- 1School of Public Health, Kunming Medical University, Kunming, China
- 2Vice Director of Center of Sports Injury Prevention, Treatment and Rehabilitation China National Institute of Sports Medicine A2 Pangmen, Beijing, China
Tuberculosis (TB) and tumor, with similarities in immune response and pathogenesis, are diseases that are prone to produce autoimmune stress response to the host immune system. With a symbiotic relationship between the two, TB can facilitate the occurrence and development of tumors, while tumor causes TB reactivation. In this review, we systematically sorted out the incidence trends and influencing factors of TB and tumor, focusing on the potential pathogenesis of TB and tumor, to provide a pathway for the co-pathogenesis of TB comorbid with tumor (TCWT). Based on this, we summarized the latest progress in the diagnosis and treatment of TCWT, and provided ideas for further exploration of clinical trials and new drug development of TCWT.
1 Background
In recent years, the stubbornly high global incidence of TB and tumor renders the two as public health problems that threaten human health and as major factors in the global disease burden (1–3). In the past two decades, the number of new TB patients stays basically high. It is estimated that the year 2022 saw 10.6 million new TB patients in the world, with an incidence rate of 133/100,000 (3). TB is also the world’s leading cause of death from a single infectious source, with 1.3 million deaths globally in 2022 (3). There are many epidemiological evidences showing that TB coexists with tumors, where TB is a susceptible factor for tumor, and tumors can augment the incidence risk of TB (4–6). TB is attributed to an increased risk of cancer mortality, and TB patients have higher comorbidity and mortality with tumor (7, 8). Many epidemiological evidences show that TB is closely related to tumors, and the risk of TB patients comorbid with lung cancer, pleural mesothelioma, Hodgkin’s lymphoma and other cancers is higher than that of the normal population (5, 9). Studies (10) have shown that cancer itself is an independent risk factor for developing active mycobacterium TB infection, however, this risk varies greatly among different cancer types, with lung cancer and hematologic malignancies having a higher risk (11, 12).
TB and tumor are bidirectional related diseases, and there is a causal relationship between them. TB facilitates the occurrence and development of tumors, and tumor causes TB reactivation, and there is a symbiotic relationship between the two. Studies have shown that both tumor patients and TB patients are prone to generate autoimmune stress reactions to the host immune system. With similarities in immune response and pathogenesis, TB and tumor can facilitate disease progression and anti-tumor or elimination of mycobacteria through immune cell reactions such as T cells (13, 14). Immunotherapy targeting T cells has also been applied to the treatment of TCWT (15). In this paper, a systematic study was conducted on the disease risk, mechanism, diagnosis and treatment of patients with TCWT, and the relationship between the two disease mechanisms was clarified to provide clinical decision-making basis for the treatment of TCWT.
2 Prevalence trend of TCWT
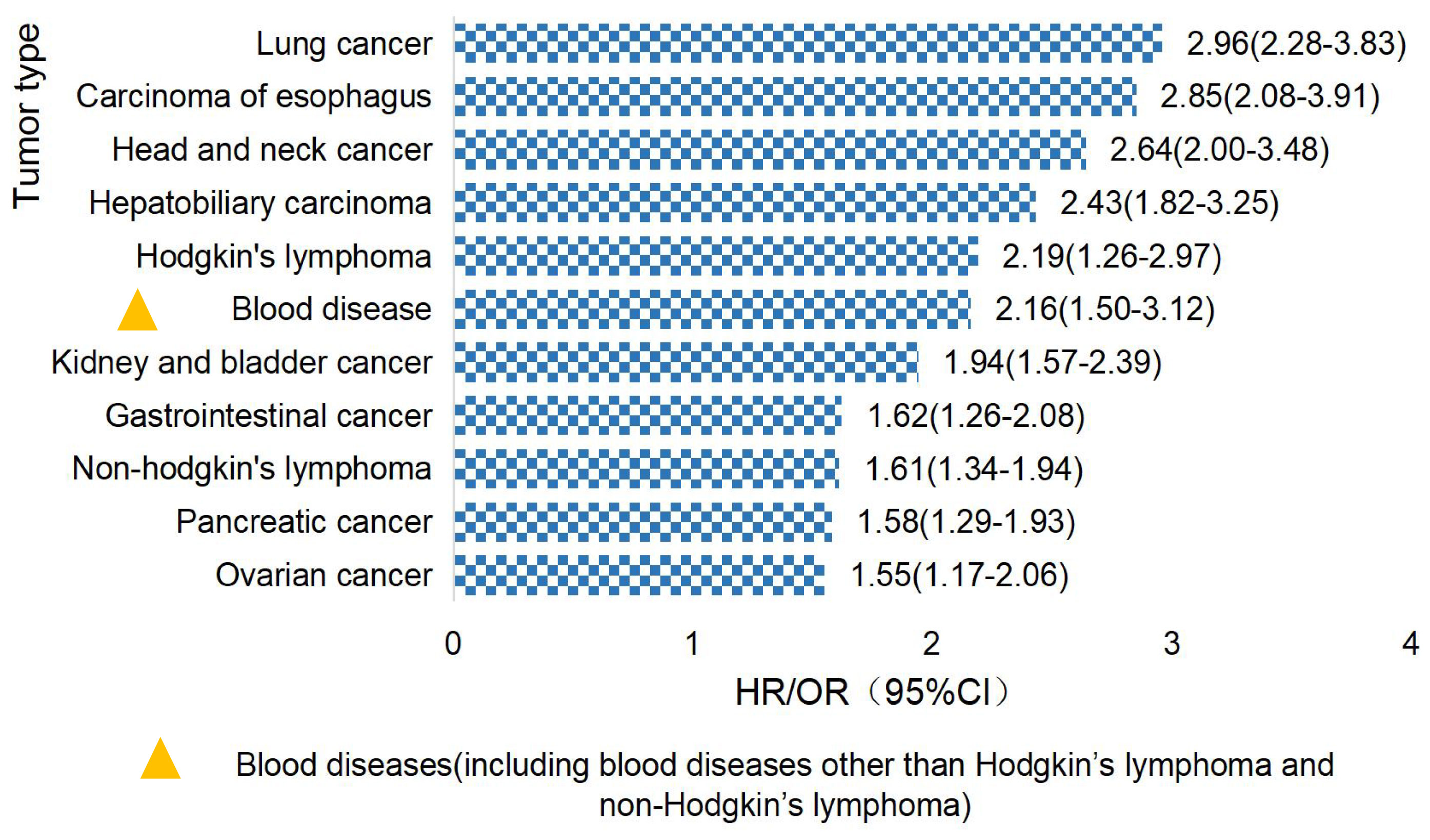
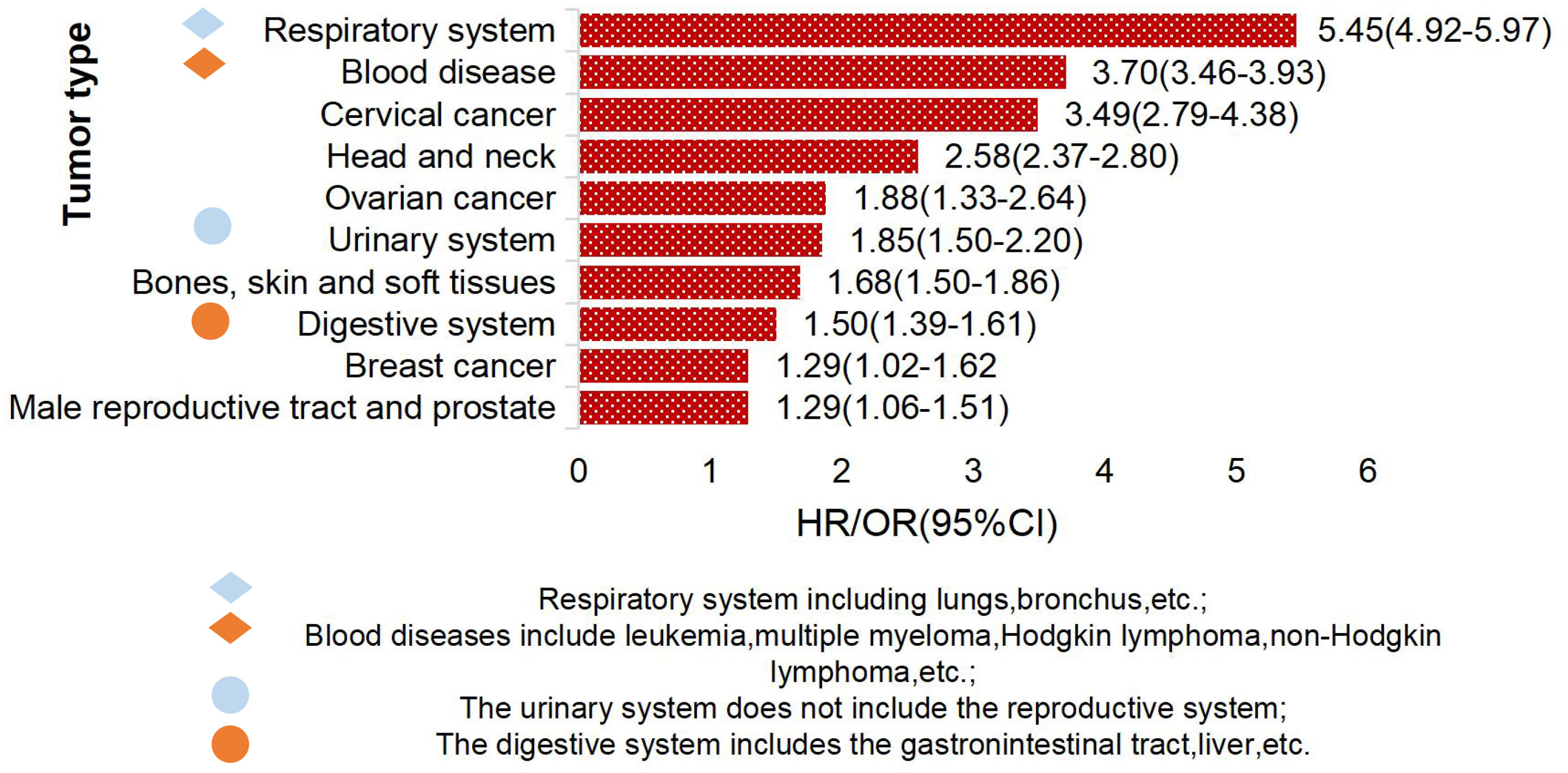
Studies have found that TCWT is on the rise, with 2.33% of global cancer (tumor) incidence attributed to TB (6, 16, 17). Studies have shown that TB patients have a high risk of developing cancer (18). The overall incidence of cancer induced by TB was 1.60(95%CI, 1.28-2.01) (19), and the incidence of lung cancer was higher in TB patients, which was about 3.0 (95%CI,2.35-3.32) (20). TCWT boosted mortality risk, with standardized mortality rates of respiratory cancer, blood cancer and head and neck cancer being 5.45, 3.70 and 2.58, respectively (21). The risk of TCWT varies in different regions. In European and American countries, the risk probability of TCWT mainly includes lung cancer, esophageal cancer, head and neck cancer, hepatobiliary cancer, Hodgkin’s lymphoma, etc. (17, 22–24), as shown in Figure 1. In Asian countries represented by China, high-risk tumors of TB patients are mostly manifested in diseases of respiratory system, blood system, cervical cancer, head and neck tumors and others (18, 25, 26), as shown in Figure 2.
