- Department of Otorhinolaryngology, The First People’s Hospital of Changzhou, The Third Affiliated Hospital of Soochow University, Soochow University, Changzhou, China
Objective: The purpose was to evaluate the relationship between peripheral eosinophilia, Japan Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC) score, and olfactory dysfunction in chronic rhinosinusitis (CRS) patients and to explore the accuracy and specific cut points of the JESREC score in predicting olfactory dysfunction.
Methods: In this cross-sectional, retrospective study, olfactory function was assessed by the Sniffin’ Sticks 12-item test and multivariate logistic regression analyses were carried out. Receiver operating characteristic curves were plotted to derive accuracy and cutoff values for the JESREC scores of the olfactory dysfunction criterion.
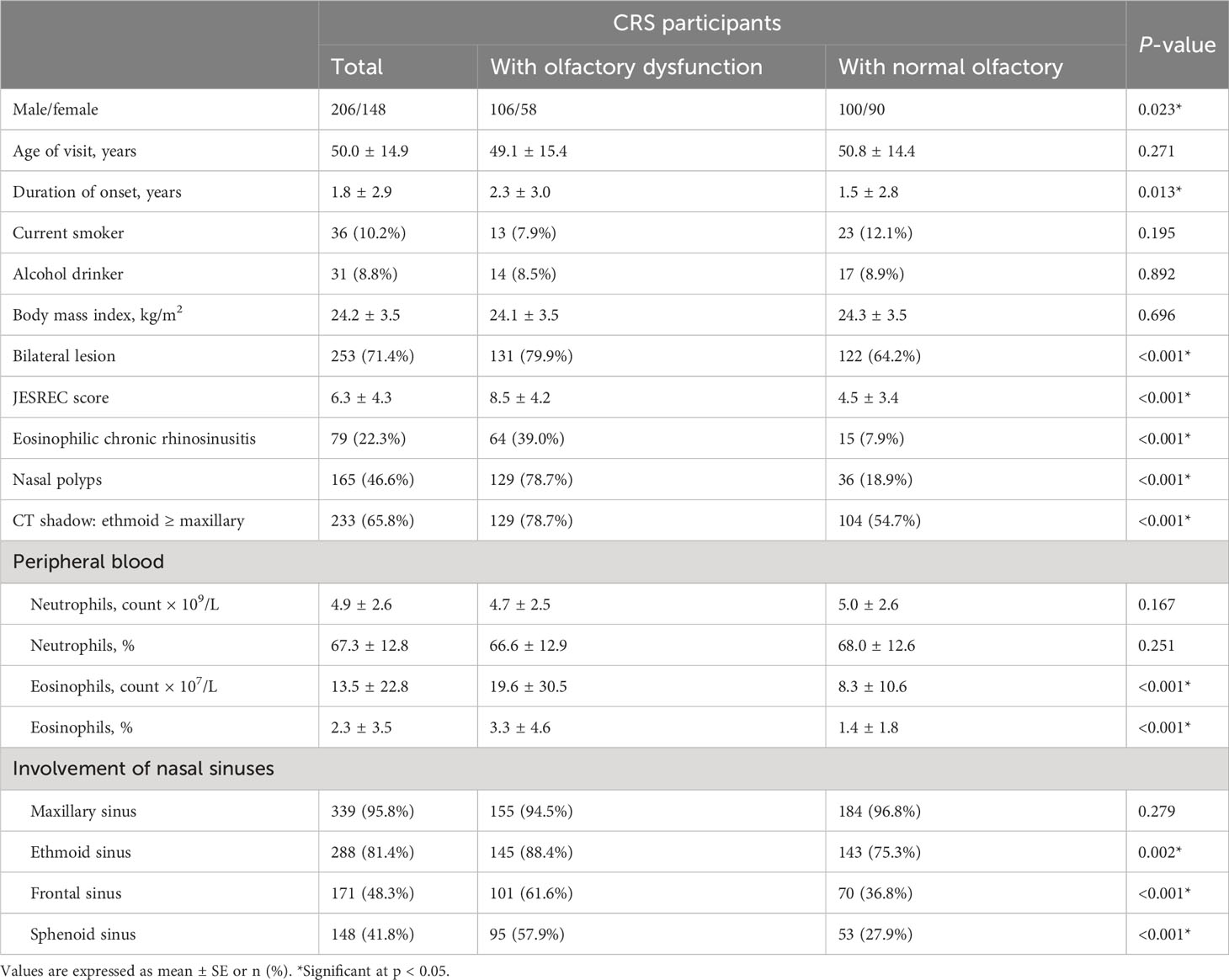
Results: A total of 354 patients [mean (SD) age, 50.0 (14.9) years; 41.8% women] were included in the final analysis. The prevalence of olfactory dysfunction was 46.3%. Individuals who had olfactory dysfunction were more likely to be male (64.6% vs. 52.6%), have eosinophilic chronic rhinosinusitis (ECRS) (39.0% vs. 7.9%), have a longer course of CRS (2.3 years vs. 1.5 years), have higher JESREC scores (8.5 vs. 4.5), and have higher proportions of nasal polyps (78.7% vs. 18.9%) and peripheral eosinophilia (3.3% vs. 1.4%). In logistic analysis, the percentage of eosinophils (1.25, 1.13–1.37), JESREC score (1.31, 1.22–1.40), bilateral lesion (2.06, 1.25–3.41), nasal polyps (15.83, 9.23–27.16), CT shadow (2.73, 1.69–4.43), and ECRS (6.86, 3.68–12.80) were associated with olfactory dysfunction in CRS patients after controlling for covariates, while peripheral neutrophils were not significant. In addition, the area under the curve was 0.778 and the cutoff value for JESREC score for olfactory dysfunction was defined as 5.5.
Conclusions: Peripheral eosinophilia and high JESREC scores were significantly associated with the risk of olfactory dysfunction in CRS patients, and special attention should be paid to patients with a JESREC score ≥6.
Introduction
Chronic rhinosinusitis (CRS) is a common chronic inflammatory disease of the nasal cavity and paranasal sinuses, which is often accompanied by olfactory dysfunction (1). Studies have shown that the incidence of olfactory dysfunction secondary to CRS is between 56% and 74%, with significant effects on the quality of life, including effects on diet, communication, and emotional wellbeing and loss of perception of dangerous odors, such as gases and toxic and harmful gases. However, the underlying mechanism remains elusive (2–5).
