- 1Department of Infectious Diseases, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Liver Disease Research, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 3Department of Pharmacy, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Background: Previous studies have suggested the potential of PD-1/PD-L1 inhibitors in the treatment of chronic HBV infection. However, since phase III clinical trials have not yet been announced, additional clinical insights may be obtained by observing changes in serum hepatitis B surface antigen (HBsAg) and HBV-DNA levels in cancer patients undergoing PD-1 inhibitor therapy.
Objective: To explore the effects of PD-1 inhibitor combinational therapy on serum HBsAg and HBV-DNA levels, investigate the incidence of HBsAg loss, HBV reactivation (HBVr), and immune-related adverse events (irAEs), and identify the risk factors associated with significant HBsAg fluctuations and HBVr.
Methods: A retrospective study including 1195 HBsAg-positive cancer patients who received PD-1 inhibitors between July 2019 and June 2023 was conducted, and 180 patients were enrolled in this study. Serum HBsAg levels before and after PD-1 inhibitor administration were compared across different subgroups. The Pearson χ2 or Fisher exact test was performed to investigate the relationships between categorical variables. Univariable and multivariable analysis were performed to identify the risk factors associated with significant HBsAg fluctuations and HBVr.
Results: With the concurrent use of antiviral agents, serum HBsAg levels decreased (Z=-3.966, P < 0.0001) in 129 patients and increased (t=-2.047, P=0.043) in 51 patients. Additionally, 7 patients (3.89%) achieved serum HBsAg loss. Virus replication was suppressed in most of the enrolled patients. When divided patients into different subgroups, significant HBsAg decreases after PD-1 inhibitor administration were discovered in lower baseline HBsAg group (Z=-2.277, P=0.023), HBeAg-seronegative group (Z=-2.200, P=0.028), non-irAEs occurrence group (Z=-2.007, P=0.045) and liver cancer group (Z=-1.987, P=0.047). Of note, 11 patients and 36 patients experienced HBVr (6.11%) and irAEs (20%), respectively, which could lead to discontinuation or delayed use of PD-1 inhibitors. After multivariable analysis, HBeAg-seropositive (OR, 7.236 [95% CI, 1.757-29.793], P=0.01) and the occurrence of irAEs (OR, 4.077 [95% CI, 1.252-13.273], P=0.02) were identified as the independent risk factors for significant HBsAg increase, the occurrence of irAEs (OR, 5.560 [95% CI, 1.252-13.273], P=0.01) was identified as the only independent risk factor for HBVr.
Conclusion: PD-1 inhibitors combined with nucleos(t)ide analogues (NAs) may exert therapeutic potential for chronic HBV infection in cancer patients. However, attention also should be paid to the risk of significant elevation in HBsAg levels, HBVr, and irAEs associated with PD-1 inhibitor combinational therapy.
Introduction
Immune checkpoint inhibitors (ICIs) have shown dramatic improvement in clinical outcomes compared with standard therapy for a range of cancer types in recent years, it enhances antitumor immunity by targeting intrinsic down regulators of immunity, such as programmed cell death 1 (PD-1) or its ligand, programmed cell death ligand 1 (PD-L1) (1). Except for the critical roles of CD8+ T cells in anti-tumor immunity upon PD-1/PD-L1 blockades (2), CD4+ T cells are also demonstrated to be required for efficacious anti-tumor responses, such as the percentages of naive CD4+ T cells secreting certain cytokines including IFN-γ and TNF-α before receiving nivolumab, were significantly higher in patients with better response to anti-PD-1 therapy (3). Similar to cancer patients, T cells are also described as”exhausted” or functionally impaired and unable to proliferate or secrete antiviral cytokines (IFN-γ) in chronic hepatitis B (CHB) (4), and emerging evidences suggest that the same checkpoint pathways may play a crucial role during acute (5) and chronic (6) hepatitis B virus (HBV) infection.
Failure to eliminate covalently closed circular DNA (cccDNA), which is the nuclear reservoir of the virus, is a major barrier to the cure of chronic HBV infection. It seems plausible that the induction of functional HBV‐specific T cells is a good approach for HBV clearance since virus-specific T cells are capable of removing cccDNA‐carrying cells in about 90% of infected patients (7). Consistent with this concept, previous studies have shown that the blockade of PD-1/PD-L1 may improve HBV-specific T-cell function in vitro (8–10). Besides, a phase Ib study in 2019 has noticed that 20 of the 22 patients (90.91%) who received nivolumab have a reduction in serum hepatitis B surface antigen (HBsAg), and nivolumab is well-tolerated in hepatitis B e antigen (HBeAg)-seronegative CHB patients (11). And in 2022, a phase IIb clinical trial (NCT04465890) of ASC22 (Envafolimab), a PD-L1 inhibitor, in patients with CHB reported that 7 patients with baseline HBsAg ≤ 500 IU/ml experienced HBsAg reduction > 0.5 log10 IU/ml under ASC22 and NAs, 3 patients even had HBsAg seroclearance (undetectable, < 0.05 IU/ml). However, more immune-related adverse events (irAEs) occurred in the ASC22 group (12). Hitherto, the implementation of phase III clinical trials of PD-1 or PD-L1 inhibitors in the treatment of CHB is yet to be announced.
Despite the exhilarating and promising study results, previous studies also have shown that PD-1 inhibitor monotherapy or combined with other ICIs (immune checkpoint inhibitors) pose a risk of HBV reactivation (HBVr) (13, 14), lack of prophylaxis antiviral treatment (15, 16), undetectable HBV-DNA (16), and combined with hepatic artery intubation chemotherapy (HAIC) (17) were identified as independent risk factor for HBVr. In addition to the impressive anti-tumor effects of ICIs, a spectrum of unique side effects referred to as irAEs have been reported (18). The mechanism of this may be that ICIs enhance the activity of T cells against antigens expressed in tumors and healthy tissues, and increase pre-existing levels of autoantibodies and inflammatory factors (1). It’s indicated that an overall incidence of irAEs ranges between 27%-78% in phase III trials of anti-PD-1/PD-L1 agents in cancer patients (19, 20). Aside from the possible permanent effects on the endocrine system, most of the irAEs are reversible. Deaths from irAEs are rare, however, deaths due to myocarditis, pneumonitis, colitis, and neurologic events, among others, can occur (1).
To improve objective responsive rate (ORR), ICI monotherapy was less received by cancer patients, and combination therapies including different types of ICIs, targeted agents, chemotherapy, and interventional therapies (21–24) were commonly used. However, the incidence of HBVr in cancer patients with ongoing PD-1 inhibitor combination therapies remains unclear, and more research is needed to validate the relationship between PD-1 inhibitors and immune-mediated clearance of HBV or serum HBsAg clearance in this context. Besides, whether there is a certain correlation between the occurrence of irAEs and changes in HBV serologic markers also needs to be clarified. In our study, each enrolled patient needed to be carefully investigated by two clinicians whether they had experienced irAEs before the first or second study endpoint, which were described in the study design, and concurrent use of NAs was required. This study aims to observe the changes in serum HBsAg and HBV-DNA levels in HBsAg-positive cancer patients, particularly significant increases or decreases in HBsAg levels. Meanwhile, investigating the incidence of HBsAg loss, irAEs, HBVr, and identifying the risk factors associated with HBsAg fluctuations and HBVr in cancer patients.
Materials and methods
Patients
This retrospective study was conducted with the approval of the institutional review board and was conducted following the Declaration of Helsinki. The requirement for written informed consent was waived because of the retrospective nature of this study. 1195 HBsAg-positive Cancer patients who were treated with PD-1 combinational therapy between July 2019 and June 2023 were identified. Data were collected through a manual review of patient electronic medical records, and laboratory and imaging results database by 2 reviewers. Patients who met the following criteria were included: (1) age ≥ 18 years old; (2) patients had cancer confirmed by pathological biopsy or two imaging techniques; (3) seropositive for HBsAg, regularly received antiviral agents and intravenous used at least one cycle of PD-1 inhibitor. According to APASL clinical practice guidelines on hepatitis B reactivation (25), taking NAs for at least one week before receiving PD-1 inhibitors was considered prior use of antiviral agents in this study. Patients were excluded if any of the following occurred during treatment: (1) HAV/HCV/HEV infection; (2) antibodies positive to human immunodeficiency virus (HIV); (3) lack of data on HBsAg quantification before and/or after administration of PD-1 inhibitors.
