- Department of Thoracic Surgery, the Second Xiangya Hospital of Central South University, Changsha, Hunan, China
Objective: To examine the correlation between SIRI and the probability of cardiovascular mortality as well as all-cause mortality in individuals with chronic kidney disease.
Methods: A cohort of 3,262 participants from the US National Health and Nutrition Examination Survey (NHANES) database were included in the study. We categorized participants into five groups based on the stage of chronic kidney disease. A weighted Cox regression model was applied to assess the relationship between SIRI and mortality. Subgroup analyses, Kaplan–Meier survival curves, and ROC curves were conducted. Additionally, restricted cubic spline analysis was employed to elucidate the detailed association between SIRI and hazard ratio (HR).
Results: This study included a cohort of 3,262 individuals, of whom 1,535 were male (weighted proportion: 42%), and 2,216 were aged 60 or above (weighted proportion: 59%). Following adjustments for covariates like age, sex, race, and education, elevated SIRI remained a significant independent risk factor for cardiovascular mortality (HR=2.50, 95%CI: 1.62-3.84, p<0.001) and all-cause mortality (HR=3.02, 95%CI: 2.03-4.51, p<0.001) in CKD patients. The restricted cubic spline analysis indicated a nonlinear relationship between SIRI and cardiovascular mortality, with SIRI>1.2 identified as an independent risk factor for cardiovascular mortality in CKD patients.
Conclusion: Heightened SIRI independently poses a risk for both all-cause and cardiovascular mortality in chronic kidney disease patients, with potentially heightened significance in the early stages (Stage I to Stage III) of chronic kidney disease.
1 Introduction
Chronic kidney disease (CKD), characterized by a low estimated glomerular filtration rate (eGFR) or elevated albuminuria, is identified as one of the top 10 contributors to poor prognosis globally (1). Epidemiology shows that approximately 15% -20% of people worldwide suffer from chronic kidney disease, and adverse outcomes caused by chronic kidney disease are an issue that cannot be ignored (2, 3). Among all the adverse outcomes caused by CKD, cardiovascular disease (CVD), as a leading cause of death, is one of the most important and severe (2, 4, 5). Patients with CKD typically present with hypertension, atherosclerosis, lipid metabolism abnormalities, mineral and bone disorders, chronic inflammation, and oxidative stress (6–12). These alterations commonly result from a decline in the glomerular filtration rate (9). Notably, chronic inflammation and oxidative stress can exacerbate endothelial damage, leading to the formation of unstable atherosclerotic plaques (6, 13–15). The intricate and complex mechanisms contributing to the onset of chronic inflammation and oxidative stress encompass the accumulation of metabolic byproducts, injury to renal tubular and glomerular cells, activation of the immune system, and disruption of endocrine regulation, among other factors (16–18). Under the collective influence of these factors, patients with CKD are more susceptible to cardiovascular events. Recent investigations reveal an upregulation in the surface expression of IL-1α on monocytes in individuals afflicted with acute myocardial infarction (AMI) and CKD. IL-1α, identified as a pivotal regulatory factor in the inflammatory processes associated with myocardial infarction and CKD, demonstrates a significant correlation with the incidence of cardiovascular events (19). Bioinformatics analysis reveals that the PI3K-AKT signaling pathway and ICAM1-mediated neutrophil infiltration are potential common pathogenic mechanisms in CKD (20). Therefore, monocytes and neutrophils play pivotal roles in the pathogenesis of CKD.
As stated below, existing research indicates that certain inflammatory markers are considered independent risk factors for mortality. In cardiovascular diseases, inflammatory markers have been employed to assess all-cause mortality in CVD (21–24) and acute coronary syndrome (ACS) (25–27). Evidence has emerged linking two-line inflammation indices, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR), to cardiovascular mortality (27–29). Systemic Inflammatory Response Index (SIRI), a novel composite index, integrates three independent white blood cell subsets (30). SIRI was initially used for the assessment of tumor prognosis (31, 32), however, the latest findings suggest that an elevated SIRI strongly correlates with unfavorable outcomes in acute coronary syndrome, cardiovascular event mortality, arrhythmias among stroke patients, and major adverse cardiovascular events following percutaneous coronary intervention (PCI) (33–35). A limited number of studies suggest that SIRI may offer distinct predictive utility in the prediction of acute kidney injury resulting from abdominal trauma (36). An analysis of SIRI elevation and cardiovascular mortality risk in hypertensive patients revealed that those in the fourth quartile of SIRI had a 2.9-fold increased risk of cardiovascular death compared to patients in the first quartile (37). Similar conclusions were confirmed in a follow-up cohort study involving 42,875 participants in the United States (30). In a cohort study conducted after peritoneal dialysis, it was observed that patients in the high SIRI level group had a cardiovascular mortality risk of approximately 2.8 times higher than that of the low-level group (38). These studies strongly suggest that an elevation in SIRI levels may be an independent risk factor for cardiovascular mortality. However, the relationship between SIRI and the risk of cardiovascular mortality in patients with CKD is unclear. Nonetheless, there is a paucity of relevant studies regarding whether SIRI can predict the risk of cardiovascular mortality in individuals afflicted by CKD.
Therefore, in order to explore the potential relationship between SIRI levels and the risk of cardiovascular and all-cause mortality in CKD individuals, we conducted a retrospective analysis using data from participants in the National Health and Nutrition Examination Survey (NHANES). This study serves as a foundational step for future research in this field.
2 Materials and methods
2.1 Data and study participants
The data utilized in this study were obtained from the National Health and Nutrition Examination Survey (NHANES, https://www.cdc.gov/nchs/nhanes/). NHANES database is a publicly accessible resource under the guidance of the National Center for Health Statistics (NCHS), which is responsible for data collection and quality control. Data extraction from the NHANES database was carried out using a rigorous stratified, multistage, and complex sampling methodology. This robust approach facilitated the acquisition of essential demographic particulars, physical assessments, biochemical blood analysis, as well as comprehensive responses to questionnaires about dietary patterns and medical histories. These data collectively provided an extensive portrayal of the health status and lifestyle factors prevalent within the U.S. population. This survey adheres to ethical guidelines, with all participants having provided informed consent before their inclusion.
Our study collected data from 2001 to 2018, encompassing a total of 91,351 participants. Initially, individuals under 18 years of age (n=37,595) and those without follow-up data (n=88) were excluded. Subsequently, a total of 5,152 individuals were excluded from the analysis due to insufficient information on complete blood cell counts, preventing the calculation of SIRI. Next, those with incomplete covariate information (n=30,128) were removed. Finally, we banned 15,126 participants who were not in CKD status. Eventually, 3262 participants were included in this study (Figure 1).
Figure 1 Flowchart of the participant’s selection from NHANES 2021-2018. In the NHANES database, each participant is given a unique SEQN identifier. Across various data collection periods, data related to the same participant is consolidated using the distinct SEQN identifier. CKD, Chronic kidney disease.
2.2 Exposure variable
Neutrophil, lymphocyte and monocyte counts were measured using automated hematology analyzing devices. SIRI is calculated by the following formula (39, 40):
2.3 Covariates
The demographic data encompassed several variables, including age(categorized into 18-39, 40–59, ≥ 60 years old, referred to literature (41)), gender, race, education level, the ratio of family income to poverty (PIR), smoking and drinking habits, dialysis, systolic blood pressure (SBP), and diastolic blood pressure (DBP). Serum albumin, serum creatinine, alanine transaminase (ALT), aspartate transaminase(AST), triglycerides (TG), glycohemoglobin(GHB) and fasting blood glucose were performed under a unified standard. The laboratory utilizes the Jaffe rate method (kinetic alkaline picrate) to determine the concentration of creatinine in serum and plasma. A solid-phase fluorescent immunoassay (FIA) was employed for the quantification of human urinary albumin, while the enzymatic method was utilized to measure urinary creatinine levels. Urinary albumin and urinary creatinine are measured in random urine collected. To calculate the urinary albumin to creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR), we employed the following formulas (41, 42):
It is important to note that Scr denotes serum creatinine concentration (mg/dL), and κ is 0.9 for males and 0.7 for females, and α is −0.411 for males and −0.329 for females.
