- 1Research Centre McGill University Health Centre, Montreal, QC, Canada
- 2Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Shanghai, China
- 3Chronic Viral Illness Service and Division of Hematology, McGill University Health Centre, Montreal, QC, Canada
Antiretroviral therapies (ART) have reduced human immunodeficiency virus (HIV) infection-associated morbidity and mortality improving the life of people with HIV (PWH). However, ART lead to residual HIV production, which in conjunction with microbial translocation and immune dysfunction contributes to chronic inflammation and immune activation. PWH on ART remain at an increased risk for cardiovascular diseases (CVDs) including myocardial infarction and stroke; which in part is explained by chronic inflammation and immune activation. Lifestyle factors and certain ART are associated with dyslipidemia characterized by an increase of low-density lipoprotein (LDL), which further contributes in the increased risk for CVDs. Lipid-lowering agents like statins are emerging as immune modulators in decreasing inflammation in a variety of conditions including HIV. The international randomized clinical trial REPRIEVE has shed light on the reduction of CVDs with statin therapy among PWH. Such reports indicate a more than expected benefit of statins beyond their lipid-lowering effects. Bempedoic acid, a first-in-class non-statin LDL-lowering drug with immune modulatory effects, may further aid PWH in combination with statins. Herein, we critically reviewed studies aimed at lipid-lowering and immune-modulating roles of statins that may benefit aging PWH.
Introduction
Antiretroviral therapies (ART) control viral replication without curing HIV infection and need to be taken for long-term. ART significantly improve the quality of life of people with HIV (PWH) by improving immune function, reducing morbidity and increasing survival (1). However, the risk for inflammatory non-AIDS events such as cancer, neurocognitive disorders, liver dysfunction and cardiovascular diseases (CVDs) remains elevated among PWH on ART (1–4). Shah et al. did a meta-analysis recently and confirmed previous findings that PWH are at a greater risk for the development of CVDs in comparison to people without HIV (5). In part, such elevated risk in PWH is explained by chronic inflammation and immune activation and a higher proportion having traditional risk factors such as smoking, diabetes, and dyslipidemia (6, 7). Factors associated with inflammation and immune activation among PWH include HIV RNA and proteins produced on ART (8), gut damage and subsequent microbial translocation coupled with gut microbial dysbiosis and co-infections such as hepatitis C virus (HCV) and cytomegalovirus (CMV) (9, 10). Increased levels of several markers of inflammation and immune activation such as C-reactive protein (CRP), interleukin (IL)-6 and soluble CD14 (sCD14) have been linked with CVD events among PWH (10). A widely studied systemic indirect marker of microbial translocation is sCD14, a monocyte activation marker which has also been associated with mortality among PWH (9, 11). Zidar et al. reported among 54 PWH versus 24 controls an association of sCD14 with oxidized low density lipoprotein (ox-LDL) (12), which is also considered a predictor of CVDs (13). We reported a correlation of sCD14 with soluble suppressor of tumorigenicity 2 (sST2) (14), a marker of cardiopulmonary dysfunction and fibrosis (15, 16), in the context of immune activation among PWH. In addition, 1,3-β-D-Glucan (βDG), a marker of microbial translocation of fungal origin contributes in chronic immune activation and has also been associated with CVDs (17, 18).
Dyslipidemia leads to CVDs and has been linked with inflammation and boosted protease inhibitors-based ART among PWH (19, 20). However, such risk is reduced with the use of integrase strand transfer inhibitor (INSTI)-based ART regimens that are increasingly being prescribed in two-drug (2-DR) or three-drug (3-DR) regimens (21–23). Increased risk for CVDs is evident among PWH versus general population even after taking into account the traditional risk factors such as smoking, physical inactivity and diabetes (4, 24, 25). Such risk is reduced with the use of statins that not only reduce dyslipidemia but also act as an immune modulator (7). Despite these benefits, statin utilization among PWH remains relatively low in part due to potential adverse effects and interactions with ART (26, 27). In addition, there is a dearth of large-scale intervention studies focusing on optimal statin utilization among PWH. The results from the NIH co-funded, multi-center Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE, NCT02344290) showed that a fixed daily dose of 4mg of pitavastatin calcium vs. placebo (in a 1:1 randomization) reduced by 35% the incidence of major CVD events over a median follow-up of 5.1 years (28). Such REPRIEVE results showing significant reduction of CVD events with statin use among PWH with low risk profile are consistent with the importance of an inflammatory aetiology for much of the CVD risk in this population. Among adverse events, muscle-related symptoms and incident diabetes mellitus (DM) respectively were 1.7 and 1.4 times more likely to be reported in pitavastatin group compared to placebo. Overall, a large sample size and a multi-ethnic study population recruited from 12 countries across continents make REPRIEVE a unique seminal study. This review aims to summarize findings from studies including REPRIEVE that evaluated statins as lipid-lowering agents and as immune modulators among people with or without HIV.
Overview of statins and their mechanism of action