TB is associated with the pathogenesis of tumors, and can lead to tumor occurrence, showing a positive correlation (23). Meta-analysis has shown that TB is an independent risk factor for cancer, and the risk of cancer in TB patients is significantly increased compared with the normal population (27). A retrospective study showed that of 3776 TB patients followed up, 86 (2.3% of the population) developed lung cancer (28). A Danish national cohort study (29) showed that TB patients had an increased long-term risk of developing cancer compared to the general population. There is a 50% increased risk of lung cancer two years after TB (9). A long-term follow-up study in Taiwan suggested that the proportion of malignant tumors in newly diagnosed TB patients increased year by year, from 9% in 2005 to 13% in 2015 (18).
Tumor has an activation effect on TB, and immunotherapy in tumor patients will cause the latent mycobacterium TB to reactivate, promote the continuous proliferation of mycobacterium TB in the body, and secrete immunosuppressive factors to inhibit the immune function of the body (30, 31). It causes a significant increase in the risk of active TB in tumor patients, with an incidence rate that is 9-13 times that in tumor free patients (11, 32, 33). A clinical study (34) showed that the median incidence of latent TB infection in patients treated with infliximab after antitumor therapy was 12.1 times higher than in patients treated with etanerceib. Multiple studies (35–37) have shown that cancer patients are at twice the risk of developing TB compared with the general population. Patients with lung cancer are 6 times more likely to develop TB than those without lung cancer (31). The risk of TB in gastric cancer patients was 2.63 times that of the general population (IRR 2.63, 95% CI 1.96-3.52) (38), and the risk of TB in adult patients with blood tumors was 3.3 times that of the general population (IRR 3.53; 95% CI 1.63-7.64) (12).
In summary, an array of epidemiological investigations have shown that TB and tumor have mutually promoting effects, and more and more epidemiological studies have proved the comorbid relationship between the two, suggesting that studies on the pathogenesis and molecular mechanism of the two should be reinforced to provide support for public health security and alleviate the global disease burden.
3 The mechanism of TB and tumors
3.1 Possible mechanisms of TB- induced tumorigenesis
3.1.1 T cells
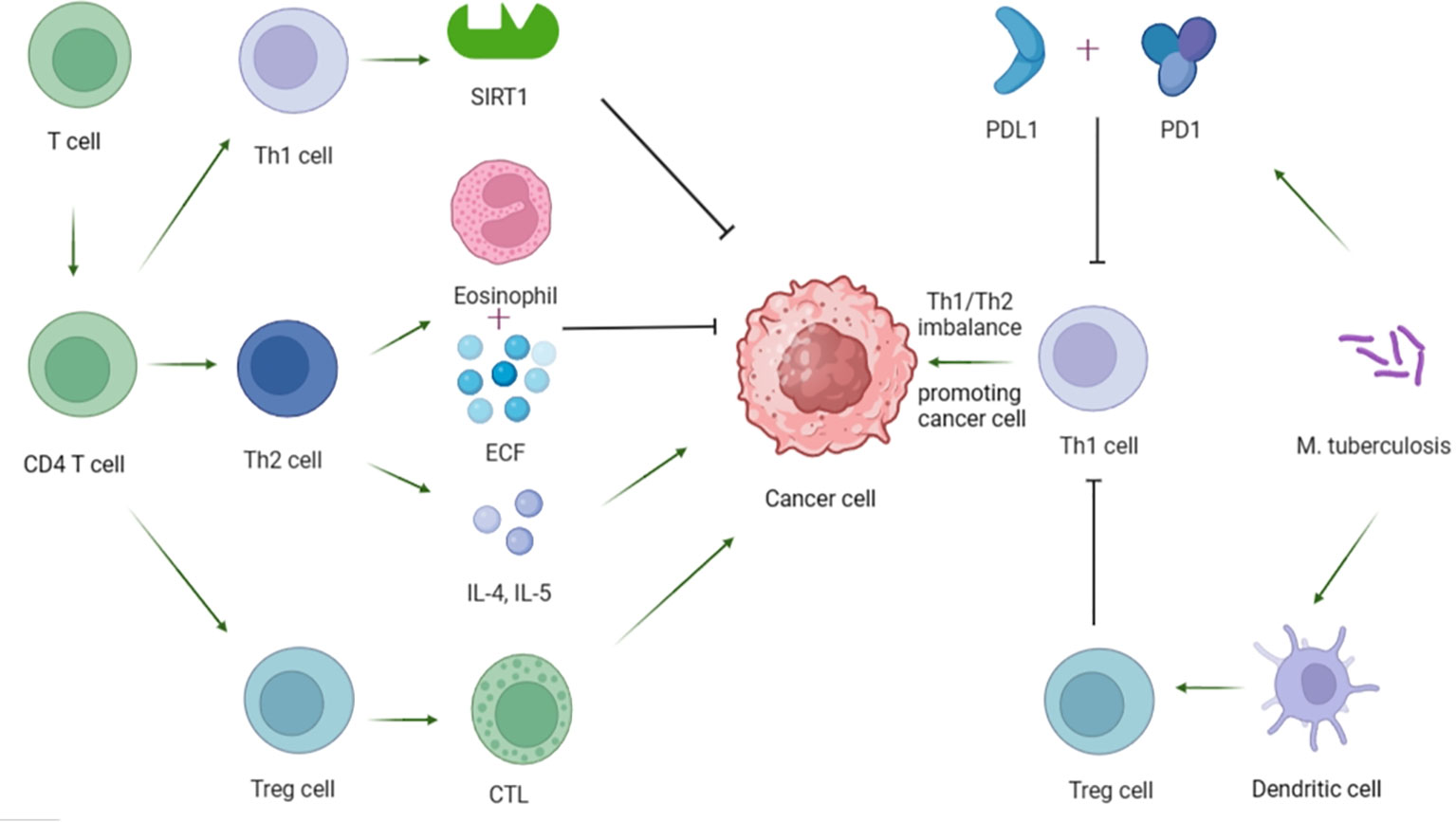
T cells play a key role in regulating tumor growth and metastasis. Among them, CD4+ T cells can be differentiated into Th1, Th2 and regulatory T cells (Tregs) according to different functions and markers. Th1 regulates immune cell function by increasing the activity of silence-message regulatory factor-associated enzyme 1 (SIRT1) dependent on nicotinamide adenine dinucleotide (NAD), thereby exerting anti-tumor effects (39). Th2 is generally believed to play an anti-tumor role through the expression of eosinophilic and eosinophilic chemotactic factor (ECF), and some scholars have also proved that TH2 can play a pro-tumor role through the secretion of cytokines IL-4 and IL-5 (40). More researchers profess the importance of Th1/Th2 imbalance leading to impaired immune function and augmented escape of tumor cells, thus promoting tumor formation (41). At the same time, studies have shown that Th1/Th2 imbalance in peripheral blood is closely related to TB nosogenesis (42). TB patients show reduced Th1 response and/or enhanced Th2 response (43), and the severity of the disease is closely related to Th1 response, the lower the Th1 response, the more severe the disease (44). To delve into the potential mechanism, Cao et al. compared the immune response of T cells in patients with active pulmonary TB and mice treated with MTB and lung cancer cells, and proved that MTB may inhibit Th1 immune response through the programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) signaling pathway. This may lead to Th1/Th2 imbalance and further promote lung cancer metastasis (45). In recent years, studies on the pathogenesis of Tregs in cancer have also attracted the attention of many researchers (41). Tregs can protect tumor cells by inhibiting cytotoxic T lymphocytes (CTL) mediated apoptosis (39, 41, 46). Furthermore, Jie et al. found that Tregs was closely related to the occurrence of TB, and the results showed that the percentage of Tregs in peripheral blood of patients with active TB was significantly higher than that in the latent TB group or control group (Tregs: 11.44 +/- 2.69% vs. 7.54 +/- 1.56% vs. 4.10 +/- 0.99%, p < 0.05) (47). The possible mechanism is that MTB can enhance the polarization ability of Tregs by driving the high expression of BTLA in dendritic cells (48). The increase of Tregs activity can lead to immunosuppression and a decrease in the number of Th1 cells, which are conducive to the occurrence and progression of skin cancer (49). In addition, it has been proved that the production of Tregs and its inhibitory properties can also be regulated through the expression of PD-1 and the binding of PD-1 to PD-L1 (46). For this reason, the treatment of immune checkpoint inhibitors (ICIs) combined with PD-1 or PD-L1, especially for cancers such as non-small cell lung cancer, has shown remarkable efficacy, and may also be of great significance for the treatment of lung cancer caused by TB (46) (Figure 3).
