- 1Department of Biomedical Engineering, College of Basic Medical Sciences, Second Military Medical University, Shanghai, China
- 2School of Gongli Hospital Medical Technology, University of Shanghai for Science and Technology, Shanghai, China
- 3Department of Biophysics, College of Basic Medical Sciences, Second Military Medical University, Shanghai, China
- 4Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5Fahe Life Science and Technology Inc., Shanghai, China
Exosomes are small extracellular vesicles (sEVs) secreted by cells. With advances in the study of sEVs, they have shown great potential in the diagnosis and treatment of disease. However, sEV therapy usually requires a certain dose and purity of sEVs to achieve the therapeutic effect, but the existing sEV purification technology exists in the form of low yield, low purity, time-consuming, complex operation and many other problems, which greatly limits the application of sEVs. Therefore, how to obtain high-purity and high-quality sEVs quickly and efficiently, and make them realize large-scale production is a major problem in current sEV research. This paper discusses how to improve the purity and yield of sEVs from the whole production process of sEVs, including the upstream cell line selection and cell culture process, to the downstream isolation and purification, quality testing and the final storage technology.
1 Introduction
sEVs are nanoscale vesicles secreted by cells with a lipid bilayer membrane structure. It was first discovered in 1983 in sheep reticulocytes in culture and was named “exosome” in 1987 (1). sEVs are generally considered to be between 30-150 nm in size (2). At first people considered cellular metabolic wastes and not taken seriously. sEVs are widely found in almost all tissue cells and body fluids (3) and are rich in lipids, proteins, and nucleic acids (4). sEVs can travel between cells and carry a wide range of substances from the parent cell for intercellular communication (5), playing an irreplaceable role in physiological and pathological situations. Compared to traditional stem cell therapies, the small size of sEVs makes it easier for them to be endocytosed by cells to transfer their cargo to recipient cells, and because sEVs are less immunogenic, they can be administered repeatedly (6).
sEVs are widely used in biomedicine in three main directions. The first is the extraction of sEVs in the pathologic microenvironment as biomarkers for disease diagnosis (7). The second is that small extracellular vesicles themselves contain a variety of cytokines, proteins, messenger RNAs (mRNAs), microRNAs (miRNA), long non-coding RNAs (IncRNAs), lipids, metabolites and even DNA fragments (8), that produce therapeutic effects (9). The third is the ability of sEVs to transport drugs (10). However, due to current technological limitations, it remains a major challenge to obtain high purity, high quality and sufficient doses of sEVs for clinical use.
sEVs were demonstrated to play vital roles in intercellular communication in normal physiological processes and in the pathogenesis of disease, including cancer neurodegenerative, diseases and cardiovascular diseases. sEVs of different origins have different roles to play (11). For example, sEVs of immune cell origin fight disease primarily by promoting immunity, whereas sEVs of stem cell origin promote tissue regeneration. In addition, due to the different purity and activity of sEVs obtained from different isolation methods, the results of their use in disease therapy are not exactly the same (12). The efficacy of sEVs obtained by immunoaffinity capture may not be as good as those obtained by other isolation methods due to the difficulty of removing the antibodies used for capture. Size exclusion chromatography is gentle, and the sEVs obtained are more pure and active, and are used for better results in disease treatment.
The purity and yield of sEVs are affected by multiple conditions, mainly the choice of cell line (sample sources), cell culture, isolation techniques, storage, etc.
2 Sample selection and cultivation methods
