- 1Jiangxi Medical College, Nanchang University, Nanchang, China
- 2Department of breast surgery, Jiangxi Cancer Hospital, Nanchang, China
Background: Previous studies have reported associations of Crohn’s disease (CD) and ulcerative colitis (UC) with the risks of extraintestinal cancers, but the causality remains unclear.
Methods: Using genetic variations robustly associated with CD and UC extracted from genome-wide association studies (GWAS) as instrumental variables. Nine types of extraintestinal cancers of European and Asian populations were selected as outcomes. We used the inverse variance weighted method as the primary approach for two-sample Mendelian randomization analysis. Sensitivity analyses were carried out to evaluate the reliability of our findings.
Results: In the European population, we found that CD showed a potential causal relationship with pancreatic cancer (OR: 1.1042; 95% CI: 1.0087-1.2088; P=0.0318). Meanwhile, both CD (outliers excluded: OR: 1.0208; 95% CI: 1.0079-1.0339; P=0.0015) and UC (outliers excluded: OR: 1.0220; 95% CI: 1.0051-1.0393; P=0.0108) were associated with a slight increase in breast cancer risk. Additionally, UC exhibited a potential causal effect on cervical cancer (outliers excluded: OR: 1.1091; 95% CI: 1.0286-1.1960; P=0.0071). In the East Asian population, CD had significant causal effects on pancreatic cancer (OR: 1.1876; 95% CI: 1.0741-1.3132; P=0.0008) and breast cancer (outliers excluded: OR: 0.9452; 95% CI: 0.9096-0.9822; P=0.0040). For UC, it exhibited significant causal associations with gastric cancer (OR: 1.1240; 95% CI: 1.0624-1.1891; P=4.7359×10–5), bile duct cancer (OR: 1.3107; 95% CI: 1.0983-1.5641; P=0.0027), hepatocellular carcinoma (OR: 1.2365; 95% CI: 1.1235-1.3608; P=1.4007×10–5) and cervical cancer (OR: 1.3941; 95% CI: 1.1708-1.6599; P=0.0002), as well as a potential causal effect on lung cancer (outliers excluded: OR: 1.1313; 95% CI: 1.0280-1.2449; P=0.0116).
Conclusions: Our study provided evidence that genetically predicted CD may be a risk factor for pancreatic and breast cancers in the European population, and for pancreatic cancer in the East Asian population. Regarding UC, it may be a risk factor for cervical and breast cancers in Europeans, and for gastric, bile duct, hepatocellular, lung, and cervical cancers in East Asians. Therefore, patients with CD and UC need to emphasize screening and prevention of site-specific extraintestinal cancers.
1 Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are the main subtypes of inflammatory bowel disease (IBD), which are chronic inflammatory disorders that primarily affect the gastrointestinal tract (1–3). It is characterized by periods of remission and flare-ups, leading to impaired quality of life for patients and substantial costs for health care (4, 5).
The risk of intestinal cancer in CD and UC has been analyzed deeply (6, 7), but the potential association of CD and UC with extraintestinal cancer has received relatively little attention. However, extraintestinal manifestations are observed in up to 35% of IBD patients (8, 9), highlighting the importance of studying the risk of extraintestinal cancer in this population.
Several observational studies have shown that IBD patients were positively associated with the risk of extraintestinal cancer. A meta-analysis of population-based cohort studies, involving 17,052 IBD patients, indicated that CD patients exhibited increased risks of cancer in the upper gastrointestinal tract, lung, and skin, while UC patients had a higher risk of liver-biliary cancer (10). Another meta-analysis suggested a significant association between IBD, especially UC, and an increased risk of cervical cancer (11). Moreover, a 20-year prospective follow-up study in Norway has reported an increased incidence of breast cancer in patients with CD and UC (12). Results from some cohort and case-control studies have reported an elevated risk of pancreatic cancer and skin cancer in patients with IBD (13–15). However, there are currently no specific guidelines for extraintestinal cancer screening or surveillance in patients with CD and UC. Additionally, it is not easy to assess the real risk and causality of extraintestinal cancer in CD and UC due to the residual confounding commonly encountered in traditional observational studies.
Mendelian randomization (MR) is a new method of etiological investigation that uses genetic variations closely linked to a particular exposure as instrumental variables (IVs) (16). This approach can avoid the limitations of traditional epidemiological studies and permit us to draw causal inferences about the impact of specific exposures on outcomes (17). Because alleles follow the principle of random allocation during gametogenesis, the offspring have random genetic variations; thus, the results are not affected by confounding factors or reverse causation (18, 19).
Our study aimed to appraise causal associations of CD and UC with extraintestinal cancers through MR analysis. Furthermore, we tried to analyze the differences between European and East Asian ethnic groups. Significantly, our findings have the potential to inform more effective and targeted cancer surveillance programs for patients with CD and UC, facilitating early cancer detection and alleviating the burden on healthcare systems.
2 Materials and methods
2.1 Study design
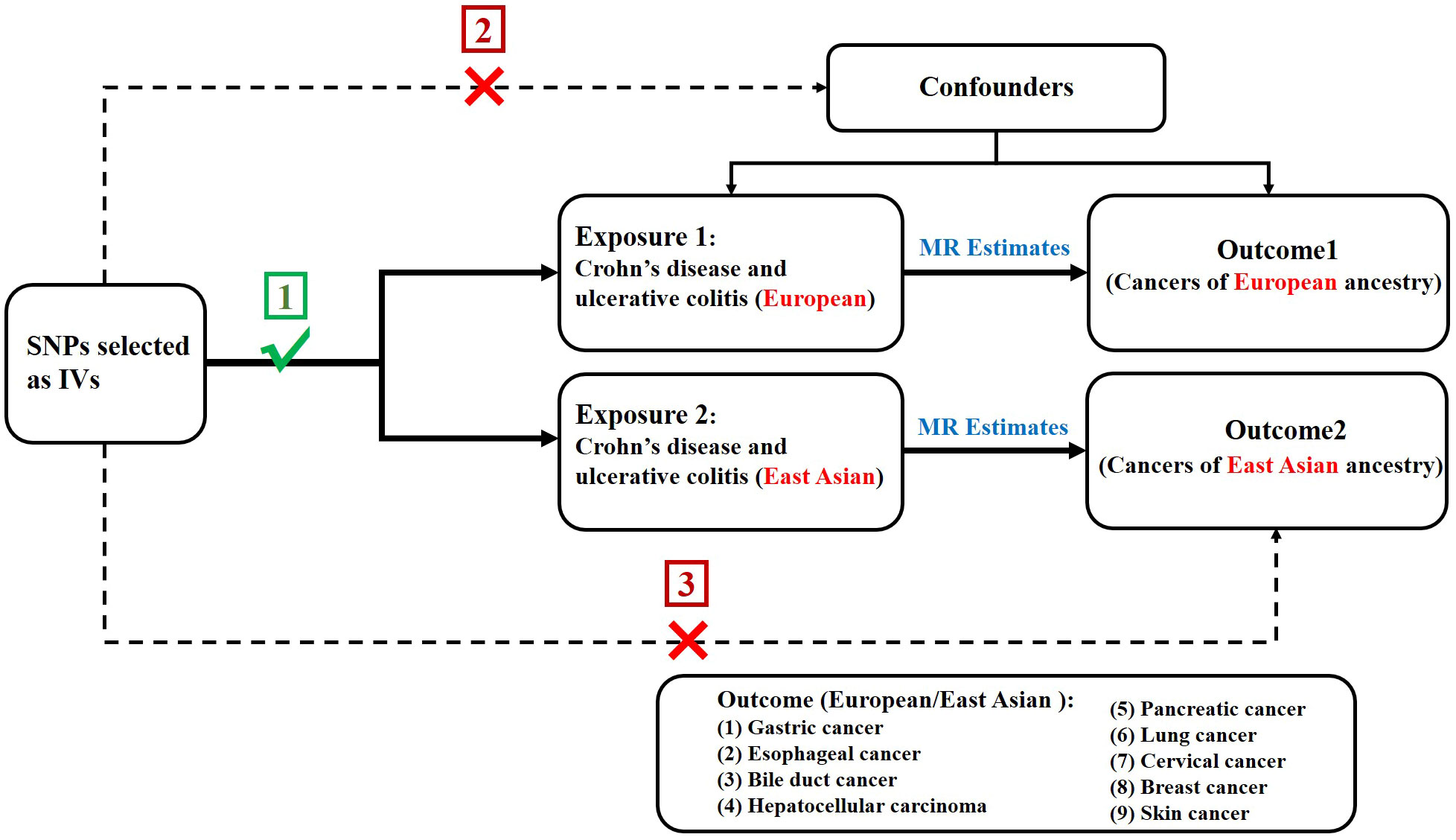
In order to assess the causal association of CD and UC with extraintestinal cancer, we conducted a two-sample MR study. The single nucleotide polymorphisms (SNPs) selected as IVs were required to meet three following key premises (20) (1): SNPs must be intensely linked to exposure; (2) SNPs must not be linked to confounding factors; and (3) SNPs should not be directly linked to outcome (Figure 1).