3.1.2 Myeloid derived suppressor cells
MDSC is a group of heterogeneous cells from bone marrow, which can inhibit immune cell response through reactive oxygen species (ROS) molecules and other pathways, and it has been proved by abundant evidences that it plays an important role in promoting tumor malignant growth and metastasis in tumor immunity (50–53). The reason: On the one hand, in the tumor microenvironment, MDSC exerts an inhibitory effect to produce excess ROS, while MDSC up-regulates the production of ROS in the high-concentration ROS environment. ROS can produce highly destructive hydroxyl radicals, resulting in the damage of DNA, proteins and lipids in the body. At the same time, oncogenes jun and fos are also activated, which eventually lead to the occurrence of tumors under the action of various factors (5, 54–56). On the other hand, in order to maintain an immunosuppressor environment, MDSC secretes a series of chemokines, thereby playing a role in the recruitment of Tregs and thus promoting the development of tumors (41, 46, 57). In addition to its role in tumors, MDSC has gradually been shown to play an important role in many chronic infectious diseases, especially in TB (58). Studies have shown (59) that under abnormal conditions of chronic infections such as TB, excessive production of MDSC will be caused to inhibit host protective T lymphocyte response. The possible mechanisms are as follows: First, MDSC changes the polarization direction of monocytes and macrophages by producing ROS, thereby inhibiting the anti-inflammatory response; On the other hand, studies have shown that MDSC inhibits the immune response of T cells and effector B cells by inducing cells such as Tregs (58). Therefore, it is very possible that MDSC is produced after infection with Mycobacterium TB, and MDSC ultimately promotes the occurrence and development of tumors through up-regulation of ROS and recruitment of Tregs.
3.1.3 Macrophages
Tumor-associated macrophages (TAMs) are one of the major tumor-infiltrating immune cell types and are generally divided into one of two functional contrast subtypes, classically activated M1 macrophages and replacement-activated M2 macrophages. The former usually exerts anti-tumor functions, including directly mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) to kill tumor cells; the latter promotes the formation and metastasis of tumor cells by inhibiting the anti-tumor immune response mediated by T cells. When the body is exposed to MTB, macrophages play an important role as the main innate immune cells (60, 61). The relevant mechanism of action may be as follows: First, it has been found that IL-37 induces the polarization of macrophages towards M2-like phenotype during MTB infection (62, 63), which may further promote the occurrence and metastasis of tumor cells. Secondly, MTB toxic factor ESAT-6 can drive the polarization of macrophages towards pro-inflammatory M1 phenotype, and subsequently towards anti-inflammatory M2 phenotype, and can also drive the transformation of activated M1 phenotype into M2 phenotype (64), which may play a role in promoting tumor. In addition, in the process of inflammation, activated macrophages will gather at the site of MTB infection and produce a large amount of active nitrogen and ROS, etc. ROS promotes the occurrence and development of tumors by activating oncogenes jun and fos (54, 55). Some scholars used a mouse model infected with MTB to test the effect of macrophage depletion on the occurrence of lung cancer, and the results showed that the incidence of lung cancer in TB mice with macrophage depletion was significantly lower than that in control TB mice without macrophage intervention (65).
3.1.4 Epidermal growth factor receptor signaling
EGFR belongs to the ERBB family of tyrosine kinase receptors. The EGFR signaling cascade is a key regulator of cell proliferation, differentiation, division, survival, and cell proliferation, and has been shown to play an important role in regulating the proliferation, survival, and differentiation of tumor cells, and is highly expressed in more than 60% of Nonsmall Cell Lung Cancer (NSCLC) (66). In recent years, more and more researchers have provided evidence that MTB can cause tumors through the EGFR pathway: Nalbandian et al. found that MTB infected macrophages induce the production of highly efficient epidermal regulatory hormone (EREG), thus activating the EGFR signaling pathway through EREG and promoting cancer progression (5, 67). Meanwhile, studies have demonstrated in mouse models that MTB infection can stimulate the expression of EREG in a toll-like receptor 2 (TLR2) -dependent manner, and EREG binds to EGFR in membrane or soluble form to stimulate downstream signal transduction, thereby inducing activation and mutation of k-ras gene, leading to the occurrence of lung cancer (67, 68) (Figure 4).
In addition, EGFR mutations are often directly or indirectly associated with the pathogenesis of some cancers with high global morbidity and mortality, such as lung cancer, pancreatic cancer and head and neck cancer (69–73). Approximately 15% of Caucasian patients and 30%-40% of Asian patients with lung adenocarcinoma carry EGFR mutations (74). Among them, the two major classical mutations are exon 19 deletion and exon 21 L858R mutation, with the incidence of 44% and 41%, respectively (75). EGFR mutations are highly prevalent in tumors, and TB can cause high EGFR mutations. Luo et al. (70) found that patients with lung scar cancer or old TB had a higher incidence of EGFR mutation (p =0.018), especially exon 19 deletion (p<0.001), in patients with lung adenocarcinoma than those without TB focal points. This was also fully demonstrated in a retrospective study of nearly 500 patients with lung adenocarcinoma (76), with a higher frequency of EGFR mutations in the TB group than in the non-TB group (56% vs 34%, p=0.038). A study in Taiwan evaluated the correlation of EGFR mutation outcomes in 275 TB patients (77), with 191 patients (69.5%) having a high EGFR mutation in their study. Although it is certain that TB can induce EGFR mutation and increase the risk of tumor development, the exact molecular mechanism remains to be further elucidated. TB not only affects the mutated status of EGFR, but also affects the treatment response of patients treated with EGFR tyrosine kinase inhibitors (TKIs). At present, EGFR-TKI targeted therapy has become the standard first-line treatment for patients with advanced EGFR-mutated NSCLC, which can significantly improve their prognosis (78). Simultaneous use of antituberculosis drugs and TKIs in a comorbidities patient for EGFR-mutated lung cancer patients with active TB has shown a safe and alternative treatment strategy (79).
3.2 Possible mechanisms of tumor-induced TB
3.2.1 Tumor effect
Cancer is largely regarded as a disease caused by gene mutation and gene alteration, and the metabolic changes of tumor cells driven by oncogenes can limit the immune response of the body by affecting the tumor microenvironment (TME), thus causing serious adverse effects on the normal immune defense function of the body (80, 81). At present, the mechanism of TB induced by tumor itself is not completely clear. The possible mechanisms are as follows: First, there is a high level of adenosine triphosphate (ATP) in TME, and CD73 decomposes ATP through dephosphorylation, and finally produces adenosine (82). Adenosine has a significant inhibitory effect on immune response (83) and can also play a protective role in extracellular bacteria and fungi, including MTB, by mediating type 3 immune effects (84). In addition, studies on lung cancer patients and mouse models have proved that lung cancer can lead to the loss of microbial diversity, the reduction of the total amount of bacteria and the change of bacterial composition, which can lead to the imbalance of microbial flora, resulting in the reduction of the stability of the body’s immune homeostasis, and thus increase the host’s susceptibility to various pathogens (85, 86). Studies have shown that up to 50%-70% of lung cancer patients are complicated by lung infection during the course of the disease (87). Wessels et al. ‘s study on children showed that the risk of TB among children with malignant tumors was indeed higher than that of ordinary children, and the risk ratio reached 11 times (88).