We defined hypertension as meeting any of the following criteria: (a) self-report of a doctor’s diagnosis of hypertension; (b) current prescription for hypertension medication; (c) three consecutive measurements of systolic blood pressure (SBP) > 140 mmHg and/or diastolic blood pressure (DBP) > 90 mmHg on different days. For diabetes, the definition included meeting any of the following conditions (43): (a) a prior diagnosis of diabetes by a healthcare professional or current use of diabetes medications; (b) fasting blood glucose (FBG) ≥ 7.0 mmol/L; (c) glycated hemoglobin (HbA1C) ≥ 6.5%. According to a comprehensive review of the literature, the assessment of CKD status is based on the following criteria (5, 41, 44–46): (a) eGFR< 60 ml/min/1.73 m2, (b) ACR > 30 mg/g. The CKD stage is based on the calculated results of eGFR (ml/min/1.73m2). The classification of CKD stage and the albuminuria grade are by international guidelines (44, 47) (CKD Stage I: eGFR≥90, Stage II: 60≤eGFR<90, Stage III:30≤eGFR<60, Stage IV: 15≤eGFR<30, Stage V: eGFR<15. Albuminuria grade: Mildly:<30mg/g, Moderately:30-300mg/g, Severely:>300mg/g).
2.4 Outcome assessment
The vital status of participants was verified by cross-referencing with the National Death Index (NDI) (https://ftp.cdc.gov/pub/Health_Statistics/NCHS/datalinkage/linked_mortality/).
All-cause mortality encompassed deaths from any cause. Cardiovascular mortality was defined as I00–I09, I11, I13, I20–I51, or I60–I69, as recorded in the National Death Index (48). This definition encompassed both cardiovascular disease (CVD) and cerebrovascular disease. For brevity, we may refer to cardiovascular mortality as CVD mortality. In the codebook for the National Center for Health Statistics (NCHS) 2019 public-use linked mortality files (Supplementary File 1), the ucode_leading for CVD mortality is represented as 001 and 005.
2.5 Statistical analysis
We performed data analysis using the R project (version 4.1.1). Given the complex, multi-stage, and multi-stratified sampling approach used in the NHANES database, we incorporated weighting variables as guided by the data provider’s usage instructions. The “gtsummary” package in the R project was employed for weighted statistical analysis. We initiated our analysis of continuous variables with a normal distribution test, which indicated a departure from a normal distribution. Consequently, we represented these non-normally distributed data by reporting the median, along with the 25% and 75% percentiles, and applied the Wilcoxon test for statistical analysis. As for categorical variables, we illustrated them through numerical values and proportions and conducted statistical analysis employing the chi-square test.
In order to mitigate potential statistical biases, we adopted a weighted Cox regression model rather than the traditional regression model for our survival analysis of the study population. We employed Hazard Ratios (HR) accompanied by 95% Confidence Intervals (CI) to evaluate the impact of SIRI on survival. Next, we conducted a proportional hazards assumption test for the Cox regression models, affirming that our models adhered to the assumption of proportional hazards. Subsequently, we established three Cox regression models with variable adjustments to assess the association between SIRI and survival. Following this, subgroup analyses were conducted. We performed weighted Cox regression analyses on the association between SIRI and the risk of mortality across seven subgroups, including age, gender, the presence of hypertension or diabetes, dialysis status, distinct CKD stages, and diverse albuminuria grades. After that, we generated Kaplan-Meier (KM) survival curves to compare the survival status among different SIRI groups. To elucidate the potential interactions between these seven subgroups and the risk of mortality, we conducted interaction analyses concurrently.
We constructed receiver operating characteristic (ROC) curves to evaluate the specificity and sensitivity of SIRI in predicting mortality. Restricted cubic spline analysis, a scientific and commonly utilized analytical method, is employed to investigate the potential nonlinear relationship between two variables (49, 50). This method enables curve fitting through restricted cubic splines. In the context of our Cox regression model, restricted cubic spline analysis is used to elucidate how the hazard ratio (HR) values vary with changes in SIRI. Statistical significance was considered at a p-value< 0.05 in all hypothesis tests.
3 Results
3.1 Population characteristics
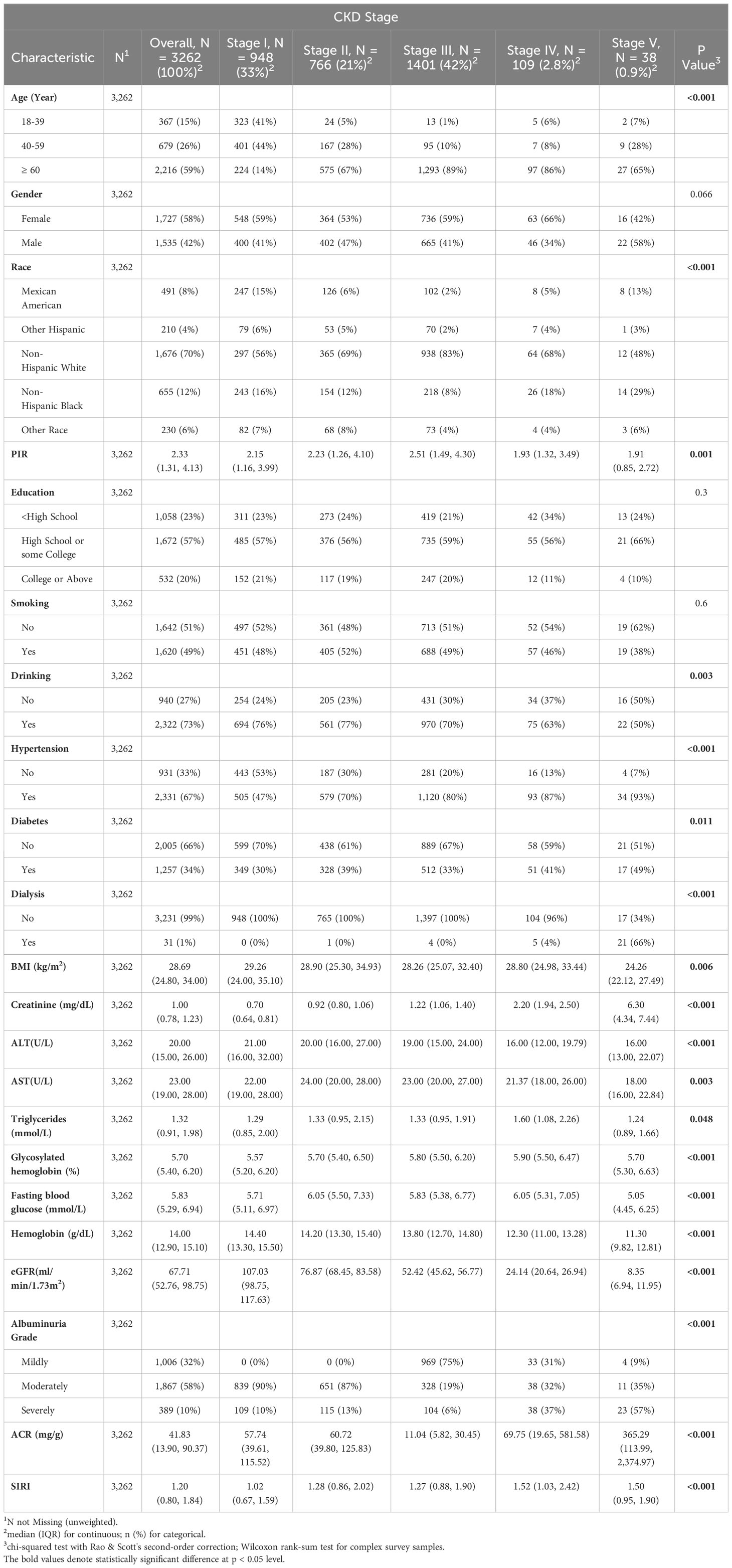
This study encompassed a cohort of 3,262 individuals. Among them, 1,535 were male (weighted proportion: 42%), and 2,216 were aged 60 or above (weighted proportion: 59%). According to the CKD staging, these individuals are classified into five groups. The populations in different groups exhibit statistical differences in terms of age, racial distribution, family income situation, drinking habits, BMI, fasting blood glucose, lipid profiles, liver function indicators, and serum creatinine levels. However, no statistically significant differences were found in terms of gender distribution, smoking habits, and educational levels (Table 1).
