
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 07 March 2024
Sec. Molecular Innate Immunity
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1335519
This article is part of the Research Topic Unraveling circRNAs and miRNAs: Key Regulators in Immune-Related Diseases View all 5 articles
 Jia-Rui You1,2†
Jia-Rui You1,2† Zeng-Jin Wen1,2†
Zeng-Jin Wen1,2† Jia-Wei Tian3
Jia-Wei Tian3 Xiao-Bing Lv1
Xiao-Bing Lv1 Rong Li1
Rong Li1 Shu-Ping Li4
Shu-Ping Li4 Hui Xin1
Hui Xin1 Pei-Feng Li2*
Pei-Feng Li2* Yin-Feng Zhang2*‡
Yin-Feng Zhang2*‡ Rui Zhang1*
Rui Zhang1*Cardiovascular diseases (CVDs) are multifactorial chronic diseases and have the highest rates of morbidity and mortality worldwide. The ubiquitin–proteasome system (UPS) plays a crucial role in posttranslational modification and quality control of proteins, maintaining intracellular homeostasis via degradation of misfolded, short-lived, or nonfunctional regulatory proteins. Noncoding RNAs (ncRNAs, such as microRNAs, long noncoding RNAs, circular RNAs and small interfering RNAs) serve as epigenetic factors and directly or indirectly participate in various physiological and pathological processes. NcRNAs that regulate ubiquitination or are regulated by the UPS are involved in the execution of target protein stability. The cross-linked relationship between the UPS, ncRNAs and CVDs has drawn researchers’ attention. Herein, we provide an update on recent developments and perspectives on how the crosstalk of the UPS and ncRNAs affects the pathological mechanisms of CVDs, particularly myocardial ischemia/reperfusion injury, myocardial infarction, cardiomyopathy, heart failure, atherosclerosis, hypertension, and ischemic stroke. In addition, we further envision that RNA interference or ncRNA mimics or inhibitors targeting the UPS can potentially be used as therapeutic tools and strategies.
Cardiovascular diseases (CVDs) are a wide spectrum of disorders affecting hearts or vessels, including myocardial ischemia/reperfusion (I/R) injury, myocardial infarction (MI), cardiomyopathy, myocarditis, heart failure (HF), atherosclerosis, hypertension (HT), and ischemic stroke (1–4). Myocardial ischemia and MI can induce excessive ventricular remodeling and the development of cardiomyocyte hypertrophy, leading to HF (2, 3, 5). Myocarditis is an inflammatory disease that damages the tissue of the heart muscle, and the main reasons are autoimmune response, viral infections, or immune reactions after infection (5). In addition, vascular diseases are progressive inflammatory pathologies, that can result in endothelial injury, atherosclerotic plaque formation, and HT, especially pulmonary arterial hypertension (PAH) (6).
The ubiquitin–proteasome system (UPS) is the main nonlysosomal pathway for targeted protein degradation in eukaryotic cells (7–9). The UPS consists of three components: a ubiquitination mechanism (ubiquitin, E1, E2 and E3 enzymes), deubiquitinating enzymes and the 26S proteasome (10). First, E1 activates the C-terminal glycine residues of ubiquitin by using ATP, and then activated ubiquitin is transferred to E2 by a high-energy thioester bond linking the C-terminal carboxyl group of ubiquitin to the active cysteine in E2. Third, E3 catalyzes the covalent attachment of ubiquitin to the lysine side chain of the substrate protein (11, 12). Subsequently, substrate proteins modified by polyubiquitin chains are degraded via the 26S proteasome. The 26S proteasome consists of two 19S regulatory complexes and one 20S complex (13, 14). E3 ubiquitin ligases regulate various aspects of eukaryotic biology by promoting ubiquitination degradation of target proteins (15). For example, smad ubiquitin regulatory factors (Smurfs) come from the HECT family of E3 ubiquitin ligases and consist of two members, Smurf1 and Smurf2 (16). Additionally, ubiquitination is invertible, and deubiquitylation can remove ubiquitin from specific substrate proteins by deubiquitinating enzymes, which maintains ubiquitin system homeostasis (10, 17, 18). The ubiquitin-specific protease (USP) family, which includes USP3, USP7, USP14, and USP28, is one of the major deubiquitinating enzymes (19). Encouragingly, both ubiquitination and deubiquitinating enzymes, such as Smurf1, Smurf2, carboxyl terminus Hsp70-interacting protein (CHIP), Nedd4, WWP2, and YOD1, are involved in the regulation of cardiovascular function and may allow for new targeted therapeutic opportunities (7). Notably, the UPS plays an essential role in many aspects of biological processes, such as cell cycle control, cell differentiation and survival, protein turnover, protein quality control and signal transduction, some of which involve ncRNAs (7–9).
Noncoding RNAs (ncRNAs), such as microRNAs (miRNAs), long noncoding RNAs (lncRNAs), circular RNAs (circRNAs) and small interfering RNAs (siRNAs) are an increasing spectrum of transcriptional regulatory factors with no coding potential but significant structural and regulatory functions (20–22). MiRNAs are single-stranded RNAs consisting of 20-22 nucleotides, and their primary function is to combine complementary sequences on mRNAs to repress their translation (5). LncRNAs are a class of ncRNAs that are more than 200 nucleotides in length and can regulate the expression of specific miRNAs and consequently modulate miRNA downstream target genes (23, 24). Long intergenic noncoding RNAs (lincRNAs) function as essential regulatory factors of gene expression (25). CircRNAs are stable single-stranded ncRNAs with closed-loop structures with miRNA sponges that interact with RNA-binding proteins as the main molecular mechanism (26, 27). Moreover, siRNAs mediated by RNA interference (RNAi) are significantly versatile tools to target corresponding mRNA and silence protein-coding genes and have been used clinically for tissue targeting based on complex chemical modifications and carrier/ligand coupling (28). One of the roles of ncRNAs is the precise control of protein stability via UPS.
There are emerging reviews about the interaction of ncRNAs and UPS on cancer, especially non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, and gastric cancer (29–31), while implications of protein ubiquitination modulated by ncRNAs in cardiovascular aspects have not been systematically elaborated and comprehensively summarized although multiple studies have demonstrated that ncRNAs have a profound impact on the genetic and proteinic programming of CVDs through their interaction with the UPS (32). Fine-regulation between ncRNAs and the UPS is critical in the epigenetics of CVDs. Overall, this review paper emphasizes the mechanisms of ubiquitination in the context of the widespread association of ncRNAs with CVDs and attempts to elucidate the perturbations of the crosstalk between the UPS, especially ubiquitin ligases, and ncRNAs in CVDs. Overall, we provide the latest advances and look forward to future cardiovascular treatment strategies targeting ncRNAs and ubiquitin ligases.
In recent years, multiple crosstalk between ncRNAs and the UPS has been demonstrated to play essential regulatory roles in various diseases, particularly CVDs (Figure 1) (33). First, the positive or negative regulation of miRNA on ubiquitination has gradually attracted considerable research attention. (1) MiRNAs directly inhibit E3 ubiquitin ligase expression and subsequently suppress ubiquitination and degradation of target proteins. (2) MiRNAs directly inhibit ubiquitin-conjugating enzyme (E2) expression. (3) MiRNAs directly inhibit the expression of deubiquitinating enzymes and disrupt the ubiquitination balance of deubiquitinating enzymes and target proteins. (4) MiRNAs regulate multiple UPS components. (5) MiRNAs target some proteins that are involved in the regulation of E3 ubiquitin ligases.
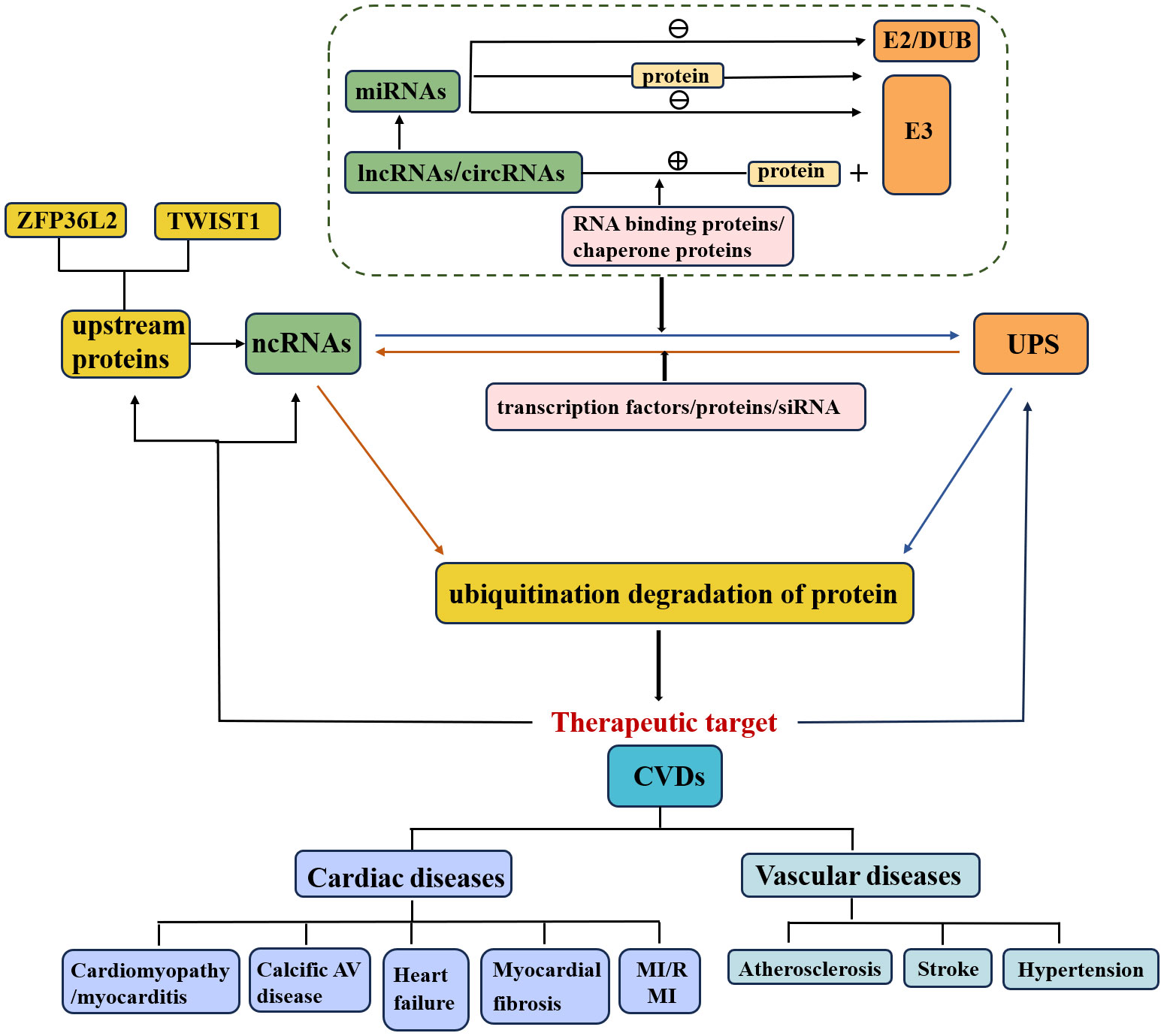
Figure 1 Relationship of ncRNAs, UPS and CVDs. The crosstalk of the UPS and ncRNAs affects the pathological mechanisms of CVDs. NcRNAs that regulate ubiquitination or are regulated by the UPS are involved in the execution of target protein stability.
Furthermore, the study of upstream regulatory pathways of miRNAs, such as lncRNAs and circRNAs, is also a meaningful research direction (29, 31). (1) LncRNAs/circRNAs promote the ubiquitination and degradation of target proteins. a) LncRNAs/circRNAs interact with target proteins and promote their ubiquitination and degradation. b) LncRNAs/circRNAs enhance the interaction between the E3 ubiquitin ligase and its target. c) LncRNAs combine with RNA binding proteins or chaperone proteins that are involved in the interaction between the E3 ubiquitin ligase and target protein (20). (2) LncRNAs inhibit the ubiquitination and degradation of target proteins. (3) LncRNAs/circRNAs act as competitive endogenous RNAs (ceRNAs) for miRNAs and thus regulate the expression of E3 ubiquitin ligases/deubiquitinating enzymes.
In addition to being regulated by ncRNAs, the UPS can also regulate ncRNA levels. On the one hand, E3 ubiquitin ligases directly regulate ncRNAs (Figure 2), thereby promoting ubiquitination and degradation of target proteins. On the other hand, the UPS indirectly regulates ncRNAs through the involvement of transcription factors/proteins. Specifically, knockdown of the E3 ubiquitin ligase by siRNA has been indicated to be involved in the pathology of CVDs via regulation of downstream protein levels and could serve as a potential therapeutic strategy.
Ischemic conditions during myocardial I/R cause anoxic damage to cardiomyocytes, reducing mitochondrial ATP synthesis via oxidative phosphorylation, altering cell membrane permeability, and finally inducing apoptosis; therefore, timely restoration of blood flow is abstractly crucial for alleviating acute MI (34). Untimely reperfusion can produce further damage and exacerbate cardiac dysfunction, which is referred to as myocardial I/R injury, anoxia/reoxygenation (A/R) injury or hypoxia/reoxygenation (H/R) injury (34, 35).
