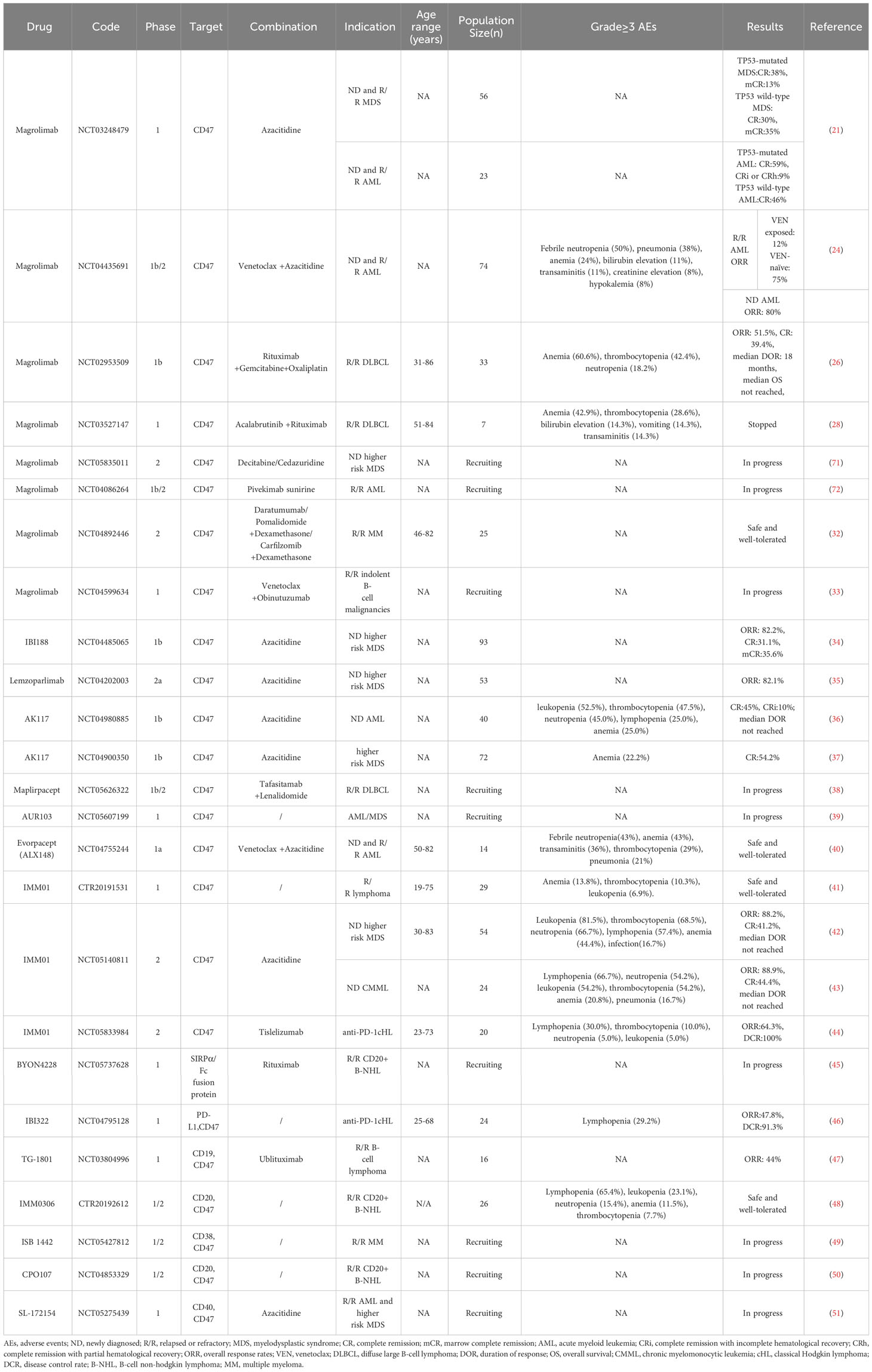
- 1Department of Hematology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Hematology, Dongyang People’s Hospital, Jinhua, China
CD47 is a cell-surface ligand that is overexpressed in various malignancies and that binds to SIRPα on macrophages to promote tumor cell evasion of phagocytosis. Blocking the CD47-SIRPα axis can increase the phagocytosis of macrophages to exert antitumor effects. CD47-based immunotherapy is a current research focus. The combination of anti-CD47 antibodies with other drugs has shown encouraging response rates in patients with hematological tumors, but side effects also occur. Bispecific antibodies and SIRPα/Fc fusion proteins appear to balance the efficacy and safety of treatment. We review the latest clinical research advances and discuss the opportunities and challenges associated with CD47-based immunotherapy for hematological malignancies.
1 Introduction
Hematological malignancies are malignant tumors originating from the lymphatic and hematopoietic systems and are characterized by high malignancy, complex treatment, and poor prognosis. The combination of multiple chemotherapeutic drugs is a classic treatment for patients with hematological malignancies (1). However, due to the strong heterogeneity of molecular characteristics, many patients still suffer relapse and resistance without personalized and precise treatment (2–5). In recent years, tremendous advances in immunotherapy have been observed (6–8). These approaches targeting the adaptive immune system have been widely used for the treatment of various hematological malignancies to improve patient prognosis. In addition, the innate immune system, which serves as the first line of defense against the external environment, plays an important role in cancer cell surveillance and elimination (9, 10). Therapies targeting the innate immune system may offer additional hope for the treatment of hematological malignancies.
CD47, recognized as an innate immune checkpoint protein, is a cell surface ligand overexpressed in various hematological and solid tumor malignancies (11–14). CD47 binds to signal-regulating protein alpha (SIRPα) on macrophages to trigger the “don’t eat me” signal that protects cancer cells from macrophage-mediated phagocytosis (Figure 1A). Blocking the CD47-SIRPα axis can increase the phagocytosis of macrophages to exert antitumor effects (Figure 1B) (15–17). Currently, inhibitors targeting the CD47-SIRPα axis are being developed worldwide, and they are in preclinical and clinical study phases. The combination of anti-CD47 antibodies and other drugs has shown encouraging response rates in patients with hematological tumors, but side effects also occur. Bispecific antibodies and SIRPα/Fc fusion proteins appear to balance the efficacy and safety of treatment. In this article, we review the new developments in CD47-based immunotherapy for hematological malignancies. In addition, we discuss the potential and challenges of targeting the CD47-SIRPα axis in the treatment of hematological malignancies.
