- 1Division of Infectious Diseases, Department of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 2Université de Franche-Comté, CHU Besançon, UMR-CNRS 6249 Chrono-environnement, Department of Infectious and Tropical Diseases, Besançon, France
- 3Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, United States
Background: We aimed to compare patient characteristics, MRSA sequence types, and biofilm production of MRSA strains that did and did not cause a foreign body infection in patients with MRSA bloodstream infections (BSI)
Methods: All adult patients with MRSA BSI hospitalized in two hospitals were identified by clinical microbiology laboratory surveillance. Only patients who had at least one implanted foreign body during the episode of BSI were included.
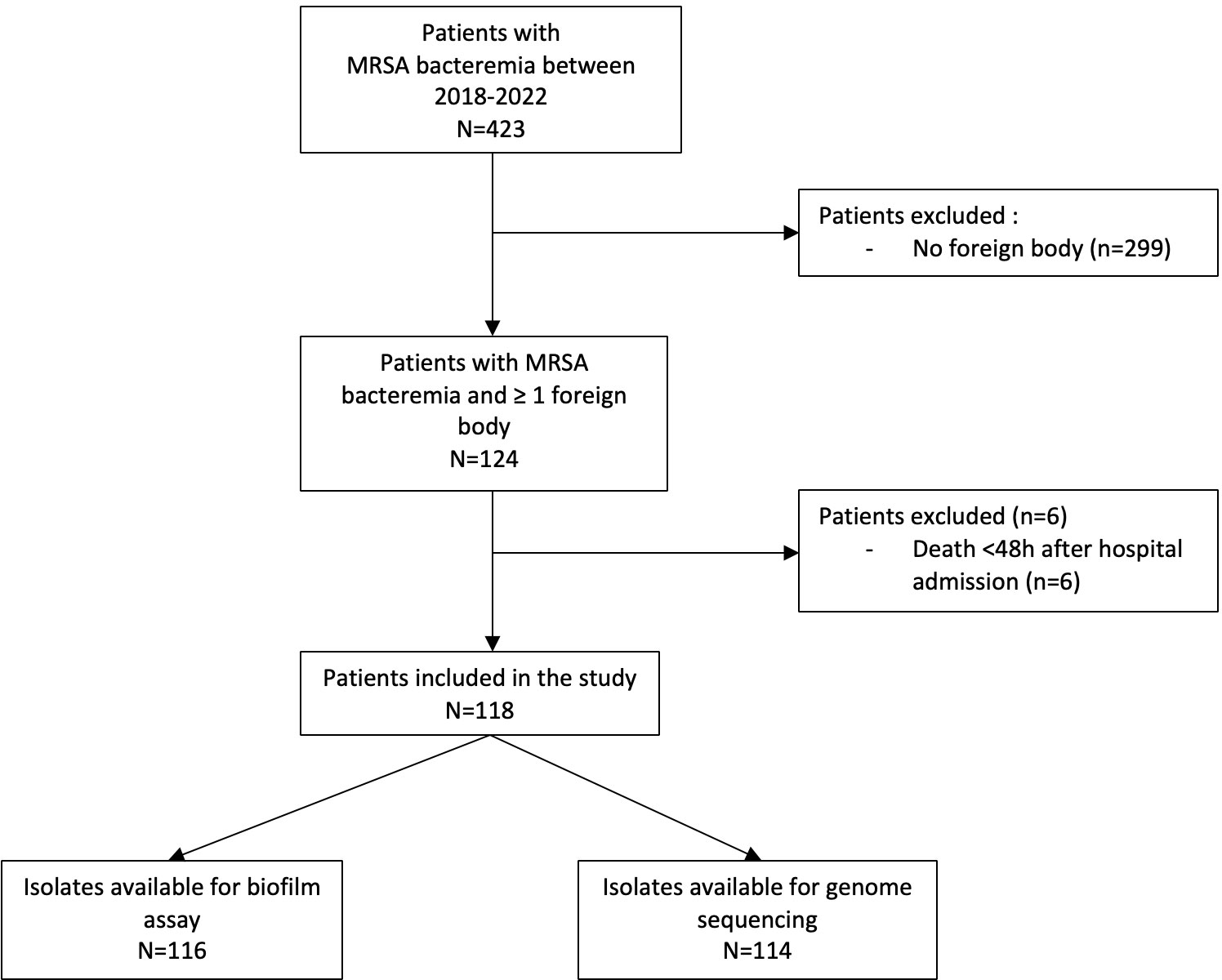
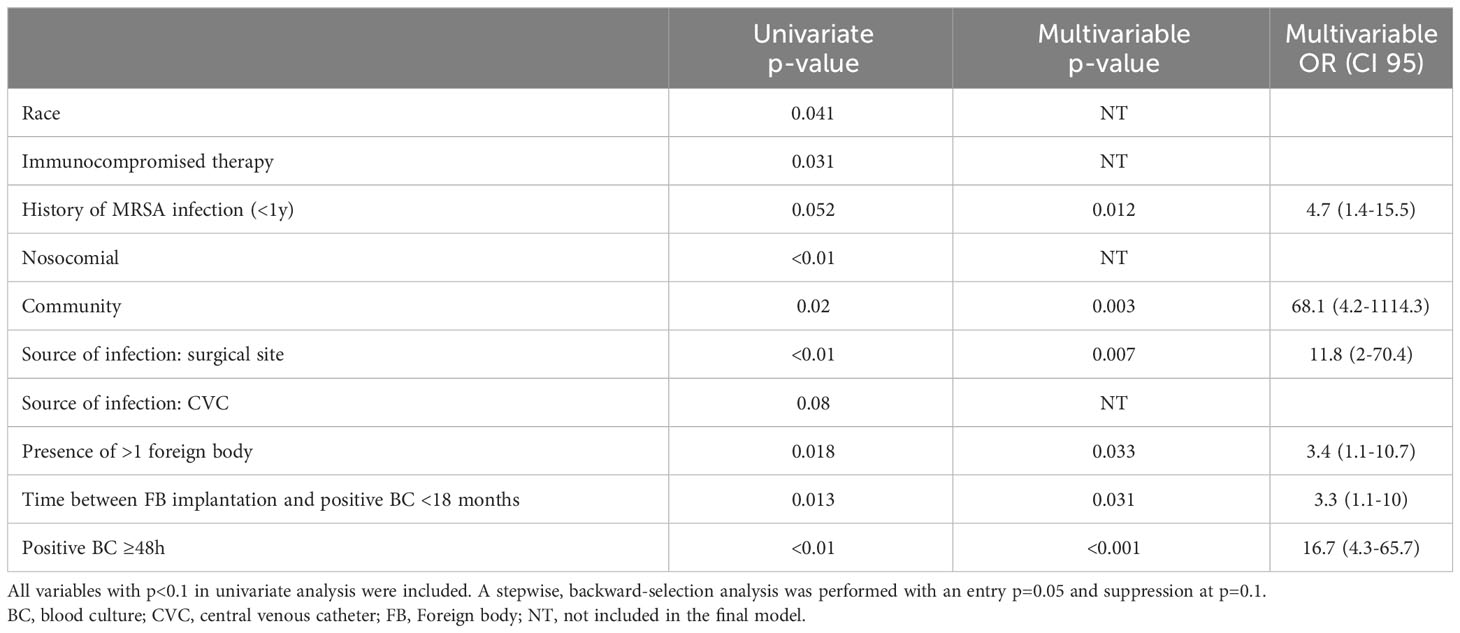
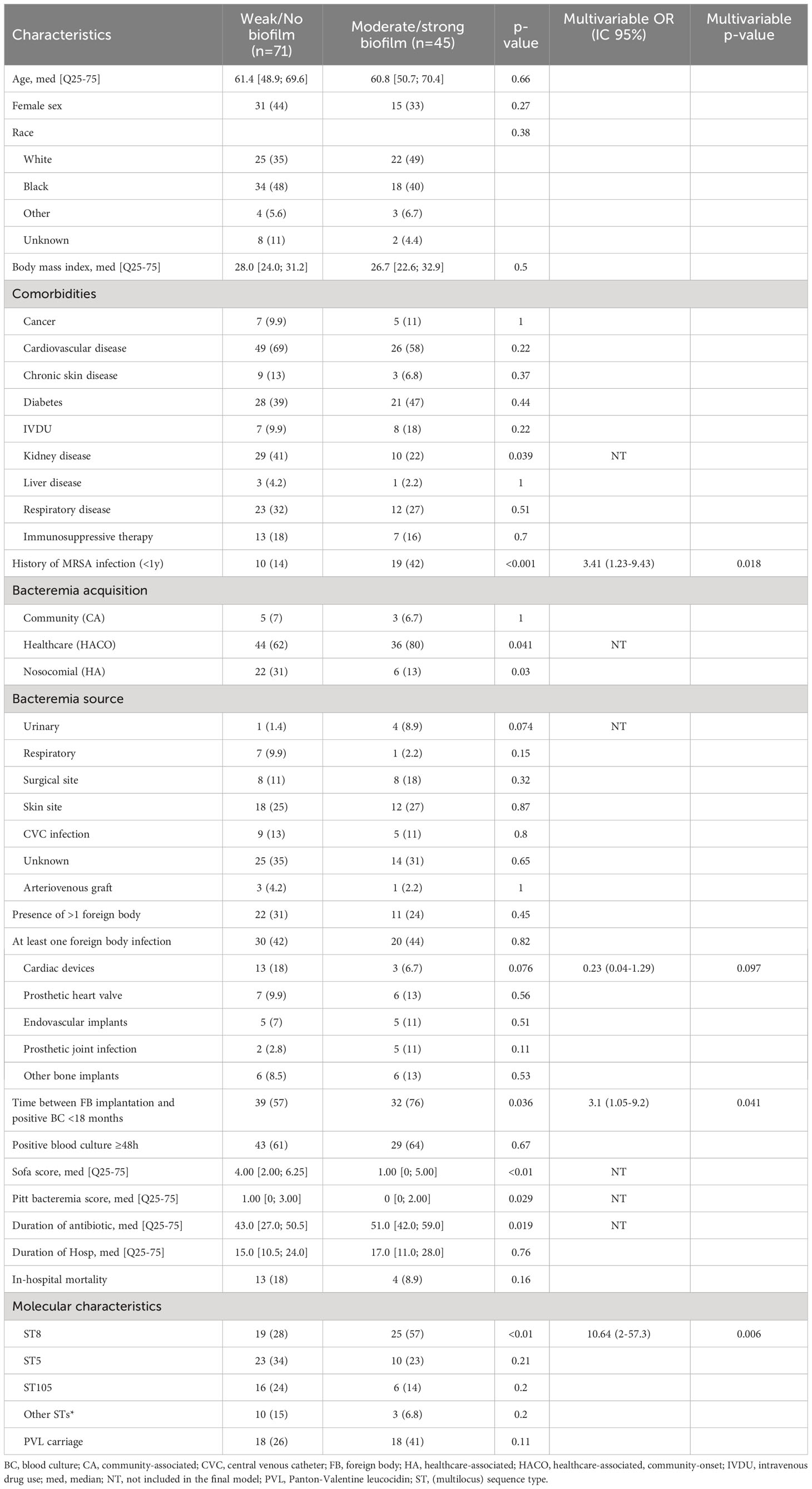
Results: In July 2018 - March 2022, of 423 patients identified with MRSA BSI, 118 (28%) had ≥1 foreign body. Among them, 51 (43%) had one or more foreign body infections. In multivariable analysis, factors associated with foreign body infection were history of MRSA infection in the last year (OR=4.7 [1.4-15.5], p=0.012) community-associated BSI (OR=68.1 [4.2-1114.3], p=0.003); surgical site infection as source of infection (OR=11.8 [2-70.4], p=0.007); presence of more than one foreign body (OR=3.4 [1.1-10.7], p=0.033); interval between foreign body implantation and infection <18 months (OR=3.3 [1.1-10], p=0.031); and positive blood culture ≥48h (OR=16.7 [4.3-65.7], p<0.001). The most prevalent sequence type was ST8 (39%), followed by ST5 (29%), and ST105 (20%) with no significant difference between patients with or without foreign body infection. Only 39% of MRSA isolates formed a moderate/strong biofilm. No significant difference was observed between patients with foreign body infection and those without foreign body infection. In multivariable analysis, subjects infected with a MRSA isolate producing moderate/strong in vitro biofilm were more likely to have a history of MRSA infection in the last year (OR=3.41 [1.23-9.43]), interval between foreign body implantation and MRSA BSI <18 months (OR=3.1 [1.05-9.2]) and ST8 (OR=10.64 [2-57.3]).
Conclusion: Most factors associated with foreign body infection in MRSA BSI were also characteristic of persistent infections. Biofilm-forming isolates were not associated with a higher risk of foreign-body infection but appeared to be associated with MRSA genetic lineage, especially ST8.