3.2.2 Chemotherapy
Platinum-based chemotherapy is recognized as the standard treatment for patients with stage II and III non-small cell lung cancer (NSCLC) and is often considered for stage IB patients with tumors ≥4cm (87). Chemotherapy drugs have an effective killing effect on tumor cells, but they also affect the immune system of patients. Investigation in TB endemic areas found that TB infection was common in systemic chemotherapy (89). On the one hand, chemotherapy can produce immunosuppressive effects on cellular immunity and immunoglobulin, affecting the normal immune function of patients. Chemotherapy, on the other hand, can cause neutropenia and leukopenia by inhibiting bone marrow function, leading to a state of severe immune deficiency with symptoms such as cough, hypoxia, dyspnea, and fever. In addition, although chemotherapy-induced pulmonary toxicity is rare in clinical practice, it can be manifested as hypersensitivity reaction, interstitial pneumonia, non-cardiogenic pulmonary edema, pleural effusion, obliterated bronchiolitis, tissue pneumonia and progressive pulmonary fibrosis. The above diseases all affect the normal immune defense function of the normal lung, thereby increasing the susceptibility of the lung to bacteria including MTB (90). Clinically, there have been reports of patients with non-small cell lung cancer infected with MTB after receiving standard chemotherapy based on bisplatin and pulmonary TB during postoperative chemotherapy for gastric cancer (91, 92). Another study, which recruited 1,809 cancer cases and 1,809 control subjects and followed them for 3 years, showed that immunosuppression of cancer or anti-cancer chemotherapy increased the risk of TB reactivation, especially in cancer patients with age-cured TB (93).
3.2.3 Immunotherapy
As new immune checkpoint inhibitors continue to enter clinical trials, ICIs is becoming one of the most important immunotherapy therapies. lCIs mainly inhibits immune checkpoint proteins by blocking CTLA-4, PD-1 and PD-L1. At present, immunotherapy of PD-1/PD-L1 has significantly prolonged the survival of patients with advanced lung cancer, and has become the standard treatment choice for the first and second line of advanced lung cancer (94). However, when ICIs are selected, they also affect immune function, which may increase susceptibility to MTB infection. There are two possible reasons for this: First, because ICIs works by blocking CTLA-4, PD-1, and PD-L1 to inhibit immune checkpoint proteins, model studies in knockout mice have demonstrated that conditions in which CTLA-4 and PD-1 are absent may lead to imbalanced immune homeostasis, resulting in reduced host immunity (95–97). Studies have shown that immunocompromised people with latent infections have a higher rate of active TB. Therefore, ICIs leads to a decrease in the body’s immunity, which may be one of the reasons for an increased susceptibility to MTB infection. In addition, pneumonia caused by ICIs is the most common pulmonary toxic reaction, which may also be one of the reasons for the increased susceptibility to MTB infection (98–100). Macrophage apoptosis plays an important role in pneumonia. Through the mutual influence and stimulation of inflammation and apoptosis, the apoptosis of macrophages is accelerated while the inflammation is aggravated (101). After MTB enters the body through the respiratory tract, the body mainly plays a defense role through macrophages and other cells. Therefore, apoptosis of macrophages increases the risk of TB and latent TB reactivation. Through comparative analysis of the function of macrophages at different stages of pneumonia in mice, it was found that macrophages’ ability to phagocytic bacteria was still poor several weeks after the inflammation subsided (102).
4 Progress in diagnosis and treatment of TCWT
4.1 Progress in the diagnosis of TCWT
At present, the research on the diagnosis technology and treatment plan of TCWT is limited by the variety of tumor types and the uncertain site of TB, and there is no standardized detection means and diagnostic technical guide for patients with TCWT. There are differences in the pathogenesis of TB combined with different tumors, which may lead to differences in detection and diagnosis techniques. For example, early clinical symptoms and CT results of TB and lung cancer are similar, and TB detection technology plus conventional detection technology cannot accurately diagnose (103–105).
Studies on the differential diagnosis of TB combined with malignant tumor FDG-PE/CT carried out in South Korea, Singapore and other countries have shown that FDG-PET/CT has a good diagnostic effect on lung cancer, lymph node and other malignant tumors. Meanwhile, FDG-PET/CT can be used for targeted screening of patients with latent TB infection before immunosuppressive therapy. A useful tool for assessing and excluding active disease sites (104, 106–108). Su Hongjian et al. detected the expression levels of LINC00665 and miR-589-3p in the serum of participants by real-time fluorescent quantitative PCR. Combined diagnosis has better diagnostic value for lung cancer complicated with TB than single detection (109). A study on the use of tumor biomarkers in the diagnosis of pulmonary TB and lung cancer found that the combined detection of CEA, CYFRA21-1 and NSE had diagnostic value for high-risk lung cancer patients with pulmonary TB, with a specificity of 89.9% and a sensitivity of 94.9%. ROC curve analysis showed that CEA+ CYFRA21-1+ NSE had the highest diagnostic accuracy (AUC=0.972) (103). In addition, Han Dongmei et al. used chemiluminescence immunoassay to detect tumor markers in participants. Serum tumor markers combined with CT have a good diagnostic effect on lung cancer complicated with TB (110). Xu Yang et al. used electrochemiluminescence to rank the clinical diagnostic value of patients, from low to high, CEA, CA125, CT and combined diagnosis, among which CT combined with CEA and CA125 in the diagnosis of lung cancer combined with TB had better clinical value (107).
In recent years, RNA and proteomics technologies have also been widely used in the diagnosis of TB and tumor (111–113). Liu et al. used miRCURYTM LAN microRNA to identify fresh peripheral blood mononuclear cells (PBMCs) from microscopic smear-positive pulmonary TB patients and healthy people, and miRNAs in patients with active TB were significantly up-regulated compared with healthy people (114). Other scholars can obtain Ts-RNA from sncRNAs of fresh peripheral blood mononuclear cells (PBMC), which is helpful for early diagnosis of lung cancer and TB (112, 115). At the same time, some studies have applied miRNA as a new biomarker to the diagnosis and prognosis of cancer diseases, and achieved good results (116). This study suggests that RNA biomarkers can be used as a new diagnostic technique in patients with TCWT. Sun et al. used proteomic techniques to compare the proteomic features and plasma protein biomarkers of patients with TB (n=15), patients with latent TB (n=15), and healthy people (n=15). A total of 31 overlapping proteins with significantly different expression levels were identified in patients with PTB compared with LTBI and healthy people. Among them, the diagnostic model composed of α1-antichymotrypsin (ACT), α1-acidic glycoprotein-1 (AGP1), and e-cadhrin (CDH1) had a sensitivity of 75.0% (21/28) and 81.8% (27/33) for PTB and lung cancer, respectively (111). This also suggests that protein can be used as a new diagnostic technology for patients with TCWT, and provides a possibility for optimizing and improving diagnostic technology for subsequent patients with TCWT.
4.2 Progress in diagnosis of TCWT
The current anti-TB treatment mainly applies WHO TB guidelines to patients with active TB, and the combination of isoniazid, rifampicin and pyrazinamide is used for 6-9 months (117). If patients are resistant to drugs, the medication regimen needs to be adjusted, and 2-5 sensitive or unused anti-TB drugs should be selected. The whole process of supervised chemotherapy management was implemented to complete treatment for 18-24 months (118, 119). Relatively speaking, cancer treatment means are more diversified, including surgery, chemotherapy and radiation therapy, targeted therapy, immunotherapy, cell therapy and other therapies, which can bring significant benefits to patients (120, 121).
Studies have shown that anti-TB drugs (isoniazid) can cause the decrease of white blood cells and platelets, resulting in liver damage, and cause chemotherapy response in tumor patients, which may act as a suppressant of the therapeutic effect (122, 123). Another study has shown that when chemotherapy and anti-TB therapy for malignant tumors are carried out simultaneously, the treatment of patients is effective and safe (124). It is suggested that the treatment of patients with TCWT is more complicated and uncertain, and the etiology, pathogenesis and therapeutic drug interaction of the two diseases should be comprehensively considered in the formulation of treatment plan (89, 124). For patients with latent TB complicated with lung cancer, anti-TB therapy is generally not required, but only anti-tumor therapy. For patients with active TB, anti-tumor chemotherapy and anti-TB therapy are required (121). Patients with urinary TB complicated with bladder cancer are prone to misdiagnosis or missed diagnosis, which is likely to delay the treatment time and lead to inappropriate treatment options (125). For patients requiring surgical resection of the tumor, preoperative tumor chemotherapy and anti-TB treatment should be synchronized, and anti-TB drugs should be continued after surgery (126), which is conducive to enhancing the therapeutic effect.
In a study of mycobacterium TB infection in mice, mice co-expressed other inhibitory receptors (including PD-1) and were effectively treated with anti-TIM-3 monoclonal antibodies against TB, suggesting that targeting TIM-3 may have significant therapeutic effects in both TB and lung cancer patients (127). In addition, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), lymphocyte-activating gene-3 (LAG-3), and glucocorticoid-induced TNF receptor (GITR) have gradually become a hot spot for drug therapy to find new targets for TCWT (128). For TCWT, the interaction between drugs should be considered when the anti-TB treatment is synchronized with the targeted therapy of tyrosine kinase inhibitors (TKI). For example, rifampicin can accelerate the metabolism of TKI drugs such as gefitinib, while isoniazid has an inhibitory effect. In order to improve clinical effect, appropriate targeted drugs or immune drugs should be selected according to the drug sensitivity results of patients (129).
