- College of Animal Science and Technology, Yangzhou University, Yangzhou, China
Introduction: This study aimed to evaluate the efficiency of tea polyphenols (TP) and medicinal plant mixtures (Astragalus membranaceus + Lonicera japonica, Rheum officinale Bail + Scutellaria baicalensis + Platycladus orientalis) combined with astaxanthin (AST), benzoic acid (BA), and yeast complex on the health status of Eriocheir sinensis.
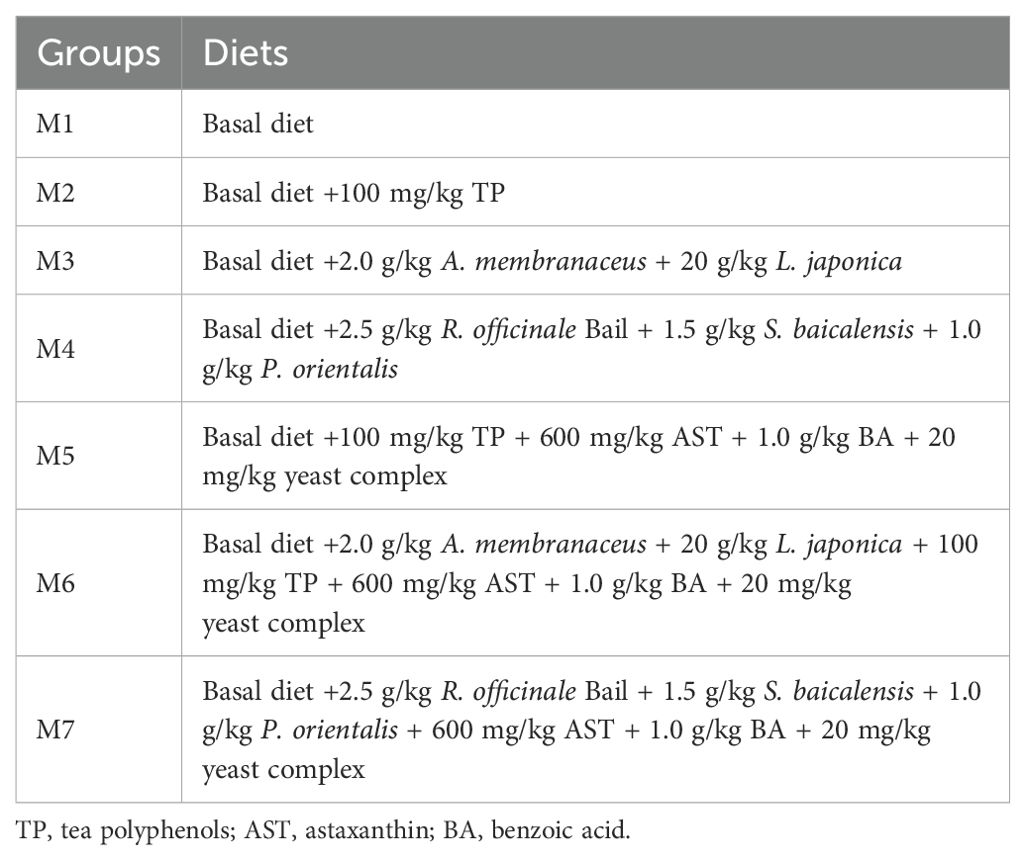
Method: A total of 630 crabs (male crabs: 41.51 ± 1.63 g; female crabs: 47.27 ± 0.79 g) were randomly distributed into seven groups with three replicates (male: female, 1:1). These crabs were fed as follows for 8 weeks: basal diet (M1), M2 (M1 + 100 mg/kg TP), M3 (M1 + 2.0 g/kg A. membranaceus + 20 g/kg L. japonica), M4 (M1 + 2.5 g/kg R. officinale Bail + 1.5 g/kg S. baicalensis + 1.0 g/kg P. orientalis), and M5, M6, M7 (M2, M3 and M4 with 600 mg/kg AST +1.0 g/kg BA + 20 mg/kg yeast complex added, respectively).
Results and discussion: The results showed that the activities of acid phosphatase (ACP), alkaline phosphatase (AKP), and lysosome (LZM) in the hemolymph were significantly increased in M5, M6, and M7 (P < 0.05), and the highest phagocytosis index (PI) and LZM activity were observed in M7 of female crabs. Moreover, the antioxidant indicators superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GPx), and catalase (CAT) of hepatopancreas were also significantly improved in M5, M6, and M7 (P < 0.05), while the malondialdehyde (MDA) contents showed an opposite trend. Furthermore, a morphological examination also showed the improved histological structure of hepatopancreas in M7, especially as seen in the clear lumens, no vacuolation, and integrity of the basal membrane of the hepatopancreatic tubule. Taken together, these results suggested that 2.5 g/kg R. officinale Bail, 1.5 g/kg S. baicalensis, and 1.0 g/kg P. orientalis in combination with 600 mg/kg AST, 1.0 g/kg BA, and 20 mg/kg yeast complex could improve the non-specific immunity, antioxidant capacity, and hepatopancreatic health of E. sinensis.
1 Introduction
The Chinese mitten crab (Eriocheir sinensis) is a commercially important freshwater species in China. In 2023, the production of E. sinensis reached 888,629 tons in China (1). However, the expansion of the E. sinensis industry has resulted in frequent disease outbreaks due to intensive aquaculture practices, leading to impaired immunity and poor oxidative stress response (2). Consequently, the application of specialized antioxidants and immunostimulants is required to boost antioxidant capacity and immune response and prevent pathogenic infections.
E. sinensis relies primarily on innate immunity, including cellular and humoral immune responses (3). Therein, hemocytes play a critical role in cellular immunity (4). The total hemocyte count (THC) and phagocytosis index (PI) are important parameters for assessing the cellular defense in crustaceans (5, 6). Moreover, enzymes such as alkaline phosphatase (AKP), acid phosphatase (ACP), and lysosome (LZM) are specific indices for evaluating the humoral immunity responses of E. sinensis as they participate in eliminating and hydrolyzing pathogen microorganisms (7–9). Additionally, the antioxidant capacity of the hepatopancreas also reflects the health status of E. sinensis (10). The total antioxidant capacity (T-AOC), catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), and glutathione peroxidase (GPx), together with the contents of malondialdehyde (MDA), are commonly used as major indicators for the evaluation of antioxidant capacity and disease surveillance in the hepatopancreas of E. sinensis (11–13). These enzymes and indicators play defensive roles against invading diseases and in detoxification in the antioxidant system.
Medicinal plants and their extracts have been used as promising alternatives to antibiotics and immunoprophylactics owing to their high efficacy, low toxicity, and environmentally friendly nature (14). Currently, studies have investigated the application of herbal extracts as feed additives in aquaculture, aimed at promoting growth, modulating immunity, and preventing disease in aquatic animals (15–18). Tea polyphenols (TP), the polyhydroxy phenolic compounds that are extracted from green tea, have been reported to enhance the immune response in fish species (19–21). In addition, Chinese medicinal plants, such as Astragalus membranaceus, Rheum officinale Bail, or their extracts also have been used as dietary supplements to enhance the immune system and antioxidant capacity of aquatic animals (22–24). Ardo et al. reported that A. membranaceus and L. japonica extracts (0.1%) enhanced the immunity of Nile tilapia (Oreochromis niloticus) (22). In addition, the anthraquinones extracted (0.1%-0.2%) from Rheum officinale Bail and Scutellaria polysaccharide (150 mg/kg) have also been demonstrated to enhance the immunity and antioxidant capacity of giant freshwater prawn (Macrobrachium rosenbergii) (23, 24). Apart from a single supplement of herb or herbal extract, the herbal mixture containing more than two species or combined with commercial products has been shown to boost the immune response of hosts. In the research by Abarike et al., it has been revealed that the combination medicinal plants (A. membranaceus, Angelica sinensis, Crataegus hupehensis) (5 g/kg) with commercial probiotic Bacillus (5 g/kg) was more effective than each separately in improving immunity in Nile tilapia (25), which suggested that the combination of herbs and probiotic provided a synergistic effect on the immune response.
Astaxanthin (AST), an oxidized form of β-carotene, is abundant in the carapace of crustaceans and marine environments (26). Studies have reported that dietary AST has benefits for E. sinensis, including non-specific immunity and antioxidant capability (11, 27, 28). Benzoic acid (BA) (0.1%) also has been used as a growth promotor in Nile tilapia (29). Moreover, it has been reported that yeast can act as an immunostimulant and antioxidant in shrimp aquaculture because of its nutritional value (30). Zhang et al. demonstrated that dietary yeast extract (5 g/kg) improved the immunity and antioxidant status of E. sinensis (31). However, the combined effect of AST, BA, and yeast complex on the antioxidant and immune response of E. sinensis has not been reported. Therefore, developing a feed additive with an optimal combination and dosage of medicinal plants and immunostimulants is essential to maintain the health of E. sinensis. In the present study, we designed six different combinations of medicinal plant mixtures with or without AST, BA, and yeast complex to evaluate the optimal formulation of feed additive for E. sinensis health, which is characterized by the innate immunity, antioxidant capacity, and hepatopancreatic histomorphology of E. sinensis. This study will provide a theoretical foundation for using medicinal plants as feed additives for E. sinensis aquaculture.