Advanced stages of CKD appear to be more prevalent in older individuals, those from economically disadvantaged households, individuals with comorbid hypertension, those undergoing dialysis, and those with elevated levels of serum creatinine, ACR, and SIRI. Participants with lower SIRI scores tend to exhibit characteristics similar to individuals with higher glomerular filtration rates (eGFR) and hemoglobin levels.
3.2 SIRI is an independent predictor of mortality
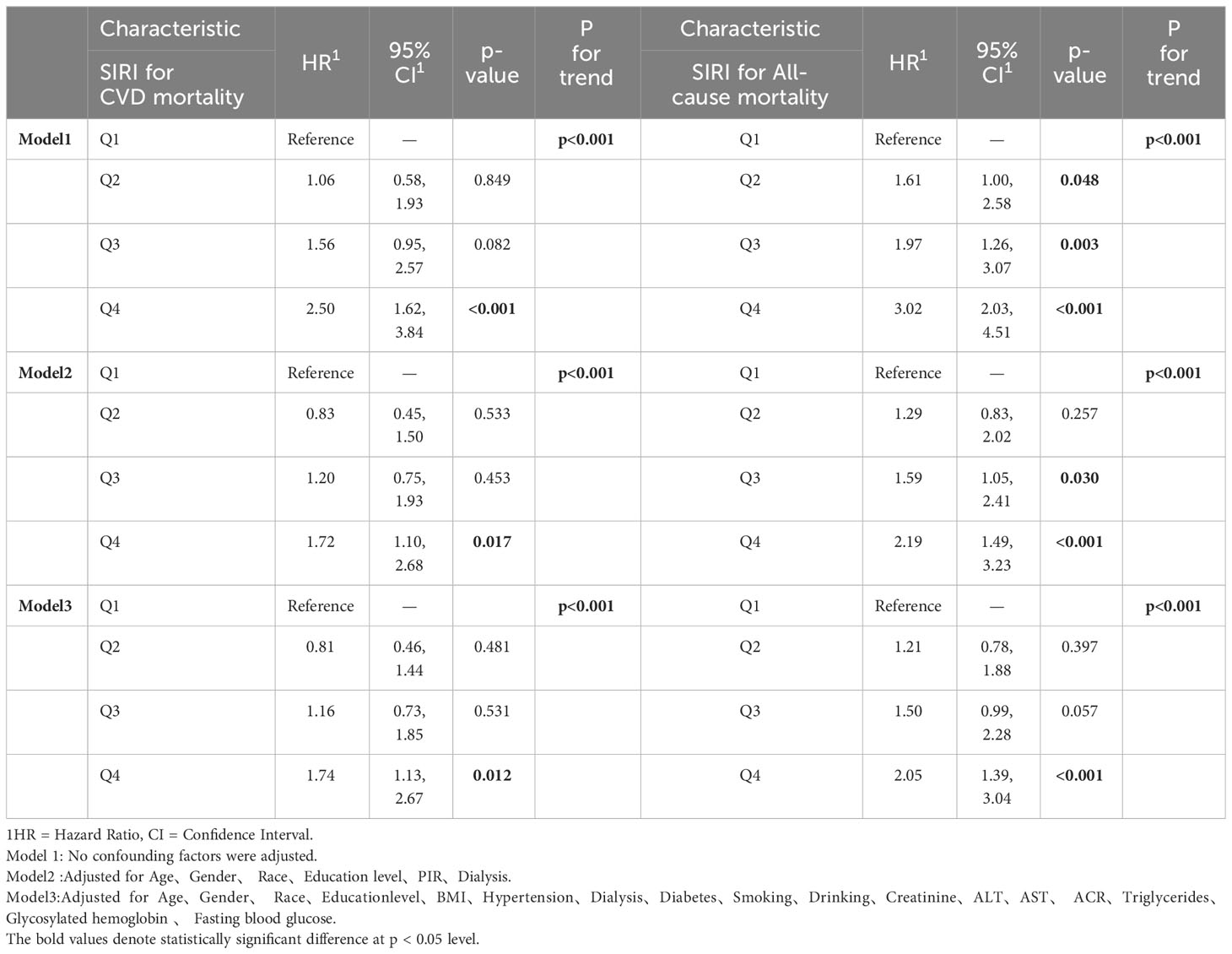
Three models were developed to assess the independent functionality of SIRI and its correlation with mortality. The investigation entailed the utilization of multivariate weighted Cox regression to examine the interconnection between SIRI and mortality, with meticulous adjustments made to account for potential confounding variables. The findings, inclusive of Hazard Ratios (HRs) and their corresponding 95% CIs, have been presented for comprehensive analysis (Table 2). It is important to note that in the weighted Cox regression analysis, all participants will be reclassified into four groups based on the quartiles of SIRI (Q1:<0.661, 0.661≤Q2<0.978, 0.978≤Q3<1.460, Q4:≥1.460).
In Model 1, no covariates were adjusted. In Model 2, we made adjustments for age, gender, race, education level, poverty status (PIR), and dialysis status. In Model 3, we further adjusted for BMI, hypertension, diabetes, alcohol consumption, smoking status, serum creatinine, fasting blood glucose, glycated hemoglobin, lipid levels, ACR, and transaminase levels. The Cox regression results indicate that higher SIRI levels are associated with an increased risk of cardiovascular mortality (Q4 vs. Q1). Compared to participants in the first quartile of SIRI, those in the fourth quartile of SIRI experienced a 2.5-fold increase in cardiovascular mortality risk and a 3.02-fold increase in all-cause mortality risk.
The Kaplan–Meier curve (Figure 2) corroborates the aforementioned conclusion. It demonstrates that individuals with higher SIRI scores experience a more significant decline in survival over a 20-year follow-up when compared to those with lower SIRI levels in CKD populations (log-rank P for trend<0.0001).
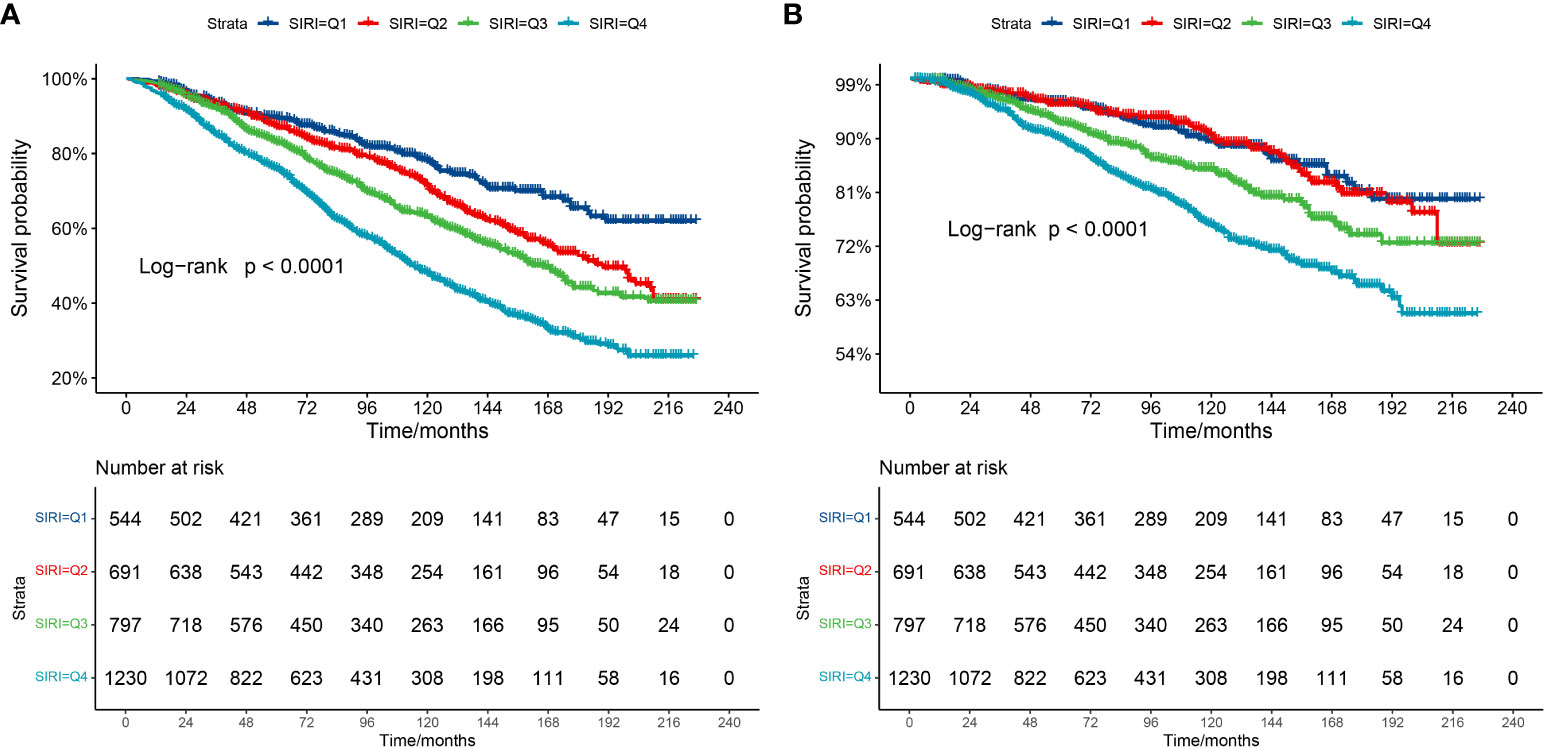
Figure 2 Kaplan-Meier survival curve. (A) Kaplan-Meier survival curve for all-cause mortality. (B) Kaplan-Meier survival curve for cardiovascular mortality. In the Kaplan-Meier curves, the population is stratified into four groups (Q1, Q2, Q3, Q4) based on the quartiles of SIRI, and statistical analysis is conducted using the log-rank test.