1 Introduction
The implantation of foreign bodies in human medicine has increased over time related to the aging population and the development of new materials, new surgical methods, and new indications (e.g., transcatheter valve replacement) (1, 2). Foreign body infections pose a significant human and economic burden (3, 4). Staphylococcus aureus is one of the most common bacterial species causing foreign body infections (1, 5–8). An accurate and timely diagnosis of foreign body infection is necessary for optimal surgical and antibiotic treatment. One of the major virulence factors contributing to S. aureus foreign body infection is the ability of some strains to form a biofilm, cause persistent infection, and develop resistance to antibiotics. Also, biofilms can enable infecting bacteria to escape human immune responses (9–11). In the event of an infection, foreign bodies usually need to be removed because antibiotics are often ineffective at killing bacteria within biofilms. Furthermore, antibiotic resistance in methicillin-resistant S. aureus (MRSA) limits antibiotic choice for treatment. However, the role of the intrinsic biofilm-forming characteristics of S. aureus strains associated with foreign body infections has been little studied. The aim of this study was to compare patient characteristics, MRSA sequence types, and biofilm production of MRSA strains that did and did not cause a foreign body infection in patients with MRSA bloodstream infections (BSIs).
2 Materials and methods
2.1 Study population
All adult patients with MRSA bacteremia hospitalized at two hospitals (Hospital of the University of Pennsylvania, and Penn Presbyterian Medical Center) were identified by clinical microbiology laboratory surveillance between July 2018 and March 2022. Only patients who had at least one implanted foreign body during the episode of BSI were included. Patients who died within 48h of hospital admission and patients receiving end-of-life care without specific directed management of MRSA BSI were excluded. Clinical data were retrospectively abstracted from the electronic medical record on a standardized case report form and entered into the online secure database REDCap (Research Electronic Data Capture), including demographics; medical history; history of MRSA infection within the last year; site of acquisition of MRSA BSI; number of days of positive blood cultures; delay between foreign body insertion and infection; initial source of BSI; management and in-hospital mortality; Acute Physiology and Chronic Health Evaluation (APACHE) II score, calculated on the day of the index positive blood culture; and the Pitt bacteremia score.
2.2 Definitions
Foreign bodies included pacemakers (PM), implantable cardiac defibrillators (ICD), cardiac resynchronization therapy (CRT) devices, left ventricular assistance devices (LVAD), prosthetic heart valves, prosthetic joints, endovascular implants and bone implants other than those related to prosthetic joints. BSI cases were categorized either as community-associated (CA), community-onset, healthcare–associated (HACO), or healthcare-associated (HA) infection as previously defined (12). Infective endocarditis was diagnosed according to the 2023 modified Duke criteria (13). Cardiac implantable electronic device infection was based on the 2020 international consensus (1). Left ventricular assist device infection related blood stream infection was defined as previously (14). Because no standard diagnostic criteria for osteosynthesis orthopedic infection existed, criteria established for PJIs were used (15). Endovascular implant infection was defined by the MAGIC consensus criteria (16).
2.3 Follow-up
Subjects with a foreign body infection were followed to the end of hospitalization or death, and subjects without a foreign body infection at the time of hospitalization were followed to the last medical follow-up visit during the study period.
2.4 In vitro mature biofilm formation
Biofilm formation was assessed using a crystal violet staining assay in a 96 well-plate, as previously described (17) (see Supplementary data). The interpretation of biofilm production was performed according to the criteria of Stepanovic et al. (17). The average optical density (OD) value of all tested strains and negative controls was calculated. Cut-off OD (ODc) was defined as three standard deviations above the mean OD of the negative control. Strains were interpreted as follows: no biofilm producer, OD≤ODc; weak biofilm producer, ODc < OD ≤ 2 × ODc; moderate biofilm producer, 2 × ODc < OD ≤ 4 × ODc; and strong biofilm producer, 4 × ODc < OD.
2.5 Molecular typing
Genomic DNA extractions were performed using a commercial kit (Qiagen). Library preparation and whole genome sequencing were performed by the Penn/Children’s Hospital of Pennsylvania Microbiome Center using Illumina MiSeq or Hiseq platforms with paired-end 150 bp reads. MLST types were identified using the Bactopia pipeline (v 1.6.5) (18). PVL genes were identified using the Bactopia subworkflow for ARIBA (v 2.14.6; https://www-microbiologyresearch-org.proxy.library.emory.edu/content/journal/mgen/10.1099/mgen.0.000131) to detect LukF and LukS genes in assembled genomes. Reference sequences were obtained from the Virulence Factor Database.
2.6 Statistical methods
Data are reported as medians (interquartile range [IQR]) for continuous variables and counts (percentages) for categorical variables. Comparisons of continuous data were performed using the Student t-test or the Mann-Whitney U test and categorical data were compared using Pearson’s chi-squared test or Fischer’s exact test, as appropriate. For statistical analysis, moderate and strong biofilm producers were compared to non/weak biofilm producers. Factors independently associated with foreign body infection and with isolates with moderate/strong biofilm formation were determined from a multivariable logistic regression model that was built using a backward elimination process. All independent variables with a p-value < 0.10 in univariable analysis were included in the multivariable model. Statistical analyses were performed using SPSS 28.0 (IBM, Armonk, NY, USA).
3 Results
3.1 Study population
In July 2018 - March 2022, of 423 patients identified with MRSA BSI, 124 (29%) had a foreign body in situ. Among them, 6 were excluded due to death in <48 hours (Figure 1). Of 118 eligible subjects, 51 (43%) had one or more foreign body infections: 16 had an intracardiac device infection (13 with PM/ICD infection, 3 with LVAD infection), 13 had a prosthetic heart valve infection, 10 had an endovascular implant infection, 7 had a PJI, and 13 had another type of bone implant infection. Among the 51 subjects with a foreign body infection, 8 had two or more foreign body infections (2 with prosthetic heart valve and PM/ICD infection; 2 with endovascular implant and a prosthetic heart valve infection; 1 with prosthetic heart valve, endovascular implant and PJI; 1 with prosthetic heart valve and PJI; and 1 with PJI and another type of bone implant infection). Moreover, 60/179 (34%) foreign bodies were infected: 13/22 (59%) prosthetic heart valves, 10/20 (50%) endovascular implants, 16/36 (44%) PM/ICD/LVAD (2 patients had 2 intracardiac devices that were not infected), 7/42 (17%) prosthetic joints (11 patients had 2 and 1 had 4 joint prostheses not infected), 13/58 (22%) other types of bone implants (5 patients had 2 and 1 patient had 4 other types of bone implants not infected).
All patients were diagnosed with one or more foreign body infection during the same hospitalization as the MRSA BSI, except for two patients: one subject diagnosed with an endovascular implant infection 2 months after the MRSA BSI diagnosis, and a second subject with PJI 3 months after MRSA BSI. In patients without a foreign body infection, the median follow-up time was 220 days (IQR 43-529). All patients with a prosthetic valve underwent a transthoracic echocardiography (TTE) study, and only 5 subjects did not undergo transesophageal echocardiography (TEE). Among these 5, one had a prosthetic valve infection diagnosed by TTE and had a contraindication to TEE. The other 4 subjects had a repeat TTE not suggestive of infective endocarditis and a follow up time of greater than 300 days without relapse of MRSA BSI or new prosthetic valve surgery. In subjects with an in situ cardiac device, all underwent a TTE and 20/34 underwent a TEE. Among 14 subjects who did not undergo TEE, 4 had a diagnosis of cardiac device infection with TTE only and 8 had a second TTE performed. In these 8 patients, blood cultures were positive for only one day.