Eosinophils are key players in the pathogenesis of allergic diseases and are increasingly recognized to play a role in the pathogenesis of CRS through the release of inflammatory mediators and tissue damage (6–8). Elevated levels of eosinophils have been observed in the nasal polyps and mucosa of patients with CRS, and their activation and release of cytokines and other mediators have been implicated in the development of CRS-associated olfactory dysfunction (9, 10). Peripheral eosinophilia is a common feature of CRS, with elevated eosinophil counts present in up to 40% of patients. Elevated peripheral eosinophil counts have been associated with more severe CRS symptoms and poorer treatment outcomes (11–13), but their relationship with olfactory dysfunction has not been widely studied. In addition, based on clinical data, the Japan Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC) study divided CRS into two subtypes, eosinophilic chronic rhinosinusitis (ECRS) and non-ECRS, which promotes precise research. Previous studies have confirmed that the JESREC scoring system was significantly correlated with prognosis (14), but few studies have focused on the relationship between JESREC score and olfactory dysfunction, and no studies have identified specific cut points. Considering the easy availability and operability of the JESREC scoring system in clinical application, it is very important and urgent to clarify the relationship between JESREC score and olfactory dysfunction and the specific cutoff point of the JESREC score in predicting olfactory dysfunction among CRS patients.
Based on these observations, the aim of our study was to investigate the potential relationship between peripheral eosinophilia, JESREC score, and olfactory dysfunction in patients with CRS. In addition, receiver operating characteristic curves were plotted to derive accuracy and cutoff values for the JESREC scores of the olfactory dysfunction criterion.
Materials and methods
Study population and design
The study population is composed of the 354 CRS patients who were assessed at the Third Affiliated Hospital of Soochow University (Changzhou, China) between March 2021 and September 2022. As described in our previous research (15), they were all Han Chinese, and none of them were infected with COVID-19. The study was approved by the Ethics Committee of Third Affiliated Hospital of Soochow University (2022CL070), and due to the use of anonymous data and the lack of any intervention, informed consent was not required.
The inclusion criteria were as follows: 1) adult patients aged over 18 years; 2) complete clinical data, including detailed medical records and nasal endoscopy, laboratory, and imaging examinations; and 3) conformity to the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2012 diagnostic criteria of CRS (16). The exclusion criteria were as follows: 1) history of medical treatment or surgery for CRS, 2) asthma and liver diseases, 3) nasal diseases that may affect olfactory sensitivity, and 4) taking glucocorticoids or hepatotoxic drugs.