2.2 Data source
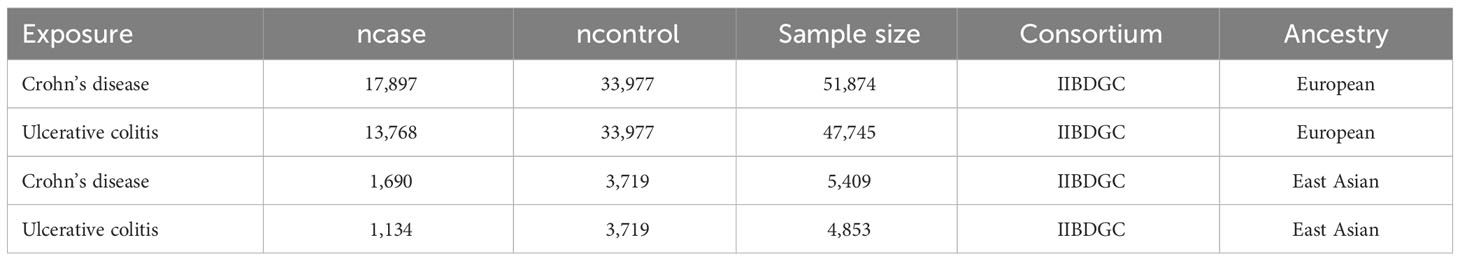
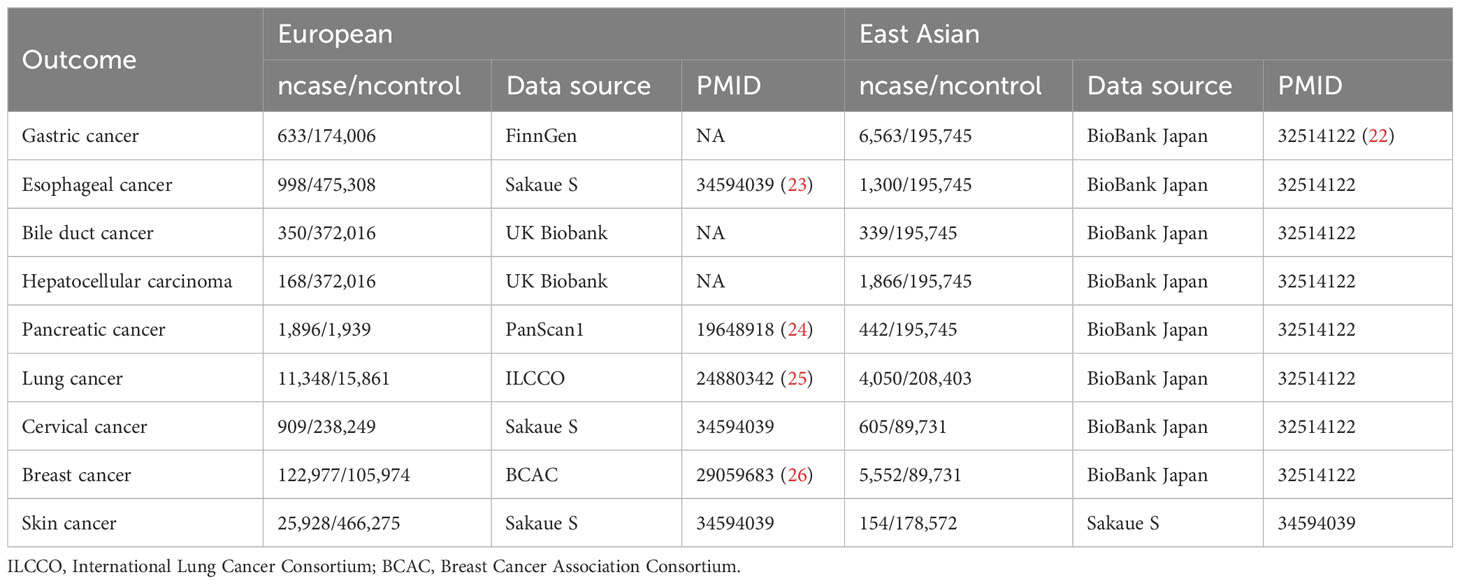
The summary genome-wide association study (GWAS) data for CD (Europeans: 17,897 cases/33,977 controls; East Asians: 1,690 cases/3,719 controls) and UC (Europeans: 13,768 cases/33977 controls; East Asians: 1,134 cases/3,719 controls) were obtained from the International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) (21). And the summary GWAS data for extraintestinal cancers included in this study were extracted directly or indirectly from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/). See Tables 1, 2 for more information about exposures and outcomes.