Data collection
Demographic data including age and sex were collected. Additional clinical information regarding liver cirrhosis, HBeAg status, serum HBsAg and HBV-DNA levels at baseline (before PD-1 inhibitor initiation) and after PD-1 inhibitor administration, cycles of PD-1 inhibitor, PD-1 inhibitor type (nivolumab, pembrolizumab, sintilimab, toripalimab, tislelizumab, and camrelizumab). The occurrence of irAEs before significant HBsAg changes or HBVr was recorded according to Version 5 of the Common Terminology Criteria for Adverse Events (CTCAE) (26). Prior use of antiviral therapy, antiviral agents (entecavir, tenofovir, tenofovir alafenamide fumarate), combined antineoplastic therapies including chemotherapy, hepatic artery intubation chemotherapy (HAIC), transcatheter arterial chemoembolization (TACE), targeted agents (apatinib, lenvatinib, regorafenib, anlotinib, sorafenib, donafenib), and radiotherapy were obtained. Oncologic factors recorded including cancer type, and ECOG (Eastern Cooperative Oncology Group) score.
Study design
After the PD-1 inhibitor therapy, eligible patients were divided into two groups based on changes in serum HBsAg levels: the HBsAg decreased group and the HBsAg increased group. The first endpoint was a significant change in serum HBsAg levels, defined as an increase or decrease of more than 0.5 log10-fold in serum HBsAg levels after PD-1 inhibition. Hence, quantification of serum HBsAg needed to be performed at least twice in this study. Most of the serum HBsAg were measured by chemoluminescence technique in the clinical laboratory of our center using an automatic chemiluminescence immunoanalyzer (I 3000; Maccura, SiChuan, China) with a detection range of 0-250 IU/ml. For patients whose serum HBsAg levels were more than 250 IU/ml, the concentrations of serum HBsAg were determined by an electrochemiluminescence immunoanalyzer (COBAS E601; Roche Diagnostics, Basel, Switzerland) with a lower limit of 10-20 IU/ml.
The secondary endpoint was the incidence of HBV reactivation (HBVr). According to the AASLD 2018 hepatitis B guidance, the occurrence of HBVr was defined as (27): for HBsAg- positive patients (1) a 2-log (100-fold) increase in HBV-DNA compared with the baseline levels; (2) HBV-DNA ≥ 3 log (1000-fold) IU/ml in a patient with previously undetectable levels (given that HBV-DNA levels fluctuate); or (3) HBV-DNA ≥ 4 log (10,000-fold) IU/ml if the baseline level was not available. For HBsAg-negative, anti-HBc-positive patients: reverse HBsAg seroconversion occurs (reappearance of HBsAg). HBV-DNA was quantified by real-time polymerase chain reaction (PCR) diagnostic kit (COBAS AmpliPrep/TaqMan; Roche Diagnostics, Basel, Switzerland) with a lower limit threshold of 10 or 20 IU/ml or real-time fluorescence quantitative PCR with a lower limit threshold of 100 IU/ml.
Statistical analysis
Normally distributed quantitative data were expressed as mean ± standard deviation, and non-normally distributed quantitative data were reported as median (range or interquartile range). Continuous variables were compared using a two-tailed Student’s t-test or Mann-Whitney U test depending on the distribution. The Pearson χ2 or Fisher exact test was performed to investigate the relationships between categorical variables. The correlation between pretreatment factors and significant HBsAg decrease or increase and HBVr were evaluated by logistic regression analysis. Factors in the univariable analysis with P < 0.2 were included in the multivariable analysis, a two-tailed P ≤ 0.05 was considered significant. Statistical analysis was performed with SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient’s characteristics
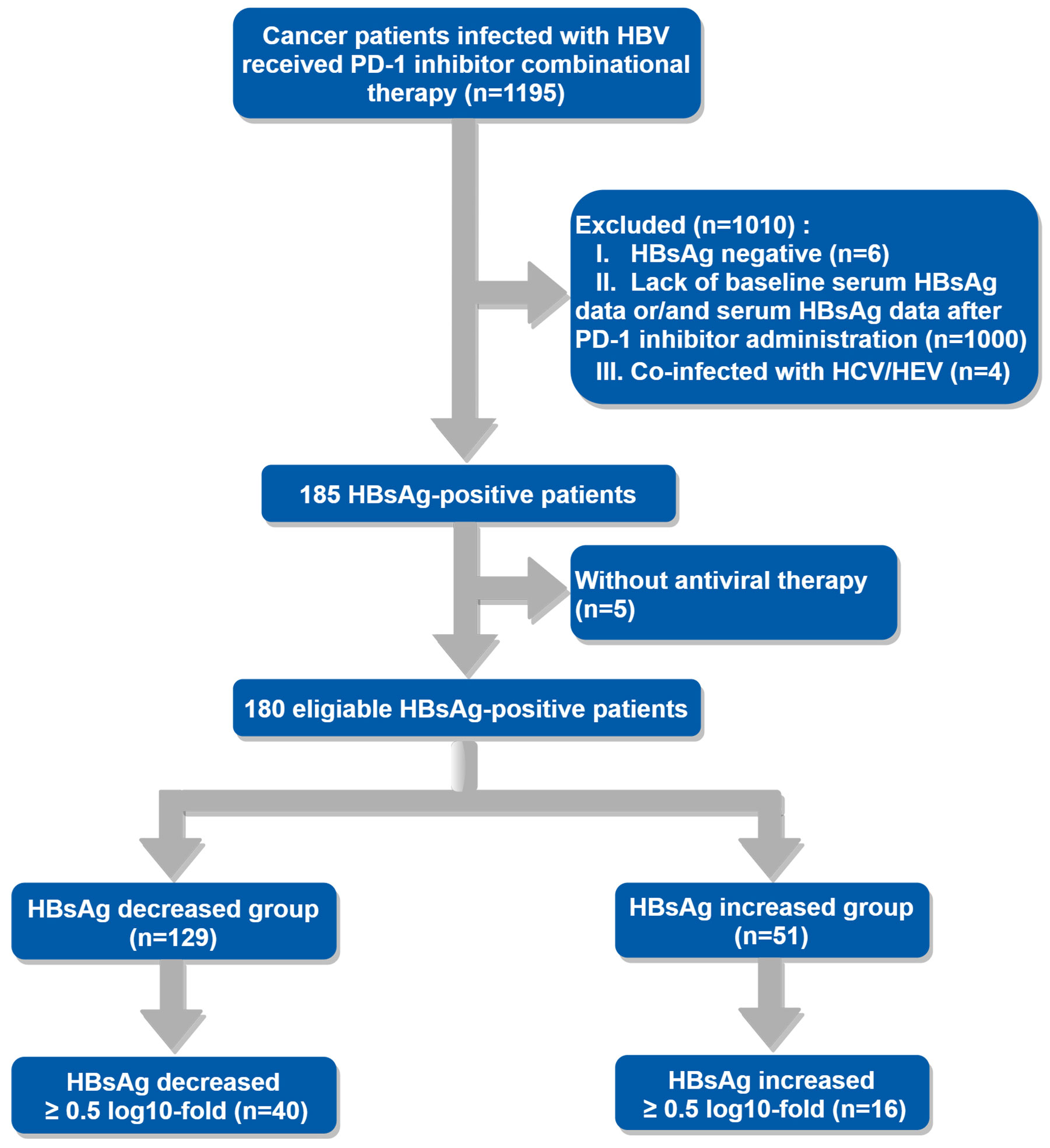
185 patients met the inclusion criteria without considering whether they received antiviral therapy or not, only 5 patients didn’t receive antiviral agents during PD-1 inhibitor combinational therapy for unknown reasons. Ultimately, 180 patients who received antiviral treatment were included in the final analysis, the enrollment process was shown in the flowchart (Figure 1). The baseline demographic and clinical characteristics of eligible patients are described in Table 1. As it presented, more patients in the HBsAg increased group were HBeAg-seropositive (21.43% VS 5.74%, P=0.02). Furthermore, there were differences in antiviral regimens between the HBsAg decreased group and the HBsAg increased group (P=0.03).