2 Materials and methods
2.1 Experimental diets
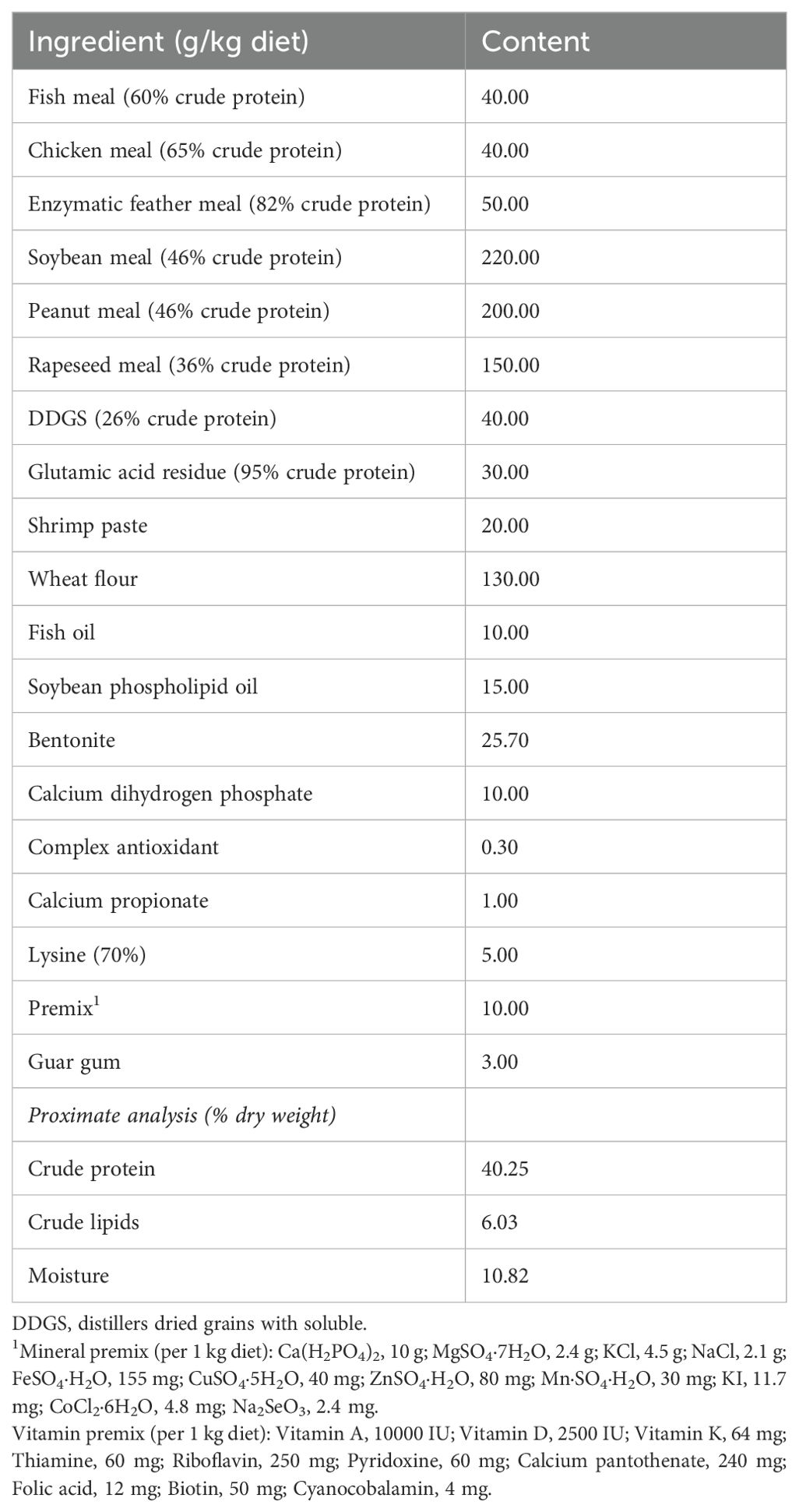
The formulation of the basal diet (M1) is shown in Table 1. Soybean meal, peanut meal, and rapeseed meal were chosen as the primary protein sources. Fish oil, shrimp paste, and soybean phospholipid oil were chosen as the primary lipid sources. The proximate composition of the basal diet was analyzed according to the method of Xu et al. (32). Three experimental diets, M2, M3, and M4, were produced by adding 100 mg/kg TP, 2.0 g/kg A. membranaceus + 20 g/kg L. japonica, and 2.5 g/kg R. officinale Bail + 1.5 g/kg S. baicalensis + 1.0 g/kg P. orientalis, respectively. Then, 600 mg/kg AST + 1.0 g/kg BA + 20 mg/kg yeast complex were added to M2, M3, and M4 diets to produce another three experimental diets named M5, M6, and M7, respectively (Table 2). All medicinal plants were supplied by Zhixin Pharmaceutical Co., Ltd., Nanjing, China. AST (purity > 98%) and BA (purity > 98%) were purchased from Yuanye Biotechnology, Shanghai, China. Yeast complex (also named as yeast extracts) was purchased from Angel Yeast Co., Ltd., Yichang, China.
Diets were prepared using the method described by Li et al. (33). All dry ingredients were finely ground using a pulverizer (HK820, Xulang Machinery Manufacturing Co., Ltd., Guangzhou, China) and then passed through a 100-mesh sieve. After thoroughly mixing the dry ingredients, the fish oil, soybean phospholipid oil, and distilled water were added to form a dough, and subsequently extruded into 1.5 mm diameter pellets by a feed mill (F-26(II), South China University of Technology Science and Technology Industrial Plant, Guangzhou, China). All diets were dried at 50°C and stored at -20°C.
2.2 Experimental animal and conditions
Healthy E. sinensis was provided by Jiangsu Haorun Group, Taizhou, China. Prior to beginning the feeding trial, crabs were fed the basal diet three times a day for 2 weeks. Then, 315 male crabs (initial weight: 41.51 ± 1.63 g) and 315 female crabs (initial weight of: 47.27 ± 0.79 g) in the same molting cycle were stored individually in each bucket (0.48 × 0.38 × 0.36 m, L: W: H) to prevent them from fighting each other, and then randomly divided into 7 groups. Each group has three replicates with 30 individuals per replicate (male: female, 1:1). The 30 buckets of each replicate were arranged along the sides of outdoor cement ponds (5.0 × 5.0 m, L: W).
The crabs were fed with experimental diets at a ratio of 2% of their average body weight twice daily (7:30, 17:00) for 8 weeks. The water temperature was 26 ± 2°C, the dissolved oxygen concentration was above 6.7-7.2 mg/L, and nitrite-N and total ammonia nitrogen were below 0.09 mg/L and 0.3 mg/L, respectively.
2.3 Samples collection and analysis
After the 8-week feeding trial, the crabs were sampled for hemolymph and hepatopancreas after 24 h fasting. The hemolymph sampling used the method Miao et al. described (34). Briefly, one part of the hemolymph samples was collected using a 1.0 mL syringe without anticoagulants from the base of the third pereiopod, stored at 4°C overnight, and centrifuged at 8000 r/min for 10 min. Then the serum was collected to analyze the activities of immune-related enzymes, including AKP, ACP, and LZM. Another part of the hemolymph samples was collected using a syringe with an anticoagulant solution (volume 1:1) for the total hemocyte count (THC) and phagocytic index (PI) determination.
One part of the hepatopancreas samples was obtained for antioxidant indices determination and preserved in Trizol regent for RNA extraction. Another part of hepatopancreas samples was stored in Bouin’s fixative for histology analysis.
The THC was determined according to the method described by Zhao et al. (35). Briefly, the uncoagulated hemolymph was fixed with fixative solution (volume 1:1, ingredients: 0.10 M sodium cacodylate and 1.5% glutaraldehyde) for 30 min to measure THC using a hemocytometer under an optical microscope (Olympus DP26, Japan). The total number of hemocytes in the square of the four corners was counted.
Cell density = (total of 4 large cells/4) ×105 cells/L
The phagocytosis index (PI) was determined according to the method used by Zhang et al. (36). The uncoagulated hemolymph was diluted saline (volume 1:1). The phagocytic activity was measured by mixing the Staphylococcus aureus suspension with hemolymph at a ratio of 10:1. The final concentration of S. aureus was 108 CFU/mL.
PI (%) = (the total number of golden grapes in phagocytes/number of active hemocytes involved in phagocytosis) × 100%
The immune-related enzymes activities of ACP, AKP, and LZM in hemolymph, and the antioxidant indices of hepatopancreas, including T-AOC, SOD, GPx, GSH, CAT, and MDA were measured by commercial kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
After 24 h of fixation, the hepatopancreas samples were embedded in paraffin, sectioned. After staining with hematoxylin and eosin (H&E), the samples were observed and photographed using an Olympus BX-50 fluorescence microscope (Olympus DP26, Japan).
The relative mRNA level of genes (proPO and crustin) was analyzed by real-time qPCR analysis. Total RNA of hepatopancreas samples was extracted by the Trizol method. 1 μg RNA was taken from each sample and transformed into cDNA using TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (Transgen Biotech, Beijing, China). ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) was used for RT-qPCR. The detailed methods were described by Li et al. (33). The CDS sequences of the target genes were acquired from the National Center for Biotechnology Information (NCBI), and the primers were designed using Primer Premier 6. The primers were listed in Table 3, and β-actin was used as the reference gene. All data was analyzed according to the 2-ΔΔCT method.
2.4 Statistical analysis
The statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). All results were presented as the means ± standard deviation (S.D.) of three replicates. Comparisons among groups were analyzed by one-way analysis of variance (ANOVA) with Duncan’s test. The statistical significance was considered when p < 0.05.