The median time between foreign body implantation and infection was greater than one year for all types of foreign body except for endovascular implants (0.39 years [IC95%, 0.16-0.61]) and other bone implants (0.98 years [0.00-2.04]) (Supplementary Figure S1).
3.2 Factors associated with foreign body infection
Patients with foreign body infections were less likely to be black (17/51, 33% vs. 35/67, 52%, p=0.041), less likely to be receiving immunosuppressive therapy (5/51, 9.8% vs. 17/67, 25%, p=0.031), more often had a history of MRSA infection within the last year (18/51, 35%, vs. 13/67, 19%, p=0.052), more often had CA, and less HA BSI (7/51, 13.7%, vs. 1/67, 1.5%, p=0.02 and 6/51, 11.8% vs. 22/67, 32.8%, p<0.01, respectively) (Table 1). In multivariable analysis, factors associated with foreign body infection were history of MRSA infection in the last year (OR=4.7 [1.4-15.5], p=0.012), CA-MRSA BSI (OR=68.1 [4.2-1114.3], p=0.003), surgical site infection as source of infection (OR=11.8 [2-70.4], p=0.007), presence of more than one foreign body (OR=3.4 [1.1-10.7], p=0.033), interval between foreign body implantation and infection <18 months (OR=3.3 [1.1-10], p=0.031), and positive blood culture ≥48h (OR=16.7 [4.3-65.7], p<0.001) (Table 2).
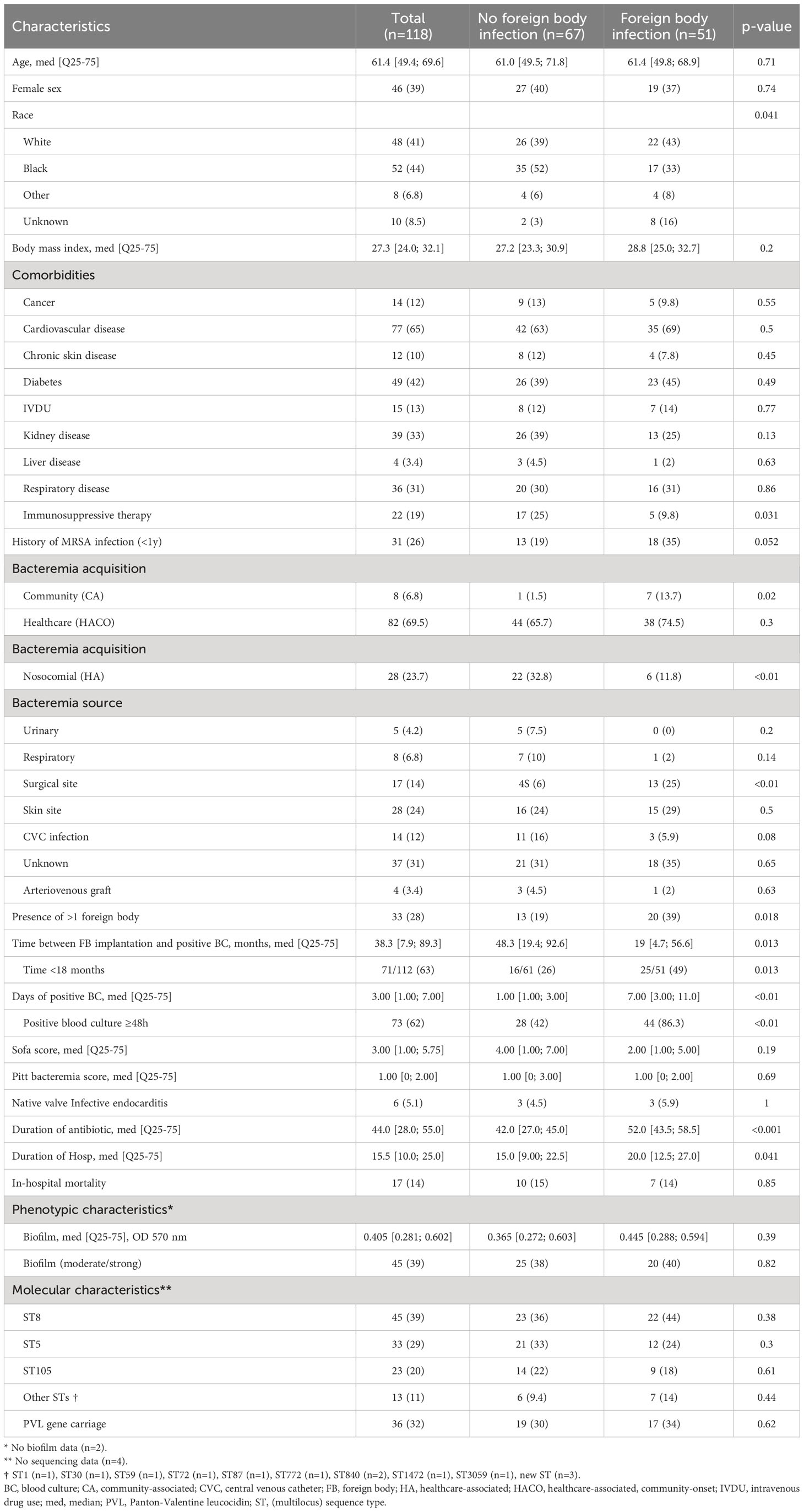
Table 1 Characteristics of 118 patients with MRSA bacteremia and at least one foreign body at time of bacteremia.