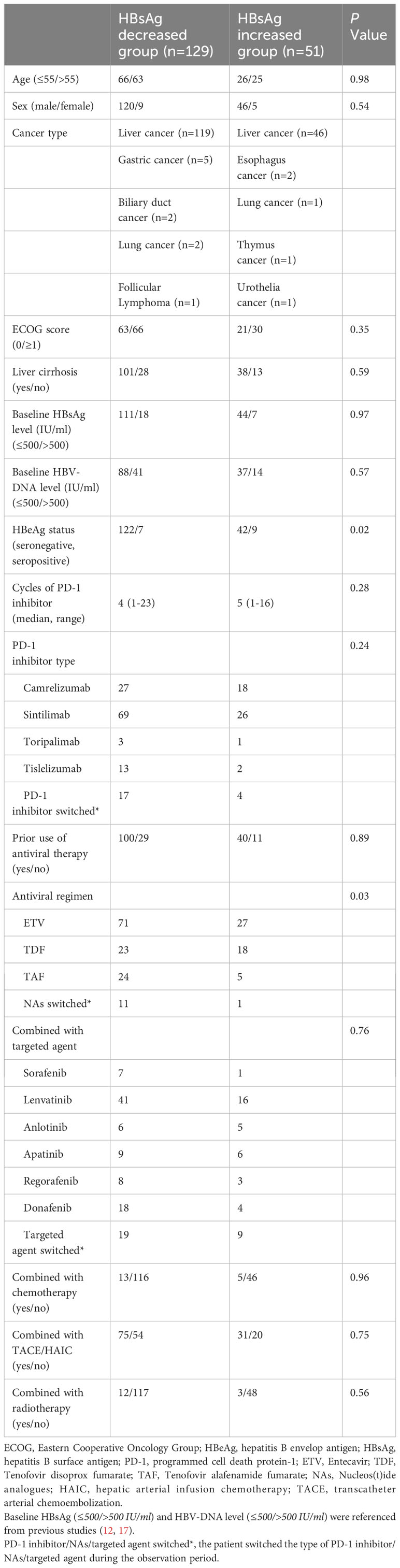
Table 1 Baseline demographic and clinical characteristics of patients under PD-1 inhibitor combinational therapy.
Patients were predominantly male (n=166, 92.22%), diagnosed with liver cancer (n=165, 91.67%), HBeAg seronegative (n=164, 91.11%), had the background of liver cirrhosis (n=139, 77.22%), and with the mean age of 54.81 ± 10.81 years old. Besides, 15 patients with other types of cancer also were included. Most of the enrolled patients (n=140, 77.78%) started antiviral therapy before PD-1 inhibitor initiation and entecavir (ETV) was selected by over half of the patients (n=98, 54.44%). Among all the patients, only 5 patients (2.78%) received PD-1 inhibitor monotherapy, while most (n=175, 97.22%) adopted PD-1 inhibitor combinational therapy, for instance, combined with chemotherapy (n=18, 10.00%), targeted agents (n=152, 84.44%), TACE or HAIC (n=106, 58.89%) and radiotherapy (n=15, 8.33%), to improve the survival rate of patients. Sintilimab (n=95, 52.78%) was a commonly used PD-1 inhibitor by cancer patients in this study.
Changes in HBsAg levels after the administration of PD-1 inhibitor under different clinical conditions
After reviewing the quantitative HBsAg data of patients before and after the initiation of PD-1 inhibitors, an overall decrease in serum HBsAg levels (log10 IU/ml) was observed [2.07 (0.87) VS 1.88 (1.07)] among all enrolled patients (Z=-2.067, P=0.039). Specifically, 129 patients exhibited a decrease [2.22 (0.62) VS 1.85 (1.01)] in serum HBsAg levels (Z=-3.966, P < 0.0001), while 51 patients showed an increase (1.44 ± 1.05 VS 1.84 ± 0.92) in serum HBsAg levels (t=-2.047, P=0.043) under the treatment of PD-1 inhibitors and NAs, as shown in Figure 2A. Notably, 40 patients within the HBsAg decreased group and 16 patients within the HBsAg increased group experienced a change in HBsAg levels exceeding 0.5 log10-fold following administration of PD-1 inhibitors.
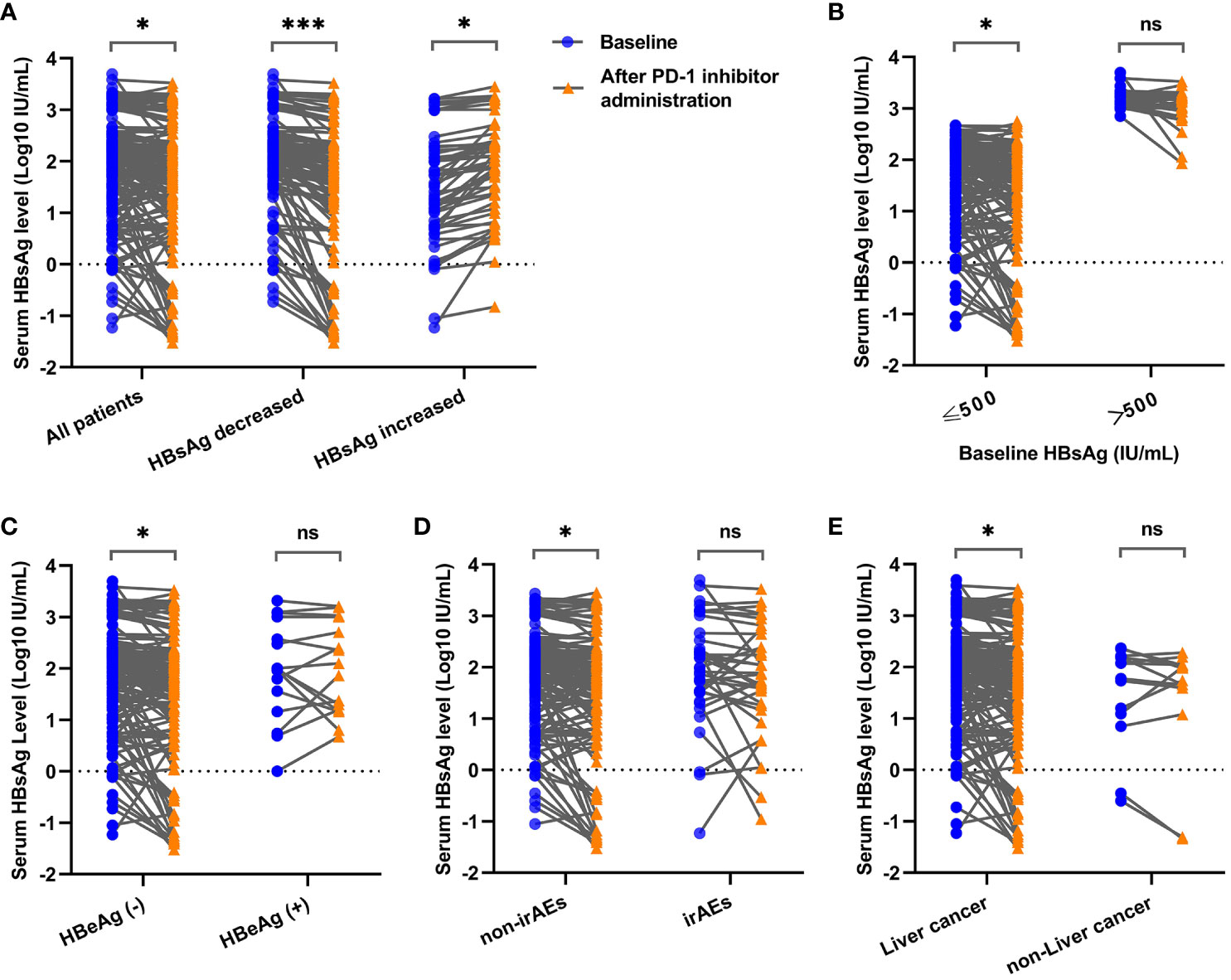
Figure 2 Comparison of serum HBsAg levels before and after PD-1 inhibitor administration in cancer patients under different clinical conditions. (A) Comparison of serum HBsAg levels among all enrolled patients, HBsAg decreased group and HBsAg increased group. (B) Comparison of serum HBsAg levels in patients with baseline HBsAg ≤ 500 IU/ml and baseline HBsAg > 500 IU/ml. (C) Comparison of serum HBsAg levels in HBeAg-seronegative group and HBeAg-seropositive group. (D) Comparison of serum HBsAg levels in non-irAEs occurrence group and irAEs occurrence group. (E) Comparison of serum HBsAg levels in liver cancer group and non-liver cancer group. * P < 0.05; *** P < 0.001; ns, not statistically significant.