Some miRNAs, particularly miRNA-424(322) and miR-378a-3p, were shown to be significantly decreased in myocardial I/R mice or rats, while overexpression of these miRNAs improved myocardial I/R-induced apoptosis and injury by inhibiting ubiquitin ligase (FBXW7, Smurf2 and TRIM55) expression and downstream protein degradation (Figure 3). In detail, Chen, Dong et al. (36) found that enhanced expression of miR322 prevented reperfusion-induced cardiomyocyte apoptosis by reducing the expression of the ubiquitin ligase FBXW7 and increasing N1-ICD protein in the hearts of mice in vivo. Similarly, miR-322/503 directly binds to and inhibits the translation of Smurf2 (an E3 ubiquitin ligase), thereby suppressing Smurf2 ubiquitination-mediated EZH2 degradation and activating the Akt/GSK3β pathway, ultimately reducing myocardial apoptosis and infarct size in the I/R rat model in vivo and in vitro (37). In addition, miR-378a-3p suppresses ubiquitinated degradation of DUSP1 by decreasing expression of the E3 ubiquitin ligase TRIM55 and then inactivates JNK1/2, eventually preventing myocardial I/R-related apoptosis and injury in rat myocardial I/R models in vivo (38). Conversely, miR-379 could facilitate cardiomyocyte apoptosis by inhibiting Smurf1 expression; meaningfully, exogenous Smurf1 protein could rescue miR379-induced apoptosis in cardiomyocytes (39). Additionally, circUSP39 promotes cardiomyocyte apoptosis and accelerates H/R-induced injury by sponging miR-362-3p and upregulating TRAF3 expression (40).
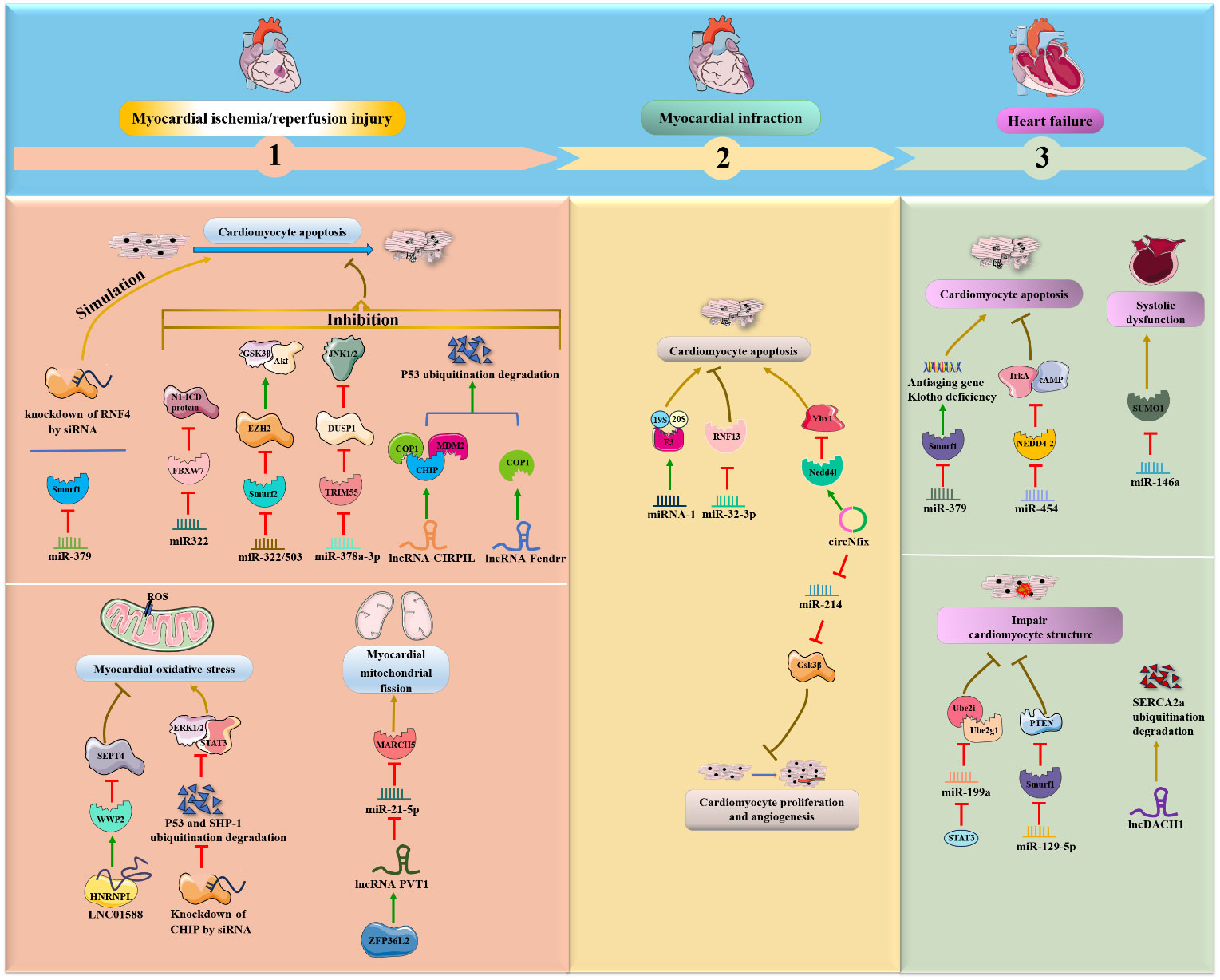
Figure 3 NcRNAs and ubiquitination in cardiac diseases. The crosstalk of ncRNAs and ubiquitination regulates cardiac diseases, such as MI/R, MI, myocardial. NcRNAs and ubiquitination are involved in cardiac diseases by regulating target protein expression.
Interestingly, lncRNAs are also emerging as an essential class of regulators in the pathology of myocardial I/R. Collectively, lncRNA-CIRPIL, lncRNA Fendrr and LINC01588 were shown to be downregulated in myocardial I/R mice or rats and to function as negative mediators of myocardial I/R-induced cardiomyocyte apoptosis by enhancing E3 ubiquitin ligases (COP1, CHIP, MDM2 and WWP2) in vivo. First, lncRNA-CIRPIL interacts with p53 and sequesters it in the cytoplasm, directly accelerating the ubiquitin-regulated degradation of p53 by upregulating the expression of E3 ubiquitin ligases COP1, CHIP and MDM2, thereby attenuating myocardial A/R-induced apoptosis and injury (41). Similar to lncRNA-CIRPIL, lncRNA Fendrr suppresses myocardial H/R-induced apoptosis by facilitating the ubiquitination degradation of p53 via COP1 (42). Moreover, LNC01588 can make direct contact with the HNRNPL protein and upregulate the E3 ubiquitin ligase WWP2, which then reduces the levels of downstream SEPT4, thereby ameliorating myocardial oxidative stress and MI during myocardial I/R damage (43). On the other hand, inhibition of p53 ubiquitination degradation and inhibition of WWP2 attenuate the protective effects of lncCIRPIL and LINC01588 (41, 43). Conversely, the ZFP36L2- lncRNA PVT1-miR-21-5p-MARCH5 axis positively affects the fission and fusion of mitochondria and cardiomyocyte apoptosis following myocardial I/R. Specifically, lncRNA PVT1 can reduce miR-21-5p expression by directly binding to and being positively regulated by zinc finger protein 36-like 2 (ZFP36L2), which then elevates the E3 ubiquitin ligase MARCH5, exerting a deteriorating effect on myocardial mitochondrial fission and myocardial I/R injury in vivo and in vitro (44).
In addition to myocardial I/R-induced apoptosis, siRNA and E3 ubiquitin ligase were also associated with ischemia and cardiotoxic-induced apoptosis. Furthermore, Qiu et al. (45) revealed that knockdown of the E3 ubiquitin ligase RNF4 by siRNA could exacerbate cardiac oxidative stress-induced apoptosis and ischemia-induced injury by upregulating the PML nuclear body and promoting p53 activity in mouse models in vivo. Moreover, the knockdown of the E3 ubiquitin ligase CHIP by siRNA promoted doxorubicin (DOX)-induced cardiac oxidative stress and injury in the mouse heart in vivo (46). Further investigations have illustrated that CHIP can ameliorate cardiac toxicity by facilitating ubiquitin-mediated degradation of SHP-1 and p53 and activating the STAT3 and ERK1/2 pathways.
In addition to ischemia- or I/R-induced cardiomyocyte apoptosis, miRNAs and circRNAs are also involved in the progression of MI through interactions with the UPS. It was demonstrated that miRNA-1 was overexpressed in male mice in vivo and in vitro during MI (47). Mechanistically, miRNA-1 extensively induces the expression of UPS components, such as 19S, 20S and ubiquitin ligase E3, and accumulates ubiquitin-positive proteins, ultimately exacerbating cardiac apoptosis and remodeling in cardiomyocytes. In contrast, suppression of miR-32-3p enhances coronary atherosclerotic plaque instability, promotes endoplasmic reticulum stress-regulated cardiomyocyte apoptosis, and aggravates acute MI by increasing ubiquitin ligase RNF13 expression in rats in vivo (48). Moreover, circNfix was validated as a proapoptotic factor for MI and inhibited cardiomyocyte proliferation and angiogenesis in rats and mice in vivo (49). Regarding the regulatory mechanism, circNfix enhances the ubiquitinated degradation of Ybx1 via the E3 ubiquitin ligase Nedd4l, and circNfix also sponges miR-214 and facilitates the expression of Gsk3β, a downstream effector associated with cardiac angiogenesis.
Cardiomyopathy can be classified as primary (genetic) and secondary, resulting in different phenotypes, including dilated, hypertrophic, diabetes and age-related cardiomyopathy (50, 51). Dilated cardiomyopathy is typically characterized by left ventricle dilation, which reduces systolic function and appears as an HF symptom (32, 52). Diabetic cardiomyopathy represents a metabolic disease associated with independent risk factors such as hyperinsulinemia, hyperglycemia and insulin resistance (53). Moreover, hypertrophic cardiomyopathy is a heterogeneous genetic disease resulting in ventricular hypertrophy, hypercontractility and fibrosis (54), while cardiac hypertrophy is an acquired compensatory reaction to both physiological and pathological overload (55).
It was verified that the miR-199/214 cluster was downregulated during dilated cardiomyopathy and that this downregulation exacerbated the loss of heart mass via the activation of the UPS, including ubiquitin E2-ligases Ube2i and Ube2g1 and E3-ligases MuRF-1 and MAFbx, in vitro (56). Notably, miR-199/214 cluster expression was positively regulated by the transcription factor TWIST1, which might be used as a preregulator for miR-199/214/UPS-involved dilated cardiomyopathy. In addition, miR‐195‐5p was speculated to inhibit SGK1 and hERG protein expression by enhancing the activity of the E3 ubiquitin ligase Nedd4‐2 during high-glucose induced cardiomyopathy in rat cardiomyocytes in vitro (57).
Moreover, miR-485-5p and lncRNAs (NONRATT054243 and NONRATT057160) have been shown to relieve myocardial apoptosis and cardiac hypertrophy through different types of ubiquitination (58, 59). Specifically, miR-485-5p can suppress mitochondrial fission and phenylephrine (PE)-induced cardiac hypertrophy by decreasing the expression of the SUMO E3 ligase MAPL and thus elevating the level of Mfn2 in an in vivo mouse model (58). In addition, the E3 ubiquitin ligase Nrdp1, which positively regulates the two core lncRNAs, NONRATT054243 and NONRATT057160, was also revealed to mitigate Ang II-induced cardiac hypertrophy by reducing downstream PIK3R3 expression and AKT phosphorylation (59). Conversely, UBE3A knockdown by siRNAs exacerbated isoproterenol-induced cardiac hypertrophy by activating the TLR4/MMP-9 pathway (60).
In addition to dilated cardiomyopathy, high glucose-induced cardiomyopathy and cardiac hypertrophy, ncRNA (especially miRNA) and the UPS are associated with septic and viral myocarditis. Remarkably, Gong, Li et al. (61) found that transcription factor sex-determining region Y (SRY)-Box 9 (SOX9) negatively regulated miR-96-5p and was significantly increased in sepsis-induced myocarditis in mice in vivo. USP7 deubiquitination modification upregulated SOX9, inhibited miR-96-5p expression and facilitated NLRP3 expression, myocardial pyroptosis and injury exacerbation. Furthermore, overexpression of miR-30a facilitates coxsackievirus B3 (CVB3) replication and induces viral myocarditis by suppressing the expression of the E3 ubiquitin ligase TRIM25, inhibiting RIG-I ubiquitination and thus reducing IFN-β activation (62). Additionally, in CVB3-infected cardiomyocytes in vivo and in vitro, upregulated miR-21 impairs cell-cell junctions via disruption of desmosome and fascial adhesion by reducing the deubiquitinating enzyme YOD1 and enhancing K48-linked polyubiquitination-mediated desmin degradation and subsequently impairing cell-cell junctions (63).
HF is a clinical syndrome that results from long-term cardiac dysfunction, cardiomyocyte hypertrophy and structural remodeling caused by hypoxia, ischemia, metabolic disorders, etc (2, 32).
The following four ncRNAs, miR-379 and miR-199a, miR-146a and lncDACH1 were shown to be upregulated during HF and to aggravate HF via inhibition of the E3 ubiquitin protein Smurf1, ubiquitin-binding enzymes Ube2i and Ube2g1, and SUMO1 and upregulation of Smurf1 in vivo and in vitro (39, 64–66). Chen’s groups suggested that miR-379 could facilitate cardiomyocyte apoptosis and possibly cause HF due to deficiency of the antiaging gene Klotho by inhibiting Smurf1 expression. Interestingly, exogenous Smurf1 protein could rescue miR379-induced apoptosis in cardiomyocytes (39). Likewise, miR-199a can be negatively regulated by signal transducer and activator of transcription 3 (STAT3), and its overexpression inhibits the expression of Ube2i and Ube2g1, thereby leading to deterioration of cardiomyocyte ultrastructure and HF (64). Specifically, miR-146a is increased in extracellular vesicles derived from failing hearts. The key regulatory pathway involves extracellular vesicle-associated miR-146a transfer into cardiomyocytes from fibroblasts and suppression of SUMO1 expression, thereby impairing cardiac contractile function and aggravating HF (65). Moreover, lncDACH1 was demonstrated to decrease cell shortening and calcium transients, impair myocardial function and consequently lead to HF by directly promoting Smurf1-induced ubiquitination degradation of sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) in mouse hearts (66).