Different samples contained different sEV content and isoforms. Different cells and cultures produce different sEVs.
2.1 sEV-producing cells and MSCs source selection
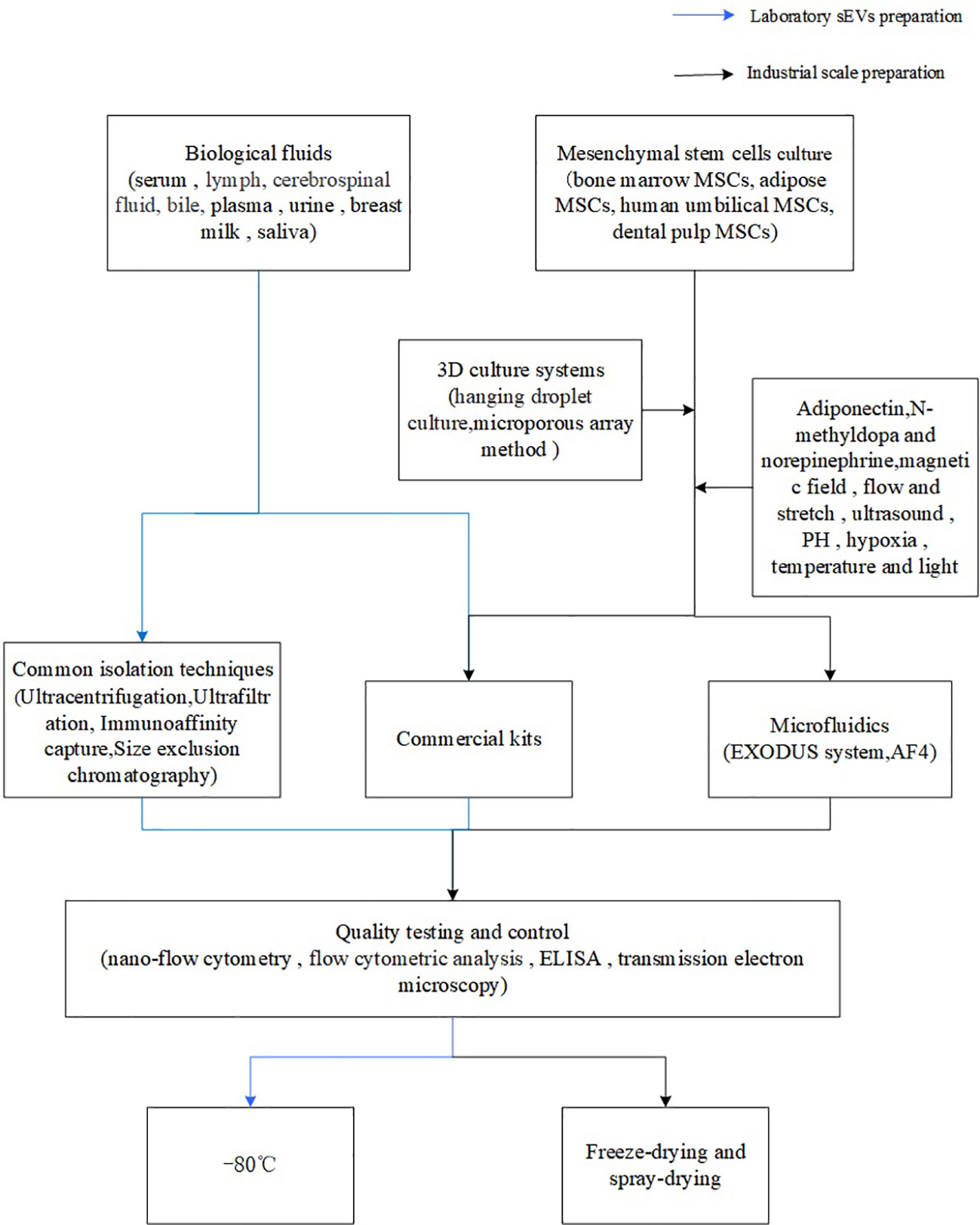
The vast majority of cells in the body can produce sEVs (13), which are found throughout our body. The sources of sEVs are mainly divided into two main categories, one is the direct extraction of sEVs from body fluids secreted by the human body, such as serum (14), lymph, cerebrospinal fluid (15), bile (16), plasma (17), urine (18), breast milk (19), saliva, etc., and the other is the extraction of sEVs from the supernatants of a variety of cell culture media (20). However, since natural body fluids, especially plasma, contain cellular debris, apoptotic vesicles, microvesicles, and plasma proteins, which are not easily separated from sEVs due to their overlapping sizes and biochemical properties, resulting in a low purity of the isolated sEVs (21). The urine has fewer interfering particles than the plasma, but a low concentration of sEVs (21). It’s obvious sEVs obtained from urine are more pure, but because of their low concentration, they require a larger volume than in plasma extraction to obtain the same mass.
In contrast, in vitro cell culture supernatants are easier to obtain and the quality of sEVs obtained is more stable, so most of the existing techniques are extracted and isolated from conditioned cell cultures (22, 23). Stem cells with high productivity have been used for the longest time for in vitro cell culture, with mesenchymal stem cells (MSCs) being the most used (24). Depending on the source, MSCs can be categorized into bone marrow MSCs, adipose MSCs, human umbilical MSCs, dental pulp MSCs and so on (25). Stem cells from different sources proliferate at different rates on their own and produce sEVs of varying quantity and quality (26), among which human umbilical MSCs produce the largest number of sEVs and the largest size (27), meanwhile, human umbilical MSCs are able to be stably cultured in serum-free medium (28), which effectively avoids the contamination by the impurities in serum in the subsequent isolation process, and is conducive to the large-scale production of sEVs.
2.2 Cell culture
The occurrence and secretion of cellular sEVs are influenced by multiple conditions. On the one hand, small molecules can influence sEV production and secretion. For example, thrombin pretreatment enhances the ability of MSCs to produce extracellular vesicles and the quality of extracellular vesicles is not affected (29). Adiponectin stimulates the production and secretion of sEVs by binding to T-cadherin on MSCs (30). N-methyldopa and norepinephrine can triple the sEV production of MSCs (31). Melatonin-treated sEVs enhance the regenerative potential of MSCs (32). In addition, external environments such as magnetic field (33), flow and stretch (34), ultrasound (35), PH (36), hypoxia (37), temperature (38), and light (39) affect the synthesis and release of sEVs.
To further obtain higher yields and quality of sEVs, Three dimensional culture systems are being used for sEVs production. The three dimensional culture improves sEVs production by increasing cell-cell-cell matrix interactions (40). Common three dimensional culture methods are hanging droplet culture and microporous array method, the hanging droplet culture is simple and easy to execute, but the yield is limited and there are limitations for sphere size adjustment, so mass production with hanging droplet culture will be time-consuming (41). The microporous array method inoculates cells into a series of small wells to which cell growth-promoting materials can be added to promote cell proliferation and sEV synthesis, In addition, the microporous array method is easier to produce three dimensional spheres than the droplet method and has a higher throughput (42), which means microporous array method more suitable for mass production. In addition, artificial sEVs with low cost, high yield and stable quality are expected to be a powerful alternative to natural sEVs (43).