Mevastatin or compactin was the first member of statins administered, which was isolated in Japan in 1976 by a biochemist, Akira Endo, from a fungal culture medium of Penicillium citrinum (29). However, the development of mevastatin was discontinued, as the drug caused lymphoma in dogs that received extremely high doses (which was about 200 times the dosage that would be used in humans). Mevastatin discovery was followed by lovastatin and simvastatin in the 1980s, both of these are also fungal metabolites. Later, the chemically modified next generation of statins such as pravastatin and fluvastatin were approved, which were comparatively more bioavailable. Rosuvastatin represents the new generation of hydrophilic statins with a longer half-life and a better potency (30). Currently, there are seven FDA approved medications in the class of statins that are commonly used, namely: atorvastatin, fluvastatin, pitavastatin, lovastatin, simvastatin, rosuvastatin, and pravastatin. These can also be grouped as natural statins such as lovastatin, simvastatin and pravastatin; and synthetic ones such as rosuvastatin, fluvastatin, atorvastatin and pitavastatin (31). Conflicting results have been reported on the superiority of hydrophilic or lipophilic statins in preventing CVDs (32). Statins are orally administered, well-tolerated and are generally considered a safe medication. Liver is the primary site of statin metabolism, while excretion mainly occurs via urinary tract.
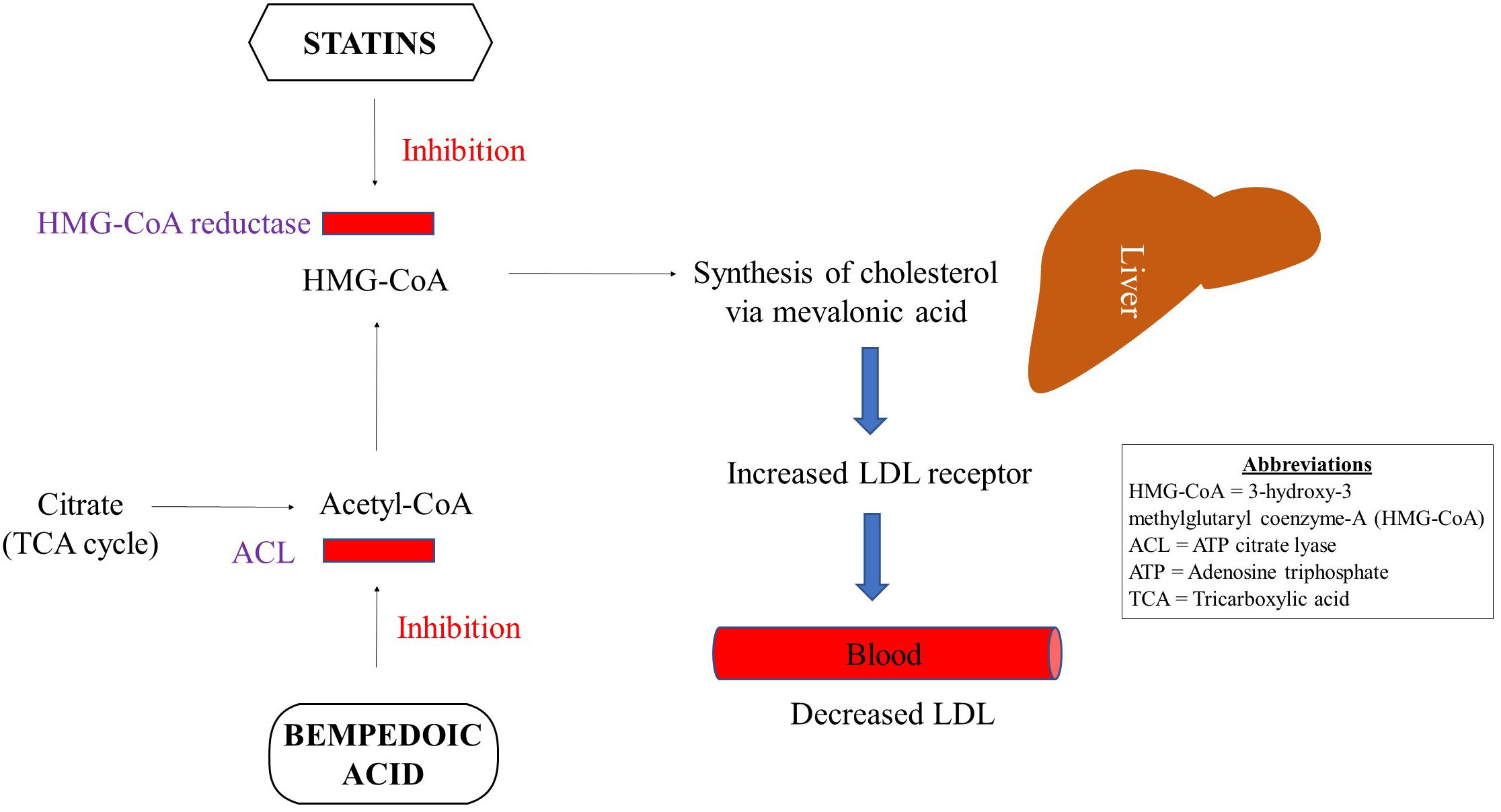
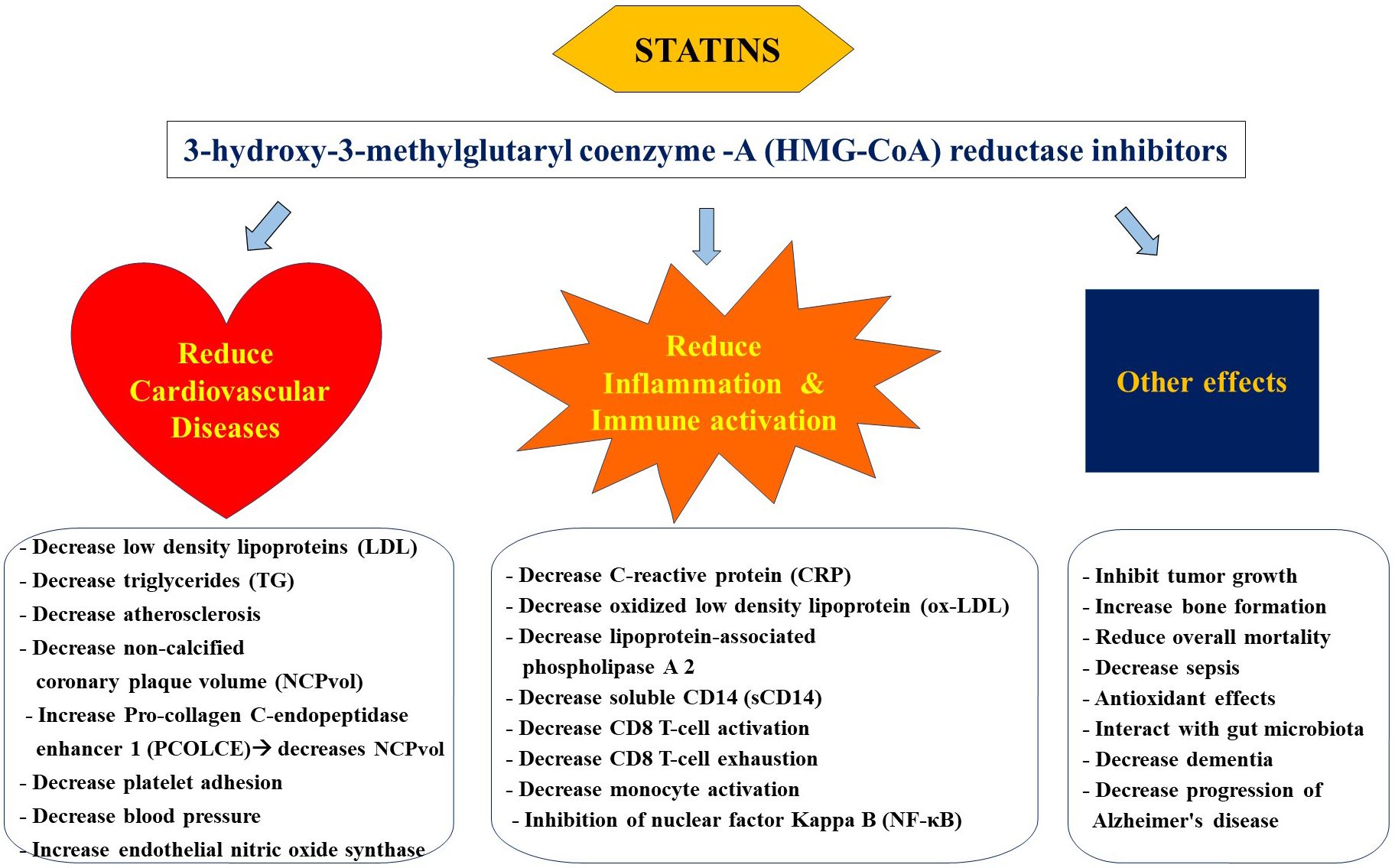
Statins regulate the concentration of plasma lipoproteins by inhibiting 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase at the proximal end of the mevalonate pathway (33) Figure 1. Inhibition of this rate-limiting step in the mevalonate pathway results in decreased endogenous production of cholesterol via overexpression of LDL receptors in the liver followed by a reduction in LDL particles in the periphery. Statins are increasingly considered a group of pleiotropic medications by being HMG-CoA reductase inhibitors that modulate the mevalonate pathway which is involved in a variety of functions such as cell proliferation and signaling, and platelet activation (34–36). Parihar and colleagues have recently reviewed the mechanism of action of statins in the context of anti-microbial therapy (30). Besides decreasing the risk for CVDs, statins are featured as immune modulatory medications that mainly decrease inflammation and immune activation (33, 37, 38) Figure 2.
Inflammation is mediated by the transcription regulator nuclear factor Kappa B (NF-κB) (39). Statins are involved in the inhibition of NF-κB activity by a variety of mechanisms ranging from: inhibition of Rho which is required for NF-κB activation and induction of kruppel like factor 2 (KLF2) which also suppresses NF-κB activation. Panigrahi and colleagues further demonstrated in vitro the protective effects of simvastatin on human aortic endothelial cells against KLF2 down-regulation induced by bacterial products and ox-LDL (40). Such functions highlight anti-inflammatory role of statins reviewed later in this manuscript.
Statins and CVDs
Statins constitute first-line therapy for the reduction of LDL and triglycerides in circulation and are known to decrease the risk for CVDs in the general population (41). The cholesterol treatment trialists collaboration further estimated a reduction in CVDs of 22% in proportion to each decrease in LDL of 38.7 mg/dL (42). Among persons with increased levels of C-reactive protein (CRP), a marker of inflammation, the Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) led by Ridker et al. reported a more than expected reduction of CVDs with the use of rosuvastatin (43). This randomized clinical trial (RCT) included 17,802 apparently healthy persons with negative history of CVDs coupled with LDL levels below 130 mg/dL, and reported that 20 mg daily rosuvastatin reduced systemic CRP levels by 37% and LDL cholesterol by 50%, leading to a reduction in the incidence of CVDs such as myocardial infarction, stroke and unstable angina (43). In addition, all-cause mortality was also significantly reduced with the use of rosuvastatin. Among adverse events, the trial reported that the statin group had a higher incidence of diabetes but did not have a significant increase in muscle weakness or cancer. Along these lines, several studies have reported statins to reduce systemic levels of CRP, which is widely used as a predictor of CVDs (44, 45). Such results suggest implication of statins beyond lipid-lowering agents in the context of inflammation.
Wong and colleagues analyzed the National Health and Nutrition Examination Survey and estimated that despite statin use up to 80% of patients did not achieve an optimal LDL level (46). They attributed such sub-optimal statin response to less potent statin use, higher LDL levels at the initiation of therapy, aggressive LDL treatment goals or poor compliance. Therefore, alternate therapies or statin in combination with another medication such as bempedoic acid has been suggested for patients who respond poorly to statin monotherapy (47). Treatment non-compliance due to statin associated muscle-related adverse effects also prompts the use of alternate treatment options such as ezetimibe and bempedoic acid, both of which can also be used in combination with statins. Of note, bempedoic acid is a medication with minimal side effects owing to its localized gut effects. Like statins, bempedoic acid is increasingly being reported to have anti-inflammatory properties coupled with improved glucose metabolism (47). Bempedoic acid has been shown to have a converging mechanism of action with that of statins (Figure 1), and is briefly discussed below.
Bempedoic acid: an emerging statin add-on
In 2020 FDA approved bempedoic acid (8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid), a novel first-in-class non-statin oral medication for the treatment of adults with atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH) who did not achieve optimal LDL reduction on statins (48, 49). Bempedoic acid is known to reduce the levels of LDL in blood by inhibiting adenosine triphosphate (ATP) citrate lyase (ACL) in the metabolic synthesis pathway upstream of statins (47) (Figure 1). ACL is an important enzyme that connects carbohydrate to lipid metabolism. Bempedoic acid is a prodrug that is converted to its active form by acyl-CoA synthetase 1, which mainly is a hepatic enzyme, and is not expressed in the skeletal muscle. Therefore, bempedoic aced is less likely to cause muscle-related adverse effects (50). Additional benefits of bempedoic acid include activation of AMP-activated protein kinase (AMPK) that confers improved insulin sensitivity. Bempedoic acid is also recently shown to have anti-inflammatory properties by decreasing CRP level via activation of AMPK (47).