2.3 SNP selection
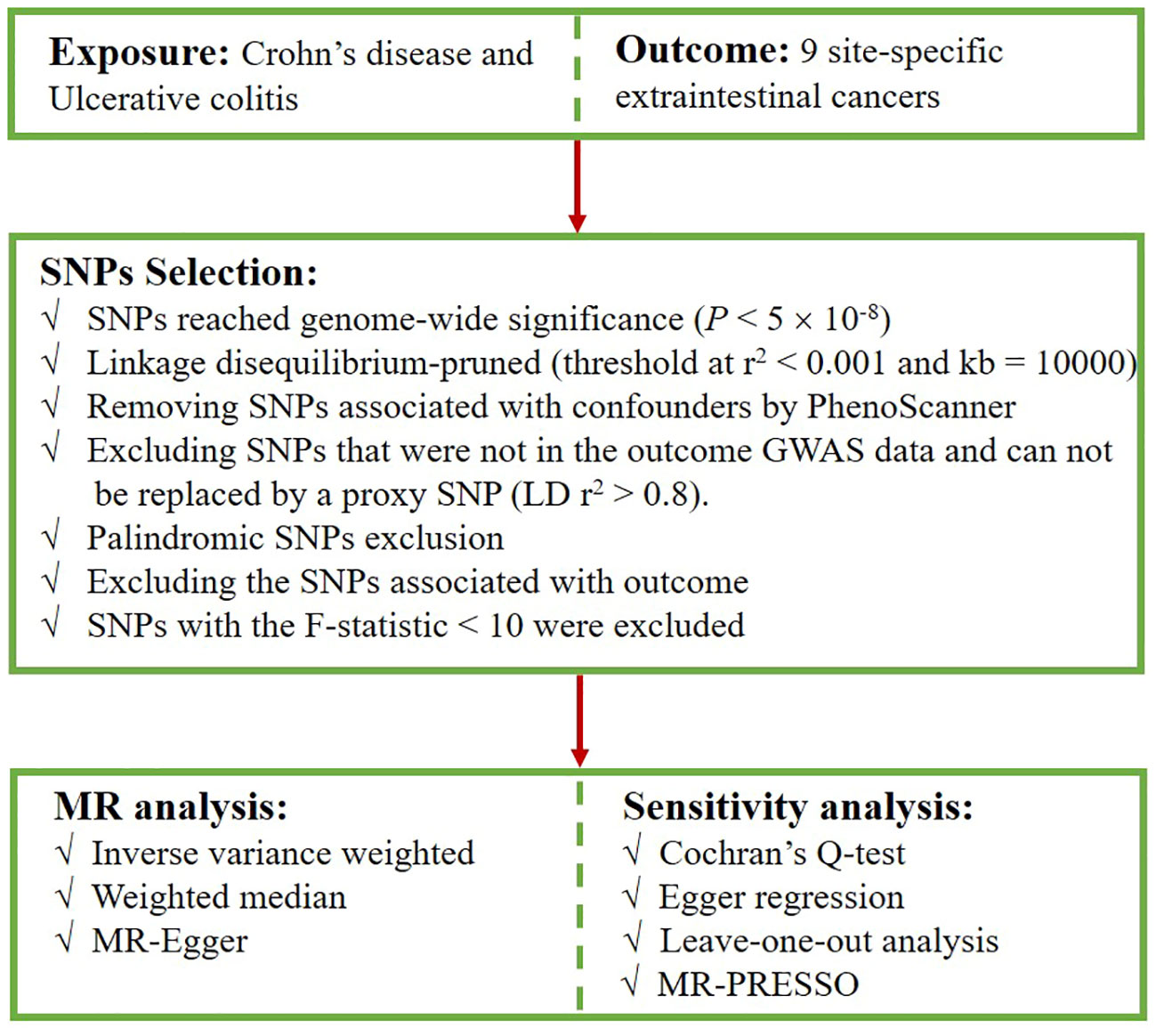
First, we extracted SNPs intensely associated with CD and UC from the corresponding datasets, with a screening condition of P < 5×10-8. Second, to ensure the independence of exposure instruments, SNPs with a low likelihood of linkage disequilibrium (LD) (r2 < 0.001, kb = 10,000) were retained (27). We checked the possible phenotypes of each SNP related to CD and UC at PhenoScanner (28), and SNPs directly linked to some recognized confounders associated with carcinogenesis were excluded, such as alcohol intake (29, 30), smoking (31), body-mass index (BMI) (32–34). The excluded SNPs and their relevant traits are presented in Supplementary Table S1. Subsequently, when the selected SNPs were not extractable from the outcome dataset, we opted for SNPs with strong correlations (r2 > 0.8) as proxies (35). Palindromic SNPs that may cause bias were removed. Furthermore, SNPs that were strongly correlated with the outcome were excluded because they deviated from the core assumption of the IVs. Finally, F-statistics were calculated (F = beta2/se2) to evaluate the potential for weak instrument bias, and any SNP with an F-statistic < 10 was excluded (36, 37). Figure 2 presents the selection flowchart.
2.4 Statistical analysis
The inverse variance weighted (IVW) method is considered to be the most powerful method for detecting causation in MR analysis (38); therefore, the results were mainly based on the IVW method, supplemented by the weighted median and MR Egger. We used odds ratios (ORs) to express the effects of CD and UC on extraintestinal cancer risk. The presence of heterogeneity was determined using Cochran’s Q test, with P < 0.05 indicating heterogeneity (39). To detect pleiotropy, we used the MR-Egger regression test, whereby a non-zero intercept was indicative of horizontal pleiotropy (P < 0.05) (40). Moreover, we identified outliers by MR-PRESSO method and repeated MR analyses after excluding these outliers (41). Additionally, leave-one-out analysis was performed to assess the impact of a single SNP’s removal on the results (42).
To determine more rigorous causalities, we used a Bonferroni-corrected significance threshold calculated as 0.0056 (0.05/9, according to the 9 types of cancer). The P value between 0.0056 and 0.05 was considered to suggest a potential relationship between exposure and outcome.
This study followed the STROBE-MR guidelines (43). All analyses were conducted using the “TwoSampleMR” and “MRPRESSO” packages in R software (version 4.3.1).
3 Results
3.1 SNP Selection
In this study, we detected 122 SNPs for CD and 88 SNPs for UC in the European population, while in the East Asian population, we observed detections of 14 SNPs for CD and 10 SNPs for UC (Supplementary Tables S2-S5). The F-statistics of all SNPs were greater than 10, avoiding weak instrumental variable bias.
3.2 Analysis of the European population
Initially, we used the GWAS data of the European population to evaluate the effects of CD on extraintestinal cancer risk. Based on IVW analysis, we observed a potential association between CD per unit increase in logOR and pancreatic cancer (OR: 1.1042; 95% CI: 1.0087-1.2088; P=0.0318) as well as skin cancer (outliers excluded: OR: 1.0267; 95% CI: 1.0062-1.0476; P=0.0103). However, the MR-Egger method in skin cancer analysis showed OR<1 (Supplementary Table S6), which was inconsistent with the direction of IVW (OR>1); thus, we could not determine the causality of CD and skin cancer. In addition, CD was found to be significantly linked to breast cancer (outliers excluded: OR: 1.0208; 95% CI: 1.0079-1.0339; P=0.0015). Regarding UC, potentially positive correlations were found between UC and the risks of cervical cancer (outliers excluded: OR: 1.1091; 95% CI: 1.0286-1.1960; P=0.0071) and breast cancer (outliers excluded: OR: 1.0220; 95% CI: 1.0051-1.0393; P=0.0108) (Table 3, Figure 3). Scatter plots of the positive results illustrated causal estimates derived from each SNP (Figure 4). Sensitivity analyses showed that the effects of CD on pancreatic cancer and UC on cervical cancer were reliable, exhibiting no heterogeneity or horizontal pleiotropy. Although the impacts of CD and UC for breast cancer remained heterogeneity after removing outliers, there was no significant pleiotropy. Moreover, leave-one-out analysis demonstrated the reliability of the findings (Supplementary Figure S1). In addition, we discovered that CD and UC were not associated with the occurrence of the other extraintestinal cancers included in this study in the European population. More results of MR analyses are presented in Supplementary Tables S6, S7.