3.3 Subgroup analysis and interaction analysis
To determine the consistency of the relationship between SIRI and mortality across various subgroups of the population, subgroup analyses were conducted within specific categories, including age, gender, the presence of hypertension and diabetes, dialysis status, CKD stage, and albuminuria grade.
The results from the forest plot (Figure 3) reveal that elevated SIRI remains an independent risk factor for all-cause mortality in individuals aged over 40, not undergoing dialysis, and with CKD stages I-IV. This association holds regardless of the presence of comorbid hypertension and diabetes or the severity of urinary albumin levels. However, the conclusion that elevated SIRI increases the risk of cardiovascular mortality appears to be established only in male participants aged over 60, with comorbid hypertension but not undergoing dialysis. Furthermore, among individuals in CKD stages, I-III, or those with mild to moderate albuminuria, elevated SIRI is associated with an increased risk of cardiovascular mortality. Interaction analysis indicates the absence of interaction between SIRI and these seven factors in jointly influencing cardiovascular or all-cause mortality. This implies that SIRI independently serves as a factor, contributing to an increased risk for both types of mortality.
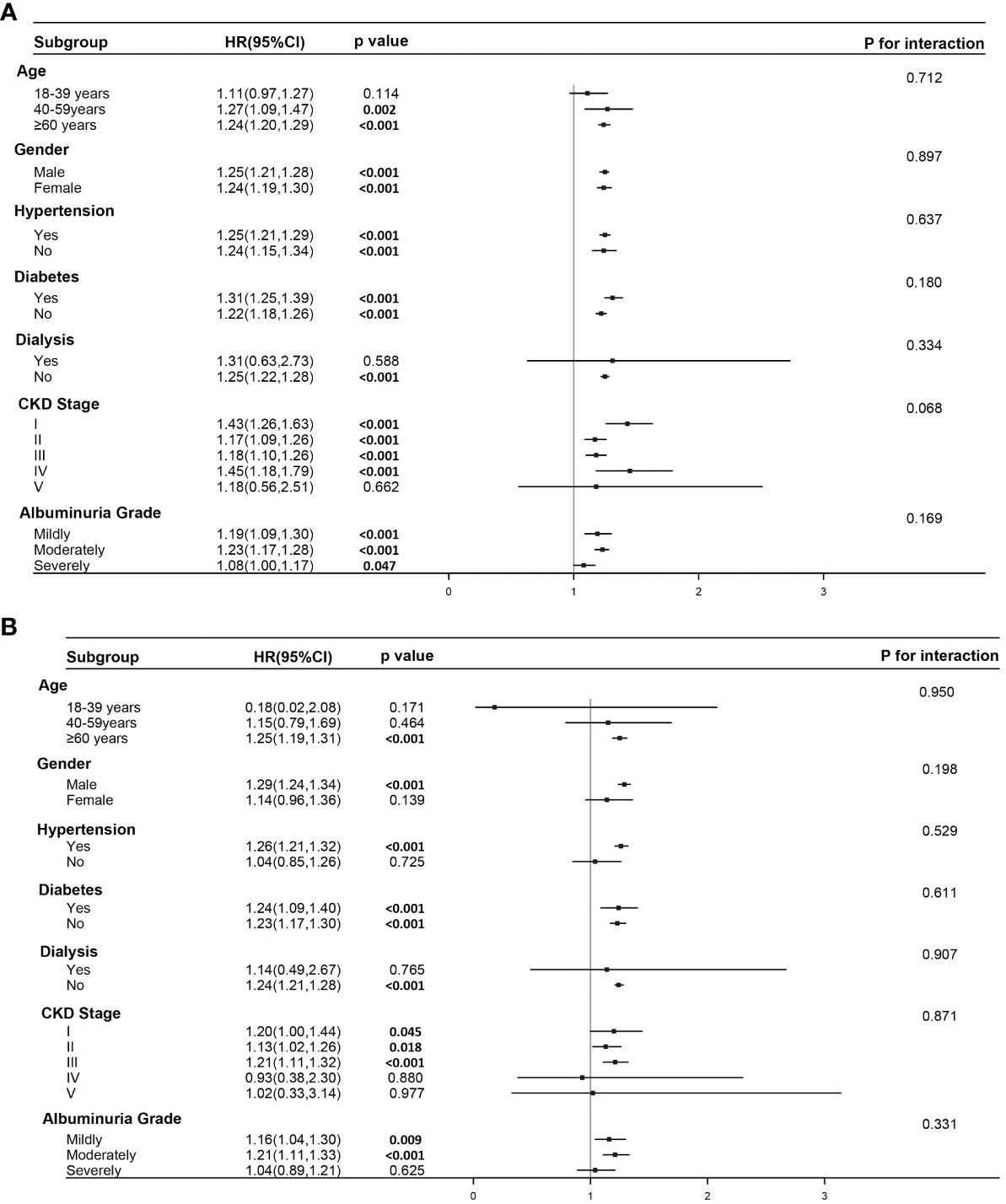
Figure 3 Subgroup analysis and interaction analysis. (A) Subgroup analysis for the association between SIRI and all-cause mortality. (B) Subgroup analysis for the association between SIRI and cardiovascular mortality. Subgroup analysis were conducted using a weighted Cox regression model. In the forest plot, a weighted Cox regression model is still employed. Interaction analysis utilizes the Likelihood Ratio Test.
3.4 Sensitivity and specificity analysis
The establishment of the Receiver Operating Characteristic curve (ROC, Figure 4) is integral for evaluating the sensitivity and specificity of SIRI as a prognostic diagnostic tool. SIRI’s predictive capacity for all-cause mortality is exemplified by an Area Under the Curve (AUC) of 0.624 (95% CI: 0.604, 0.644). Likewise, the predictive performance of SIRI for cardiovascular mortality yields an AUC of 0.593 (95% CI: 0.564, 0.622).