Bempedoic acid is prescribed in a single daily oral dose of 180mg which is generally well-tolerated. When given in combination with statins and/or ezetimibe in comparison to placebo, bempedoic acid significantly reduced LDL and CRP in patients with or at risk for ASCVD or HeFH (51). Ray et al. showed in a RCT involving 2230 patients with ASCVD and/or HeFH, with LDL ≥70mg/dl and receiving maximally tolerated statin dose that further reduction in LDL was achieved with the use of bempedoic acid versus placebo (49). They showed a reduction in LDL by 19.2mg/dl was achieved following 12 weeks of daily 180 mg dose of bempedoic acid. Both adverse events and serious adverse events were comparable in both arms of the trial with the exception of almost four times higher incidence of gout in the treatment arm. Bempedoic acid is also known to enhance the plasma levels of statins such as pravastatin and simvastatin and may require patient monitoring (47). In addition, it also offers a safer alternate in patients who are unable to tolerate statins. Therefore, it represents a promising medication in the context of HIV infection as a statin add-on or as an independent lipid-lowering and immune modulatory agent. Among ART-treated PWH on statins with poor treatment compliance owing to undesirable statin effects such as muscle issues, bempedoic acid could be a better option once evaluated for its safety and efficacy in large-scale randomized clinical trials.
Statin use among PWH
Improvements in ART coupled with its early initiation are leading the evolution of HIV infection into a chronic condition with increased risks for co-morbidities in comparison to the general population. CVDs including myocardial infarction, unstable angina and stroke constitute the major portion of such co-morbidities and are overrepresented among PWH even after years being treated with ART. Shah et al. estimated in 2018 the global prevalence of HIV-associated CVDs to have tripled over the past two decades in a meta-analysis of 80 studies representing about 0.8 million participants and 3.5 million person-years of follow-up (5). HIV itself and associated chronic immune activation and inflammation jointly contribute to the risk of CVDs besides traditional factors such as smoking, diabetes and dyslipidemia (52). Ladapo et al. reported a lower prescription of statins among PWH versus their HIV-uninfected counterparts in a survey of nationally representative sample of adults at high risk for CVDs in the United States (26). Similarly, Wu et al. reported among PWH who met statin prescription criteria, only about 39% were actually prescribed statins (53). Statins among PWH are primarily prescribed to decrease dyslipidemia; thereby, reducing the risk for CVDs. However, Phan et al. reported a lack of association between statin use and carotid atherosclerosis progression or total mortality among PWH at high risk for CVDs (54). The authors further discussed the limitations of their study such as an observational study design, a small sample size, an overall small number of deaths and an overall small number of statin users which may have influenced their findings.
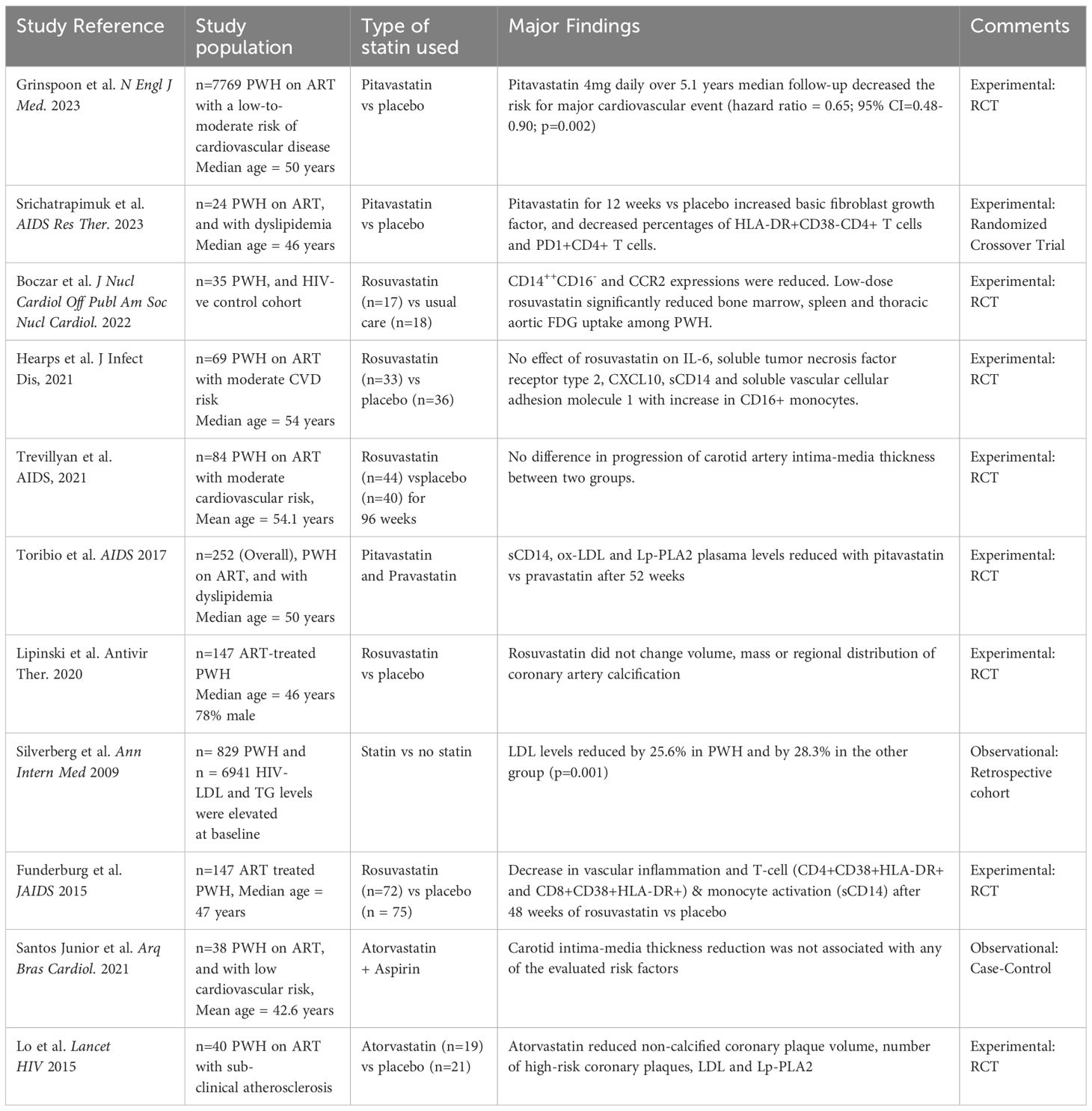
Grinspoon et al. recently reported results from much awaited REPRIEVE study, a phase 3 randomized controlled cosmopolitan clinical trial, largest ever conducted in PWH from March 2015 to July 2019 at 145 sites in 12 countries (28, 55, 56). Among 7,769 study participants 40-75 years of age, ART-treated, without CVD, renal or liver diseases, and with CD4 T-cell count > 100 cells/ul, nearly half were randomly assigned 4mg per day of pitavastatin calcium orally, while the rest were given an identical placebo. The median age of the study participants was 50 years and they did not have any past medical history of ASCVDs and were at a low to moderate risk of such conditions (57). The ASCVDs risk score was assessed by the 2013 American College of Cardiology/American Heart Association criteria (58). The trial was stopped earlier than planned as the statin therapy showed clear benefits which outweighed risks after a median follow-up of 5.1 years. The statin group in comparison to placebo had significantly lower incidence of major CVD events (4.8 vs 7.3 per 1000 person-years) with a hazard ratio of 0.65 (95% CI: 0.48-0.90). However, the intervention group also developed muscle-related symptoms and incident DM 1.7 and 1.4 times respectively, more than the placebo group, which remain lower than expected. Of note, pitavastatin comparatively remains less accessible owing to being expensive under patent. Along these lines, the extension of study findings to other statins remains a study limitation until determined, as also noted by Vergallo and Patrono (59). Grinspoon et al. further discuss that pitavastatin will soon be off-patent paving the way for the availability of its cheaper generic forms (60). In a mechanistic sub-study of REPRIEVE to evaluate the biological pathways implicated in statin response, using a targeted discovery proteomics approach among 542 participants, pitavastatin versus placebo was associated with a reduction in non-calcified plaque via Pro-collagen C-endopeptidase enhancer 1 (PCOLCE or PCPE-1), the rate-limiting enzyme in collagen deposition in interstitial tissue (61). Of note, changes in non-calcified coronary plaque volume were independent of changes in LDL. However, in another analysis Grinspoon et al. did not find an association between non-calcified or total coronary plaque volume and inflammatory markers in a representative sample of 604 study participants (62). Taken together, REPRIEVE is a benchmark study among PWH owing to its global representation, large multi-ethnic sample, strong study design and a prominent team of investigators.