In contrast, Wang et al. (67) observed that miR-454 was significantly decreased in HF rat models in vivo and oxidative stress-damaged cardiomyocyte H9c2 cells in vitro. Specifically, miR-454 can suppress cardiomyocyte apoptosis by inhibiting TrkA ubiquitination and degradation and activating the cAMP signaling pathway by negatively targeting the ubiquitin E3 ligase NEDD4-2. Similarly, downregulation of miR-129-5p impairs myocardial structure and function during chronic HF by promoting Smurf1 expression and then accelerating ubiquitination degradation of PTEN in chronic HF rat models (68).
In addition to MI, cardiomyopathy and HF, several research groups have demonstrated a combined role for ncRNAs and ubiquitination in cardiac diseases such as heart aging, myocardial fibrosis, and calcified aortic valve (AV) disease.
In mouse models in vivo, lncRNA LOC105378097 suppressed cardiomyocyte mitophagy and induced heart aging by promoting Parkin ubiquitination and reducing Parkin protein stability (69) (Figure 4). CircHIPK3 attenuates heart aging by promoting the binding of E3 ubiquitin ligase β-TrCP to HuR, enhancing ubiquitination and degradation of HuR and ultimately decreasing the activity of p21 in mice in vivo and in vitro (70). Additionally, it was verified that circ-sh3rf3 inhibited fibroblast-to-myofibroblast differentiation and consequently alleviated myocardial fibrosis by directly enhancing GATA-4 ubiquitination degradation via the E3 ubiquitin–protein ligase sh3rf3 and then abolishing GATA-4 inhibition of miR-29a expression (71). Furthermore, loss of miR-483 contributes to endothelial inflammation and consequently calcific AV disease and exerts deteriorating effects by increasing the expression of Ube2c, enhancing ubiquitination-induced pVHL degradation and activating the HIF-1α pathway (72).
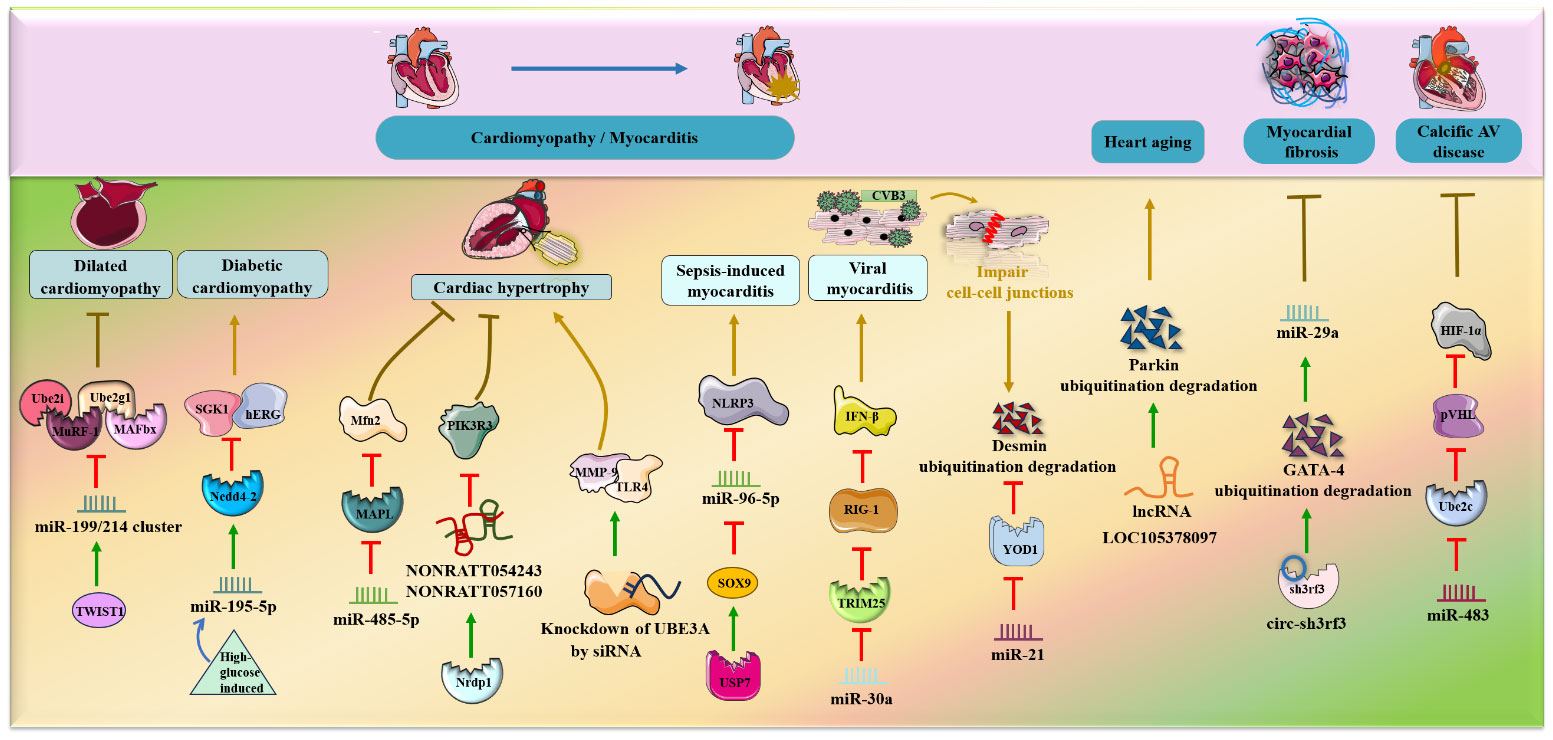
Figure 4 NcRNAs and ubiquitination in other cardiac diseases. The crosstalk of ncRNAs and ubiquitination regulates cardiac diseases, such as hypertrophy, myocarditis, heart failure, heart aging, myocardial fibrosis and calcific AV disease. NcRNAs and ubiquitination are involved in cardiac diseases by regulating target protein expression.
Atherosclerosis is a chronic inflammatory disease of the blood vessel wall characterized by maladaptive endothelial responses, VSMC structural changes, and macrophage lipid deposition (3, 5, 28). During atherosclerosis development, the major pathological processes are endothelial cell inflammation injury and overgrowth and migration of human VSMCs, in which ubiquitination and ncRNAs are implicated.
The lncRNA NEAT1 and miR-19b mentioned below were shown to aggravate endothelial cell injury and induce an inflammatory response via upregulation of the expression of the deubiquitinating enzyme BRCC3 and inhibition of the expression of the deubiquitinating enzyme CYLD (73, 74) (Figure 5). In detail, Yao, Song et al. (73) elucidated that lncRNA NEAT1, as a powerful miR-204 sponge, promoted BRCC3 expression in human umbilical vein endothelial cells (HUVECs) and significantly aggravated H/R-mediated NLRP3 inflammasome-activated endothelial cell injury and pyroptosis. In addition, miR-19b exerted a proinflammatory effect on vascular endothelial cells by reducing the expression of CYLD and further enhancing the accumulation of TRAF6 in spontaneously hypertensive rats in vivo (74).
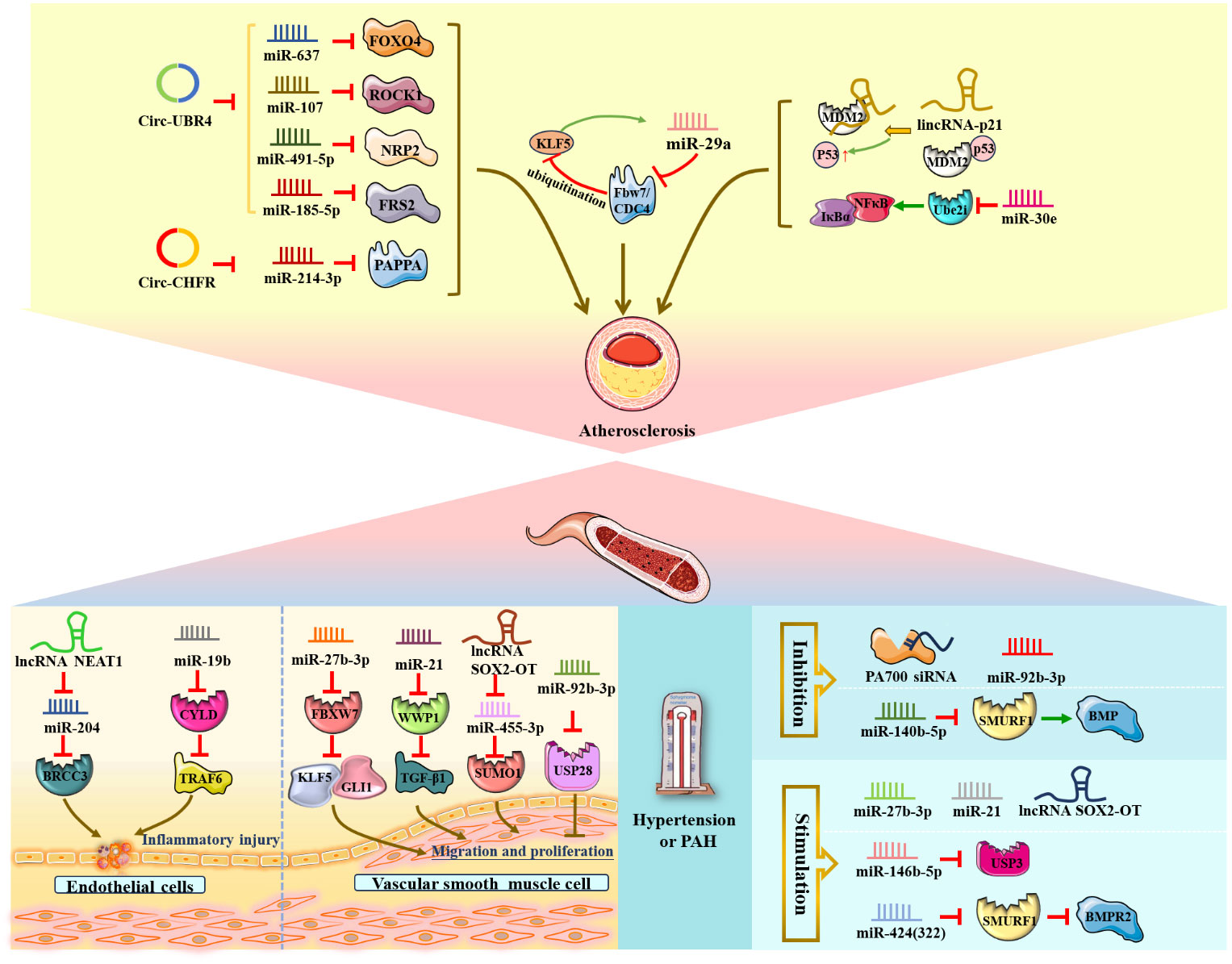
Figure 5 NcRNAs and ubiquitination in vascular diseases. The crosstalk of ncRNAs and ubiquitination regulates vascular diseases, such as endothelial cell inflammatory injury, VSMC proliferation and migration, atherosclerosis, stroke, and hypertension. NcRNAs and ubiquitination are involved in vascular diseases by regulating target protein expression.
Additionally, circular RNA ubiquitin protein ligase E3 component N-recognin 4 (circ-UBR4), circRNA E3 ubiquitin-protein ligase (circ-CHFR) and circRNA ubiquitin-specific peptidase 36 (circ_USP36) were shown to be upregulated in ox-LDL-induced human VSMCs. Circ-UBR4 sponges miR-637 and reduces the expression of miR-637, thereby promoting FOXO4 production (75, 76). Similarly, circ-UBR4 also promotes human VSMC proliferation by sponging miR-107, miR-491-5p and miR-185-5p and then increasing the expression of ROCK1, NRP2 and FRS2 (77–79). Moreover, circ-CHFR could contribute to human VSMC growth and migration as well as atherosclerosis deterioration via the circ-CHFR/miR-214-3p/PAPPA axis (80). Circ_USP36 exacerbates ox-LDL-induced human endothelial cell dysfunction via circ_USP36/miR-197-3p/ROBO1 axis (81). Notably, Zheng et al. (82) illustrated that miR-29a, Fbw7/CDC4 and KLF5 form a regulatory crosstalk and positive feedback loop that promotes the development of atherosclerosis. Mechanistically, miR-29a could be regulated by KLF5 to reduce Fbw7/CDC4 expression and subsequently enhance the stability of KLF5 by decreasing Fbw7/CDC4-dependent ubiquitination.
Conversely, Zong et al. (83) verified that miR-30e could exert an inhibitory effect on the proliferation and migration of human VSMCs and act as an antiatherosclerotic agent by negatively targeting Ube2i and reducing the IκBα/NFκB signaling pathway in rats in vivo. Similarly, lincRNA-p21 functioned as a negative regulator of VSMC proliferation during atherosclerosis and induced VSMC apoptosis by directly binding to the E3 ubiquitin ligase MDM2, decreasing MDM2-p53 interactions and ubiquitination degradation, ultimately promoting p53 activity in mice in vivo and in vitro (84).