Data collection and covariates
Data collection was performed by medical staff in the department of otolaryngology according to a standard protocol when the patient first came to our hospital. Detailed information on demographic characteristics, medical records, and laboratory testing was obtained. Current drinkers were defined as those who were drinking or had stopped drinking for less than 6 months, and smokers were defined as those who were currently smoking or had stopped smoking for less than 6 months. The duration of CRS (unit: year) was defined as years between the first diagnosis and baseline evaluation in a diagnosed patient. Nasal polyps were identified by nasal endoscopy or sinus CT. Peripheral blood collection and complete blood count were performed in the central clinical laboratory of Third Affiliated Hospital of Soochow University by automated analyzers.
The criteria of the JESREC score (14) were as follows: 1) disease side: both sides vs. one side (3 vs. 0); 2) nasal polyps: presence vs. absence (2 vs. 0); 3) CT shadow: ethmoid ≥ maxillary: positive vs. negative (2 vs. 0); and 4) percentage of eosinophils of peripheral blood: ≤2 vs. >2 to ≤5 vs. >5 to ≤10 vs. >10 (0 vs. 4 vs. 8 vs. 10). A JESREC score ≥11 indicated ECRS and <11 indicated non-ECRS.
Assessment of olfactory dysfunction
Olfactory function was assessed by the Sniffin’ Sticks 12-item test (SST-12). Briefly, participants were asked to smell for 3 to 4 s in turn and identify correct odors using a multiple-choice format. Correctly identified odorants were assigned 1 point, and the total of the SST-12 score ranges from 0 to 12. An SST-12 score <11 indicated olfactory dysfunction (17, 18).
Statistical analysis
IBM SPSS Statistics 24.0 (SPSS Inc., Chicago, IL, United States) was used for all statistical analyses. Continuous variables were expressed as mean with SD, and categorical variables were presented as frequency (percentage). The characteristics of the participants were separated by olfactory function, and comparison between the two groups was performed by two-tailed unpaired t-tests or chi-squared tests (χ2-test) or Wilcoxon signed-rank tests. Multivariate logistic regression analyses were carried out to examine the associations of eosinophils of peripheral blood, JESREC score, and CRS subtype with the risk of olfactory dysfunction. Odds ratios (ORs) were estimated from two models: model 1 was adjusted for age and sex; model 2 was further adjusted for smoking and alcohol consumption. In addition, to derive cutoff values for the JESREC scores of olfactory dysfunction criterion, we constructed receiver operating characteristic (ROC) curves. Significance was accepted at P < 0.05.
Results
General characteristics
Table 1 shows the general characteristics of the participants. A total of 354 patients [mean (SD) age, 50.0 (14.9) years; 41.8% women; mean (SD) duration of CRS, 1.8 (2.9) years] were included in the final analysis. Compared with patients with normal olfactory function, individuals who had olfactory dysfunction were more likely to be male (64.6% vs. 52.6%), have ECRS (39.0% vs. 7.9%), have a longer course of CRS (2.3 years vs. 1.5 years), have higher JESREC scores (8.5 vs. 4.5), and have higher proportions of nasal polyps (78.7% vs. 18.9%), bilateral lesion (79.9% vs. 64.2%), and peripheral blood eosinophils (3.3% vs. 1.4%).
Association between peripheral eosinophilia, JESREC score, and olfactory dysfunction
Table 2 shows the multivariate logistic regression analysis with olfactory dysfunction among adult CRS patients. The adjusted OR for olfactory dysfunction was not significant with peripheral blood neutrophils, which were displayed as counts (0.94, 0.86–1.03) or percentages (0.99, 0.97–1.01). The JESREC score (1.31, 1.22–1.40) and its various components (bilateral lesion: 2.06, 1.25–3.41; nasal polyps: 15.83, 9.23–27.16; CT shadow: 2.73, 1.69–4.43; percentage of eosinophils of peripheral blood: 1.25, 1.13–1.37) were associated with olfactory dysfunction after adjusting for covariates. In addition, compared with non-ECRS, ECRS determined by the JESREC score was also risk factor of olfactory function (model 2, 6.86, 3.68–12.80).