Lo and colleagues reported a reduction of non-calcified coronary plaque volume, LDL and lipoprotein associated phospholipase 2 (LAP-A2) with one year use of atorvastatin in comparison to placebo in a small RCT with 40 ART-treated PWH with subclinical atherosclerosis (63) (Table 1). Several RCTs have further assessed the role of statins in decreasing inflammation, and LDL among treated PWH, as summarized in Table 1 (28, 63–72). However, in comparison to the general population, a smaller decrease in LDL and triglycerides is achieved by the use of statins in PWH (73). Such sub-optimal statin response among PWH could partly be explained by co-administration with different ART regimens, different statin dosages, compliance, or the complexity of dyslipidemia in this population.
Dyslipidemia among PWH is characterized by elevated low density lipoproteins with a smaller particle size, decreased high density lipoproteins and hypertriglyceridemia (74). Of note, the smaller dense low-density lipoproteins are highly atherogenic, and are further intensified by the high level of triglycerides in circulation. Stopping Atherosclerosis and Treating Unhealthy bone with RosuvastatiN in HIV (SATURN-HIV) is the first double-blind RCT that studied markers of cardiovascular risk, skeletal health, and immune activation following statin therapy among ART-treated PWH (71). The study reported a reduction in several markers of inflammation and lymphocyte and monocyte activation after a 48-week rosuvastatin administration at a daily dose of 10mg (Table 1). Of note, the study found significant reductions in lipoprotein-associated phospholipase A2 (Lp-PLA2) which is a specific marker of vascular inflammation. Such observation further supports the non-lipid-lowering endothelial protective function of statins. Erlandson et al. conducted a secondary analysis of SATURN-HIV study and reported a worsening of insulin resistance in the statin group vs placebo from baseline to week 96 (75). However, the incidence of DM was similar in the two arms, which could be attributed to a relatively smaller sample size and a relatively short duration of the study. In contrast, HIV Outpatient Study (HOPS) reported an increased risk of incident DM with statin use among 4692 PWH which mirrored the findings in the general population (76). Such data suggest a risk-benefit consideration prior to statin use, especially in patients with a lower CVD risk.
While fluvastatin, rosuvastatin and pravastatin are generally considered safer statins, others such as simvastatin and atorvastatin interact with certain type of ART such as protease inhibitor (PI)-based regimens and may require dose adjustments or contraindication (77). PIs and other antiretrovirals such as efavirenz inhibit cytochrome P450 3A4 (CYP3A4) enzyme, which is predominantly required in the metabolism of statins. In addition, Malvestutto and colleagues showed pitavastatin devoid of interactions with contemporary ART including efavirenz (78). Among the newer ART classes, integrase strand transfer inhibitors (INSTIs) such as dolutegravir and bictegravir are preferred as a first-line therapy (79). INSTIs in general are not associated with unfavorable changes in lipids nor they are known to result in harmful interactions with statins (27, 79, 80). However, data from prospective RESPOND study of 17 European and Australin cohorts of about 30,000 PWH reported an increased risk of CVDs in the first 2 years of INSTI versus no-INSTI exposure after accounting for known CVD risk factors (80). Encouragingly, similar CVD risk levels were observed in the two groups beyond 2 years; thereby suggesting further confirmation of such results in long-term studies. In addition, the authors acknowledged the potential for unmeasured confounding and channeling bias influencing their study findings. Currently INSTI-based 2DR (such as dolutegravir+lamivudine) versus 3-DR are increasingly being employed which is not only a cost-effective strategy, but also reduces the burden of medications increasing compliance and reducing adverse effects while showing non-inferiority in several RCTs (21–23). Cahn et al. reported in secondary analysis of GEMINI-1 and GEMINI-2 RCTs with ART-naïve participants from 187 centres in 21 countries that 2DR dolutegravir+lamivudine was not inferior to 3DR through 144 weeks of the study period (22). Two cardiac related fatal adverse events in the 2DR group were not drug related, and the changes in lipids at 144 weeks from the baseline generally favored 3DR group. They further reported no differences in the levels of IL-6 and CRP at week 144 from baseline among the two groups. Similarly, Cossarizza et al. reported no differences in IL-6 levels between 2-DR and 3-DR arms in an open label RCT after 12 months of treatment switch (81). Contrastingly, Lombardi et al. showed decreased CRP levels after 1 year of treatment switch to 2-DR group versus treatment continuation in 3-DR group in a longitudinal analysis (23). Along these lines, data from SALSA RCT with ART-experienced virologically suppressed participants showed reduction in one (sCD14) out of four biomarkers of inflammation in the 2-DR versus 3-DR group over 48 weeks (82). The clinical significance of such change remains to be determined. On the other hand, TANGO RCT assessed random assignment to 2DR versus continuation of a tenofovir alafenamide (TAF)–based 3-or 4-drug regimen in ART-experienced virologically suppressed participants and showed no such benefits in inflammatory biomarkers (83, 84). However, their results favored INSTI-based 2DR in terms of overall changes in lipids such as total cholesterol, LDL and triglycerides. Taken together, more large-scale prospective assessments are required to determine the effect of INSTI based 2-DR in the reduction of inflammation and of CVD risk, which may further contribute in the reduction of clinical events. Such information would be useful as further guidance to the most correct place for statin administration among PWH.
Singh et al. reported on the strategy for the management of interactions which include prescription of appropriate statin and at a lower than recommended dose (85). However, the dose reduction has also been associated with reduced efficacy. On the other hand, a higher dose is linked with adverse effects such as a higher risk for the development of DM (86). Nearly 10% increase in the onset of DM following statin therapy has been reported in both observational and interventional studies (87, 88). A likely explanation of such increase in DM following statin therapy is owing to an increase in insulin resistance and/or β-cell dysfunction (88). Along these lines, risk-benefit analysis needs to be given a key consideration prior to statin administration among PWH owing to adverse effects such as DM. In 2019, the scientific committee of American Heart Association recommended preventive therapy among PWH with elevated CVD risk profile including presence of HIV-associated and/or more general factors such as a positive family history and chronic kidney disease (89). While REPRIEVE data strongly support statin use; others concluded a lower but non-significant decrease in the risk of all-cause mortality, any adverse effect and DM with statin treatment versus placebo/control (54, 90). Of note, study by Phan et al. included 127 participants and showed no difference in the progression of carotid artery intima-media thickness (cIMT) with statin use (54).
Riestenberg et al. have reported in an American cohort of 5,039 PWH and 10.011 controls that the former group was more likely to have ever taken pravastatin or atorvastatin (91). They further showed differences in statin prescription by ethnicity among PWH and such disparity was less pronounced among controls. Statins also provide benefits in decreasing chronic inflammation and immune activation which may decrease the risk for other non-AIDS events alongside CVDs (77).
Statins as immune modulators: beyond their lipid-lowering effect
Beyond the lipid-lowering traditional role of statins, emerging studies have reported on their multifaceted effects ranging from anti-inflammatory immune modulatory functions (37), as briefly introduced earlier. They have been shown to inhibit NF-KB activity that further leads to a reduction of inflammation and of activated immune cells. Similarly, nitric oxide (NO) production is also increased by statins mainly via upregulation of endothelial NO synthase (eNOS) leading to a decrease in platelet aggregation and inflammation (33). Several mechanisms have been implicated in statin induced eNOS expression such as by activation of either the Phosphoinositide 3-kinase (PI3k)/Protein kinase B (Akt) pathway or the adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway or by the inhibition of the Rho/ROCK pathway.
Statins are implicated in both innate and adaptive immune response including in reduced T-cell activation (92, 93). They are reported to induce resistance of CD4 T cells to HIV-1 infection via upregulation of cyclin-dependent kinase inhibitor p21, together with inhibition of immune cell activation and proliferation in vitro (94). In the context of HIV infection, systemic immune activation further adds to the risk for CVDs thereby suggesting a pleiotropic role of statins (77). Two markers of immune activation have been extensively studied along these lines; 1) the soluble CD14 (sCD14), a circulatory marker of monocyte activation, also a surrogate marker of microbial translocation; and 2) the oxidized low-density lipoprotein (ox-LDL), a type of low density lipoprotein that may exacerbate atherosclerotic plaques and activate innate and adaptive immune responses (95). We and others have shown in PWH that sCD14 is linked with several markers of immune dysfunction (9, 14). Soluble CD14 is an indirect marker of microbial translocation which is related to lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria that is a direct marker of microbial translocation (96). LPS is known to induce immune activation in chronic HIV infection (97) and has been inconsistently associated with clinical events (98–100). Such conflicting results could in part be explained by its low precision, association with serum lipid levels and methodological challenges encountered during its quantification, and potential cross-reactivity with βDG (17, 96, 101). LPS activates monocytes/macrophage via lipopolysaccharide binding protein together with toll-like receptor 4 resulting in the release of sCD14 (102, 103). Both sCD14 and ox-LDL have been associated with an increased risk for CVDs (103, 104). Encouragingly, statins have been shown to reduce the systemic level of both of these immune activation markers (20, 68, 105). Hileman et al. were the first to report a significant decrease in ox-LDL levels following a 10 mg daily dose of rosuvastatin versus placebo for 24-weeks among PWH on ART (106). Similarly, Nou et al. showed effectiveness of atorvastatin in decreasing ox-LDL levels among PWH on ART (107). A recent meta-analysis further reported variations in ox-LDL decrease with different types of statins (108). However, all statins analyzed were associated with a significant decrease in ox-LDL levels. Besides ox-LDL, Toribio et al. also attributed a reduction in sCD14 and Lp-PLA2 levels to pitavastatin vs pravastatin after 52 weeks of therapy (68). Of note, Lp-PLA2 is increasingly considered a novel biomarker for vascular wall inflammation.