3.3 Sequence type
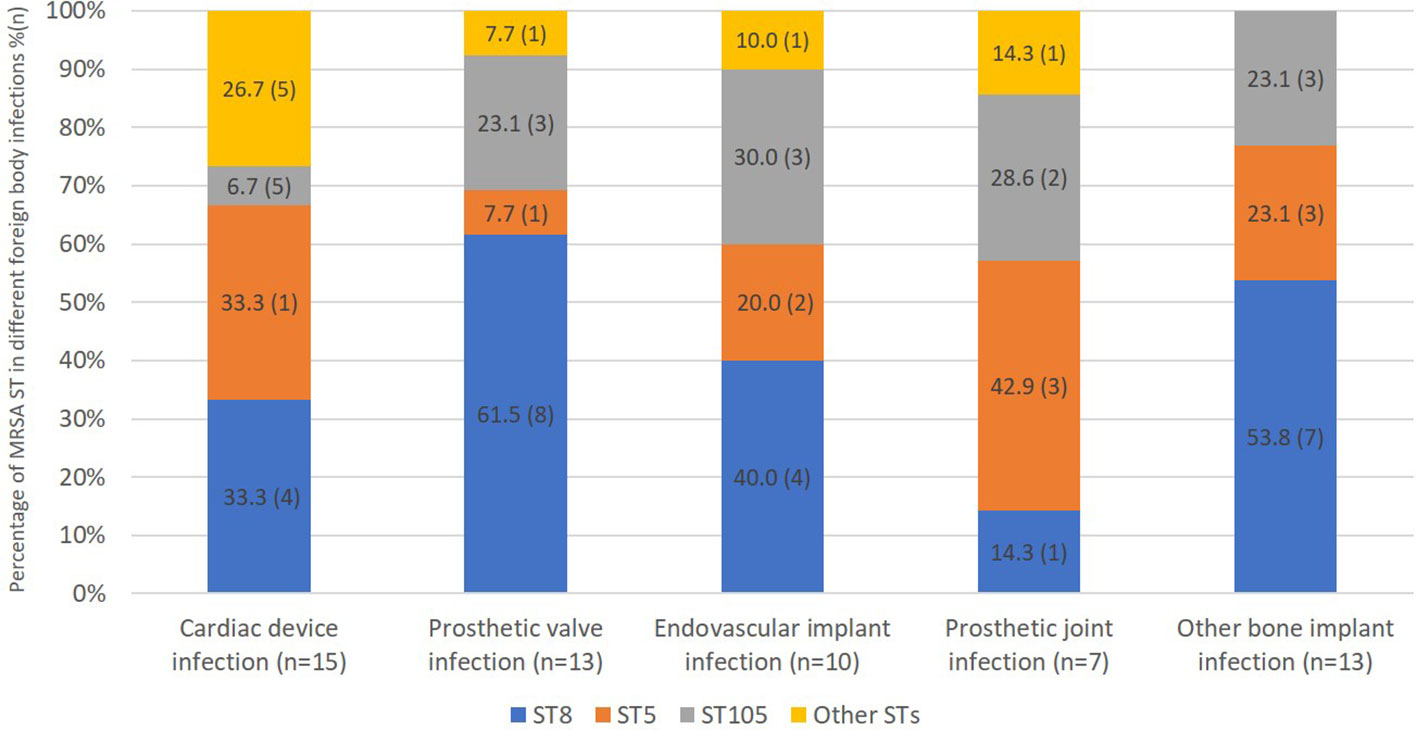
We were able to obtain whole genome sequence data for 114/118 isolates (Table 1). The most prevalent sequence type was ST8 (39%), followed by ST5 (29%), and ST105 (20%). There was no significant difference in ST comparing patients with or without foreign body infection (Table 1). Moreover, 36 isolates carried Panton Valentin leucocidin (PVL) genes, 34 in MRSA ST8, 1 in MRSA ST772 (CC1) and 1 in a novel, previously unassigned ST belonged to CC8. MRSA ST8 tended to be more frequent in patients with prosthetic valve infection than patients with PJI, and MRSA ST5 tended to be more frequent in patients with PJI than prosthetic valve infection (8/13, 62% vs. 1/7, 14%, p=0.07 and 3/7, 43% vs. 1/13, 7.7%, p=0.1, respectively) although these associations were not significant (Figure 2).
3.4 Biofilm formation
In vitro biofilm formation assays were performed for 116/118 MRSA isolates (Table 1). Only 39% (45/116) of isolates formed a moderate/strong biofilm. No significant difference was observed in moderate/strong biofilm formation between patients with foreign body infection and those without foreign body infection (20/45, 44% vs. 30/71, 42%, p=0.82) (Table 3). Among subjects with a prosthetic heart valve (n=22), those with a prosthetic heart valve infection were more likely to have an isolate with moderate/severe biofilm production than subjects without prosthetic heart valve infection (6/13, 43% vs. 0/9, p=0.046). Moreover, MRSA BSI isolates in subjects with PJI produced more biofilm than those from subjects with a cardiac device infection (5/7, 71% vs. 3/16, 19%, p=0.022) (Figure 3). In multivariable analysis, subjects infected with a MRSA isolate producing moderate/strong in vitro biofilm were more likely to have a history of MRSA infection in the last year (OR=3.41 [1.23-9.43]), interval between foreign body implantation and MRSA BSI <18 months (OR=3.1 [1.05-9.2]), and ST8 (OR=10.64 [2-57.3]) (Table 3).
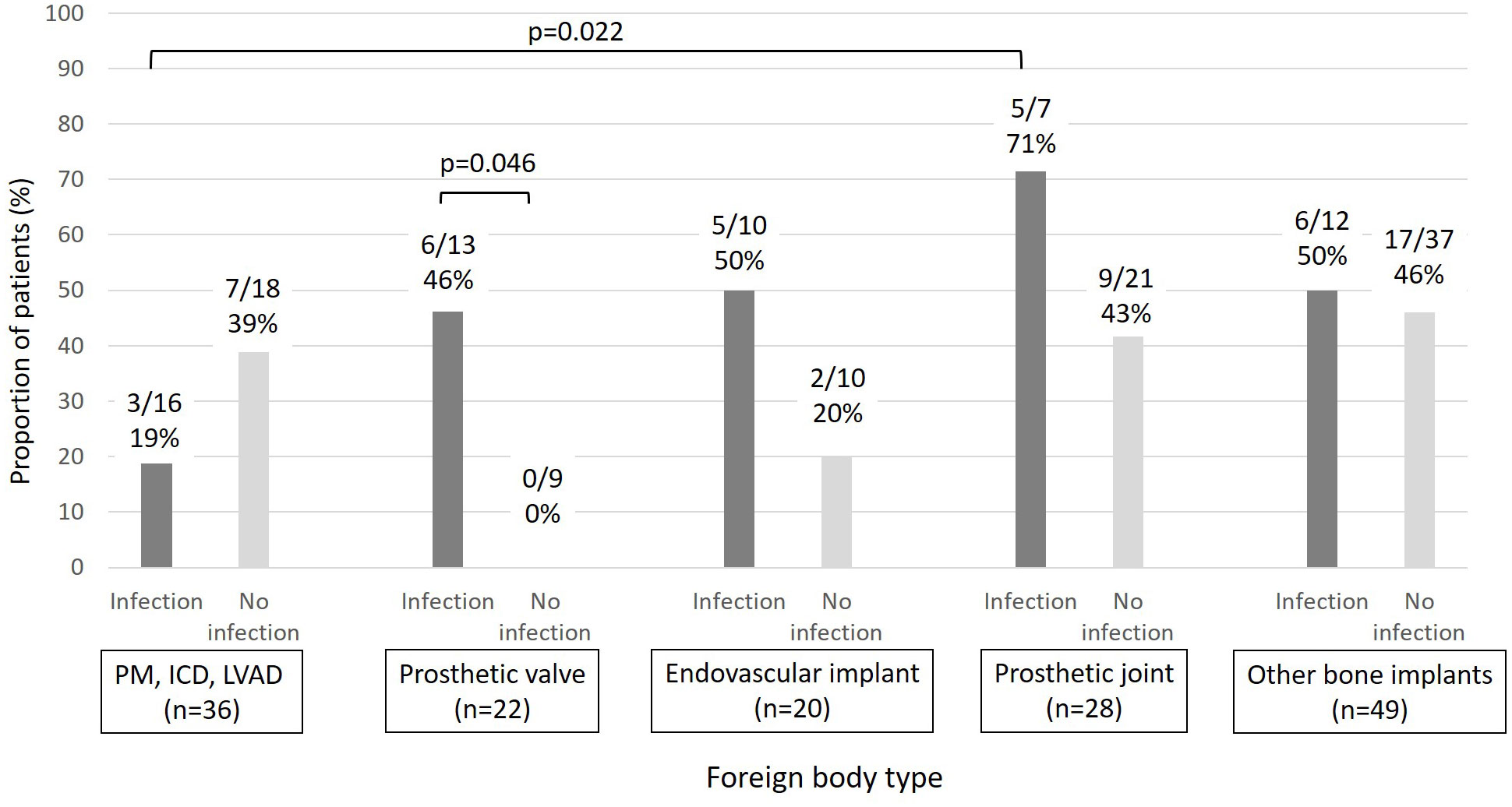
Figure 3 Proportion of patients with biofilm-forming MRSA (moderate/strong) by foreign body type and foreign body infection (PM: pacemaker, ICD: implantable cardiac defibrillators, LVAD: Left ventricular assistance devices).
4 Discussion
We studied clinical and microbiologic risk factors for foreign body infections complicating MRSA BSI in a cohort of 124 adult patients with a foreign body in situ at the time of BSI diagnosis. One-third of all 423 patients with a MRSA BSI had a foreign body, and 43% of these 124 subjects had a foreign body infection complicating the BSI. Patients with bone implants were rarely infected. In vitro level of biofilm formation by MRSA isolates was not associated with risk of foreign body infection, but high-level biofilm formation was more common in patients with a history of MRSA infection and MRSA ST8 infection.
Many studies have analyzed foreign body infection in patients with MRSA infection and the presence of at least one foreign body at the time of BSI (19–27). However few studies have analyzed the role of biofilm and genetic characteristics of MRSA in this population. Interestingly, in our study neither comorbidities nor demographic characteristics were associated with risk of foreign body infection. These results contrast with previously identified risk factors for metastatic infection in MRSA BSI, probably because only patients with a foreign body in situ were included in this study. In previous studies, CA-MRSA was found to be associated with metastatic infection and persistent bacteremia, as we found (19, 28). Moreover, persistence of positive blood cultures after initiation of antimicrobial therapy was identified as a risk factor for complicated bacteremia, cardiac device infection, and infective endocarditis, but also 90 day-mortality (19, 25, 29, 30).