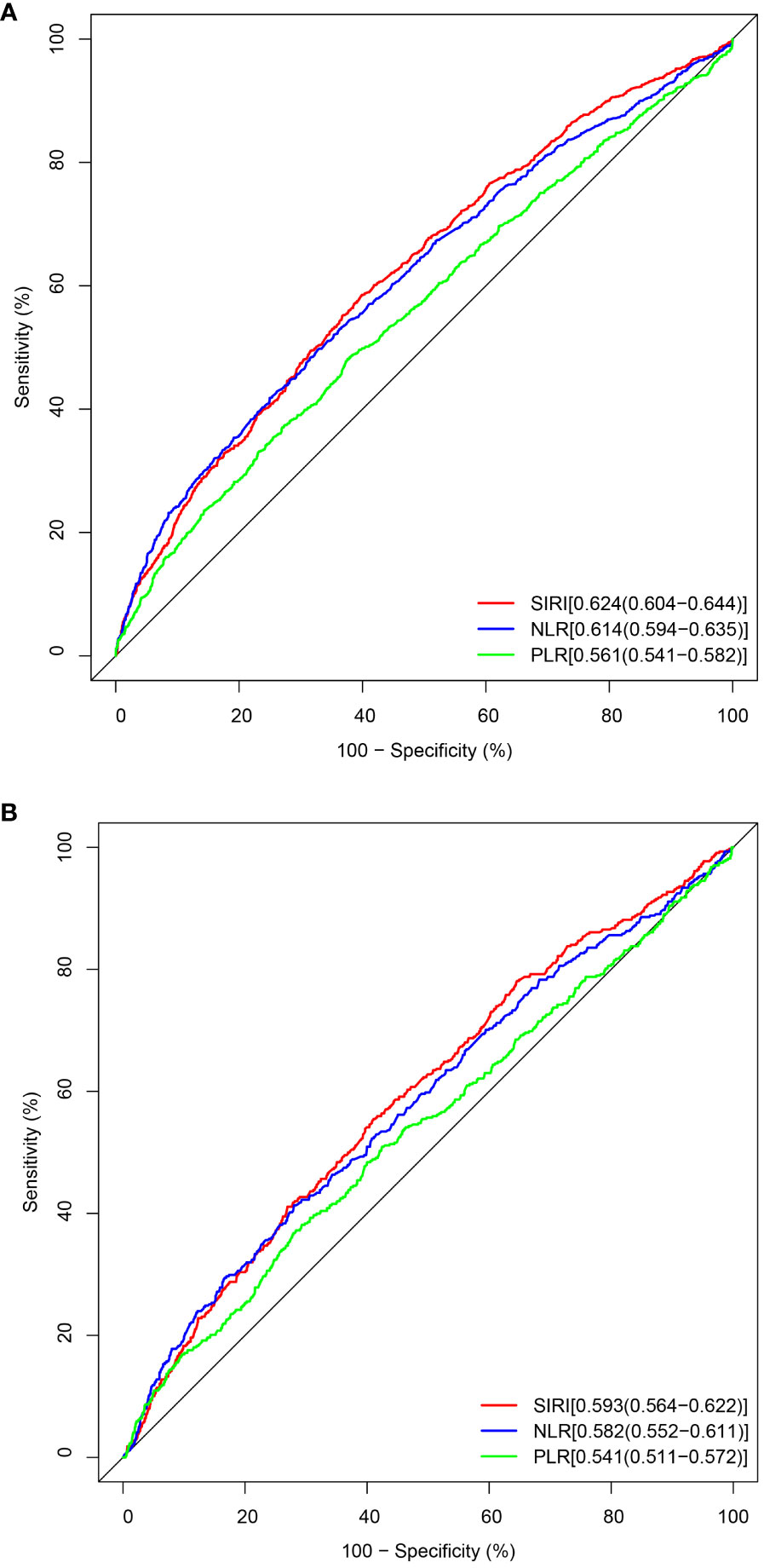
Figure 4 The ROC value of SIRI, NLR, and PLR in predicting outcomes in CKD patients. (A) ROC curve analysis of SIRI and all-cause mortality. The AUC of SIRI was 0.624 with a sensitivity of 0.585 and a specificity of 0.601. (B) ROC curve analysis of SIRI and cardiovascular mortality. The AUC of SIRI was 0.593 with a sensitivity of 0.555 and a specificity of 0.589.
3.5 Restricted cubic spline regression analysis
In order to elucidate the specific relationship between SIRI and HR (Hazard Ratio), the restricted cubic spline regression analysis was conducted (Figure 5). In the examination of the relationship between SIRI and the Hazard Ratio (HR) for cardiovascular mortality, we discovered a non-linear correlation (Nonlinear p-value = 0.0389, which is<0.05, indicating a significant nonlinear relationship between SIRI and HR for CVD). Therefore, we utilized a non-linear model to elucidate their association. The SIRI value corresponding to an HR of 1 is 1.2. When SIRI is<1.2, there is a decrease in cardiovascular mortality risk within a certain range as SIRI increases. However, when SIRI > 1.2, there is an increase in cardiovascular mortality risk, and as SIRI continues to increase, the HR for cardiovascular mortality changes gradually and levels off.
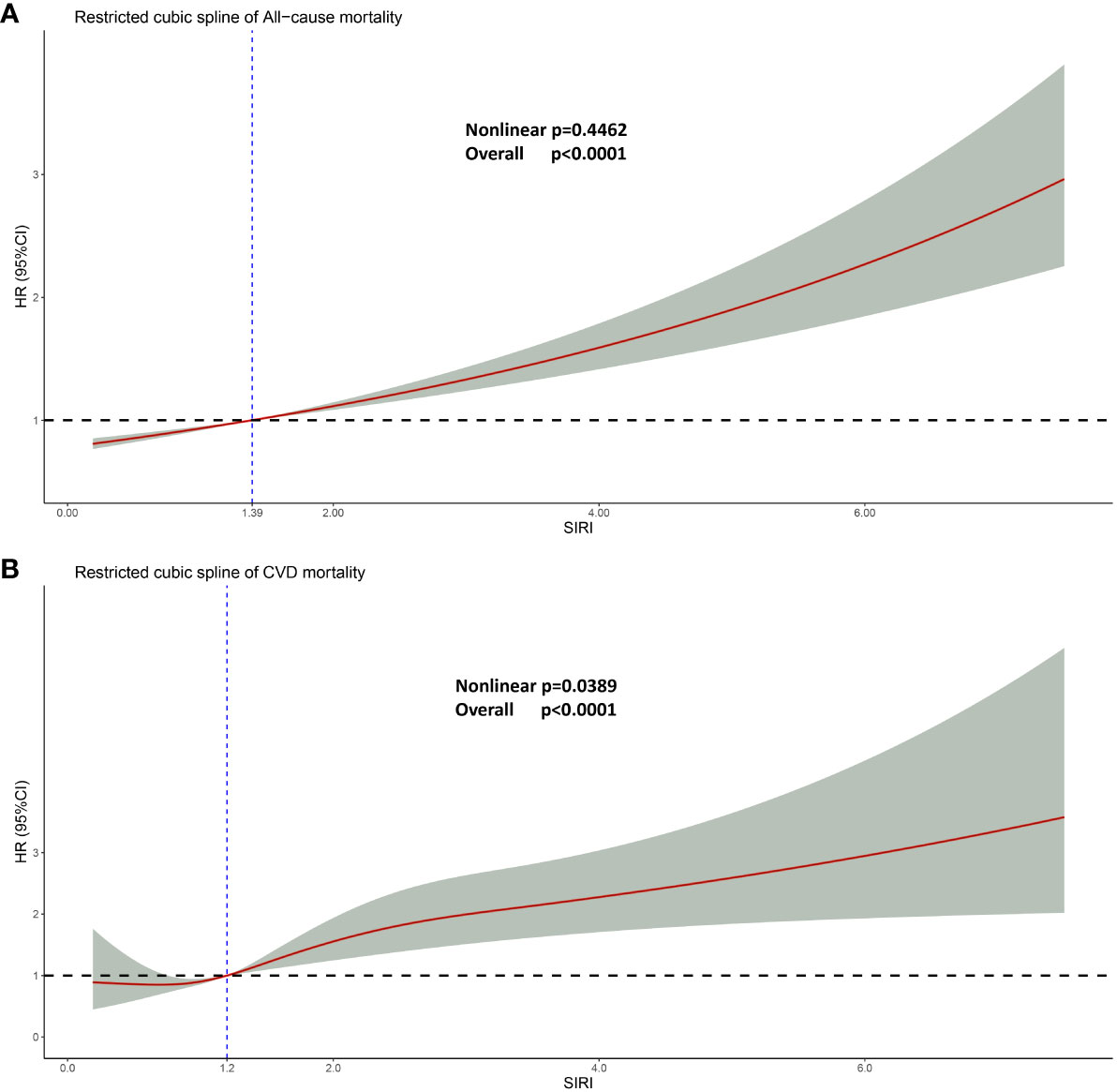
Figure 5 Restricted cubic spline regression analysis. (A) Linear relationship between SIRI and all-cause mortality. (B) Non-linear relationship between SIRI and cardiovascular mortality. For both (A, B), adjusted for age, gender, race, education, smoking, drinking, hypertension, diabetes, ALT, AST, fasting blood glucose, serum creatinine, total cholesterol, and glycosylated hemoglobin. Likelihood Ratio Test was used in the Restricted cubic spline regression analysis analysis.