Another emerging marker of cardiac dysfunction is soluble suppressor of tumorigenicity 2 (sST2), which is encoded by interleukin (IL)-1 receptor family gene ST2 (109, 110). Besides expressing the soluble isoform, ST2 gene also expresses a transmembrane ST2L isoform in a variety of cells such as dendritic cells, mast cells, macrophages, and T-helper cells (110, 111). The functional ligand of ST2 is the inflammation induced factor IL-33, an alarmin cytokine which is also released by myocardial endothelial cells. IL-33/ST2 axis plays an important role in acute and local inflammation as well as in issue repair. It is being increasingly employed as a prognostic marker in a variety of diseases including cardiac insufficiency and atherosclerosis. In a mouse model, Miller et al. (111) showed IL-33/ST2 signaling reduced the development of atherosclerosis. Secemsky et al. first reported sST2 to be linked with cardiac dysfunction and all-cause mortality among 332 PWH, of whom 79% were on long-term ART (15). We and others have shown sST2 to be associated with markers of inflammation and immune activation in the context of HIV infection (14). Interestingly, deFilippi et al. reported atorvastatin to be associated with a reduction in the levels of sST2 among PWH on ART which indicated a fibrosis mitigation effect (112). They observed a strong positive correlation of sST2 with sCD14 and monocyte chemoattractant protein 1 (MCP-1). Of note, the correlation between sST2 and sCD14 had previously been unearthed by our team (14). In addition, deFilippi et al. also reported a positive correlation between sST2 and ox-LDL (112), levels of both of these markers are reduced with statin use. Besides bacterial translocation from the gut to periphery, the translocation of fungal products such as the major cell wall component 1,3-β-D-Glucan (βDG), has also been shown to contribute to the chronic inflammation and immune activation and has been associated with cardiopulmonary dysfunction (17, 113, 114). We recently reported correlation of plasma βDG with markers of disease progression, gut damage, bacterial translocation, and inflammation among PWH (17). Whether statin use reduces βDG is yet to be explored, especially when statins are known to influence the systemic levels of markers of bacterial translocation to decrease the level of chronic inflammation and immune activation.
Besides translocation of microbial products, gut microbial dysbiosis has also been linked with chronic inflammation and immune activation and is not restored on long term ART (115–117), and thus could contribute to the risk for adverse events such as CVDs. Encouragingly, growing body of literature supports the notion that statins can regulate gut microbiome (118–120). On the other hand, gut microbiome has also been shown to impact statin efficacy by converting it into secondary metabolites as is the case with other medications. Such association may partly explain a compromised statin response and/or associated adverse effects in some patients (121, 122). Wilmanski et al. recently linked the composition of gut microbiome with heterogeneity of statin response (120). Notably, they showed more intense on/off target statin response in a diversity-depleted gut microbiome with abundance of Bacteroides. However, their study was limited by factors such as a cross-sectional study design and a pre-dominantly white study population from the US west coast and Western Europe.
Statins are implicated in the reduced prevalence of gut microbiota dysbiosis (118) and in the maintenance of homeostasis of gut microbiota by regulating intestinal innate immunity, exhibiting antibacterial activity and inhibiting cell membrane biosynthesis (119). In addition, statins influence bile acid metabolism that leads to changes in the composition of gut microbiota. Moreover, NF-κB signaling is also impacted by statins leading to changes in the composition of gut microbiota. Khan et al. reported in hypercholesterolemic patients an association of atorvastatin with relative abundance of anti-inflammation associated Faecalibacterium prausnitzii, Akkermansia muciniphila, and genus Oscillospira coupled with decreased abundance of proinflammatory Desulfovibrio species (123). Further studies evaluating the effects of statins on gut microbiota in the context of HIV-associated chronic inflammation and immune activation may highlight the therapeutic potential of these traditionally lipid-lowering agents. Therefore, targeting gut microbiota dysbiosis among PWH on ART represents a promising strategy for the reduction of CVDs (124).
In addition, statins are observed to be associated with cell growth, proliferation, secretion and apoptosis, bone formation and angiogenesis (125–127) (Figure 2). Such immune modulatory effects in conditions such as sepsis, pneumonia, influenza, cancer and HIV warrant further research to explore the pleiotropic role of statins (128–132).
Conclusions and future directions
CVDs are known as the major contributor to morbidity and mortality among aging HIV-infected and uninfected populations. Such CVD risk is predicted to increase in the absence of targeted interventions to further jeopardize the quality of life of PWH, an infection which has evolved as a chronic disease. In the light of improvements in contemporary ART and guidelines suggesting prompt ART initiation following an HIV diagnosis, the quantification of CVD risk remains to be evaluated, followed by an assessment of need for interventions. Targeting traditional risk factors, particularly among PWH, with healthy life-style approaches such as smoking cessation and promotion of physical activity is of utmost importance, but may not be sufficient. Lipid-lowering statins can play a major role in the prevention of CVDs and such role remains less appreciated among PWH. Recent evidence suggests a broader implication of statins in the context of ART-treated HIV infection highlighted by a reduction in chronic inflammation and immune activation, which otherwise are fueled in part by microbial translocation and microbial dysbiosis owing to persistent gut damage. Thereby, ART-recipient PWH even with a low or median risk for CVDs can benefit from the pleiotropic effects of statins targeting not only dyslipidemia, but also chronic inflammation and immune activation. Along these lines, emerging areas include further elucidation of statin-gut microbiome interactions which may translate into a reduction of adverse events such as CVDs. The results from the large-scale, multi-centered, multi-national REPRIEVE study clearly show a more than expected reduction in the risk for major cardiovascular events with the use of pitavastatin. Such results can guide policy to lessen CVDs among PWH taking long-term ART.
Author contributions
VM: Writing – review & editing, Writing – original draft, Conceptualization. JC: Writing – review & editing, Writing – original draft. JR: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Our work was funded by the Fonds de la Recherche Québec-Santé (FRQ-S): Réseau SIDA/Maladies Infectieuses and Thérapie Cellulaire; the Canadian Institutes of Health Research (CIHR; Grants HOP 103230, PTJ 166049, and DC0190GP); the Vaccines and Immunotherapies Core of the CIHR Canadian HIV Trials Network (CTN; Grant CTN 247); the Canadian Foundation for AIDS Research (CANFAR; Grant 02-512); the CIHR-funded Canadian HIV Cure Enterprise (CanCURE) Team Grant HB2-164064. JR is the holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University.
Acknowledgments
Authors thank Angie Massicotte for her administrative support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. (2016) 214:S44–50. doi: 10.1093/infdis/jiw275
2. Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. (2021) 9:e161–9. doi: 10.1016/S2214-109X(20)30459-9
3. Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. J Am Heart Assoc. (2019) 8:e012241. doi: 10.1161/JAHA.119.012241
4. Gooden TE, Gardner M, Wang J, Jolly K, Lane DA, Benjamin LA, et al. Incidence of cardiometabolic diseases in people with and without human immunodeficiency virus in the United Kingdom: a population-based matched cohort study. J Infect Dis. (2022) 225:1348–56. doi: 10.1093/infdis/jiab420
5. Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. (2018) 138:1100–12. doi: 10.1161/CIRCULATIONAHA.117.033369
6. Saves M, Chene G, Ducimetiere P, Leport C, Le Moal G, Amouyel P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. (2003) 37:292–8. doi: 10.1086/375844
7. Avgousti H, Feinstein MJ. Prevention and treatment of cardiovascular disease in HIV: practical insights in an evolving field. Top Antivir Med. (2023) 31:559–65.