In recent years, people have paid more and more attention to the immune evasion of tumor cells and the immunotherapy of Mycobacterium TB, and immunotherapy has gradually become the main treatment methods in the field of tumor, and immune checkpoint inhibitors (ICI) and PD-1/PD-L1 inhibitors have been applied to the treatment of tumor patients with TB (130, 131). Studies have shown that PD-1 is highly expressed in pathological sections of patients with TB complicated with lung cancer (132). Blocking PD-1 may activate T cell function and enhance immune response to tumor and MTB, and PD-1 blocking therapy has important clinical significance in improving the prognosis of patients with TCWT. At the same time, patients with tumor combined with TB should be cautious about using immune checkpoint inhibitor (ICI) or PD-1/PD-L1 treatment. Recent evidence suggests that tumor patients treated with immune checkpoint inhibitors (ICI) may promote TB reactivation or accelerated progression of TB infection (133). Another literature review discussed the incidence of TB caused by PD-1/PD-L1 therapy in tumor patients. Compared with tumor patients receiving PD-1/PD-L1 blocking therapy, the incidence of TB was 35 times that of the general population, reaching every 2,000 cases/100,000 people (134). Clinical use of PD-1/PD-L1 inhibitors may significantly increase the risk of TB reactivation and death in patients (135). Based on this, in clinical practice, clinicians should take comprehensive consideration and risk assessment to develop immunotherapy programs for TCWT, so as to improve the quality and safety of patient diagnosis and treatment. In summary, in the clinical treatment of patients with TCWT, it is necessary to consider the comorbidities to develop joint treatment strategies, and select appropriate treatment methods to play a synergistic or positive role in TB and tumors.
At present, a number of nanomedicine (preparations) have been approved for the treatment of advanced non-small cell lung cancer and other tumors (136, 137). Studies have also shown that nanoparticles have a higher biological distribution in the tumor region and a stronger tumor inhibitory effect (138). In addition, nanotherapy has also been applied to anti-TB therapy, and the nano drug delivery system can further improve the efficacy of anti-TB drugs (139, 140). A variety of nanotechnology has been carried out to study the diagnosis and treatment of TB, and the research results show that the efficacy is good (141–143). Nanotechnology offers more possible strategies for the diagnosis and treatment of TCWT. Currently, nano-dose inhalers have been developed for the treatment of lung cancer and TB, and can also be applied in patients with TB and lung cancer (144). In the future, these new technologies will play an important role in the treatment of patients with TCWT, especially for patients with TB combined with malignant tumor, which will help improve the treatment effect of patients and bring greater benefits to patients.
5 Summary
With the progress of science and technology and the diversification of treatment methods, more and more diagnostic methods and advanced treatment technologies have been applied to clinical diagnosis and treatment. The diagnosis, treatment and prevention of TCWT should also be forward-looking, and the application of new diagnostic technologies such as single-cell technology and proteomics in the diagnosis of TB and tumor patients should be actively explored. In the treatment of TB and cancer patients, it is also necessary to actively explore the clinical practice of advanced therapies such as cell therapy and nanotechnology in their diagnosis and treatment, and carry out efficient scientific treatment technologies that are leading, improving and popular. Through systematic literature analysis, we mainly studied the pathogenesis, mechanism, diagnosis and treatment of patients with TCWT. The results showed that TB and tumors interact and influence each other, suggesting that TB and tumor should be diagnosed and treated from a holistic perspective of comorbidities. Integrated and individualized treatment protocols should be developed for patients with an eye to effectively improve the therapeutic effects, and provide ideas for the follow-up clinical research of TCWT, the development of diagnostic technology and the improvement of integrated treatment protocols.
Author contributions
CW: Data curation, Formal Analysis, Investigation, Writing – original draft. R-QZ: Writing – review & editing. G-ZH: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors acknowledge the support of 1.National Natural Science Foundation of China(Approval No.: 72264021);2.the Major Research Project of Philosophy and Social Sciences by the Ministry of Education of the People’s Republic of China (Approval No.: 21JZD039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Auguste P, Tsertsvadze A, Pink J, Court R, Seedat F, Gurung T, et al. Accurate diagnosis of latent tuberculosis in children, people who are immunocompromised or at risk from immunosuppression and recent arrivals from countries with a high incidence of tuberculosis: systematic review and economic evaluation. Health Technol Assess. (2016) 20(38):1–678. doi: 10.3310/hta20380
2. Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis (2015) 61 Suppl 3(Suppl 3):S179–87. doi: 10.1093/cid/civ581
3. World Health Organization Global Tubeclosis report. Geneva: World Health Organization (2023). Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023.
4. Marais BJ, Loennroth K, Lawn SD, Migliori GB, Mwaba P, Glaziou P, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis (2013) 13:436–48. doi: 10.1016/S1473-3099(13)70015-X
5. Nalbandian A, Yan BS, Pichugin A, Bronson RT, Kramnik I. Lung carcinogenesis induced by chronic tuberculosis infection: the experimental model and genetic control. Oncogene (2009) 28(17):1928–38. doi: 10.1038/onc.2009.32
6. Gupta PK, Tripathi D, Kulkarni S, Rajan MG. Mycobacterium tuberculosis H37Rv infected THP-1 cells induce epithelial mesenchymal transition (EMT) in lung adenocarcinoma epithelial cell line (A549). Cell Immunol (2016) 300:33–40. doi: 10.1016/j.cellimm.2015.11.007
7. Leung CC, Hui L, Lee RS, Lam TH, Yew WW, Hui DS, et al. Tuberculosis is associated with increased lung cancer mortality. Int J tuberculosis Lung Dis (2013) 17(5):687–92. doi: 10.5588/ijtld.12.0816
8. Zhou Y, Cui Z, Zhou X, Chen C, Jiang S, Hu Z. The presence of old pulmonary tuberculosis is an independent prognostic factor for squamous cell lung cancer survival. J Cardiothor Surg (2013) 8(1):123. doi: 10.1186/1749-8090-8-123
9. Simonsen DF, Farkas DK, Søgaard M, Horsburgh CR, Sørensen HT, Thomsen RW. Tuberculosis and risk of cancer: a Danish nationwide cohort study. Int J Tuberculosis Lung Dis (2014) 18(10):1211–9. doi: 10.5588/ijtld.14.0161
10. Anastasopoulou A, Ziogas DC, Samarkos M, Kirkwood JM, Gogas H. Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: current evidence and clinical practice recommendations. J immunother Cancer (2019) 7(1):1–13. doi: 10.1186/s40425-019-0717-7
11. Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li CP, et al. Aerodigestive tract, lung and haematological cancers are risk factors for tuberculosis: an 8-year population-based study. Int J Tuberc Lung Dis (2011) 15(1):125–30.
12. Dai G, Phalen S, McMurray DN. Nutritional modulation of host responses to mycobacteria. Front Biosci (1998) 3:e110–22. doi: 10.2741/a371
13. Lamplugh Z, Fan Y. Vascular microenvironment, tumor immunity and immunotherapy. Front Immunol (2021) 12:811485. doi: 10.3389/fimmu.2021.811485
14. Bickett TE, Karam SD. Tuberculosis-cancer parallels in immune response regulation. Int J Mol Sci (2020) 21(17):6136. doi: 10.3390/ijms21176136
15. Kuo CH, Lo CY, Chung FT, Lee KY, Lin SM, Wang CH, et al. Concomitant active tuberculosis prolongs survival in non-small cell lung cancer: a study in a tuberculosis-endemic country. PloS One (2012) 7(3):e33226. doi: 10.1371/journal.pone.0033226
16. Wong JY, Zhang H, Hsiung CA, Shiraishi K, Yu K, Matsuo K, et al. Tuberculosis infection and lung adenocarcinoma: Mendelian randomization and pathway analysis of genome-wide association study data from never-smoking Asian women. Genomics (2020) 112(2):1223–1232. doi: 10.1016/j.ygeno.2019.07.008
17. Leung CY, Huang HL, Rahman MM, Nomura S, Krull Abe S, Saito E. Shibuya K.Cancer incidence attributable to tuberculosis in 2015: global, regional, and national estimates. BMC Cancer (2020) 20(1):412. doi: 10.1186/s12885-020-06891-5
18. Shu CC, Liao KM, Chen YC, Wang JJ, Ho CH. The burdens of tuberculosis on patients with Malignancy: incidence, mortality and relapse. Sci Rep (2019) 9:11901. doi: 10.1038/s41598-019-48395-8
19. Kuo SC, Hu YW, Liu CJ, Lee YT, Chen YT, Chen TL, et al. Association between tuberculosis infections and non-pulmonary Malignancies: a nationwide population-based study. Br J Cancer (2013) 109(1):229–34. doi: 10.1038/bjc.2013.220
20. Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li CP, et al. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer (2011) 117:618–24. doi: 10.1002/cncr.25616
21. Cha SI, Shin KM, Lee JW, Lee SY, Kim CH, Park JY, et al. The clinical course of respiratory tuberculosis in lung cancer patients. Int J Tuberc Lung Dis (2009) 13:1002–7.