The pathogenicity of S. aureus in foreign body infection is based on its ability to adhere, colonize, and persist on a foreign body. The impact of S. aureus biofilm on the clinical characteristics and outcome of foreign body infection has little been studied. Previous studies showed contradictory results on an association between in vitro biofilm production and clinical outcome. Alonso et al. analyzed patients with S. aureus bacteremia and infective endocarditis, catheter-related bacteremia or non-device associated bacteremia. No association was found between biofilm production (using a crystal violet assay), biofilm metabolic activity (using XTT solution) and the 3 different groups of infection (31). Mesrati et al. evaluated biofilm formation among S. aureus isolates responsible for foreign body infections. The foreign bodies included central venous catheters (n=24), indwelling urethral catheters (n=3), hemodialysis catheters (n=3), prosthetic joint (n=8), tracheal catheters (n=1), and nasogastric tubes (n=1). Among the 87 S. aureus isolates, 5 produced strong, 20 moderate and 35 weak biofilm. Biofilm production was statistically greater in the foreign body group compared with the non-foreign body group (80% vs. 60%; P=0.04). However, MRSA strains of S. aureus were reported in only 9/40 in the device group and 23/47 in the non-device group (32).
Although studies of in vitro biofilm production comparing S. aureus isolates from different types of infections have been contradictory, biofilm production has previously been associated with certain clonal lineages of S. aureus. Fernández-Hidalgo et al. analyzed 209 S. aureus strains causing infective endocarditis and found that strains belonging to CC5 and CC22 had greater biofilm formation, measured as significantly higher optical densities (1.369 [1.18] vs. 0.920 [0.93], p = 0.008). However, only 20% of isolates were MRSA, and CC5 was the most prevalent (22%) followed by CC30 (19%) and CC8 (11%). Similarly, Naicker et al. showed that in 30 genotypically varied strains isolated from blood culture, only 5 isolates were strong biofilm producers, and all belonged to one spa type clonal cluster (spa-CC 064) related to CC5 and CC8 (33).
The results of our study may be impacted by the molecular epidemiology of MRSA at the medical center studied. However, the distribution of STs in our study was similar to previous studies on MRSA infections in the U.S. MRSA ST5 and ST105 belonging to CC5 were reported to be the most prevalent CC in MRSA infection in the US (69%), followed by CC8 and CC30 (28). In our study, only one ST30 isolate was found. The specific ST8 MRSA clone USA300 commonly carries the PVL and the Arginine Catabolic Mobile Element (ACME). It is likely that most of the ST8 isolates were USA300. In MRSA BSI, the USA300 lineage has been associated with a higher risk of metastatic infection, emboli, and persistent bacteremia (28). USA300 has been shown to be an excellent biofilm former in two biofilm models. In an in vitro study of 76 MRSA isolates classified into 13 clones, static biofilm (microtiter plate) and dynamic biofilm with confocal laser-scanning and time-lapse microscopy were performed. In the two models, the USA300 clone formed more biofilm than others. In fact, strains carrying SCCmec type IV generally showed greater biofilm production than those carrying other SCCmec (I-III) types. Thus, both SCCmec type IV and ACME carriage seem to be associated with increased biofilm formation (34), although there is very likely confounding in the relationship between SCCmec type and ST and there may not be an independent association with SCCmec type. In any case, it is likely that differences in genotype are a critical factor in observed variation in biofilm formation.