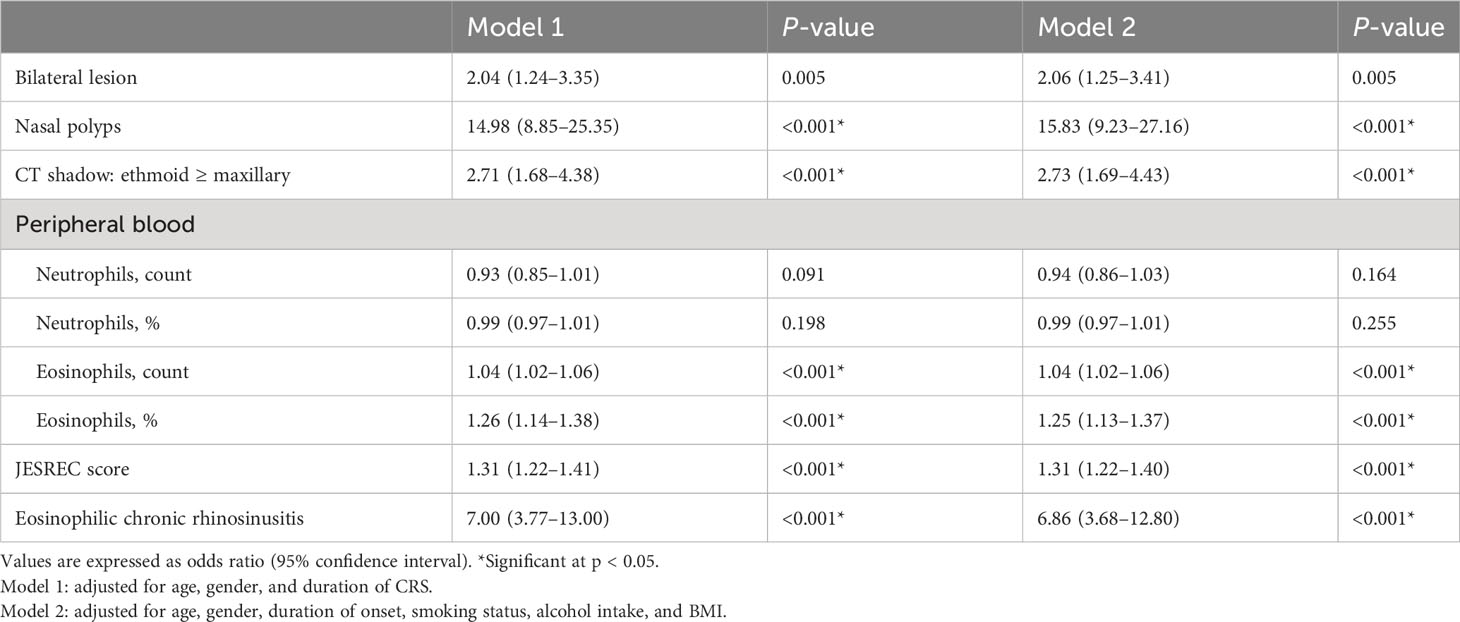
Table 2 Multivariate logistic regression analysis with olfactory dysfunction as a dependent variable in CRS patients.
To clarify the specific cut points of JESREC score for predicting olfactory dysfunction in patients with CRS, the ROC curve was plotted (Figure 1A). The area under the curve (AUC) was 0.778, and the cutoff value for the JESREC score for olfactory dysfunction was defined as 5.5 (Figure 1B). If the JESREC score was 6 or higher, the case was more likely to have a decreased sense of smell, which requires attention and active treatment from doctors. Sensitivity and specificity were 70.7% and 74.7%, respectively.