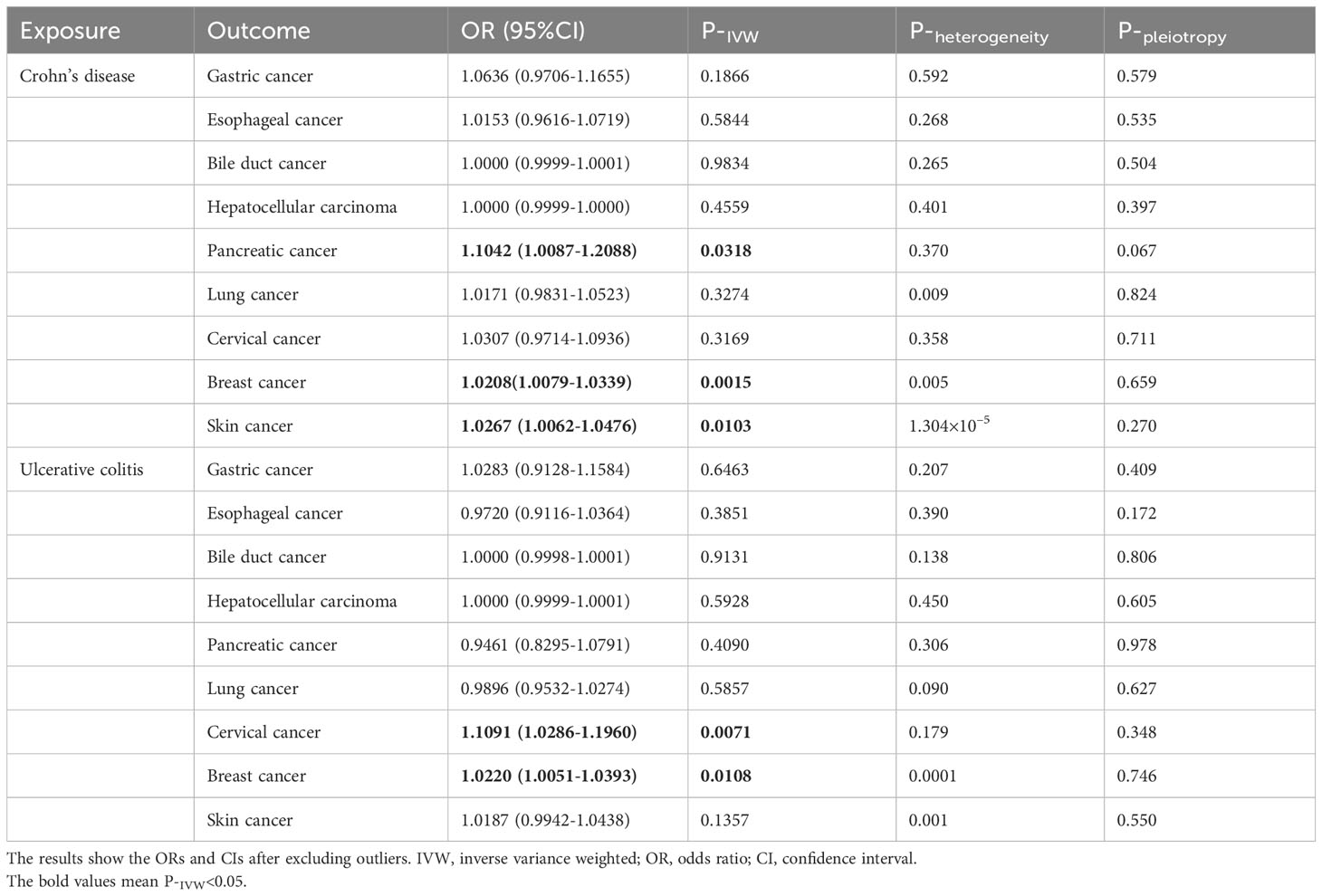
Table 3 Causal effects of Crohn’s disease and ulcerative colitis on the risk of extraintestinal cancers in the European population.
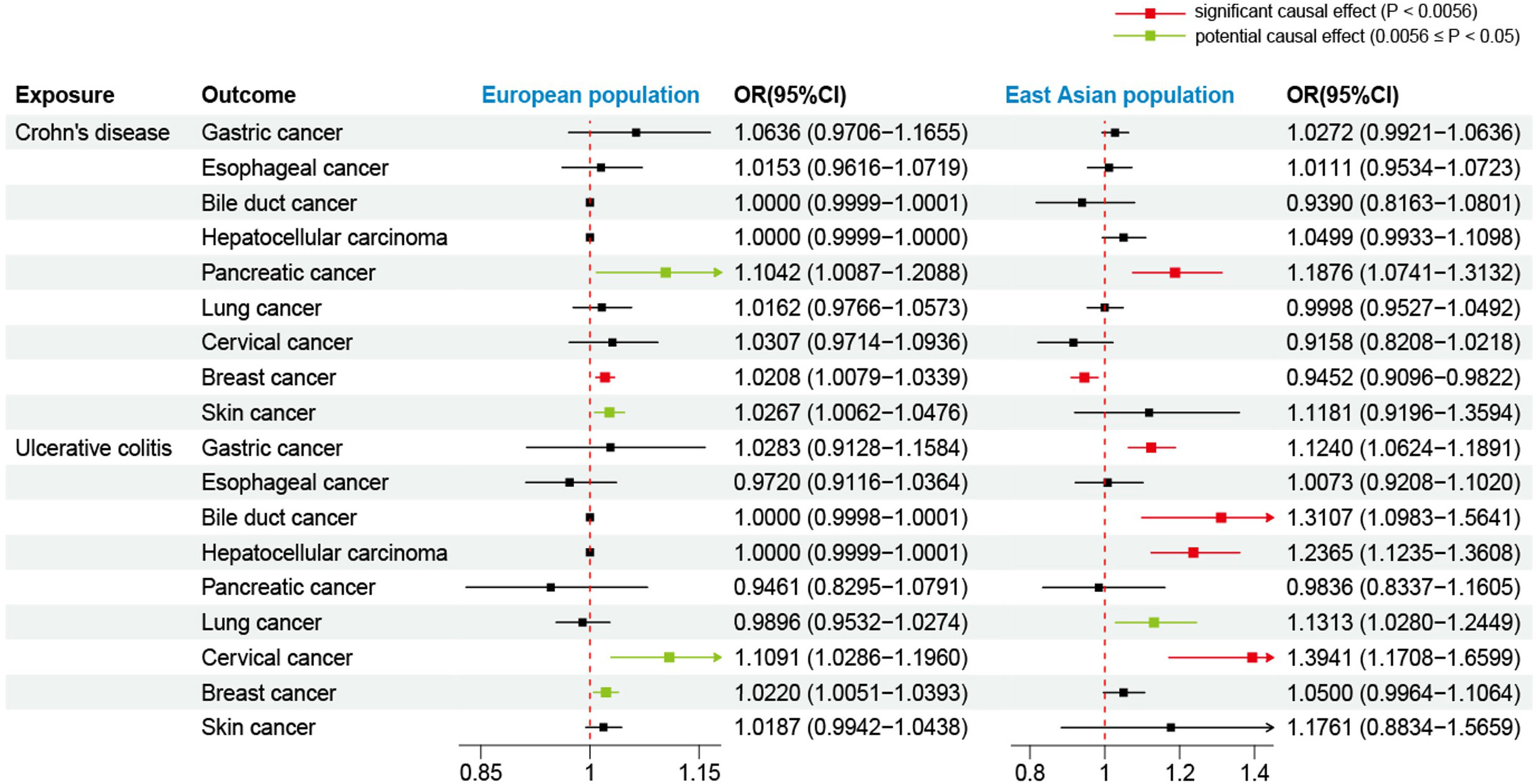
Figure 3 Forest plot for effects of Crohn’s disease and ulcerative colitis on the risk of extraintestinal cancers based on the IVW. The results show the ORs and CIs after excluding outliers. OR, odds ratio; CI, confidence interval.

Figure 4 Scatter plots of the positive results for effects of Crohn’s disease and ulcerative colitis on the risk of extraintestinal cancers in the European population. (A) Crohn’s disease on pancreatic cancer (B) Crohn’s disease on breast cancer (C) ulcerative colitis on cervical cancer (D) ulcerative colitis on breast cancer.
3.3 Analysis of the East Asian population
Using the GWAS data from the East Asian population, genetic prediction indicated a significant causal association between CD and an increased risk of pancreatic cancer (P=0.0008), with an OR of 1.1876 (95% CI: 1.0741-1.3132). Surprisingly, CD was found to be significantly negatively related to breast cancer risk (outliers excluded: OR: 0.9452; 95% CI: 0.9096-0.9822; P=0.0040). Regarding UC, we identified a significant causal effect between UC per unit increase in logOR and four types of extraintestinal cancer that are gastric cancer (OR: 1.1240; 95% CI: 1.0624-1.1891; P=4.7359×10–5), bile duct cancer (OR: 1.3107; 95% CI: 1.0983-1.5641; P=0.0027), hepatocellular carcinoma (OR: 1.2365; 95% CI: 1.1235-1.3608; P=1.4007×10–5), and cervical cancer (OR: 1.3941; 95% CI: 1.1708-1.6599; P=0.0002). At the genetic level, these findings indicate that UC increases the risk of developing the four types of cancer (Table 4, Figure 3). In addition, UC was found to be potentially positively associated with a higher risk of lung cancer (outliers excluded: OR: 1.1313; 95% CI: 1.0280-1.2449; P=0.0116). Scatter plots of the positive results illustrated causal estimates derived from each SNP (Figure 5). Sensitivity analyses showed that the above associations were robust in the East Asian population, displaying no significant heterogeneity or horizontal pleiotropy. Furthermore, leave-one-out analysis further illustrated the robustness of the results (Supplementary Figure S2). More results of MR analyses are presented in Supplementary Tables S8, S9.