3 sEV isolation and purification
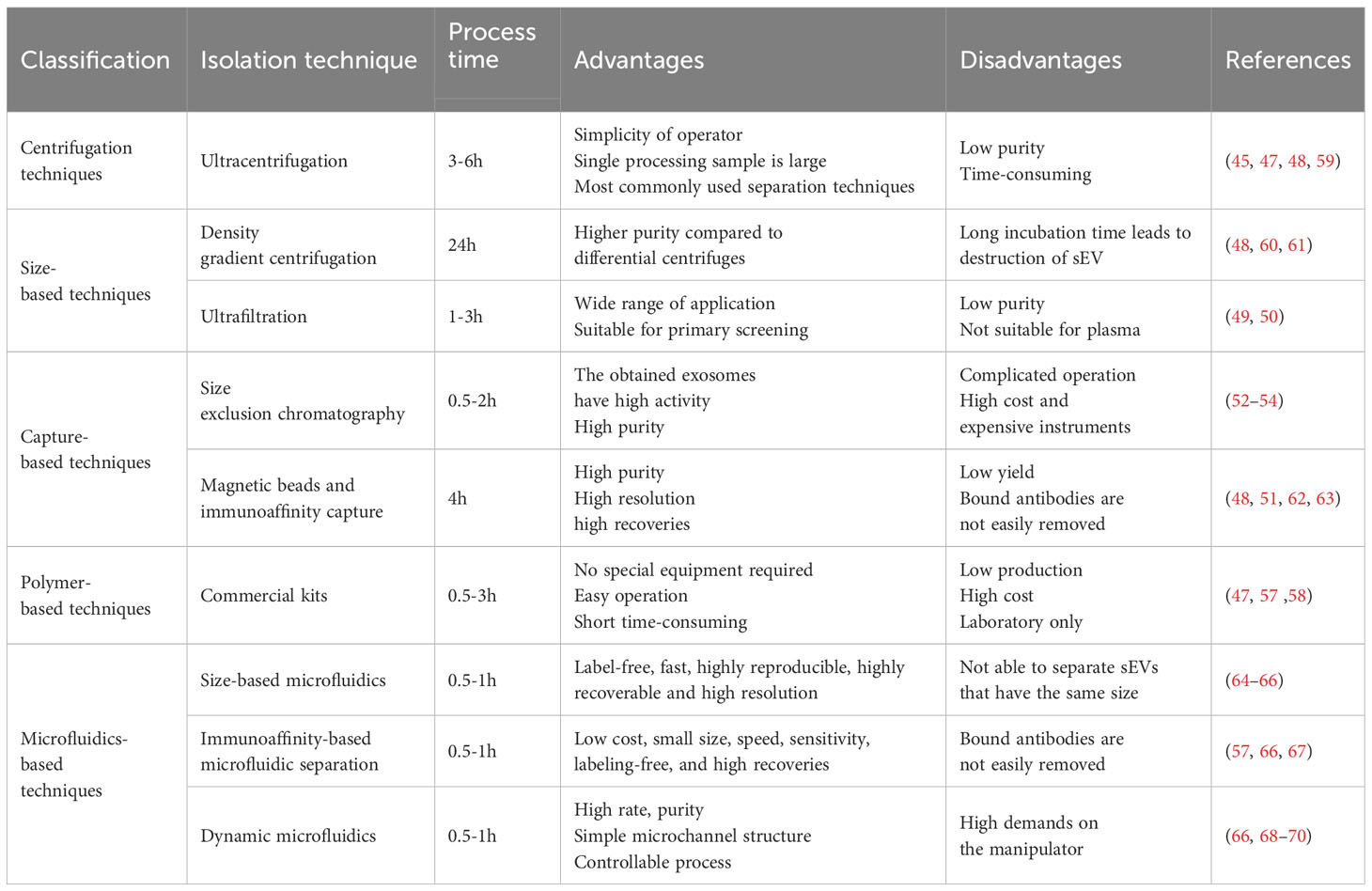
3.1 Common isolation techniques
Common isolation techniques include ultracentrifugation, ultrafiltration, immunoaffinity capture, size exclusion chromatography and precipitation.
Ultracentrifugation is the classical method for sEV isolation (44). At the same time, it is also the most widely used isolation technique (45). Ultracentrifugation is based on the separation of sEVs and impurities in the samples with different densities and sizes (46), the dead cells, cellular debris, and large extracellular vesicles in the samples are successively removed by different rotational speeds, and finally obtain the sEVs in the supernatant (45). Ultracentrifugation can process a large number of samples at one time and is easy to perform, but produces less pure and time-consuming sEVs because of the different subtypes of sEVs have overlapping density ranges (47). Formation of a density gradient medium with sucrose or iodixanol in combination with ultracentrifugation improves the purity of sEVs, but prolonged incubation with high sucrose concentrations impairs the structure of sEVs (48). In addition, the polymer density layer is expensive and not suitable for sEV scale-up.
Ultrafiltration is based on the isolation of different extracellular vesicles with different sizes, which can only pass through a series of semi-permeable membranes with defined pore sizes (49). However, since extracellular vesicles are deformable, vesicles that do not conform to the size can also be deformed to pass through the pore resulting in sEV impurity, so they are only used for preliminary isolation (50).
Immunoaffinity capture is an sEV isolation technique based on antigen-antibody interactions, in which immobilization of a specific antibody allows for specific binding of an antigen unique to the surface of the sEV, and thus capturing the sEVs (51). However, the overlap of antigens between different subpopulations and the difficulty of removing capture antibodies can affect later functional assay studies of sEVs, that not conducive to subsequent sEV research and applications.