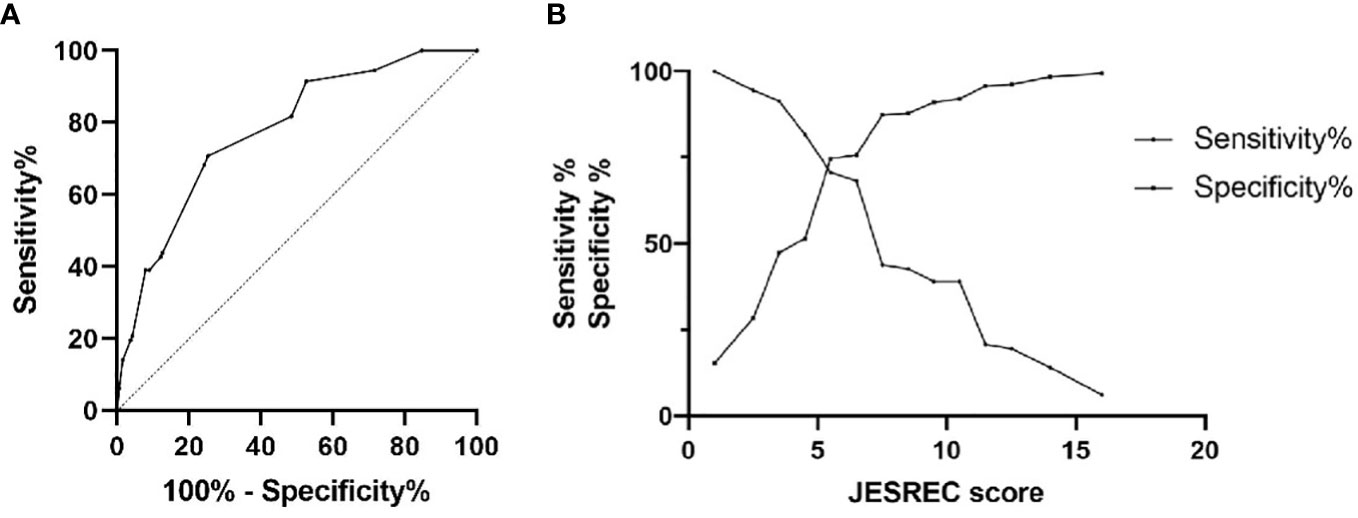
Figure 1 ROC curve analysis for JESREC score on olfactory dysfunction among adult CRS patients. (A) ROC curve for olfactory dysfunction: AUC was 0.778. (B) Sensitivity‐Specificity Plot: Cutoff value of JESREC score was 5.5, intersection point.
Discussion
This study indicated that peripheral eosinophilia and JESREC score were strongly associated with olfactory dysfunction among adult CRS patients. A JESREC score ≥6 should be a warning of the risk of olfactory dysfunction.
In the present study, 46.3% patients suffered from olfactory dysfunction, and among them, 64.6% were male, which was similar to a previous study (19, 20). In recent years, a growing number of studies have confirmed that CRS subtypes classified according to JESREC score and components of JESREC score, including nasal polyps, peripheral eosinophilia, and lesion location, were closely related with olfactory function (14, 21–23). Eosinophilia in peripheral blood and the nasal mucosa were common in CRS, especially in ECRS patients (24), and compared with CRS patients with normal olfactory function, patients with olfactory dysfunction had higher proportions of ECRS subtype and eosinophilia in our study, which was consistent with previous results (25, 26). Ahn et al. (27) reported that the olfactory score of ECRS patients was lower than that of non-ECRS patients. In addition, neutrophils, as the most common inflammatory cells in non-ECRS (28, 29), did not differ in the two groups in this study and multivariate logistic analysis also indicated that peripheral neutrophilia was not associated with olfactory dysfunction.
In the clinical analysis, logistic regression results revealed a risk relationship between peripheral eosinophilia; JESREC score; bilateral lesion; nasal polyps; CT shadow: ethmoid ≥ maxillary; and ECRS and olfactory dysfunction. It is important to emphasize that despite alcohol and tobacco consumption not demonstrating any statistical association in the bivariate analysis of this study, considering the potential effects of smoking and alcohol consumption on CRS patients in previous studies, we still included them in the analysis of model 2. Specifically, in our previous article analyzing the relationship between olfactory dysfunction and metabolic syndrome in CRS patients (15), alcohol and tobacco consumption were significantly different between the different groups. Furthermore, we also referred to models from other scholars’ studies (30–33), which also demonstrated the potential impact of smoking and alcohol consumption on CRS. Of course, it should be emphasized that the influence of smoking and alcohol consumption on CRS patients is still controversial, but considering the consistency of the model 1 and model 2 results in this study, we still show the results of the two models in the final result.
