- 1Department of Orthopedic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Orthopedics Research Institute of Zhejiang University, Hangzhou, Zhejiang, China
- 3Key Laboratory of Motor System Disease Research and Precision Therapy of Zhejiang Province, Hangzhou, Zhejiang, China
- 4Clinical Research Center of Motor System Disease of Zhejiang Province, Hangzhou, Zhejiang, China
Bone is a common organ for solid tumor metastasis. Malignant bone tumor becomes insensitive to systemic therapy after colonization, followed by poor prognosis and high relapse rate. Immune and bone cells in situ constitute a unique immune microenvironment, which plays a crucial role in the context of bone metastasis. This review firstly focuses on lymphatic cells in bone metastatic cancer, including their function in tumor dissemination, invasion, growth and possible cytotoxicity-induced eradication. Subsequently, we examine myeloid cells, namely macrophages, myeloid-derived suppressor cells, dendritic cells, and megakaryocytes, evaluating their interaction with cytotoxic T lymphocytes and contribution to bone metastasis. As important components of skeletal tissue, osteoclasts and osteoblasts derived from bone marrow stromal cells, engaging in ‘vicious cycle’ accelerate osteolytic bone metastasis. We also explain the concept tumor dormancy and investigate underlying role of immune microenvironment on it. Additionally, a thorough review of emerging treatments for bone metastatic malignancy in clinical research, especially immunotherapy, is presented, indicating current challenges and opportunities in research and development of bone metastasis therapies.
1 Introduction
Bone metastasis is a common target organ of metastasis for several solid tumor types, including lung, breast, prostate, colorectal, thyroid, and gynecological tumors, and melanoma. Statistically, approximately 70% of patients with metastatic prostate and breast cancer develop bone metastases (1). Once cancer has spread to the bone, it is usually difficult to cure and is accompanied by a variety of accompanying complications such as pain, increased risk of fractures, and hypercalcemia (2).
Early studies of bone metastatic cancer present a phenomenon known as the “vicious cycle,” in which interactions between tumor cells and bone cells exacerbate the development of bone metastatic cancer (3). Tumor cells release substances such as parathyroid hormone-related protein (PTHrP), which stimulates osteoblasts to produce nuclear factor B receptor-activated ligand (RANKL), which further activates osteoclasts and leads to osteolysis. In turn, the multiple factors produced by osteolysis further promote tumor growth and more bone loss (4). This study reveals the impact of the bone microenvironment on the interactions between tumor cells.
The tumor microenvironment (TME) is a composite of various components (5), including the immune microenvironment. Meanwhile, the bone plays an important role as an immune organ in the body. The immune microenvironment of bone metastatic cancer is characterized by immune cells, such as T cells, macrophages, dendritic cells (DC), megakaryocytes, and myeloid-derived suppressor cells (MDSCs) (6). MDSCs are derived from immature myeloid progenitor cells and inhibit the immune function of T cells and NK cells in the TME (7). In addition, the bone microenvironment contains two key cell types: osteoblasts and osteoclasts. Osteoblasts are derived from multiple potential mesenchymal stem cells in the bone marrow stroma (8). Most prostate cancer bone metastases are osteogenic, with tumor cells tending to promote osteogenic activation of osteoblasts, whereas osteoclasts are derived from monocytes and are responsible for bone resorption (9). In osteolytic tumors such as bone metastases of the breast, lung, and kidney, tumor cells tend to promote the osteolytic function of osteoclasts.
Key factors in the immune microenvironment may include the local cytokine environment, the presence of helper stromal cells, specific types of immune cells, all of which play an important role in tumor-specific interactions (10). Different types of immune cells exert different functions in the immune microenvironment of metastatic bone cancer. Immune cells, such as NK cells and cytotoxic T cells, are capable of directly killing tumor cells using different mechanisms, whereas other immune cell subtypes, such as regulatory T cells (Tregs), a subtypes of CD4+ T helper cells, M2-type macrophages, tolerogenic DC, and MDSCs, inhibit adaptive immune responses to tumors, thereby promoting tumor progression and metastasis (11).
In metastatic cancer of the bone, specific cells in the bone and immune cells share a common environment (12), therefore this paper reviews the different types of cells and their effects in the immune microenvironment, and discusses areas for future development in this field.
2 Lymphatic immune cells
2.1 T cells
T cells originate from hematopoietic stem cells and lymphoid progenitors stored in the bone marrow and differentiate into primary lymphoid organs waiting to be activated by antigens. Partial tumor-infiltrating lymphocytes (TIL) in tumors have tumor cell-killing function (13). However, in the immunosuppressive microenvironment of bone metastatic cancer, T cells can be suppressed, leading to depletion or inactivation of T cells (14). Furthermore, the microenvironment of bone metastatic cancer also attracts other T cell subtypes, such as Tregs and other CD4+ T cells, which can support tumor growth and metastasis (11). For example, T cells can promote the development and maturation of osteoclasts, which can lead to the malignant cycle of bone metastatic cancer. In bone metastatic cancer, T cells are recruited and activated by tumor secreted factors such as PTHrP, interleukin (IL)-7, and IL-8, and recruited T cells can secrete tumor necrosis factor-α (TNF-α) or RANKL to induce bone resorption (15), which allows T cells to also participate in the vicious cycle process. Of course, this osteoclast-promoting effect is unique to nonactivated T cells (16).
T cells can be classified according to their function as cytotoxic T lymphocytes (CTL) and helper T cells (Th), where Th cells can be further classified as Th1, Th2, Th9, Th17, Th22, Tfh, and Tregs, depending on their function. Recent studies have shown that Th1, Th2, Th17, and Tregs are involved in the occurrence and development of tumor cells in bone metastases (17–21).
2.1.1 Cytotoxic T lymphocytes
CTLs are closely associated with the anticancer immune response, as these cells expresses a CD8 glycoprotein on its surface, and are also known as CD8+ T cells (22). They are activated to kill tumor cells by interacting with DC that present tumor-specific antigens (23).
Interferon-γ (IFN-γ) produced by CTL plays a key role in determining the antitumor ability of CTLs. Production of IFN-γ contributes to the tumor expression of MHC-I, making them more easily recognizable by CTLs (24), and directly inhibits tumor cell proliferation and induces apoptosis, thus exerts a direct role in the fight against cancer (25). IFN-γ from other sources also plays an important role in CTLs. PD-L1 deficiency in myeloid cells in bone metastatic cancers upregulates immunostimulatory genes, thereby contributing to macrophage polarization towards the M1 type and enhances IFN-γ signaling, which promotes the recruitment and activation of CTLs (26). Additionally, the presence of IFN-γ reduces tumor-associated bone loss and inhibits osteoblast development (27). However, IFN-γ also inhibits tumor cell killing by immune cells. Specifically, IFN-γ inhibits CTL function by upregulating the expression of programmed death ligand 1 (PD-L1) on the surface of tumor cells; thus, increasing its binding to the programmed cell death-1 (PD-1) receptor on the surface of CTL cells (28). IFN-γ can also activate interferon regulatory factor 2 (IRF2), a CTL transcription factor in the TME, thus changing CTL from an activated state to a depleted state and forcing tumor cells to evade immune surveillance (29).
CTLs are also affected by osteoclasts in bone metastatic cancer. Osteoclasts have been shown to undergo apoptosis and produce apoptotic vesicles during cyclic bone remodeling in bone metastatic cancer, thus inhibiting the infiltration and activity of CTLS (30). Conversely, osteoclasts exert a positive regulatory effect on CTLs. Lyn(-/-) mice, with more numerous osteoclasts, had reduced bone tumor growth despite enhanced osteolysis, due to the increased tumor-killing function of CTLs as a result of the increase in osteoclasts (31).
Furthermore, in addition to playing an important role in tumor metastasis to bone, CTLs also exert multiple critical functions in different types of bone metastatic cancers. Overexpression of estrogen-related receptor alpha (ERRα) in breast cancer bone metastases activates the tumor-killing effect of CTLs, through the production of chemokines C-C chemokine receptor type 17 (CCL17) and C-C chemokine receptor type 20 (CCL20), which allows CTLs to evade the control of transforming growth factor-β (TGF-β) (32). The up-regulation of IL-27 in bone metastasis of prostate cancer can lead to up-regulation of genes related to T-cell activation (33). The signal transducer and activator of the transcription 6 (STAT6) pathway is important in CTL immune suppression, which can be independent of Tregs (34). Snail(+) tumor cells can secrete Human follistatin-like protein 1 (FSTL1) not only to directly promote tumor bone metastasis, but also to generate CD45(-) activated leukocyte cell adhesion molecule (ALCAM)(+) cells, which can be surrounded by CD8+ T cells with weak CTL activity that contribute to the development of bone metastatic cancer (35).
2.1.2 Th cells
Th cells are immune T cells that produce cytokines involved in the adaptive immune response (36). Th cells play an important role in the mechanisms regulating of entry tumors into the bone environment and subsequent adaptive immune processes. Activated Th cells also release a variety of factors, such as IL-6, IL-11, IL-15, RANKL, and TNF-α, which promote osteoclastogenesis and bone resorption and provide a favorable environment for tumor bone metastasis (37, 38) (Figure 1). IL-7 is involved in T-cell proliferation and activation and can prompt CD8+ and CD4+ T cells to produce factors such as RANKL and TNF-α (39). These factors play a role in bone metastasis in the bone environment. However, activated CD4+ T cells also produce IFN-γ, which can inhibit osteoclast activity (40). Thus, Th cells influence the bone environment by releasing multiple factors and are also involved in regulating immune infiltration of tumors. Some studies have also implicated Th cells in the process of bone metastasis (41). Of particular note, in breast cancer, inhibition of poly ADP-ribose polymerase 2 (PARP2) increases the risk of bone metastasis, as this leads to an increase in immature myeloid cells in the bone marrow, which inhibits Th cell recruitment and creates an immunosuppressive microenvironment (42).
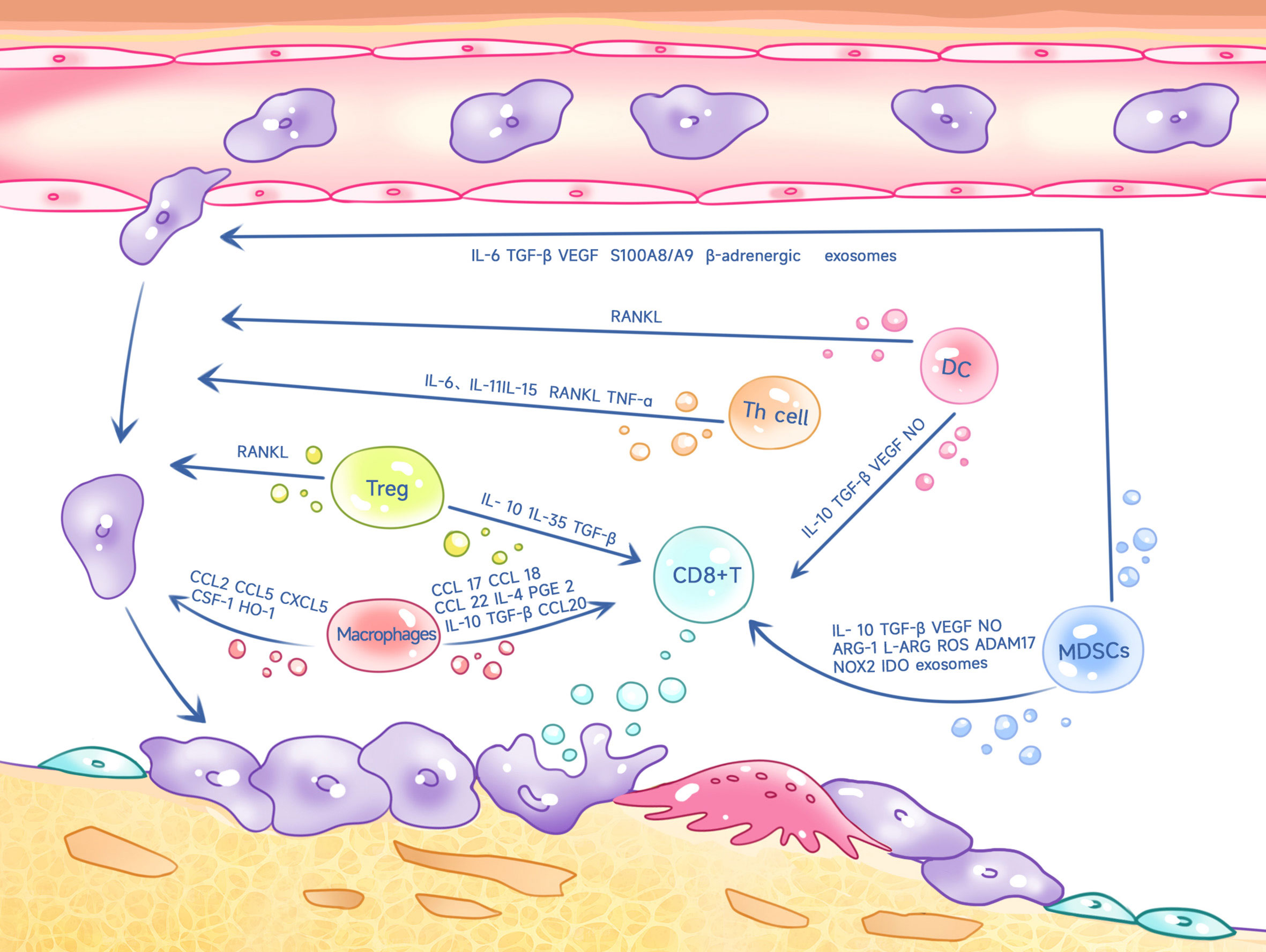
Figure 1 Th cells, Tregs, macrophages, MDSCs, DC cells inhibit CD8+ T cell killing on tumors and promote tumor cell metastasis to bone through multiple factors.