Size exclusion chromatography (SEC) is also an isolation method based on sEV size, where large particles are unable to enter the gel pores and small sEVs are allowed to enter, which is a milder separation method that yields sEVs with higher purity and activity (52). However, the resolution of SEC decreases when the particles are close to or larger than the upper limit of the pore size, and so SEC is often used in conjunction with ultracentrifugation (53) and ultrafiltration (54) to improve the purity of sEVs.
Precipitation is sEVs under the action of polyethylene glycol usually, the solubility decreases leading to the precipitation of sEVs, and then sEVs can be obtained by low-speed centrifugation (55), which is easy to operate, does not require special equipment, and is conducive to the preparation of large-scale, but is prone to the introduction of impurities leading to the sEVs are not high purity (56).
3.2 sEVs isolation kits
As the demand for sEVs increased, a variety of commercial kits rapidly emerged. Kits principle is based on traditional sEV separation methods such as ultrafiltration, sedimentation and so on. Commercial kits do not require special equipment, with the advantages of simple operation and short time consuming, and can isolate sEVs from most common body fluids and cell culture supernatants (57). However, kits are expensive and cannot process a large number of samples at once, so they are not suitable for high-volume processing. Furthermore here are significant differences in the purity and quantity of sEVs collected by different kits (47). For example, the yield of sEVs obtained with the invitrogen kit is dozens of times more than that obtained with conventional ultracentrifugation, but the purity of the output sEVs is unsatisfactory, and the sEV isolation kit requires pre-separation before use, which makes the experimental process cumbersome (58).
3.3 Emerging sEVs isolation technologies
Although there are many techniques for sEV isolation and purification, all of the above techniques have significant drawbacks and are not suitable for large-scale production of sEVs (Table 1). Microfluidics is an emerging method for sEV isolation, which is a technique for controlling fluids in micrometer-sized channels that relies on a range of sEV properties including immunoaffinity, density, and size, and it overcomes the limitations of traditional methods by offering advantages such as low cost, small size, speed, sensitivity, labeling-free, and high recoveries (57). It is expected to replace traditional methods in the future and play an important role in the industrialized mass production of sEVs in the future.
The EXODUS system separates and purifies sEVs by two coupled oscillators generating a dual-frequency transverse wave on the membrane, which produces sEVs at a rate, purity, and throughput that are far superior to the others (68). Asymmetric-flow field-flow fractionation technology (AF4) is label-free, fast, highly reproducible, highly recoverable and high resolution, which helps to separate different sEV isoforms (64). However, because AF4 separates based on particle size, it is not able to separate sEVs that have the same size, but are actually different. Double tangential flow size screening-based microfluidic chip greatly improves sEV recovery rate and purity. Its sEV recovery rate up to 77.8, acquired sEVs can be directly used for sEV analysis (65). Capture of sEVs by altering temperature was devised by Kenichi Nagase (71).
Whether it is the traditional separation technology or the microfluidic technology in the last two years, it has not fully achieved the ideal separation effect. Based on the properties of sEVs and downstream applications, it may be a useful idea to combine different isolation methods to get better separation effect. The combination of different techniques may offer the possibility of efficiently obtaining high-purity and high-yield sEVs.
4 Quality testing and control of sEVs
Since the present technologies does not allow for a good isolation of sEVs from other impurities, the subsequent assessment of the purity and quality of the sEVs obtained is particularly important. Different subtypes of sEVs contain different proteins, lipids, and nucleic acids because of the different cells of origin of the sEVs (72).
These differences of different sEVs have an important role in the assessment of sEV subtypes, such as the tetraspanins (CD9, CD63, CD81) that are often used to differentiate subpopulations of extracellular vesicles and to assess sEV purification (73). Commonly used techniques for sEV detection include nano-flow cytometry (74), flow cytometric analysis (75), ELISA (76), transmission electron microscopy and so on. These techniques are used to assess the quantity and purity of sEVs in samples. To provide a better platform for the use of sEVs in the clinic.
5 Storage of sEVs
From the current study, the storage conditions of sEVs including temperature, storage time and even the number of freezing and thawing cycles have a great impact on the concentration, purity and function of sEVs (77). Common storage conditions in the laboratory are 4°C, -20°C, and -80°C., the concentration and purity of sEVs decreases accordingly with increasing temperature and storage time. For commercial and clinical use, long-term storage of sEVs is generally required. Because -80°C can effectively inhibits biologically active proteins and reduces the loss of sEVs, -80°C is usually considered to be the optimal temperature for sEV storage (78). However, this storage method usually makes sEVs susceptible to “frostbite”, mainly due to osmotic imbalance, so cryoprotectants are usually added during the freezing process to maintain protein stability and prevent osmotic damage (79). Commonly used cryoprotectants such as trehalose prevent aggregation by avoiding internal icing of sEVs, while the addition of trehalose contributes to the colloidal stability of sEVs (80). It has been shown that the addition of human albumin and trehalose during storage helps to improve long-term storage of sEVs, maintain freeze-thaw stability, and increase sEV recovery when diluents are used downstream (81).
However, -80°C is not suitable for the transportation and application of sEVs, and not all factories and laboratories have -80°C storage conditions. Therefore, freeze-drying and spray-drying are used for the preservation of sEVs, and studies have shown that freeze-drying can be used for long-term preservation at 4 °C (82), reducing storage requirements and costs for sEVs. In addition, repeated freezing and thawing can lead to a decrease in the number of sEVs and an increase in their size (83), so we should avoid repeated freezing and thawing process as much as possible to ensure the quality of sEVs during storage and transportation.