22. Matsuo M. Development of active tuberculosis during treatment of head and neck carcinoma: a case series. J Med Case Rep (2019) 13(1):162. doi: 10.1186/s13256-019-2055-2
23. Luczynski P, Poulin P, Romanowski K, Johnston JC. Tuberculosis and risk of cancer: A systematic review and meta-analysis. PloS One (2022) 17(12):e0278661. doi: 10.1371/journal.pone.0278661
24. Askling J, Ekbom A. Risk of non-Hodgkin’s lymphoma following tuberculosis. Br J Cancer (2001) 84(1):113–5. doi: 10.1054/bjoc.2000.1551
25. Chen GL, Xia ZG, Jin J, Yu BH, Cao J. Characterization of artificial pneumothorax-unrelated Pyothorax-associated lymphoma. J Oncol (2021) 2021:3869438. doi: 10.1155/2021/3869438
26. Chen GL, Guo L, Yang S, Ji DM. Cancer risk in tuberculosis patients in a high endemic area. BMC Cancer (2021) 21(1):679. doi: 10.1186/s12885-021-08391-6
27. Hwang SY, Kim JY, Lee HS, Lee S, Kim D, Kim S, et al. Pulmonary tuberculosis and risk of lung cancer: Asystematic review and meta-analysis. J.Clin.Med (2022) 11:765. doi: 10.3390/jcm11030765
28. An SJ, Kim YJ, Han SS, Heo J. Effects of age on the association between pulmonary tuberculosis and lung cancer in a South Korean cohort. J Thorac Dis (2020) 12:375–382.14. doi: 10.21037/jtd.2020.01.38
29. Shiels MS, Albanes D, Virtamo J, Engels EA. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev (2011) 20(4):672–8. doi: 10.1158/1055-9965.EPI-10-1166
30. Amaral EP, Vinhaes CL, Oliveira-De-Souza D, Nogueira B, Akrami KM, Andrade BB. The interplay between systemic inflammation, oxidative stress, and tissue remodeling in tuberculosis. Antioxid Redox Signal (2021) 34(6):471–85. doi: 10.1089/ars.2020.8124
31. Di W, Shen B. Mechanism of action and clinical research status of immunotherapy for non-small cell lung cancer. J Nanjing Med Univ (Natural Sci Edition) (2020) 40(11):1739–46. doi: 10.7655/NYDXBNS20201130
32. Kumar DS, Ronald LA, Romanowski K, Rose C, Shulha HP, Cook VJ, et al. Risk of active tuberculosis in migrants diagnosed with cancer: a retrospective cohort study in British Columbia, Canada. BMJ Open (2021) 11(3):e037827. doi: 10.1136/bmjopen-2020-037827
33. Cheng MP, Abou Chakra CN, Yansouni CP, Cnossen S, Shrier I, Menzies D, et al. Risk of active tuberculosis in patients with cancer: A systematic review and meta-analysis. Clin Infect Dis (2017) 64(5):635–44. doi: 10.1093/cid/ciw838
34. Wallis RS. Mathematical modeling of the cause of tuberculosis during tumor necrosis factor blockade. Arthritis Rheumatol (2008) 58(4):947–52. doi: 10.1002/art.23285
35. Liu C-J, Hong Y-C, Teng C-J, Hung M-H, Hu Y-W, Ku F-C, et al. Risk and impact of tuberculosis in patients with chronic myeloid leukemia: a nationwide population-based study in Taiwan. Int J Cancer (2015) 136(8):1881–7. doi: 10.1002/ijc.29201
36. Dobler CC, Cheung K, Nguyen J, Martin A. Risk of tuberculosis in patients with solid cancers and haematological Malignancies: a systematic review and meta-analysis. Eur Respir J (2017) 50(2):1700157. doi: 10.1183/13993003.00157-2017
37. Seo GH, Kim MJ, Seo S, Hwang B, Lee E, Yun Y, et al. Cancer-specific incidence rates of tuberculosis: A 5-year nationwide population-based study in a country with an intermediate tuberculosis burden. Med (Baltim) (2016) 95(38):e4919. doi: 10.1097/MD.0000000000004919
38. Simonsen DF, Farkas DK, Horsburgh CR, Thomsen RW, Sørensen HT. Increased risk of active tuberculosis after cancer diagnosis. J Infect (2017) 74(6):590–8. doi: 10.1016/j.jinf.2017.03.012
39. Chatterjee S, Daenthanasanmak A. CD38-NAD+Axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab (2018) 27(1):85–100.e8. doi: 10.1016/j.cmet.2017.10.006
40. Schreiber S, Hammers CM, Kaasch AJ, Schraven B, Dudeck A. Kahlfuss S.Metabolic interdependency of th2 cell-mediated type 2 immunity and the tumor microenvironment. Front Immunol (2021) 12:632581. doi: 10.3389/fimmu.2021.632581
41. Duan MC, Zhong XN, Liu GN, Wei JR. The Treg/Th17 paradigm in lung cancer. J Immunol Res (2014) 2014:730380. doi: 10.1155/2014/730380
42. da Silva MV, Tiburcio MG, MaChado JR, Silva DA, Rodrigues DB, Rodrigues V, et al. Complexity and controversies over the cytokine profifiles of T helper cell subpopulations in tuberculosis. J Immunol Res (2015) 2015:639107. doi: 10.1155/2015/639107
43. Zhang L, Jiang Y, Cui Z, Yang W, Yue L, Ma Y, et al. Mycobacterium vaccae induces a strong Th1 response that subsequently declines in C57BL/6 mice. J Vet Sci (2016) 17:505–13. doi: 10.4142/jvs.2016.17.4.505
44. Handzel ZT, Barak V, Altman Y, Bibi H, Lidgi M, Iancovici-Kidon M, et al. Increased Thl and Th2 type cytokine production in patients with active tuberculosis. Isr. Med Assoc J (2007) 9:479–83.
45. Cao SH, Li JW, Lu J, Zhong R, Zhong H. Mycobacterium tuberculosis antigens repress Th1 immune response suppression and promotes lung cancer metastasis through PD-1/PDl-1 signaling pathway. Cell Death Dis (2019) 10(2):44. doi: 10.1038/s41419-018-1237-y
46. Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol (2017) 47(5):765–79. doi: 10.1002/eji.201646875
47. Luo J, Zhang M, Yan B, Zhang K, Chen M, Deng S. Imbalance of Th17 and Treg in peripheral blood mononuclear cells of active tuberculosis patients. Braz J Infect Dis (2017) 21(2):155–61. doi: 10.1016/j.bjid.2016.10.011
48. Zhang JA, Lu YB, Wang WD, Liu GB. BTLA-expressing dendritic cells in patients with tuberculosis exhibit reduced production of IL-12/IFN-α and increased production of IL-4 and TGF-β, favoring th2 and foxp3 treg polarization. Front Immunol (2020) 11:518. doi: 10.3389/fimmu.2020.00518
49. Gianchecchi E, Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in treg development and their involvement in autoimmunity onset and cancer progression. Front Immunol (2018) 9:2374. doi: 10.3389/fimmu.2018.02374
50. Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol (2018) 9:2499. doi: 10.3389/fimmu.2018.02499
51. Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, et al. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol (2009) 9:937–48. doi: 10.1016/j.intimp.2009.03.021
52. Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res (2017) 5(1):3–8. doi: 10.1158/2326-6066.CIR-16-0297
53. Umansky V, Blattner C, Gebhardt C, Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines (2016) 4:36. doi: 10.3390/vaccines4040036
54. Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, et al. HIF-1αregulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med (2010) 207:2439–53. doi: 10.1084/jem.20100587
55. Zhai W, Wu F, Zhang Y, Fu Y, Liu Z. The immune escape mechanisms of mycobacterium tuberculosis. Int J Mol Sci (2019) 20(2):340. doi: 10.3390/ijms20020340
56. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol (2018) 80:50–64. doi: 10.1016/j.semcdb.2017.05.023
57. Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest (2015) 125(9):3365–76. doi: 10.1172/JCI80006
58. Munansangu BSM, Kenyon C, Walzl G, Loxton AG, Kotze LA, du Plessis N. Immunometabolism of myeloid-derived suppressor cells: implications for mycobacterium tuberculosis infection and insights from tumor biology. Int J Mol Sci (2022) 23(7):3512. doi: 10.3390/ijms23073512
59. Ray A, Chakraborty K, Ray P. Immunosuppressive MDSCs induced by TLR signaling during infection and role in resolution of inflammation. Front Cell Infect Microbiol (2013) 3:52. doi: 10.3389/fcimb.2013.00052
60. Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol (2011) 4(3):288–93. doi: 10.1038/mi.2011.10
61. Khan A, Singh VK, Hunter RL, Jagannath C. Macrophage heterogeneity and plasticity in tuberculosis. J Leukoc Biol (2019) 106(2):275–82. doi: 10.1002/JLB.MR0318-095RR
62. Ward PS, Thompson CB. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell (2012) 21:297–308. doi: 10.1016/j.ccr.2012.02.014
63. Huang Z, Gao C, Chi X, Hu YW, Zheng L, Zeng T, et al. L-37 expression is upregulated in patients with tuberculosis and induces macrophages towards an M2-like phenotype. Scand J Immunol (2015) 82(4):370–9. doi: 10.1111/sji.12326
64. Refai A, Gritli S, Barbouche M-R, Essafi M. Mycobacterium tuberculosis virulent factor ESAT-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front Cell Infect Microbiol (2018) 8:327. doi: 10.3389/fcimb.2018.00327
65. Li J, Pan Y, Zhang B, Chen Q. Macrophages are needed in the Progression of tuberculosis into lungcancer. Tumour Biol (2015) 36(8):6063–6. doi: 10.1007/s13277-015-3283-8
66. Da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
67. Cheng WL, Feng PH, Lee KY, Chen KY, Sun WL, Van Hiep N, et al. The role of EREG/EGFR pathway in tumor progression. Int J Mol Sci (2021) 22(23):12828. doi: 10.3390/ijms222312828