Ischemic stroke is a typical clinical condition of cerebrovascular disease due to neuronal damage and destruction of supporting structures caused by cerebrovascular embolism and cerebral infarction (85).
Several research groups have studied and confirmed the role of miRNAs, circRNAs and lncRNAs in ischemic stroke (Figure 6). For example, miR-124 participated in poststroke neurovascular remodeling by directly downregulating the expression of the deubiquitinating enzyme USP14 and reducing REST levels. MiR−129−5p could improve poststroke neurovascular injury via inhibition of the E3 ubiquitin ligase SIAH1 and activation of the mTOR signaling pathway (86–88). Additionally, circDLGAP4 expression was significantly downregulated in both stroke mouse models and plasma from ischemic stroke patients, but overexpression of circDLGAP4 sponged miR-143 and reduced E3 ubiquitin ligase HECTD1 expression, thereby ameliorating poststroke neurovascular injury and reducing infarct size (89). Zhuang, Chen et al. found that circUCK2 suppresses endothelial-mesenchymal transition and protects the blood-brain barrier in ischemic stroke by interacting with FUS to increase the expression of the E3 ubiquitin ligase HECTD1 in mice in vivo and in vitro (90). Moreover, circSCMH1 promotes translocation of obesity-associated protein into the endothelial cell nucleus via ubiquitination and subsequently facilitates m6A demethylation of Plpp3 mRNA and upregulates Plpp3 expression, thereby enhancing vascular repair after stroke in mice in vivo and in vitro (91). LncRNA SNHG15 suppressed K63-linked TRAF2 ubiquitination to improve acute ischemic stroke (92). Notably, in mouse ischemic stroke models (middle cerebral artery occlusion) in vivo, miR-181b expression is downregulated in response to ischemic exposure, thus ameliorating poststroke neurovascular injury by increasing ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL1) and HSPA5 expression (93). However, circRNA HECT domain E3 ubiquitin-protein ligase 1 (circ_HECTD1) could aggravate poststroke neurovascular injury via the circ_HECTD1/miR-27a-3p/FSTL1 axis and circ_HECTD1/miR-133b/TRAF3 axis (94, 95). Furthermore, overexpression of lncRNA MIAT and lncRNA-Fendrr reduced ubiquitination and degradation of REDD1 and EGLN2, NLRC4, lncRNA SNHG3 and lncRNA NEAT1 reduced ubiquitination of HDAC3 and EGR1, and lncRNA-Nespas silencing promoted TAK1 polyubiquitination, eventually aggravating cerebral I/R injury and acute ischemic stroke (96–101).
Therapeutically, lithium improved neurovascular remodeling by upregulating miR-124 expression, decreasing REST abundance and inhibiting deubiquitination of peri-infarct brain tissues and proteins on the 4th day post-stroke (102). Inhibition of FBXO3 by siRNA alleviates cerebral I/R injury by suppressing ubiquitination and degradation of HIPK2 via the UPS (103), while TGR5 siRNA reduces the inhibitory effect of the E3 ubiquitin ligase Pellino3 on caspase-8 and NLRP3, thereby exacerbating cerebrovascular stroke (104, 105).
Presented as an increase in localized pulmonary arterial blood pressure, PAH is characterized by endothelial cell dysfunction, VSMC proliferation, vascular remodeling, and right ventricular overload (106, 107).
VSMCs form the middle layer of arteries and have regulatory functions in the vasculature, such as regulating blood flow, blood pressure and vascular tension (108). Accumulating evidence has illustrated that several miRNAs and lncRNAs are associated with the progression of PAH through the involvement of ubiquitination. Three ncRNAs, miR-27b-3p, miR-21 and lncRNA SOX2-OT, promoted the proliferation of pulmonary artery smooth muscle cells (PASMCs) via downregulation of FBXW7 and WWP1 and upregulation of SUMO1 (109–111). The researchers revealed that overexpression of miR-27b-3p could significantly elevate GLI1 expression through inhibition of ubiquitin ligase FBXW7 expression and accelerating KLF5 accumulation by attenuating its ubiquitination degradation in rats in vivo (109). Notably, miR-21 overexpression facilitated chronic hypoxia-induced PASMC proliferation and pulmonary vascular remodeling, and the possible mechanism was that miR-21 negatively regulated the E3 ubiquitin ligase WWP1 and then activated TGF-β1 signaling in mice in vivo and in vitro (110). Furthermore, Jiang, Hei, et al. (111) identified that increased lncRNA SOX2-OT promoted PASMC growth and migration by negatively reducing downstream miR-455-3p expression and elevating SUMO1 levels. In contrast, miR-92b-3P suppressed the proliferation of PASMCs under hypoxic conditions and improved PAH by inhibiting USP28 expression in vitro (112).
In addition, miR-140-5p expression was reduced in rat models in vivo and in PAH patients, and knockdown of miR-140-5p could facilitate the expression of the E3 ubiquitin ligase SMURF1 and activate BMP signaling, thereby promoting PASMC proliferation and pulmonary artery endothelial dysfunction and possibly exacerbating PAH (113). Conversely, miR-146-5p facilitated hypoxia-induced proliferation of pulmonary artery endothelial cells and PASMCs by negatively regulating USP3 (114). Specifically, Xu et al. (115) performed a control experiment—knockdown of the 26S proteasome regulatory subunit PA700 by siRNA and modifications of PA700 by tyrosine nitration in vitro—and the results indicated that PA700 nitration could promote the combination with the 20S proteasome, activate the 26S proteasome, and reduce GTPCH I and vascular protective enzyme BH4 expression, ultimately damaging endothelial cells in Ang II-induced hypertension. Notably, Baptista et al. discovered that miR-424(322) functioned as a messenger connecting PAH and right ventricle hypertrophy; mechanistically, overexpression of miR-424(322) suppressed SMURF1 expression and activated the BMPR2 pathway in a PAH rat model in vivo and in PAH patients, thereby inducing right ventricle overload (116).
Increasing evidence has revealed that ncRNAs are in different biofluids, which suggests that ncRNAs have the potential to be used as diagnostic indicators by detecting their dynamic expression (117, 118). Unlike sampling ncRNAs via invasive techniques such as biopsy, it is easy and inexpensive to detect ncRNAs from blood samples (119). However, the current obstacle is the lack of some ncRNAs with sensitivity and specificity (120).
LncRNA LOC105378097, circDLGAP4, miR-140-5p, miR-424(322), miR-146-5p, circ-UBR4 and miR-29a are considered diagnostic indicators of heart aging, ischemic stroke, PAH and atherosclerosis (Table 1). LncRNA LOC105378097 is upregulated in the serum of patients with heart aging, which indicates a high diagnostic value (69). Downregulated circDLGAP4 has potential diagnostic significance by sampling plasma from ischemic stroke patients (89). Moreover, reduced expression of miR-140-5p but enhanced miR-424(322) and miR-146-5p expression can be detected in whole blood samples from PAH patients, suggesting their diagnostic significance (113, 114, 116). Additionally, circ-UBR4 and miR-29a, as diagnostic biomarkers, are increased in serum from atherosclerosis patients (75, 77–79, 82).
Intriguingly, numerous clinical studies have shown that ncRNAs serve as diagnostic markers for CVDs. By using various screening approaches, such as single-cell sequencing and functional high-throughput screening, ncRNA molecules identified in body fluids can be validated in large cohorts of patient samples and used as novel biomarkers for CVDs (121). For example, miR-423-5p and lncRNA LIPCAR might be biomarkers for heart failure in patients’ plasma; miR-1, miR-133, miR-423, miR-208b, miR-499 and lncRNA UCA1, LIPCAR might be used as biomarkers for acute myocardial infarction; miR-155 and miR-17 might serve as biomarkers for coronary artery disease in blood or urine (28, 122, 123). Circ-STAT3 might be a predictor for stroke functional outcomes in 982 patients’ analysis for stroke recovery (124). However, ncRNA-based diagnostic strategies for CVDs are still in the experimental and preclinical stages due to ncRNA variability, analytical and technical factors, relevant isolation methods, cross-platform standardization and accuracy (28), therefore, ncRNA-based diagnostic markers for CVDs may be a supplement and development program for early screening and accurate and comprehensive diagnosis of CVDs in the future.
Advanced studies have shown that therapeutic strategies based on specific ncRNA modulation are promising candidates to improve CVDs (28, 125, 126). NcRNAs are also considered to be key novel regulators of cardiovascular function and risk factors, potentially serving as small-molecule targets (e.g., miRNAs, lncRNAs, circRNAs and lincRNAs) or gene silencing tools (e.g., siRNAs) for cardiovascular treatment and prognosis assessment (23, 28). Specifically, RNA interference (RNAi) drugs and further novel drugs that mimic or inhibit endogenous ncRNAs may lay the blueprint for the treatment of CVDs at the small molecule level by utilizing molecular interactions, particularly ubiquitination or deubiquitination.
On the first level, ncRNAs are involved in the pathological processes of CVDs and function as potential therapeutic targets by directly regulating E2/E3/deubiquitinating enzyme levels and affecting downstream protein expression (Table 2). Various ncRNAs exert cardioprotective effects by inhibiting downstream proteins of ubiquitination degradation (miR322, miR-322/503, miR-378a-3p, miR-454, miR-129-5p) or promoting ubiquitination degradation (lncCIRPIL, LNC01588) (36–38, 41, 43, 67, 68). For example, miR322, miR-322/503, miR-378a-3p, lncCIRPIL, and LNC01588 were shown to exert anti-apoptotic effects to alleviate myocardial I/R progression by inhibiting downstream proteins of FBXW7/Smurf2/TRIM55 ubiquitination or promoting ubiquitination degradation of p53/SEP4 (36–38, 41, 43). MiR322/503 (homolog of human miR-424) reduces myocardial I/R-induced apoptosis and infarct size via the miR-424(322)/503/FBXW7/N1-ICD/HIF-1α and miR-424(322)/503/Smurf2/EZH2 axes (36, 37). Additionally, lncCIRPIL can protect cardiomyocytes from A/R-induced apoptosis by promoting ubiquitination degradation of p53 (41). Moreover, LNC01588 was found to inhibit cardiomyocyte apoptosis and oxidative stress via the LNC01588/HNRNPL/WWP2/SEP4 pathway (43). Furthermore, miR-454 exerts anti-apoptosis effects and improves HF via the miR-454/NEDD4-2/TrkA/cAMP axis (67), while miR-129-5p provides potential therapeutic benefits in CHF via modulation of the miR-129-5p/Smurf1/PTEN axis (68).
In addition, several ncRNAs are directly involved in improving vascular diseases by inhibiting the ubiquitination of downstream proteins (miR-30e, circDLGAP4, lncRNA-Nespas, SNHG15, miR-92b-3p) or promoting binding to the ubiquitin ligase(lincRNA-p21) (83, 84, 89, 92, 101, 112). MiR-30e and lincRNA-p21 act as potential targets for the treatment of atherosclerosis by inhibiting human VSMC proliferation and migration, respectively, via the miR-30e/Ube2i/IκBα/NFκB and lincRNA-p21/MDM2/p53 axes (83, 84). Notably, circDLGAP4, lncRNA-Nespas, and SNHG15 can exert neuroprotective effects to alleviate ischemic stroke by decreasing the expression of the E3 ubiquitin ligase HECTD1 or inhibiting the ubiquitination of TAK1/TRAF2 (89, 92, 101). Interestingly, circDLGAP4 serves as a therapeutic target for ischemic stroke and a biomarker of disease activity via regulation of the circDLGAP4/miR-143/HECTD1 axis and plasma detection (89). LncRNA-Nespas can improve the prognosis of ischemic stroke via the lncRNA-Nespas/TAK1/NF-κB pathway (101). Furthermore, miR-92b-3P can be a potential therapeutic benefit for PAH treatment by suppressing the expression of the deubiquitinating enzyme USP28 (112).
Unlike the ncRNAs mentioned above, which directly exert protective effects on hearts and blood vessels, many ncRNAs are suppressed to improve CVDs by regulating UPS components. For instance, knockdown of ncRNAs was considered a promising therapeutic strategy for the treatment of heart diseases by suppressing ubiquitination degradation of proteins (miRNA-1, circNfix, miR‐195‐5p, lncRNA LOC105378097, lncDACH1) or promoting ubiquitination enzyme expression (miR-30a, miR-21) (47, 49, 57, 62, 63, 66, 69). Silencing of miRNA-1 and circNfix plays important roles in inhibiting cardiomyocyte apoptosis and alleviating the MI process by inhibiting UPS components/Nedd4l (47, 49). Interestingly, a reduction in SE-regulated circNfix has proliferative and proangiogenic effects and improves MI prognosis via the SE/circNfix/Nedd4l/Ybx1 signaling pathway and circNfix/miR-214/Gsk3β axis (49). In addition, knockdown of miR‐195‐5p and lncDACH1 can prevent and treat diabetic cardiomyopathy and HF, respectively, by regulating the miR‐195‐5p/SGK1/Nedd4‐2 axis or enhancing SERCA2 ubiquitination and degradation (57, 66). Silencing of lncRNA LOC105378097 has protective effects against heart aging by facilitating Parkin ubiquitination.
Moreover, inhibition of miR-30a and miR-21 can suppress CVB3 replication and reduce CVB3 infection-induced injury by promoting the expression of the E3 ubiquitin ligase TRIM25/deubiquitinating enzyme YOD1 (62, 63).