3 Results
3.1 Hematologic non-specific immune parameters of crabs
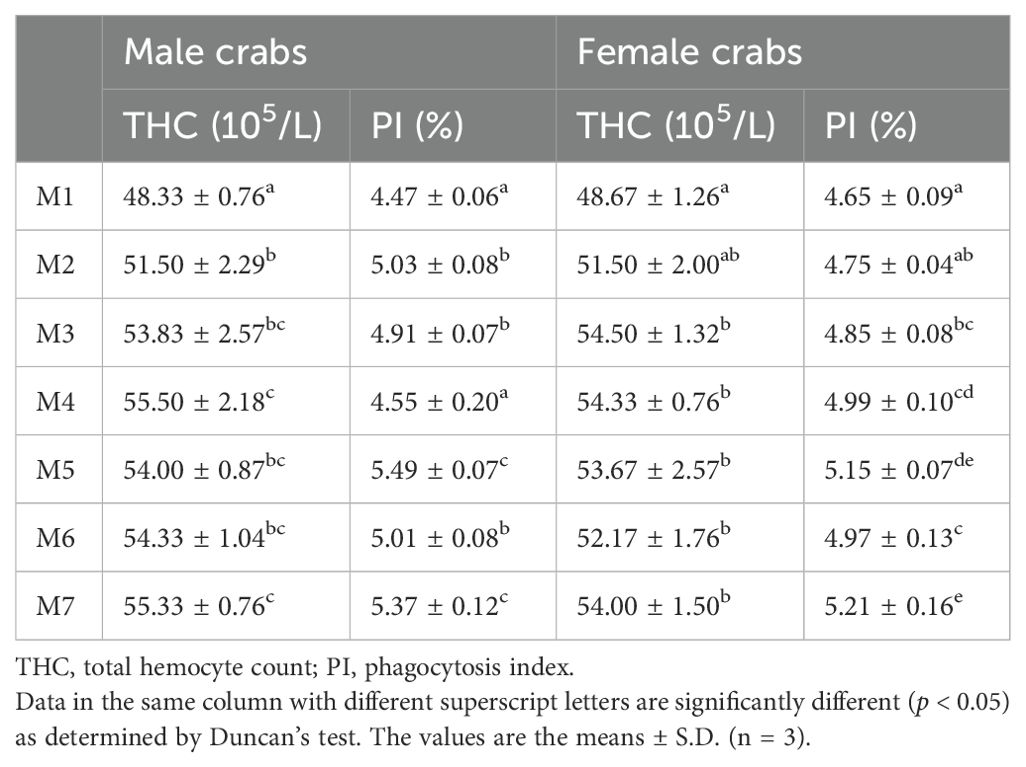
In male crabs, the THC was significantly increased in medicinal plant treatment groups (M2, M3, M4, M5, M6, and M7) compared with M1, and reached the highest in M4 and M7 (p < 0.05), and the PI was significantly improved in M5 and M7 compared with other groups (p < 0.05). In female crabs, the THC also significantly increased in M3, M4, M5, M6, and M7 compared with M1 (p < 0.05). Moreover, the PI in M5 and M7 was significantly increased compared to M2 and M7, respectively (p < 0.05) (Table 4).
Furthermore, we examined the activities of ACP, AKP, and LZM in hemolymph. In male crabs, the activity of ACP was significantly increased in M2 compared with other treatments (p < 0.05). The activity of AKP was significantly increased in medicinal plant treatment groups (M2, M3, M4, M5, M6, and M7) compared with M1 (p < 0.05), and the highest AKP activity of M5 was significantly increased compared to M2. For the LZM activity, it was significantly increased in M3, M4, M5, M6, and M7 compared with M1, as well as significantly increased in the AST, BA, and yeast complex treatment groups (M5, M6, and M7) compared with M2, M3, and M4, respectively (p < 0.05) (Figures 1A–C).

Figure 1. Hematologic immune-related enzyme activity of E. sinensis fed seven experimental diets (means ± S.D., n = 3). (A) Acid phosphatase (ACP) activity of male crabs; (B) Alkaline phosphatase (AKP) activity of male crabs; (C) Lysosome (LZM) activity of male crabs; (D) ACP activity of female crabs; (E) AKP activity of female crabs; (F) LZM activity of female crabs. Bars with different letters indicate significance among the treatments (p < 0.05).
In female crabs, the activity of ACP was significantly increased in M2, M3, M5, and M6 compared with others (p < 0.05), and the ACP activity in M5 and M6 was significantly increased compared to M2 and M3, respectively. Moreover, the activity of LZM was significantly increased in M4 and M7 when compared with others (p < 0.05), reaching the highest in M7 (Figures 1D–F).
3.2 Immune-related genes of hepatopancreas
The proPO expression showed an increasing trend from M2 to M6 and reached the highest in M6 (Figures 2A, C). Similarly, the expression of crustin also showed an increasing trend from M2 to M7, which was significantly higher in M2-M7 for male crabs and M3-M7 for female crabs (p < 0.05) (Figures 2B, D).
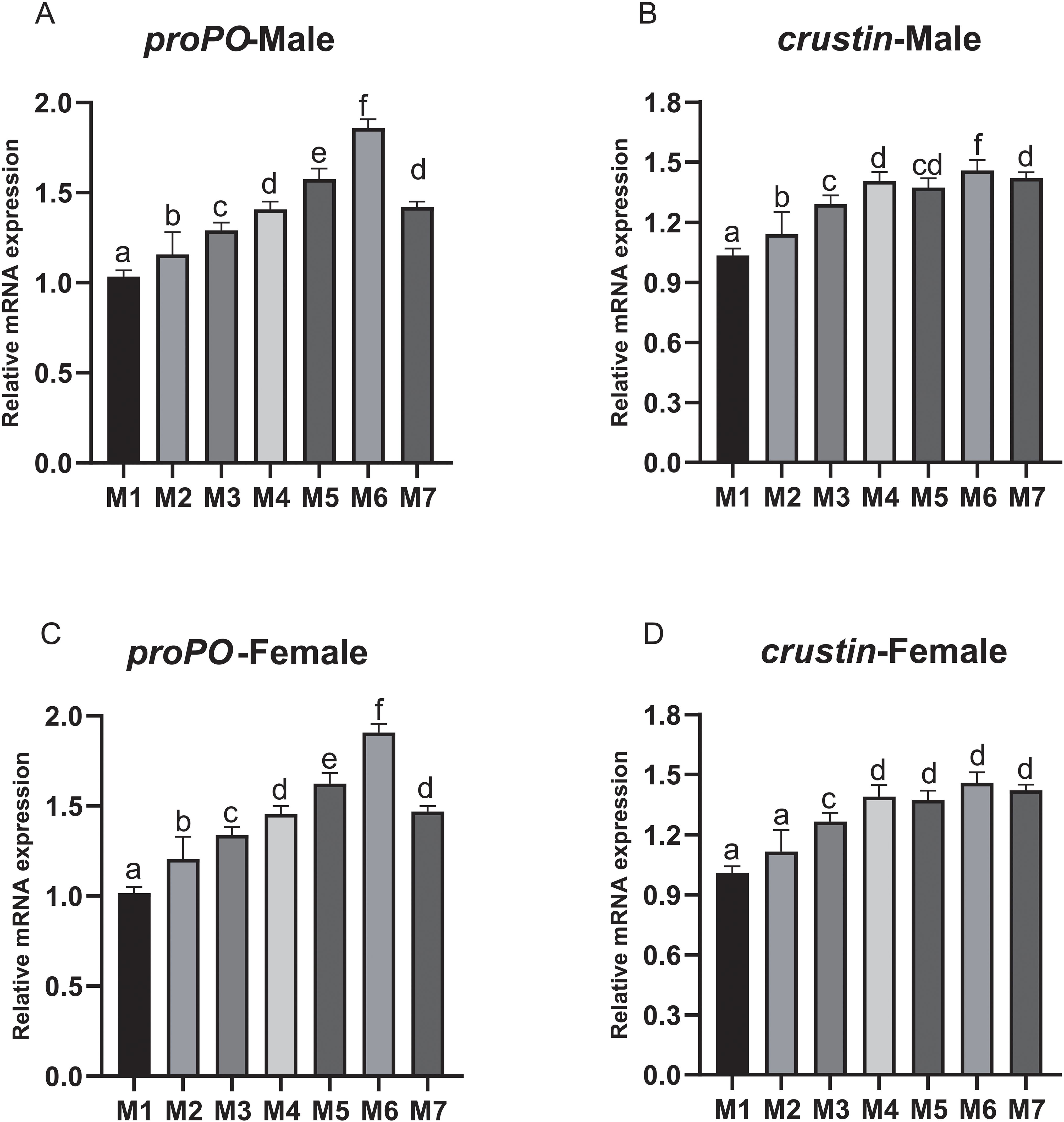
Figure 2. The relative expression levels of proPO and crustin in hemolymph of E. sinensis fed seven experimental diets (means ± S.D., n = 3). (A) Prophenoloxidase (proPO) of male crabs; (B) crustin of male crabs; (C) proPO of female crabs; (D) crustin of female crabs. Bars with different letters indicate significantly among the treatments (p < 0.05).
3.3 Antioxidant capacity of hepatopancreas in crabs
To evaluate the antioxidant capacity of crab hepatopancreas, we measured the antioxidant indices, including T-AOC, SOD, GSH, CAT, GPx, and MDA. In male crabs, no significant difference in T-AOC was found among all treatment groups. The activity of SOD was significantly improved in crabs receiving M3, M4, M5, M6, and M7 compared with M1 and M2, and the SOD activity in M7 was significantly higher compared with other groups (p < 0.05). A similar trend was also found in the GSH contents and CAT activities, which were significantly increased in M5, M6 and M7 when compared to M2, M3, and M4, respectively (p < 0.05), and reached their highest level in M7 and M6, respectively. The activity of GPx was significantly higher in M5, M6, and M7 than that of other groups, and reached the highest in M7 (p < 0.05). Correspondingly, the MDA content was significantly reduced in crabs receiving medicinal plant treatments (M2, M3, M4, M5, M6, and M7) compared to the control. Furthermore, the MDA value in the groups receiving the combined AST, BA, and yeast complex (M5, M6, and M7) was significantly decreased compared with M1-M4, and the lowest was in M7 (p < 0.05) (Figure 3).