68. Bauer AK, Velmurugan K, Xiong KN, Alexander CM, Xiong J, Brooks R. Epiregulin is required for lung tumor promotion in a murine two-stage carcinogenesis model. Mol Carcinog (2017) 56(1):94–105. doi: 10.1002/mc.22475
69. Wang ZX. ErbB receptors and cancer. Methods Mol Biol (2017) 1652:3–35. doi: 10.1007/978-1-4939-7219-7_1
70. Luo YH, Wu CH, Wu WS, Huang CY, Su WJ, Tsai CM. Association between tumor epidermal growth factor receptor mutation and pulmonary tuberculosis in patients with adenocarcinoma of the lungs. J Thorac Oncol (2012) 7(2):299–305. doi: 10.1097/JTO.0b013e31823c588d
71. Roskoski R Jr. he ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res (2014) 79:34–74. doi: 10.1016/j.phrs.2013.11.002
72. Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell (2014) 25(3):282–303. doi: 10.1016/j.ccr.2014.02.025
73. Jett JR, Carr LL. Targeted therapy for non-small cell lung cancer. Am J Respir Crit Care Med (2013) 188:907–12. doi: 10.1164/rccm.201301-0189PP
74. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol (2014) 9:154–62. doi: 10.1097/JTO.0000000000000033
75. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer, role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene (2009) 28:S24–31. doi: 10.1038/onc.2009.198
76. Hwang IK, Paik SS, Lee SH. Impact of pulmonary tuberculosis on the EGFR mutational status and clinical outcome in patients with lung adenocarcinoma. Cancer Res Treat (2019) 51(1):158–68. doi: 10.4143/crt.2018.084
77. Xie YL, Su N, Zhou W, Lei A, Li X, Li WW, et al. Concomitant pulmonary tuberculosis impair survival in advanced epidermal growth factor receptor (EGFR) mutant lung adenocarcinoma patients receiving EGFR-tyrosine kinase inhibitor. Cancer Manag Res (2021) 13:7517–26. doi: 10.2147/CMAR.S326349
78. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer. Int J Oncol (2021) 59(5):90. doi: 10.3892/ijo.2021.5270
79. Jin CB, Yang B. A case of delayed diagnostic pulmonary tuberculosis during targeted therapy in an EGFR mutant non-small cell lung cancer patient. Case Rep Onco (2021) 14(1):659–63. doi: 10.1159/000514050
80. Vaupel P, Mayer A. Hypoxia in tumors: Pathogenesis-related classification, characterization of hypoxia subtypes, and associated biological and clinical implications. Adv Exp Med Biol (2014) 812:19–24. doi: 10.1007/978-1-4939-0620-8_3
81. Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell (2020) 78(6):1019–33. doi: 10.1016/j.molcel.2020.05.034
82. Pansy K, Uhl B, Krstic J, Szmyra M, Fechter K, Santiso A, et al. Immune regulatory processes of the tumor microenvironment under malignant conditions. Int J Mol Sci (2021) 22(24):13311. doi: 10.3390/ijms222413311
83. Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer (2018) 6(1):57. doi: 10.1186/s40425-018-0360-8
84. Haskó G, Antonioli L, Cronstein BN. Adenosine metabolism, immunity and joint health. Biochem Pharmacol (2018) 151:307–13. doi: 10.1016/j.bcp.2018.02.002
85. Dong Q, Chen ES, Zhao C, Jin C. Host-microbiome interaction in lung cancer. Front Immunol (2021) 12:679829. doi: 10.3389/fimmu.2021.679829
86. Goto T. Microbiota and lung cancer. Semin Cancer Biol (2022) 86(Pt 3):1–10. doi: 10.1016/j.semcancer.2022.07.006
87. Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Am Soc Clin Oncol Educ Book (2017) 37:630–639. doi: 10.1200/EDBK_175188
88. Wessels G, Hesseling PB, Gie RP, Nel E. The increased risk of developing tuberculosis in children with Malignancy. Ann Trop Paediatr (1992) 12(3):277–81. doi: 10.1080/02724936.1992.11747585
89. Kim DK, Lee SW, Yoo CG, Kim YW, Han SK, Shim YS, et al. Clinical characteristics and treatment responses of tuberculosis in patients with Malignancy receiving anticancer chemotherapy. Chest (2005) 128:2218–22. doi: 10.1378/chest.128.4.2218
90. Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg Med Clin North Am (2014) 32(1):167–203. doi: 10.1016/j.emc.2013.09.002
91. Jacobs RE, Gu P, Chachoua A. Reactivation of pulmonary tuberculosis during cancer treatment. Int J Mycobacteriol (2015) 4(4):337–40. doi: 10.1016/j.ijmyco.2015.05.015
92. Nakajima T, Fujikawa T, Takagi Y, Shiroiwa H, Fujiwara H. A case of concomitant administration of anti-cancer drug and anti-tuberculosis drug for pulmonary tuberculosis developed during postoperative chemotherapy for gastric cance. Gan To Kagaku Ryoho (2021) 48(7):959–62.
93. Kim HR, Hwang SS, Ro YK, Jeon CH, Ha DY, Park SJ, et al. Solid-organ Malignancy as a risk factor for tuberculosis. Respirology (2008) 13(3):413–9. doi: 10.1111/j.1440-1843.2008.01282.x
94. Wu F, Zhou C. Current status and future prospects of immunotherapy for lung cancer. Chin J Surg Oncol (2020) 12(3):185–187. doi: 10.3969/j.issn.1674-4136.2020
95. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity (1995) 3(5):541– 7. doi: 10.1016/1074-7613(95)90125-6
96. Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science (1995) 270(5238):985– 8. doi: 10.1126/science.270.5238.985
97. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science (2001) 291:319–22. doi: 10.1126/science.291.5502.319
98. Cappelli LC, Shah AA, Bingham CO 3rd. Immune-related adverse effects of cancer immunotherapy implications for rheumatology. Rheum Dis Clin North Am (2017) 43:65–78. doi: 10.1016/j.rdc.2016.09.007
99. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
100. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
101. Fan EKY, Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir Res (2018) 19(1):50. doi: 10.1186/s12931-018-0756-5
102. Roquilly A, Jacqueline C, Davieau M, Mollé A, Sadek A, Fourgeux C, et al. Alveolar macrophages are epigenetically altered after in flammation, leading to long-term lung immunoparalysis. Immunol (2020) 21(6):636–48. doi: 10.1038/s41590-020-0673-x
103. Jia H, Zhang L, Wang B. The value of combination analysis of tumor biomarkers for early differentiating diagnosis of lung cancer and pulmonary tuberculosis. Ann Clin Lab Sci (2019) 49(5):645–9.
104. Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis: presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther (2005) 22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x
105. Hofmeyr A, Lau WF, Slavin MA. Mycobacterium tuberculosis infection in patients with cancer, the role of 18-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring treatment response. Tuberculosis (Edinb) (2007) 87(5):459–63. doi: 10.1016/j.tube.2007.05.013
106. Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis (2018) 18(7):e199–210. doi: 10.1016/S1473-3099(18)30111-7
107. Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology (2000) 216:117–21. doi: 10.1148/radiology.216.1.r00jl19117
108. Low SY, Eng P, Keng GHW, Ng DCE. Positron emission tomography with CT in the evaluation of non-small cell lung cancer in populations with a high prevalence of tuberculosis. Respirology (2006) 11:84–9. doi: 10.1111/j.1440-1843.2006.00789.x
109. Han D, Lai Y, Li L, Gu M. Analysis of the diagnostic effect of CT imaging features combined with tumor markers on pulmonary tuberculosis complicated with lung cancer. Chin Med Equip (2023) 20(05):59–63. doi: 10.3969/J.ISSN.1672-8270.2023.05.011
110. Xu Y, Cai S, Zhou J. Clinical diagnostic value of CT combined with CEA and CA125 in patients with pulmonary tuberculosis complicated with lung cancer. Chin J CT MRI (2022) 20(09):50–1 + 4. doi: 10.3969/j.issn.1672-5131.2022.09.018
111. Sun H, Pan L, Jia H, Zhang Z, Gao M, Huang M, et al. Label-free quantitative proteomics identifies novel plasma biomarkers for distinguishing pulmonary tuberculosis and latent infection. Front Microbiol (2018) 9:1267. doi: 10.3389/fmicb.2018.01267
112. Balatti V, Nigita G, Veneziano D, Drusco A, Stein GS, Messier TL, et al. tsRNA signatures in cancer. Proc NatlAcad Sci USA (2017) 114:8071–6. doi: 10.1073/pnas.1706908114
113. Fan NJ, Gao JL, Liu Y, Song W, Zhang ZY, Gao CF. Labelfree quantitative mass pectrometry reveals a panel of differentially expressedproteins in colorectal cancer. Biomed Res Int (2015) 2015:365068. doi: 10.1155/2015/365068
114. Liu Y, Wang X, Jiang J, Cao Z, Yang B, Cheng X. Modulation of T cell cytokine production by miR-144* with elevated expression in patients with pulmonary tuberculosis. Mol Immunol (2011) 48(9-10):1084–90. doi: 10.1016/j.molimm.2011.02.001
115. Farina NH, Scalia S, Adams CE, Hong D, Fritz AJ, Messier TL, et al. Identification of tRNA-derived small RNA (tsRNA) responsive to the tumor suppressor, RUNX1, in breast cancer. J Cell Physiol (2020) 235:5318–27. doi: 10.1002/jcp.29419
116. Jay C, Nemunaitis J, Chen P, Fulgham P, Tong AW. miRNA profiling for diagnosis and prognosis of human cancer. DNA Cell Biol (2007) 26:293–300. doi: 10.1089/dna.2006.0554
117. Chinese center for disease control and prevention. Tech Guidelines Tuberculosis Prev Control China (2021 Edition) (2021).