To investigate the changes of HBsAg under different clinical conditions, multiple subgroups were conducted in the present study. It showed that significant HBsAg decreases were observed in lower baseline HBsAg group (Z=-2.277, P=0.023) (Figure 2B), HBeAg-seronegative group (Z=-2.200, P=0.028) (Figure 2C), non-irAEs occurrence group (Z=-2.007, P=0.045) (Figure 2D) and liver cancer group (Z=-1.987, P=0.047) (Figure 2E), while no difference of HBsAg changes was found when patients were divided into groups according to the types of NAs, baseline HBV-DNA levels, liver cirrhosis, prior use of antiviral therapy, the cycles of PD-1 inhibitors, and the types of PD-1 inhibitors (Supplementary Figure 1).
The incidence of serum HBsAg loss in cancer patients
HBsAg loss, defined as a change from positive at baseline to negative at any postbaseline visit within the targeted time window, occurred in 7 patients (7/180, 3.89%), as shown in Table 2. All of these patients were male, HBeAg seronegative, and had low baseline HBsAg levels (0.19 to 57.20 IU/ml). 6 patients were diagnosed with liver cancer and liver cirrhosis, and all received antiviral treatment before PD-1 inhibitor. Except for patient 2, who had gastric cancer with no background of liver cirrhosis and without the prior use of antiviral agents. It took 9.29 to 42.86 weeks to achieve HBsAg loss in these patients, only patient 3 experienced HBsAg seroconversion, during which anti-HBs reached 26.30 IU/ml.
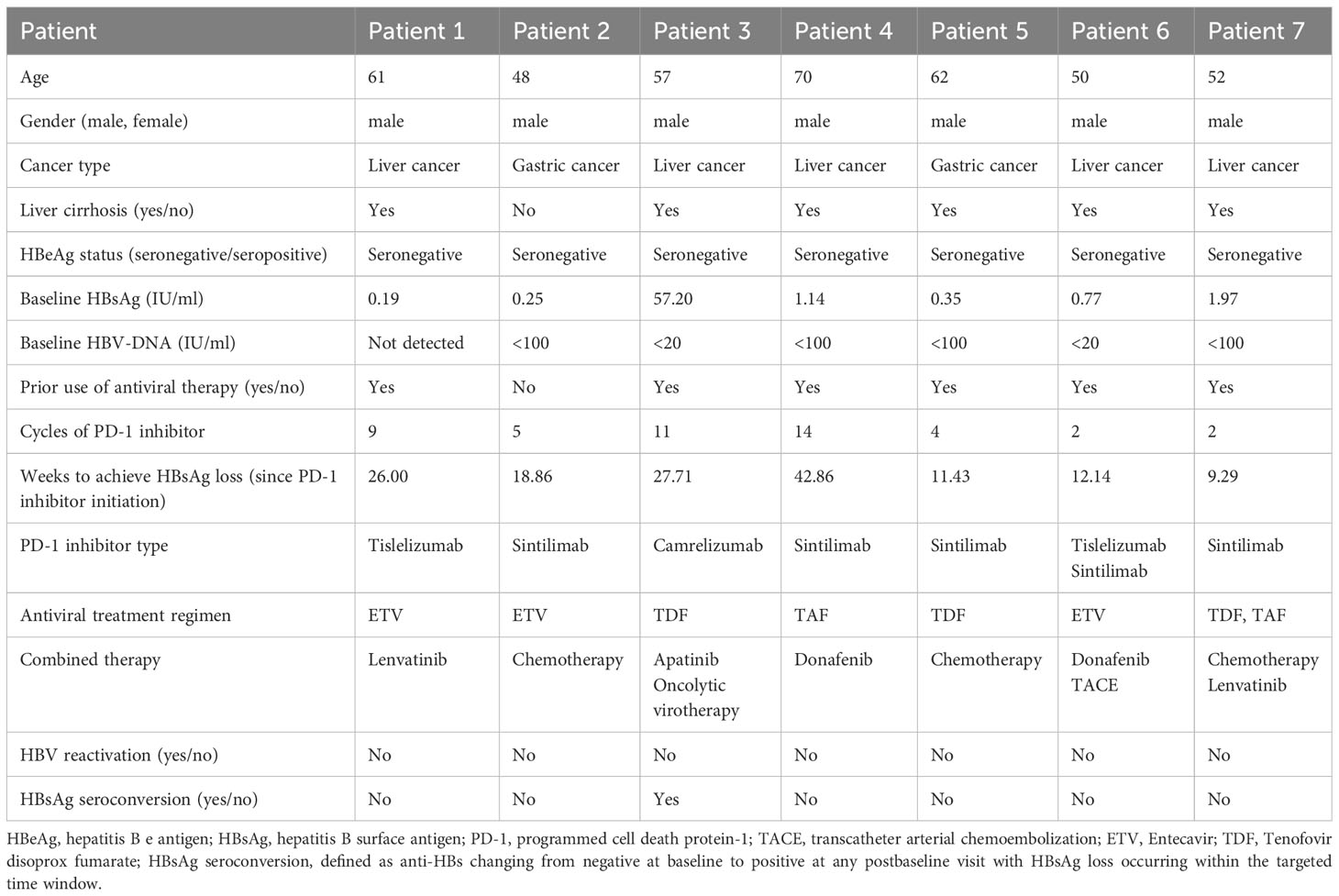
Table 2 Clinical characteristics of patients with serum HBsAg loss during PD-1 inhibitor combinational therapy.
The incidence of HBV reactivation under PD-1 inhibitor combinational therapy
With concurrent use of NAs, HBV-DNA levels were kept undetectable, remained stable at a low level, or decreased in most of the enrolled cancer patients (167/180, 92.78%) in this study. However, there were 11 patients (11/180, 6.11%) developed HBVr within 4.57 to 81.29 weeks under PD-1 inhibitor therapy. The details of these HBV-reactivated patients are listed in Supplementary Table 1. HBV-DNA levels of 9 patients increased by at least 100-fold compared to baseline, and the highest HBV-DNA level was 2.54×108 IU/ml at the diagnosis of HBVr. Of note, two patients achieved serum HBsAg loss after receiving antiviral agents and PD-1 inhibitors, however, serum HBsAg returned to positive afterward when the PD-1 inhibitor was still being used.