Methods used to evaluate in vitro biofilm formation in S. aureus have differed among published studies, and this may have affected observed study results in the literature. Whereas Christensen et al. was one of first authors to describe the microtiter plate biofilm method in coagulase negative staphylococci, Stepanovic et al. reported a more precise protocol, and explained the variability of such different protocols (17, 35). The main elements of biofilm production assay protocols that Stepanovic et al. standardized and that we utilized were 1) a well-defined concentration of bacteria (0.5 McFarland) before filling the 96-well plate, 2) the use of tryptic soy broth (TSB) or brain-heart infusion (BHI) with supplementation of 1% glucose to increase biofilm formation, 3) rinsing the plate at least 3 times after bacterial culture, and 4) the method used to calculate the ODc, using the negative control (17). Furthermore, our experiment were performed by a single person, which limited inter-variability, especially when it came to rinsing the plate with the same pressure. Although we applied the protocol of Stepanovic et al., we did not demonstrate an association between MRSA biofilm formation and foreign body infection.
There are certain limitations to our study. First, we had a relatively small sample size and included different types of foreign bodies, with distinct pathophysiology of infection. Thus, we may not have had adequate power to demonstrate a significant association between level of biofilm formation and risk of foreign body infection. Future studies including only one type of foreign body in MRSA BSI patients may show different results. Second, S. aureus biofilm formation in foreign body infection may not be replicated by the microtiter plate biofilm assay. Study of biofilm formation in vitro or in vivo on a relevant tissue and/or a foreign body surface more similar to those implanted in patients may be warranted to better evaluate the role of biofilm in foreign body infection. Third, it is possible that there were BSI subjects in our study with a prosthetic joint and no local symptoms who had a missed diagnosis of a true PJI. However, our patients were followed up after BSI to avoid this misclassification. Moreover, the absence of clinical symptoms in patients with an orthopedic device and a S. aureus BSI usually rules out a PJI (23). In a study by Dufour et al., among patients with S. aureus BSI and a prosthetic joint 27/143 (19%) had at least one PJI, and for all PJI except one, diagnosis was made during the same hospitalization as MRSA BSI (20).
5 Conclusion
Most factors associated with foreign body infection in MRSA BSI were also characteristic of persistent infections. Patients with bone implants were rarely infected. Biofilm-forming isolates were not associated with a higher risk of foreign-body infection. However, high-level in vitro biofilm formation was associated with MRSA genetic lineage, especially ST8. Animal studies may be needed to confirm that in vitro biofilm formation corresponds to in vivo biofilm formation and is not simply a lineage-specific marker of MRSA in vitro.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: Sequence Read Archive (Bioproject PRJNA751847).
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the University of Pennsylvania. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study was exempt from the need for informed consent by the Institutional Review Board of the University of Pennsylvania.
Author contributions
KB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft. NJ: Investigation, Project administration, Writing – review & editing. MS: Investigation, Writing – review & editing. BT: Formal analysis, Writing – review & editing. TR: Formal analysis, Writing – review & editing. MD: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. KB is funded by ACAI (Association de Chimiothérapie Anti-Infectieuse) and by University Hospital of Besancon (France) with post-doctoral research grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank the French Association of Anti-Infective Chemotherapy (ACAI, association de chimiothérapie anti-infectieuse) and University Hospital of Besancon for providing personal funding for KB. We thank the Penn/CHOP Microbiome Center for sequencing of MRSA isolates.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The supplementary file contains a more detailed section of the method.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1335867/full#supplementary-material
References
1. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace. (2020) 22:515–49. doi: 10.1093/europace/euz246
2. Nemes S, Rolfson O, W-Dahl A, Garellick G, Sundberg M, Kärrholm J, et al. Historical view and future demand for knee arthroplasty in Sweden. Acta Orthopaedica. (2015), 426–31. doi: 10.3109/17453674.2015.1034608
3. Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR, et al. Impact of cardiac implantable electronic device infection: A clinical and economic analysis of the WRAP-IT trial. Circ Arrhythm Electrophysiol. (2020) 13:e008280. doi: 10.1161/CIRCEP.119.008280
4. Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ, Huddleston PM. Trends in the epidemiology of osteomyelitis. J Bone Joint Surg Am. (2015) 97:837–45. doi: 10.2106/JBJS.N.01350
5. Lemaignen A, Bernard L, Marmor S, Ferry T, Grammatico-Guillon L, Astagneau P. Epidemiology of complex bone and joint infections in France using a national registry: The CRIOAc network. J Infection. (2021) 82:199–206. doi: 10.1016/j.jinf.2020.12.010
6. Kuehl R, Tschudin-Sutter S, Morgenstern M, Dangel M, Egli A, Nowakowski A, et al. Time-dependent differences in management and microbiology of orthopaedic internal fixation-associated infections: an observational prospective study with 229 patients. Clin Microbiol Infection. (2018). doi: 10.1016/j.cmi.2018.03.040
7. Revest M, Camou F, Senneville E, Caillon J, Laurent F, Calvet B, et al. Medical treatment of prosthetic vascular graft infections: Review of the literature and proposals of a Working Group. Int J Antimicrob Agents. (2015) 46:254–65. doi: 10.1016/j.ijantimicag.2015.04.014
8. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2015) 36:2921–64. doi: 10.1093/eurheartj/ehv318
9. Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. (2015) 28:603–61. doi: 10.1128/CMR.00134-14
10. Figueiredo AMS, Ferreira FA, Beltrame CO, Côrtes MF. The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit Rev Microbiol. (2017) 43:602–20. doi: 10.1080/1040841X.2017.1282941
11. Ricciardi BF, Muthukrishnan G, Masters E, Ninomiya M, Lee CC, Schwarz EM. Staphylococcus aureus evasion of host immunity in the setting of prosthetic joint infection: biofilm and beyond. Curr Rev Musculoskelet Med. (2018) 11:389–400. doi: 10.1007/s12178-018-9501-4