Interestingly, in the context of all-cause mortality, SIRI and HR exhibit a linear association (non-linear p = 0.4462). As SIRI increases, HR follows suit, and this trend persists beyond the threshold of 1.39, at which point HR surpasses 1.0.
4 Discussion
This is a multicenter, large-scale, and complex sampling cross-sectional study that investigates the relationship between SIRI levels and cardiovascular mortality as well as all-cause mortality in 3262 interviewed individuals with chronic kidney disease in the United States. To the best of our knowledge, this is the first report to explore the association between these two factors. Using a weighted Cox regression model, we have identified a linear relationship between SIRI and all-cause mortality in patients with chronic kidney disease, while a non-linear relationship was observed with cardiovascular mortality (Figure 5). Notably, SIRI has emerged as an independent risk factor for both all-cause mortality and cardiovascular mortality in patients with chronic kidney disease. These findings strongly support the notion that SIRI plays a significant role in predicting mortality outcomes in this patient population.
Inflammation is regarded as a fundamental component in the pathophysiological processes associated with chronic kidney disease (51). Inflammation additionally triggers abnormal activation of neutrophils and the monocyte-macrophage system, subsequently inducing the release of inflammatory factors. These factors contribute to vascular endothelial damage and platelet aggregation (52–55). Among these factors, the differentiation of monocytes into macrophages plays a crucial role in the pathogenesis of ischemic heart disease (56). Pro-inflammatory cytokines such as TNF, IL-1β, and IL-6, secreted by monocytes, enhance their migratory potential towards tissue sites of inflammation and are associated with an increased risk of cardiovascular disease in patients with CKD (57). A study of a mouse myocardial infarction model reveals that after myocardial infarction, despite the predominance of N1 neutrophils, the percentage of N2 neutrophils increased post-MI from 2.4 ± 0.6% on Day 1 to 18.1 ± 3.0% on Day 7 (58). This implies that within the first week after a heart attack, the total number of neutrophils increases. A cross-sectional study revealed a significant reduction in the total number of lymphocytes in the peripheral blood of patients with end-stage renal disease compared to the healthy population (59). In essence, the increased counts of monocytes and neutrophils, coupled with reduced lymphocyte numbers, may offer a plausible explanation for the heightened risk of all-cause and cardiovascular mortality associated with elevated SIRI levels in patients with CKD. Numerous studies have illustrated immune system dysregulation, heightened inflammatory signaling, diminished release of anti-inflammatory factors, and persistent accumulation of chronic inflammation in individuals with chronic kidney disease (60–63). Therefore, anti-inflammatory therapy is also recommended for patients in CKD stages I to III (64). Interestingly, inflammatory activities may be more pronounced during CKD stages IV and V than during stages I to III (65), which is consistent with our findings(advanced CKD stage with a higher level of SIRI). However, in patients with advanced stages of chronic kidney disease (IV-V), the primary causes of cardiovascular death are sudden cardiac death and myocardial infarction rather than inflammatory reactions (66, 67). This might be a reason that in our subgroup analysis, an elevation in SIRI appears to be unrelated to an increased risk of cardiovascular death in participants in CKD stages IV and V.
Several factors can impact the mortality of individuals with CKD, including age, gender, race, hypertension, diabetes, and lifestyle habits like smoking and drinking (68). To mitigate the influence of these variables, three Cox models were employed to elucidate the association between elevated SIRI and mortality risk while adjusting for different factors. Even after accounting for confounding variables, increased SIRI persisted as an independent risk factor for both cardiovascular and all-cause mortality in chronic kidney disease patients. Subgroup analyses further revealed that elevated SIRI retained its independent association with all-cause mortality in various CKD subgroups. The simplicity of the SIRI definition and the accessibility of data through laboratory analysis of a patient’s complete blood count enhance its applicability as a novel biomarker, offering broad prospects and significant economic benefits in clinical settings. C-reactive protein (CRP) is a commonly employed marker for inflammation detection. However, our analysis of the NHANES database revealed that CRP levels were not assessed for every participant every year (the indicator was not measured from 2011 to 2014). This suggests that a higher prevalence of missing values could introduce notable statistical analysis errors. Consequently, we opted not to incorporate this variable into our study. Additionally, it’s worth noting that the cost associated with CRP measurement is higher compared to that of a complete blood count assessment. While SIRI exhibits a slightly superior predictive ability compared to NLR and PLR in the ROC curve, the overall predictive performance of SIRI is still constrained (AUC approximately 0.6). This result is consistent with the AUC reported in previous literature (37). We speculate that this may be related to the relatively small number of individuals experiencing cardiovascular death events during the follow-up period. In pursuit of practicality, we conducted a restricted cubic spline analysis (Figure 5) to elucidate the relationship between SIRI and hazard ratio (HR). Our findings reveal a non-linear association between SIRI and the risk of cardiovascular mortality, indicating that SIRI levels exceeding 1.2 are linked to an elevated risk of cardiovascular death in individuals with chronic kidney disease. This underscores the importance of dynamic monitoring of SIRI levels during the treatment of CKD patients to mitigate the risk of cardiovascular mortality.
Our study possesses several notable strengths. Firstly, we assessed a substantial sample size of 3,262 participants, each with comprehensive clinical information and survival data. The data collection process was meticulously designed and executed by the NCHS team in the United States, ensuring a high level of reliability following rigorous quality testing. Secondly, our utilization of a weighted Cox regression model enhances the generalizability of our findings. Despite the seemingly modest sample size, the participants effectively represented diverse social strata in the United States from 2001 to 2018, rendering our analysis results widely applicable and representative. Thirdly, we identified a specific threshold for controlling SIRI levels to mitigate the risk of cardiovascular and all-cause mortality in individuals with CKD. Nevertheless, our study has certain limitations. Firstly, its cross-sectional nature means that dynamic follow-up data are lacking. Additionally, the availability of survival data from the NCHS is limited to 2018 and earlier, precluding access to more recent follow-up information. Finally, the relatively low percentage of cardiovascular deaths among the 3,262 participants introduces some statistical error, necessitating larger cohort studies for more robust conclusions in the future.
5 Conclusion
In summary, our study indicates that in patients with chronic kidney disease, increased SIRI level is an independent risk factor for cardiovascular mortality(SIRI>1.20) and all-cause mortality(SIRI>1.39), with potentially heightened significance in the early stages (Stage I to Stage III) of chronic kidney disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LG: Writing – original draft. ZX: Data curation, Writing – review & editing. BQ: Data curation, Writing – review & editing. WW: Data curation, Writing – review & editing. HC: Data curation, Writing – review & editing. JW: Methodology, Writing – review & editing. YC: Methodology, Writing – review & editing. ZG: Methodology, Writing – review & editing. RH: Methodology, Writing – review & editing. YY: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The data analysis for this study required the use of a paid R software package, and funding was provided by the National Natural Science Foundation (Project Title: “The mechanism of DsbA-L in bleomycin-induced pulmonary fibrosis by upregulating AKT1 and NLRP3 expression”. Funding project number: 82070069). We express our deep appreciation for their support.
Acknowledgments
We express our sincere gratitude to Mr. Liu Wenwu from the Department of Cardiology, Xiangya Second Hospital, Central South University, for his assistance in data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1338025/full#supplementary-material
Abbreviations
SIRI, Systemic Inflammatory Response Index; NHANES, National Health and Nutrition Examination Survey; ROC, Receiver Operating Characteristics; CKD, Chronic kidney disease; PIR, Poverty income ratio; CVD, Cardiovascular events; BMI, Body Mass Index; CI, Confidence Interval; HR, Hazard Ratio; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; eGFR, Estimated glomerular filtration rate; ACR, Urinary albumin to creatinine ratio.
