EJ, Kim YM, Kim HY, Lee YK, et al. Hepatitis B virus reactivation in a primary central nervous system lymphoma patient following intrathecal rituximab treatment. Acta Haematol (2011) 125(3):121–4. doi: 10.1159/000321792
32. Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive elemen. Proc Natl Acad Sci USA (1986) 83(6):1627–31. doi: 10.1073/pnas.83.6.1627
33. Chow N, Wong D, Lai C-L, Mak L-Y, Fung J, Ma H-T, et al. Effect of antiviral treatment on hepatitis B virus integration and hepatocyte clonal expansion. Clin Infect Dis (2023) 76(3):e801–9. doi: 10.1093/cid/ciac383
34. Wong GL, Yuen BW, Chan HL, Tse YK, Yip TC, Lam KL, et al. Impact of dose and duration of corticosteroid on the risk of hepatitis flare in patients with chronic hepatitis B. Liver Int (2019) 39(2):271–9. doi: 10.1111/liv.13953
35. Frampton JE. Inebilizumab: first approval. Drugs (2020) 80(12):1259–64. doi: 10.1007/s40265-020-01370-4
36. Kümpfel T, Giglhuber K, Aktas O, Ayzenberg I, Bellmann-Strobl J, Häußler V, et al. Update on the diagnosis and treatment of neuromyelitis optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part II: Attack therapy and long-term management. J Neurol (2024) 271(1):141–76. doi: 10.1007/s00415-023-11910-z
37. Graf J, Mares J, Barnett M, Aktas O, Albrecht P, Zamvil SS, et al. Targeting B cells to modify MS, NMOSD, and MOGAD: Part 2. Neurol Neuroimmunol Neuroinflamm (2021) 8(1):e919. doi: 10.1212/NXI.0000000000000919
38. Gou B, Yang P, Feng J, Li Y, Huang G, Shi J, et al. The case report of AQP4 and MOG IgG double positive NMOSD treated with subcutaneous Ofatumumab. J Neuroimmunol (2023) 376:578035. doi: 10.1016/j.jneuroim.2023.578035
39. Zhang W, Jiao Y, Jiao J, Jin M, Peng D. Successful treatment of rituximab-unresponsive elderly-onset neuromyelitis optica spectrum disorder and hypogammaglobulinemia with ofatumumab plus intravenous immunoglobulin therapy in a patient with mutant FCGR3A genotype: A case report. Front Immunol (2022) 13:1047992. doi: 10.3389/fimmu.2022.1047992
40. Maillart E, Renaldo F, Papeix C, Deiva K, Bonheur J, Kwon T, et al. Dramatic efficacy of ofatumumab in refractory pediatric-onset AQP4-IgG neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm (2020) 7(3):e683. doi: 10.1212/NXI.0000000000000683
41. Zhan Y, Zhao M, Li X, Ouyang H, Du C, Chen G, et al. A meaningful exploration of ofatumumab in refractory NMOSD: a case report. Front Immunol (2023) 14:1208017. doi: 10.3389/fimmu.2023.1208017
42. Moren C, Montillo M, Panayiotidis P, Dimou M, Bloor A, Dupuis J, et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: a phase IV, non-interventional, observational study from the European Research Initiative on Chronic Lymphocytic Leukemia. Haematologica (2015) 100(4):511–6. doi: 10.3324/haematol.2014.118158
Keywords: neuromyelitis optica spectrum disorders, hepatitis B virus, ofatumumab, inebilizumab, case report
Citation: Cai L, Liu X, Zhou H, Li J, Zhou D and Hong Z (2024) Case report: Identification of Hepatitis B Virus in the cerebrospinal fluid of neuromyelitis optica spectrum disorders and successful treatment with ofatumumab and inebilizumab. Front. Immunol. 15:1351782. doi: 10.3389/fimmu.2024.1351782
Received: 07 December 2023; Accepted: 24 January 2024;
Published: 15 February 2024.
Edited by:
Hans-Hartmut Peter, Independent researcher, GermanyReviewed by:
Gerson D. Keppeke, Universidad Católica del Norte, ChileMohamed Tharwat Hegazy, Cairo University, Egypt
Copyright © 2024 Cai, Liu, Zhou, Li, Zhou and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Hong, aG9uZ3poZW5nb29nQGFsaXl1bi5jb20=
†ORCID: Hongyu Zhou, orcid.org/0000-0002-7637-1305
Jinmei Li, orcid.org/0000-0002-7411-8269
Dong Zhou, orcid.org/0000-0001-7101-4125
Zhen Hong, orcid.org/0000-0002-0014-6873
 Linjun Cai
Linjun Cai Xu Liu1
Xu Liu1 Hongyu Zhou
Hongyu Zhou Jinmei Li
Jinmei Li Dong Zhou
Dong Zhou Zhen Hong
Zhen Hong