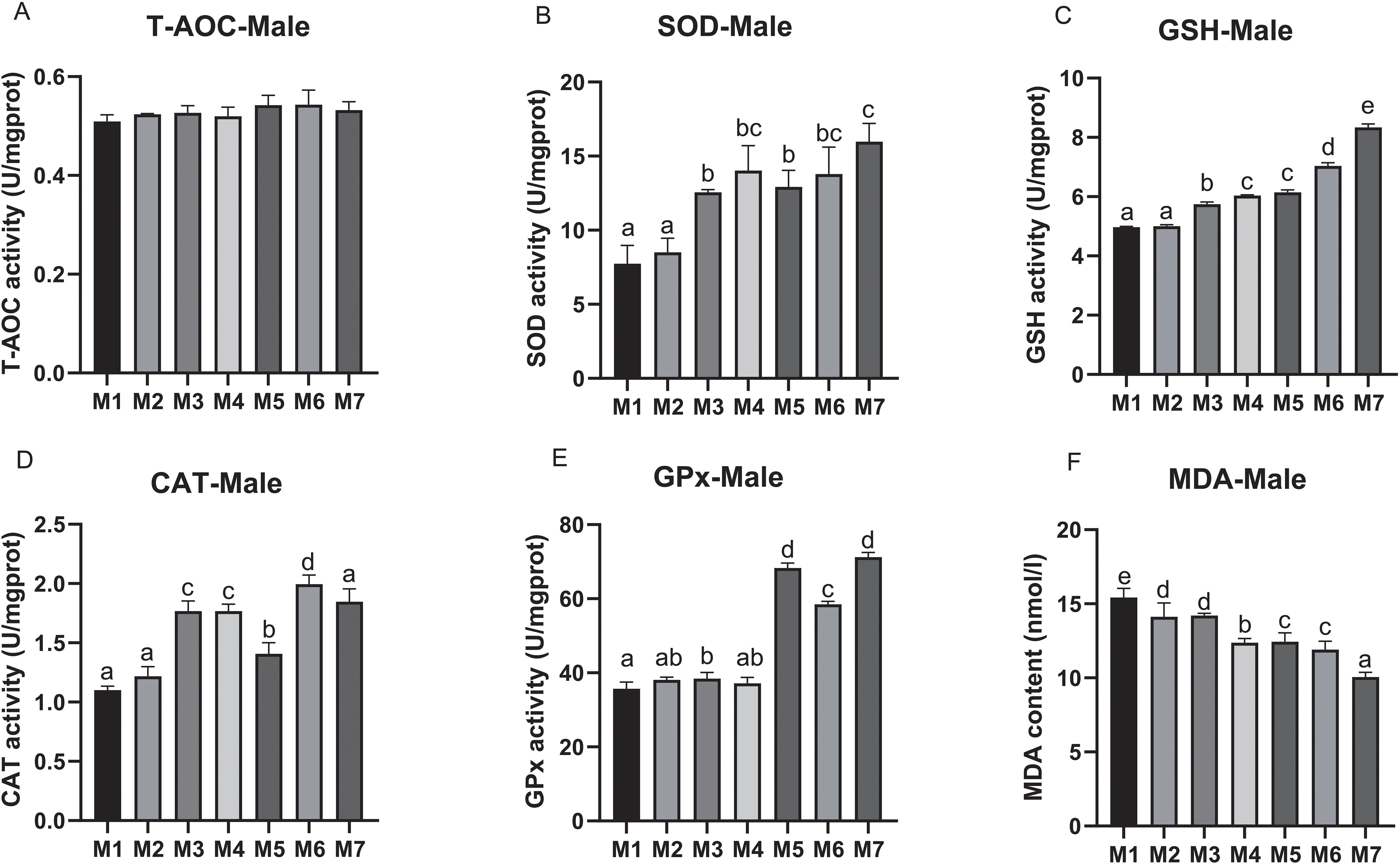
Figure 3. The hepatopancreatic antioxidant parameters of male E. sinensis fed seven experimental diets (means ± S.D., n = 3). (A) Total antioxidant capacity (T-AOC); (B) Superoxide dismutase (SOD); (C) Glutathione (GSH); (D) Catalase (CAT); (E) Glutathione peroxidase (GPx); (F) Malondinaldehyde (MDA). Bars with different letters indicate significance among the treatments (p < 0.05).
In female crabs, the T-AOC activity was significantly increased in M4 compared to M1(p < 0.05), while other groups did not show a significant difference. The activity of SOD in M5, M6, and M7 was significantly increased compared to M2, M3, and M4, respectively, and reached the highest in M7 (p < 0.05). For the GSH contents, it was significantly increased in medicinal plant treatment groups (M2, M3, M4, M5, M6, and M7) compared to the control. Moreover, the M5, M6, and M7 treatment groups had significantly higher GSH contents than M2, M3, and M4, respectively, and M7 showed the highest GSH contents (p < 0.05). For the activity of GPx, it was significantly increased in M5, M6, and M7 compared with other groups, and the highest was found in M5 (p < 0.05). As compared to M2 and M3, M5 and M6 had higher CAT activity (p < 0.05). Similarly, the MDA level in crabs in the medicinal plant treatment groups (M2, M3, M4, M5, M6, and M7) was also significantly decreased compared to M1, and M5 showed a significantly lower MDA value than M2 (p < 0.05) (Figure 4).
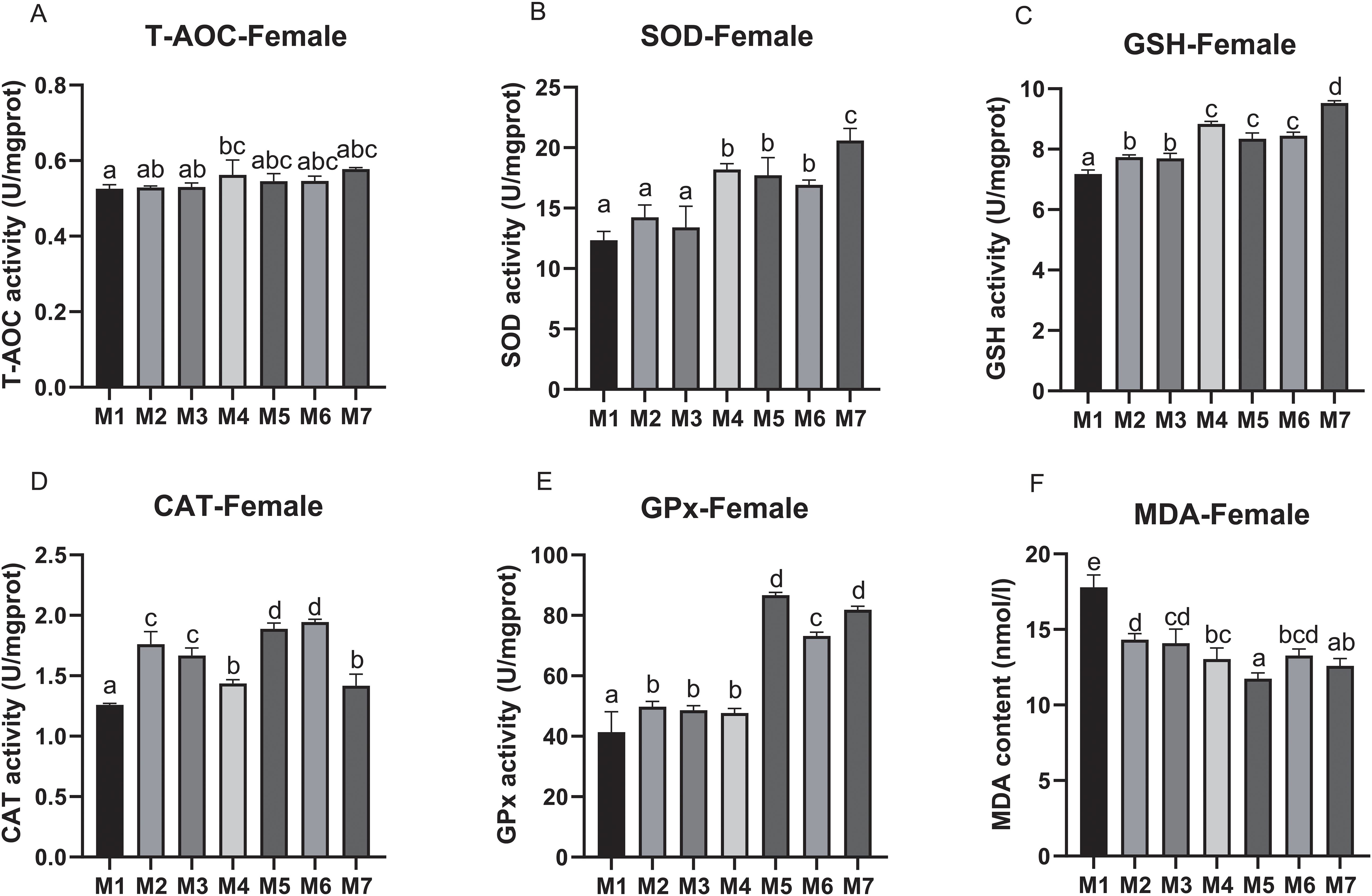
Figure 4. The hepatopancreatic antioxidant parameters of female E. sinensis fed seven experimental diets (means ± S.D., n = 3). (A) Total antioxidant capacity (T-AOC); (B) Superoxide dismutase (SOD); (C) Glutathione (GSH); (D) Catalase (CAT); (E) Glutathione peroxidase (GPx); (F) Malondinaldehyde (MDA). Bars with different letters indicate significance among the treatments (p < 0.05).