Of all the 11 patients, 7 cases experienced HBVr during PD-1 inhibitor therapy, while HBVr occurred 4.14 to 16 weeks after the last dose of PD-1 inhibitors in the other 4 cases. 3 out of 11 cases were diagnosed with HBV-associated hepatitis, and 2 of them discontinued PD-1 inhibitors due to hepatitis flare and HBV-related acute-on-chronic liver failure (ACLF), respectively. Moreover, we noticed that 5 cases experienced irAEs before HBVr, and 2 of them discontinued PD-1 inhibitors as a result of immunotherapy intolerance. In addition, some patients had withdrawn immunotherapy owing to cancer progression (n=1) and personal willingness (n=3). With the concurrent use of NAs, HBV-DNA levels of 3 cases achieved undetectable, and 7 cases remained detectable in the latest viral quantification, the patient’s condition with HBV-related ACLF worsened and gave up treatment eventually.
The occurrence of immune-related adverse events, and safety evaluation of PD-1 inhibitors
As confirmed by two physicians, there were 36 (20.00%) patients who had experienced at least one irAEs of any grade during PD-1 inhibitor combinational therapy, and 13 patients (7.22%) developed grade 3/4 adverse events. As shown in Supplementary Table 2, the most common adverse event in the present study was rash (n=12, 6.67%), and then followed by hepatitis (n=9, 5.00%), fever (n=4, 2.22%) and hypothyroidism (n=4, 2.22%). The most common grade 3/4 adverse event was hepatitis (n=9, 5.00%). 20 patients received glucocorticoids after the occurrence of irAEs according to clinical guidelines. However, 3 patients didn’t improve due to acute liver failure (ALF), ACLF and acute myocarditis, respectively. During treatment, 11 patients discontinued PD-1 inhibitors permanently due to irAEs, one patient discontinued PD-1 inhibitors due to irAEs and cancer progression. In addition, irAEs didn’t disturb the administration of PD-1 inhibitors in 12 patients but delayed in the rest 12 patients.
To investigate whether the safety of PD-1 inhibitor combinational therapy was related to the baseline HBV-DNA and HBsAg levels, we regrouped patients with reference to previous studies (12, 17), and found that patients with baseline HBV-DNA > 500 IU/ml had a higher percentage of discontinuation of PD-1 inhibitors due to irAEs (OR 1.688 [95% CI, 0.460-6.195], P=0.048). However, there was no difference in the incidence of all-grade irAEs, 3/4 irAEs, HBVr, and HBV-related hepatitis between high and low groups based on baseline HBV-DNA or HBsAg levels as shown in Table 3.
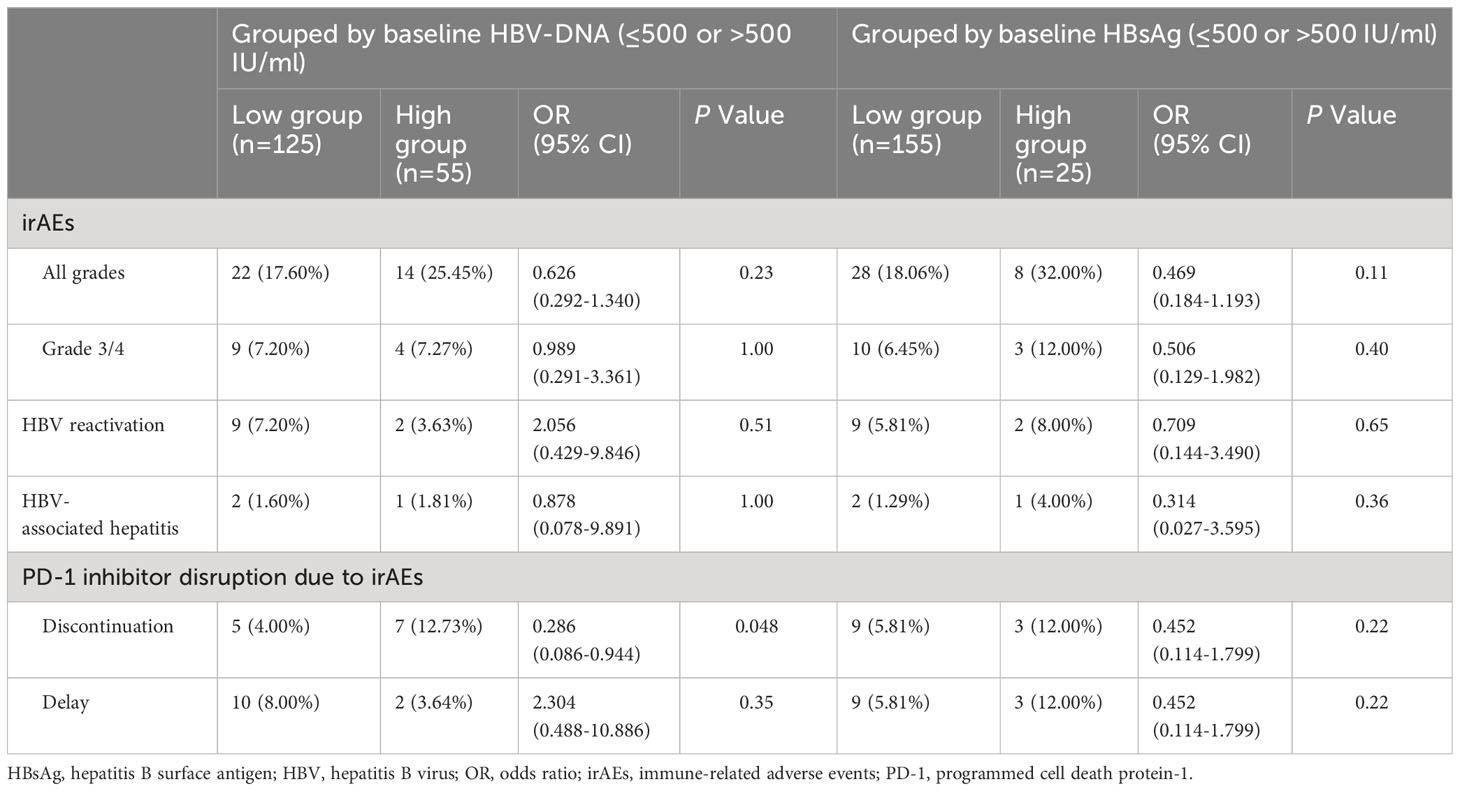
Table 3 Safety comparison of PD-1 Inhibitor combinational therapy under different grouping conditions.
Risk factors associated with significant serum HBsAg fluctuation and HBV reactivation
Considering that there may be minor detection errors or fluctuations in serum HBsAg quantification, we established criteria for defining clinically significant fluctuations in HBsAg levels by referring to a previous study (12). The results of the risk factor analysis are presented in Tables 4–6.