For the treatment of vascular diseases, silencing of ncRNAs provides potential therapeutic strategies by inducing ubiquitination degradation (miR-29a, miR-181b, lncRNA-Fendrr, lncRNA MIAT, lncRNA SNHG, miR-21, miR-424(322)) or inhibiting the deubiquitinating enzyme BRCC3/SUMO1 (lncRNA NEAT1, lncRNA SOX2-OT) (73, 82, 93, 96, 98, 100, 110, 111, 116). First, knocking down lncRNA NEAT1 can exert anti-inflammatory effects and protect endothelial cells by regulating the miR−204/BRCC3 axis (73). To attenuate atherosclerosis, inhibition of miR-29a is an effective therapeutic approach by modulating the KLF5/miR-29a/Fbw7/CDC4/KLF5 positive feedback loop (82). Additionally, inhibition of miR-181b, lncRNA-Fendrr, lncRNA MIAT and lncRNA SNHG3 was determined to play neuroprotective roles in treating cerebral I/R injury and ischemic stroke by promoting UCHL1/HERC2 expression or accelerating REDD1/HDAC3 ubiquitination degradation (93, 96, 98, 100). Specifically, knockdown of miR-181b and lncRNA-Fendrr can enhance ischemic injury-induced neuroprotection by the miR-181b/UCHL1/HSPA5 axis and lncRNA-Fendrr/HERC2/NLRC4 axis (93, 100). Furthermore, suppression of miR-21, miR-424(322) and lncRNA SOX2-OT offers potential therapeutic benefits in PAH by upregulating WWP1/SMURF1 or downregulating SUMO1 (110, 111, 116). Decreased miR-21 and lncRNA SOX2-OT prevent PASMC proliferation and pulmonary vascular remodeling, respectively, by the miR-21/WWP1/TGF-β1 signaling pathway and SOX2-OT/miR-455-3p/SUMO1 axis, and lncRNA SOX2-OT also serves as a novel biomarker for the diagnosis of PAH (110, 111). Interestingly, miR-424(322) detection in plasma can function as a diagnostic biomarker for PAH and improve prognosis via the miR-424(322)/SMURF1/BMPR2 pathway (116).
This is particularly noteworthy that circRNA, as an emerging ncRNA, has received much attention in recent years. The mechanism is that circRNAs are involved in CVD progression by sponging miRNAs and regulating downstream proteins (circRNA/miRNA/ubiquitin ligase or deubiquitinating enzymes/protein axis) via ubiquitination, for example, circDLGAP4/miR-143/HECTD1 axis improved stroke (89), circ-UBR4/miR-637/FOXO4, circ-UBR4/miR-107/ROCK1, circ-UBR4/miR-491-5p/NRP2, circ-UBR4/miR-185-5p/FRS2, and circ-CHFR/miR-214-3p/PAPPA axes as well as inhibition of circ_USP36/miR-197-3p/ROBO1 axis could ameliorate atherosclerosis (75–81), circ-HECTD1/miR-133b/TRAF3 and circ_HECTD1/miR-27a-3p/FSTL1 axes as well as inhibition of circNfix/Nedd4l/Ybx1 and circNfix/miR-214/Gsk3β axes could reduce myocardial infarction and CVD (49, 94, 95), circ-sh3rf3/sh3rf3/GATA-4/miR-29a alleviated cardiac fibrosis (71).
On the second level, some ubiquitin ligase mimics or inhibitors serve as therapeutic targets, providing new perspectives for the treatment of CVDs. Nrdp1, TRIM55 inhibitors and Circ_HECTD1 inhibitors can improve CVDs by being negatively regulated by miR-378a-3p, positively regulating NONRATT054243 and NONRATT057160 or negatively regulating miR-27a-3p and miR-133b (38, 59, 94, 95). For instance, Nrdp1 may have potential therapeutic effects on cardiomyocyte hypertrophy via the Nrdp1/NONRATT054243 and NONRATT057160 axes (59). Conversely, TRIM55 silencing inhibits cardiomyocyte apoptosis via the downstream DUSP1/JNK pathway for the treatment of myocardial I/R injury (38). Circ_HECTD1 deficiency offers a novel preclinical basis for cerebral ischemia injury and cerebral infarction via the circ_HECTD1/miR-27a-3p/FSTL1 axis and circ_HECTD1/miR-133b/TRAF3 axis (94, 95). In addition, many studies determined the mechanism and manifestation of CVD after ubiquitin ligases RNF4, CHIP, UBE3A and FBXO3 knockdown by siRNA, suggesting that these ubiquitin ligases can function as targets for CVD treatment. RNF4 suppresses ischemia-induced cardiomyocyte apoptosis via the PML/p53 axis (45). Similarly, CHIP provides a therapeutic method for DOX-induced HF via CHIP/SHP-1 and p53/STAT3 and ERK1/2 (46). UBE3A is vital for alleviating cardiac hypertrophy via the UBE3A/TLR4/MMP-9 pathway (60). In contrast, inhibition of FBXO3 exerts anti-inflammatory effects and relieves ischemic stroke by suppressing of HIPK2 ubiquitination and degradation (103).
On the third level, therapeutic targets are not only ncRNAs or UPS, and there are several ncRNAs, UPS components or downstream proteins that work together as targets for the treatment of CVDs. The details are as follows: miR379 and Smurf1, miR-199a and specific UPS components, miR-146a and SUMOylation were shown to participate in the treatment of HF (39, 64, 65); HIF-1α pathway inhibitors and miR-483 mimics can be potential therapeutic agents for calcific AV disease (72); miR-140-5p and SMURF1 are emerging as crucial regulators and therapeutic targets for PAH (113). Additionally, miR-129-5p and SIAH1 can be therapeutic and preventive targets in cerebral ischemic injury via the miR−129−5p/SIAH1/mTOR signalling pathway (88). Similarly, miR-124 and REST provide therapeutic benefits in protecting against stroke via miR-124/USP14/REST, while other studies have demonstrated that lithium or M2 microglia-derived exosomes can regulate miR-124 and REST to play a neuroprotective role (86, 87, 102). Cardiomyocyte hypertrophy and myocardial fibrosis can be treated via the regulation of the miR-485-5p/MAPL/Mfn2 axis and Circ-sh3rf3/GATA-4/miR-29a cascade (58, 71). Moreover, five notable crosstalk pathways, circ-UBR4/miR-637/FOXO4, circ-UBR4/miR-107/ROCK1, circ-UBR4/miR-491-5p/NRP2, circ-UBR4/miR-185-5p/FRS2 and circ-CHFR/miR-214-3p/PAPPA axis, regulate VSMC migration and growth to improve atherosclerosis (75, 77–80). Furthermore, regulating the MIAT/EGLN2 axis and NEAT1/EGR1/RBM25 axis provides therapeutic strategies for ischemic stroke therapy (97, 99), and ETAR/miR-27b-3p/FBXW7/KLF5/GLI1 axis and miRNA-146-5p/USP3 axis may act as potential targets for PAH prevention and treatment (109, 114).
On the fourth level, targeting upstream proteins, which are regulators of crosstalk between ncRNA and the UPS, represents a promising and novel approach to CVD treatment. Specifically, ZFP36L2 silencing improves myocardial I/R injury via regulation of the lncRNA PVT1/miR-21-5p/MARCH5 axis (44). In the treatment of dilated cardiomyopathy, TWIST1 acts as an advantageous target to improve heart quality by increasing miR-199/214 cluster expression and decreasing UPS activity (56). Interestingly, SOX9 is regulated by USP7 and has therapeutic value for sepsis-induced myocardial damage by regulating the downstream miR-96-5p/NLRP3 pathway (61). For vascular diseases, soybean-derived vasoactive peptide protects vascular endothelial cells from inflammatory damage via manipulation of the miR-19b/CYLD/TRAF6 axis (74). TGR5 has an anti-neuroinflammatory role in the clinical treatment of poststroke via the TGR5/Pellino3/caspase-8 and NLRP3 axes (104). Furthermore, PA700 inhibitors are effective for Ang II-induced hypertension therapy via regulation of 20S,26S proteasomes/GTPCH I and BH4 (115).
Nucleic acid drugs based on various disease-associated ncRNAs provide a new perspective in disease therapy. NcRNA-based drugs are designed to exert therapeutic effects by interacting with proteins or nucleic acids (127). However, this type of drug has two notable limitations. First, RNA molecules may evoke the host’s immune system and result in adverse immune responses. Second, because of the general nature of their targets, ncRNA-based drugs might not target specific sites (125). Therefore, current studies have proposed that ncRNAs combined with biocompatible nanoparticles (NPs) can offer better therapeutic properties. Using NPs as delivery vectors can reduce drug toxicity, facilitate targeted drug release, and increase drug bioavailability, which provides an optimized therapeutic strategy for the cardiovascular field (125, 128–130). In addition, with the production of proteasome inhibitors, the UPS offers a new therapeutic frontier. Drugs targeting ubiquitin ligases were found to have few side effects and significant treatment outcomes (15).
Notably, there have been various clinical trials using ncRNA or ubiquitin ligases for the treatment of CVDs. For instance, MiR-132 inhibitor CDR132L as a novel antisense therapy drug was demonstrated to alleviate heart failure and cardiac remodelling in first clinical trial (131). In the PREDIMED randomized controlled trial, a high-unsaturated fat medicine diet intervention, decreasing triglycerides, possibly reducing stroke risk in miRNA-410 target rs13702 C allele carriers (132). Moreover, the randomized controlled study found that the concentrations of HDL-carried miR-223 and miR-135a were elevated in healthy men following intake of trans fatty acids, preventing worsening of cardiovascular risk (133). Furthermore, siRNA therapy, Olpasiran, was applied to decrease lipoprotein(a) concentrations, thereby effectively ameliorating atherosclerotic CVDs (134). Similarly, Nissen, Wolski et al. revealed that plasma lipoprotein(a) concentrations are dose-related reduced in the siRNA SLN360 single ascending dose study (135). In addition, the ubiquitin E3 ligase NEDD4 rs4149601 can serve as a predictor of adverse cardiovascular consequences in whites without hydrochlorothiazide intervention, and NEDD4 rs292449, rs75982813 and rs4149601 can act as a predictor of blood pressure response after hydrochlorothiazide administration in Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) clinical trial (136).
In conclusion, known clinical trials using ncRNAs or ubiquitin ligases for the treatment of CVDs may provide a basis for emerging relevant laboratory studies of crosstalk between ncRNAs and ubiquitin ligases for CVD treatment, moving towards further clinical trials. For example, NEDD4 was found to predict adverse cardiovascular outcomes in PEAR clinical trial (136), and miR-454/NEDD4-2/TrkA/cAMP axis was demonstrated to exert anti-apoptosis effects and improves HF in rats, thus potentially laying the foundation for clinical trials.
Undoubtedly, CVDs constitute enormous health and socioeconomic burdens worldwide. The UPS is involved in the posttranscriptional modification of proteins. NcRNAs are unique RNA transcripts and play crucial roles in cellular processes and the development of various diseases, including CVDs. In this review, we creatively focused on the role of crosstalk between ncRNAs and the UPS in CVD processes. The main mechanism is that ncRNAs regulate E3 ubiquitin ligase levels and ubiquitination degradation of targeted proteins, and vice versa. A small number of E3 ubiquitin ligases can also regulate ncRNA expression. The crosstalk between ncRNAs and the UPS can affect the development of CVDs, such as myocardial I/R injury, MI, cardiomyopathy, HF, myocarditis, atherosclerosis, HT, and ischemic stroke.
A better knowledge of the role of ncRNAs and the UPS can finally provide promising therapeutic strategies for the treatment of CVDs. (1) Targeting ncRNAs can regulate E3/E2/deubiquitinating enzyme expression and subsequently promote or inhibit ubiquitination degradation of targeted proteins. (2) E3 ubiquitin ligases can act as therapeutic targets by regulating ncRNA expression or directly promoting ubiquitination and degradation of downstream proteins. (3) NcRNAs, UPS components and target proteins can serve as cotherapeutic targets. (4) Targeting upstream regulatory proteins or directly knocking down of E3 ubiquitin ligases by siRNA can have a therapeutic impact on CVD. Specifically, the levels of miR-424(322), miR-140-5p, circDLGAP4, and lncRNA LOC105378097, as well as SMURF1, HECTD1, and Parkin ubiquitination can be measured in clinical PAH, stroke, and cardiac aging patient blood, suggesting their diagnostic value.
In addition to the UPS, ncRNAs interact with other protein degeneration pathways involved in CVD development, such as calpains and autophagy. MiR-124-3 reduces cardiomyocyte viability by increasing the expression of the target calpain1, enhancing caspase-3 activity and down-regulating Bcl-2 (137). MiR-137 suppresses the proliferation of hypoxia-induced PASMC and improved PAH by upregulating calpain-2 expression (138). Moreover, Gao, Chen et al. (139) found that many miRNAs promote cardiomyocyte autophagy and improve cardiac function by upregulating ATGs (LC3 and beclin-1) and generating autophagosomes. Hsa_circ_0030042 sponges target eIF4A3 and regulates abnormal autophagy to enhance atherosclerotic plaque stability (140).