6 Laboratory and scale-up of sEVs
In the past few years, the clinical application of sEVs has become more and more widespread, however, in order to achieve significant clinical therapeutic effects require a certain dose of sEVs, but with the current production technology of sEVs, the production of sEVs is not high (84). With the increasing demand for sEVs, the traditional method of isolating sEVs from body fluids, such as human plasma, is obviously unable to meet the demand for the use of sEVs, so the large-scale production of sEVs is crucial. Less mature cells such as MSCs are often used in industry for culture to obtain sEVs (85). In reality, serum-free medium is usually used for stem cell culture due to the high content of endogenous extracellular vesicles in fetal bovine serum (86), which is not conducive to later isolation and purification. Studies show that switching from serum-containing to serum-free media produces sEVs that exhibit stronger therapeutic effects (87). However, it has been shown that the use of serum-free media leads to an increase in reactive oxygen species and emergency-related proteins (88). However, it is undeniable that serum-free medium it is favorable to improve the purity of sEVs and reduce the unknown side effects of sEVs during clinical application.
In addition sEV production is related to the surface area to which cells can attach in the bioreactor. Therefore microcarriers are particularly important in bioreactors, which are generally spherical in shape to provide a larger value-added area for adhesion (89). A variety of bioreactor systems are used for large-scale production, such as hollow-fiber membranes (90), three-dimensional stirred-tank bioreactors (91).
Of course, the production of sEVs from the general laboratory to industrial mass production is not a simple process, which not only involves the selection of cell lines in the early stage, cell culture, but also includes the isolation and purification of the latter, quality control, storage (Figures 1, 2). And importantly tighter control of lot-to-lot consistency of sEVs is not easy (92). There is still a long way to go for sEV purification and scale-up.
7 Discussion
sEVs have a wide range of applications and are currently used in the diagnosis and treatment of diseases, cosmetic skincare and scalp care for hair regrowth. In particular, stem cell-derived sEVs show great potential for clinical therapy. sEVs can travel between cells for the purpose of intercellular communication. In addition, due to the small size of sEVs, it is easier to transfer cargo to recipient cells by endocytosis, and the low immunogenicity allows repeated administration. These properties determine that sEVs are more likely to produce good therapeutic results.
Despite the widespread use of sEVs, their use is limited in several ways. Currently, a major challenge in the field of sEV research focuses on the isolation and purification of sEVs and how to achieve large-scale production to meet the needs of society. Due to technological limitations, various methods currently have drawbacks, initially people tried to build on their strengths and avoid their weaknesses by combining different isolation methods, and then emerging technologies such as microfluidics were invented and incorporated into the existing isolation techniques, which resulted in an effective improvement of sEV purity and yield. In addition, with the use of advanced technologies such as serum-free media and bioreactors for sEV production, the yield of sEVs has been effectively increased, but nevertheless, there are still many challenges in the large-scale production of sEVs.
Author contributions
SH: Writing – review & editing. XZ: Writing – original draft. HA: Writing – review & editing. KQ: Writing – review & editing. GL: Writing – review & editing. SZ: Writing – review & editing. YZ: Writing – review & editing. WF: Writing – review & editing. CL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant numbers 82322055, 81773261, 31970882, 81903140, 82041012 and 92169115); the Shanghai Rising-Star Program (grant number 19QA1411400); the Shanghai Sailing Program (19YF1438600); the Shanghai Chenguang Program (grant number 17CG35); and the Shanghai Biomedical Technology Support Project (20S11906600) and the Open Project Grant from Engineering Research Center of Cell and Therapeutic Antibody, Ministry of Education, Shanghai Jiao Tong University.