8. Dubé M, Tastet O, Dufour C, Sannier G, Brassard N, Delgado GG, et al. Spontaneous HIV expression during suppressive ART is associated with the magnitude and function of HIV-specific CD4+ and CD8+ T cells. Cell Host Microbe. (2023) 31:1507–22.e5. doi: 0.1016/j.chom.2023.08.006
9. Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. (2013) 21:6–13. doi: 10.1016/j.tim.2012.09.001
10. Hsu DC, Sereti I. Serious non-AIDS events: therapeutic targets of immune activation and chronic inflammation in HIV infection. Drugs. (2016) 76:533–49. doi: 10.1007/s40265-016-0546-7
11. Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. (2011) 203:780–90. doi: 10.1093/infdis/jiq118
12. Zidar DA, Juchnowski S, Ferrari B, Clagett B, Pilch-Cooper HA, Rose S, et al. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr 1999. (2015) 69:154–60. doi: 10.1097/QAI.0000000000000566
13. Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. (2005) 112:651–7. doi: 10.1161/CIRCULATIONAHA.104.529297
14. Mehraj V, Jenabian MA, Ponte R, Lebouché B, Costiniuk C, Thomas R, et al. The plasma levels of soluble ST2 as a marker of gut mucosal damage in early HIV infection. AIDS. (2016) 30:1617–27. doi: 10.1097/QAD.0000000000001105
15. Secemsky EA, Scherzer R, Nitta E, Wu AHB, Lange DC, Deeks SG, et al. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. JACC Heart Fail. (2015) 3:591–9. doi: 10.1016/j.jchf.2015.03.007
16. Zhang T, Xu C, Zhao R, Cao Z. Diagnostic value of sST2 in cardiovascular diseases: a systematic review and meta-analysis. Front Cardiovasc Med. (2021) 8:697837. doi: 10.3389/fcvm.2021.697837
17. Mehraj V, Ramendra R, Isnard S, Dupuy FP, Ponte R, Chen J, et al. Circulating (1→3)-β-D-glucan is associated with immune activation during human immunodeficiency virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am. (2020) 70:232–41. doi: 10.1093/cid/ciz212
18. Isnard S, Fombuena B, Sadouni M, Lin J, Richard C, Routy B, et al. Circulating β-d-glucan as a marker of subclinical coronary plaque in antiretroviral therapy-treated people with human immunodeficiency virus. Open Forum Infect Dis. (2021) 8:ofab109. doi: 10.1093/ofid/ofab109
19. Stein JH. Dyslipidemia in the era of HIV protease inhibitors. Prog Cardiovasc Dis. (2003) 45:293–304. doi: 10.1053/pcad.2003.4
20. Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS Lond Engl. (2016) 30:1495–509. doi: 10.1097/QAD.0000000000001109
21. Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. (2019) 393:143–55. doi: 10.1016/S0140-6736(18)32462-0
22. Cahn P, Sierra Madero J, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy - naive adults with HIV-1 infection. AIDS Lond Engl. (2022) 36:39–48. doi: 10.1097/QAD.0000000000003070
23. Lombardi F, Belmonti S, Moschese D, Fabbiani M, Borghetti A, Ciccullo A, et al. Inflammation markers in virologically suppressed HIV-Infected patients after switching to dolutegravir plus lamivudine vs continuing triple therapy: 48-week results in real-life setting. HIV Res Clin Pract. (2022) 23:28–36.
24. Webel AR, Perazzo J, Longenecker CT, Jenkins T, Sattar A, Rodriguez M, et al. The influence of exercise on cardiovascular health in sedentary adults with human immunodeficiency virus. J Cardiovasc Nurs. (2018) 33:239–47. doi: 10.1097/JCN.0000000000000450
25. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. (2007) 92:2506–12. doi: 10.1210/jc.2006-2190
26. Ladapo JA, Richards AK, DeWitt CM, Harawa NT, Shoptaw S, Cunningham WE, et al. Disparities in the quality of cardiovascular care between HIV-infected versus HIV-uninfected adults in the United States: A cross-sectional study. J Am Heart Assoc. (2017) 6:e007107. doi: 10.1161/JAHA.117.007107
27. Sarkar S, Brown TT. Lipid disorders in people with HIV. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, editors. Endotext. MDText.com, Inc, South Dartmouth (MA (2023). Available at: http://www.ncbi.nlm.nih.gov/books/NBK567198/.
28. Grinspoon SK, Fitch KV, Zanni MV, Fichtenbaum CJ, Umbleja T, Aberg JA, et al. Pitavastatin to prevent cardiovascular disease in HIV infection. N Engl J Med. (2023) 389:687–99. doi: 10.1056/NEJMoa2304146
29. Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci. (2010) 86:484–93. doi: 10.2183/pjab.86.484
30. Parihar SP, Guler R, Brombacher F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat Rev Immunol. (2019) 19:104–17. doi: 10.1038/s41577-018-0094-3
31. Koushki K, Shahbaz SK, Mashayekhi K, Sadeghi M, Zayeri ZD, Taba MY, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol. (2021) 60:175–99. doi: 10.1007/s12016-020-08791-9
32. Climent E, Benaiges D, Pedro-Botet J. Hydrophilic or lipophilic statins? Front Cardiovasc Med. (2021) 8:687585. doi: 10.3389/fcvm.2021.687585
33. Zhang Q, Dong J, Yu Z. Pleiotropic use of Statins as non-lipid-lowering drugs. Int J Biol Sci. (2020) 16:2704–11. doi: 10.7150/ijbs.42965
34. Gong L, Xiao Y, Xia F, Wu P, Zhao T, Xie S, et al. The mevalonate coordinates energy input and cell proliferation. Cell Death Dis. (2019) 10:327. doi: 10.1038/s41419-019-1544-y
35. Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. (2016) 16:718–31. doi: 10.1038/nrc.2016.76
36. Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering–are they clinically relevant? Eur Heart J. (2003) 24:225–48. doi: 10.1016/S0195-668X(02)00419-0
37. Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. (2005) 45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748
38. Kim SW, Kang HJ, Jhon M, Kim JW, Lee JY, Walker AJ, et al. Statins and inflammation: new therapeutic opportunities in psychiatry. Front Psychiatry. (2019) 10:103. doi: 10.3389/fpsyt.2019.00103
39. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. (2018) 18:309–24. doi: 10.1038/nri.2017.142
40. Panigrahi S, Freeman ML, Funderburg NT, Mudd JC, Younes SA, Sieg SF, et al. SIV/SHIV infection triggers vascular inflammation, diminished expression of Krüppel-like factor 2 and endothelial dysfunction. J Infect Dis. (2016) 213:1419–27. doi: 10.1093/infdis/jiv749
41. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 140(11):e563–95. doi: 10.1161/CIR.0000000000000677
42. Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet Lond Engl. (2010) 376:1670–81. doi: 10.1016/S0140-6736(10)61350-5
43. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. (2008) 359:2195–207. doi: 10.1056/NEJMoa0807646
44. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. (2001) 344:1959–65. doi: 10.1056/NEJM200106283442601
45. Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. (2005) 352:29–38. doi: 10.1056/NEJMoa042000
46. Wong ND, Young D, Zhao Y, Nguyen H, Caballes J, Khan I, et al. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011-2012. J Clin Lipidol. (2016) 10:1109–18. doi: 10.1016/j.jacl.2016.06.011
47. Biolo G, Vinci P, Mangogna A, Landolfo M, Schincariol P, Fiotti N, et al. Mechanism of action and therapeutic use of bempedoic acid in atherosclerosis and metabolic syndrome. Front Cardiovasc Med. (2022) 9:1028355. doi: 10.3389/fcvm.2022.1028355
48. ACC News Story: FDA Approves Bempedoic Acid for Treatment of Adults With HeFH or Established ASCVD (2020). Available online at: https://www.acc.org/latest-in-cardiology/articles/2020/02/24/10/09/fda-approves-bempedoic-acid-for-treatment-of-adults-with-hefh-or-established-ascvd.
49. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. (2019) 380:1022–32. doi: 10.1056/NEJMoa1803917
50. Laufs U, Ballantyne CM, Banach M, Bays H, Catapano AL, Duell PB, et al. Efficacy and safety of bempedoic acid in patients not receiving statins in phase 3 clinical trials. J Clin Lipidol. (2022) 16:286–97. doi: 10.1016/j.jacl.2022.03.001
51. Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. (2019) 8:e011662. doi: 10.1161/JAHA.118.011662
52. Fitch KV, Fulda ES, Grinspoon SK. Statins for primary cardiovascular disease prevention among people with HIV: emergent directions. Curr Opin HIV AIDS. (2022) 17:293–300. doi: 10.1097/COH.0000000000000752
53. Wu PY, Sun HY, Huang YS, Liu WD, Lin KY, Luo YZ, et al. Under-utilization of statins among people with HIV who were aged 40 years or older. J Microbiol Immunol Infect. (2024) 57:200–3. doi: 10.1016/j.jmii.2024.01.003
54. Phan BAP, Ma Y, Scherzer R, Deeks SG, Hsue PY. Association between statin use, atherosclerosis, and mortality in HIV-infected adults. PLoS One. (2020) 15:e0232636. doi: 10.1371/journal.pone.0232636
55. Grinspoon SK, Fitch KV, Overton ET, Fichtenbaum CJ, Zanni MV, Aberg JA, et al. Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE). Am Heart J. (2019) 212:23–35. doi: 10.1016/j.ahj.2018.12.016
56. Grinspoon SK, Douglas PS, Hoffmann U, Ribaudo HJ. Leveraging a landmark trial of primary cardiovascular disease prevention in human immunodeficiency virus: introduction from the REPRIEVE coprincipal investigators. J Infect Dis. (2020) 222:S1–7. doi: 10.1093/infdis/jiaa098
57. Gilbert JM, Fitch KV, Grinspoon SK. HIV-related cardiovascular disease, statins, and the REPRIEVE trial. Top Antivir Med. (2015) 23:146–9.
58. Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. (2014) 129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98
59. Vergallo R, Patrono C. Cardiovascular disease prevention in people living with HIV: from REPRIEVE to a statin of grace. Eur Heart J. (2023) 44(41):4308–9. doi: 10.1093/eurheartj/ehad594
60. Grinspoon SK, Ribaudo HJ, Douglas PS. Pitavastatin and cardiovascular disease in HIV. Reply N Engl J Med. (2023) 389:e46. doi: 10.1056/NEJMc2311117
61. Kolossváry M, Schnittman SR, Zanni MV, Fitch KV, Fichtenbaum CJ, Aberg JA, et al. Pitavastatin reduces non-calcified plaque via pro-collagen PCOLCE independently of LDL in REPRIEVE. [CROI 2024 Oral Abstract# 151]. In Denver, Colorado. From the 2024 Conference on Retroviruses and Opportunistic Infections. (2024).
62. Grinspoon SK, Lu MT, McCallum S, Zanni MV, Foldyna B, Paradis K, et al. Relating pitavastatin effects on inflammatory biomarkers to plaque changes in REPRIEVE. [CROI 2024 Poster Abstract# 773]. In Denver, Colorado. From the 2024 Conference on Retroviruses and Opportunistic Infections. (2024).
63. Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. (2015) 2:e52–63. doi: 10.1016/S2352-3018(14)00032-0
64. Srichatrapimuk S, Wongsa A, Sungkanuparph S, Kiertiburanakul S, Tassaneetrithep B, Phuphuakrat A. Effects of pitavastatin on atherosclerotic-associated inflammatory biomarkers in people living with HIV with dyslipidemia and receiving ritonavir-boosted atazanavir: a randomized, double-blind, crossover study. AIDS Res Ther. (2023) 20:13. doi: 10.1186/s12981-023-00506-2
65. Boczar KE, Faller E, Zeng W, Wang J, Small GR, Corrales-Medina VF, et al. Anti-inflammatory effect of rosuvastatin in patients with HIV infection: An FDG-PET pilot study. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. (2022) 29:3057–68. doi: 10.1007/s12350-021-02830-4
66. Hearps AC, Angelovich TA, Trevillyan JM, Wong ME, Calmy A, Hoy JF, et al. Effect of rosuvastatin therapy on biomarkers of inflammation and immune activation in people with human immunodeficiency virus at intermediate cardiovascular risk. J Infect Dis. (2021) 224:667–72. doi: 10.1093/infdis/jiaa775
67. Trevillyan JM, Dart A, Paul E, Cavassini M, Fehr J, Staehelin C, et al. Impact of rosuvastatin on atherosclerosis in people with HIV at moderate cardiovascular risk: a randomised, controlled trial. AIDS. (2021) 35:619–24. doi: 10.1097/QAD.0000000000002764
68. Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS Lond Engl. (2017) 31:797–806. doi: 10.1097/QAD.0000000000001427
69. Lipinski J, Margevicius S, Schluchter MD, Wilson DL, McComsey GA, Longenecker CT. Statin effect on coronary calcium distribution, mass and volume scores and associations with immune activation among HIV+ Persons on antiretroviral therapy. Antivir Ther. (2020) 25:419–24. doi: 10.3851/IMP3389
70. Silverberg MJ, Leyden W, Hurley L, Go AS, Quesenberry CP, Klein D, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med. (2009) 150:301–13. doi: 10.7326/0003-4819-150-5-200903030-00006
71. Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. JAIDS J Acquir Immune Defic Syndr. (2015) 68:396–404. doi: 10.1097/QAI.0000000000000478
72. Santos Junior GGD, Araújo PSR, Leite KME, Godoi ET, Vasconcelos AF, Lacerda HR. The effect of atorvastatin + Aspirin on the endothelial function differs with age in patients with HIV: A case-control study. Arq Bras Cardiol. (2021) 117:365–75. doi: 10.36660/abc.20190844
73. Hürlimann D, Chenevard R, Ruschitzka F, Flepp M, Enseleit F, Béchir M, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart Br Card Soc. (2006) 92:110–2. doi: 10.1136/hrt.2004.056523
74. Bittar R, Giral P, Aslangul E, Assoumou L, Valantin MA, Kalmykova O, et al. Effects of rosuvastatin versus pravastatin on low-density lipoprotein diameter in HIV-1-infected patients receiving ritonavir-boosted protease inhibitor. AIDS Lond Engl. (2012) 26:1801–5. doi: 10.1097/QAD.0b013e328357063c
75. Erlandson KM, Jiang Y, Debanne SM, McComsey GA. Rosuvastatin worsens insulin resistance in HIV-infected adults on antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. (2015) 61:1566–72. doi: 10.1093/cid/civ554
76. Lichtenstein KA, Hart RLD, Wood KC, Bozzette S, Buchacz K, Brooks JT, et al. Statin use is associated with incident diabetes mellitus among patients in the HIV outpatient study. J Acquir Immune Defic Syndr 1999. (2015) 69:306–11. doi: 10.1097/QAI.0000000000000581
77. Eckard AR, McComsey GA. The role of statins in the setting of HIV infection. Curr HIV/AIDS Rep. (2015) 12:305–12. doi: 10.1007/s11904-015-0273-9
78. Malvestutto CD, Ma Q, Morse GD, Underberg JA, Aberg JA. Lack of pharmacokinetic interactions between pitavastatin and Efavirenz or Darunavir/Ritonavir. JAIDS J Acquir Immune Defic Syndr. (2014) 67:390–6. doi: 10.1097/QAI.0000000000000333
79. Guidelines for the use of antiretroviral agents in adults and adolescents with HIVS. HHS panel on antiretroviral guidelines for adults and adolescents—A working group of the office of AIDS research advisory council (OARAC). In: ClinicalInfo.HIV.gov. US Department of Health and Human Services, Rockville (MD). Available at: https://www.ncbi.nlm.nih.gov/books/NBK586306/.
80. Neesgaard B, Greenberg L, Miró JM, Grabmeier-Pfistershammer K, Wandeler G, Smith C, et al. Associations between integrase strand-transfer inhibitors and cardiovascular disease in people living with HIV: a multicentre prospective study from the RESPOND cohort consortium. Lancet HIV. (2022) 9:e474–85. doi: 10.1016/S2352-3018(22)00094-7
81. Cossarizza A, Cozzi-Lepri A, Mattioli M, Paolini A, Neroni A, De Biasi S, et al. Evaluating immunological and inflammatory changes of treatment-experienced people living with HIV switching from first-line triple cART regimens to DTG/3TC vs. B/F/TAF: the DEBATE trial. Front Immunol. (2023) 14:1279390. doi: 10.3389/fimmu.2023.1279390
82. Llibre JM, Brites C, Cheng CY, Osiyemi O, Galera C, Hocqueloux L, et al. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1 (HIV-1): week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis. (2023) 76:720–9. doi: 10.1093/cid/ciac130
83. Van Wyk J, Ajana F, Bisshop F, De Wit S, Osiyemi O, Portilla Sogorb J, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide–based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis. (2020) 71:1920–9. doi: 10.1093/cid/ciz1243
84. Osiyemi O, De Wit S, Ajana F, Bisshop F, Portilla J, Routy JP, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide–based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis. (2022) 75:975–86. doi: 10.1093/cid/ciac036
85. Singh S, Willig JH, Mugavero MJ, Crane PK, Harrington RD, Knopp RH, et al. Comparative effectiveness and toxicity of statins among HIV-infected patients. Clin Infect Dis Off Publ Infect Dis Soc Am. (2011) 52:387–95. doi: 10.1093/cid/ciq111
86. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet Lond Engl. (2010) 375:735–42. doi: 10.1016/S0140-6736(09)61965-6
87. Chrysant SG. New onset diabetes mellitus induced by statins: current evidence. Postgrad Med. (2017) 129:430–5. doi: 10.1080/00325481.2017.1292107
88. Zhao W, Zhao SP. Different effects of statins on induction of diabetes mellitus: an experimental study. Drug Des Devel Ther. (2015) 9:6211–23. doi: 10.2147/DDDT
89. Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: A scientific statement from the American heart association. Circulation. (2019) 140(2):e98–124. doi: 10.1161/CIR.0000000000000695
90. Vigny NN, Bonsu KO, Kadirvelu A. Effectiveness and safety of statins on outcomes in patients with HIV infection: a systematic review and meta-analysis. Sci Rep. (2022) 12:18121. doi: 10.1038/s41598-022-23102-2
91. Riestenberg RA, Furman A, Cowen A, Pawlowksi A, Schneider D, Lewis AA, et al. Differences in statin utilization and lipid lowering by race, ethnicity, and HIV status in a real-world cohort of persons with human immunodeficiency virus and uninfected persons. Am Heart J. (2019) 209:79–87. doi: 10.1016/j.ahj.2018.11.012
92. Mira E, Mañes S. Immunomodulatory and anti-inflammatory activities of statins. Endocr Metab Immune Disord Drug Targets. (2009) 9:237–47. doi: 10.2174/187153009789044383
93. Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discovery. (2005) 4:977–87. doi: 10.1038/nrd1901