As mentioned previously, naïve Th cells can differentiate into different subtypes, including Th1, Th2, Th17, and Tregs, which exert different in the tumor immune response. This further highlights the importance of Th cells in bone metastatic cancer. Th1 cells in bone metastases of melanoma are affected by intestinal microbes, and when intestinal microbes are depleted, Th1 cell growth is inhibited, accelerating tumor growth and osteolysis (17). Despite the increase in CD4+ T cells within prostate cancer bone metastases, they are insensitive to immune checkpoint therapy, because CD4+ T cells differentiate into the Th17 rather than the Th1 line, mechanically because bone tumors promote osteoclast-mediated bone resorption, releasing TGF-β, which inhibits the development of the Th1 line (18). Wang et al. used genetically engineered hematopoietic stem cells (HSC) to deliver a small molecule inhibitor of TGF-β to the bone marrow, which resulted in the differentiation of CD4+T into Th1 and Th2 cells (19). LysM(Cre)/Tgfbr2 knockdown significantly inhibits the proliferation, angiogenesis, and osteoclast activation of metastatic cancer (43).
2.1.3 Tregs
Regulatory T cells (Tregs) are an important subpopulation of CD4+ T cells that are essential for the induction and maintenance of normal peripheral tolerance and the prevention of autoimmunity (20). Tregs can inhibit the function of many cells in bone metastatic cancers, including CD8+ T cells and Th1 cells, thus generating an immunosuppressive microenvironment (21). The specific mechanism is exerted through IL-10, IL-35, and TGF-β activity (44, 45) (Figure 1). Additionally, Tregs significantly inhibited the proliferation of CD4+CD25- T cells by directly contacting and thus blocking the delivery of costimulatory signals (46).
Increased Treg infiltration in prostate cancer often leads to a poor prognosis. In the immune microenvironment of bone metastasis from prostate cancer, Tregs can translocate to the bone marrow through C-X-C chemokine receptor 4 (CXCR4)/C-X-C Motif Chemokine 12 (CXCL12) (47). In addition to its immunosuppressive effects, Forkhead box protein P3 (Foxp3)+ Tregs are a key source of RANKL (48). As mentioned previously, RANKL produced by T cells can promote osteolysis to bone metastasis (37) (Figure 1). Bone marrow DC, in turn, promote the proliferation of Tregs through the receptor activator of NF-KappaB (RANK)-RANKL axis (47, 49). Thus, Tregs form a positive feedback axis for tumor bone metastasis and osteolysis through the RANK-RANKL axis.
Expression of CD73 on the surface of Tregs can also promote tumor metastasis (50). During the immune response, Th17 cells can be transformed into Tregs, which results from activation of the aromatic hydrocarbon receptor (AHR) by TGF-β (51). Activation of TNFR2 in Tregs by TNF can promote the expansion of immunosuppressive Tregs (52). The Tregs bone metastatic cancer microenvironment also interacts with osteoblasts and osteoclasts. For instance, osteoblasts can inhibit the function of CTLs by creating a suitable environment for Tregs through aerobic glycolysis (53). Tregs regulate osteoclast differentiation through cytotoxic T lymphocyte antigen (CTLA-4) in cell-to-cell contacts (54), thus altering the immune microenvironment of bone metastatic cancer.
2.2 Natural killer cells
Natural killer cells (NK cells) rapidly recognize and destroy cancer cells (55). For example, NK cells can release perforin and granzyme, mechanisms that lead to apoptosis of cancer cells (56).
However, several factors can influence the function of NK cells. In the early stages of bone metastasis, estrogen receptor (ER)-positive luminal cancers release signal peptide, CUB domain and EGF-like domain containing 2 (SCUBE2), which may help to induce osteoblasts to differentiate into osteoclasts, thereby inhibiting NK cells activity and providing favorable conditions for tumor colonization (57). In breast cancer, overactivation of the Janus kinase (JAK)/STAT signaling pathway has been widely reported (58, 59). However, inhibition of the JAK/STAT signaling pathway decreases the antitumor immune function of NK cells in metastatic tumors (60). Gut microbial deprivation also inhibits the proliferation of NK cells in bone metastatic tumors (17). This phenomenon suggests that intestinal microbes may play a role in modulating the immune response. Furthermore, in the case of neuroblastoma bone metastases, IL-2 therapy has been shown to be effective, suppressing tumors by increasing NK cell activity (61). Overexpression of PTHrP promoted bone metastasis of small-cell lung cancer in a mouse model, which may be related to NK cell depletion, but the exact mechanism needs to be further investigated (62).
3 Myeloid immune cells
3.1 Macrophages
Macrophages are an important component of the mononuclear phagocyte system (MPS) and are key cells in the tumor immune system (63). The main immune cells that infiltrate tumors are macrophages, also known as tumor-associated macrophages (TAM) (64). There is growing evidence that these macrophages play an important role in the immune microenvironment of bone metastatic cancer and play a key regulatory role in tumor progression, angiogenesis, invasion, and metastasis (65–67).
3.1.1 Impact on adaptive immunity
Macrophages can polarize into M1 and M2 types in the TME (68–70). M1 macrophages can produce reactive oxygen species (ROS), have high antigen presentation potential, and can recruit CTLs (71–73). Although M2 macrophages are usually considered pro-tumorigenic, M2 macrophages can recruit Tregs and Th2 cells by secreting anti-inflammatory factors that induce adaptive immune incompetence of the body against tumors (74, 75).
The chemokine CCL20 is highly expressed in macrophages, as is the homologous C–C chemokine receptor type 6 (CCR6) expressed on T cells. Macrophages in bone metastatic cancers inhibit the immune response of T cells to tumors through regulation of the CCL20-CCR6 axis (76). Furthermore, TAM-derived CCL17, CCL18, CCL22, IL-4, IL-10, TGF-β, and prostaglandin E2 (PGE 2) can inhibit the antitumor function of T cells (77–83). In bone metastases of prostate cancer, fusion of tumor cells with myeloid cells, including macrophages, further suppresses the immune response while promoting tumor growth (84) (Figure 1).
3.1.2 Impact on tumor metastasis
Macrophages can promote the growth of tumor cells in bone in several ways (85), and inhibition of macrophages reduces the incidence of tumor bone metastasis (86). Targeting anti-CD115 antibodies reduces the number of macrophages in tumors and therefore reduces osteolytic lesions in transplanted breast cancer cells (87). The interaction between macrophages and prostate cancer cells contributes to upregulation of cathepsin K expression in macrophages, which promotes tumor progression within metastases (88). CD137, a member of the TNF receptor superfamily, can promote macrophage migration into the TME and stimulate macrophage transformation into osteoclasts by enhancing Fra1 expression, thus promoting tumor bone metastasis (89). Furthermore, CCL5 secreted by TAMs contributes to bone metastasis of prostate cancer (90). CCL2 can help prostate tumor growth and bone metastasis by recruiting macrophages and osteoclasts (91) and macrophages also promote breast cancer bone metastases in an IL-4R-dependent manner, and inhibition of IL-4R effectively reduces the occurrence of bone metastases (92). Furthermore, CXCL5 and colony stimulating factor 1 (CSF-1) are associated with macrophage-driven bone metastasis (87, 93–95) Cyclooxygenase-1 (COX-1) positive macrophages can play an important role in prostate cancer bone metastasis (96) (Figure 1).
These studies highlight the important role of macrophages in immunomodulation and bone metastasis and provide useful information to better understand the onset and progression of bone metastatic cancer.
3.2 Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSC) are distinct immunosuppressive cells in tumors. MDSCs consist of a heterogeneous population of immature myeloid cells (IMCs) with immunosuppressive functions (97). MDSCs have been found to be widely infiltrated in a wide variety of cancers and have a significant ability to suppress T cell responses, leading to a poor prognosis. Such cells have attracted increasing attention in the academic community (81). Furthermore, MDSCs play a crucial role in bone (98).
3.2.1 Impact on adaptive immunity
MDSCs inhibit T cell proliferation by suppressing the immune response of T cells in several ways: (i) by generating arginase-1 (ARG-1)-dependent depletion and chelating L-cysteine depletion of L-arginine to inhibit T cell proliferation; (ii) by interfering with the signaling of the IL-2 receptor and generating ROS and NO to inhibit T-cell function; (iii) by expressing ADAM 17 (which contains structural domains of de-integrins and metalloproteinases17) and galactose lectin 9 that interfere with T-cell metastasis and pro-apoptosis activity; and (iv) by inducing Treg proliferation, which promotes bone metastasis growth (99–101). MDSCs also inhibit NK cell function via TGF-β, and IL-10 (102–104) inhibits dendritic cell differentiation and antigen presentation through IL-10, vascular endothelial growth factor (VEGF), NADPH oxidases (NOX2), and ROS (103, 105–108). Recent studies have shown that MDSC-produced exosomes can overactivate or deplete CD8+ T cells, thus suppressing immune function (109). In bone metastasis of breast cancer, MDSCs express PD-L1, which not only inhibits T cell function, but also promotes osteoclastogenesis, thus facilitating the progression of bone metastatic cancer (16) (Figure 1).
3.2.2 Impact on tumor metastasis
In the metastatic bone cancer microenvironment, MDSCs not only have immunosuppressive effects, but also accelerate bone lysis and destruction. MDSCs can promote bone tumor metastasis through a variety of mechanisms (98, 110). Tumors recruit MDSCs through the CCL2/CCL12-CCR2, CCL3/4/5-CCR5, CCL15-CCR1, CX3CL1/CCL26-CX3CR2, CXCL5/CXCL2/CXCL1-CXCR2, CXCL8 (IL-8)-CXCR1/CXCR2, CCL21-CCR7, CXCL13-CXCR5 pathways, promoting immunosuppression in the TME, while, MDSCs also promote tumor metastasis via the CCL5/CCR5,CCL15-CCR1,CXCL5/CXCL1-CCR2, CXCL8 (IL-8)-CXCR1/CXCR2 pathways (111). MDSCs also secrete TGF-β, S100A8/A9, VEGF and exosomes to interact with the immune system, endothelial cells, fibroblasts, and liver stellate cells, thus making the bone microenvironment suitable for tumor implantation (112). Furthermore, in a 4T1 mouse metastasis model, inhibition of interferon regulatory factor 7 (IRF7) enhanced the prometastatic activity of MDSCs. Conversely, IRF7 overexpression can counteract the effects of MDSCs and restore the activity of CD8+ T cells and NK cells to reduce metastasis (113). MDSCs can also promote tumor metastasis by enhancing β-adrenergic signaling and the IL-6/STAT3 pathway (114). The prometastatic effects of MDSCs are also closely related to osteoclasts. MDSCs derived from bone metastatic cancers can be induced to become osteoclast progenitors and can differentiate into osteoclasts (115). In addition to becoming osteoclasts themselves, they can also induce osteoclastogenesis, and in bone metastatic cancers, MDSC-produced nitric oxide (NO) not only mediates immunosuppression, but also mediates osteoclast generation (98, 99). Due to the fact that bone metastasized tumors express hypoxia-inducible factor (HIF)-1α at a higher level than primary tumors, which plays an important role in osteoclast formation (98, 99), and NO in turn up-regulates HIF-1α through various mechanisms, such as phosphatidylinositol 3-kinase and schizogen-activated protein kinase (116). Tumor cell levels of PTHrP and GL I-Kruppel 2(Gli 2) can be induced by MDSCs, which are also involved in osteoclastogenesis (110) (Figure 1).