The mechanisms underlying CRS-associated olfactory loss are not fully understood, and the possible mechanisms are as follows: bilateral lesion and nasal polyps work by blocking the nasal cavity to prevent the odor molecules from reaching the olfactory region (34, 35); an increase in peripheral eosinophils is closely related to the percentage of infiltration of eosinophils in the sinuses, and numerous infiltrated eosinophils in the olfactory mucosa and nasal polyps may promote c-Jun N-terminal kinase pathway activation by secreting their stored granule proteins and cytokines, including interleukin 5 (IL-5), transforming growth factor α (TGF-α), and IL-2, leading to apoptosis and death of olfactory sensory neurons (36–38). In addition, previous studies have shown that the JESREC score can be used to distinguish between ECRS and non-ECRS and was useful for predicting CRS endotypes (14, 39). Our study was the first to describe a cutoff value for the JESREC score for olfactory dysfunction, which could assist doctors in more accurate prediction of long-term changes in olfaction among adult CRS patients and provide more targeted treatment.
Our study has several limitations. Firstly, limited by the cross-sectional study design, further research is needed to clarify the causal relationship between peripheral blood eosinophilia, JESREC score, and olfactory dysfunction. Secondly, all participants in this study were Han Chinese, and caution is still required in generalizing the conclusions to other ethnicities. Thirdly, being limited to a retrospective cross-sectional study and having incomplete clinical data, we lack information on skin testing or blood allergy testing that can reflect the atopic status of the patients, which may have potential impact on the results. In addition, although this article explored the potential mechanism of peripheral eosinophilia and JESREC score on olfactory decline in CRS patients, further research is needed on the specific pathophysiology.
In summary, peripheral eosinophilia and high JESREC scores were significantly associated with the risk of olfactory dysfunction in patients with chronic rhinosinusitis, and special attention should be paid to patients with JESREC score ≥6.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Third Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because of anonymous data and the lack of any intervention.
Author contributions
LZ: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. HL: Methodology, Software, Writing – original draft. TW: Data curation, Software, Validation, Writing – original draft. ZW: Formal Analysis, Project administration, Writing – original draft. YW: Funding acquisition, Resources, Visualization, Writing – original draft. SG: Data curation, Investigation, Methodology, Writing – original draft. WL: Data curation, Methodology, Resources, Writing – original draft. YZ: Investigation, Project administration, Validation, Writing – original draft. HX: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. JY: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Png LH, Kalish L, Campbell RG, Seresirikachorn K, Albrecht T, Raji N, et al. Predictors of persistent disease in biologic treated type 2 diffuse/eosinophilic chronic rhinosinusitis undergoing surgery. Int Forum Allergy Rhinol (2023). doi: 10.1002/alr.23282
2. Passali GC, Passali D, Cingi C, Ciprandi G. Smell impairment in patients with chronic rhinosinusitis: a real-life study. Eur Arch Otorhinolaryngol (2022) 279(2):773–7. doi: 10.1007/s00405-021-06848-9
3. Gong X, Han Z, Fan H, Wu Y, He Y, Fu Y, et al. The interplay of inflammation and remodeling in the pathogenesis of chronic rhinosinusitis: current understanding and future directions. Front Immunol (2023) 14:1238673. doi: 10.3389/fimmu.2023.1238673
4. Sedaghat AR, Kuan EC, Scadding GK. Epidemiology of chronic rhinosinusitis: prevalence and risk factors. J Allergy Clin Immunol Pract (2022) 10(6):1395–403. doi: 10.1016/j.jaip.2022.01.016