94. Elahi S, Weiss RH, Merani S. Atorvastatin restricts HIV replication in CD4+ T cells by upregulation of p21. AIDS Lond Engl. (2016) 30:171–83. doi: 10.1097/QAD.0000000000000917
95. Rhoads JP, Major AS. How oxidized low-density lipoprotein activates inflammatory responses. Crit Rev Immunol. (2018) 38:333–42. doi: 10.1615/CritRevImmunol.v38.i4
96. Ramendra R, Isnard S, Mehraj V, Chen J, Zhang Y, Finkelman M, et al. Circulating LPS and (1→3)-β-D-glucan: a Folie à Deux contributing to HIV-associated immune activation. Front Immunol. (2019) 10:465. doi: 10.3389/fimmu.2019.00465
97. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. (2006) 12:1365–71. doi: 10.1038/nm1511
98. Marchetti G, Cozzi-Lepri A, Merlini E, Bellistrì GM, Castagna A, Galli M, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. (2011) 25:1385–94. doi: 10.1097/QAD.0b013e3283471d10
99. Merlini E, Cozzi-lepri A, Castagna A, Costantini A, Caputo SL, Carrara S, et al. Inflammation and microbial translocation measured prior to combination antiretroviral therapy (cART) and long-term probability of clinical progression in people living with HIV. BMC Infect Dis. (2021) 21:557. doi: 10.1186/s12879-021-06260-y
100. for the Icona Foundation Study Group, Marchetti G, Cozzi-Lepri A, Tincati C, Calcagno A, Ceccherini-Silberstein F, et al. Immune activation and microbial translocation in liver disease progression in HIV/hepatitis co-infected patients: results from the Icona Foundation study. BMC Infect Dis. (2014) 14:79. doi: 10.1186/1471-2334-14-79
101. Wong J, Zhang Y, Patidar A, Vilar E, Finkelman M, Farrington K. Is endotoxemia in stable hemodialysis patients an artefact? Limitations of the limulus amebocyte lysate assay and role of (1→3)-β-D glucan. PLoS One. (2016) 11:e0164978. doi: 10.1371/journal.pone.0164978
102. Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. (2002) 23:301–4. doi: 10.1016/S1471-4906(02)02233-0
103. Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS Lond Engl. (2014) 28:969–77. doi: 10.1097/QAD.0000000000000158
104. Duong M, Petit JM, Martha B, Galland F, Piroth L, Walldner A, et al. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV Clin Trials. (2006) 7:41–7. doi: 10.1310/7381-M1YD-RTV5-4RYT
105. Jamialahmadi T, Baratzadeh F, Reiner Ž, Simental-Mendía LE, Xu S, Susekov AV, et al. The effects of statin dose, lipophilicity, and combination of statins plus ezetimibe on circulating oxidized low-density lipoprotein levels: A systematic review and meta-analysis of randomized controlled trials. Mediators Inflamm. (2021) 2021:9661752. doi: 10.1155/2021/9661752
106. Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS Lond Engl. (2016) 30:65–73. doi: 10.1097/QAD.0000000000000885
107. Nou E, Lu MT, Looby SE, Fitch KV, Kim EA, Lee H, et al. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with HIV. AIDS Lond Engl. (2016) 30:583–90. doi: 10.1097/QAD.0000000000000946
108. Jamialahmadi T, Baratzadeh F, Reiner Ž, Mannarino MR, Cardenia V, Simental-Mendía LE, et al. The effects of statin therapy on oxidized LDL and its antibodies: a systematic review and meta-analysis. Oxid Med Cell Longev. (2022) 2022:7850659. doi: 10.1155/2022/7850659
109. Ghali R, Altara R, Louch WE, Cataliotti A, Mallat Z, Kaplan A, et al. IL-33 (Interleukin 33)/sST2 axis in hypertension and heart failure. Hypertens Dallas Tex 1979. (2018) 72:818–28. doi: 10.1161/HYPERTENSIONAHA.118.11157
110. Mehraj V, Ponte R, Routy JP. The dynamic role of the IL-33/ST2 axis in chronic viral-infections: alarming and adjuvanting the immune response. EBioMedicine. (2016) 9:37–44. doi: 10.1016/j.ebiom.2016.06.047
111. Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. (2008) 205:339–46. doi: 10.1084/jem.20071868
112. deFilippi C, Christenson R, Joyce J, Park EA, Wu A, Fitch KV, et al. Brief report: statin effects on myocardial fibrosis markers in people living with HIV. JAIDS J Acquir Immune Defic Syndr. (2018) 78:105–10. doi: 10.1097/QAI.0000000000001644
113. Morris A, Hillenbrand M, Finkelman M, George MP, Singh V, Kessinger C, et al. Serum (1→3)-β-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. JAIDS J Acquir Immune Defic Syndr. (2012) 61:462–8. doi: 10.1097/QAI.0b013e318271799b
114. Hoenigl M, Faria De Oliveira M, Pérez-Santiago J, Zhang Y, Woods SP, Finkelman M, et al. Correlation of (1→3)-β-D-glucan with other inflammation markers in chronically HIV infected persons on suppressive antiretroviral therapy. GMS Infect Dis 3Doc03. (2015). doi: 10.3205/id000018
115. Ancona G, Merlini E, Tincati C, Barassi A, Calcagno A, Augello M, et al. Long-term suppressive cART is not sufficient to restore intestinal permeability and gut microbiota compositional changes. Front Immunol. (2021) 12:639291. doi: 10.3389/fimmu.2021.639291
116. Serrano-Villar S, Rojo D, Martínez-Martínez M, Deusch S, Vázquez-Castellanos JF, Bargiela R, et al. Gut bacteria metabolism impacts immune recovery in HIV-infected individuals. EBioMedicine. (2016) 8:203–16. doi: 10.1016/j.ebiom.2016.04.033
117. Vyboh K, Jenabian MA, Mehraj V, Routy JP. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J Immunol Res. (2015) 2015:1–9. doi: 10.1155/2015/614127
118. MetaCardis Consortium, Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. (2020) 581:310–5. doi: 10.1038/s41586-020-2269-x
119. Chen P, Li K. Statin therapy and gut microbiota. In: Liu D, editor. Statins - From Lipid-Lowering Benefits to Pleiotropic Effects. IntechOpen (2023). doi: 10.5772/intechopen.1001098
120. Wilmanski T, Kornilov SA, Diener C, Conomos MP, Lovejoy JC, Sebastiani P, et al. Heterogeneity in statin responses explained by variation in the human gut microbiome. Med. (2022) 3:388–405.e6. doi: 10.1016/j.medj.2022.04.007
121. Karlson BW, Wiklund O, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Variability of low-density lipoprotein cholesterol response with different doses of atorvastatin, rosuvastatin, and simvastatin: results from VOYAGER. Eur Heart J - Cardiovasc Pharmacother. (2016) 2:212–7. doi: 10.1093/ehjcvp/pvw006
122. Tuteja S, Ferguson JF. Gut microbiome and response to cardiovascular drugs. Circ Genomic Precis Med. (2019) 12:e002314. doi: 10.1161/CIRCGEN.119.002314
123. Khan TJ, Ahmed YM, Zamzami MA, Siddiqui AM, Khan I, Baothman OAS, et al. Atorvastatin treatment modulates the gut microbiota of the hypercholesterolemic patients. Omics J Integr Biol. (2018) 22:154–63. doi: 10.1089/omi.2017.0130
124. El-Far M, Tremblay CL. Gut microbial diversity in HIV infection post combined antiretroviral therapy: a key target for prevention of cardiovascular disease. Curr Opin HIV AIDS. (2018) 13:38–44. doi: 10.1097/COH.0000000000000426
125. Demierre MF, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. (2005) 5:930–42. doi: 10.1038/nrc1751
126. Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. (1999) 286:1946–9. doi: 10.1126/science.286.5446.1946
127. Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. (2000) 6:1004–10. doi: 10.1038/79510
128. Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. (2013) 187:743–50. doi: 10.1164/rccm.201209-1718OC
129. Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. (2009) 169:1658–67. doi: 10.1001/archinternmed.2009.286
130. Dulak J, Józkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. (2005) 5:579–94. doi: 10.2174/156800905774932824
131. Vosper J, Masuccio A, Kullmann M, Ploner C, Geley S, Hengst L. Statin-induced depletion of geranylgeranyl pyrophosphate inhibits cell proliferation by a novel pathway of Skp2 degradation. Oncotarget. (2015) 6:2889–902. doi: 10.18632/oncotarget.v6i5
Keywords: statins, HIV, cardiovascular diseases, inflammation, immune activation, REPRIEVE randomized clinical trial
Citation: Mehraj V, Chen J and Routy J-P (2024) Effects of statins beyond lipid-lowering agents in ART-treated HIV infection. Front. Immunol. 15:1339338. doi: 10.3389/fimmu.2024.1339338
Received: 16 November 2023; Accepted: 22 March 2024;
Published: 09 April 2024.
Edited by:
Guido Poli, Vita-Salute San Raffaele University, ItalyReviewed by:
Camilla Muccini, San Raffaele Hospital (IRCCS), ItalyGiulia Carla Marchetti, University of Milan, Italy
Copyright © 2024 Mehraj, Chen and Routy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Pierre Routy, amVhbi1waWVycmUucm91dHlAbWNnaWxsLmNh
 Vikram Mehraj
Vikram Mehraj Jun Chen
Jun Chen Jean-Pierre Routy
Jean-Pierre Routy