3.3 Dendritic cells
DC are a class of immune cells that originate in the bone marrow and are widely distributed in various tissues (117–119). They play a key role in the induction and regulation of innate and adaptive immune responses by antigen presentation (117–120). DC efficiently phagocytose apoptotic cells and cross-present viral, tumor, and autoantigens to CD8(+) T cells (121, 122). In tumors, the role of DC is crucial, as they are capable of initiating an effective T cell response, attracting T cells to the tumor site, and maintaining the function of effector memory T cells (22, 123). Circulating DC readily migrate to the bone marrow due to the high expression of vascular cell adhesion molecule-1 (VCAM-1) and endothelial selectin in the microvasculature of the bone marrow, which is critical for metastatic bone cancers (124).
3.3.1 Impact on adaptive immunity
DC can differentiate into two subpopulations: myeloid DC (mDC) and plasmacytoid DC (pDC) (123). Although the importance of DC in antitumor immune responses is well known, cancer cells can still promote an immunosuppressive phenotype by affecting DC. In metastatic bone cancer, DC in the TME inhibit the tumor-killing activity of CD8+ T cells by producing cytokines such as IL-10, VEGF, TGF-β, and NO (125). IL-6 produced by tumor cells can contribute to the differentiation of hematopoietic stem and progenitor cells (HSPC) into monocyte-dendritic progenitor cells (MDPs) (126). Furthermore, high expression of CD1a(+) and CD83(+) has been reported to be negatively correlated with the development of bone metastases (127). In breast cancer bone metastases, pDC can persistently activate Th2, increase infiltration of Tregs and MDSCs, and produce osteolytic cytokines, leading to severe bone destruction (128). Mitochondrial transcription factor A (TFAM) deletion improves the presentation of antigens by DC through the cGAS-STING pathway, reversing immunosuppression in the TME (129). Furthermore, DC can induce the production of the PTHrP-derived peptide, PTR-4, which maintains CTL activation and thus improves tumor killing (130).
3.3.2 Impact on tumor metastasis
DC also play a key role in promoting tumor metastasis. TGF-β produced by tumors inhibits dendritic cell migration from the tumor site to lymphatic drainage, increasing the risk of tumor metastasis (131) (Figure 1).
3.3.3 RANK-RANKL and dendritic cells
The RANK-RANKL axis is closely related to DC. RANKL was first identified in 1997 using human bone marrow-derived DC (132), and RANK signaling in DC leads to immune tolerance in many cases (132–135). For example, RANKL from tumors of the genital tract induces an immature and tolerogenic phenotype in DC (136). DC are critical in antitumor combination therapies with anti-CTLA-4 and anti-RANKL antibodies (137). Thus, RANK signaling in DC may contribute to immune tolerance in bone metastatic cancers. RANKL produced by pDC can directly affect MDSCs by inducing their differentiation into osteoclasts, which promotes bone destruction and growth of breast cancer cells (138) (Figure 1). Furthermore, infiltration of pDC in cancer is associated with elevated levels of chemokines and cytokines that are directly or indirectly related to immunosuppression and osteoclastogenesis (138). These soluble factors also induce RANKL expression, which further stimulates osteoclastogenesis.
In conclusion, the dual role of DC in metastatic bone cancer is important for understanding the dynamic balance of the immune microenvironment and the metastatic mechanism of tumors. These findings are expected to provide new ideas for future immunotherapeutic strategies to improve immune system control of bone metastatic cancer.
3.4 Megakaryocytes
Megakaryocytes (MKs) are a class of cells derived from bone marrow-resident hematopoietic stem cells (HSC), which play a role in platelet production by responding to thrombopoietin (TPO) and exert a regulatory role in platelet production through their response to TPO. In addition to their effects on platelet production, MKs also affect osteoclasts and osteoblasts, thus regulating the bone microenvironment (139–142). Therefore, MKs also play a key role in bone metastatic cancer. MKs can inhibit osteoclast function while promoting osteoblast proliferation (143). In a mouse model, intracardiac injection of TPO-treated prostate cancer cells reduced the formation of bone metastases (144). Furthermore, the number of MKs in the bone marrow increased after intracardiac injection of highly osteogenic breast cancer cells (145, 146), suggesting that MKs play a key role in bone metastasis.
4 Osteoclasts and osteoblasts in bone metastatic cancer
4.1 Osteoclasts
Osteoclasts are a specialized class of cell types derived from monocytic macrophages (147). Their development and function are regulated, in part, by CSF-1 and RANKL (148). RANKL and CSF-1 bind to RANK in mature osteoclasts to induce the process of bone resorption. Furthermore, the balance between RANKL and its osteoprotegerin receptor (OPG) plays a key role in the regulation of osteoclast function. Knockdown of OPG in mice resulted in a decrease in bone density, while overexpression of OPG increased bone density (149).
During osteolytic bone metastasis, tumor cells continuously secrete a variety of osteoclastogenic cytokines in the bone, including CSF-1, PTHrP, RANKL, IL-8, IL-11, prostaglandin E, matrix metalloproteinase 1 (MMP-1), stromal cell communication network (CCN), and TNF-α (150–156). These factors directly stimulate osteoclast-mediated bone resorption and lead to the release of bone-derived tumor growth factors such as TGF-β, insulin-like growth factors (IGF), platelet-derived growth factor (PDGF), and bone morphogenetic protein (BMP) in the bone matrix, which promotes tumor growth in bone metastases (157, 158). TGF-β, a factor released after bone matrix lysis, stimulates the tumor’s secretion of PTHrP directly (159). This osteolytic cascade response is driven by the production of PTHrP. PTHrP plays a dual role in bone reconstruction. First, PTHrP upregulates monocyte chemoattractant protein-1(MCP-1) in osteoblasts, a key mediator of osteoclastogenesis, leading to the formation of osteoblastic lesions (160). Second, PTHrP stimulates osteoclast formation by improving osteoblast production of RANKL and CCL2 (142). Thus, a mutual promotion between tumor cells and osteoblasts, in which tumor cells promote osteolysis, and osteolysis then releases tumor growth factors that promote tumor growth, becomes a key therapeutic challenge. Breast cancer cells secrete an integrin-binding sialoprotein (IBSP) in bone, which attracts osteoclasts and creates an osteoclast-rich bone microenvironment (161). The R-responsive protein 2 (RSPO2) ligand in breast cancer cells interacts with RANK to promote osteoclast-mediated osteolysis (162). Furthermore, an ATP-dependent transporter protein called ABCC5 also mediates osteoclast-mediated bone resorption in breast cancer bone metastases (163). Inhibition of AEPase activity in breast cancer cells reduces osteoclast differentiation while attenuating osteolytic lesions caused by breast cancer bone metastases from breast cancer (164). Furthermore, early growth response-1 (EGR1) plays a direct role in the regulation of angiogenesis and osteoclastogenic factors in prostate cancer bone metastasis (165). Induction of tumor cells stimulates osteoclasts to secrete the IL-20RB ligand IL-19, which activates JAK1/STAT3 signaling and thus promotes proliferation of bone metastatic cancer cells (166). Furthermore, galactose lectin-3 (Gal-3) is located on the surface of osteoclasts and regulates the microenvironment of osteolytic bone metastatic cancer in the presence of RANKL (167). In bone metastatic cancers, CD47 on the surface of multiple cells regulates osteoclasts by modulating nitric oxide synthase activity, thus increasing the risk of tumor bone metastasis (168).
Exosomes secreted by tumor cells promote osteoclast differentiation and activation, leading to bone damage and remodeling of the bone metastasis microenvironment. The exosome miR-21 from SCP28 cells promotes osteoclast formation by regulating the expression of PDCD4 protein (155). In breast cancer bone metastasis, the miR-124/IL-11 axis plays a crucial role in the survival and differentiation of osteoclast progenitor cells (169). Furthermore, osteoblastic tumor exosomes can also induce osteoclast differentiation (170). For prostate cancer cells, extracellular vesicles (EVs) promote osteoclast formation in the presence of RANKL (171). Furthermore, tumor EVs of atypical cancer origin can also promote bone metastasis in hepatocellular carcinoma (172).
The mechanical environment of the bone is also critical for bone metastatic cancer. The activities of osteoblasts and cancer cells are regulated by the mechanical environment. Early changes in the mechanical environment can activate osteoclasts, which can lead to extensive osteolytic bone loss triggered by advanced bone metastatic cancer (173).
However, it is important to note that tumors also exert a dual effect on osteoclasts in the bone environment. In bone metastasis, tumor cell-secreted CST6 enters osteoclasts and inhibits the activity of the cysteine protease cathepsin B (CTSB), leading to the up-regulation of sphingosinekinase1 (SPHK1), which inhibits RANKL-induced activation of p38 and suppresses osteoclast maturation (174) (Figure 2).
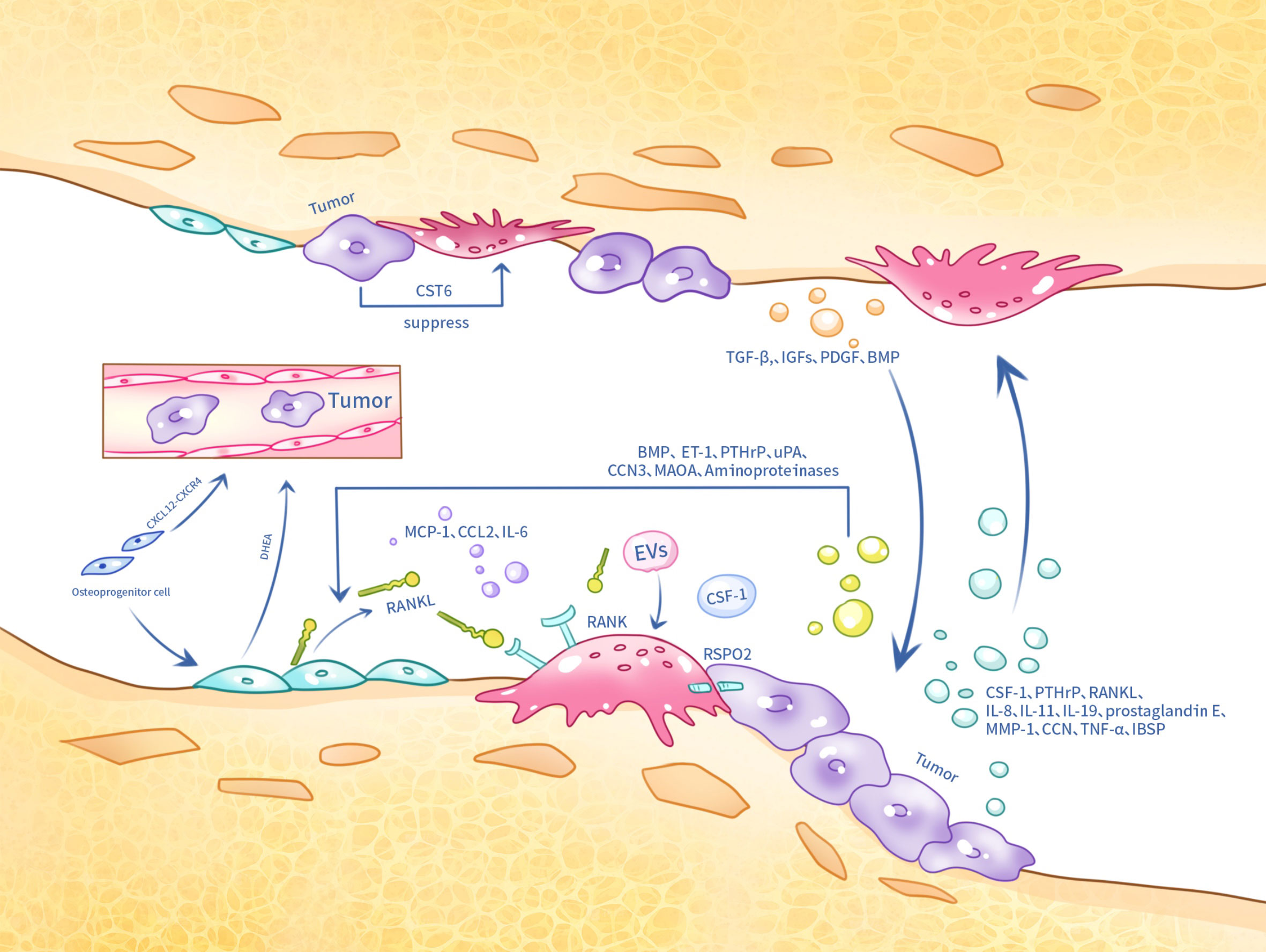
Figure 2 Tumor cells, osteoclasts and osteoblasts in metastatic bone cancer interact with each other at the skeletal site by several means.