5. Hong HY, Chen TY, Yang QT, Sun YQ, Chen FH, Lou HF, et al. Chinese expert consensus on the use of biologics in patients with chronic rhinosinusitis (2022, Zhuhai). ORL J Otorhinolaryngol Relat Spec (2023) 85(3):128–40. doi: 10.1159/000529918
6. Lin YT, Lin CF, Liao CK, Yeh TH. Comprehensive evaluation of type 2 endotype and clinical features in patients with chronic rhinosinusitis with nasal polyps in Taiwan: a cross-sectional study. Eur Arch Otorhinolaryngol (2023) 280(12):5379–89. doi: 10.1007/s00405-023-08118-2
7. Yang HW, Park JH, Jo MS, Shin JM, Kim DW, Park IH. Eosinophil-derived osteopontin induces the expression of pro-inflammatory mediators and stimulates extracellular matrix production in nasal fibroblasts: the role of osteopontin in eosinophilic chronic rhinosinusitis. Front Immunol (2022) 13:777928. doi: 10.3389/fimmu.2022.777928
8. Gevaert P, Han JK, Smith SG, Sousa AR, Howarth PH, Yancey SW, et al. The roles of eosinophils and interleukin-5 in the pathophysiology of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol (2022) 12(11):1413–23. doi: 10.1002/alr.22994
9. Gan W, Xiang Y, Wei B, Liu S, Liu F. The inflammatory microenvironment of nasal polyps in patients with chronic rhinosinusitis and the relationship of this microenvironment with the nasal microbiome. Asian J Surg (2024) 47(1):124–33. doi: 10.1016/j.asjsur.2023.08.096
10. Ma L, Deng Y, Wang K, Shi J, Sun Y. Relationship between eosinophilic and neutrophilic inflammation in Chinese chronic rhinosinusitis with nasal polyps. Int Arch Allergy Immunol (2023) 184(6):576–86. doi: 10.1159/000528946
11. Bayer K, Hamidovic S, Brkic FF, Besser G, Mueller CA, Liu DT. Peripheral eosinophil count and eosinophil-to-lymphocyte ratio are associated with revision sinus surgery. Eur Arch Otorhinolaryngol (2023) 280(1):183–90. doi: 10.1007/s00405-022-07497-2
12. Hu Y, Cao PP, Liang GT, Cui YH, Liu Z. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope (2012) 122(3):498–503. doi: 10.1002/lary.22507
13. Matsuwaki Y, Ookushi T, Asaka D, Mori E, Nakajima T, Yoshida T, et al. Chronic rhinosinusitis: risk factors for the recurrence of chronic rhinosinusitis based on 5-year follow-up after endoscopic sinus surgery. Int Arch Allergy Immunol (2008) 146 Suppl 1:77–81. doi: 10.1159/000126066
14. Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy (2015) 70(8):995–1003. doi: 10.1111/all.12644
15. Zhang L, Wang T, Wang Z, Li H, Wu Y, Guo S, et al. Analysis of risk factors affecting olfactory dysfunction in patients with chronic rhinosinusitis: Highlighting the role of metabolic syndrome. Laryngoscope Investig Otolaryngol (2023) 8(3):615–20. doi: 10.1002/lio2.1061
16. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology (2012) 50(1):1–12. doi: 10.4193/Rhino12.000
17. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 'Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses (1997) 22(1):39–52. doi: 10.1093/chemse/22.1.39
18. Van Regemorter V, Dollase J, Coulie R, et al. Olfactory dysfunction predicts frailty and poor postoperative outcome in older patients scheduled for elective non-cardiac surgery. J Nutr Health Aging (2022) 26(11):981–6. doi: 10.1007/s12603-022-1851-3
19. Barroso B, Valverde-Monge M, Betancor D, Gómez-López A, Villalobos-Vildas C, González-Cano B, et al. Smell improvement in chronic rhinosinusitis with nasal polyps with monoclonal antibodies: a systematic review. J Investig Allergol Clin Immunol (2023) 33(6):419–30. doi: 10.18176/jiaci.0939
20. Song J, Wang M, Wang C, Zhang L. Olfactory dysfunction in chronic rhinosinusitis: insights into the underlying mechanisms and treatments. Expert Rev Clin Immunol (2023) 19(8):993–1004. doi: 10.1080/1744666X.2023.2235891
21. Tsuda T, Suzuki M, Kato Y, Kidoguchi M, Kumai T, Fujieda S, et al. The current findings in eosinophilic chronic rhinosinusitis. Auris Nasus Larynx (2023) 51(1):51–60. doi: 10.1016/j.anl.2023.08.002
22. Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features of various criteria of eosinophilic chronic rhinosinusitis: A systematic review and meta-analysis. Clin Exp Otorhinolaryngol (2022) 15(3):230–46. doi: 10.21053/ceo.2022.00052