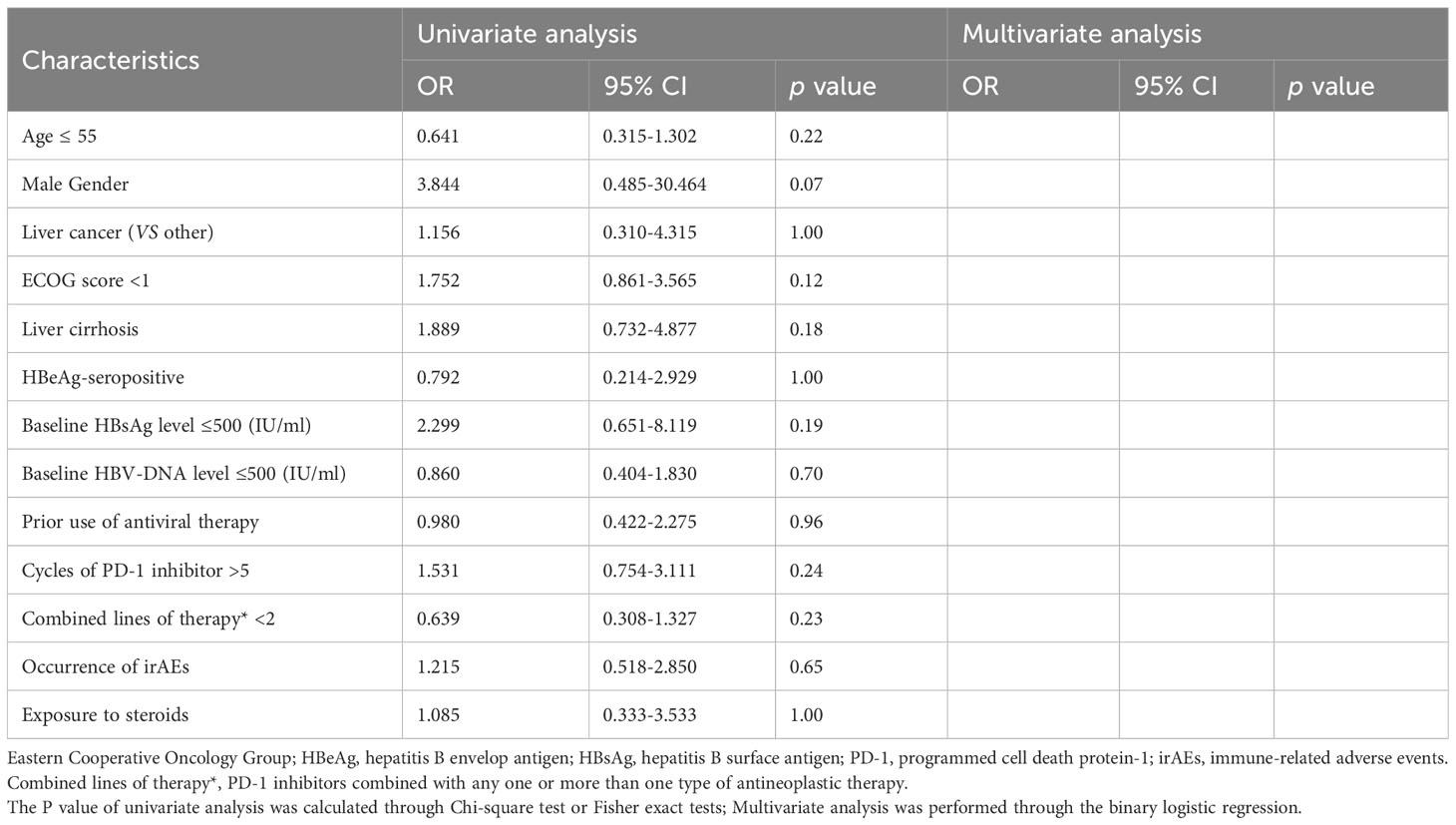
Table 4 Analysis of risk factors associated with significant serum HBsAg decrease during PD-1 inhibitor combinational therapy.
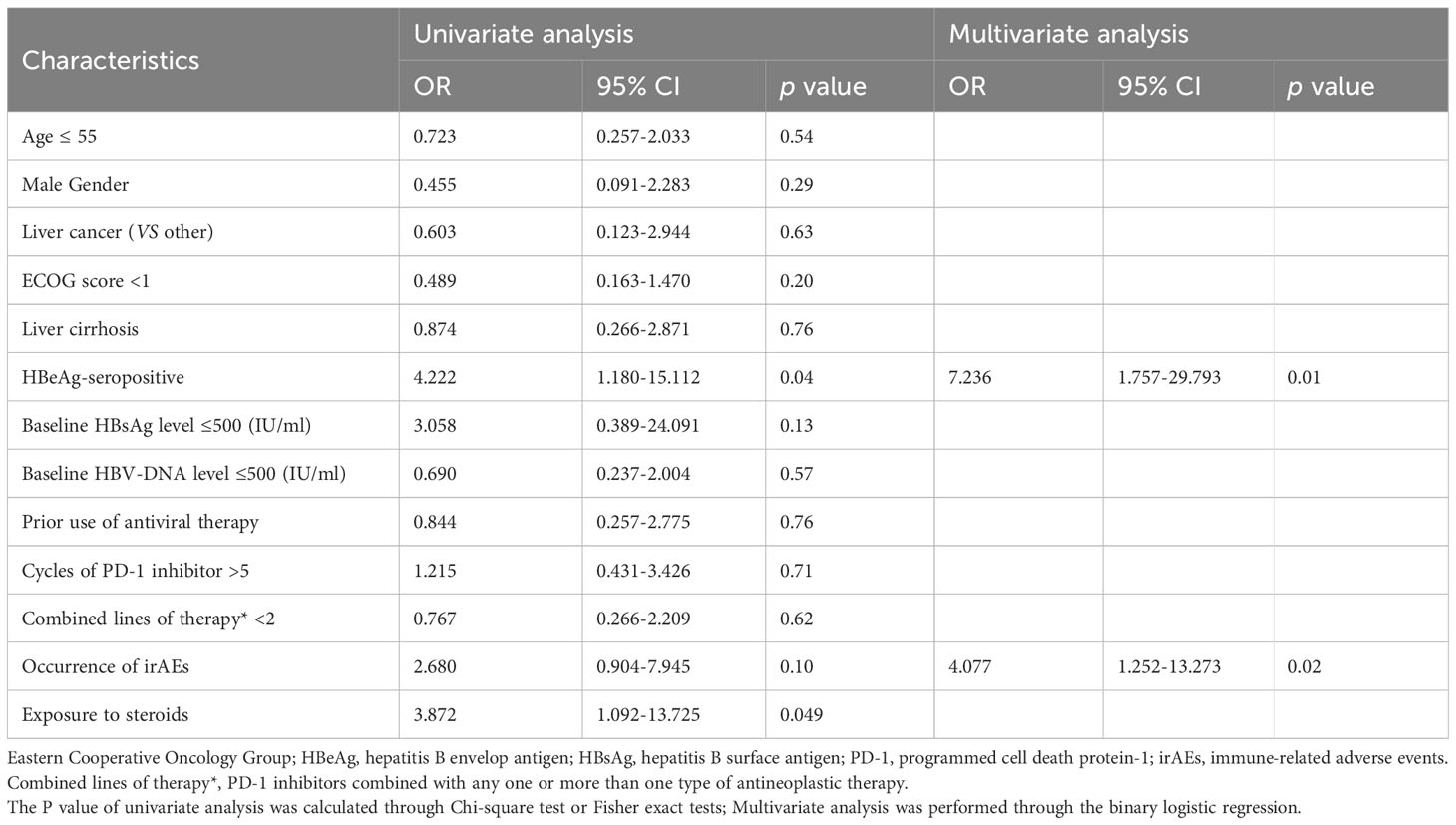
Table 5 Analysis of risk factors associated with significant serum HBsAg increase during PD-1 inhibitor combinational therapy.