Conflict of interest
Author WF is a shareholder at Fahe Life Science and Technology Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. (1987) 262:9412–20. doi: 10.1016/S0021-9258(18)48095-7
2. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. (2016) 113:E968–77. doi: 10.1073/pnas.1521230113
3. Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. (2022) 9:811971. doi: 10.3389/fbioe.2021.811971
4. Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany KV, Liang NW, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci (Weinh). (2022) 9:e2103222. doi: 10.1002/advs.202103222
5. Valadi H, Ekström K, Bossios A, Sjöstrand M, JJ L, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007) 9:654–9. doi: 10.1038/ncb1596
6. Safari B, Aghazadeh M, Davaran S, Roshangar L. Exosome-loaded hydrogels: A new cell-free therapeutic approach for skin regeneration. Eur J Pharm Biopharm. (2022) 171:50–9. doi: 10.1016/j.ejpb.2021.11.002
7. Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. (2020) 182:1044–1061.e18. doi: 10.1016/j.cell.2020.07.009
8. Zhu L, Sun HT, Wang S, Huang SL, Zheng Y, Wang CQ, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. (2020) 13:152. doi: 10.1186/s13045-020-00987-y
9. Hu S, Li Z, Shen D, Zhu D, Huang K, Su T, et al. Exosome-eluting stents for vascular healing after ischaemic injury. Nat BioMed Eng. (2021) 5:1174–88. doi: 10.1038/s41551-021-00705-0
10. Kar R, Dhar R, Mukherjee S, Nag S, Gorai S, Mukerjee N, et al. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Eng. (2023) 9:577–94. doi: 10.1021/acsbiomaterials.2c01329
11. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
12. Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. (2019) 11:eaav8521. doi: 10.1126/scitranslmed.aav8521
13. Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, et al. Regulation of exosome production and cargo sorting. Int J Biol Sci. (2021) 17:163–77. doi: 10.7150/ijbs.53671
14. Vázquez-Mera S, Martelo-Vidal L, Miguéns-Suárez P, Saavedra-Nieves P, Arias P, González-Fernández C, et al. Serum exosome inflamma-miRs are surrogate biomarkers for asthma phenotype and severity. Allergy. (2023) 78:141–55. doi: 10.1111/all.15480
15. Li C, Qin T, Jin Y, Hu J, Yuan F, Cao Y, et al. Cerebrospinal fluid-derived extracellular vesicles after spinal cord injury promote vascular regeneration via PI3K/AKT signaling pathway. J Orthop Translat. (2023) 39:124–34. doi: 10.1016/j.jot.2023.02.001
16. Severino V, Dumonceau JM, Delhaye M, Moll S, Annessi-Ramseyer I, Robin X, et al. Extracellular vesicles in bile as markers of Malignant biliary stenoses. Gastroenterology. (2017) 153:495–504.e8. doi: 10.1053/j.gastro.2017.04.043
17. Khan NZ, Cao T, He J, Ritzel RM, Li Y, Henry RJ, et al. Spinal cord injury alters microRNA and CD81+ exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain Behav Immun. (2021) 92:165–83. doi: 10.1016/j.bbi.2020.12.007
18. Sun Z, Wu J, Bi Q, Wang W. Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis. Stem Cell Res Ther. (2022) 13:297. doi: 10.1186/s13287-022-02986-x
19. Melnik BC, Stremmel W, Weiskirchen R, John SM, Schmitz G. Exosome-derived microRNAs of human milk and their effects on infant health and development. Biomolecules. (2021) 11:851. doi: 10.3390/biom11060851
20. Burgio S, Noori L, Marino Gammazza A, Campanella C, Logozzi M, Fais S, et al. Extracellular vesicles-based drug delivery systems: A new challenge and the exemplum of Malignant pleural mesothelioma. Int J Mol Sci. (2020) 21:5432. doi: 10.3390/ijms21155432
21. Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci. (2014) 369:20130502. doi: 10.1098/rstb.2013.0502
22. Li P, Kaslan M, SH L, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. (2017) 7:789–804. doi: 10.7150/thno.18133
23. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. (2019) 8:307. doi: 10.3390/cells8040307
24. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Int Soc Cell Ther position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
25. Álvarez-Viejo M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J Stem Cells. (2020) 12:100–9. doi: 10.4252/wjsc.v12.i2.100
26. Mucientes A, Herranz E, Moro E, González-Corchón A, Peña-Soria MJ, Abasolo L, et al. Influence of mesenchymal stem cell sources on their regenerative capacities on different surfaces. Cells. (2021) 10:481. doi: 10.3390/cells10020481
27. Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. (2018) 26:2838–47. doi: 10.1016/j.ymthe.2018.09.015
28. Haraszti RA, Miller R, Dubuke ML, Rockwell HE, Coles AH, Sapp E, et al. Serum deprivation of mesenchymal stem cells improves exosome activity and alters lipid and protein composition. iScience. (2019) 16:230–41. doi: 10.1016/j.isci.2019.05.029
29. Sung DK, Sung SI, Ahn SY, Chang YS, Park WS. Thrombin preconditioning boosts biogenesis of extracellular vesicles from mesenchymal stem cells and enriches their cargo contents via protease-activated receptor-mediated signaling pathways. Int J Mol Sci. (2019) 20:2899. doi: 10.3390/ijms20122899
30. Nakamura Y, Kita S, Tanaka Y, Fukuda S, Obata Y, Okita T, et al. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol Ther. (2020) 28:2203–19. doi: 10.1016/j.ymthe.2020.06.026
31. Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. (2020) 9:660. doi: 10.3390/cells9030660
32. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. (2020) 11:259. doi: 10.1186/s13287-020-01756-x