References
1. Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/s0140-6736(20)30752-2
2. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. (2021) 143:1157–72. doi: 10.1161/circulationaha.120.050686
3. Li X, Lindholm B. Cardiovascular risk prediction in chronic kidney disease. Am J Nephrol. (2022) 53:730–9. doi: 10.1159/000528560
4. Lim YJ, Sidor NA, Tonial NC, Che A, Urquhart BL. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: mechanisms and therapeutic targets. Toxins. (2021) 13. doi: 10.3390/toxins13020142
5. Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. (2022) 18:696–707. doi: 10.1038/s41581-022-00616-6
6. Reiss AB, Miyawaki N, Moon J, Kasselman LJ, Voloshyna I, D'Avino R Jr., et al. Ckd, arterial calcification, atherosclerosis and bone health: inter-relationships and controversies. Atherosclerosis. (2018) 278:49–59. doi: 10.1016/j.atherosclerosis.2018.08.046
7. Sinha AD, Agarwal R. Clinical pharmacology of antihypertensive therapy for the treatment of hypertension in ckd. Clin J Am Soc Nephrol CJASN. (2019) 14:757–64. doi: 10.2215/cjn.04330418
8. Gilligan S, Raphael KL. Hyperkalemia and hypokalemia in ckd: prevalence, risk factors, and clinical outcomes. Adv chronic Kidney Dis. (2017) 24:315–8. doi: 10.1053/j.ackd.2017.06.004
9. Evenepoel P, Stenvinkel P, Shanahan C, Pacifici R. Inflammation and gut dysbiosis as drivers of ckd-mbd. Nat Rev Nephrol. (2023) 19:646–57. doi: 10.1038/s41581-023-00736-7
10. Baaten C, Schröer JR, Floege J, Marx N, Jankowski J, Berger M, et al. Platelet abnormalities in ckd and their implications for antiplatelet therapy. Clin J Am Soc Nephrol CJASN. (2022) 17:155–70. doi: 10.2215/cjn.04100321
11. Ebert T, Neytchev O, Witasp A, Kublickiene K, Stenvinkel P, Shiels PG. Inflammation and oxidative stress in chronic kidney disease and dialysis patients. Antioxidants Redox Signaling. (2021) 35:1426–48. doi: 10.1089/ars.2020.8184
12. Baek J, He C, Afshinnia F, Michailidis G, Pennathur S. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol. (2022) 18:38–55. doi: 10.1038/s41581-021-00488-2
13. Baker M, Perazella MA. Nsaids in ckd: are they safe? Am J Kidney. (2020) 76:546–57. doi: 10.1053/j.ajkd.2020.03.023
14. Ku E, Lee BJ, Wei J, Weir MR. Hypertension in ckd: core curriculum 2019. Am J Kidney Dis. (2019) 74:120–31. doi: 10.1053/j.ajkd.2018.12.044
15. Yang C, Wang H, Zhao X, Matsushita K, Coresh J, Zhang L, et al. Ckd in China: evolving spectrum and public health implications. Am J Kidney Dis. (2020) 76:258–64. doi: 10.1053/j.ajkd.2019.05.032
16. Ding WY, Gupta D, Wong CF, Lip GYH. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc Res. (2021) 117:1046–59. doi: 10.1093/cvr/cvaa258
17. Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ (Clinical Res ed). (2020) 370:m2687. doi: 10.1136/bmj.m2687
18. Yanai H, Adachi H, Hakoshima M, Katsuyama H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22179221
19. Schunk SJ, Triem S, Schmit D, Zewinger S, Sarakpi T, Becker E, et al. Interleukin-1α Is a central regulator of leukocyte-endothelial adhesion in myocardial infarction and in chronic kidney disease. Circulation. (2021) 144:893–908. doi: 10.1161/circulationaha.121.053547
20. Liang Z, Hu X, Lin R, Tang Z, Ye Z, Mao R, et al. Identification of shared gene signatures and molecular mechanisms between chronic kidney disease and ulcerative colitis. Front Immunol. (2023) 14:1078310. doi: 10.3389/fimmu.2023.1078310
21. Verdoia M, Nardin M, Gioscia R, Negro F, Marcolongo M, Suryapranata H, et al. Higher neutrophil-to-lymphocyte ratio (Nlr) increases the risk of suboptimal platelet inhibition and major cardiovascular ischemic events among acs patients receiving dual antiplatelet therapy with ticagrelor. Vasc Pharmacol. (2020) 132:106765. doi: 10.1016/j.vph.2020.106765
22. Mureşan AV, Russu E, Arbănaşi EM, Kaller R, Hosu I, Arbănaşi EM, et al. The predictive value of nlr, mlr, and plr in the outcome of end-stage kidney disease patients. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10061272
23. Qiu C, Liu S, Li X, Li W, Hu G, Liu F. Prognostic value of monocyte-to-lymphocyte ratio for 90-day all-cause mortality in type 2 diabetes mellitus patients with chronic kidney disease. Sci Rep. (2023) 13:13136. doi: 10.1038/s41598-023-40429-6
24. Urbanowicz T, Olasińska-Wiśniewska A, Michalak M, Rodzki M, Witkowska A, Straburzyńska-Migaj E, et al. The prognostic significance of neutrophil to lymphocyte ratio (Nlr), monocyte to lymphocyte ratio (Mlr) and platelet to lymphocyte ratio (Plr) on long-term survival in off-pump coronary artery bypass grafting (Opcab) procedures. Biology. (2021) 11. doi: 10.3390/biology11010034
25. Kaiser H, Wang X, Kvist-Hansen A, Krakauer M, Gørtz PM, McCauley BD, et al. Biomarkers of subclinical atherosclerosis in patients with psoriasis. Sci Rep. (2021) 11:21438. doi: 10.1038/s41598-021-00999-9
26. Trtica Majnarić L, Guljaš S, Bosnić Z, Šerić V, Wittlinger T. Neutrophil-to-lymphocyte ratio as a cardiovascular risk marker may be less efficient in women than in men. Biomolecules. (2021) 11. doi: 10.3390/biom11040528
27. Wang P, Guo X, Zhou Y, Li Z, Yu S, Sun Y, et al. Monocyte-to-high-density lipoprotein ratio and systemic inflammation response index are associated with the risk of metabolic disorders and cardiovascular diseases in general rural population. Front Endocrinol. (2022) 13:944991. doi: 10.3389/fendo.2022.944991
28. Fan Z, Ji H, Li Y, Jian X, Li L, Liu T. Relationship between monocyte-to-lymphocyte ratio and coronary plaque vulnerability in patients with stable angina. Biomarkers Med. (2017) 11:979–90. doi: 10.2217/bmm-2017-0235
29. Balta S, Ozturk C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. (2015) 26:680–1. doi: 10.3109/09537104.2014.979340
30. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation index (Sii), system inflammation response index (Siri) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 us adults. J Clin Med. (2023) 12. doi: 10.3390/jcm12031128
31. Cai H, Chen Y, Zhang Q, Liu Y, Jia H. High preoperative cea and systemic inflammation response index (C-siri) predict unfavorable survival of resectable colorectal cancer. World J Surg Oncol. (2023) 21:178. doi: 10.1186/s12957-023-03056-z
32. Wang T, Zhang D, Tang D, Heng Y, Lu LM, Tao L. The role of systemic inflammatory response index (Siri) and tumor-infiltrating lymphocytes (Tils) in the prognosis of patients with laryngeal squamous cell carcinoma. J Cancer Res Clin Oncol. (2023) 149:5627–36. doi: 10.1007/s00432-022-04469-1
33. Dziedzic EA, Gąsior JS, Tuzimek A, Paleczny J, Junka A, Dąbrowski M, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (Sii) and systemic inflammatory response index (Siri)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23179553
34. Lin KB, Fan FH, Cai MQ, Yu Y, Fu CL, Ding LY, et al. Systemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur J Med Res. (2022) 27:106. doi: 10.1186/s40001-022-00733-9
35. Li Q, Ma X, Shao Q, Yang Z, Wang Y, Gao F, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. (2022) 9:811790. doi: 10.3389/fcvm.2022.811790
36. Vunvulea V, Budişcă OA, Arbănaşi EM, Mureşan AV, Arbănaşi EM, Brînzaniuc K, et al. The predictive role of systemic inflammatory markers in the development of acute kidney failure and mortality in patients with abdominal trauma. J personalized Med. (2022) 12. doi: 10.3390/jpm12122045