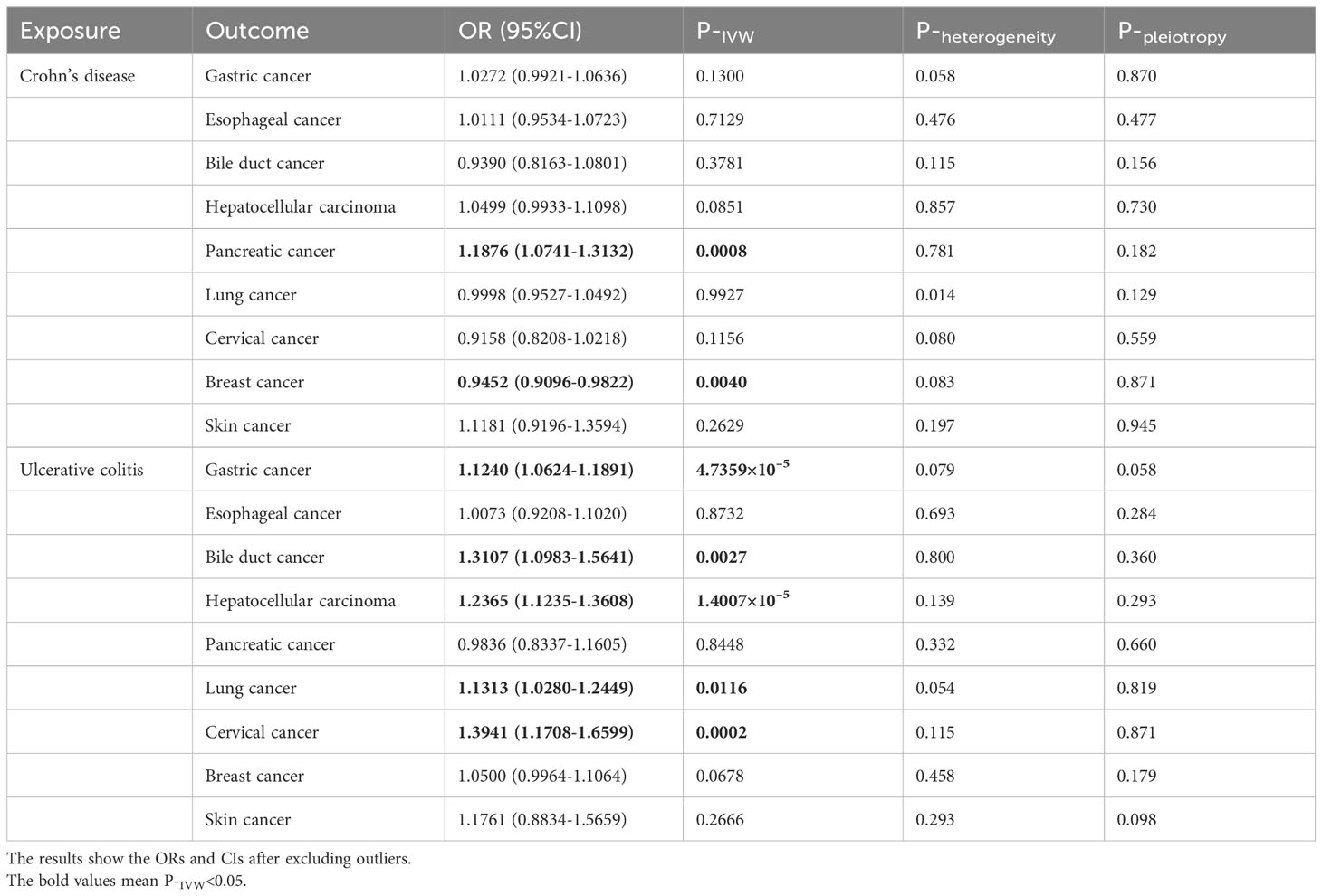
Table 4 Causal effects of Crohn’s disease and ulcerative colitis on the risk of extraintestinal cancers in the East Asian population.
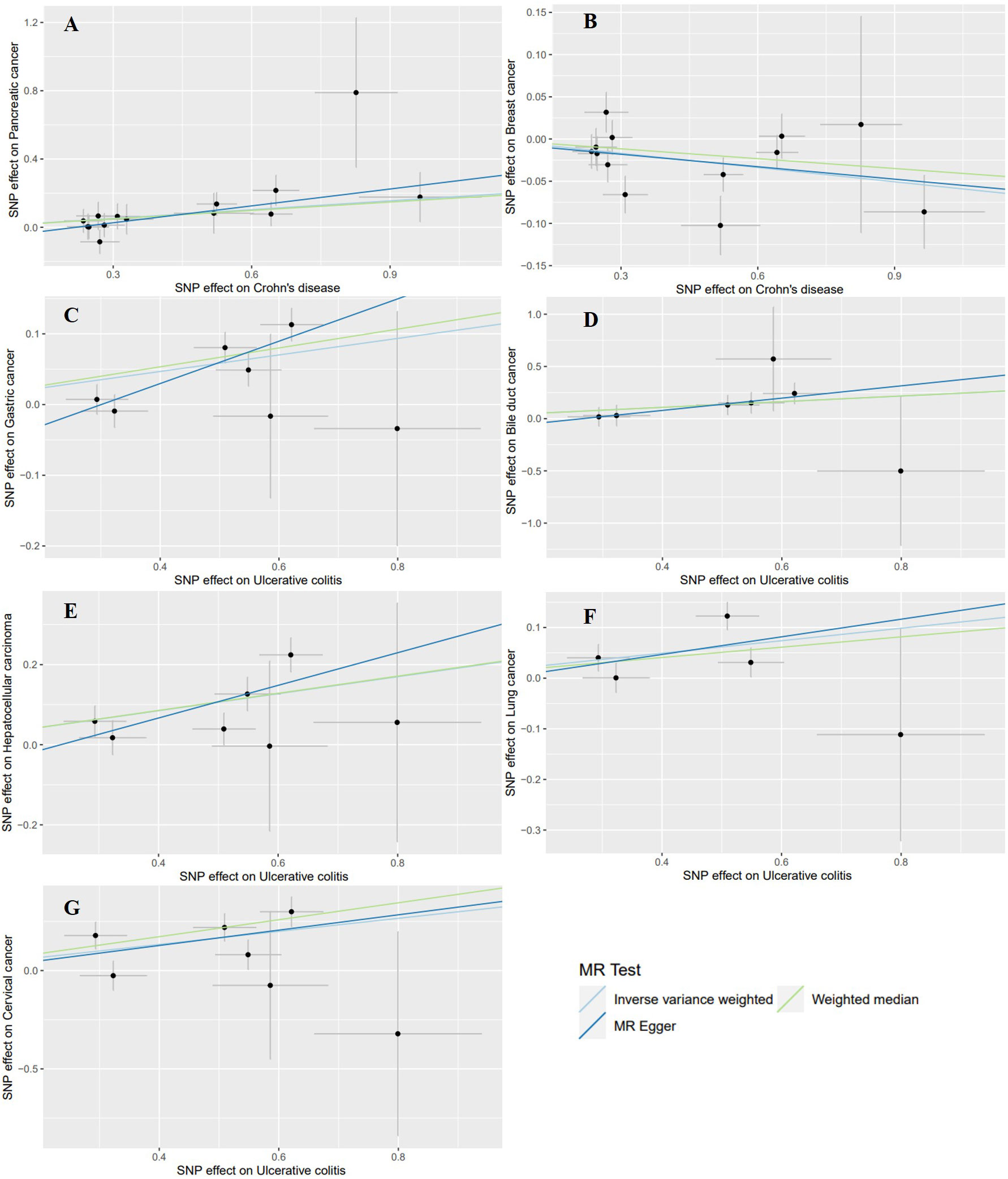
Figure 5 Scatter plots of the positive results for effects of Crohn’s disease and ulcerative colitis on the risk of extraintestinal cancers in the East Asian population. (A) Crohn’s disease on pancreatic cancer (B) Crohn’s disease on breast cancer (C) ulcerative colitis on gastric cancer (D) ulcerative colitis on bile duct cancer (E) ulcerative colitis on hepatocellular carcinoma (F) ulcerative colitis on lung cancer (G) ulcerative colitis on cervical cancer.