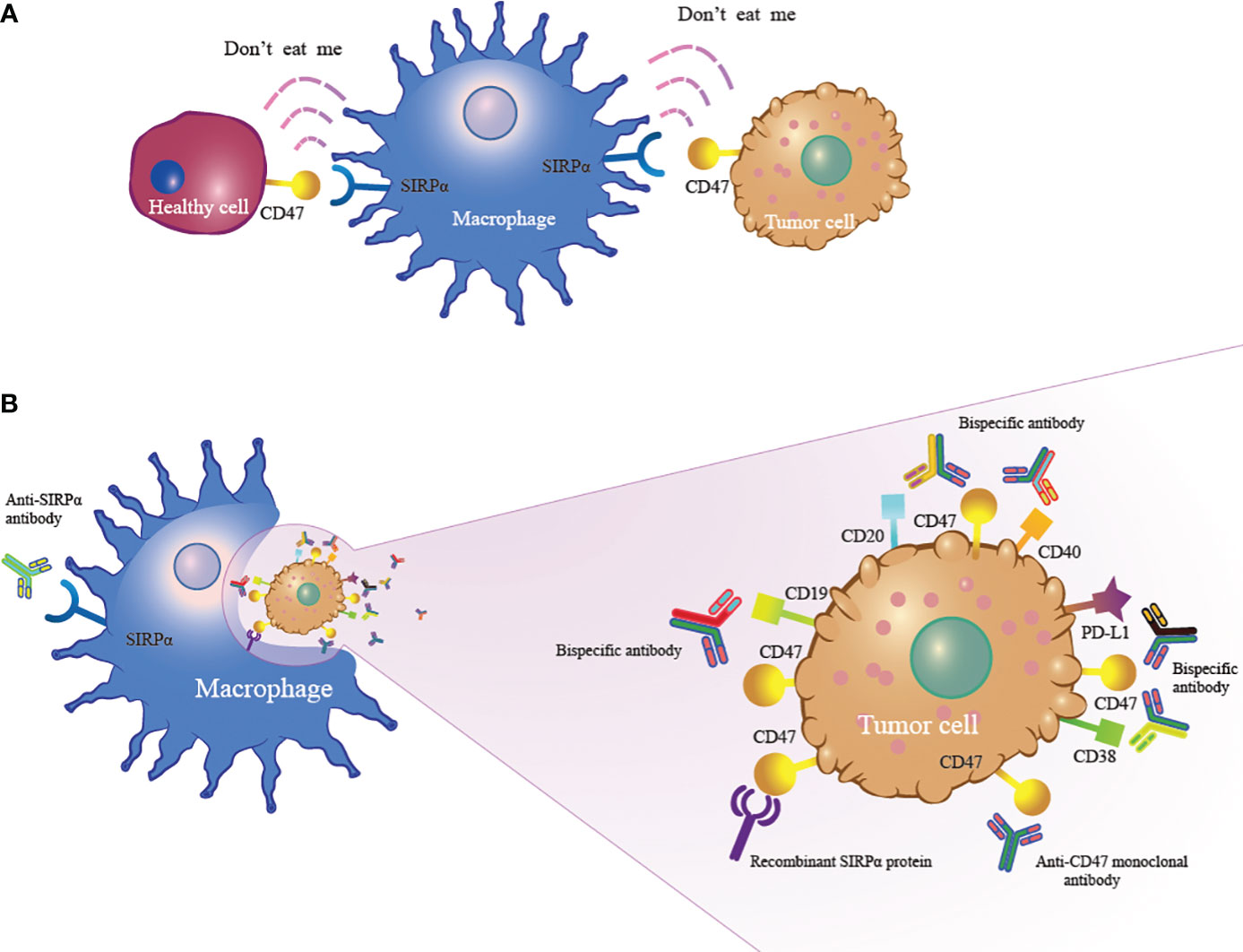
Figure 1 The mechanism of CD47-based immunotherapy. (A) CD47 expressed on tumor cells binds to SIRPα on macrophages to activate the “don’t eat me” signal to enable tumor cells to escape macrophage-mediated phagocytosis. (B) Anti-CD47 monoclonal antibody, anti-SIRPα antibody, recombinant SIRPα protein and bispecific antibody inhibit the CD47-SIRPα interaction, leading to macrophage phagocytosis of tumor cells.
2 CD47 monoclonal antibody
2.1 Magrolimab
Magrolimab, a humanized monoclonal antibody against CD47, is currently being evaluated in several clinical trials for hematological malignancies. A phase 1 trial of magrolimab with azacitidine had meaningful efficacy, with an overall response rate (ORR) of 75% and a complete remission (CR) rate of 33% in patients with higher-risk myelodysplastic syndrome (MDS) (18), while the ORR and CR rates were much lower with the single agent azacitidine in pivotal trials (ORR< 60%, CR rates< 20%) (19, 20). Encouraging results were also observed in TP53-mutated patients receiving combined treatment comprising magrolimab and azacitidine, and the CR and marrow complete remission (mCR) rates of TP53-mutated MDS patients were 38% and 13%, respectively. The CR rate of TP53-mutated acute myeloid leukemia (AML) patients was 59% (21). These results are in good agreement with those of previous clinical trials in which TP53-mutated MDS and AML patients were treated with azacitidine, for which the CR rate was < 22% (22, 23). Magrolimab was shown to decrease the frequency of TP53 mutation alleles in this clinical trial, which led to improved drug response rates. Triple therapy with magrolimab, azacitidine and venetoclax was evaluated in 74 AML patients. This triple combination has an ORR of 75% in patients with relapsed or refractory (R/R) AML previously not treated with venetoclax, with greater responses (ORR 80%) in newly diagnosed (ND) AML patients. However, for patients who were previously exposed to venetoclax, the ORR was only 12%. In this study, 24% of patients (18/74) experienced ≥ Grade 3 anemia, but no anemia-related life-threatening events or deaths occurred (24). However, Gilead Sciences announced the discontinuation of the phase 3 enhance study of magrolimab plus azacitidine in patients with higher-risk MDS in July 2023 suddenly for undisclosed reasons. Gilead Sciences announced that its phase 3 enhance study should be stopped in AML patients with TP53 mutations. Compared with standard of care, magrolimab is unlikely to demonstrate a survival benefit in patients with AML harboring TP53 mutations.