118. Liebenberg D, Gordhan BG, Kana BD. Drug resistant tuberculosis: Implications for transmission, diagnosis, and disease management. Front Cell Infection Microbiol (2022) 12:943545. doi: 10.3389/fcimb.2022.943545
119. Khawbung JL, Nath D, Chakraborty S. Drug resistant Tuberculosis: A review. Comp Immunol Microbiol Infect Dis (2021) 74:101574. doi: 10.1016/j.cimid.2020.101574
120. Yang L, Zhuang L, Ye Z, Li L, Guan J, Gong W. Immunotherapy and biomarkers in patients with lung cancer with tuberculosis: Recent advances and future Directions. iScience (2023) 26(10):107881. doi: 10.1016/j.isci.2023.107881
121. Ye M-F, Su S, Huang Z-H, Zou J-J, Su D-H, Chen X-H, et al. Efficacy and safety of concurrent anti-tuberculosis treatment and chemotherapy in lung cancer patients with co-existent tuberculosis. Ann Trans Med (2020) 8(18):1143. doi: 10.21037/atm-20-5964
122. Lima GC, Silva EV, Magalhães PO, Naves JS. Efficacy and safety of a four-drug fixed-dose combination regimen versus separate drugs for treatment of pulmonary tuberculosis: a systematic review and meta-analysis. Braz J Microbiol (2017) 48(2):198–207. doi: 10.1016/j.bjm.2016.12.003
123. Pontali E, Centis R, D’Ambrosio L, Toscanini F, Migliori GB. Recent evidence on delamanid use for rifampicin-resistant tuberculosis. J Thorac Dis (2019) 11(Suppl 3):S457–60. doi: 10.21037/jtd.2018.11.26
124. Hirashima T, Tamura Y, Han Y, Hashimoto S, Tanaka A, Shiroyama T, et al. Efficacy and safety of concurrent anti-Cancer and anti-tuberculosis chemotherapy in Cancer patients with active Mycobacterium tuberculosis: a retrospective study. BMC Cancer (2018) 18(1):975. doi: 10.1186/s12885-018-4889-1
125. Kamyshan IS, Klimenko IA, Kirichenko SA. Urinary bladder cancer in patients with tuberculous and post-tuberculous cystitis. Urologiia (Moscow Russia: 1999) 2000(2):21–4.
126. Kadono Y, Koizumi H. A case of transitional cell carcinoma of the bladder with active urinary tract tuberculosis. Nihon Hinyokika Gakkai Zasshi Japanese J Urol (2002) 93(1):58–61. doi: 10.5980/jpnjurol1989.93.58
127. Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, et al. Tim3 mediates t cell exhaustion during mycobacterium tuberculosisinfection. PloS Pathog (2016) 12(3):e1005490. doi: 10.1371/journal.ppat.1005490
128. Hughes PE, Caenepeel S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol (2016) 37:462–76. doi: 10.1016/j.it.2016.04.010
129. Tostmann A, Boeree MJ, Aarnoutse RE, De Lange WCM, van der Ven AJAM, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol (2008) 23(2):192–202. doi: 10.1111/j.1440-1746.2007.05207.x
130. Li B, Chan HL, Chen P. Immune checkpoint inhibitors: Basics and challenges. Curr Med Chem (2019) 26:3009–25. doi: 10.2174/0929867324666170804143706
131. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpointimmunotherapy for non-small cell lung cancer: Benefits and pulmonary toxicities. Chest (2018) 154:1416–23. doi: 10.1016/j.chest.2018.08.1048
132. Shi J, Li J, Wang Q, Cheng X, Du H, Han R, et al. The safety and efficacy of immunotherapy with anti-programmed cell death 1 monoclonal antibody for lung cancer complicated with mycobacterium tuberculosis infection. Transl Lung Cancer Res (2021) 10:3929–42. doi: 10.21037/tlcr-21-524
133. Morelli T, Fujita K, Redelman-Sidi G, Elkington PT. Infections due to dysregulated immunity: An emerging complication of cancer immunotherapy. Thorax (2022) 77:304–11. doi: 10.1136/thoraxjnl-2021-217260
134. Liu K, Wang D, Yao C, Qiao M, Li Q, Ren W, et al. Increased tuberculosis incidence due to immunotherapy based on pd-1 and pd-l1 blockade: A systematic review and metaanalysis. Front Immunol (2022) 13:727220. doi: 10.3389/fimmu.2022.727220
135. Wykes MN, Lewin SR. Immune checkpoint blockade in infectiousdiseases. Nat Rev Immunol (2018) 18:91–104. doi: 10.1038/nri.2017.112
136. Barenholz Y. Doxil®–the first FDA-approved nano-drug: Lessons learned. J Control Release (2012) 160:117–34. doi: 10.1016/j.jconrel.2012.03.020
137. Gupta N, Hatoum H, Dy GK. First line treatment of advanced non-small-cell lung cancer-specific focus on albumin bound paclitaxel. Int J Nanomed (2014) 9:209–21. doi: 10.2147/IJN.S41770
138. Iyer R, Nguyen T, Padanilam D, Xu CC, Saha D, Nguyen KT, et al. Glutathione-responsive biodegradable polyurethane nanoparticles for lung cancer treatment. J Control Release (2020) 321:363–71. doi: 10.1016/j.jconrel.2020.02.021
139. Anusha S, Baburajeev CP, Mohan CD, Mathai J, Rangappa S, Mohan S, et al. A nano-MgO and ionic liquid-catalyzed ‘green’ synthesis protocol for the development of adamantyl-imidazolo-thiadiazoles as antituberculosis agents targeting sterol14α-demethylase (CYP51). PloS One (2015) 10(10):e0139798. doi: 10.1371/journal.pone.0139798
140. Sharma R, Kaur A, Sharma AK, Dilbaghi N, Sharma AK. Nano-based anti-tubercular drug delivery and therapeutic interventions in tuberculosis. Curr Drug Targets (2017) 18:72–86. doi: 10.2174/1389450116666150804110238
141. Shojaei TR, Mohd Salleh MA, Tabatabaei M, Ekrami A, Motallebi R, Rahmani-Cherati T, et al. Development of sandwich-form biosensor to detect Mycobacterium tuberculosiscomplex in clinical sputum specimens. Braz J Infect Dis (2014) 18:600–8. doi: 10.1016/j.bjid.2014.05.015
142. Wang SQ, Inci F, De Libero G, Singhal A, Demirci U. Point-of-care assays for tuberculosis: Role of nanotechnology/microfluidics. Biotechnol Adv (2013) 31:438–49. doi: 10.1016/j.biotechadv.2013.01.006
143. Yang H, Qin LH, Wang YL, Zhang BB, Liu ZH, Ma H, et al. Detection of Mycobacterium tuberculosis based on H37Rv binding peptides using surface functionalized magnetic microspheres coupled with quantum dots—a nano detection method for Mycobacterium tuberculosis. Int J Nanomed (2015) 10:77–88. doi: 10.2147/IJN.S71700
Keywords: tuberculosis (TB), tumor, cancer, tuberculosis comorbid with tumor (TCWT), pathogenesis, immunotherapy
Citation: Wang C, Zou R-Q and He G-Z (2024) Progress in mechanism-based diagnosis and treatment of tuberculosis comorbid with tumor. Front. Immunol. 15:1344821. doi: 10.3389/fimmu.2024.1344821
Received: 26 November 2023; Accepted: 02 January 2024;
Published: 17 January 2024.
Edited by:
Geng Chen, Stemirna Therapeutics Co., Ltd., ChinaReviewed by:
Suresh Kalathil, University at Buffalo, United StatesDongsheng Yue, Tianjin Medical University Cancer Institute and Hospital, China
Copyright © 2024 Wang, Zou and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Zhong He, aGVndW96aG9uZ0BrbW11LmVkdS5jbg==
 Chuan Wang
Chuan Wang Rong-Qi Zou
Rong-Qi Zou Guo-Zhong He1*
Guo-Zhong He1*