Despite growing evidence from experimental studies demonstrating the potential mechanism and significant implication for ncRNAs and the UPS in CVDs, there are still many limitations. First, the specific mechanism is still incomplete, and there are few evidence-based epidemiologic and clinical studies. Second, individual ncRNA or the UPS has a large number of regulatory roles in CVDs, but some of them remain unclear. Third, compared to the crosstalk between miRNAs and the UPS, knowledge on the relationship between lncRNAs/circRNAs/siRNAs and the UPS is far scarcer. Fourth, it is also unknown whether the mechanism of the interaction between ncRNAs, the UPS and CVDs is indeed widely effective in cardiomyocytes. Fifth, further studies are needed to examine the effects of genetic variability among individuals (e.g. DNA and RNA methylation, ATP-dependent chromatin remodelling, and post-translational histone modifications) on the relationship between ncRNAs, ubiquitin ligases, and CVD progression (141). Sixth, the role of other protein degradation pathways regulated by ncRNAs (e.g. calpain and autophagy) in CVDs needs to be further summarized and expanded. Finally, therapy targeting ncRNAs or the UPS alone is not yet mature, and cross-linking CVD therapy with the two seems far off.
On the one hand, ncRNA-based therapy offers the advantage of targeting traditionally undruggable targets and a potential role in biomarker-guided therapy. However, there are key challenges to overcome in developing effective ncRNA therapies, such as (1) obtaining sufficient stability and delivery in vivo, (2) acquiring high specificity of therapeutic ncRNA for molecular targets, and (3) attaining the ability to target intended cell types or tissues via appropriate vectors and transcriptional targeting and minimizing the side effects of the therapeutic ncRNA itself or drug delivery systems. To reduce these shortcomings, lipid NPs and viral vectors have been widely studied and serve as novel vectors for the delivery of nucleic acid drugs. On the other hand, proteasome, ubiquitin ligase E3 inhibitors and deubiquitinating enzyme inhibitors are undergoing clinical trials and have promising roles in CVD treatment. Nevertheless, proteasome inhibitors may cause selective downstream cell death, the mechanism of which is unclear. The effectiveness of proteasome inhibitor treatment is impaired by innate or acquired resistance mechanisms.
In addition to basic research on signaling pathways, the specific crosstalk of ubiquitin ligases and ncRNAs in CVDs needs to be further investigated with epidemiological evidence and clinical trials. We believe that advanced therapy based on ncRNAs and ubiquitination or deubiquitination can be more effective in treating CVDs and reducing the high mortality rate of CVD for the benefit of all humankind.
J-RY: Writing – original draft, Writing – review & editing. Z-JW: Writing – original draft, Writing – review & editing. J-WT: Investigation, Writing – review & editing. X-BL: Investigation, Writing – review & editing. RL: Investigation, Writing – review & editing. S-PL: Investigation, Writing – review & editing. HX: Funding acquisition, Writing – review & editing. P-FL: Funding acquisition, Writing – review & editing. Y-FZ: Funding acquisition, Investigation, Writing – review & editing. RZ: Investigation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Integrated Project of Major Research Plan of National Natural Science Foundation of China (92249303); National Natural Science Foundation of China (22006084); Science and Technology Development Planning Project of Qingdao (Shinan District), China (2022-2-004-YY); and Clinical Medicine + X Research Project of the Affiliated Hospital of Qingdao University (X202101027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16:203–12. doi: 10.1038/s41569-018-0119-4
2. Kalayinia S, Arjmand F, Maleki M, Malakootian M, Singh CP. MicroRNAs: roles in cardiovascular development and disease. Cardiovasc Pathol. (2021) 50:107296. doi: 10.1016/j.carpath.2020.107296
3. van Blokland IV, Groot HE, Franke LH, van der Wijst MGP, van der Harst P. Translational insights from single-cell technologies across the cardiovascular disease continuum. Trends Cardiovasc Med. (2022) 32:127–35. doi: 10.1016/j.tcm.2021.02.009
4. Liu Y, Ding W, Wang J, Ao X, Xue J. Non-coding RNA-mediated modulation of ferroptosis in cardiovascular diseases. BioMed Pharmacother. (2023) 164:114993. doi: 10.1016/j.biopha.2023.114993
5. Siasos G, Bletsa E, Stampouloglou PK, Oikonomou E, Tsigkou V, Paschou SA, et al. MicroRNAs in cardiovascular disease. Hellenic J Cardiol. (2020) 61:165–73. doi: 10.1016/j.hjc.2020.03.003
6. Schrottmaier WC, Mussbacher M, Salzmann M, Assinger A. Platelet-leukocyte interplay during vascular disease. Atherosclerosis. (2020) 307:109–20. doi: 10.1016/j.atherosclerosis.2020.04.018
7. Patterson C, Ike C, Willis PW4, Stouffer GA, Willis MS. The bitter end: the ubiquitin-proteasome system and cardiac dysfunction. Circulation. (2007) 115:1456–63. doi: 10.1161/CIRCULATIONAHA.106.649863
8. Powell SR, Herrmann J, Lerman A, Patterson C, Wang X. The ubiquitin-proteasome system and cardiovascular disease. Prog Mol Biol Transl Sci. (2012) 109:295–346. doi: 10.1016/B978-0-12-397863-9.00009-2
9. Zhao X, Nogawa A, Matsunaga T, Takegami T, Nakagawa H, Ishigaki Y. Proteasome inhibitors and knockdown of SMG1 cause accumulation of Upf1 and Upf2 in human cells. Int J Oncol. (2014) 44:222–8. doi: 10.3892/ijo.2013.2149
10. Basu B, Ghosh MK. Ubiquitination and deubiquitination in the regulation of epithelial-mesenchymal transition in cancer: Shifting gears at the molecular level. Biochim Biophys Acta Mol Cell Res. (2022) 1869:119261. doi: 10.1016/j.bbamcr.2022.119261
11. Tang M, Li S, Chen J. Ubiquitylation in DNA double-strand break repair. DNA Repair (Amst). (2021) 103:103129. doi: 10.1016/j.dnarep.2021.103129
12. Schreiner S, Wimmer P, Dobner T. Adenovirus degradation of cellular proteins. Future Microbiol. (2012) 7:211–25. doi: 10.2217/fmb.11.153
13. Wójcik C, Yano M, DeMartino GN. RNA interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J Cell Sci. (2004) 117:281–92. doi: 10.1242/jcs.00841
14. Fussi N, Höllerhage M, Chakroun T, Nykänen NP, Rösler TW, Koeglsperger T, et al. Exosomal secretion of α-synuclein as protective mechanism after upstream blockage of macroautophagy. Cell Death Dis. (2018) 9:757. doi: 10.1038/s41419-018-0816-2
15. Zheng N, Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem. (2017) 86:129–57. doi: 10.1146/annurev-biochem-060815-014922
16. David D, Nair SA, Pillai MR. Smurf E3 ubiquitin ligases at the cross roads of oncogenesis and tumor suppression. Biochim Biophys Acta. (2013) 1835:119–28. doi: 10.1016/j.bbcan.2012.11.003
17. Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. (2014) 20:1242–53. doi: 10.1038/nm.3739
18. Cao Y, Xie L, Shi F, Tang M, Li Y, Hu J, et al. Targeting the signaling in Epstein-Barr virus-associated diseases: mechanism, regulation, and clinical study. Signal Transduct Target Ther. (2021) 6:15. doi: 10.1038/s41392-020-00376-4
19. Si X, Gao G, Wong J, Wang Y, Zhang J, Luo H. Ubiquitination is required for effective replication of coxsackievirus B3. PloS One. (2008) 3:e2585. doi: 10.1371/journal.pone.0002585
20. Zhou X, Ao X, Jia Z, Li Y, Kuang S, Du C, et al. Non-coding RNA in cancer drug resistance: Underlying mechanisms and clinical applications. Front Oncol. (2022) 12:951864. doi: 10.3389/fonc.2022.951864
21. Ao X, Ding W, Li X, Xu Q, Chen X, Zhou X, et al. Non-coding RNAs regulating mitochondrial function in cardiovascular diseases. J Mol Med (Berl). (2023) 101:501–26. doi: 10.1007/s00109-023-02305-8
22. Liu Y, Wang Y, Li X, Jia Y, Wang J, Ao X. FOXO3a in cancer drug resistance. Cancer Lett. (2022) 540:215724. doi: 10.1016/j.canlet.2022.215724
23. Liu Y, Ao X, Wang Y, Li X, Wang J. Long non-coding RNA in gastric cancer: mechanisms and clinical implications for drug resistance. Front Oncol. (2022) 12:841411. doi: 10.3389/fonc.2022.841411
24. Liu Y, Ding W, Yu W, Zhang Y, Ao X, Wang J. Long non-coding RNAs: Biogenesis, functions, and clinical significance in gastric cancer. Mol Ther Oncolytics. (2021) 23:458–76. doi: 10.1016/j.omto.2021.11.005
25. Atianand MK, Hu W, Satpathy AT, Shen Y, Ricci EP, Alvarez-Dominguez JR, et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell. (2016) 165:1672–85. doi: 10.1016/j.cell.2016.05.075
26. Wen ZJ, Xin H, Wang YC, Liu HW, Gao YY, Zhang YF. Emerging roles of circRNAs in the pathological process of myocardial infarction. Mol Ther Nucleic Acids. (2021) 26:828–48. doi: 10.1016/j.omtn.2021.10.002
27. Liu Y, Ao X, Yu W, Zhang Y, Wang J. Biogenesis, functions, and clinical implications of circular RNAs in non-small cell lung cancer. Mol Ther Nucleic Acids. (2022) 27:50–72. doi: 10.1016/j.omtn.2021.11.013
28. Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M, et al. Landmesser, Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. (2018) 39:2704–16. doi: 10.1093/eurheartj/ehx165
29. Ma X, Dang Y, Shao X, Chen X, Wu F, Li Y. Ubiquitination and long non-coding RNAs regulate actin cytoskeleton regulators in cancer progression. Int J Mol Sci. (2019) 20:2997. doi: 10.3390/ijms20122997
30. Sun Y, He P, Li L, Ding X. The significance of the crosstalk between ubiquitination or deubiquitination and ncRNAs in non-small cell lung cancer. Front Oncol. (2022) 12:969032. doi: 10.3389/fonc.2022.969032
31. Zhou J, Liu J, Xing H, Shen Y, Xie M, Chai J, et al. Implications of protein ubiquitination modulated by lncRNAs in gastrointestinal cancers. Biochem Pharmacol. (2021) 188:114558. doi: 10.1016/j.bcp.2021.114558
32. Fischer MA, Vondriska TM. Clinical epigenomics for cardiovascular disease: Diagnostics and therapies. J Mol Cell Cardiol. (2021) 154:97–105. doi: 10.1016/j.yjmcc.2021.01.011
33. Qu J, Lin Z. Autophagy regulation by crosstalk between miRNAs and ubiquitination system. Int J Mol Sci. (2021) 22:11912. doi: 10.3390/ijms222111912
34. Altesha MA, Ni T, Khan A, Liu K, Zheng X. Circular RNA in cardiovascular disease. J Cell Physiol. (2019) 234:5588–600. doi: 10.1002/jcp.27384
35. Wu Y, Liu H, Wang X. Cardioprotection of pharmacological postconditioning on myocardial ischemia/reperfusion injury. Life Sci. (2021) 264:118628. doi: 10.1016/j.lfs.2020.118628
36. Chen Z, Su X, Shen Y, Jin Y, Luo T, Kim IM, et al. MiR322 mediates cardioprotection against ischemia/reperfusion injury via FBXW7/notch pathway. J Mol Cell Cardiol. (2019) 133:67–74. doi: 10.1016/j.yjmcc.2019.05.020
37. Dong W, Xie F, Chen XY, Huang WL, Zhang YZ, Luo WB, et al. Inhibition of Smurf2 translation by miR-322/503 protects from ischemia-reperfusion injury by modulating EZH2/Akt/GSK3β signaling. Am J Physiol Cell Physiol. (2019) 317:C253–c261. doi: 10.1152/ajpcell.00375.2018
38. Tan J, Shen J, Zhu H, Gong Y, Zhu H, Li J, et al. miR-378a-3p inhibits ischemia/reperfusion-induced apoptosis in H9C2 cardiomyocytes by targeting TRIM55 via the DUSP1-JNK1/2 signaling pathway. Aging (Albany NY). (2020) 12:8939–52. doi: 10.18632/aging.v12i10
39. Chen K, Zhang B, Sun Z. MicroRNA 379 regulates klotho deficiency-induced cardiomyocyte apoptosis via repression of smurf1. Hypertension. (2021) 78:342–52. doi: 10.1161/HYPERTENSIONAHA.120.16888
40. Wang J, Wang X, Cao M, Zhang L, Lin J. CircUSP39/miR-362-3p/TRAF3 axis mediates hypoxia/reoxygenation-induced cardiomyocyte oxidative stress, inflammation, and apoptosis. Int Heart J. (2023) 64:263–73. doi: 10.1536/ihj.22-232
41. Jiang Y, Yang Y, Zhang Y, Yang J, Zhang MM, Li S, et al. Cytoplasmic sequestration of p53 by lncRNA-CIRPILalleviates myocardial ischemia/reperfusion injury. Commun Biol. (2022) 5:716. doi: 10.1038/s42003-022-03651-y
42. Li X, Ni L, Wang W, Zong L, Yao B. LncRNA Fendrr inhibits hypoxia/reoxygenation-induced cardiomyocyte apoptosis by downregulating p53 expression. J Pharm Pharmacol. (2020) 72:1211–20. doi: 10.1111/jphp.13298
43. Song Y, Ren X, Gao F, Li F, Zhou J, Chen J, et al. LINC01588 regulates WWP2-mediated cardiomyocyte injury by interacting with HNRNPL. Environ Toxicol. (2022) 37:1629–41. doi: 10.1002/tox.23512
44. Wu F, Huang W, Tan Q, Guo Y, Cao Y, Shang J, et al. ZFP36L2 regulates myocardial ischemia/reperfusion injury and attenuates mitochondrial fusion and fission by LncRNA PVT1. Cell Death Dis. (2021) 12:614. doi: 10.1038/s41419-021-03876-5
45. Qiu F, Han Y, Shao X, Paulo P, Li W, Zhu M, et al. Knockdown of endogenous RNF4 exacerbates ischaemia-induced cardiomyocyte apoptosis in mice. J Cell Mol Med. (2020) 24:9545–59. doi: 10.1111/jcmm.15363
46. Wang L, Zhang TP, Zhang Y, Bi HL, Guan XM, Wang HX, et al. Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein. Sci Rep. (2016) 6:28399. doi: 10.1038/srep28399
47. Wei L, Zhang Y, Qi X, Sun X, Li Y, Xu Y. Ubiquitin−proteasomes are the dominant mediators of the regulatory effect of microRNA−1 on cardiac remodeling after myocardial infarction. Int J Mol Med. (2019) 44:1899–907. doi: 10.3892/ijmm
48. Huang D, Liu Y, Gao L, Wei X, Xu Y, Cai R, et al. MiR-32-3p regulates myocardial injury induced by microembolism and microvascular obstruction by targeting RNF13 to regulate the stability of atherosclerotic plaques. J Cardiovasc Transl Res. (2022) 15:143–66. doi: 10.1007/s12265-021-10150-8
49. Huang S, Li X, Zheng H, Si X, Li B, Wei G, et al. Loss of super-enhancer-regulated circRNA nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation. (2019) 139:2857–76. doi: 10.1161/CIRCULATIONAHA.118.038361
50. Yamada T, Nomura S. Recent findings related to cardiomyopathy and genetics. Int J Mol Sci. (2021) 22:12522. doi: 10.3390/ijms222212522
51. Mallavarapu A, Taksande A. Dilated cardiomyopathy in children: early detection and treatment. Cureus. (2022) 14:e31111. doi: 10.7759/cureus.31111