The promising efficacy of magrolimab plus rituximab was shown in R/R non-Hodgkin lymphoma (NHL) patients. Among 15 patients with diffuse large B-cell lymphoma (DLBCL), the ORR and CR rate were 40% and 33%, respectively. Among the 7 patients with follicular lymphoma, the ORR and CR rate were 71% and 43%, respectively. The median duration of response (DOR) was not reached at a median follow-up of 6.2 months and 8.1 months (25). In addition, the combination of magrolimab, rituximab, gemcitabine and oxaliplatin produced encouraging results in R/R DLBCL patients, with an ORR of 51.5% and a CR rate of 39.4% (26). After a median follow-up of 11.3 months, the median DOR was 18 months, and the median overall survival (OS) was not reached. Similar results were observed in a historical study of 196 patients with R/R DLBCL treated with rituximab plus gemcitabine and oxaliplatin (R-GemOx), for which the ORR was 54%. With a median follow-up of 22 months, the median OS was 10 months. However, the CR rate for these patients was only 23% (27). The poor ORR of R/R DLBCL patients receiving combination therapy comprising magrolimab, rituximab and acalabrutinib was 28%, and the study was stopped early due to the lack of significant clinical synergy between the three drugs (28). In addition to tumor cells, erythrocytes also highly express CD47 (29, 30), which leads to accelerated clearance of erythrocytes in patients treated with magrolimab, resulting in severe hemolytic anemia. However, these adverse events were mitigated by administering a lower priming dose of magrolimab (31). Many possibilities have been demonstrated for the use of magrolimab in the treatment of hematological malignancies, and multiple combinations of magrolimab and other drugs are currently undergoing clinical trials (32, 33).
2.2 Letaplimab (IBI188)
Letaplimab is another traditional humanized anti-CD47 monoclonal antibody that has certain antitumor effects but inevitably leads to anemia. For 45 evaluable patients treated with letaplimab and azacitidine in a phase 1b trial, 82.2% of patients achieved an ORR, with 31.1% achieving CR. The incidence of anemia among these patients was 48% (34).
2.3 Lemzoparlimab
Lemzoparlimab is an anti-CD47 antibody screened using human-derived natural bacteriophage technology that can specifically target tumor cells to circumvent hematological adverse events by reducing binding to erythrocytes. Lemzoparlimab is now being tested in ND higher-risk MDS patients in a phase 2a trial (35). Among 28 evaluable patients who received ≥ 3 cycles of treatment with lemzoparlimab and azacitidine, the ORR was 82.1%.
2.4 Ligufalimab (AK117)
Ligufalimab is now being investigated in ND AML patients in a phase 1b trial (36). A total of 40 patients were enrolled and received combination therapy comprising ligufalimab and azacitidine. The most frequently reported Grade ≥ 3 adverse events (AEs) were leukopenia (52.5%), thrombocytopenia (47.5%), neutropenia (45.0%), lymphopenia (25.0%), and anemia (25.0%). Among the 20 evaluable patients, 9 patients achieved CR, and 2 achieved complete remission with incomplete hematological recovery (CRi). After a median follow-up of 6.7 months, the median DOR was not reached. Another phase 1b study conducted from the ligufalimab study conducted in ND high-risk MDS patients showed that among 27 evaluable patients, the CR rate was 48.1%. AK117 was also well tolerated and was associated with a low incidence of anemia in MDS patients, and 22.2% of patients experienced Grade ≥ 3 anemia (37).
2.5 Maplirpacept (PF-07901801)
Maplirpacept is currently being tested in a phase 1b/2 study in patients with R/R DLBCL (38). In phase 1b, researchers will determine the maximum tolerable dose of maplirpacept and determine the doses of tafasitamab and lenalidomide. In phase 2, researchers will explore the objective response of patients receiving this triple combination treatment.
2.6 AUR103
AUR103 is an oral small molecule inhibitor of CD47 and is currently in a phase 1 trial (39). There are currently no publicly available data.
3 SIRPα/Fc fusion protein
3.1 Evorpacept (ALX148)
Evorpacept is a high-affinity CD47-blocking fusion protein with an inactive human immunoglobulin Fc region. It can promote macrophage phagocytosis of tumor cells but has almost no effect on normal blood cells. The results from phase 1a in ND and R/R AML showed that evorpacept in combination with venetoclax and azacitidine was safe and tolerable (40). However, in August 2023, ALX Oncology announced the termination of the recombinant protein ALX148 in MDS and AML due to poor efficacy. This may be because ALX148 engineered with an inactive Fc effector has fewer side effects but attenuates the effect on tumor cells.
3.2 IMM01
IMM01, a recombinant human SIRPα fusion protein, can bind to CD47 on the tumor cell membrane to mediate macrophage phagocytosis of tumor cells. Preclinical data revealed that IMM01 has the unique characteristic of weak human erythrocyte conjugates that prevent severe hemolytic anemia.
The preliminary results of a phase 1 trial showed that IMM01 monotherapy was well tolerated in R/R lymphoma patients, with only four patients (13.8%) experiencing anemia (Grade ≥3) (41). In addition, a phase 2 trial of IMM01 with azacitidine demonstrated its efficacy, with an ORR of 88.2% and a CR rate of 41.2% in patients with ND higher risk MDS. With a median follow-up of 5.6 months, the median DOR was not reached (42). Encouraging results were also observed in ND chronic myelomonocytic leukemia (CMML) patients receiving combined treatment comprising IMM01 and azacitidine; the ORR was 88.9%, and the CR rate was 44.4%. The CR rate increases with increasing treatment time, but the median DOR was not reached (43).