To inhibit tumor growth, some clinical strategies can be achieved by breaking this “vicious cycle”. For example, procoxacin reduces prostate cancer bone metastasis by disrupting the feedback loop of the TGF-β/C-Raf/MAPK pathway and inhibiting osteoblast and osteoclast activity (175). Denosumab is a fully human IgG2 monoclonal antibody that specifically targets RANKL. It binds to RANKL with high affinity and specificity and inhibits the binding of RANKL to osteoclast precursors and osteoclast surface RANK, thus inhibiting osteoclast differentiation and activity, and disrupting the “vicious cycle” in tumor bone metastasis. This helps to inhibit excessive bone resorption and reduce bone destruction (176).
Bisphosphonates are a class of drugs that are absorbed by bone at sites of active bone metabolism (177). Bisphosphonates inhibit osteoclast activity and survival, reducing osteoclast-mediated bone resorption. Furthermore, they can also cause osteoclast apoptosis and have a direct apoptotic effect on tumor cells (178). Therefore, bisphosphonate therapy is now the standard of care for patients with malignant bone disease in a variety of tumor types, including prostate, breast, lung, and multiple myeloma (179). Furthermore, STING agonists can also modulate osteoclast function in the TME and reduce osteolysis, thus slowing tumor progression (180).
4.2 Osteoblasts
Osteoblasts are derived from mesenchymal stem cells whose primary function is bone formation. The Wnt and Runt-related transcription factor 2 (Runx2) pathways play a key role in the maturation and directed differentiation of osteoblasts (181). A hallmark of osteoblast differentiation is the formation of type 1 collagen, and this process becomes critical when mediated by Shh signaling directed at prostate cancer. The stromal collagen and Shh signaling pathways act synergistically and are essential for osteoblast formation. Although cancer bone metastasis is generally presented as osteolytic metastasis, the main mechanism of cancer bone metastasis is osteogenic metastasis (182).
Prostate cancers secrete a variety of factors, such as BMP and endothelin-1 (ET-1), which promote the maturation of osteogenic precursor cells, PTHrP, which inhibits osteoblast apoptosis, aminoproteinases, which indirectly promote bone formation, and urinary fibrinogen activator (uPA) (183). These conditions contribute to the enhanced deposition of a new bone matrix. Furthermore, prostate cancer secreted CCN3 improves the expression of BMP, Runx2, and osterix in osteoblasts through glycogen synthase kinase3β (GSK3β) and β-catenin signaling pathways (184).
Although cancer bone metastasis is predominantly osteolytic, CD137 has been reported to recruit monocytes/macrophages to migrate into the TME and promote the differentiation of monocytes or macrophages into osteoblasts during bone metastasis (89). Hypoxic conditions activate HIF-1α, a specific signaling factor for osteoblasts. Activation of HIF-1α signaling increases CXCL12 blood levels, which directly activates the CXCR4 receptor and promotes the migration of breast cancer cells to bone (185).
Furthermore, in clinical practice, the reduction of androgen levels is one of the main approaches in prostate cancer treatment. Osteoblasts have been reported to secrete the adrenal androgen precursor dehydroepiandrosterone (DHEA), which does not induce the androgen receptor (AR), but promotes cancer progression and metastasis (186). Monoamine oxidase A (MAOA) also plays an important role in prostate cancer bone metastasis. MAOA stimulates the release of IL-6 from osteoblasts, which creates a bone microenvironment conducive to the homing, growth and survival of cancer cells, and also activates osteoclastogenesis through the production of RANKL and IL-6 by osteoblasts, which contributes to the development of bone metastases from cancer (187). Furthermore, VCAM1 has been reported to activate painless micrometastases by recruiting osteoblast progenitor cells (188). Studies in animal models have shown that Plumbagin successfully inhibited breast cancer cell metastasis and osteolysis by significantly altering the RANKL/OPG ratio in osteoblasts (189) (Figure 2).
5 Tumor dormancy in tumor metastasis to bone
Tumor metastasis formation is a complex process that includes local invasion and infiltration of tumor cells, survival, and extravasation of tumor cells after entering the circulation, as well as survival and proliferation in target organs (190). After invading the bloodstream, tumor cells are defined circulating tumor cells (CTCs) (191). A small percentage of tumor cells can reach distant organs to colonize (192). Once they reach a distant site, tumor cells remain dormant until that environment can support tumor growth and proliferation (193, 194). Numerous clinical studies have found that tumors have metastasized to bone early in their development, entering a dormant state in preparation for future growth (195). Clinical observations have found that for tumors that are susceptible to bone metastasis, the number of patients with skeletal lesions is less than the number of patients with diffuse tumor cells (DTC) detectable in the bone marrow, a finding that supports the idea that the bone microenvironment supports tumor dormancy (196, 197). Therefore, understanding the relationship between the bone microenvironment and tumor dormancy can contribute to subsequent treatment and research.
5.1 Metastasis
Perivascular cells highly expressing CXCL12 in vascular microhabitats in bone marrow sinuses can keep breast cancer cells dormant in the vasculature through CXCL-12/CXCR4 interaction (198). Immunohistochemical analysis of bone marrow from breast cancer patients showed that dormant breast cancer cells preferentially localize in CXCL12-rich vascular regions (198). CXCR4/CXCL12 also plays a crucial role in bone metastasis of prostate cancer (199). However, unlike breast cancer, prostate cancer cells may benefit from this supportive environment that maintains dormancy, but does not contribute to tumor growth (193). Furthermore, growth-arrest specific 6 (GAS6) can induce tumor dormancy in cancer (200).
5.2 Influence of immune factors
The bone microenvironment is also an immune-privileged site that protects dormant tumor cells from environmental damage and resulting immune responses. Tregs in the bone immune microenvironment can create an immune microenvironment that supports the growth of dormant tumor cells, allowing them to evade immune attacks (201). MDSCs in the bone microenvironment can prevent the removal of dormant tumor cells by inhibiting the activity of anti-TME CTLs and NK cells (10); Furthermore, bone marrow mesenchymal stem cells can also protect dormant tumor cells (195).
6 Immunotherapy for metastatic bone cancer
Bone metastatic cancers are resistant to a variety of immunotherapies due to a specific immunosuppressive microenvironment (10, 202). As a result, current treatment for patients with bone metastases has focused primarily on palliative therapies to reduce pain and improve quality of life. Due to the specificity and importance of multiple immune cells in bone metastatic cancer, it is particularly crucial to find effective immunotherapy methods for bone metastatic cancer.
Human PD-1 (CD279), encoded by the PDCD1 gene, is a transmembrane protein that is expressed as an immunosuppressive receptor predominantly on monocytes, B cells, NK cells, macrophages, and activated T-cells (203–206). Its ligands PD-L1 and PD-L2 are expressed in DC, macrophages, and tumor cells (207, 208). PD-1 activation can mediate T cell inactivation and block signaling downstream of T-cell receptor (TCR) activation (209, 210). In immunotherapy, α-PD-1 drugs, such as nivolumab, can bind to immune cells such as T cells, B cells, NK cells, macrophages, and monocytes expressing PD-1, thus blocking PD-1 signaling (203–205). This helps to keep T cells continuously activated to fight off tumors. In patients with bone metastases from non-small cell lung cancer, overall survival increased by 7.9 months in patients with nivolumab (211), suggesting that α-PD-1 therapy may be useful to reduce tumor burden in patients with bone metastases.
Combining a PD-1 blocker (nivolumab) with a CTLA-4 blocker (ipilimumab) is more effective than PD-1 blockers alone and is a standard of care for many different cancers (211). In a study of advanced renal clear cell carcinoma, a lower 12 month OS rate was found in patients with bone metastases treated with ipilimumab/nivolumab (41.7%) compared to patients without bone metastases (82.7%) (212). Another retrospective study of patients with renal cell carcinoma (RCC) bone metastases treated with ipilimumab/nivolumab found relatively low efficacy (21%) and median OS (25.6 months) (213), and, generally, patients with bone metastases responded poorly to the combination of PD-1 and CTLA-4 inhibitors (213, 214). This may be related to the fact that bone metastatic cancers present an immunologically “cold” phenotype (215) and an immunosuppressive microenvironment (including infiltration of multiple immunosuppressive cells such as Tregs). Thus, eliminating Tregs in the bone metastatic microenvironment is a promising aspect of immunotherapy. Furthermore, anti-PD-1 immunotherapy can also produce long-term benefits in preventing bone destruction and relieving pain in bone cancer by inhibiting osteoclastogenesis (216).
NK cells kill tumors through multiple mechanisms, including granzyme B and perforin-mediated apoptosis or Fas-Fas ligand interactions. When IRF7 levels are restored, the response of host NK cells can be reactivated (113). Furthermore, gut microbial supplementation also helps promote NK cell proliferation in metastatic bone tumors (17). The ability of modified NK cells to produce IL-2 and IL-15, stimulate proliferation, and increase resistance to tumors makes them a new option for the treatment of bone metastatic cancer (217).
Macrophages play a crucial role in the immunotherapy of bone metastatic cancer.M2-type macrophages inhibit CD8+ T-cell resistance to tumors through multiple pathways. The use of anti-CD115 antibodies, trabectedin, clodronic acid, and zoledronic acid reduces the number of macrophages within the tumor (218). Macrophages are recruited to tumor sites primarily through the CCL2/CCR2 axis and CSF-1/CSF-1R signaling, so blocking these two signaling axes also reduces macrophage infiltration (219–221).
MDSCs are extensively infiltrated in metastatic bone cancers and have a significant ability to suppress T cell responses. Based on available evidence, the use of CXCR4 antagonists and indoleamine2,3-dioxygenase1 (IDO1) inhibitors activates CD8+ T cells and suppresses MDSCs, thus delaying bone metastasis in mouse breast cancer disease (222). Dickkopf-1 (Dkk1), a secreted Wnt antagonist, modulates the number and function of MDSCs in bone metastases in mice (223). Furthermore, multiple chemokine axes, such as CCR2/CCL2, CXCR2/CXCL5, and CXCR4/CXCL12, and inhibition of these signaling pathways prevents the entry of MDSCs from the bone marrow into the TME (10).