23. Kim JY, Han YE, Seo Y, Choe G, Kim MK, Huh G, et al. Revisiting the clinical scoring system for the prognosis of chronic rhinosinusitis with nasal polyps. Yonsei Med J (2019) 60(6):578–84. doi: 10.3349/ymj.2019.60.6.578
24. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol (2016) 137(5):1449–56.e4. doi: 10.1016/j.jaci.2015.12.1324
25. Smith KA, Gill AS, Pollard CE, Sumsion JS, Saffari H, Ashby S, et al. An eosinophil peroxidase activity assay accurately predicts eosinophilic chronic rhinosinusitis. J Allergy Clin Immunol (2023) 152(2):400–7. doi: 10.1016/j.jaci.2023.04.012
26. McHugh T, Snidvongs K, Xie M, Banglawala S, Sommer D. High tissue eosinophilia as a marker to predict recurrence for eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol (2018) 8(12):1421–9. doi: 10.1002/alr.22194
27. Ahn SH, Lee EJ, Ha JG, Hwang CS, Yoon JH, Kim CH, et al. Comparison of olfactory and taste functions between eosinophilic and non-eosinophilic chronic rhinosinusitis. Auris Nasus Larynx (2020) 47(5):820–7. doi: 10.1016/j.anl.2020.04.006
28. Wang X, Sima Y, Zhao Y, Zhang N, Zheng M, Du K, et al. Endotypes of chronic rhinosinusitis based on inflammatory and remodeling factors. J Allergy Clin Immunol (2023) 151(2):458–68. doi: 10.1016/j.jaci.2022.10.010
29. Wang H, Pan L, Liu Z. Neutrophils as a protagonist and target in chronic rhinosinusitis. Clin Exp Otorhinolaryngol (2019) 12(4):337–47. doi: 10.21053/ceo.2019.00654
30. Wee JH, Min C, Park MW, Byun SH, Lee HJ, Song CM, et al. Association between dyslipidemia and chronic rhinosinusitis in a Korean population. Diagnostics (Basel) (2020) 11(1):26. doi: 10.3390/diagnostics11010026
31. Tint D, Kubala S, Toskala E. Risk factors and comorbidities in chronic rhinosinusitis. Curr Allergy Asthma Rep (2016) 16(2):16. doi: 10.1007/s11882-015-0589-y
32. Hutson K, Clark A, Hopkins C, Ahmed S, Kumar N, Carrie S, et al. Evaluation of smoking as a modifying factor in chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg (2021) 147(2):159–65. doi: 10.1001/jamaoto.2020.4354
33. Wang X, Chen Y, Zhu X, Zhou Y, Su H, Zhao Y. Associations of alcohol consumption with the risk and surgical outcomes of chronic rhinosinusitis in China: A case-control study. Clin Otolaryngol (2022) 47(6):664–71. doi: 10.1111/coa.13970
34. Kwah JH, Peters AT. Nasal polyps and rhinosinusitis. Allergy Asthma Proc (2019) 40(6):380–4. doi: 10.2500/aap.2019.40.4252
35. Kato A, Schleimer RP, Bleier BS. Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol (2022) 149(5):1491–503. doi: 10.1016/j.jaci.2022.02.016
36. Victores AJ, Chen M, Smith A, Lane AP. Olfactory loss in chronic rhinosinusitis is associated with neuronal activation of c-Jun N-terminal kinase. Int Forum Allergy Rhinol (2018) 8(3):415–20. doi: 10.1002/alr.22053
37. Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy (2014) 28(3):192–8. doi: 10.2500/ajra.2014.28.4033
38. Kim DK, Choi SA, Eun KM, Kim SK, Kim DW, Phi JH. Tumour necrosis factor alpha and interleukin-5 inhibit olfactory regeneration via apoptosis of olfactory sphere cells in mice models of allergic rhinitis. Clin Exp Allergy (2019) 49(8):1139–49. doi: 10.1111/cea.13401
Keywords: chronic rhinosinusitis, olfactory dysfunction, peripheral eosinophilia, JESREC score, eosinophilic chronic rhinosinusitis
Citation: Zhang L, Li H, Wang T, Wang Z, Wu Y, Guo S, Li W, Zhou Y, Xue H and You J (2024) Association between peripheral eosinophilia, JESREC score, and olfactory dysfunction in patients with chronic rhinosinusitis. Front. Immunol. 15:1334656. doi: 10.3389/fimmu.2024.1334656
Received: 07 November 2023; Accepted: 04 January 2024;
Published: 24 January 2024.
Edited by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoReviewed by:
Gandhi Fernando Pavon, Independent Researcher, Mexico City, MexicoMarcos Alejandro Jimenez Chobillon, National Institute of Respiratory Diseases-Mexico (INER), Mexico
Copyright © 2024 Zhang, Li, Wang, Wang, Wu, Guo, Li, Zhou, Xue and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianqiang You, dWpxaWFuZ0AxNjMuY29t; Haixiang Xue, eGh4YndjQDEyNi5jb20=; Ling Zhang, bGluZ3poYW5nMDMyOUAxNjMuY29t
 Ling Zhang
Ling Zhang Haifeng Li
Haifeng Li