Furthermore, IMM01 can present tumor antigens to T cells through MHC molecules to exert dual antitumor effects. The combination of IMM01 and tislelizumab (an anti-PD-L1 antibody) had synergistic effects on more patients with classic Hodgkin lymphoma (cHL), with an ORR of 64.3% and a disease control rate (DCR) of 100%. IMM01 demonstrated good tolerability and safety among these patients, with no reported hemolytic anemia or hemolysis (44).
3.3 BYON4228
BYON4228 is a novel humanized SIRPα antibody with high specificity for SIRPα that maximizes the antitumor immune response and silences the Fc backbone to reduce toxic effects. BYON4228 is currently in a phase 1 trial to evaluate its safety and efficacy in R/R B-cell NHL (45). No clinical data on BYON4228 have been reported thus far.
4 Bispecific antibody
4.1 IBI322
IBI322, an anti-CD47/PD-L1 bispecific antibody, is highly selective for tumor cells but mitigates the effects of other cells. It enables macrophages to phagocytose lymphoma cells and promotes antitumor cytotoxic T-cell immune responses. A phase 1 study revealed that IBI322 monotherapy was safe and effective for anti-PD-1or PD-L1 treatment-resistant cHL patients. Among the 23 evaluable patients, the ORR and DCR were 47.8% and 91.3%, respectively. Lymphopenia is the most common AE (Grade ≥3) and occurs in approximately 29.2% of patients, while no patients experienced AE-induced discontinuation or death (46).
4.2 TG-1801
TG-1801 is a bispecific antibody designed with one arm blocking CD47 and the other arm binding to CD19 to accurately identify tumor cells. A combination treatment of TG-1801and ublituximab (an anti-CD20 antibody) was evaluated in 16 R/R B-cell lymphoma patients (47). The ORR was 44%, with one patient achieving CR and 6 patients achieving partial response (PR).
4.3 IMM0306
IMM0306 is a bispecific antibody that targets CD47 and CD20, and a higher affinity for CD20 results in a better binding preference to malignant B cells and more effective anti-lymphoma activity. IMM0306 monotherapy therapy is currently in a phase 1/2 trial to evaluate its safety and efficacy in R/R B-cell NHL (48).
Moreover, many CD47-targeted bispecific antibodies that are engineered to specifically target other surface proteins on tumor cells while blocking the CD47-SIRP axis can produce synergistic antitumor effects. These agents are currently in phase 1 trials to evaluate their safety and effectiveness (49–51).
5 Discussion
The CD47-SIRPα axis is a novel antitumor target that has shown promising results in clinical studies for the treatment of hematological malignancies. However, as related studies have progressed, questions about CD47-based immunotherapy have emerged, such as about the limited efficacy of single agent therapy and biosafety issues.
Compared with those designed on a human IgG1 scaffold, anti-CD47 antibiotics engineered on a human IgG4 scaffold can minimize the Fc-dependent effector functions of innate immunity, such as antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) (52, 53). CD47 is widely expressed in normal cells, so many companies have chosen to develop human IgG4-type antibiotics to reduce damage to normal cells, which weakens the antitumor effect of these anti-CD47 antibody monotherapies (53, 54). Therefore, it is necessary to combine an anti-CD47 antibody with other drugs to enhance antitumor activity. The selectivity of anti-CD47 antibodies for tumors depends not only on blocking antiphagocytic signals but also on the extensive expression of prophagocytic signals. Azacitidine is a cytotoxic agent that induces the endogenous expression of cell surface calreticulin in AML and MDS cell lines. Cell surface calreticulin serves as an identified prophagocytic signal that binds to its macrophage receptor, low-density lipoprotein-related protein, resulting in phagocytosis of target cells (55, 56). The combination of azacitidine and magolizumab not only blocks the “don’t eat me” signal but also activates the “eat me” signal, resulting in significantly greater macrophage-mediated cellular phagocytosis of cells than that of cells treated with either drug alone. In addition to cytotoxic agents, other drugs that can induce cell apoptosis, such as the combination of magrolimab, venetoclax and azacitidine, also have synergistic effects on cells treated with anti-CD47 antibodies. Moreover, Dr. Boasman reported that the combination of a Jak inhibitor (ruxolitinib) and an anti-CD47 antibody increased the expression of calreticulin, signaling a much stronger prophagocytic message in cells derived from primary myelofibrosis patients (57). The tumor microenvironment is complex, and in addition to calreticulin, abnormal expression of the regulatory protein macrophage inhibitory factor (MIF) can also have a great impact on the survival of tumor cells (58). MIF, which is associated with most cancers in all stages, can modify the activation, adherence, and phagocytosis of macrophages. In addition to its immunological functions, MIF is considered to play a role in cell proliferation and differentiation. Dr. Li studied the tumor microenvironment of multiple myeloma (MM) patients and reported that one of the significant changes was the reprogramming of macrophages, which results in phagocytic dysfunction (59). An MIF inhibitor can correct this effect. A dual-macrophage-targeted therapeutic strategy involving the combination of an MIF inhibitor and an anti-CD47 antibody activated phagocytosis and repolarized macrophages to a functional phenotype and demonstrated potent antitumor effects in vitro and in vivo. In addition, anti-CD47 antibody-mediated phagocytosis can be enhanced by combination with tumor-targeting antibodies. The anti-CD20 antibody rituximab exerts effects by binding to Fc receptors on natural killer (NK) cells (16). The Fc domain of rituximab provides a potent prophagocytic signal for macrophages by stimulating ADCP. In rituximab-resistant patients, the combination of magrolimab plus rituximab improves antitumor activity through blockade of the antiphagocytic CD47 signaling pathway combined with rituximab-mediated activation of ADCP via the Fc domain (25). Although CD47 has been recognized as an innate immune checkpoint, studies have shown that blockade of the CD47-SIRPα axis can increase the cross-presentation of antigens, leading to adaptive antitumor immune responses initiated and activated by T cells. Thus, T-cell responses could be enhanced by the combination of T-cell checkpoint inhibitors (anti-PD-1 and PD-L1 antibodies) and anti-CD47 antibodies (60). A clinical trial has been initiated to evaluate drugs targeting the CD47-SIRα axis and tislelizumab (an anti-PD-L1 antibody) in lymphoma patients (44).