52. Brieler J, Breeden MA, Tucker J. Cardiomyopathy: an overview. Am Fam Physician. (2017) 96:640–6.
53. Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. (2018) 61:21–8. doi: 10.1007/s00125-017-4390-4
54. Tuohy CV, Kaul S, Song HK, Nazer B, Heitner SB. Hypertrophic cardiomyopathy: the future of treatment. Eur J Heart Fail. (2020) 22:228–40. doi: 10.1002/ejhf.1715
55. Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. (2014) 114:565–71. doi: 10.1161/CIRCRESAHA.114.300507
56. Baumgarten A, Bang C, Tschirner A, Engelmann A, Adams V, von Haehling S, et al. TWIST1 regulates the activity of ubiquitin proteasome system via the miR-199/214 cluster in human end-stage dilated cardiomyopathy. Int J Cardiol. (2013) 168:1447–52. doi: 10.1016/j.ijcard.2012.12.094
57. Shi Y, Yan C, Li Y, Zhang Y, Zhang G, Li M, et al. Expression signature of miRNAs and the potential role of miR-195-5p in high-glucose-treated rat cardiomyocytes. J Biochem Mol Toxicol. (2020) 34:e22423. doi: 10.1002/jbt.22423
58. Zhao Y, Ponnusamy M, Liu C, Tian J, Dong Y, Gao J, et al. MiR-485-5p modulates mitochondrial fission through targeting mitochondrial anchored protein ligase in cardiac hypertrophy. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:2871–81. doi: 10.1016/j.bbadis.2017.07.034
59. Zhang Y, Su L, Zhang K. Transcriptional effects of E3 ligase nrdp1 on hypertrophy in neonatal rat cardiomyocytes by microarray and integrated gene network analysis. Cardiology. (2016) 135:203–15. doi: 10.1159/000447235
60. Li Y, Ma L, Gu S, Tian J, Cao Y, Jin Z, et al. UBE3A alleviates isoproterenol-induced cardiac hypertrophy through the inhibition of the TLR4/MMP-9 signaling pathway. Acta Biochim Biophys Sin (Shanghai). (2020) 52:58–63. doi: 10.1093/abbs/gmz119
61. Gong X, Li Y, He Y, Zhou F. USP7-SOX9-miR-96-5p-NLRP3 network regulates myocardial injury and cardiomyocyte pyroptosis in sepsis. Hum Gene Ther. (2022) 33:1073–90. doi: 10.1089/hum.2022.078
62. Li J, Xie Y, Li L, Li X, Shen L, Gong J, et al. MicroRNA-30a modulates type I interferon responses to facilitate coxsackievirus B3 replication via targeting tripartite motif protein 25. Front Immunol. (2020) 11:603437. doi: 10.3389/fimmu.2020.603437
63. Ye X, Zhang HM, Qiu Y, Hanson PJ, Hemida MG, Wei W, et al. Coxsackievirus-induced miR-21 disrupts cardiomyocyte interactions via the downregulation of intercalated disk components. PloS Pathog. (2014) 10:e1004070. doi: 10.1371/journal.ppat.1004070
64. Haghikia A, Missol-Kolka E, Tsikas D, Venturini L, Brundiers S, Castoldi M, et al. Signal transducer and activator of transcription 3-mediated regulation of miR-199a-5p links cardiomyocyte and endothelial cell function in the heart: a key role for ubiquitin-conjugating enzymes. Eur Heart J. (2011) 32:1287–97. doi: 10.1093/eurheartj/ehq369
65. Oh JG, Watanabe S, Lee A, Gorski PA, Lee P, Jeong D, et al. miR-146a suppresses SUMO1 expression and induces cardiac dysfunction in maladaptive hypertrophy. Circ Res. (2018) 123:673–85. doi: 10.1161/CIRCRESAHA.118.312751
66. Cai B, Zhang Y, Zhao Y, Wang J, Li T, Zhang Y, et al. Long noncoding RNA-DACH1 (Dachshund homolog 1) regulates cardiac function by inhibiting SERCA2a (Sarcoplasmic reticulum calcium ATPase 2a). Hypertension. (2019) 74:833–42. doi: 10.1161/HYPERTENSIONAHA.119.12998
67. Wang Y, Pan W, Bai X, Wang X, Wang Y, Yin Y. microRNA-454-mediated NEDD4-2/TrkA/cAMP axis in heart failure: Mechanisms and cardioprotective implications. J Cell Mol Med. (2021) 25:5082–98. doi: 10.1111/jcmm.16491
68. Qi Y, Tang Y, Yin L, Ding K, Zhao C, Yan W, et al. miR-129-5p restores cardiac function in rats with chronic heart failure by targeting the E3 ubiquitin ligase Smurf1 and promoting PTEN expression. Bioengineered. (2022) 13:2371–86. doi: 10.1080/21655979.2021.2024335
69. Liu X, Bai X, Liu H, Hong Y, Cui H, Wang L, et al. LncRNA LOC105378097 inhibits cardiac mitophagy in natural ageing mice. Clin Transl Med. (2022) 12:e908. doi: 10.1002/ctm2.908
70. Ding F, Lu L, Wu C, Pan X, Liu B, Zhang Y, et al. circHIPK3 prevents cardiac senescence by acting as a scaffold to recruit ubiquitin ligase to degrade HuR. Theranostics. (2022) 12:7550–66. doi: 10.7150/thno.77630
71. Ma CX, Wei ZR, Sun T, Yang MH, Sun YQ, Kai KL, et al. Circ-sh3rf3/GATA-4/miR-29a regulatory axis in fibroblast-myofibroblast differentiation and myocardial fibrosis. Cell Mol Life Sci. (2023) 80:50. doi: 10.1007/s00018-023-04699-7
72. Fernandez Esmerats J, Villa-Roel N, Kumar S, Gu L, Salim MT, Ohh M, et al. Disturbed Flow Increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, Inducing Aortic Valve Calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1α (Hypoxia-Inducible Factor-1α) Pathway in Endothelial Cells. Arterioscler Thromb Vasc Biol. (2019) 39:467–81. doi: 10.1161/ATVBAHA.118.312233
73. Yao T, Song Y, Li S, Gu J, Yan X. Inhibition of lncRNA NEAT1 protects endothelial cells against hypoxia/reoxygenation−induced NLRP3 inflammasome activation by targeting the miR−204/BRCC3 axis. Mol Med Rep. (2022) 25:32. doi: 10.3892/mmr.2021.12548
74. Song T, Zhou M, Li W, Lv M, Zheng L, Zhao M. The anti-inflammatory effect of vasoactive peptides from soybean protein hydrolysates by mediating serum extracellular vesicles-derived miRNA-19b/CYLD/TRAF6 axis in the vascular microenvironment of SHRs. Food Res Int. (2022) 160:111742. doi: 10.1016/j.foodres.2022.111742
75. Ding Y, Tang T, Lu J, Wang J. Circ_UBR4 knockdown alleviates oxidized low-density lipoprotein-provoked growth and migration of human vascular smooth muscle cells by acting on the miR-637/FOXO4 pathway. J Cardiovasc Pharmacol. (2021) 78:534–43. doi: 10.1097/FJC.0000000000001098
76. Zhang Y, Yu W, Chang W, Wang M, Zhang L, Yu F. Light chain amyloidosis-induced autophagy is mediated by the foxo3a/Beclin-1 pathway in cardiomyocytes. Lab Invest. (2023) 103:100001. doi: 10.1016/j.labinv.2022.100001
77. Zhang Y, Zhang C, Chen Z, Wang M. Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis. Open Life Sci. (2021) 16:419–30. doi: 10.1515/biol-2021-0044
78. Peng H, Liu S, Li Y, Wang C, Zhong Y. A Novel circUBR4/miR-491-5p/NRP2 ceRNA Network Regulates Oxidized Low-density Lipoprotein-induced Proliferation and Migration in Vascular Smooth Muscle Cells. J Cardiovasc Pharmacol. (2022) 79:512–22. doi: 10.1097/FJC.0000000000001204
79. Sun C, Li J, Li Y, Li L, Huang G. Circular RNA circUBR4 regulates ox-LDL-induced proliferation and migration of vascular smooth muscle cells through miR-185-5p/FRS2 axis. Mol Cell Biochem. (2021) 476:3899–910. doi: 10.1007/s11010-021-04207-0
80. Lu Q, Li Y, Lou J, Li P, Gu Y, Wang X. Circ-CHFR modulates the proliferation, migration, and invasion of ox-LDL-induced human aorta vascular smooth muscle cells through the miR-214-3p/PAPPA axis. Clin Hemorheol Microcirc. (2022) 80:399–412. doi: 10.3233/CH-211288
81. Zhang Y, Li W, Li H, Zhou M, Zhang J, Fu Y, et al. Circ_USP36 silencing attenuates oxidized low-density lipoprotein-induced dysfunction in endothelial cells in atherosclerosis through mediating miR-197-3p/ROBO1 axis. J Cardiovasc Pharmacol. (2021) 78:e761–72. doi: 10.1097/FJC.0000000000001124
82. Zheng B, Zheng CY, Zhang Y, Yin WN, Li YH, Liu C, et al. Regulatory crosstalk between KLF5, miR-29a and Fbw7/CDC4 cooperatively promotes atherosclerotic development. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:374–86. doi: 10.1016/j.bbadis.2017.10.021
83. Zong Y, Wu P, Nai C, Luo Y, Hu F, Gao W, et al. Effect of microRNA-30e on the behavior of vascular smooth muscle cells via targeting ubiquitin-conjugating enzyme E2I. Circ J. (2017) 81:567–76. doi: 10.1253/circj.CJ-16-0751
84. Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. (2014) 130:1452–65. doi: 10.1161/CIRCULATIONAHA.114.011675
86. Doeppner TR, Doehring M, Bretschneider E, Zechariah A, Kaltwasser B, Müller B, et al. MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol. (2013) 126:251–65. doi: 10.1007/s00401-013-1142-5
87. Song Y, Li Z, He T, Qu M, Jiang L, Li W, et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. (2019) 9:2910–23. doi: 10.7150/thno.30879
88. Lei Y, Jin X, Sun M, Ji Z. miR-129-5p ameliorates ischemic brain injury by binding to SIAH1 and activating the mTOR signaling pathway. J Mol Neurosci. (2021) 71:1761–71. doi: 10.1007/s12031-021-01872-0
89. Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, et al. Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci. (2018) 38:32–50. doi: 10.1523/JNEUROSCI.1348-17.2017
90. Zhuang JH, Chen HX, Gao N, Sun RD, Xiao CY, Zeng DH, et al. CircUCK2 regulates HECTD1-mediated endothelial-mesenchymal transition inhibition by interacting with FUS and protects the blood-brain barrier in ischemic stroke. Kaohsiung J Med Sci. (2023) 39:40–51. doi: 10.1002/kjm2.12611
91. Li B, Xi W, Bai Y, Liu X, Zhang Y, Li L, et al. FTO-dependent m(6)A modification of Plpp3 in circSCMH1-regulated vascular repair and functional recovery following stroke. Nat Commun. (2023) 14:489. doi: 10.1038/s41467-023-36008-y
92. Sun H, Li S, Xu Z, Liu C, Gong P, Deng Q, et al. SNHG15 is a negative regulator of inflammation by mediating TRAF2 ubiquitination in stroke-induced immunosuppression. J Neuroinflamm. (2022) 19:1. doi: 10.1186/s12974-021-02372-z
93. Peng Z, Li J, Li Y, Yang X, Feng S, Han S, et al. Downregulation of miR-181b in mouse brain following ischemic stroke induces neuroprotection against ischemic injury through targeting heat shock protein A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1. J Neurosci Res. (2013) 91:1349–62. doi: 10.1002/jnr.v91.10
94. Zhang Z, He J, Wang B. Circular RNA circ_HECTD1 regulates cell injury after cerebral infarction by miR-27a-3p/FSTL1 axis. Cell Cycle. (2021) 20:914–26. doi: 10.1080/15384101.2021.1909885
95. Dai Q, Ma Y, Xu Z, Zhang L, Yang H, Liu Q, et al. Downregulation of circular RNA HECTD1 induces neuroprotection against ischemic stroke through the microRNA-133b/TRAF3 pathway. Life Sci. (2021) 264:118626. doi: 10.1016/j.lfs.2020.118626
96. Guo X, Wang Y, Zheng D, Cheng X, Sun Y. LncRNA-MIAT promotes neural cell autophagy and apoptosis in ischemic stroke by up-regulating REDD1. Brain Res. (2021) 1763:147436. doi: 10.1016/j.brainres.2021.147436
97. Li S, Fu J, Wang Y, Hu C, Xu F. LncRNA MIAT enhances cerebral ischaemia/reperfusion injury in rat model via interacting with EGLN2 and reduces its ubiquitin-mediated degradation. J Cell Mol Med. (2021) 25:10140–51. doi: 10.1111/jcmm.16950