4 Discussion
There is evidence from many studies that patients with CD and UC are at an increased risk of developing intestinal cancer (6, 7, 44, 45). Interestingly, a growing body of research evidence indicates that these patients are at significantly elevated risk of extraintestinal cancers (46). Our MR study may provide some new evidence on the extraintestinal cancer risk in patients with CD and UC.
During analyses of causal effects between CD and extraintestinal cancers, we discovered that the incidence of pancreatic cancer in patients with CD is 1.10 times higher in the European population and 1.19 times higher in the East Asian population compared to individuals without CD. Similarly, Yu’s MR study showed a causal effect between CD and pancreatic cancer risk (OR: 1.111; 95% CI: 1.015-1.213) in Europeans (47). Two studies, one involving Scandinavians and the other Koreans, also consistently reported that CD increases the risk of pancreatic cancer (48, 49). A previous study found that IL-18 played a key role in the pathogenesis of both CD and pancreatic cancer through a common pathogenic pathway, affecting the immune response and tumor microenvironment by activating immune cells (50). As for breast cancer, our MR analysis showed that the risk was slightly increased in CD patients in Europe (OR: 1.0208; 95% CI: 1.0079-1.0339; P=0.0015). Pellino et al. reported that CD was an independent risk factor for developing breast cancer (OR: 2.76; 95% CI: 1.2-6.2; P=0.017) (51). Existing studies indicated that CD and breast cancer may share common molecular mechanisms (52–54). However, we found that the risk of breast cancer was reduced by 5.48% in CD patients (OR: 0.9452; 95% CI: 0.9096-0.9822) in East Asia. Although previous cohort studies have shown a decreased risk of breast cancer in patients with CD (55, 56), future research is needed to explore the underlying mechanisms that influence cancer risk variation across diverse populations. The genetic profiles of CD patients may differ between European and East Asian populations, leading to varied susceptibility to developing breast cancer.
In our MR study, the effect size of CD on the risk of breast cancer in Europeans was relatively small (OR=1.0208), suggesting that the elevated risk is just modest. Furthermore, in East Asians, the risk of breast cancer may even be reduced. Therefore, it is not recommended for CD patients to conduct earlier or more frequent breast cancer screening compared to the general screening programs currently in place.
In addition, the result from the IVW method indicated a potential association between CD and skin cancer in the European population (OR: 1.0267; 95% CI: 1.0062-1.0476). The pathogenesis of the enhanced risk of skin cancer in CD is poorly understood and may be associated with underlying immune dysfunction in CD patients, leading to altered tumor surveillance (14, 57). However, the MR-Egger method showed OR<1, inconsistent with the direction of IVW. Consequently, the causal association between CD and skin cancer cannot be definitively established in this study. More studies are needed to further determine the causality.
When analyzing causal associations between UC and extraintestinal cancers, we found that the risk of cervical cancer in UC patients is 1.11‐fold higher in the European population and 1.39‐fold higher in the East Asian population compared to individuals without UC. Similar results were reported in several previous studies, suggesting that UC increased the risk of cervical cancer and recommending that women with UC receive regular cervical cancer screening (11, 49, 58, 59).
In Europeans, similar to CD, UC only slightly increased the risk of breast cancer (OR: 1.0220; 95% CI: 1.0051-1.0393). In East Asians, we did not find any correlation between UC and breast cancer. Therefore, we also do not recommend UC patients to undergo breast cancer screening earlier or more frequently than the general screening programs. Interestingly, UC was significantly positively associated with gastric cancer in the East Asian population. Nissen et al. conducted two case-control studies and found that UC was more likely than CD to be a risk factor for gastric cancer (60). Since studies on the relationship between UC and the risk of gastric cancer are limited in the Asian population, more relevant research should be conducted in the future (61). For bile duct and hepatocellular cancers, a large number of studies have suggested that IBD is positively related to the risk of hepatobiliary cancers (62–64). However, our MR study only identified the effect of UC in increasing the risk of bile duct and hepatocellular cancers in the East Asian population. Notably, a previous study involving 17,052 patients with IBD reported that the risk of hepatobiliary cancers was elevated only in patients with UC (10). Another study also reported a significant positive association between UC and bile duct cancer (65). This observation may be associated with the fact that up to 5% of UC patients develop primary sclerosing cholangitis (PSC), a condition known to carry a lifetime risk of developing bile duct cancer ranging from 10% to 15% (66, 67). As for the potential mechanism of the association between IBD and hepatocellular carcinoma, it may be related to the sharing of immune-related biomarkers (68).
Interestingly, our MR study found a potential positive association between UC and lung cancer risk in the East Asian population. A recent study has demonstrated that the lungs and colon can jointly regulate inflammation and immunity through the lung-gut axis, particularly via the transport of gut microbiota and metabolites (69). This provides a possible mechanism for the correlation between UC and lung cancer. Regrettably, two meta-analyses reported an increased risk of lung cancer in CD, but not in UC (10, 70). Although traditional observational studies can offer some initial insight into the association between IBD and cancer, their findings may be influenced by confounders (71, 72). Moreover, our findings diverged from previous observational studies, potentially attributed to the limited sample size of the GWAS dataset.
Our MR study revealed some differences in the risk of developing extraintestinal cancer in CD and UC between European and East Asian populations. In the European population, we found that both CD and UC slightly increased the risk of breast cancer. However, these findings were not found in East Asians. In addition, we found varying degrees of associations between UC and gastric cancer, bile duct cancer, hepatocellular carcinoma, and lung cancer in the East Asian population, but not in the European population. The reasons for these differences in causality remain unclear. It has been reported that there are significant differences in the phenotypes of IBD between Western and Eastern populations (73). Different ethnic groups may have unique genetic markers and susceptibility genes that influence complex genetic diseases (74). These genetic variants can lead to differences in cancer risk among diverse populations.
The strengths of our study lie in the direct assessment of the causal effects of CD and UC on the risk of extraintestinal cancer using the MR method. This approach allows us to avoid the interference of confounding factors in traditional observational studies. Furthermore, our findings provide new evidence for site-specific cancer screening and intervention in patients with CD and UC.
Nevertheless, this study has several limitations. First, the GWAS data for this study are derived from European and East Asian populations, which limits the application of our findings to other populations. Hence, future studies are required to verify the applicability of our results to different populations. Second, we cannot stratify the analysis by sex due to the lack of sex-specific GWAS data. Finally, the MR study can only analyze the causality and cannot explain the specific biological pathways. Further research is necessary to investigate the mechanisms behind the associations of CD and UC with the risk of extraintestinal cancer.