3.4 Hepatopancreas histology
The histology of the crab hepatopancreas was examined by H&E staining. In male crabs, the epithelial cells of hepatopancreas were highly vacuolated in M1 and M3, and slight vacuolation appeared in the M2, M4, and M5 groups. The vacuolar structures disappeared in M6 and M7, and the basal laminae of hepatopancreas were thickened in M5, M6, and M7 compared with M1, M2, M3, and M4 (Figure 5). In female crabs, the basal laminae of hepatopancreas were separated with honeycomb-like pores in M1, while the hepatopancreas exhibited a few vacuoles in M5 and M6. Collectively, the hepatopancreas of both male and female crabs in M7 showed clear lumens with no vacuolation and an intact basement membrane (Figure 6).
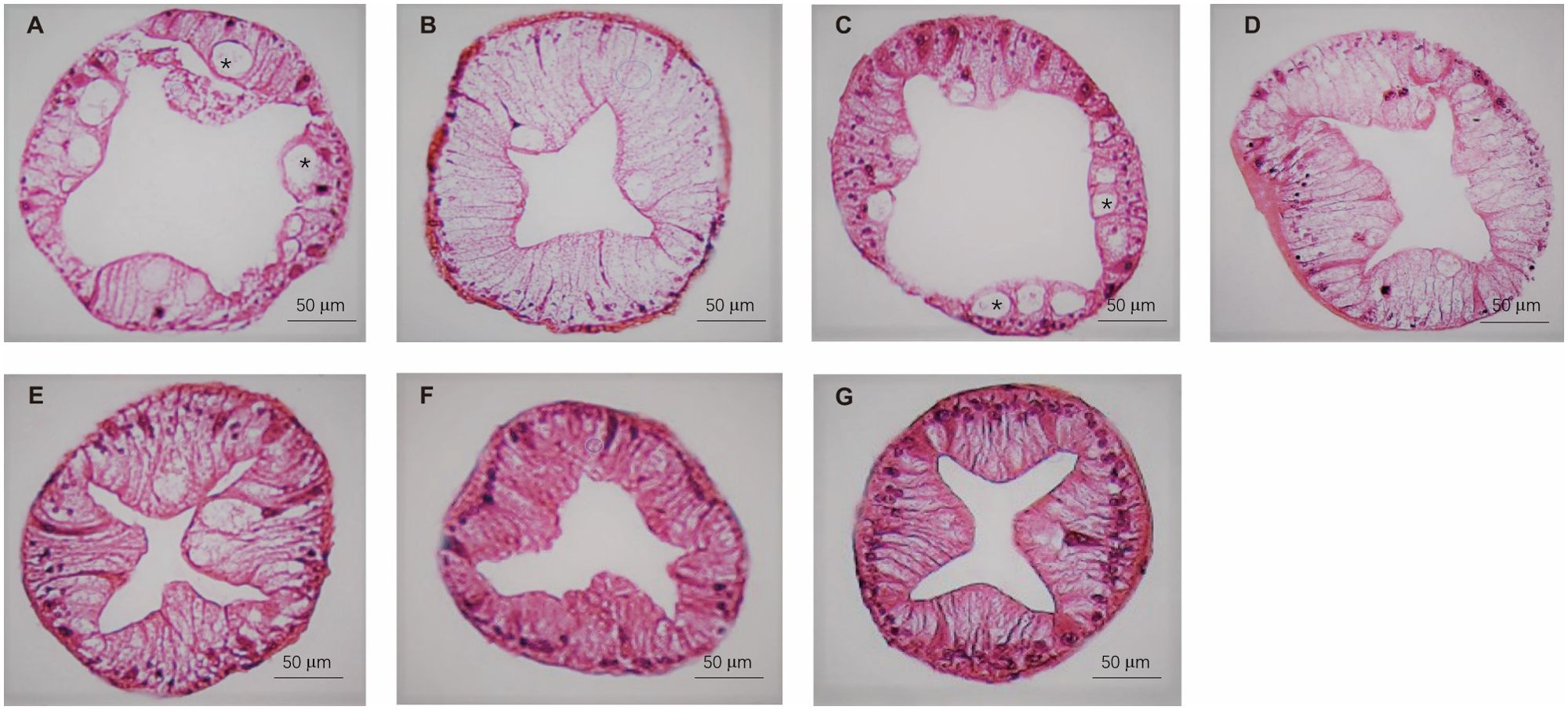
Figure 5. The hepatopancreas histological structure of male crabs fed seven experimental diets. (A: M1, B: M2, C: M3, D: M4, E: M5, F: M6, and G: M7). The asterisk indicates vacuoles within the hepatic tubules.
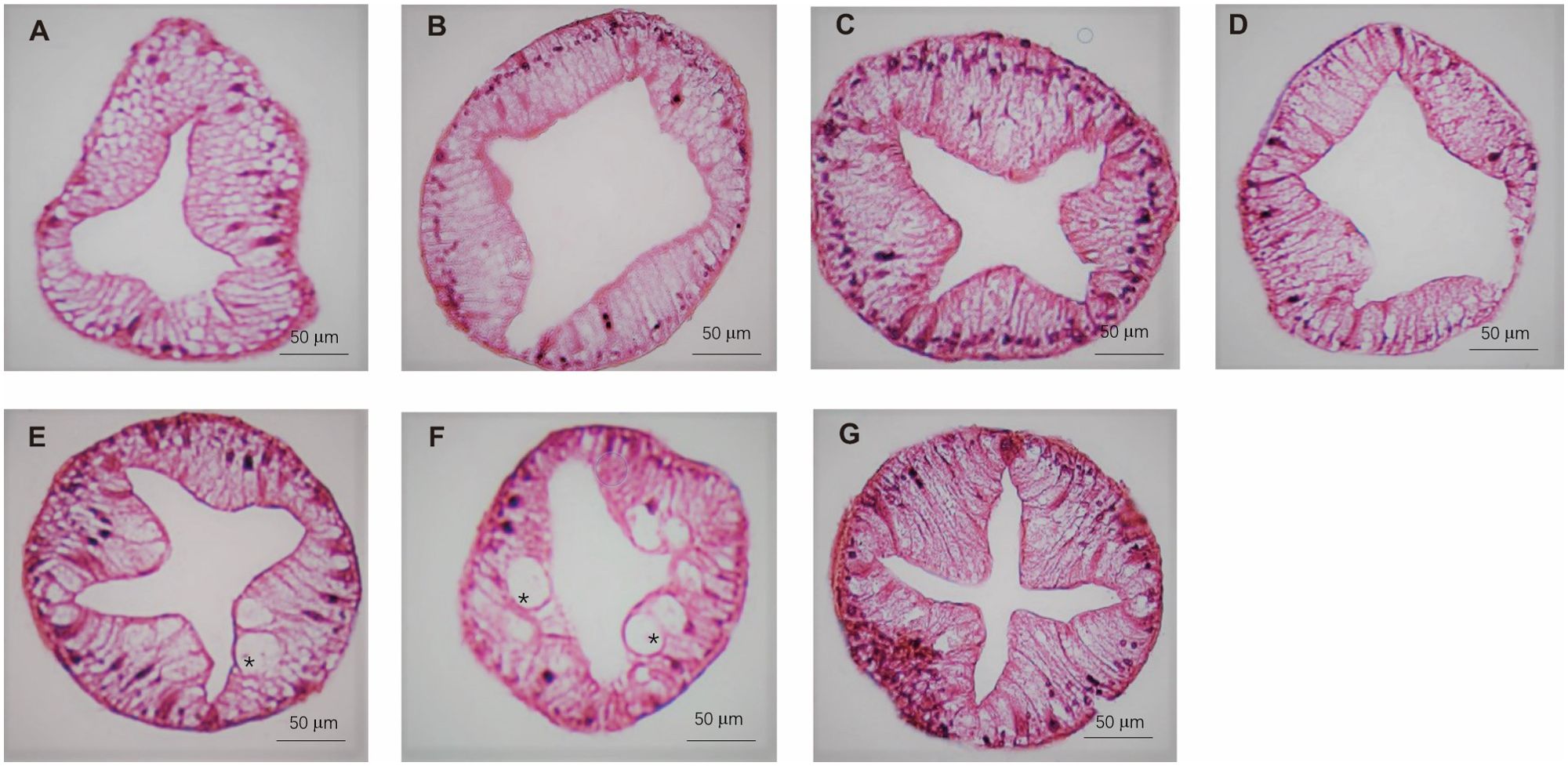
Figure 6. The hepatopancreas histological structure of female crabs fed seven experimental diets. (A: M1, B: M2, C: M3, D: M4, E: M5, F: M6, and G: M7). The asterisk indicates vacuoles within the hepatic tubules.
4 Discussion
Medicinal plants, including A. membranaceus, L. japonica, R. officinale Bail, S. baicalensis, and P. orientalis, have been applied as feed additives for aquatic animals. They can be used individually in a mixture, or in combination with other immunostimulants. In many aquatic species, studies have demonstrated that the combination of medicinal plants improves the immune response better than a single additive (25, 37, 38). In the current study, we evaluated the efficacy of dietary single (TP) or medicinal plant mixtures (A. membranaceus + L. japonica, R. officinale Bail + S. baicalensis + P. orientalis) in combination with AST, BA, and yeast complex or not, for the innate immunity, antioxidant capacity, and histomorphology of hepatopancreas E. sinensis.