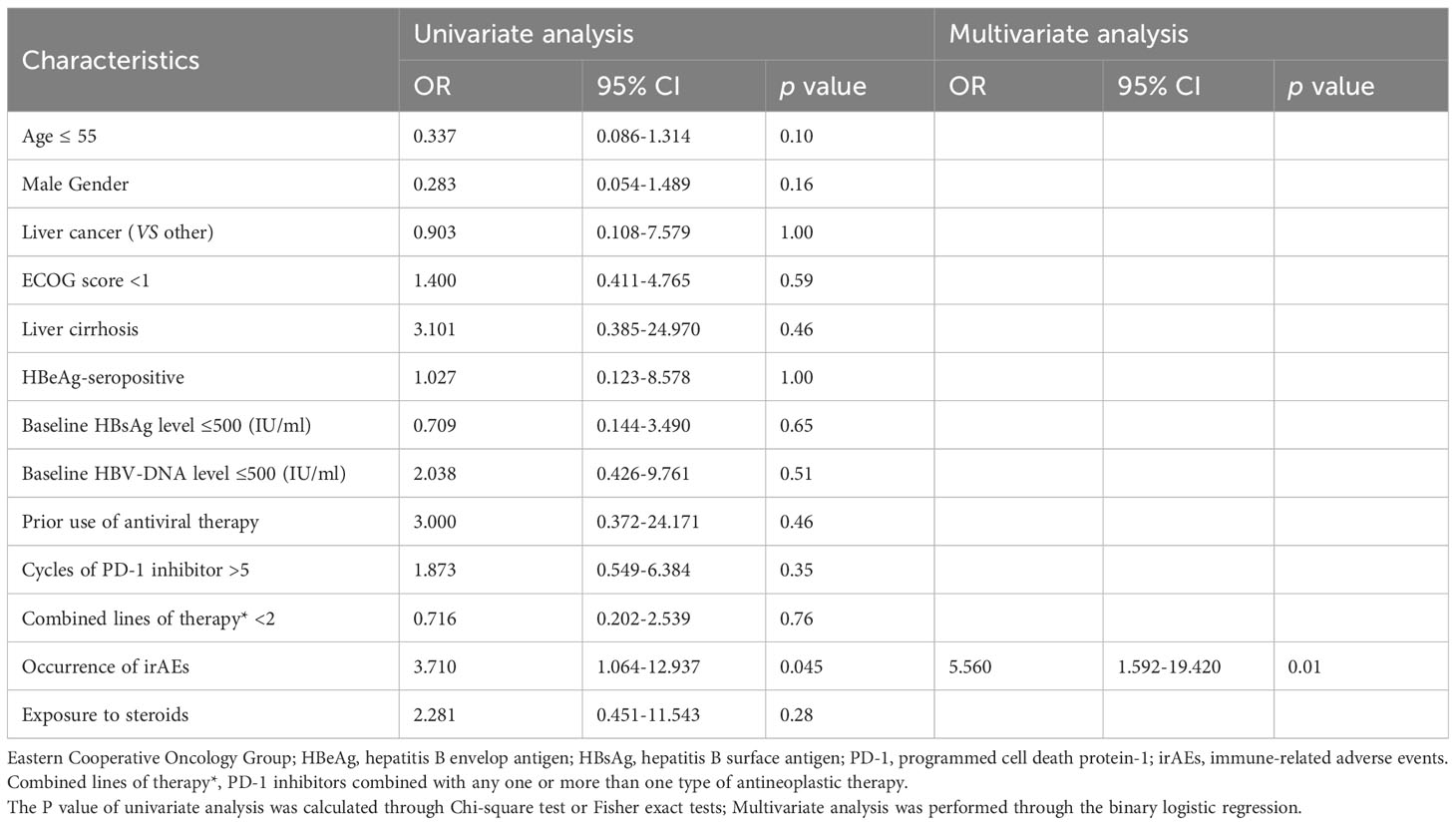
Table 6 Analysis of risk factors associated with HBV reactivation during PD-1 inhibitor combinational therapy.
In the univariable analysis, HBeAg-seropositive (OR, 4.222 [95% CI, 1.180-15.112], P=0.04), and exposure to steroids during treatment (OR, 3.872 [95% CI, 1.092-13.725]; P=0.049) were significant risk factors for HBsAg increase, the occurrence of irAEs (OR, 3.710 [95% CI, 1.064-12.937], P=0.045) was a significant risk factor for HBVr. In the multivariable analysis, HBeAg-seropositive (OR, 7.236 [95% CI, 1.757-29.793], P=0.01) and the occurrence of irAEs (OR, 4.077 [95% CI, 1.252-13.273]; P=0.02) were identified as the independent risk factor for HBsAg increase, the occurrence of irAEs (OR, 5.560 [95% CI, 1.252-13.273], P=0.01) was identified as the only independent risk factor for HBVr. Of note, no significant risk factors were discovered to be associated with significant HBsAg decrease both in univariable and multivariable analysis.
Discussion
It’s well known that a HBV-DNA decline directly reflects a reduction of viral replication, while HBsAg decline signifies a reduction of transcriptional activity of intranuclear cccDNA and integrated DNA sequences (28). The clearance of HBsAg is regarded as the closest correlate of cure and the ultimate goal of CHB therapy (29). However, only a few clinical trials (11, 12) have attempted to clarify the potential of PD-1/PD-L1 inhibitors in the treatment of CHB. Retrospectively observing changes in HBsAg and HBV-DNA levels in HBsAg-positive cancer patients undergoing PD-1 inhibitor combination therapy may yield more relevant clinical information.
In the present study, we noticed that viral replication could be effectively inhibited in 92.78% (167/180) of enrolled patients, and overall serum HBsAg levels decreased under PD-1 inhibitor and antiviral therapy (P=0.04), which was consistent with the study of Zeng et al. (30), it revealed that HBV targeting gRNA/cas9 induced a decrease in the expression of HBsAg in vitro, combined anti-HBV and anti-PD-1 CRISPR/Cas9 exhibited a stronger antiviral effect than either treatment alone. In another WHV study of woodchucks receiving entecavir, anti-PD-L1 mAb prevented viral rebound following withdrawal of entecavir (31). Taken together, it indicated that PD-1 inhibitor combined with NAs played a certain role in inhibiting viral replication and inducing HBsAg decrease. Upon PD-1 blockade, patients with baseline HBsAg ≤ 500 IU/ml were found to have a statistically significant decrease (P=0.02) in serum HBsAg in this study, which was in line with a previous study (32), it demonstrated that HBV-specific T cell functions were better preserved in CHB patients with lower serum HBsAg levels, and PD-L1 blockade improved HBV-specific CD4+ T cell function only in HBslo patients (serum HBsAg < 500 IU/ml). Meanwhile, we noticed that there were 7 patients (7/180, 3.89%) who achieved HBsAg loss, the rate of which was similar to a previous clinical trial (1/24, 4.17%) on CHB (11). However, a recent retrospective study reported that HBsAg seroclearance occurred in only 2 patients (0.39%) out of 511 HBsAg-positive cancer patients undergoing ICIs (13). The discrepancy among studies may be related to the limited patients included in our study, or cancer patients who failed to monitor serum HBsAg regularly in other studies.
It has been reported that the cumulative HBsAg loss rate of HBeAg-positive patients after 7 years of TDF treatment is higher than HBeAg-negative patients (11.8% VS 0.3%) (33), which makes CHB patients, especially HBeAg-negative patients, have to take medication for life. On the contrary, HBeAg-negative patients were prone to experience a decrease in HBsAg levels (P=0.03) in our study, and patients who achieved HBsAg loss were all HBeAg-negative, which may be attributed to the enhancement of HBV-specific T cell function by PD-1 inhibitors (8–10), In addition, the HBsAg levels decreased in the liver cancer group (P=0.047) when compared with the non-liver cancer group (P=0.36), which may be owing to patients with HBV-related liver cancer pay more attention to the regular follow-up of HBV serologic markers, making it easier to observe changes in serum HBsAg levels. Another undeniable fact was that most of the patients included in this study were HBeAg-negative (164/180, 91.11%) and had liver cancer (165/180, 91.67%), resulting in a more significant statistical difference in these patients.
Consistent with other studies, HBVr (11/180, 6.11%) was also discovered in this study. However, the incidence of HBVr varied greatly (0-30.05%) in different studies (34). The discrepancy may lie in the differences in the proportion of patients who received PD-1 inhibitor monotherapy versus combination therapy. Additionally, unlike the present study, other studies also included HBsAg-negative cancer patients. Even though no correlation was found between HBVr and combined lines of therapies in both univariate and multivariate analysis in this study, PD-1 inhibitor itself, chemotherapy, targeted agent, TACE (35), HAIC (17), and radiotherapy (36) had all been reported to pose a risk of HBVr in cancer patients. Of note, two patients first experienced HBsAg loss, followed by a re-positivity of HBsAg. This suggests that the stability of HBsAg loss induced by PD-1 inhibitors may be unstable or susceptible to other combination therapies. Besides, one patient experienced PD-1 inhibitor discontinuation due to HBV-related ACLF and had a poor prognosis, which reflected that HBVr posed unique challenges to the oncologic population including the possibility of treatment delays or discontinuation of systemic therapies that may affect overall survival. However, with additional awareness, screening, and appropriate antiviral prophylactic, most cases of HBVr can be prevented and well managed (37).