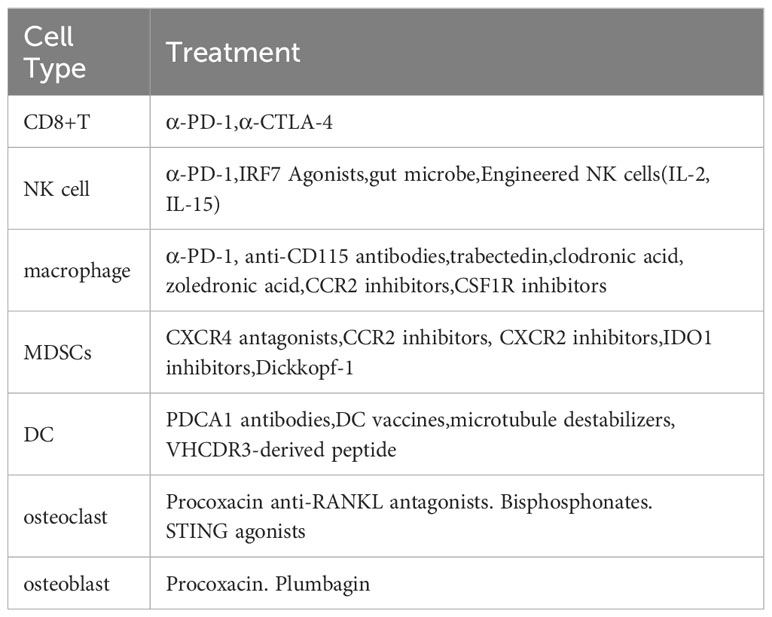
In immunotherapy, tolerogenic DC or pDC in tumors can affect the killing function of CD8+ T cells. By using PDCA1 antibodies, pDC can be reduced, thus reducing the load of bone metastases in breast cancer (128). Furthermore, microtubule destabilizers (e.g., dolastatin 10 and ansamitocin P3) can convert tolerant DC into activated DC that stimulate the killing effect of CD8+ T cells, which in turn fight the tumor (224). DC vaccines have also been considered a new approach to treating bone metastases by injecting DC-carrying tumor antigens to activate the immune response within the tumor (38). In a model of melanoma metastasis, stimulation of DC with cyclic VHCDR3-derived peptide (Rb9) inhibited melanoma metastasis (225). CD103+cDC1 vaccine inhibited primary and metastatic tumor growth, and IL-12 produced by CD103+DC was critical for NK cell-mediated tumor control (226, 227) (Table 1).
7 Discussion
Survival of patients with multiple solid tumors that metastasize to the bone is a great challenge. Previous studies have thoroughly explained the “vicious cycle” between tumor cells that metastasize to bone and osteoclasts and osteoblasts, and there are various therapeutic approaches, including the use of deslumab and bisphosphonates, that can break the “vicious cycle”. Meanwhile, the role of various types of immune cells and non-immune cells in the bone microenvironment during the transfer of tumor cells from the primary site to the bone has also been well studied. However, the multiple effects of multiple immune cells on adaptive immunity, i.e., on the specific tumor-killing effects of CTLs, after tumor cells colonize the immune microenvironment following bone are still not well reviewed, and thus a better understanding of the immune microenvironment of metastatic bone cancer is crucial for multiple effects. How to balance the immune cell effects on tumor-killing function and tumor growth promotion is a central question for the subsequent exploration of therapeutic approaches for bone metastatic cancer. Breaking the suppressive function of immune cells on adaptive immunity and enhancing the tumor-killing effect of immune cells on promoting CTLs will be the direction of future research on the immune microenvironment of bone metastatic cancer. In this review, we describe the multiple effects of various immune cells in the bone immune microenvironment, including osteoclasts and osteoblasts, on tumor metastasis and on adaptive immunity, highlighting the specific mechanisms by which the various types of immune cells function.
We also discuss tumor dormancy at the skeletal site, including the various types of immune factors that may influence tumor dormancy. Many solid tumors develop bone metastases at an early stage, but the tumor cells are dormant and the bone microenvironment protects the dormant tumor cells. Studying the effects of immune cells in the bone microenvironment on dormant tumor cells can guide clinical treatment for preventing bone metastasis in solid tumors. How to kill dormant tumor cells while avoiding harmful effects on the body’s normal bone immune microenvironment is still a question that needs to be explored.
We also reviewed current and future therapeutic approaches for the treatment of bone metastatic cancers. Within conventional immunotherapeutic agents, α-PD-1 agents have been shown to be helpful in reducing the tumor burden in patients with bone metastases from non-small cell lung cancers, and because of the special microenvironment of bone, α-PD-1 immunotherapy also has an impact on other factors such as osteoclasts, making the future of α-PD-1 in bone metastatic cancers also worthy of explore., as our understanding of the signaling mechanisms between tumor cells and cells in the bone immune microenvironment increases, several emerging therapeutic approaches, such as modification of NK cells, targeting of MDSCs and macrophages, and DC vaccines, can also be effective and efficient in halting the progression of skeletal lesions.
In conclusion, the interplay between the intrinsic cells of the bone, the immune cells, the bone matrix, and the tumor cells is critical for the progression of the tumor. Once the tumor invades the bone, how to prevent the immune cells from being called “accomplices” of tumor progression is still a question. What factors can break the “vicious cycle” between the four also needs to be further investigated. What factors promote tumor dormancy in metastatic bone cancer, and what factors cause dormant tumor cells to awaken and proliferate. Traditional immunotherapy is not effective in metastatic bone cancer, and it is worth exploring how to improve the effectiveness of immunotherapy in metastatic bone cancer by targeting various types of immune cells. The future of many emerging therapies is bright, but further research is needed to exploit the specificities of the bone microenvironment to combat bone tumors.
Author contributions
CS: Writing – original draft, Writing – review & editing. LJ: Writing – original draft, Writing – review & editing. MH: Writing – original draft, Writing – review & editing. ZW: Writing – original draft. JL: Writing – original draft. LS: Writing – review & editing. YE: Writing – review & editing. XY: Writing – original draft, Writing – review & editing. ZS: Writing – review & editing. TC: Writing – review & editing. WF: Writing – review & editing. ZS: Writing – review & editing. CX: Writing – review & editing. WZ: Writing – original draft. ZJ: Writing – original draft. ZZ: Writing – original draft. ZY: Writing – review & editing. LB: Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (NO. 82172688).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. (2018) 18:44. doi: 10.1186/s12885-017-3922-0.
2. Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J Bone Miner Res. (2018) 33:2099–113. doi: 10.1002/jbmr.3618.
3. Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. (1994) 32:73–84. doi: 10.1007/BF00666208.
4. Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. (2003) 3:453–8. doi: 10.1038/nrc1098.
5. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013.
6. Fu T, Dai LJ, Wu SY, Xiao Y, Ma D, Jiang YZ, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. (2021) 14:98. doi: 10.1186/s13045-021-01103-4.
7. Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. (2020) 17:1–12. doi: 10.1038/s41423-019-0306-1.
8. Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. (2011) 7:208–18. doi: 10.1038/nrendo.2010.227.
9. Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. (2008) 27:41–55. doi: 10.1007/s10555-007-9109-4.
10. Xiang L, Gilkes DM. The contribution of the immune system in bone metastasis pathogenesis. Int J Mol Sci. (2019) 20:999. doi: 10.3390/ijms20040999.
11. Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl). (2013) 91:411–29. doi: 10.1007/s00109-013-1021-5.
12. Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. (2019) 19:626–42. doi: 10.1038/s41577-019-0178-8.
13. Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. (2010) 29:1093–102. doi: 10.1038/onc.2009.416.
14. Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. (2013) 25:214–21. doi: 10.1016/j.coi.2012.12.003.
15. Fournier PGJ, Chirgwin JM, Guise TA. New insights into the role of T cells in the vicious cycle of bone metastases. Curr Opin Rheumatol. (2006) 18:396–404. doi: 10.1097/01.bor.0000231909.35043.da.
16. Arellano DL, Juárez P, Verdugo-Meza A, Almeida-Luna PS, Corral-Avila JA, Drescher F, et al. Bone microenvironment-suppressed T cells increase osteoclast formation and osteolytic bone metastases in mice. J Bone Miner Res. (2022) 37:1446–63. doi: 10.1002/jbmr.4615.
17. Pal S, Perrien DS, Yumoto T, Faccio R, Stoica A, Adams J, et al. The microbiome restrains melanoma bone growth by promoting intestinal NK and Th1 cell homing to bone. J Clin Invest. (2022) 132:e157340. doi: 10.1172/JCI157340.
18. Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell. (2019) 179:1177–1190.e13. doi: 10.1016/j.cell.2019.10.029
19. Wang B, Bai J, Tian B, Chen H, Yang Q, Chen Y, et al. Genetically engineered hematopoietic stem cells deliver TGF-β Inhibitor to enhance bone metastases immunotherapy. Adv Sci (Weinh). (2022) 9:e2201451. doi: 10.1002/advs.202201451.
20. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. (2006) 6:295–307. doi: 10.1038/nri1806.
21. Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. (2006) 16:124–36. doi: 10.1016/j.semcancer.2005.11.006.
22. Owen KL, Parker BS. Beyond the vicious cycle: The role of innate osteoimmunity, automimicry and tumor-inherent changes in dictating bone metastasis. Mol Immunol. (2019) 110:57–68. doi: 10.1016/j.molimm.2017.11.023.
23. Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. (2007) 21:1580–4. doi: 10.1038/sj.leu.2404658.
24. Martini M, Testi MG, Pasetto M, Picchio MC, Innamorati G, Mazzocco M, et al. IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine. (2010) 28:3548–57. doi: 10.1016/j.vaccine.2010.03.007.
25. Kakuta S, Tagawa Yi, Shibata S, Nanno M, Iwakura Y. Inhibition of B16 melanoma experimental metastasis by interferon-gamma through direct inhibition of cell proliferation and activation of antitumour host mechanisms. Immunology. (2002) 105:92–100. doi: 10.1046/j.0019-2805.2001.01342.x.
26. Zuo H, Wan Y. Inhibition of myeloid PD-L1 suppresses osteoclastogenesis and cancer bone metastasis. Cancer Gene Ther. (2022) 29:1342–54. doi: 10.1038/s41417-022-00446-5.
27. Mendoza-Reinoso V, McCauley LK, Fournier PGJ. Contribution of macrophages and T cells in skeletal metastasis. Cancers (Basel). (2020) 12:1014. doi: 10.3390/cancers12041014.
28. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. (2002) 8:793–800. doi: 10.1038/nm730.
29. Lukhele S, Rabbo DA, Guo M, Shen J, Elsaesser HJ, Quevedo R, et al. The transcription factor IRF2 drives interferon-mediated CD8+ T cell exhaustion to restrict anti-tumor immunity. Immunity. (2022) 55:2369–2385.e10. doi: 10.1016/j.immuni.2022.10.020
30. Wu Y, Ai H, Xi Y, Tan J, Qu Y, Xu J, et al. Osteoclast-derived apoptotic bodies inhibit naive CD8+ T cell activation via Siglec15, promoting breast cancer secondary metastasis. Cell Rep Med. (2023) 4:101165. doi: 10.1016/j.xcrm.2023.101165.
31. Zhang K, Kim S, Cremasco V, Hirbe AC, Collins L, Piwnica-Worms D, et al. CD8+ T cells regulate bone tumor burden independent of osteoclast resorption. Cancer Res. (2011) 71:4799–808. doi: 10.1158/0008-5472.CAN-10-3922.
32. Bouchet M, Lainé A, Boyault C, Proponnet-Guerault M, Meugnier E, Bouazza L, et al. ERRα Expression in bone metastases leads to an exacerbated antitumor immune response. Cancer Res. (2020) 80:2914–26. doi: 10.1158/0008-5472.CAN-19-3584.
33. Zolochevska O, Diaz-Quiñones AO, Ellis J, Figueiredo ML. Interleukin-27 expression modifies prostate cancer cell crosstalk with bone and immune cells in vitro. J Cell Physiol. (2013) 228:1127–36. doi: 10.1002/jcp.24265.
34. Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol. (2000) 165:6015–9. doi: 10.4049/jimmunol.165.11.6015.
35. Kudo-Saito C, Fuwa T, Murakami K, Kawakami Y. Targeting FSTL1 prevents tumor bone metastasis and consequent immune dysfunction. Cancer Res. (2013) 73:6185–93. doi: 10.1158/0008-5472.CAN-13-1364.
36. Zhu J. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. (2018) 10:a030338. doi: 10.1101/cshperspect.a030338.
37. Monteiro AC, Leal AC, Gonçalves-Silva T, Mercadante ACT, Kestelman F, Chaves SB, et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PloS One. (2013) 8:e68171. doi: 10.1371/journal.pone.0068171.
38. Luo G, He Y, Zhao Q, Yu X. Immune cells act as promising targets for the treatment of bone metastasis. Recent Pat Anticancer Drug Discovery. (2017) 12:221–33. doi: 10.2174/1574892812666170606123113.
39. Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, et al. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. (2002) 100:4615–21. doi: 10.1182/blood-2002-04-1121.
40. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. (2007) 96:41–101. doi: 10.1016/S0065-2776(07)96002-2.
41. Meng F, Han X, Min Z, He X, Zhu S. Prognostic signatures associated with high infiltration of Tregs in bone metastatic prostate cancer. Aging (Albany NY). (2021) 13:17442–61. doi: 10.18632/aging.v13i13.
42. Zuo H, Yang D, Yang Q, Tang H, Fu YX, Wan Y. Differential regulation of breast cancer bone metastasis by PARP1 and PARP2. Nat Commun. (2020) 11:1578. doi: 10.1038/s41467-020-15429-z.
43. Meng X, Vander Ark A, Lee P, Hostetter G, Bhowmick NA, Matrisian LM, et al. Myeloid-specific TGF-β signaling in bone promotes basic-FGF and breast cancer bone metastasis. Oncogene. (2016) 35:2370–8. doi: 10.1038/onc.2015.297.
44. Jarnicki AG, Lysaght J, Todryk S, Mills KHG. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. (2006) 177:896–904. doi: 10.4049/jimmunol.177.2.896.
45. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. (2007) 450:566–9. doi: 10.1038/nature06306.
46. Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. (1998) 188:287–96. doi: 10.1084/jem.188.2.287.
47. Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. (2004) 64:8451–5. doi: 10.1158/0008-5472.CAN-04-1987.
48. Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. (2011) 470:548–53. doi: 10.1038/nature09707.
49. Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. (2012) 1:152–61. doi: 10.4161/onci.1.2.18480.
50. Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MWL, Darcy PK, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. (2011) 71:2892–900. doi: 10.1158/0008-5472.CAN-10-4246.
51. Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. (2015) 523:221–5. doi: 10.1038/nature14452.
52. Chopra M, Riedel SS, Biehl M, Krieger S, von Krosigk V, Bäuerlein CA, et al. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis. (2013) 34:1296–303. doi: 10.1093/carcin/bgt038.
53. Muscarella AM, Aguirre S, Hao X, Waldvogel SM, Zhang XHF. Exploiting bone niches: progression of disseminated tumor cells to metastasis. J Clin Invest. (2021) 131:e143764. doi: 10.1172/JCI143764.
54. Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PloS One. (2012) 7:e46342. doi: 10.1371/journal.pone.0046342.
55. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. (2011) 331:44–9. doi: 10.1126/science.1198687.
56. Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SEA, Yagita H, et al. Activation of NK cell cytotoxicity. Mol Immunol. (2005) 42:501–10. doi: 10.1016/j.molimm.2004.07.034.
57. Wu Q, Tian P, He D, Jia Z, He Y, Luo W, et al. SCUBE2 mediates bone metastasis of luminal breast cancer by modulating immune-suppressive osteoblastic niches. Cell Res. (2023) 33:464–78. doi: 10.1038/s41422-023-00810-6.
58. Walker SR, Nelson EA, Zou L, Chaudhury M, Signoretti S, Richardson A, et al. Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol Cancer Res. (2009) 7:966–76. doi: 10.1158/1541-7786.MCR-08-0238.
59. Marotta LLC, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24– stem cell-like breast cancer cells in human tumors. J Clin Invest. (2011) 121:2723–35. doi: 10.1172/JCI44745.
60. Bottos A, Gotthardt D, Gill JW, Gattelli A, Frei A, Tzankov A, et al. Decreased NK-cell tumour immunosurveillance consequent to JAK inhibition enhances metastasis in breast cancer models. Nat Commun. (2016) 7:12258. doi: 10.1038/ncomms12258.
61. Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. (1998) 91:1706–15. doi: 10.1182/blood.V91.5.1706.1706_1706_1715.
62. Sone S, Yano S. Molecular pathogenesis and its therapeutic modalities of lung cancer metastasis to bone. Cancer Metastasis Rev. (2007) 26:685–9. doi: 10.1007/s10555-007-9081-z.
63. Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. (2006) 18:49–53. doi: 10.1016/j.coi.2005.11.008.
64. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. (2004) 4:71–8. doi: 10.1038/nrc1256.
65. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. (2013) 496:445–55. doi: 10.1038/nature12034.
66. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. (2010) 11:889–96. doi: 10.1038/ni.1937.
67. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. (2019) 12:76. doi: 10.1186/s13045-019-0760-3.
68. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. (2005) 5:953–64. doi: 10.1038/nri1733.
69. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. (2002) 23:549–55. doi: 10.1016/S1471-4906(02)02302-5.
70. Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. (2014) 40:274–88. doi: 10.1016/j.immuni.2014.01.006.
71. Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front Immunol. (2015) 6:263. doi: 10.3389/fimmu.2015.00263
72. Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. (2019) 10:2035. doi: 10.3389/fimmu.2019.02035.
73. Haloul M, Oliveira ERA, Kader M, Wells JZ, Tominello TR, El Andaloussi A, et al. mTORC1-mediated polarization of M1 macrophages and their accumulation in the liver correlate with immunopathology in fatal ehrlichiosis. Sci Rep. (2019) 9:14050. doi: 10.1038/s41598-019-50320-y.
74. Mehta AK, Kadel S, Townsend MG, Oliwa M, Guerriero JL. Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol. (2021) 12:643771. doi: 10.3389/fimmu.2021.643771.
75. Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. (2015) 125:3365–76. doi: 10.1172/JCI80006.
76. Kfoury Y, Baryawno N, Severe N, Mei S, Gustafsson K, Hirz T, et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell. (2021) 39:1464–1478.e8. doi: 10.1016/j.ccell.2021.09.005.
77. Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J BioMed Sci. (2019) 26:78. doi: 10.1186/s12929-019-0568-z.
78. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. (2002) 196:254–65. doi: 10.1002/path.1027.
79. Annacker O, Asseman C, Read S, Powrie F. Interleukin-10 in the regulation of T cell-induced colitis. J Autoimmun. (2003) 20:277–9. doi: 10.1016/S0896-8411(03)00045-3.
80. Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. (2005) 8:369–80. doi: 10.1016/j.ccr.2005.10.012.
81. Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. (2014) 26:623–37. doi: 10.1016/j.ccell.2014.09.006.
82. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. (2012) 188:21–8. doi: 10.4049/jimmunol.1101029.
83. Kim OH, Kang GH, Noh H, Cha JY, Lee HJ, Yoon JH, et al. Proangiogenic TIE2(+)/CD31 (+) macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes. Mol Cells. (2013) 36:432–8. doi: 10.1007/s10059-013-0194-7.
84. Ye X, Huang X, Fu X, Zhang X, Lin R, Zhang W, et al. Myeloid-like tumor hybrid cells in bone marrow promote progression of prostate cancer bone metastasis. J Hematol Oncol. (2023) 16:46. doi: 10.1186/s13045-023-01442-4.
85. Cho HJ, Jung JI, Lim DY, Kwon GT, Her S, Park JH, et al. Bone marrow-derived, alternatively activated macrophages enhance solid tumor growth and lung metastasis of mammary carcinoma cells in a Balb/C mouse orthotopic model. Breast Cancer Res. (2012) 14:R81. doi: 10.1186/bcr3195.
86. Hiraoka K, Zenmyo M, Watari K, Iguchi H, Fotovati A, Kimura YN, et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Sci. (2008) 99:1595–602. doi: 10.1111/j.1349-7006.2008.00880.x.
87. Fend L, Accart N, Kintz J, Cochin S, Reymann C, Le Pogam F, et al. Therapeutic effects of anti-CD115 monoclonal antibody in mouse cancer models through dual inhibition of tumor-associated macrophages and osteoclasts. PloS One. (2013) 8:e73310. doi: 10.1371/journal.pone.0073310.
88. Herroon MK, Rajagurubandara E, Rudy DL, Chalasani A, Hardaway AL, Podgorski I. Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene. (2013) 32:1580–93. doi: 10.1038/onc.2012.166.
89. Jiang P, Gao W, Ma T, Wang R, Piao Y, Dong X, et al. CD137 promotes bone metastasis of breast cancer by enhancing the migration and osteoclast differentiation of monocytes/macrophages. Theranostics. (2019) 9:2950–66. doi: 10.7150/thno.29617.
90. Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. (2020) 11:234. doi: 10.1038/s41419-020-2435-y.
91. Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. (2009) 11:1235–42. doi: 10.1593/neo.09988.
92. Ma RY, Zhang H, Li XF, Zhang CB, Selli C, Tagliavini G, et al. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J Exp Med. (2020) 217:e20191820. doi: 10.1084/jem.20191820.
93. Roca H, Jones JD, Purica MC, Weidner S, Koh AJ, Kuo R, et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J Clin Invest. (2018) 128:248–66. doi: 10.1172/JCI92466.
94. Sullivan AR, Pixley FJ. CSF-1R signaling in health and disease: a focus on the mammary gland. J Mammary Gland Biol Neoplasia. (2014) 19:149–59. doi: 10.1007/s10911-014-9320-1.
95. Drapkin BJ, Farago AF. Unexpected synergy reveals new therapeutic strategy in SCLC. Trends Pharmacol Sci. (2019) 40:295–7. doi: 10.1016/j.tips.2019.03.005.
96. Halin Bergström S, Nilsson M, Adamo H, Thysell E, Jernberg E, Stattin P, et al. Extratumoral heme oxygenase-1 (HO-1) expressing macrophages likely promote primary and metastatic prostate tumor growth. PloS One. (2016) 11:e0157280. doi: 10.1371/journal.pone.0157280.
97. Ling Z, Yang C, Tan J, Dou C, Chen Y. Beyond immunosuppressive effects: dual roles of myeloid-derived suppressor cells in bone-related diseases. Cell Mol Life Sci. (2021) 78:7161–83. doi: 10.1007/s00018-021-03966-9.
98. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. (2009) 9:162–74. doi: 10.1038/nri2506.
99. Sawant A, Ponnazhagan S. Myeloid-derived suppressor cells as osteoclast progenitors: a novel target for controlling osteolytic bone metastasis. Cancer Res. (2013) 73:4606–10. doi: 10.1158/0008-5472.CAN-13-0305.
100. Ryan MR, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ, et al. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci U S A. (2005) 102:16735–40. doi: 10.1073/pnas.0505168102.
101. Keskinov AA, Shurin MR. Myeloid regulatory cells in tumor spreading and metastasis. Immunobiology. (2015) 220:236–42. doi: 10.1016/j.imbio.2014.07.017.
102. Wu MY, Li CJ, Yiang GT, Cheng YL, Tsai APY, Hou YT, et al. Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem. (2018) 46:1423–38. doi: 10.1159/000489184.
103. Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. (2011) 46:156–64. doi: 10.3109/00365521.2010.516450.
104. Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. (2009) 182:240–9. doi: 10.4049/jimmunol.182.1.240.
105. Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR–/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. (2010) 72:540–7. doi: 10.1111/sji.2010.72.issue-6.
106. Won WJ, Deshane JS, Leavenworth JW, Oliva CR, Griguer CE. Metabolic and functional reprogramming of myeloid-derived suppressor cells and their therapeutic control in glioblastoma. Cell Stress. (2019) 3:47–65. doi: 10.15698/cst.
107. Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med. (2016) 13:206–14. doi: 10.20892/j.issn.2095-3941.2015.0070.
108. Wang SH, Lu QY, Guo YH, Song YY, Liu PJ, Wang YC. The blockage of Notch signalling promoted the generation of polymorphonuclear myeloid-derived suppressor cells with lower immunosuppression. Eur J Cancer. (2016) 68:90–105. doi: 10.1016/j.ejca.2016.08.019.
109. Rashid MH, Borin TF, Ara R, Piranlioglu R, Achyut BR, Korkaya H, et al. Critical immunosuppressive effect of MDSC−derived exosomes in the tumor microenvironment. Oncol Rep. (2021) 45:1171–81. doi: 10.3892/or.
110. Danilin S, Merkel AR, Johnson JR, Johnson RW, Edwards JR, Sterling JA. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology. (2012) 1:1484–94. doi: 10.4161/onci.21990.
111. Li BH, Garstka MA, Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol. (2020) 117:201–15. doi: 10.1016/j.molimm.2019.11.014.