Due to the lack of a specific immune system, hemocytes play a crucial role in crustaceans’ immune response (39). Phagocytosis is an important cellular defense reaction against invading pathogens (40). Ardó et al. found that feeding tilapia with A. membranaceus and L. japonica significantly enhanced phagocytic activity (22). Moreover, studies also showed that dietary supplementation with R. officinale extract and S. baicalensis have a positive effect on the phagocytic activity of orange-spotted grouper (Epinephelus coioides) and red drum (Sciaenops ocellatus), respectively (41, 42). In alignment with these results, the present study has shown that PI was significantly higher in the combined medicinal plant treatment groups (M2 and M3 for male crabs, M3 and M4 for female crabs) than in the M1 group. Furthermore, we observed that the PI of M5 and M7 was significantly increased compared with M2 and M4, respectively. AST, BA, and yeast complex have been applied as immunostimulants to improve the immune status of aquatic animals (11, 27–29, 31). Studies have reported that dietary AST (50 mg/kg) increased the phagocytic activity of Pacific white shrimp (Penaeus vannamei) (43) and Asian seabass (Lates calcarifer) (44). Moreover, Abu-Elala et al. demonstrated that dietary 0.2% yeast cell wall supplementation improved the PI of Nile tilapia (45). These results suggested that a combination of medicinal plant mixtures with AST, BA, and yeast complex would augment the phagocytic activity of crabs.
ACP and AKP are two important nonspecific phosphohydrolases that can destroy and eliminate invading bacteria and are of great importance in maintaining the health of crustaceans. LZM is a bactericidal factor that plays a key role in preventing pathogenic bacteria by degrading the peptidoglycan layer in their cell walls (46). In this study, we observed that the activities of hemolymph ACP in female crabs and the LZM activity in male crabs were significantly increased in M5, M6, and M7 treatment groups compared with M2, M3, and M4, respectively, and the highest LZM activity was found in M7 for female crabs. Consistently, the effects of chemical ingredients from Scutellaria and Rheum officinale Bail on enhancing ACP, AKP, and LZM activities have been reported in the study of giant freshwater prawn (M. rosenbergii) (23, 24). In E. sinensis, studies have also demonstrated that dietary AST or yeast improves the ACP, AKP, and LZM activities (27, 47). These results demonstrated that medicinal plant mixture (R. officinale Bail, S. baicalensis, P. orientalis) combined with AST, BA, and yeast complex boost the immune response of crabs. Thus, it can be inferred that the improved immune status in crabs may be attributed to the consequence of synergy among the active molecules contained in the medicinal plants (e.g. anthraquinones, polysaccharides) and immunostimulants. However, the mechanisms or modes of action of these effective ingredients in crabs require further investigation. The proPO system and Crustin are major components in the innate immunity of E. sinensis (48, 49), especially participating in defense against pathogens. The results showed that the expression levels of proPO and crustin were significantly increased in the M5 and M6 groups compared with M2 and M3, respectively. These results demonstrated that AST, BA, and yeast complex enhanced the immune response of crabs and were associated with the pathogen defense system.
For crustaceans, the antioxidant capability of hepatopancreas is vital for their health status (50). High levels of ROS will cause oxidative damage to tissues (51), and antioxidant enzymes, such as SOD, GPx, and the lipid peroxidation product MDA, are commonly used as indicators of antioxidant capacity (52, 53). SOD catalyzes the process of superoxide dismutation, and hydrogen peroxide is decomposed into water and oxygen by GPx (54). Moreover, GSH can directly scavenge ROS and becomes oxidized glutathione (GSSG) (55). In this study, we observed that the activity of SOD and GSH contents were significantly increased in M4 for both male and female crabs, indicating that the combination of R. officinale Bail, S. baicalensis, and P. orientalis exhibited better antioxidant effects than that of the M1-M3 groups, which may be attributed to the natural components of these medicinal plants. Previous studies demonstrated that anthraquinone extract from rhubarb can resist oxidant stress in many aquatic animals (56–59). Similarly, the chemical constituents of S. baicalensis, including baicalein and polysaccharide, have also shown antioxidant capacity in koi carp (Cyprinus carpio) (60), GIFT tilapia (Oreochromis niloticus) (61), and M. rosenbergii (23). Moreover, activity of SOD and GSHcontents in female crabs and the activity of GPx both in male and female crabs were significantly improved in M5, M6, and M7 treatment groups compared with M2, M3, and M4, respectively. However, the trend of hepatopancreatic MDA level, a marker of endogenous oxidative damage (62), showed an inverted tendency in male crabs. Consistently, these findings suggested that medicinal plant mixtures combined with AST, BA, and yeast complex might promote synergistic effects on the antioxidant capacity of crabs. As feed additives, dietary supplementation with AST, BA, and yeast complex have shown positive effects on the antioxidant function in animals. The antioxidant activity of AST has been reported in coral trout (Plectropomus leopardus) (63), loach (Paramisgurnus dabryanus) (64), E.sinensis (27), red swamp crayfish (Procambarus clarkii) (65), and pufferfish (Takifugu obscurus) (66). Moreover, BA supplementation also has been reported to increase antioxidant capacity in animals (67, 68). The antioxidant properties of yeast have been reported to improve bacterial resistance in aquatic animal diseases (30, 69, 70). The research of Zhang et al. demonstrated that feeding gibel carp with yeast culture improved their plasma SOD activity after exposure to A. hydrophila (71). Similarly, Zhang et al. found that dietary yeast extract supplementation increased CAT and SOD activities in E. sinensis (31). Collectively, these results further verified that dietary supplementation with mixtures of R. officinale Bail, S. baicalensis, P. orientalis, AST, BA, and yeast complex is beneficial for improving antioxidant capacity of E. sinensis.
Furthermore, combined with the histological characteristic analysis of the hepatopancreas, we observed that the hepatopancreatic tubular structure was improved in the M7 group, which exhibited fewer vacuolar structures and thickened basal lamiae. These results further demonstrated that medicinal plants together with AST, BA, and yeast complex could maintain the health of crab hepatopancreas.
In conclusion, although it is difficult to clarify the mechanism of the synergic action due to the multiple effective ingredients contained in medicinal plants, the present study demonstrated the positive effect of the combination of medicinal plant mixtures and immunostimulants on the immunopotentiation and antioxidation in crabs. In comparison to chemical drugs, herbal preparations have the potential to enhance the growth performance and nutritional quality of aquatic animals, while simultaneously safeguarding their tissue structure. Furthermore, they offer the additional benefits of low cost, low drug resistance, and straightforward operation, which collectively make them an attractive proposition for application in aquaculture. This study provided a feasible formulation of medicinal plant mixture containing 2.5 g/kg R. officinale Bail, 1.5 g/kg S. baicalensis, and 1.0 g/kg P. orientalis, in combination with 600 mg/kg AST, 1.0 g/kg BA, and 20 mg/kg yeast complex, which significantly enhanced the non-specific immunity, antioxidant capacity, and hepatopancreatic health of E. sinensis. In future, research should be conducted to investigate more possible combinations of feed additives that are suitable for E. sinensis with bio-safety concerns.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Animal Care Advisory Committee of Yangzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
AW: Data curation, Writing – original draft. JX: Data curation, Writing – review & editing. XZ: Formal analysis, Writing – review & editing. XL: Formal analysis, Writing – review & editing. ML: Formal analysis, Writing – review & editing. XD: Writing – review & editing. SM: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by grant from the ‘JBGS’ Project of Seed Industry Revitalization in Jiangsu Province [JBGS (2021) 126], National Natural Science Foundation of China (31972799).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yearbook C. Fishery Administration Bureau of the Ministry of Agriculture and Villages. China Agricultural Press (2024).