Specifically, erythrocytes are a significant exception to normal cells, as they express prophagocytic signals in certain environments. Moreover, erythrocytes also highly express CD47, which is involved in the protection against erythrocyte clearance. After receiving anti-CD47 antibodies, senescent erythrocytes acquire CD47 blockade in the presence of enhanced prophagocytic signals, leading to accelerated clearance and ultimately to anemia. This adverse event was mitigated by administering a lower priming dose of magrolimab, which eliminated older erythrocytes and preserved younger erythrocytes lacking prophagocytic signals. Although this procedure still resulted in transient mild anemia, the patient’s anemia was relieved to some extent with a compensatory increase in reticulocytes. Moreover, erythrocytes exposed to the priming dose rapidly shed CD47 from the cell surface through a process called erythrocyte pruning, shielding erythrocytes from the effects of subsequent doses of magrolimab (61, 62). Furthermore, increasing the selectivity of antibodies for tumor cells is an option for reducing anemia. Lemzoparlimab, a novel anti-CD47 antibody, did not cause severe anemia to develop when it mediated the phagocytosis of tumor cells. This is due to glycosylation near the binding epitope on erythrocyte CD47, which “protects” erythrocytes from lemzoparlimab binding. In addition, 82.1% of MDS patients treated with lemzoparlimab achieved an ORR without serious anemia (35). In addition to modifying anti-CD47 antibodies to target a distinct CD47 epitope, recombinant SIRPα can also reduce hematological adverse events. Among these recombinant proteins, ALX 148 and IMM01 are the most promising and are currently being evaluated in clinical studies of hematological malignancies. However, ALX Oncology announced the termination of the recombinant protein ALX148 in MDS and AML due to poor efficacy. Although an inactive Fc effector reduces biosafety issues, it also limits the effectiveness of treatment. In addition, SIRPα is highly expressed on central and peripheral nervous system cells, and some anti-SIRPα antibodies may lead to the loss or dysfunction of nerve cells, resulting in neurological dysfunction (63, 64).
How to avoid accidental injury to normal cells while exerting antitumor effects is the most important problem that needs to be considered in the development of CD47-based immunotherapy in the future. In this situation, many bispecific antibodies have emerged; one arm blocks CD47, while the other arm binds to common cancer antibody targets. In addition, an “imbalanced” design with a decreased binding affinity to CD47 and increased binding affinity to tumor cell surface proteins can retain tumor-specific phagocytic stimulation activity while retaining host cells to limit toxicity. It is crucial to identify and select surface biomarkers for hematological malignancies. In addition, novel drug delivery carriers based on nanoparticles are also good choices because of their small molecular weight, precise targeting, and easy modification (65). Multifunctionalized iron oxide magnetic nanoparticles, which are carriers of anti-CD47 antibodies, not only help to retain their targeting activity but also achieve a short-term increase in delivery to cancer cells, accelerating cancer cell apoptosis (66). Nanoparticles loaded with an anti-CD47 antibody achieved antitumor effects in a 4T1 tumor-bearing mouse model by continuously releasing the antibody to block the CD47-SIRPα axis (67). In addition, identifying the optimal timing and approach for introducing drugs may also lead to greater efficacy and toxicity reduction (68–70). All of these questions require further exploration.
In conclusion, the CD47-SIRPα axis is a promising antitumor target, and multiple CD47-targeted drugs have entered clinical trials. The latest clinical research advances are listed in Table 1. Although there are difficulties in the development of CD47-based immunotherapy for hematological malignancies, such as poor efficacy and hematological side effects, these issues may be solved by the development of bispecific antibodies and the establishment of new drug delivery systems. However, further results are worthy of our anticipation.
Author contributions
YX: Writing – original draft. PJ: Writing – original draft. ZX: Writing – original draft. HY: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LY20H080002), and the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2019KY452).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tang L, Huang Z, Mei H, Hu Y. Immunotherapy in hematologic Malignancies: achievements, challenges and future prospects. Signal Transduction Targeted Ther (2023) 8:306. doi: 10.1038/s41392-023-01521-5
2. Kayser S, Levis MJ. The clinical impact of the molecular landscape of acute myeloid leukemia. Haematologica (2023) 108:308–20. doi: 10.3324/haematol.2022.280801
3. Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of hodgkin lymphoma in the modern era. Br J Haematol (2019) 184:45–59. doi: 10.1111/bjh.15614
4. Ansell SM. Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc (2015) 90:1574–83. doi: 10.1016/j.mayocp.2015.07.005
5. Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-hodgkin lymphoma. Lancet (London England) (2017) 390:298–310. doi: 10.1016/s0140-6736(16)32407-2
6. Kansara RR, Speziali C. Immunotherapy in hematologic Malignancies. Curr Oncol (Toronto Ont) (2020) 27:S124–s31. doi: 10.3747/co.27.5117
7. Craddock C, Friedberg JW. Immunotherapy for hematologic Malignancies. J Clin Oncol (2021) 39:343–5. doi: 10.1200/jco.20.03106
8. Noh JY, Seo H, Lee J, Jung H. Immunotherapy in hematologic Malignancies: emerging therapies and novel approaches. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21218000
9. Yi T, Li J, Chen H, Wu J, An J, Xu Y, et al. Splenic dendritic cells survey red blood cells for missing self-cd47 to trigger adaptive immune responses. Immunity (2015) 43:764–75. doi: 10.1016/j.immuni.2015.08.021
10. Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer (2019) 19:568–86. doi: 10.1038/s41568-019-0183-z
11. Bian HT, Shen YW, Zhou YD, Nagle DG, Guan YY, Zhang WD, et al. Cd47: beyond an immune checkpoint in cancer treatment. Biochim Biophys Acta Rev Cancer (2022) 1877:188771. doi: 10.1016/j.bbcan.2022.188771
12. Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: A 50-kd plasma membrane antigen physically and functionally associated with integrins. J Cell Biol (1990) 111:2785–94. doi: 10.1083/jcb.111.6.2785
13. Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (Cd47). J Cell Sci (1995) 108:3419–25. doi: 10.1242/jcs.108.11.3419
14. Maute R, Xu J, Weissman IL. Cd47-sirpα-targeted therapeutics: status and prospects. Immuno-oncol Technol (2022) 13:100070. doi: 10.1016/j.iotech.2022.100070
15. Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, et al. Therapeutic antibody targeting of cd47 eliminates human acute lymphoblastic leukemia. Cancer Res (2011) 71:1374–84. doi: 10.1158/0008-5472.Can-10-2238
16. Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-cd47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-hodgkin lymphoma. Cell (2010) 142:699–713. doi: 10.1016/j.cell.2010.07.044
17. Yang H, Xun Y, You H. The landscape overview of cd47-based immunotherapy for hematological Malignancies. biomark Res (2023) 11:15. doi: 10.1186/s40364-023-00456-x
18. Sallman DA, Al Malki MM, Asch AS, Wang ES, Jurcic JG, Bradley TJ, et al. Magrolimab in combination with azacitidine in patients with higher-risk myelodysplastic syndromes: final results of a phase Ib study. J Clin Oncol (2023) 41:2815–26. doi: 10.1200/jco.22.01794
19. Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the cancer and leukemia group B. J Clin Oncol (2006) 24:3895–903. doi: 10.1200/jco.2005.05.4346
20. Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol (2002) 20:2429–40. doi: 10.1200/jco.2002.04.117
21. Johnson L, Zhang Y, Li B, Aviles L, Lal I, Ramsingh G, et al. Nature of clinical response and depth of molecular response in patients with tp53 mutant myelodysplastic syndromes (Mds) and acute myeloid leukemia (Aml) treated with magrolimab with azacitidine. Blood (2022) 140:6934–5. doi: 10.1182/blood-2022-160337
22. Bally C, Adès L, Renneville A, Sebert M, Eclache V, Preudhomme C, et al. Prognostic value of tp53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leukemia Res (2014) 38:751–5. doi: 10.1016/j.leukres.2014.03.012
23. Bories P, Prade N, Lagarde S, Cabarrou B, Largeaud L, Plenecassagnes J, et al. Impact of tp53 mutations in acute myeloid leukemia patients treated with azacitidine. PloS One (2020) 15:e0238795. doi: 10.1371/journal.pone.0238795
24. Daver N, Senapati J, Maiti A, Loghavi S, Kadia TM, DiNardo CD, et al. Phase I/ii study of azacitidine (Aza) with venetoclax (Ven) and magrolimab (Magro) in patients (Pts) with newly diagnosed (Nd) older/unfit or high-risk acute myeloid leukemia (Aml) and relapsed/refractory (R/R) aml. Blood (2022) 140:141–4. doi: 10.1182/blood-2022-170188
25. Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. Cd47 blockade by hu5f9-G4 and rituximab in non-hodgkin's lymphoma. New Engl J Med (2018) 379:1711–21. doi: 10.1056/NEJMoa1807315
26. Maakaron J, Asch AS, Popplewell LL, Collins GP, Flinn IW, Ghosh N, et al. Magrolimab in combination with rituximab + Chemotherapy in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (Dlbcl). Blood (2022) 140:3728–30. doi: 10.1182/blood-2022-167772
27. Cazelles C, Belhadj K, Vellemans H, Camus V, Copie C, Veresezan L, et al. Rituximab plus gemcitabine and oxaliplatin (R-gemox) in refractory/relapsed (R/R) dlbcl. A real life study in patients ineligible for autologous transplantation. Blood (2019) 134:4115–. doi: 10.1182/blood-2019-124143
28. de Vos S, Reagan PM, Patel MR, Saba NS, Mortlock A, Cerec V, et al. Magrolimab, rituximab and acalabrutinib for relapsed/refractory (R/R) diffuse large B-cell lymphoma (Dlbcl): results from the phase 1 prism trial. Blood (2022) 140:6635–7. doi: 10.1182/blood-2022-162447
29. Khandelwal S, van Rooijen N, Saxena RK. Reduced expression of cd47 during murine red blood cell (Rbc) senescence and its role in rbc clearance from the circulation. Transfusion (2007) 47:1725–32. doi: 10.1111/j.1537-2995.2007.01348.x
30. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of cd47 as a marker of self on red blood cells. Sci (New York NY) (2000) 288:2051–4. doi: 10.1126/science.288.5473.2051
31. Chao MP, Takimoto CH, Feng DD, McKenna K, Gip P, Liu J, et al. Therapeutic targeting of the macrophage immune checkpoint cd47 in myeloid Malignancies. Front Oncol (2019) 9:1380. doi: 10.3389/fonc.2019.01380
32. Paul B, Minarík J, Cottini F, Gasparetto C, Khouri J, Gandhi M, et al. Safety and tolerability of magrolimab combinations in patients with relapsed/refractory multiple myeloma (Rrmm): safety run-in results from a phase 2 study. Blood (2023) 142:3383–. doi: 10.1182/blood-2023-186997
33. Lakhotia R, Melani C, Pittaluga S, Phelan JD, Pradhan A, Tadese A, et al. Phase I study of response-adapted treatment with venetoclax, obinutuzumab, and magrolimab (Venom) in relapsed and refractory indolent B-cell Malignancies. Blood (2023) 142:6172–. doi: 10.1182/blood-2023-178766
34. Miao M, Wu D, Xie S, Hong M, Chang C, Yu L, et al. A phase 1b study to evaluate safety and efficacy of ibi188 in combination with azacitidine (Aza) as a first-line treatment in subjects with newly diagnosed higher risk myelodysplastic syndrome. Blood (2022) 140:4045–6. doi: 10.1182/blood-2022-155901
35. Xiao Z, Chang C, Li Q, Yan X, Qin T, Tong H, et al. Molecular biomarker analyses for exploring the therapeutic mechanism of lemzoparlimab and azacitidine (Aza) in newly diagnosed higher risk myelodysplastic syndrome (Hr-mds). Blood (2022) 140:8821–2. doi: 10.1182/blood-2022-163880
36. Wang H, Zhang Q, Teng Q, Li Z, Liu H, Wang ZM, et al. A phase 1b study evaluating the safety and efficacy of ak117 (Anti-cd47 monoclonal antibody) in combination with azacitidine in patients with treatment-naïve acute myeloid leukemia. Blood (2023) 142:4280–. doi: 10.1182/blood-2023-180618
37. Miao M, Teng Q, Wu D, Jiang Z, Jiang S, Li F, et al. Ak117 (Anti-cd47 monoclonal antibody) in combination with azacitidine for newly diagnosed higher risk myelodysplastic syndrome (Hr-mds): ak117-103 phase 1b results. Blood (2023) 142:1865–. doi: 10.1182/blood-2023-179099
38. Hoyle M, Paccagnella L. A multicenter, open-label, phase 1b/2 study to evaluate the effects of maplirpacept in combination with tafasitamab and lenalidomide in people with relapsed or refractory diffuse large B-cell lymphoma. Blood (2023) 142:6249–. doi: 10.1182/blood-2023-181202
39. Krishnappa M, Mandavia D, Jain M, Mukesh S, Patel A, Agarwal A, et al. Pk, pd and safety of first-in-human, first-in-class phase I trial (Aur103-101; bharat) of aur103, an oral cd47 inhibitor, in patients with advanced Malignancies. EHA Congress (2023). doi: 10.1097/01.HS9.0000969864.18372.7f.