112. Wang Y, Ding Y, Guo N, Wang S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol. (2019) 10:172. doi: 10.3389/fimmu.2019.00172.
113. Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. (2012) 18:1224–31. doi: 10.1038/nm.2830.
114. An J, Feng L, Ren J, Li Y, Li G, Liu C, et al. Chronic stress promotes breast carcinoma metastasis by accumulating myeloid-derived suppressor cells through activating β-adrenergic signaling. Oncoimmunology. (2021) 10:2004659. doi: 10.1080/2162402X.2021.2004659.
115. Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, et al. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. (2013) 73:672–82. doi: 10.1158/0008-5472.CAN-12-2202.
116. Papachristou DJ, Basdra EK, Papavassiliou AG. Bone metastases: molecular mechanisms and novel therapeutic interventions. Med Res Rev. (2012) 32:611–36. doi: 10.1002/med.20224.
117. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. (1998) 392:245–52. doi: 10.1038/32588.
118. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. (2007) 449:419–26. doi: 10.1038/nature06175.
119. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. (2012) 30:1–22. doi: 10.1146/annurev-immunol-100311-102839.
120. Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. (1973) 137:1142–62. doi: 10.1084/jem.137.5.1142.
121. Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. (1998) 188:1359–68. doi: 10.1084/jem.188.7.1359.
122. Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. (2001) 19:47–64. doi: 10.1146/annurev.immunol.19.1.47.
123. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. (2012) 12:265–77. doi: 10.1038/nrc3258.
124. Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AWM, et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat Immunol. (2005) 6:1029–37. doi: 10.1038/ni1249.
125. Capietto AH, Faccio R. Immune regulation of bone metastasis. Bonekey Rep. (2014) 3:600. doi: 10.1038/bonekey.2014.95.
126. Magidey-Klein K, Cooper TJ, Kveler K, Normand R, Zhang T, Timaner M, et al. IL-6 contributes to metastatic switch via the differentiation of monocytic-dendritic progenitors into prometastatic immune cells. J Immunother Cancer. (2021) 9:e002856. doi: 10.1136/jitc-2021-002856.
127. Giorello MB, Matas A, Marenco P, Davies KM, Borzone FR, Calcagno M de L, et al. CD1a- and CD83-positive dendritic cells as prognostic markers of metastasis development in early breast cancer patients. Breast Cancer. (2021) 28:1328–39. doi: 10.1007/s12282-021-01270-9.
128. Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. (2012) 189:4258–65. doi: 10.4049/jimmunol.1101855.
129. Lu T, Zhang Z, Bi Z, Lan T, Zeng H, Liu Y, et al. TFAM deficiency in dendritic cells leads to mitochondrial dysfunction and enhanced antitumor immunity through cGAS-STING pathway. J Immunother Cancer. (2023) 11:e005430. doi: 10.1136/jitc-2022-005430.
130. Correale P, Micheli L, Vecchio MT, Sabatino M, Petrioli R, Pozzessere D, et al. A parathyroid-hormone-related-protein (PTH-rP)-specific cytotoxic T cell response induced by in vitro stimulation of tumour-infiltrating lymphocytes derived from prostate cancer metastases, with epitope peptide-loaded autologous dendritic cells and low-dose IL-2. Br J Cancer. (2001) 85:1722–30. doi: 10.1054/bjoc.2001.2136.
131. Imai K, Minamiya Y, Koyota S, Ito M, Saito H, Sato Y, et al. Inhibition of dendritic cell migration by transforming growth factor-β1 increases tumor-draining lymph node metastasis. J Exp Clin Cancer Res. (2012) 31:3. doi: 10.1186/1756-9966-31-3.
132. Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. (1997) 390:175–9. doi: 10.1038/36593.
133. Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. (1999) 13:2412–24. doi: 10.1101/gad.13.18.2412.
134. Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, et al. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med. (2000) 191:495–502. doi: 10.1084/jem.191.3.495.
135. Totsuka T, Kanai T, Nemoto Y, Tomita T, Okamoto R, Tsuchiya K, et al. RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol. (2009) 182:6079–87. doi: 10.4049/jimmunol.0711823.
136. Demoulin SA, Somja J, Duray A, Guénin S, Roncarati P, Delvenne PO, et al. Cervical (pre)neoplastic microenvironment promotes the emergence of tolerogenic dendritic cells via RANKL secretion. Oncoimmunology. (2015) 4:e1008334. doi: 10.1080/2162402X.2015.1008334.
137. Ahern E, Harjunpää H, Barkauskas D, Allen S, Takeda K, Yagita H, et al. Co-administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated antitumor immunity in mice. Clin Cancer Res. (2017) 23:5789–801. doi: 10.1158/1078-0432.CCR-17-0606.
138. Esposito M, Kang Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol Ther. (2014) 141:222–33. doi: 10.1016/j.pharmthera.2013.10.006.
139. Thiede MA, Smock SL, Petersen DN, Grasser WA, Thompson DD, Nishimoto SK. Presence of messenger ribonucleic acid encoding osteocalcin, a marker of bone turnover, in bone marrow megakaryocytes and peripheral blood platelets. Endocrinology. (1994) 135:929–37. doi: 10.1210/endo.135.3.8070388.
140. Kelm RJ, Hair GA, Mann KG, Grant BW. Characterization of human osteoblast and megakaryocyte-derived osteonectin (SPARC). Blood. (1992) 80:3112–9. doi: 10.1182/blood.V80.12.3112.bloodjournal80123112.
141. Breton-Gorius J, Clezardin P, Guichard J, Debili N, Malaval L, Vainchenker W, et al. Localization of platelet osteonectin at the internal face of the alpha-granule membranes in platelets and megakaryocytes. Blood. (1992) 79:936–41. doi: 10.1182/blood.V79.4.936.bloodjournal794936.
142. Chenu C, Delmas PD. Platelets contribute to circulating levels of bone sialoprotein in human. J Bone Miner Res. (1992) 7:47–54. doi: 10.1002/jbmr.5650070108.
143. Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, et al. Megakaryocyte-mediated inhibition of osteoclast development. Bone. (2006) 39:991–9. doi: 10.1016/j.bone.2006.05.004.
144. Li X, Koh AJ, Wang Z, Soki FN, Park SI, Pienta KJ, et al. Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J Bone Miner Res. (2011) 26:125–34. doi: 10.1002/jbmr.204.
145. Previdi S, Maroni P, Matteucci E, Broggini M, Bendinelli P, Desiderio MA. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and beta-catenin/Wnt pathways. Eur J Cancer. (2010) 46:1679–91. doi: 10.1016/j.ejca.2010.02.036.
146. Jackson W, Sosnoski DM, Ohanessian SE, Chandler P, Mobley A, Meisel KD, et al. Role of megakaryocytes in breast cancer metastasis to bone. Cancer Res. (2017) 77:1942–54. doi: 10.1158/0008-5472.CAN-16-1084.
147. Cappariello A, Maurizi A, Veeriah V, Teti A. Reprint of: The Great Beauty of the osteoclast. Arch Biochem Biophys. (2014) 561:13–21. doi: 10.1016/j.abb.2014.08.009.
148. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. (2003) 423:337–42. doi: 10.1038/nature01658.
149. Buenrostro D, Mulcrone PL, Owens P, Sterling JA. The bone microenvironment: a fertile soil for tumor growth. Curr Osteoporos Rep. (2016) 14:151–8. doi: 10.1007/s11914-016-0315-2.
150. Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. (2002) 62:5571–9.
151. Boucharaba A, Serre CM, Grès S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. (2004) 114:1714–25. doi: 10.1172/JCI22123.
152. Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. (1996) 98:1544–9. doi: 10.1172/JCI118947.
153. Ouellet V, Tiedemann K, Mourskaia A, Fong JE, Tran-Thanh D, Amir E, et al. CCN3 impairs osteoblast and stimulates osteoclast differentiation to favor breast cancer metastasis to bone. Am J Pathol. (2011) 178:2377–88. doi: 10.1016/j.ajpath.2011.01.033.
154. Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. (1999) 140:4451–8. doi: 10.1210/en.140.10.4451.
155. Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. (2011) 11:411–25. doi: 10.1038/nrc3055.
156. Welm AL, Sneddon JB, Taylor C, Nuyten DSA, van de Vijver MJ, Hasegawa BH, et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A. (2007) 104:7570–5. doi: 10.1073/pnas.0702095104.
157. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. (2014) 5:511. doi: 10.3389/fimmu.2014.00511.
158. Miao D, He B, Jiang Y, Kobayashi T, Sorocéanu MA, Zhao J, et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest. (2005) 115:2402–11. doi: 10.1172/JCI24918.
159. Ma Q, Liang M, Wu Y, Dou C, Xu J, Dong S, et al. Small extracellular vesicles deliver osteolytic effectors and mediate cancer-induced osteolysis in bone metastatic niche. J Extracell Vesicles. (2021) 10:e12068. doi: 10.1002/jev2.12068.
160. Mulholland BS, Forwood MR, Morrison NA. Monocyte chemoattractant protein-1 (MCP-1/CCL2) drives activation of bone remodelling and skeletal metastasis. Curr Osteoporos Rep. (2019) 17:538–47. doi: 10.1007/s11914-019-00545-7.
161. Wu K, Feng J, Lyu F, Xing F, Sharma S, Liu Y, et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat Commun. (2021) 12:5196. doi: 10.1038/s41467-021-25473-y.
162. Yue Z, Niu X, Yuan Z, Qin Q, Jiang W, He L, et al. RSPO2 and RANKL signal through LGR4 to regulate osteoclastic premetastatic niche formation and bone metastasis. J Clin Invest. (2022) 132:e144579. doi: 10.1172/JCI144579.
163. Mourskaia AA, Amir E, Dong Z, Tiedemann K, Cory S, Omeroglu A, et al. ABCC5 supports osteoclast formation and promotes breast cancer metastasis to bone. Breast Cancer Res. (2012) 14:R149. doi: 10.1186/bcr3361.
164. Chen J, Xu W, Song K, Da LT, Zhang X, Lin M, et al. Legumain inhibitor prevents breast cancer bone metastasis by attenuating osteoclast differentiation and function. Bone. (2023) 169:116680. doi: 10.1016/j.bone.2023.116680.
165. Li L, Ameri AH, Wang S, Jansson KH, Casey OM, Yang Q, et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene. (2019) 38:6241–55. doi: 10.1038/s41388-019-0873-8.
166. He Y, Luo W, Liu Y, Wang Y, Ma C, Wu Q, et al. IL-20RB mediates tumoral response to osteoclastic niches and promotes bone metastasis of lung cancer. J Clin Invest. (2022) 132:e157917. doi: 10.1172/JCI157917.
167. Nakajima K, Kho DH, Yanagawa T, Harazono Y, Hogan V, Chen W, et al. Galectin-3 cleavage alters bone remodeling: different outcomes in breast and prostate cancer skeletal metastasis. Cancer Res. (2016) 76:1391–402. doi: 10.1158/0008-5472.CAN-15-1793.
168. Uluçkan O, Becker SN, Deng H, Zou W, Prior JL, Piwnica-Worms D, et al. CD47 regulates bone mass and tumor metastasis to bone. Cancer Res. (2009) 69:3196–204. doi: 10.1158/0008-5472.CAN-08-3358.
169. Cai WL, Huang WD, Li B, Chen TR, Li ZX, Zhao CL, et al. microRNA-124 inhibits bone metastasis of breast cancer by repressing Interleukin-11. Mol Cancer. (2018) 17:9. doi: 10.1186/s12943-017-0746-0.
170. Yu L, Sui B, Fan W, Lei L, Zhou L, Yang L, et al. Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p. J Extracell Vesicles. (2021) 10:e12056. doi: 10.1002/jev2.12056.
171. Urabe F, Kosaka N, Yamamoto Y, Ito K, Otsuka K, Soekmadji C, et al. Metastatic prostate cancer-derived extracellular vesicles facilitate osteoclastogenesis by transferring the CDCP1 protein. J Extracell Vesicles. (2023) 12:e12312. doi: 10.1002/jev2.12312.