37. Zhao S, Dong S, Qin Y, Wang Y, Zhang B, Liu A. Inflammation index siri is associated with increased all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. (2022) 9:1066219. doi: 10.3389/fcvm.2022.1066219
38. Li J, Li Y, Zou Y, Chen Y, He L, Wang Y, et al. Use of the systemic inflammation response index (Siri) as a novel prognostic marker for patients on peritoneal dialysis. Renal Failure. (2022) 44:1228–36. doi: 10.1080/0886022x.2022.2100262
39. Wang RH, Wen WX, Jiang ZP, Du ZP, Ma ZH, Lu AL, et al. The clinical value of neutrophil-to-lymphocyte ratio (Nlr), systemic immune-inflammation index (Sii), platelet-to-lymphocyte ratio (Plr) and systemic inflammation response index (Siri) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. (2023) 14:1115031. doi: 10.3389/fimmu.2023.1115031
40. Wang X, Ni Q, Wang J, Wu S, Chen P, Xing D. Systemic inflammation response index is a promising prognostic marker in elderly patients with heart failure: A retrospective cohort study. Front Cardiovasc Med. (2022) 9:871031. doi: 10.3389/fcvm.2022.871031
41. Kang H, Lee JP, Choi K. Exposure to phthalates and environmental phenols in association with chronic kidney disease (Ckd) among the general us population participating in multi-cycle nhanes (2005–2016). Sci Total Environ. (2021) 791. doi: 10.1016/j.scitotenv.2021.148343
42. Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis : an individual participant-based meta-analysis. Ann Internal Med. (2020) 173:426–35. doi: 10.7326/m20-0529
43. 2. Classification and diagnosis of diabetes:Standards of medical care in diabetes—2020. Diabetes Care. (2020) 43:S14–31. doi: 10.2337/dc20-S002
44. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet (London England). (2017) 389:1238–52. doi: 10.1016/s0140-6736(16)32064-5
45. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: A review. Jama. (2019) 322:1294–304. doi: 10.1001/jama.2019.14745
46. Wiles K, Chappell LC, Lightstone L, Bramham K. Updates in diagnosis and management of preeclampsia in women with ckd. Clin J Am Soc Nephrol CJASN. (2020) 15:1371–80. doi: 10.2215/cjn.15121219
47. Chapter 1: definition and classification of ckd. Kidney Int Suppl. (2013) 3:19–62. doi: 10.1038/kisup.2012.64
48. Tan QH, Huang YQ, Liu XC, Liu L, Lo K, Chen JY, et al. A U-shaped relationship between selenium concentrations and all-cause or cardiovascular mortality in patients with hypertension. Front Cardiovasc Med. (2021) 8:671618. doi: 10.3389/fcvm.2021.671618
49. Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: A guide for clinicians. Bone Marrow Transplant. (2019) 55:675–80. doi: 10.1038/s41409-019-0679-x
50. Deng Y-T, Kuo K, Wu B-S, Ou Y-N, Yang L, Zhang Y-R, et al. Associations of resting heart rate with incident dementia, cognition, and brain structure: A prospective cohort study of uk biobank. Alzheimer's Res Ther. (2022) 14. doi: 10.1186/s13195-022-01088-3
51. Fu Y, Xiang Y, Li H, Chen A, Dong Z. Inflammation in kidney repair: mechanism and therapeutic potential. Pharmacol Ther. (2022) 237:108240. doi: 10.1016/j.pharmthera.2022.108240
52. Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscl Rep. (2014) 16:435. doi: 10.1007/s11883-014-0435-z
53. Hirsch K, Nolley S, Ralph DD, Zheng Y, Altemeier WA, Rhodes CJ, et al. Circulating markers of inflammation and angiogenesis and clinical outcomes across subtypes of pulmonary arterial hypertension. J Heart Lung Transplant. (2023) 42:173–82. doi: 10.1016/j.healun.2022.10.026
54. Mills RJ, Humphrey SJ, Fortuna PRJ, Lor M, Foster SR, Quaife-Ryan GA, et al. Bet inhibition blocks inflammation-induced cardiac dysfunction and sars-cov-2 infection. Cell. (2021) 184:2167–82.e22. doi: 10.1016/j.cell.2021.03.026
55. Jin HY, Weir-McCall JR, Leipsic JA, Son JW, Sellers SL, Shao M, et al. The relationship between coronary calcification and the natural history of coronary artery disease. JACC Cardiovasc Imaging. (2021) 14:233–42. doi: 10.1016/j.jcmg.2020.08.036
56. Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. (2006) 98:1177–85. doi: 10.1161/01.Res.0000220106.64661.71
57. Bishop NC, Burton JO, Graham-Brown MPM, Stensel DJ, Viana JL, Watson EL. Exercise and chronic kidney disease: potential mechanisms underlying the physiological benefits. Nat Rev Nephrol. (2023) 19:244–56. doi: 10.1038/s41581-022-00675-9
58. Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res. (2016) 110:51–61. doi: 10.1093/cvr/cvw024
59. Freitas GRR, da Luz Fernandes M, Agena F, Jaluul O, Silva SC, Lemos FBC, et al. Aging and end stage renal disease cause a decrease in absolute circulating lymphocyte counts with a shift to a memory profile and diverge in treg population. Aging Dis. (2019) 10:49–61. doi: 10.14336/ad.2018.0318
60. Speer T, Dimmeler S, Schunk SJ, Fliser D, Ridker PM. Targeting innate immunity-driven inflammation in ckd and cardiovascular disease. Nat Rev Nephrol. (2022) 18:762–78. doi: 10.1038/s41581-022-00621-9
61. Wu W, Wang X, Yu X, Lan HY. Smad3 signatures in renal inflammation and fibrosis. Int J Biol Sci. (2022) 18:2795–806. doi: 10.7150/ijbs.71595
62. Brennan E, Kantharidis P, Cooper ME, Godson C. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat Rev Nephrol. (2021) 17:725–39. doi: 10.1038/s41581-021-00454-y
63. Gluba-Brzózka A, Franczyk B, Olszewski R, Rysz J. The influence of inflammation on anemia in ckd patients. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21030725
64. Cantero-Navarro E, Rayego-Mateos S, Orejudo M, Tejedor-Santamaria L, Tejera-Munoz A, Sanz AB, et al. Role of macrophages and related cytokines in kidney disease. Front Med (Lausanne). (2021) 8:688060. doi: 10.3389/fmed.2021.688060
65. Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke H-D, Tribouilloy C, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. (2010) 77:550–6. doi: 10.1038/ki.2009.503
66. Tonelli M, Karumanchi SA, Thadhani R. Epidemiology and mechanisms of uremia-related cardiovascular disease. Circulation. (2016) 133:518–36. doi: 10.1161/CIRCULATIONAHA.115.018713
67. Sidhu MS, Alexander KP, Huang Z, Mathew RO, Newman JD, O’Brien SM, et al. Cause-specific mortality in patients with advanced chronic kidney disease in the ischemia-ckd trial. JACC: Cardiovasc Interventions. (2023) 16:209–18. doi: 10.1016/j.jcin.2022.10.062
Keywords: SIRI, CKD, mortality, cardiovascular risk, NHANES
Citation: Gu L, Xia Z, Qing B, Wang W, Chen H, Wang J, Chen Y, Gai Z, Hu R and Yuan Y (2024) Systemic Inflammatory Response Index (SIRI) is associated with all-cause mortality and cardiovascular mortality in population with chronic kidney disease: evidence from NHANES (2001–2018). Front. Immunol. 15:1338025. doi: 10.3389/fimmu.2024.1338025
Received: 14 November 2023; Accepted: 19 February 2024;
Published: 15 March 2024.
Edited by:
Jason C. O’Connor, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Mustafa Mithaiwala, University of Arkansas Medical Center, United StatesAfaf Saliba, The University of Texas Health Science Center at San Antonio, United States
Subrata Debnath, The University of Texas Health Science Center at San Antonio, United States
Copyright © 2024 Gu, Xia, Qing, Wang, Chen, Wang, Chen, Gai, Hu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunchang Yuan, eXVhbnljQGNzdS5lZHUuY24=
 Linguo Gu
Linguo Gu Zhenkun Xia
Zhenkun Xia Yunchang Yuan
Yunchang Yuan