40. Garcia-Manero G, Przespolewski A, Abaza Y, Byrne M, Fong AP, Jin F, et al. Evorpacept (Alx148), a cd47-blocking myeloid checkpoint inhibitor, in combination with azacitidine and venetoclax in patients with acute myeloid leukemia (Aspen-05): results from phase 1a dose escalation part. Blood (2022) 140:9046–7. doi: 10.1182/blood-2022-157606
41. Qi J, Sun M, Ji D, Zhou P, Xing S, Huang C, et al. A first-in-human phase I dose escalation study of imm01, sirpα Fc protein in patients with relapsed or refractory lymphoma. Blood (2022) 140:3651–2. doi: 10.1182/blood-2022-158359
42. Yang W, Gao S, Yan X, Guo R, Han L, Li F, et al. Preliminary results of a phase 2 study of imm01 combined with azacitidine (Aza) as the first-line treatment in adult patients with higher risk myelodysplastic syndromes (Mds). Blood (2023) 142:320–. doi: 10.1182/blood-2023-174420
43. Tong H, Gao S, Yang W, Li J, Yin Q, Zhao X, et al. Preliminary results of a phase 2 study of imm01 combined with azacitidine (Aza) as the first-line treatment in adult patients with chronic myelomonocytic leukemia (Cmml). Blood (2023) 142:1859–. doi: 10.1182/blood-2023-181501
44. Zhou K, Song Y, Yi T, Hou S, Liu X, Lin N, et al. Imm01 plus tislelizumab in prior anti-pd-1 failed classic hodgkin lymphoma: an open label, multicenter, phase 2 study (Imm01-04) evaluating safety as well as preliminary anti-tumor activity. Blood (2023) 142:609–. doi: 10.1182/blood-2023-174829
45. Klaassen W, van den Berg TK, van den Tweel E, Hooren HV, Schellens J. First-in-human dose-escalation and -expansion trial with sirpα Antibody byon4228 in R/R B-cell nhl. Blood (2023) 142:3144–. doi: 10.1182/blood-2023-177895
46. Zhang H, Yu J, Li H, Qian W, Xiao X, Cai Q, et al. Cd47/pd-L1 bispecific antibody (Ibi322) in anti-pd-1 or pd-L1 treatment-resistant classical hodgkin lymphoma: A phase I study. EHA Congress (2023). doi: 10.1097/01.HS9.0000967776.81028.41.
47. Hawkes E, Lewis KL, Wong Doo N, Patil SS, Miskin HP, Sportelli P, et al. First-in-human (Fih) study of the fully-human kappa-lambda cd19/cd47 bispecific antibody tg-1801 in patients (Pts) with B-cell lymphoma. Blood (2022) 140:6599–601. doi: 10.1182/blood-2022-169171
48. Shi Y, Song Y, Zhang M, Jing H, Wang Z, Li Z, et al. Preliminary safety and efficacy evaluation of imm0306, a cd47 and cd20 bispecific monoclonal antibody-trap (Mab-trap), from an ongoing phase I dose-escalation study in patients with relapsed or refractory B-cell non- hodgkin's lymphoma (R/R B-nhl). Blood (2022) 140:9323–4. doi: 10.1182/blood-2022-157862
49. Kazandjian D, Quach H, Sia H, Ho PJ, Spencer A, Schroeder MA, et al. Initial dose escalation of isb 1442, a novel cd38 biparatopic X cd47 bispecific antibody, in patients with relapsed / refractory multiple myeloma (Rrmm). Blood (2023) 142:4707–. doi: 10.1182/blood-2023-186241
50. Skarbnik AP, Chen FL, Mountjoy L, Myint ZW, Young PA, Whiteley AR, et al. Trial in progress: first report of the phase 1/2 study of the safety and efficacy of cpo107, a bispecific agent targeting cd20/cd47 in cd20 expressing non-hodgkin lymphoma (Nhl). Blood (2022) 140:12059–60. doi: 10.1182/blood-2022-164746
51. Daver N, Stein AS, Bixby D, Chai-Ho W, Zeidner JF, Maher K, et al. Safety, pharmacodynamic, and anti-tumor activity of sl-172154 as monotherapy and in combination with azacitidine (Aza) in relapsed/refractory (R/R) acute myeloid leukemia (Aml) and higher-risk myelodysplastic syndromes/neoplasms (Hr-mds) patients (Pts). Blood (2023) 142:4278–. doi: 10.1182/blood-2023-173991
52. Xu B, Tian L, Chen J, Wang J, Ma R, Dong W, et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma. Nat Commun (2021) 12:5908. doi: 10.1038/s41467-021-26003-6
53. Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-clinical development of a humanized anti-cd47 antibody with anti-cancer therapeutic potential. PloS One (2015) 10:e0137345. doi: 10.1371/journal.pone.0137345
54. Pietsch EC, Dong J, Cardoso R, Zhang X, Chin D, Hawkins R, et al. Anti-leukemic activity and tolerability of anti-human cd47 monoclonal antibodies. Blood Cancer J (2017) 7:e536. doi: 10.1038/bcj.2017.7
55. Matsusaka K, Azuma Y, Kaga Y, Uchida S, Takebayashi Y, Tsuyama T, et al. Distinct roles in phagocytosis of the early and late increases of cell surface calreticulin induced by oxaliplatin. Biochem Biophysics Rep (2022) 29:101222. doi: 10.1016/j.bbrep.2022.101222