12. Morrison MA, Hageman JC, Klevens RM. Case definition for community-associated methicillin-resistant Staphylococcus aureus. J Hosp Infect. (2006) 62:241. doi: 10.1016/j.jhin.2005.07.011
13. Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-international society for cardiovascular infectious diseases criteria for infective endocarditis: updating the modified duke criteria. Clin Infect Dis. (2023), ciad271. doi: 10.1093/cid/ciad271
14. Hannan MM, Husain S, Mattner F, Danziger-Isakov L, Drew RJ, Corey GR, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. (2011) 30:375–84. doi: 10.1016/j.healun.2011.01.717
15. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of Periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. (2018) 33:1309–14.e2. doi: 10.1016/j.arth.2018.02.078
16. Lyons OTA, Baguneid M, Barwick TD, Bell RE, Foster N, Homer-Vanniasinkam S, et al. Diagnosis of aortic graft infection: A case definition by the management of aortic graft infection collaboration (MAGIC). Eur J Vasc Endovascular Surg. (2016) 52:758–63. doi: 10.1016/j.ejvs.2016.09.007
17. Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. (2007) 115:891–9. doi: 10.1111/j.1600-0463.2007.apm_630.x
18. Petit RA, Read TD. Bactopia: a flexible pipeline for complete analysis of bacterial genomes. mSystems. (2020) 5:e00190–20. doi: 10.1128/mSystems.00190-20
19. Berge A, Carlsén C, Petropoulos A, Gadler F, Rasmussen M. Staphylococcus aureus bacteraemia, cardiac implantable electronic device, and the risk of endocarditis: a retrospective population-based cohort study. Eur J Clin Microbiol Infect Dis. (2023) 42:583–91. doi: 10.1007/s10096-023-04585-x
20. Dufour S, Piroth L, Chirouze C, Tattevin P, Becker A, Braquet P, et al. Staphylococcus aureus bloodstream infection in patients with prosthetic joints in the prospective VIRSTA cohort study: frequency and time of occurrence of periprosthetic joint infection. Open Forum Infect Dis. (2019) 6:ofz515. doi: 10.1093/ofid/ofz515
21. Honkanen M, Jämsen E, Karppelin M, Huttunen R, Eskelinen A, Syrjänen J. Periprosthetic joint infections as a consequence of Bacteremia. Open Forum Infect Dis. (2019) 6:ofz218. doi: 10.1093/ofid/ofz218
22. Kaasch AJ, Kern WV, Joost I, Hellmich M, Seifert H, Rieg S. Effect of clinically uninfected orthopedic implants and pacemakers/AICDs in low-risk Staphylococcus aureus bloodstream infection on crude mortality rate: A post hoc analysis of a large cohort study. Open Forum Infect Dis. (2019) 6:ofz170. doi: 10.1093/ofid/ofz170
23. Kouijzer IJE, Speijker LTD, Aarntzen EHJG, Rijnen WHC, Somford MP, Maat I, et al. Clinically unsuspected orthopedic implants during S. aureus bacteremia do not require additional diagnostic work-up. Infection. (2023) 51:743–7. doi: 10.1007/s15010-022-01913-9
24. Maskarinec SA, Thaden JT, Cyr DD, Ruffin F, Souli M, Fowler VG. The risk of cardiac device-related infection in bacteremic patients is species specific: results of a 12-year prospective cohort. Open Forum Infect Dis. (2017) 4:ofx132. doi: 10.1093/ofid/ofx132
25. Boix-Palop L, Dietl B, Calbo E, Di Marco A, Xercavins M, Pérez-Crespo PMM, et al. Risk of cardiac device-related infection in patients with late-onset bloodstream infection. Anal Natl Cohort. J Infect. (2022) 85:123–9. doi: 10.1016/j.jinf.2022.05.022
26. Joost I, Bothe W, Pausch C, Kaasch A, Lange B, Peyerl-Hoffmann G, et al. Staphylococcus aureus bloodstream infection in patients with ventricular assist devices-Management and outcome in a prospective bicenter cohort. J Infect. (2018) 77:30–7. doi: 10.1016/j.jinf.2018.05.002
27. Nakajima I, Narui R, Tokutake K, Norton CA, Stevenson WG, Richardson TD, et al. Staphylococcus bacteremia without evidence of cardiac implantable electronic device infection. Heart Rhythm. (2021) 18:752–9. doi: 10.1016/j.hrthm.2020.12.011
28. Souli M, Ruffin F, Choi S-H, Park LP, Gao S, Lent NC, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis. (2019) 69:1868–77. doi: 10.1093/cid/ciz112
29. Tubiana S, Duval X, Alla F, Selton-Suty C, Tattevin P, Delahaye F, et al. The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J Infection. (2016) 72:544–53. doi: 10.1016/j.jinf.2016.02.003
30. van der Vaart TW, Prins JM, Soetekouw R, van Twillert G, Veenstra J, Herpers BL, et al. All-cause and infection-related mortality in Staphylococcus aureus bacteremia, a multicenter prospective cohort study. Open Forum Infect Dis. (2022) 9:ofac653. doi: 10.1093/ofid/ofac653
31. Alonso B, Pérez-Granda MJ, Latorre MC, Sánchez-Carrillo C, Bouza E, Muñoz P, et al. Production of biofilm by Staphylococcus aureus: Association with infective endocarditis? Enferm Infecc Microbiol Clin (Engl Ed). (2022) 40:418–22. doi: 10.1016/j.eimce.2021.03.009
32. Mesrati I, Saidani M, Jemili M, Ferjeni S, Slim A, Boubaker IB-B. Virulence determinants, biofilm production and antimicrobial susceptibility in Staphylococcus aureus causing device-associated infections in a Tunisian hospital. Int J Antimicrob Agents. (2018) 52:922–9. doi: 10.1016/j.ijantimicag.2018.05.004
33. Naicker PR, Karayem K, Hoek KGP, Harvey J, Wasserman E. Biofilm formation in invasive Staphylococcus aureus isolates is associated with the clonal lineage. Microb Pathog. (2016) 90:41–9. doi: 10.1016/j.micpath.2015.10.023
34. Vanhommerig E, Moons P, Pirici D, Lammens C, Hernalsteens J-P, De Greve H, et al. Comparison of biofilm formation between major clonal lineages of methicillin resistant Staphylococcus aureus. PLoS One. (2014) 9:e104561. doi: 10.1371/journal.pone.0104561
35. Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. (1985) 22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985
Keywords: Staphylococcus aureus, biofilm, foreign bodies, methicillin resistance, bacteremia
Citation: Bouiller K, Jacko NF, Shumaker MJ, Talbot BM, Read TD and David MZ (2024) Factors associated with foreign body infection in methicillin-resistant Staphylococcus aureus bacteremia. Front. Immunol. 15:1335867. doi: 10.3389/fimmu.2024.1335867
Received: 17 November 2023; Accepted: 05 February 2024;
Published: 16 February 2024.
Edited by:
Rachel McLoughlin, Trinity College Dublin, IrelandReviewed by:
Ata Nevzat Yalcin, Akdeniz University, TurkeySrishtee Arora, Texas A and M University, United States
Copyright © 2024 Bouiller, Jacko, Shumaker, Talbot, Read and David. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Z. David, bWljaGRhdkBwZW5ubWVkaWNpbmUudXBlbm4uZWR1
 Kevin Bouiller
Kevin Bouiller Natasia F. Jacko1
Natasia F. Jacko1 Brooke M. Talbot
Brooke M. Talbot Timothy D. Read
Timothy D. Read Michael Z. David
Michael Z. David