98. Huang D, Cao Y, Zu T, Ju J. Interference with long noncoding RNA SNHG3 alleviates cerebral ischemia-reperfusion injury by inhibiting microglial activation. J Leukoc Biol. (2022) 111:759–69. doi: 10.1002/JLB.1A0421-190R
99. Cao JW, Tang ZB, Zhao JW, Zhao JK, Yao JL, Sheng XM, et al. LncRNA nuclear-enriched abundant transcript 1 aggravates cerebral ischemia/reperfusion injury through activating early growth response-1/RNA binding motif protein 25 axis. J Neurochem. (2022) 163:500–16. doi: 10.1111/jnc.15692
100. Wang LQ, Zheng YY, Zhou HJ, Zhang XX, Wu P, Zhu SM. LncRNA-Fendrr protects against the ubiquitination and degradation of NLRC4 protein through HERC2 to regulate the pyroptosis of microglia. Mol Med. (2021) 27:39. doi: 10.1186/s10020-021-00299-y
101. Deng Y, Chen D, Wang L, Gao F, Jin B, Lv H, et al. Silencing of long noncoding RNA nespas aggravates microglial cell death and neuroinflammation in ischemic stroke. Stroke. (2019) 50:1850–8. doi: 10.1161/STROKEAHA.118.023376
102. Doeppner TR, Kaltwasser B, Sanchez-Mendoza EH, Caglayan AB, Bähr M, Hermann DM. Lithium-induced neuroprotection in stroke involves increased miR-124 expression, reduced RE1-silencing transcription factor abundance and decreased protein deubiquitination by GSK3β inhibition-independent pathways. J Cereb Blood Flow Metab. (2017) 37:914–26. doi: 10.1177/0271678X16647738
103. Gao Y, Xiao X, Luo J, Wang J, Peng Q, Zhao J, et al. E3 ubiquitin ligase FBXO3 drives neuroinflammation to aggravate cerebral ischemia/reperfusion injury. Int J Mol Sci. (2022) 23:13648. doi: 10.3390/ijms232113648
104. Liang H, Matei N, McBride DW, Xu Y, Zhou Z, Tang J, et al. TGR5 activation attenuates neuroinflammation via Pellino3 inhibition of caspase-8/NLRP3 after middle cerebral artery occlusion in rats. J Neuroinflamm. (2021) 18:40. doi: 10.1186/s12974-021-02087-1
105. Zhang Y, Yu W, Flynn C, Chang W, Zhang L, Wang M, et al. Interplay between gut microbiota and NLRP3 inflammasome in intracerebral hemorrhage. Nutrients. (2022) 14:5251. doi: 10.3390/nu14245251
106. Luna RCP, de Oliveira Y, Lisboa JVC, Chaves TR, de Araújo TAM, de Sousa EE, et al. Insights on the epigenetic mechanisms underlying pulmonary arterial hypertension. Braz J Med Biol Res. (2018) 51:e7437. doi: 10.1590/1414-431x20187437
107. Li M, Dong X, Liu Y, Sun X, Li Z, He J. Inhibition of ubiquitin proteasome function suppresses proliferation of pulmonary artery smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. (2011) 384:517–23. doi: 10.1007/s00210-011-0678-y
108. Shi J, Yang Y, Cheng A, Xu G, He F. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. (2020) 319:H613–h631. doi: 10.1152/ajpheart.00220.2020
109. Wang Q, Chai L, Zhang Q, Wang J, Liu J, Chen H, et al. Induction of GLI1 by miR-27b-3p/FBXW7/KLF5 pathway contributes to pulmonary arterial hypertension. J Mol Cell Cardiol. (2022) 171:16–29. doi: 10.1016/j.yjmcc.2022.06.012
110. Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, et al. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. (2012) 302:L521–529. doi: 10.1152/ajplung.00316.2011
111. Jiang Y, Hei B, Hao W, Lin S, Wang Y, Liu X, et al. Clinical value of lncRNA SOX2-OT in pulmonary arterial hypertension and its role in pulmonary artery smooth muscle cell proliferation, migration, apoptosis, and inflammatory. Heart Lung. (2022) 55:16–23. doi: 10.1016/j.hrtlng.2022.04.002
112. Hao X, Ma C, Chen S, Dang J, Cheng X, Zhu D. Reverse the down regulation of miR-92b-3p by hypoxia can suppress the proliferation of pulmonary artery smooth muscle cells by targeting USP28. Biochem Biophys Res Commun. (2018) 503:3064–77. doi: 10.1016/j.bbrc.2018.08.095
113. Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. (2016) 126:2495–508. doi: 10.1172/JCI83361
114. Zhang W, Qi Y, Wu B. MicroRNA-146-5p Promotes Pulmonary Artery Endothelial Cell Proliferation under Hypoxic Conditions through Regulating USP3. Dis Markers. (2021) 2021:3668422. doi: 10.1155/2021/3668422
115. Xu J, Wang S, Wu Y, Song P, Zou MH. Tyrosine nitration of PA700 activates the 26S proteasome to induce endothelial dysfunction in mice with angiotensin II-induced hypertension. Hypertension. (2009) 54:625–32. doi: 10.1161/HYPERTENSIONAHA.109.133736
116. Baptista R, Marques C, Catarino S, Enguita FJ, Costa MC, Matafome P, et al. MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovasc Res. (2018) 114:53–64. doi: 10.1093/cvr/cvx187
117. Castellano JJ, Díaz-Beyá M, Navarro A. Clinic and therapeutic potential of non-coding RNAs in cancer. Transl Cancer Res. (2021) 10:3087–9. doi: 10.21037/tcr
118. Meng F, Yan J, Ma Q, Jiao Y, Han L, Xu J, et al. Expression status and clinical significance of lncRNA APPAT in the progression of atherosclerosis. PeerJ. (2018) 6:e4246. doi: 10.7717/peerj.4246
119. Budakoti M, Panwar AS, Molpa D, Singh RK, Büsselberg D, Mishra AP, et al. Micro-RNA: The darkhorse of cancer. Cell Signal. (2021) 83:109995. doi: 10.1016/j.cellsig.2021.109995
120. Han Q, Wang M, Dong X, Wei F, Luo Y, Sun X. Non-coding RNAs in hepatocellular carcinoma: Insights into regulatory mechanisms, clinical significance, and therapeutic potential. Front Immunol. (2022) 13:985815. doi: 10.3389/fimmu.2022.985815
121. Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol. (2019) 16:661–74. doi: 10.1038/s41569-019-0218-x
122. Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res. (2017) 120:381–99. doi: 10.1161/CIRCRESAHA.116.308434
123. Bär C, Chatterjee S, Thum T. Long noncoding RNAs in cardiovascular pathology. Diagnosis Therapy Circ. (2016) 134:1484–99. doi: 10.1161/CIRCULATIONAHA.116.023686
124. Liu X, Wang Q, Zhao J, Chang H, Zhu R. Inflammation-related circRNA polymorphism and ischemic stroke prognosis. J Mol Neurosci. (2021) 71:2126–33. doi: 10.1007/s12031-021-01889-5
125. Laura Francés J, Musolino E, Papait R, Pagiatakis C. Non-coding RNAs in cell-to-cell communication: exploiting physiological mechanisms as therapeutic targets in cardiovascular pathologies. Int J Mol Sci. (2023) 24:2205. doi: 10.3390/ijms24032205
126. Gomes CPC, Schroen B, Kuster GM, Robinson EL, Ford K, Squire IB, et al. Regulatory RNAs in heart failure. Circulation. (2020) 141:313–28. doi: 10.1161/CIRCULATIONAHA.119.042474
127. Wang J, Tian T, Li X, Zhang Y. Noncoding RNAs emerging as drugs or drug targets: their chemical modification, bio-conjugation and intracellular regulation. Molecules. (2022) 27:6717. doi: 10.3390/molecules27196717
128. Musolino E, Pagiatakis C, Serio S, Borgese M, Gamberoni F, Gornati R, et al. The Yin and Yang of epigenetics in the field of nanoparticles. Nanoscale Adv. (2022) 4:979–94. doi: 10.1039/D1NA00682G
129. Zhang Y, Yu W, Chen M, Zhang B, Zhang L, Li P. The applications of nanozymes in cancer therapy: based on regulating pyroptosis, ferroptosis and autophagy of tumor cells. Nanoscale. (2023) 15:12137–56. doi: 10.1039/D3NR01722B
130. Zhang Y, Zhang L, Wang M, Li P. The applications of nanozymes in neurological diseases: From mechanism to design. Theranostics. (2023) 13:2492–514. doi: 10.7150/thno.83370
131. Täubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. (2021) 42:178–88. doi: 10.1093/eurheartj/ehaa898
132. Corella D, Sorlí JV, Estruch R, Coltell O, Ortega-Azorín C, Portolés O, et al. MicroRNA-410 regulated lipoprotein lipase variant rs13702 is associated with stroke incidence and modulated by diet in the randomized controlled PREDIMED trial. Am J Clin Nutr. (2014) 100:719–31. doi: 10.3945/ajcn.113.076992
133. Desgagné V, Guay SP, Guérin R, Corbin F, Couture P, Lamarche B, et al. Variations in HDL-carried miR-223 and miR-135a concentrations after consumption of dietary trans fat are associated with changes in blood lipid and inflammatory markers in healthy men - an exploratory study. Epigenetics. (2016) 11:438–48. doi: 10.1080/15592294.2016.1176816
134. O'Donoghue ML, Rosenson RS, Gencer B, López JAG, Lepor NE, Baum SJ, et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N Engl J Med. (2022) 387:1855–64. doi: 10.1056/NEJMoa2211023
135. Nissen SE, Wolski K, Balog C, Swerdlow DI, Scrimgeour AC, Rambaran C, et al. Single ascending dose study of a short interfering RNA targeting lipoprotein(a) production in individuals with elevated plasma lipoprotein(a) levels. Jama. (2022) 327:1679–87. doi: 10.1001/jama.2022.5050
136. McDonough CW, Burbage SE, Duarte JD, Gong Y, Langaee TY, Turner ST, et al. Association of variants in NEDD4L with blood pressure response and adverse cardiovascular outcomes in hypertensive patients treated with thiazide diuretics. J Hypertens. (2013) 31:698–704. doi: 10.1097/HJH.0b013e32835e2a71
137. Xue X, Xi W, Li W, Xiao J, Wang Z, Zhang Y. Hydrogen-rich saline alleviates cardiomyocyte apoptosis by reducing expression of calpain1 via miR-124-3p. ESC Heart Fail. (2023) 10:3077–90. doi: 10.1002/ehf2.14492
138. Ge XY, Zhu TT, Yao MZ, Liu H, Wu Q, Qiao J, et al. MicroRNA-137 inhibited hypoxia-induced proliferation of pulmonary artery smooth muscle cells by targeting calpain-2. BioMed Res Int. (2021) 2021:2202888. doi: 10.1155/2021/2202888
139. Gao J, Chen X, Shan C, Wang Y, Li P, Shao K. Autophagy in cardiovascular diseases: role of noncoding RNAs. Mol Ther Nucleic Acids. (2021) 23:101–18. doi: 10.1016/j.omtn.2020.10.039
140. Yu F, Zhang Y, Wang Z, Gong W, Zhang C. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics. (2021) 11:5404–17. doi: 10.7150/thno.48389
Keywords: miRNA, lncRNA, circRNA, ubiquitination, deubiquitination, UPS
Citation: You J-R, Wen Z-J, Tian J-W, Lv X-B, Li R, Li S-P, Xin H, Li P-F, Zhang Y-F and Zhang R (2024) Crosstalk between ubiquitin ligases and ncRNAs drives cardiovascular disease progression. Front. Immunol. 15:1335519. doi: 10.3389/fimmu.2024.1335519
Received: 09 November 2023; Accepted: 26 February 2024;
Published: 07 March 2024.
Edited by:
Zulqarnain Baloch, Kunming University of Science and Technology, ChinaReviewed by:
Usman Alizai, The Ohio State University, United StatesCopyright © 2024 You, Wen, Tian, Lv, Li, Li, Xin, Li, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Zhang, emhhbmdydWlAcWR1LmVkdS5jbg==; Yin-Feng Zhang, emhhbmd5aW5mZW5nQHFkdS5lZHUuY24=; Pei-Feng Li, cGVpZmxpQHFkdS5lZHUuY24=
†These authors have contributed equally to this work
‡ORCID: Yin-Feng Zhang, orcid.org/0000-0003-2457-7712
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.