5 Conclusion
In summary, based on MR analyses and large-scale GWAS data, our study indicated that genetically predicted CD may be a risk factor for pancreatic and breast cancers in the European population, and for pancreatic cancer in the East Asian population. Regarding UC, it may be a risk factor for cervical and breast cancers in Europeans, and for gastric, bile duct, hepatocellular, lung, and cervical cancers in East Asians. Therefore, patients with CD and UC need to emphasize screening and prevention of site-specific extraintestinal cancers.
Data availability statement
In this study, all GWAS data were extracted from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/).
Author contributions
CY: Conceptualization, Investigation, Methodology, Writing – original draft. JX: Data curation, Writing – original draft. SX: Data curation, Writing – review & editing. LT: Writing – review & editing. QH: Writing – review & editing. XZ: Writing – review & editing. YH: Writing – review & editing. TY: Formal analysis, Writing – original draft. ZS: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by the National Natural Science Foundation of China (82260565), Wu Jieping Medical Foundation (320.6750.2020-20-17), and Open Fund for Scientific Research of Jiangxi Cancer Hospital (2021K04).
Acknowledgments
Thanks to the MRC Integrative Epidemiology Unit (IEU) at the University of Bristol for developing the IEU open GWAS project. Thank them for extracting relevant GWAS data from FinnGen biobank, UK Biobank, BioBank Japan, IIBDGC, PanScan, ILCCO, BCAC and published articles.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1339207/full#supplementary-material
References
1. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut (2019) 68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484
2. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet (2017) 389(10080):1741–55. doi: 10.1016/S0140-6736(16)31711-1
3. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet (2017) 389(10080):1756–70. doi: 10.1016/S0140-6736(16)32126-2
4. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol (2015) 12(12):720–7. doi: 10.1038/nrgastro.2015.150
5. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (2017) 390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0
6. Olen O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in Crohn's disease: a Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol (2020) 5(5):475–84. doi: 10.1016/S2468-1253(20)30005-4
7. Olen O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet (2020) 395(10218):123–31. doi: 10.1016/S0140-6736(19)32545-0
8. Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am (1999) 28(2):445–58. doi: 10.1016/S0889-8553(05)70064-9
9. Kilic Y, Kamal S, Jaffar F, Sriranganathan D, Quraishi MN, Segal JP. Prevalence of extraintestinal manifestations in inflammatory bowel disease: A systematic review and meta-analysis. Inflammation Bowel Dis (2023) 12:izad061. doi: 10.1093/ibd/izad061
10. Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol (2010) 105(7):1480–7. doi: 10.1038/ajg.2009.760
11. Kim J, Jung JH, Jo H, Kim MH, Kang DR, Kim HM. Risk of uterine cervical cancer in inflammatory bowel disease: a systematic review and meta-analysis. Scand J Gastroenterol (2023) 58(12):1412–21. doi: 10.1080/00365521.2023.2238101
12. Hovde O, Hoivik ML, Henriksen M, Solberg IC, Smastuen MC, Moum BA. Malignancies in patients with inflammatory bowel disease: results from 20 years of follow-up in the IBSEN study. J Crohns Colitis (2017) 11(5):571–7. doi: 10.1093/ecco-jcc/jjw193
13. Yuan F, Pfeiffer RM, Julian-Serrano S, Arjani S, Barrett MJ, Koshiol J, et al. Autoimmune conditions and pancreatic cancer risk in older American adults. Int J Cancer (2023) 152(2):172–82. doi: 10.1002/ijc.34235
14. Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology (2012) 143(2):390–9 e1. doi: 10.1053/j.gastro.2012.05.004
15. Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol (2010) 8(3):268–74. doi: 10.1016/j.cgh.2009.11.024
16. Richmond RC, Davey Smith G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb Perspect Med. (2022) 12(1):a040501. doi: 10.1101/cshperspect.a040501
17. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
18. Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol (2016) 27(11):3253–65. doi: 10.1681/ASN.2016010098
19. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (2018) 362:k601. doi: 10.1136/bmj.k601
20. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol (2013) 37(7):658–65. doi: 10.1002/gepi.21758
21. Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet (2015) 47(9):979–86. doi: 10.1038/ng.3359
22. Ishigaki K, Akiyama M, Kanai M, Takahashi A, Kawakami E, Sugishita H, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet (2020) 52(7):669–79. doi: 10.1038/s41588-020-0640-3
23. Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet (2021) 53(10):1415–24. doi: 10.1038/s41588-021-00931-x
24. Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet (2009) 41(9):986–90. doi: 10.1038/ng.429
25. Wang Y, McKay JD, Rafnar T, Wang Z, Timofeeva MN, Broderick P, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet (2014) 46(7):736–41. doi: 10.1038/ng.3002
26. Michailidou K, Lindstrom S, Dennis J, Beesley J, Hui S, Kar S, et al. Association analysis identifies 65 new breast cancer risk loci. Nature (2017) 551(7678):92–4. doi: 10.1038/nature24284
27. Nounu A, Kar SP, Relton CL, Richmond RC. Sex steroid hormones and risk of breast cancer: a two-sample Mendelian randomization study. Breast Cancer Res (2022) 24(1):66. doi: 10.1186/s13058-022-01553-9
28. Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics (2016) 32(20):3207–9. doi: 10.1093/bioinformatics/btw373
29. Jun S, Park H, Kim UJ, Choi EJ, Lee HA, Park B, et al. Cancer risk based on alcohol consumption levels: a comprehensive systematic review and meta-analysis. Epidemiol Health (2023) 45:e2023092. doi: 10.4178/epih.e2023092
30. Rumgay H, Shield K, Charvat H, Ferrari P, Sornpaisarn B, Obot I, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol (2021) 22(8):1071–80. doi: 10.1016/S1470-2045(21)00279-5
31. Hecht SS, Hatsukami DK. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat Rev Cancer (2022) 22(3):143–55. doi: 10.1038/s41568-021-00423-4
32. Collaborators GBDCRF. The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (2022) 400(10352):563–91. doi: 10.1016/S0140-6736(22)01438-6
33. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet (2014) 384(9945):755–65. doi: 10.1016/S0140-6736(14)60892-8
34. The Lancet Diabetes E. The obesity-cancer link: of increasing concern. Lancet Diabetes Endocrinol (2020) 8(3):175. doi: 10.1016/S2213-8587(20)30031-0
35. Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics (2008) 24(24):2938–9. doi: 10.1093/bioinformatics/btn564
36. Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol (2013) 42(4):1134–44. doi: 10.1093/ije/dyt093
37. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol (2011) 40(3):740–52. doi: 10.1093/ije/dyq151
38. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol (2017) 46(6):1985–98. doi: 10.1093/ije/dyx102
39. Cohen JF, Chalumeau M, Cohen R, Korevaar DA, Khoshnood B, Bossuyt PM. Cochran's Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J Clin Epidemiol (2015) 68(3):299–306. doi: 10.1016/j.jclinepi.2014.09.005
40. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
41. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
42. Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet (2018) 27(R2):R195–208. doi: 10.1093/hmg/ddy163
43. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA (2021) 326(16):1614–21. doi: 10.1001/jama.2021.18236
44. Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2017) 2(4):269–76. doi: 10.1016/S2468-1253(17)30004-3
45. Samadder NJ, Valentine JF, Guthery S, Singh H, Bernstein CN, Leighton JA, et al. Family history associates with increased risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol (2019) 17(9):1807–13 e1. doi: 10.1016/j.cgh.2018.09.038
46. Piovani D, Hassan C, Repici A, Rimassa L, Carlo-Stella C, Nikolopoulos GK, et al. Risk of cancer in inflammatory bowel diseases: umbrella review and reanalysis of meta-analyses. Gastroenterology (2022) 163(3):671–84. doi: 10.1053/j.gastro.2022.05.038
47. Min Y, Liu Z, Li R, Jin J, Wei Z, Pei Y, et al. Association between inflammatory bowel disease and pancreatic cancer: results from the two-sample Mendelian randomization study. Front Oncol (2023) 13:1155123. doi: 10.3389/fonc.2023.1155123
48. Everhov AH, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Inflammatory bowel disease and pancreatic cancer: a Scandinavian register-based cohort study 1969-2017. Aliment Pharmacol Ther (2020) 52(1):143–54. doi: 10.1111/apt.15785
49. Jung YS, Han M, Park S, Kim WH, Cheon JH. Cancer risk in the early stages of inflammatory bowel disease in korean patients: A nationwide population-based study. J Crohns Colitis (2017) 11(8):954–62. doi: 10.1093/ecco-jcc/jjx040
50. Li Z, Yu X, Werner J, Bazhin AV, D'Haese JG. The role of interleukin-18 in pancreatitis and pancreatic cancer. Cytokine Growth Factor Rev (2019) 50:1–12. doi: 10.1016/j.cytogfr.2019.11.001
51. Pellino G, Sciaudone G, Patturelli M, Candilio G, De Fatico GS, Landino I, et al. Relatives of Crohn's disease patients and breast cancer: an overlooked condition. Int J Surg (2014) 12 Suppl 1:S156–8. doi: 10.1016/j.ijsu.2014.05.022
52. Zhou J, Yang R. Identification of key pathways and genes shared between Crohn's disease and breast cancer using bioinformatics analysis. Oncol Lett (2020) 20(4):119. doi: 10.3892/ol.2020.11981
53. Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut (2009) 58(8):1152–67. doi: 10.1136/gut.2008.163667
54. Ma K, Yang L, Shen R, Kong B, Chen W, Liang J, et al. Th17 cells regulate the production of CXCL1 in breast cancer. Int Immunopharmacol (2018) 56:320–9. doi: 10.1016/j.intimp.2018.01.026
55. van den Heuvel TR, Wintjens DS, Jeuring SF, Wassink MH, Romberg-Camps MJ, Oostenbrug LE, et al. Inflammatory bowel disease, cancer and medication: Cancer risk in the Dutch population-based IBDSL cohort. Int J Cancer (2016) 139(6):1270–80. doi: 10.1002/ijc.30183
56. Hemminki K, Liu X, Ji J, Forsti A, Sundquist J, Sundquist K. Effect of autoimmune diseases on risk and survival in female cancers. Gynecol Oncol (2012) 127(1):180–5. doi: 10.1016/j.ygyno.2012.07.100
57. Singh S, Nagpal SJ, Murad MH, Yadav S, Kane SV, Pardi DS, et al. Inflammatory bowel disease is associated with an increased risk of melanoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol (2014) 12(2):210–8. doi: 10.1016/j.cgh.2013.04.033
58. Hutfless S, Fireman B, Kane S, Herrinton LJ. Screening differences and risk of cervical cancer in inflammatory bowel disease. Aliment Pharmacol Ther (2008) 28(5):598–605. doi: 10.1111/j.1365-2036.2008.03766.x
59. Kim J, Jo H, Ha MC, Kim H, Lee JK, Han JH, et al. Elevated risk of cervical cancer in elderly women with incident ulcerative colitis in South Korea. Sci Rep (2023) 13(1):8323. doi: 10.1038/s41598-023-33476-6
60. Nissen LH, Assendorp EL, van der Post RS, Derikx LA, de Jong DJ, Kievit W, et al. Impaired gastric cancer survival in patients with inflammatory bowel disease. J Gastrointestin Liver Dis (2016) 25(4):431–40. doi: 10.15403/jgld.2014.1121.254.nis
61. Wan Q, Zhao R, Xia L, Wu Y, Zhou Y, Wang Y, et al. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: a meta-analysis of 26 observational studies. J Cancer Res Clin Oncol (2021) 147(4):1077–87. doi: 10.1007/s00432-020-03496-0
62. Faye AS, Holmer AK, Axelrad JE. Cancer in inflammatory bowel disease. Gastroenterol Clin North Am (2022) 51(3):649–66. doi: 10.1016/j.gtc.2022.05.003
63. Erichsen R, Jepsen P, Vilstrup H, Ekbom A, Sorensen HT. Incidence and prognosis of cholangiocarcinoma in Danish patients with and without inflammatory bowel disease: a national cohort study, 1978-2003. Eur J Epidemiol (2009) 24(9):513–20. doi: 10.1007/s10654-009-9365-4
64. Yu J, Refsum E, Helsingen LM, Folseraas T, Ploner A, Wieszczy P, et al. Risk of hepato-pancreato-biliary cancer is increased by primary sclerosing cholangitis in patients with inflammatory bowel disease: A population-based cohort study. United Eur Gastroenterol J (2022) 10(2):212–24. doi: 10.1002/ueg2.12204
65. Castro FA, Liu X, Forsti A, Ji J, Sundquist J, Sundquist K, et al. Increased risk of hepatobiliary cancers after hospitalization for autoimmune disease. Clin Gastroenterol Hepatol (2014) 12(6):1038–45 e7. doi: 10.1016/j.cgh.2013.11.007
66. Saich R, Chapman R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J Gastroenterol (2008) 14(3):331–7. doi: 10.3748/wjg.14.331
67. Loftus EV Jr., Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut (2005) 54(1):91–6. doi: 10.1136/gut.2004.046615
68. Nguyen TB, Do DN, Nguyen TTP, Nguyen TL, Nguyen-Thanh T, Nguyen HT. Immune-related biomarkers shared by inflammatory bowel disease and liver cancer. PloS One (2022) 17(4):e0267358. doi: 10.1371/journal.pone.0267358
69. Tang Q, Liu R, Chu G, Wang Y, Cui H, Zhang T, et al. A comprehensive analysis of microflora and metabolites in the development of ulcerative colitis into colorectal cancer based on the lung-gut correlation theory. Molecules (2022) 27(18):5838. doi: 10.3390/molecules27185838
70. Lo B, Zhao M, Vind I, Burisch J. The risk of extraintestinal cancer in inflammatory bowel disease: A systematic review and meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol (2021) 19(6):1117–38 e19. doi: 10.1016/j.cgh.2020.08.015
71. Wang MT, Bolland MJ, Grey A. Reporting of limitations of observational research. JAMA Intern Med (2015) 175(9):1571–2. doi: 10.1001/jamainternmed.2015.2147
72. Nguyen VT, Engleton M, Davison M, Ravaud P, Porcher R, Boutron I. Risk of bias in observational studies using routinely collected data of comparative effectiveness research: a meta-research study. BMC Med (2021) 19(1):279. doi: 10.1186/s12916-021-02151-w
73. Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol (2014) 20(33):11525–37. doi: 10.3748/wjg.v20.i33.11525
Keywords: Crohn’s disease, ulcerative colitis, extraintestinal cancer, mendelian randomization, genetic association
Citation: Yu C, Xu J, Xu S, Tang L, Han Q, Zeng X, Huang Y, Yu T and Sun Z (2024) Exploring genetic associations of Crohn’s disease and ulcerative colitis with extraintestinal cancers in European and East Asian populations. Front. Immunol. 15:1339207. doi: 10.3389/fimmu.2024.1339207
Received: 15 November 2023; Accepted: 19 January 2024;
Published: 08 February 2024.
Edited by:
Shahanshah Khan, University of Texas Southwestern Medical Center, United StatesReviewed by:
Vincent Salvatore Gallicchio, Clemson University, United StatesMarcos Edgar Herkenhoff, University of São Paulo, Brazil
Copyright © 2024 Yu, Xu, Xu, Tang, Han, Zeng, Huang, Yu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengkui Sun, sunzhengkui@sohu.com
†These authors have contributed equally to this work
 Chengdong Yu
Chengdong Yu