2. Ding Z, Cao M, Zhu X, Xu G, Wang R. Changes in the gut microbiome of the Chinese mitten crab (Eriocheir sinensis) in response to white spot syndrome virus (wssv) infection. J Fish Dis. (2017) 40:1561–71. doi: 10.1111/jfd.12624
3. Yang X, Song X, Zhang C, Pang Y, Song Y, Cheng Y, et al. Effects of dietary melatonin on hematological immunity, antioxidant defense and antibacterial ability in the Chinese mitten crab. Eriocheir sinensis. Aquaculture. (2020) 529:735671. doi: 10.1016/j.aquaculture.2020.735671
4. Zhang C, Zhang Q, Pang Y, Song X, Zhou N, Wang J, et al. The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci Total Environ. (2019) 653:1426–34. doi: 10.1016/j.scitotenv.2018.11.063
5. Xu Z, Guan W, Xie D, Lu W, Ren X, Yuan J, et al. Evaluation of immunological response in shrimp Penaeus vannamei submitted to low temperature and air exposure. Dev Com Immunol. (2019) 100:103413. doi: 10.1016/j.dci.2019.103413
6. Yang W, Tran NT, Zhu C, Zhang M, Yao D, Aweya JJ, et al. Enhanced immune responses and protection against the secondary infection in mud crab (Scylla paramamosain) primed with formalin-killed Vibrio parahemolyticus. Aquaculture. (2020) 529:735671. doi: 10.1016/j.aquaculture.2020.735671
7. Hong Y, Yin H, Huang Y, Huang Q, Yang X. Immune response to abamectin-induced oxidative stress in Chinese mitten crab. Eriocheir sinensis. Ecotoxicol Environ Saf. (2020) 188:109889. doi: 10.1016/j.ecoenv.2019.109889
8. Bao J, Li X, Xing Y, Feng C, Jiang H. Effects of hypoxia on immune responses and carbohydrate metabolism in the Chinese mitten crab. Eriocheir sinensis. Aquac Res. (2020) 51:2735–44. doi: 10.1111/are.1461
9. Chen KT, Malo MS, Beasley-Topliffe LK, Poelstra K, Millan JL, Mostafa G, et al. A role for intestinal alkaline phosphatase in the maintenance of local gut immunity. Digest Dis Sci. (2011) 56:1020–7. doi: 10.1007/s10620-010-1396-x
10. Song X, Yang C, Tang J, Yang H, Xu X, Wu K, et al. A combination of vitamins c and e alleviates oxidized fish oil-induced hepatopancreatic injury in juvenile Chinese mitten crab. Eriocheir sinensis. Aquacult Res. (2019) 50:1585–98. doi: 10.1111/are.14036
11. Wu X, Zhao L, Long X, Liu J, Su F, Cheng Y. Effects of dietary supplementation of haematococcus pluvialis powder on gonadal development, coloration and antioxidant capacity of adult male Chinese mitten crab (Eriocheir sinensis). Aquacult Res. (2017) 48:5214–23. doi: 10.1111/are.13333
12. Jiang H, Cai YM, Chen LQ, Zhang XW, Hu SN, Wang Q. Functional annotation and analysis of expressed sequence tags from the hepatopancreas of mitten crab (Eriocheir sinensis). Mar Biotechnol. (2009) 11:317–26. doi: 10.1007/s10126-008-9146-1
13. Liu JD, Liu WB, Zhang DD, Xu CY, Zhang CY, Zheng XC, et al. Dietary reduced glutathione supplementation can improve growth, antioxidant capacity, and immunity on Chinese mitten crab. Eriocheir sinensis. Fish Shellfish Immunol. (2020) 100:300–8. doi: 10.1016/j.fsi.2020.02.064
14. Bone K, Mills S. Principles and practice of phytotherapy: Modern herbal medicine. Elsevier Health Sci. (2012).
15. Zheng X, Chi C, Xu C, Liu J, Zhang C, Zhang L, et al. Effects of dietary supplementation with icariin on growth performance, antioxidant capacity and non-specific immunity of Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. (2019) 90:264–73. doi: 10.1016/j.fsi.2019.04.296
16. Fan Y, Wang X, Wang Y, Liu H, Yu X, Li L, et al. Potential effects of dietary probiotics with Chinese herb polysaccharides on the growth performance, immunity, disease resistance, and intestinal microbiota of rainbow trout (Oncorhynchus mykiss). J World Aquaculture Soc. (2021) 52:1194–208. doi: 10.1111/jwas.12757
17. Adeshina I, Abdel-Tawwab M, Tijjani ZA, Tiamiyu LO, Jahanbakhshi A. Dietary Tridax procumbens leaves extract stimulated growth, antioxidants, immunity, and resistance of Nile tilapia, Oreochromis niloticus, to monogenean parasitic infection. Aquaculture. (2021) 532:736047. doi: 10.1016/j.aquaculture.2020.736047
18. Heydari M, Firouzbakhsh F, Paknejad H. Effects of Mentha longifolia extract on some blood and immune parameters, and disease resistance against yersiniosis in rainbow trout. Aquaculture. (2020) 515. doi: 10.1016/j.aquaculture.2019.73458
19. Harikrishnan R, Balasundaram C, Heo MS. Influence of diet enriched with green tea on innate humoral and cellular immune response of kelp grouper (Epinephelus bruneus) to Vibrio carchariae infection. Fish Shellfish Immunol. (2011) 30:972–9. doi: 10.1016/j.fsi.2011.01.029
20. Sheikhzadeh N, Nofouzi K, Delazar A, Oushani AK. Immunomodulatory effects of decaffeinated green tea (Camellia sinensis) on the immune system of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. (2011) 31:1268–9. doi: 10.1016/j.fsi.2011.09.010
21. Hwang JH, Lee SW, Rha SJ, Yoon HS, Park ES, Han KH, et al. Dietary green tea extract improves growth performance, body composition, and stress recovery in the juvenile black rockfish. Sebastes schlegeli. Aquacult Int. (2013) 21:525–38. doi: 10.1007/s10499-012-9586-5
22. Ardo L, Yin G, Xu P, Varadi L, Szigeti G, Jeney Z, et al. Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. . Aquaculture. (2008) 275:26–33. doi: 10.1016/j.aquaculture.2007.12.022
23. Liu B, Ge X, He Y, Xie J, Xu P, He Y, et al. Effects of anthraquinones extracted from Rheum officinale Bail on the growth, non-specific immune response of Macrobrachium rosenbergii. Aquaculture. (2010) 310:13–9. doi: 10.1016/j.aquaculture.2010.09.020SunL
24. Sun L, Lin F, Sun B, Qin Z, Chen K, Zhao L, et al. Scutellaria polysaccharide mediates the immunity and antioxidant capacity of giant freshwater prawn (Macrobrachium rosenbergii). Dev Com Immunol. (2023) 143. doi: 10.1016/j.aquaculture.2007.12.022
25. Abarike ED, Jian J, Tang J, Cai J, Sakyi EM, Kuebutornye FK. A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of hsp70 and hif-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia. Oreochromis niloticus. Aquac Rep. (2020) 18:100438. doi: 10.1016/j.aqrep.2020.100438
26. Asker D, Awad TS, Beppu T, Ueda K. A novel radio-tolerant astaxanthin-producing bacterium reveals a new astaxanthin derivative: Astaxanthin dirhamnoside. Methods Mol Biol (Clifton NJ). (2012) 892:61–97. doi: 10.1007/978-1-61779-879-5_4
27. Jiang X, Zu L, Wang Z, Cheng Y, Yang Y, Wu X. Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab. Eriocheir sinensis. Fish Shellfish Immunol. (2020) 102:499–510. doi: 10.1016/j.fsi.2020.05.021
28. Long X, Wu X, Zhao L, Liu J, Cheng Y. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab. Eriocheir sinensis. Aquaculture. (2017) 473:545–53. doi: 10.1016/j.aquaculture.2017.03.010
29. Santos GG, Libanori MCM, Pereira SA, Ferrarezi JVS, Ferreira MB, Soligo TA, et al. Probiotic mix of Bacillus spp. and benzoic organic acid as growth promoter against Streptococcus agalactiae in Nile tilapia. Aquaculture. (2023) 566:739212. doi: 10.1016/j.aquaculture.2022.739212
30. Cesena CE, Vega-Villasante F, Aguirre-Guzman G, Luna-Gonzalez A, Isidro Campa-Cordova A. Update on the use of yeast in shrimp aquaculture: A mini review. Int Aquat Res. (2021) 13:1–16. doi: 10.22034/IAR.2021.1904524.1066
31. Zhang R, Jiang Y, Zhou L, Chen Y, Wen C, Liu W, et al. Effects of dietary yeast extract supplementation on growth, body composition, non-specific immunity, and antioxidant status of Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. (2019) 86:1019–25. doi: 10.1016/j.fsi.2018.12.052
32. Xu J, Xu X, Hu J, Zhou Z, Wan W, Zhou Y, et al. Effects of dietary arachidonic acid on the growth performance, feed utilization and fatty acid metabolism of Chinese mitten crab (Eriocheir sinensis). Aquac Rep. (2022) 24:101170. doi: 10.1016/j.aqrep.2022.101170
33. Li M, Wang A, Lin X, Miao H, Liu X, Xu J, et al. Effects of dietary trimetlylamine oxide on the growth performance, feed utilization and appetite regulation of Chinese mitten crab (Eriocheir sinensis). Aquaculture. (2023) 575:739754. doi: 10.1016/j.aquaculture.2023.739754
34. Miao S, Hu J, Wan W, Xia S, Han B, Zhou Y, et al. Effects of graded levels of starch on the non-specific immune responses, antioxidant capacities and intestinal health in Chinese mitten crab. Eriocheir inensis. Fish Shellfish Immunol. (2020) 104:402–9. doi: 10.1016/j.fsi.2020.06.035
35. Zhao C, Zhu J, Hu J, Dong X, Sun L, Zhang X, et al. Effects of dietary Bacillus pumilus on growth performance, innate immunity and digestive enzymes of giant freshwater prawns (Macrobrachium rosenbergii). Aquacult Nutr. (2019) 25:712–20. doi: 10.1111/anu.12894
36. Zhang X, Chang E, Fu Y, Liu X, Xu J, Wu Y, et al. Tryptophan can alleviate the inhibition in growth and immunity of tilapia (Gift Oreochromis spp.) induced by high dietary soybean meal level. Aquac Rep. (2023) 31:101646. doi: 10.1016/j.aqrep.2023.101646
37. Harikrishnan R, Kim MC, Kim JS, Balasundaram C, Heo MS. Probiotics and herbal mixtures enhance the growth, blood constituents, and nonspecific immune response in Paralichthys olivaceus against Streptococcus parauberis. Fish Shellfish Immunol. (2011) 31:310–7. doi: 10.1016/j.fsi.2011.05.020
38. Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture. (2014) 433:50–61. doi: 10.1016/j.aquaculture.2014.05.048
39. Hauton C. The scope of the crustacean immune system for disease control. J Invertebr Pathol. (2012) 110:251–60. doi: 10.1016/j.jip.2012.03.005
40. Tsaplina OA. Phagocytosis of bacterial pathogens: Modification of cellular processes by bacterial factors. Tsitologiia. (2013) 55:83–91.