33. Xia B, Gao X, Qian J, Li S, Yu B, Hao Y, et al. A novel superparamagnetic multifunctional nerve scaffold: remote actuation strategy to boost in situ extracellular vesicles production for enhanced peripheral nerve repair. Adv Mater. (2024) 36(3):e2305374. doi: 10.1002/adma.202305374
34. Guo S, Debbi L, Zohar B, Samuel R, Arzi RS, Fried AI, et al. Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett. (2021) 21:2497–504. doi: 10.1021/acs.nanolett.0c04834
35. Zhao Z, Qu L, Shuang T, Wu S, Su Y, Lu F, et al. Low-intensity ultrasound radiation increases exosome yield for efficient drug delivery. J Drug Delivery Sci Technol. (2020) 57:101713. doi: 10.1016/j.jddst.2020.101713
36. Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PloS One. (2014) 9:e88193. doi: 10.1371/journal.pone.0088193
37. Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. (2013) 110:7312–7. doi: 10.1073/pnas.1220998110
38. Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PloS One. (2011) 6:e16899. doi: 10.1371/journal.pone.0016899
39. Ruan S, Erwin N, He M. Light-induced high-efficient cellular production of immune functional extracellular vesicles. J Extracell Vesicles. (2022) 11:e12194. doi: 10.1002/jev2.12194
40. Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. (2019) 9:13012. doi: 10.1038/s41598-019-49671-3
41. de Groot TE, Veserat KS, Berthier E, Beebe DJ, Theberge AB. Surface-tension driven open microfluidic platform for hanging droplet culture. Lab Chip. (2016) 16:334–44. doi: 10.1039/C5LC01353D
42. Manzoor AA, Romita L, Hwang DK. A review on microwell and microfluidic geometric array fabrication techniques and its potential applications in cellular studies. Can J Chem Eng. (2021) 99:61–96. doi: 10.1002/cjce.23875
43. Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. (2021) 19:242. doi: 10.1186/s12951-021-00986-2
44. Gardiner C, Di Vizio D, Sahoo S, Théry C, Witwer KW, Wauben M, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. (2016) 5:32945. doi: 10.3402/jev.v5.32945
45. Momen-Heravi F. Isolation of extracellular vesicles by ultracentrifugationy. Methods Mol Biol. (2017) 1660:25–32. doi: 10.1007/978-1-4939-7253-1_3
46. Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. (2015) 5:17319. doi: 10.1038/srep17319
47. Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. (2019) 9:5335. doi: 10.1038/s41598-019-41800-2
48. Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. (2012) 56:293–304. doi: 10.1016/j.ymeth.2012.01.002
49. Xu R, Simpson RJ, Greening DW. A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol Biol. (2017) 1545:91–116. doi: 10.1007/978-1-4939-6728-5_7
50. Benedikter BJ, Bouwman FG, Vajen T, Heinzmann ACA, Grauls G, Mariman EC, et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep. (2017) 7:15297. doi: 10.1038/s41598-017-15717-7
51. Staubach S, Bauer FN, Tertel T, Börger V, Stambouli O, Salzig D, et al. Scaled preparation of extracellular vesicles from conditioned media. Adv Drug Delivery Rev. (2021) 177:113940. doi: 10.1016/j.addr.2021.113940
52. Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine. (2017) 13:2061–5. doi: 10.1016/j.nano.2017.03.011
53. Guan S, Yu H, Yan G, Gao M, Sun W, Zhang X. Characterization of urinary exosomes purified with size exclusion chromatography and ultracentrifugation. J Proteome Res. (2020) 19:2217–25. doi: 10.1021/acs.jproteome.9b00693
54. Oeyen E, Van Mol K, Baggerman G, Willems H, Boonen K, Rolfo C, et al. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine. J Extracell Vesicles. (2018) 7:1490143. doi: 10.1080/20013078.2018.1490143
55. Börger V, Staubach S, Dittrich R, Stambouli O, Giebel B. Scaled isolation of mesenchymal stem/stromal cell-derived extracellular vesicles. Curr Protoc Stem Cell Biol. (2020) 55:e128. doi: 10.1002/cpsc.128
56. Ryu KJ, Lee JY, Park C, Cho D, Kim SJ. Isolation of small extracellular vesicles from human serum using a combination of ultracentrifugation with polymer-based precipitation. Ann Lab Med. (2020) 40:253–8. doi: 10.3343/alm.2020.40.3.253
57. Shirejini SZ, Inci F. The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits. Biotechnol Adv. (2022) 54:107814. doi: 10.1016/j.biotechadv.2021.107814
58. Boriachek K, Masud MK, Palma C, Phan HP, Yamauchi Y, Hossain MSA, et al. Avoiding pre-isolation step in exosome analysis: direct isolation and sensitive detection of exosomes using gold-loaded nanoporous ferric oxide nanozymes. Anal Chem. (2019) 91:3827–34. doi: 10.1021/acs.analchem.8b03619
59. Momen-Heravi F. Isolation of extracellular vesicles by ultracentrifugation. Methods Mol Biol. (2017) 1660:25–32. doi: 10.1007/978-1-4939-7253-1_3
60. D'Acunzo P, Kim Y, Ungania JM, Pérez-González R, Goulbourne CN, Levy E. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat Protoc. (2022) 17:2517–49. doi: 10.1038/s41596-022-00719-1
61. Chen BY, Sung CW, Chen C, Cheng CM, Lin DP, Huang CT, et al. Advances in exosomes technology. Clin Chim Acta. (2019) 493:14–9. doi: 10.1016/j.cca.2019.02.021
62. Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. (2018) 7:1435138. doi: 10.1080/20013078.2018.1435138
63. Kim H, Shin S. ExoCAS-2: rapid and pure isolation of exosomes by anionic exchange using magnetic beads. Biomedicines. (2021) 9:28. doi: 10.3390/biomedicines9010028
64. Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc. (2019) 14:1027–53. doi: 10.1038/s41596-019-0126-x
65. Hua X, Zhu Q, Liu Y, Zhou S, Huang P, Li Q, et al. A double tangential flow filtration-based microfluidic device for highly efficient separation and enrichment of exosomes. Anal Chim Acta. (2023) 1258:341160. doi: 10.1016/j.aca.2023.341160
66. Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J. (2017) 12:1600699. doi: 10.1002/biot.201600699
67. Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. (2016) 16:489–96. doi: 10.1039/C5LC01117E
68. Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q, et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods. (2021) 18:212–8. doi: 10.1038/s41592-020-01034-x
69. Shi N, Mohibullah M, Easley CJ. Active flow control and dynamic analysis in droplet microfluidics. Annu Rev Anal Chem (Palo Alto Calif). (2021) 14:133–53. doi: 10.1146/annurev-anchem-122120-042627
70. Täuber S, von Lieres E, Grünberger A. Dynamic environmental control in microfluidic single-cell cultivations: from concepts to applications. Small. (2020) 16:e1906670. doi: 10.1002/smll.201906670
71. Nagase K, Yamazaki K, Maekawa Y, Kanazawa H. Thermoresponsive bio-affinity interfaces for temperature-modulated selective capture and release of targeted exosomes. Mater Today Bio. (2022) 18:100521. doi: 10.1016/j.mtbio.2022.100521
72. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977
73. Karimi N, Dalirfardouei R, Dias T, Lötvall J, Lässer C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma - Contributions of platelet extracellular vesicles in plasma samples. J Extracell Vesicles. (2022) 11:e12213. doi: 10.1002/jev2.12213
74. Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. (2019) 9:1697028. doi: 10.1080/20013078.2019.1697028
75. Libregts SFWM, Arkesteijn GJA, Németh A, Nolte-'t Hoen ENM, Wauben MHM. Flow cytometric analysis of extracellular vesicle subsets in plasma: impact of swarm by particles of non-interest. J Thromb Haemost. (2018) 16:1423–36. doi: 10.1111/jth.14154
76. Lee J, Kim H, Heo Y, Yoo YK, Han SI, Kim C, et al. Enhanced paper-based ELISA for simultaneous EVs/exosome isolation and detection using streptavidin agarose-based immobilization. Analyst. (2019) 145:157–64. doi: 10.1039/C9AN01140D
77. Yuan F, Li YM, Wang Z. Preserving extracellular vesicles for biomedical applications: consideration of storage stability before and after isolation. Drug Deliv. (2021) 28:1501–9. doi: 10.1080/10717544.2021.1951896
78. Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M, et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles. (2017) 6:1359478. doi: 10.1080/20013078.2017.1359478
79. Kusuma GD, Barabadi M, JL T, Morton DAV, Frith JE, Lim R. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol. (2018) 9:1199. doi: 10.3389/fphar.2018.01199
80. Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chrétien D, et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. (2016) 6:36162. doi: 10.1038/srep36162
81. Görgens A, Corso G, Hagey DW, Jawad Wiklander R, Gustafsson MO, Felldin U, et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J Extracell Vesicles. (2022) 11:e12238. doi: 10.1002/jev2.12238
82. Charoenviriyakul C, Takahashi Y, Nishikawa M, Takakura Y. Preservation of exosomes at room temperature using lyophilization. Int J Pharm. (2018) 553:1–7. doi: 10.1016/j.ijpharm.2018.10.032
83. Gelibter S, Marostica G, Mandelli A, Siciliani S, Podini P, Finardi A, et al. The impact of storage on extracellular vesicles: A systematic study. J Extracell Vesicles. (2022) 11:e12162. doi: 10.1002/jev2.12162
84. Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. BioMed Pharmacother. (2020) 128:110237. doi: 10.1016/j.biopha.2020.110237
85. Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol. (2016) 7:231. doi: 10.3389/fphar.2016.00231
86. Shelke GV, Lässer C, Gho YS, Lötvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. (2014) 3:24783. doi: 10.3402/jev.v3.24783
87. Kim JY, Rhim WK, Seo HJ, Lee JY, Park CG, Han DK. Comparative analysis of MSC-derived exosomes depending on cell culture media for regenerative bioactivity. Tissue Eng Regener Med. (2021) 18:355–67. doi: 10.1007/s13770-021-00352-1
88. Li J, Lee Y, Johansson HJ, Mäger I, Vader P, Nordin JZ, et al. Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles. J Extracell Vesicles. (2015) 4:26883. doi: 10.3402/jev.v4.26883
89. Fernandes AM, Fernandes TG, Diogo MM, da Silva CL, Henrique D, Cabral JM. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. (2007) 132:227–36. doi: 10.1016/j.jbiotec.2007.05.031
90. Yan L, Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. (2020) 36:165–78. doi: 10.1007/s10565-019-09504-5
91. Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. (2020) 11:206. doi: 10.1186/s13287-020-01719-2
Keywords: small extracellular vesicles, purification, scale-up, industrialization, therapeutics
Citation: Zheng X, Ai H, Qian K, Li G, Zhang S, Zou Y, Lei C, Fu W and Hu S (2024) Small extracellular vesicles purification and scale-up. Front. Immunol. 15:1344681. doi: 10.3389/fimmu.2024.1344681
Received: 26 November 2023; Accepted: 06 February 2024;
Published: 26 February 2024.
Edited by:
Sina Naserian, Hôpital Paul Brousse, FranceReviewed by:
Juan Antonio Fafian Labora, University of A Coruña, SpainSara Shamdani, Hôpital Paul Brousse, France
Copyright © 2024 Zheng, Ai, Qian, Li, Zhang, Zou, Lei, Fu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Hu, aHVzQHNtbXUuZWR1LmNu; Wenyan Fu, ZnV3ZW55YW5AZmVuZ21lZC5jb20=
 Xinya Zheng
Xinya Zheng Hongru Ai
Hongru Ai Kewen Qian
Kewen Qian Guangyao Li3
Guangyao Li3 Shuyi Zhang
Shuyi Zhang Yitan Zou
Yitan Zou Changhai Lei
Changhai Lei Wenyan Fu
Wenyan Fu Shi Hu
Shi Hu