172. Zhang S, Liao X, Chen S, Qian W, Li M, Xu Y, et al. Large oncosome-loaded VAPA promotes bone-tropic metastasis of hepatocellular carcinoma via formation of osteoclastic pre-metastatic niche. Adv Sci (Weinh). (2022) 9:e2201974. doi: 10.1002/advs.202201974.
173. Verbruggen ASK, McNamara LM. Mechanoregulation may drive osteolysis during bone metastasis: A finite element analysis of the mechanical environment within bone tissue during bone metastasis and osteolytic resorption. J Mech Behav BioMed Mater. (2023) 138:105662. doi: 10.1016/j.jmbbm.2023.105662.
174. Li X, Liang Y, Lian C, Peng F, Xiao Y, He Y, et al. CST6 protein and peptides inhibit breast cancer bone metastasis by suppressing CTSB activity and osteoclastogenesis. Theranostics. (2021) 11:9821–32. doi: 10.7150/thno.62187.
175. Kong D, Ye C, Zhang C, Sun X, Wang F, Chen R, et al. Procoxacin bidirectionally inhibits osteoblastic and osteoclastic activity in bone and suppresses bone metastasis of prostate cancer. J Exp Clin Cancer Res. (2023) 42:45. doi: 10.1186/s13046-023-02610-7.
176. de Castro LF, Michel Z, Pan K, Taylor J, Szymczuk V, Paravastu S, et al. Safety and efficacy of denosumab for fibrous dysplasia of bone. N Engl J Med. (2023) 388:766–8. doi: 10.1056/NEJMc2214862.
177. Omata D, Munakata L, Maruyama K, Suzuki R. Ultrasound and microbubble-mediated drug delivery and immunotherapy. J Med Ultrason. (2001). doi: 10.1007/s10396-022-01201-x
178. Petitdemange C, Becquart P, Wauquier N, Béziat V, Debré P, Leroy EM, et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PloS Pathog. (2011) 7:e1002268. doi: 10.1371/journal.ppat.1002268.
179. Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. (2021) 9:e002591. doi: 10.1136/jitc-2021-002591.
180. Wang K, Donnelly CR, Jiang C, Liao Y, Luo X, Tao X, et al. STING suppresses bone cancer pain via immune and neuronal modulation. Nat Commun. (2021) 12:4558. doi: 10.1038/s41467-021-24867-2.
181. Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. (2000) 289:1501–4. doi: 10.1126/science.289.5484.1501.
182. Zunich SM, Valdovinos M, Douglas T, Walterhouse D, Iannaccone P, Lamm MLG. Osteoblast-secreted collagen upregulates paracrine Sonic hedgehog signaling by prostate cancer cells and enhances osteoblast differentiation. Mol Cancer. (2012) 11:30. doi: 10.1186/1476-4598-11-30.
183. Jin JK, Dayyani F, Gallick GE. Steps in prostate cancer progression that lead to bone metastasis. Int J Cancer. (2011) 128:2545–61. doi: 10.1002/ijc.26024.
184. Chen PC, Liu SC, Lin TH, Lin LW, Wu HC, Tai HC, et al. Prostate cancer-secreted CCN3 uses the GSK3β and β-catenin pathways to enhance osteogenic factor levels in osteoblasts. Environ Toxicol. (2021) 36:425–32. doi: 10.1002/tox.23048.
185. Devignes CS, Aslan Y, Brenot A, Devillers A, Schepers K, Fabre S, et al. HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc Natl Acad Sci U S A. (2018) 115:E992–1001. doi: 10.1073/pnas.1718009115.
186. Moon HH, Clines KL, O’Day PJ, Al-Barghouthi BM, Farber EA, Farber CR, et al. Osteoblasts generate testosterone from DHEA and activate androgen signaling in prostate cancer cells. J Bone Miner Res. (2021) 36:1566–79. doi: 10.1002/jbmr.4313.
187. Wu JB, Yin L, Shi C, Li Q, Duan P, Huang JM, et al. MAOA-dependent activation of shh-IL6-RANKL signaling network promotes prostate cancer metastasis by engaging tumor-stromal cell interactions. Cancer Cell. (2017) 31:368–82. doi: 10.1016/j.ccell.2017.02.003.
188. Kan C, Vargas G, Pape FL, Clézardin P. Cancer cell colonisation in the bone microenvironment. Int J Mol Sci. (2016) 17:1674. doi: 10.3390/ijms17101674.
189. Li Z, Xiao J, Wu X, Li W, Yang Z, Xie J, et al. Plumbagin inhibits breast tumor bone metastasis and osteolysis by modulating the tumor-bone microenvironment. Curr Mol Med. (2012) 12:967–81. doi: 10.2174/156652412802480871.
190. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. (2017) 168:670–91. doi: 10.1016/j.cell.2016.11.037.
191. Badia-Ramentol J, Linares J, Gómez-Llonin A, Calon A. Minimal residual disease, metastasis and immunity. Biomolecules. (2021) 11:130. doi: 10.3390/biom11020130.
192. Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer. (2020) 1:672–80. doi: 10.1038/s43018-020-0088-5.
193. Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. (2011) 121:1298–312. doi: 10.1172/JCI43414.
194. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. (2014) 14:611–22. doi: 10.1038/nrc3793.
195. Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. (2014) 14:306–21. doi: 10.1016/j.stem.2014.02.002.
196. Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. (2005) 353:793–802. doi: 10.1056/NEJMoa050434.
197. Melchior SW, Corey E, Ellis WJ, Ross AA, Layton TJ, Oswin MM, et al. Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clin Cancer Res. (1997) 3:249–56.
198. Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med. (2016) 8:340ra73. doi: 10.1126/scitranslmed.aad4059.
199. Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. (2005) 20:318–29. doi: 10.1359/JBMR.041109.
200. Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PloS One. (2013) 8:e61873. doi: 10.1371/journal.pone.0061873.
201. Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. (2011) 474:216–9. doi: 10.1038/nature10160.
202. Reinstein ZZ, Pamarthy S, Sagar V, Costa R, Abdulkadir SA, Giles FJ, et al. Overcoming immunosuppression in bone metastases. Crit Rev Oncol Hematol. (2017) 117:114–27. doi: 10.1016/j.critrevonc.2017.05.004.
203. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. (2009) 114:1537–44. doi: 10.1182/blood-2008-12-195792.
204. Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. (2010) 207:2175–86. doi: 10.1084/jem.20100637.
205. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. (2018) 8:86. doi: 10.3389/fonc.2018.00086.
206. Wen Y, Tang F, Tu C, Hornicek F, Duan Z, Min L. Immune checkpoints in osteosarcoma: Recent advances and therapeutic potential. Cancer Lett. (2022) 547:215887. doi: 10.1016/j.canlet.2022.215887.
207. Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. (1996) 8:765–72. doi: 10.1093/intimm/8.5.765.
208. Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. (2002) 84:57–62. doi: 10.1016/S0165-2478(02)00142-6.
209. Mizuno R, Sugiura D, Shimizu K, Maruhashi T, Watada M, Okazaki IM, et al. PD-1 primarily targets TCR signal in the inhibition of functional T cell activation. Front Immunol. (2019) 10:630. doi: 10.3389/fimmu.2019.00630.
210. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. (2005) 25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005.
211. Joseph GJ, Johnson DB, Johnson RW. Immune checkpoint inhibitors in bone metastasis: Clinical challenges, toxicities, and mechanisms. J Bone Oncol. (2023) 43:100505. doi: 10.1016/j.jbo.2023.100505.
212. Pham F, Belkaid S, Maillet D, Confavreux CB, Dalle S, Péron J. Impact of bone metastases on patients with renal cell carcinoma or melanoma treated with combotherapy ipilimumab plus nivolumab. Biomedicines. (2022) 10:2758. doi: 10.3390/biomedicines10112758.
213. Desai K, Brown L, Wei W, Tucker M, Kao C, Kinsey E, et al. A multi-institutional, retrospective analysis of patients with metastatic renal cell carcinoma to bone treated with combination ipilimumab and nivolumab. Target Oncol. (2021) 16:633–42. doi: 10.1007/s11523-021-00832-3.
214. Landi L, D’Incà F, Gelibter A, Chiari R, Grossi F, Delmonte A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. (2019) 7:316. doi: 10.1186/s40425-019-0793-8.
215. Zhu YJ, Chang XS, Zhou R, Chen YD, Ma HC, Xiao ZZ, et al. Bone metastasis attenuates efficacy of immune checkpoint inhibitors and displays “cold” immune characteristics in Non-small cell lung cancer. Lung Cancer. (2022) 166:189–96. doi: 10.1016/j.lungcan.2022.03.006.
216. Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest. (2020) 130:3603–20. doi: 10.1172/JCI133334.
217. Dahlberg CIM, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. (2015) 6:605. doi: 10.3389/fimmu.2015.00605.
218. Xavier KB. Bacterial interspecies quorum sensing in the mammalian gut microbiota. C. R Biologies. (2018) 341:300. doi: 10.1016/j.crvi.2018.03.006
219. Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. (2017) 5:53. doi: 10.1186/s40425-017-0257-y.
220. Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. (2015) 212:1043–59. doi: 10.1084/jem.20141836.
221. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217.
222. Zhang J, Pang Y, Xie T, Zhu L. CXCR4 antagonism in combination with IDO1 inhibition weakens immune suppression and inhibits tumor growth in mouse breast cancer bone metastases. Onco Targets Ther. (2019) 12:4985–92. doi: 10.2147/OTT.
223. Johansson M, Giger FA, Fielding T, Houart C. Dkk1 controls cell-cell interaction through regulation of non-nuclear β-catenin pools. Dev Cell. (2019) 51:775–786.e3. doi: 10.1016/j.devcel.2019.10.026.
224. Müller P, Martin K, Theurich S, von Bergwelt-Baildon M, Zippelius A. Cancer chemotherapy agents target intratumoral dendritic cells to potentiate antitumor immunity. Oncoimmunology. (2014) 3:e954460. doi: 10.4161/21624011.2014.954460.
225. MaChado FC, Girola N, Maia VSC, Bergami-Santos PC, Morais AS, Azevedo RA, et al. Immunomodulatory protective effects of rb9 cyclic-peptide in a metastatic melanoma setting and the involvement of dendritic cells. Front Immunol. (2019) 10:3122. doi: 10.3389/fimmu.2019.03122.
226. Mittal D, Vijayan D, Putz EM, Aguilera AR, Markey KA, Straube J, et al. Interleukin-12 from CD103+ Batf3-dependent dendritic cells required for NK-cell suppression of metastasis. Cancer Immunol Res. (2017) 5:1098–108. doi: 10.1158/2326-6066.CIR-17-0341.
Keywords: bone metastatic cancer, tumor immune microenvironment, TME, immune cell, immunosuppression, metastasizing
Citation: Chen S, Lei J, Mou H, Zhang W, Jin L, Lu S, Yinwang E, Xue Y, Shao Z, Chen T, Wang F, Zhao S, Chai X, Wang Z, Zhang J, Zhang Z, Ye Z and Li B (2024) Multiple influence of immune cells in the bone metastatic cancer microenvironment on tumors. Front. Immunol. 15:1335366. doi: 10.3389/fimmu.2024.1335366
Received: 08 November 2023; Accepted: 07 February 2024;
Published: 23 February 2024.
Edited by:
Selvarangan Ponnazhagan, University of Alabama at Birmingham, United StatesReviewed by:
Heather Marie Gibson, Wayne State University, United StatesSubhashis Pal, Emory University, United States
Copyright © 2024 Chen, Lei, Mou, Zhang, Jin, Lu, Yinwang, Xue, Shao, Chen, Wang, Zhao, Chai, Wang, Zhang, Zhang, Ye and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binghao Li, bGliaW5naGFvQHpqdS5lZHUuY24=; Zhaoming Ye, eWV6aGFvbWluZ0B6anUuZWR1LmNu; Zengjie Zhang, emVuZ2ppZXpoYW5nQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Shixin Chen
Shixin Chen Jiangchu Lei1,2,3,4†
Jiangchu Lei1,2,3,4† Eloy Yinwang
Eloy Yinwang Zhenxuan Shao
Zhenxuan Shao Tao Chen
Tao Chen Xupeng Chai
Xupeng Chai Zenan Wang
Zenan Wang Zengjie Zhang
Zengjie Zhang Zhaoming Ye
Zhaoming Ye Binghao Li
Binghao Li