41. Kuo IP, Lee PT, Nan FH. Rheum officinale extract promotes the innate immunity of orange-spotted grouper (Epinephelus coioides) and exerts strong bactericidal activity against six aquatic pathogens. Fish Shellfish Immunol. (2020) 102:117–24. doi: 10.1016/j.fsi.2020.04.024
42. Pan T, Yan M, Chen S, Wang X. Effects of ten traditional Chinese herbs on immune response and disease resistance of Sciaenops ocellatus (Actinopterygii: Perciformes: Sciaenidae). Acta Ichthyologica Et Piscatoria. (2013) 43:41–9. doi: 10.3750/AIP2013.43.1.06
43. Lin YJ, Chang JJ, Huang HT, Lee CP, Hu YF, Wu ML, et al. Improving red-color performance, immune response and resistance to Vibrio parahaemolyticus on white shrimp Penaeus vannamei by an engineered astaxanthin yeast. Sci Rep. (2023) 13:2248–8. doi: 10.1038/s41598-023-29225-4
44. Lim KC, Yusoff FM, Shariff M, Kamarudin MS, Nagao N. Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, lates calcarifer (bloch, 1790). Aquaculture. (2019) 512:734339. doi: 10.1016/j.aquaculture.2019.734339
45. Abu-Elala NM, Younis NA, AbuBakr HO, Ragaa NM, Borges LL, Bonato MA. Efficacy of dietary yeast cell wall supplementation on the nutrition and immune response of Nile tilapia. Egyptian J Aquat Res. (2018) 44:333–41. doi: 10.1016/j.ejar.2018.11.001
46. Fu L, Zhou G, Pan J, Li Y, Lu Q, Zhou J, et al. Effects of astragalus polysaccharides on antioxidant abilities and non-specific immune responses of Chinese mitten crab. Eriocheir sinensis. Aquacult Int. (2017) 25:1333–43. doi: 10.1007/s10499-017-0117-2
47. Wang X, Shen Z, Wang C, Li E, Qin JG, Chen L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish Shellfish Immunol. (2019) 87:22–31. doi: 10.1016/j.fsi.2018.12.076
48. Cerenius L, Lee BL, Soderhall K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. (2008) 29:263–71. doi: 10.1016/j.it.2008.02.009
49. Huang P, Gao J, Du J, Nie Z, Li Q, Sun Y, et al. Prometryn exposure disrupts the intestinal health of Eriocheir sinensis: Physiological responses and underlying mechanism. Comp Biochem Phys C. (2024) 277:109820. doi: 10.1016/j.cbpc.2023.109820
50. Vogt G. Cytopathology and immune response in the hepatopancreas of decapod crustaceans. Dis Aquat Org. (2020) 138:41–88. doi: 10.3354/dao03443
51. Valavanidis A, Vlahogianni T, Dassenakis M. Scoullos M.Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf. (2006) 64:178–89. doi: 10.1016/j.ecoenv.2005.03.013
52. Shan H, Wang T, Dong Y, Ma S. Effects of dietary Ampithoe sp. supplementation on the growth, energy status, antioxidant capacity, and ammonia-N tolerance of the shrimp Litopenaeus vannamei: Continuous versus interval feeding. Aquaculture. (2019) 509:32–9. doi: 10.1016/j.aquaculture.2019.05.021
53. Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. (1990) 186:421–31. doi: 10.1016/0076-6879(90)86135-I
54. Li Y, Wei L, Cao J, Qiu L, Jiang X, Li P, et al. Oxidative stress, DNA damage and antioxidant enzyme activities in the Pacific white shrimp (Litopenaeus vannarnei) when exposed to hypoxia and reoxygenation. Chemosphere. (2016) 144:234–40. doi: 10.1016/j.chemosphere.2015.08.051
55. Wang X, Xu W, Zhou H, Zhang Y, Gao W, Zhang W, et al. Reduced glutathione supplementation in practical diet improves the growth, anti-oxidative capacity, disease resistance and gut morphology of shrimp. Litopenaeus vannamei. Fish Shellfish Immunol. (2018) 73:152–7. doi: 10.1016/j.fsi.2017.11.043
56. Xie J, Liu B, Zhou Q, Su Y, He Y, Pan L, et al. Effects of anthraquinone extract from rhubarb Rheum officinale bail on the crowding stress response and growth of common carp Cyprinus carpio var. Jian. Aquaculture. (2008) 281:5–11. doi: 10.1016/j.aquaculture.2008.03.038
57. Liu B, Xie J, Ge X, Xu P, Wang A, He Y, et al. Effects of anthraquinone extract from Rheum officinale bail on the growth performance and physiological responses of Macrobrachium rosenbergii under high temperature stress. Fish Shellfish Immunol. (2010) 29:49–57. doi: 10.1016/j.fsi.2010.02.018
58. Song C, Liu B, Jiang S, Xiong Y, Sun C, Zhou Q, et al. Anthraquinone extract from Rheum officinale bail improves growth performance and toll-relish signaling-regulated immunity and hyperthermia tolerance in freshwater prawn Macrobrachium nipponense. 3 Biotech. (2020) 10:526. doi: 10.1007/s13205-020-02519-4
59. Liu B, Ge X, Xie J, Xu P, He Y, Cui Y, et al. Effects of anthraquinone extract from Rheum officinale bail on the physiological responses and hsp70 gene expression of Megalobrama amblycephala under Aeromonas hydrophila infection. Fish Shellfish Immunol. (2012) 32:1–7. doi: 10.1016/j.fsi.2011.02.015
60. Du Y, Han Y, Zhang R, Zhang Y, Bao S, Cao Y. Dietary baicalein improves growth performance, antioxidant activity, and intestinal flora of koi carp (Cyprinus carpio). Aquac Rep. (2020) 27:101421. doi: 10.1016/j.aqrep.2022.101421
61. Jia R, Du J, Cao L, Feng W, Xu P, Yin G. Effects of dietary baicalin supplementation on growth performance, antioxidative status and protection against oxidative stress-induced liver injury in Gift tilapia (Oreochromis niloticus). Comp Biochem Physiol C-Toxicology Pharmacol. (2021) 240. doi: 10.1016/j.cbpc.2020.108914
62. Sumida S, Tanaka K, Kitao H, Nakadomo F. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. Int J Biochem. (1989) 21:835–8. doi: 10.1016/0020-711x(89)90280-2
63. Zhu X, Hao R, Zhang J, Tian C, Hong Y, Zhu C, et al. Dietary astaxanthin improves the antioxidant capacity, immunity and disease resistance of coral trout (Plectropomus leopardus). Fish Shellfish Immunol. (2022) 122:38–47. doi: 10.1016/j.fsi.2022.01.037
64. Chen XM, Gao CS, Du XY, Yao JM, He FF, Niu XT, et al. Effects of dietary astaxanthin on the growth, innate immunity and antioxidant defense system of Paramisgurnus dabryanus. Aquaculture Nutr. (2020) 26:1453–62. doi: 10.1111/anu.13093
65. Cheng Y, Wu S. Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish. Procambarus clarkii. Aquaculture. (2019) 512:734341. doi: 10.1016/j.aquaculture.2019.734341
66. Cheng CH, Guo ZX, Ye CX, Wang AL. Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifugu obscurus) under high temperature stress. Fish Physiol Biochem. (2018) 44:209–18. doi: 10.1007/s10695-017-0425-5
67. Saravanan N, Rajasankar S, Nalini N. Antioxidant effect of 2-hydroxy-4-methoxy benzoic acid on ethanol-induced hepatotoxicity in rats. J Pharm Pharmacol. (2007) 59:445–53. doi: 10.1211/jpp.59.3.0015
68. Wang F, Yin Y, Yang M, Chen J, Fu C, Huang K. Effects of combined supplementation of macleaya cordata extract and benzoic acid on the growth performance, immune responses, antioxidant capacity, intestinal morphology, and microbial composition in weaned piglets. Front Vet Sci. (2021) 8:708597. doi: 10.3389/fvets.2021.708597
69. Kheirabadi EP, Shekarabi PH, Yadollahi F, Soltani M, Najafi E, von Hellens J, et al. Red yeast (Phaffia rhodozyma) and its effect on growth, antioxidant activity and color pigmentation of rainbow trout (Oncorhynchus mykiss). Aquac Rep. (2022) 23. doi: 10.1016/j.aqrep.2022.101082
70. Xv Z, Zhong Y, Wei Y, Zhang T, Zhou W, Jiang Y, et al. Yeast culture supplementation alters the performance and health status of juvenile largemouth bass (Micropterus salmoides) fed a high-plant protein diet. Aquacult Nutr. (2021) 27:2637–50. doi: 10.1111/anu.13391
Keywords: medicinal plants, immunostimulants, immune response, antioxidant capacity, Eriocheir sinensis
Citation: Wang A, Xu J, Zhang X, Liu X, Li M, Dong X and Miao S (2024) Effects of dietary supplementation with medicinal plant mixtures and immunostimulants on the immune response, antioxidant capacity, and hepatopancreatic health of Chinese mitten crab (Eriocheir sinensis). Front. Immunol. 15:1347736. doi: 10.3389/fimmu.2024.1347736
Received: 01 December 2023; Accepted: 30 July 2024;
Published: 02 September 2024.
Edited by:
Mengqiang Wang, Ocean University of China, ChinaReviewed by:
Min Jin, Ningbo University, ChinaXiaodan Wang, East China Normal University, China
Youji Wang, Shanghai Ocean University, China
Copyright © 2024 Wang, Xu, Zhang, Liu, Li, Dong and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyan Miao, bXN5dG91Z2FvQDEyNi5jb20=
†These authors have contributed equally to this work
 Anran Wang
Anran Wang Jie Xu†
Jie Xu† Shuyan Miao
Shuyan Miao