56. Luo JQ, Liu R, Chen FM, Zhang JY, Zheng SJ, Shao D, et al. Nanoparticle-mediated cd47-sirpα Blockade and calreticulin exposure for improved cancer chemo-immunotherapy. ACS Nano (2023) 17:8966-79. doi: 10.1021/acsnano.2c08240
57. Boasman K, Simmonds MJ, Rinaldi CR. Combination of ruxolitinib and magrolimab significantly increases calreticulin expression in myelofibrosis cd34+ Cells in vitro. Proof of concept for combination therapy. Blood (2022) 140:12165–6. doi: 10.1182/blood-2022-163769
58. Javeed A, Zhao Y, Zhao Y. Macrophage-migration inhibitory factor: role in inflammatory diseases and graft rejection. Inflammation Res (2008) 57:45–50. doi: 10.1007/s00011-007-7110-6
59. Li J, Yang Y, Wang W, Xu J, Liu P. Single-cell analyses of immune microenvironment informs potential benefits of dual macrophage-targeted therapy in multiple myeloma. Blood (2022) 140:4255–6. doi: 10.1182/blood-2022-166274
60. Younes A, Ansell S, Fowler N, Wilson W, de Vos S, Seymour J, et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol (2017) 14:335–46. doi: 10.1038/nrclinonc.2016.205
61. Chen JY, McKenna KM, Choi TS, Duan J, Brown L, Stewart JJ, et al. Rbc-specific cd47 pruning confers protection and underlies the transient anemia in patients treated with anti-cd47 antibody 5f9. Blood (2018) 132:2327–. doi: 10.1182/blood-2018-99-115674
62. Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, et al. First-in-human, first-in-class phase I trial of the anti-cd47 antibody hu5f9-G4 in patients with advanced cancers. J Clin Oncol (2019) 37:946–53. doi: 10.1200/jco.18.02018
63. Shui M, Sun Y, Lin D, Xue Z, Liu J, Wu A, et al. Anomalous levels of cd47/signal regulatory protein alpha in the hippocampus lead to excess microglial engulfment in mouse model of perioperative neurocognitive disorders. Front Neurosci (2022) 16:788675. doi: 10.3389/fnins.2022.788675
64. Ding X, Wang J, Huang M, Chen Z, Liu J, Zhang Q, et al. Loss of microglial sirpα Promotes synaptic pruning in preclinical models of neurodegeneration. Nat Commun (2021) 12:2030. doi: 10.1038/s41467-021-22301-1
65. Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the cd47/sirpα Axis. Front Immunol (2020) 11:18. doi: 10.3389/fimmu.2020.00018
66. Trabulo S, Aires A, Aicher A, Heeschen C, Cortajarena AL. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim Biophys Acta Gen Subj (2017) 1861:1597–605. doi: 10.1016/j.bbagen.2017.01.035
67. Huang L, Zhang Y, Li Y, Meng F, Li H, Zhang H, et al. Time-programmed delivery of sorafenib and anti-cd47 antibody via a double-layer-gel matrix for postsurgical treatment of breast cancer. Nano-micro Lett (2021) 13:141. doi: 10.1007/s40820-021-00647-x
68. Ko YJ, Lee JW, Kim H, Cho E, Yang Y, Kim IS, et al. Versatile activatable vsirpα-probe for cancer-targeted imaging and macrophage-mediated phagocytosis of cancer cells. J Controlled Release (2020) 323:376–86. doi: 10.1016/j.jconrel.2020.04.037
69. Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: A nonrandomized, open-label, phase ii study. Cancer Discovery (2019) 9:370–83. doi: 10.1158/2159-8290.Cd-18-0774
70. Ye L, Lv W, He W, Li S, Min Z, Gong L, et al. Reduced Malignant Glioblastoma Recurrence Post-Resection through the Anti-Cd47 Antibody and Temozolomide Co-Embedded in-Situ Hydrogel System. J Controlled Release (2023) 359:224–33. doi: 10.1016/j.jconrel.2023.05.046
71. Zeidan AM, Mosher K, Souza S, Mirakhur B, Keer HN, Taylor JA. Phase 2 study of oral decitabine/cedazuridine in combination with magrolimab for previously untreated subjects with intermediate to very high-risk myelodysplastic syndromes (Mds). Blood (2022) 140:9779–80. doi: 10.1182/blood-2022-158276
72. Daver N, Montesinos P, Aribi A, Martinelli G, Wang E, Altman J, et al. Trial in progress: phase 1b/2 study of pivekimab sunirine (Pvek, imgn632) in combination with venetoclax/azacitidine or magrolimab for patients with cd123-positive acute myeloid leukemia (Aml). EHA Congress (2023). doi: 10.1200/JCO.2023.41.16_suppl.TPS7073.
Keywords: cd47, anti-CD47 antibody, immunotherapy, hematological malignancies, magrolimab
Citation: Xu Y, Jiang P, Xu Z and Ye H (2024) Opportunities and challenges for anti-CD47 antibodies in hematological malignancies. Front. Immunol. 15:1348852. doi: 10.3389/fimmu.2024.1348852
Received: 03 December 2023; Accepted: 07 February 2024;
Published: 23 February 2024.
Edited by:
Si Zhang, Fudan University, ChinaReviewed by:
Yucai Wang, Mayo Clinic, United StatesCopyright © 2024 Xu, Jiang, Xu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haige Ye, aGFpZ2V5ZUB3emhvc3BpdGFsLmNu
 Yilan Xu1
Yilan Xu1 Haige Ye
Haige Ye