To the best of our knowledge, the present study first identified the occurrence of irAEs as the only independent risk factor for HBVr, while failed to find any factors associated with HBVr that had been reported in other studies including male sex, younger age, HBeAg-seropositive, the presence of cirrhosis (38, 39) and PD-1 inhibitor combined with HAIC (17), etc. The reason for this discrepancy may be attributed to an imbalanced gender distribution in our study, as well as the older age, predominantly HBeAg-seronegative status, and presence of liver cirrhosis among patients with HBVr in the present study. Additionally, a larger proportion of patients received HAIC in the previous study. Furthermore, researchers rarely considered the possible causal relationship between irAEs and HBVr. The possible mechanism of HBVr triggered by PD-1 inhibitor might be that: i) blocking the PD-1/PD-L1 axis may lead to the destruction of hepatocytes and the release of previously latent virus into circulation (40). ii) PD-1 blockade may promote the proliferation of T regulatory cells (Tregs) (41) and myeloid-derived suppressor cells (MDSCs) (42), increasing immuno-suppression and then the reactivation of HBV; iii) MDSC levels were considered as a novel biomarker for related immune dysfunction, such as irAEs (43), and inflammatory Treg reprogramming was suggested a feature of immunotherapy-induced irAEs (44), this may explain that irAEs occurrence was a risk factor for HBVr.
What also can’t be ignored in the present study was that serum HBsAg levels increased (P=0.043) in 51 cancer patients, HBeAg-seropositive and the occurrence of irAEs were identified as the independent risk factors for significant HBsAg increase. The underlying mechanism for this may be: i) T cells, B cells, NK cells, and DCs were associated with the clearance of serum HBsAg (45), impairing these immune cells through cytotoxic drugs, which were used in combinational therapies such as chemotherapy, TACE and HAIC, may lead to the increase of HBsAg; ii) The HBeAg-seropositive patients included in this study were mostly in the immune clearance phase, a typical feature of this phase was the occurrence of spontaneous flares, which were often preceded by an increase in the HBV-DNA level (46), and a positive correlation between pHBsAg (the percentage of immunohistochemical HBsAg) and serum levels of HBV-DNA and HBsAg were observed by another study (47), especially in HBeAg-seropositive group. iii) as the suppression of excessive functions of Tregs and MDSC may be one of the proposed immune mechanisms for HBsAg seroclearance (45), the involvement of these cells in irAEs may lead to an increase in HBsAg levels. However, a negative correlation between the Treg frequency and irAEs was discovered by preclinical models of irAEs (48), and the frequency of peripheral Tregs between irAEs group and non-irAEs group showed no significant differences in patients with advanced metastatic melanoma who were receiving PD-1 inhibitors (44), which implied the controversial role that Tregs played in irAEs. Therefore, more detailed studies should be conducted to explore the immune mechanisms underlying HBVr or HBsAg increase under PD-1 inhibitor therapy, as well as to elucidate the paradoxical role of Tregs in irAEs.
With the increasing use of ICIs, cancer patients are at risk of a series of irAEs that can present at any time, including after cessation of immune checkpoint blockade therapy, and may wax and wane over time (1). In this study, 20% (36/180) of patients experienced all-grade irAEs and 7.22% (13/180) of patients developed severe irAEs (grade 3/4), which resulted in delayed and discontinued use of PD-1 inhibitors. Inconsistent with previous studies (19, 20), our study showed a lower prevalence of irAEs in cancer patients. This may be related to the difficulty of evaluating profiles of irAEs and obtaining accurate data on incidence or prevalence, due to selection criteria, relatively small sample sizes, strict diagnosis standards, and limited duration of follow-ups. In addition, we noticed that HBsAg levels were decreased (P=0.045) in the non-irAEs group compared to the irAEs group, which indirectly supported that the occurrence of irAEs was a risk factor for elevated serum HBsAg levels. Interestingly, we also noticed that patients with baseline HBV-DNA > 500 IU/ml had a higher rate of discontinuation of PD-1 inhibitors (P=0.048) due to irAEs. This may be partially attributed to the higher irAEs incidence in patients with baseline HBV-DNA > 500 IU/ml in this study, meanwhile, the patient’s acceptance and tolerance of irAEs also should be considered. Although studies (11, 21, 22, 49) have shown that PD-1/PD-L1 inhibitors are relatively safe and effective for cancer patients, we should still be cautious of the irAEs they may cause. Given the high immunogenicity and long half-life of PD-1 or PD-L1 therapeutic blocking mAbs, they are more likely to cause higher levels of irAEs and are difficult to be timely removed (50). Recently, Zhai et al. (51) have demonstrated a newly screened cyclic peptide C8, which can be removed in a shorter period of time to reverse the irAEs due to its reasonable half-life, could work as a blocker for PD-1 and reactivate CD8+ T cells to treat cancers. It may have the potential as a drug candidate not only for cancer immunotherapy but also for treating chronic hepatitis B in the future.
Conclusion
Under the concurrent use of NAs, we observed an overall decrease in the levels of serum HBsAg in cancer patients receiving PD-1 inhibitor combinational therapy, with a small number of patients achieving HBsAg loss, and the viral replication of most patients can also be effectively inhibited. It suggested that PD-1 inhibitors combined with NAs may have therapeutic effects on chronic HBV infection, and may contribute to the clinical cure of hepatitis B. However, due to the influence of the PD-1 inhibitor itself or other combined antineoplastic therapies, the state of HBsAg loss in some patients cannot be stably maintained.
Except that HBeAg-positive was identified independent risk factor for significant HBsAg increase, our study first identified the occurrence of irAEs as the independent risk factor both for significant HBsAg increase and HBVr, and patients may discontinue PD-1 inhibitors as a result of HBVr or irAEs. This may provide some risk implications for researchers conducting clinical trials using PD-1 or PD-L1 inhibitors to treat CHB, and clinicians need to pay more attention to the safety of PD-1 inhibitors.
Limitations
However, there are several limitations in this study. First, most of the cancer patients with HBV infection are excluded for lacking the awareness of monitoring serum HBsAg or HBV-DNA regularly, which may lead to selection bias, more eligible patients should be enrolled in future studies. Second, more well-designed, large-scale prospective and retrospective studies on cancer patients with HBV infection are needed before any definitive conclusions can be reached. Third, there were few patients with other types of cancer included in this study, more patients diagnosed with other types of cancer should be enrolled in future studies. Fourth, although the quantitation of serum HBsAg and HBV-DNA levels, particularly serum HBsAg levels, were mostly performed using the same quantitative methods before and after PD-1 inhibitor administration in this study, it is essential for the quantitative methods of serum HBV-DNA and HBsAg to remain consistent throughout the treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the institutional review board of the Third Affiliated Hospital of Sun Yat-sen University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of this study.
Author contributions
YZ: Data curation, Investigation, Methodology, Visualization, Writing – original draft. JH: Data curation, Investigation, Resources, Writing – review & editing. JP: Data curation, Investigation, Methodology, Writing – review & editing. SP: Data curation, Investigation, Methodology, Writing – review & editing. YJ: Data curation, Resources, Writing – review & editing. YW: Data curation, Resources, Writing – review & editing. XL: Conceptualization, Formal analysis, Project administration, Supervision, Writing – review & editing. YC: Conceptualization, Formal analysis, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science and Technology Planning Project of Guangdong Province, China (Grant number 2019B020228001), Sun Yat-sen University Clinical Research 5010 Programme (Grant number 2016009) and Wu Jieping Medical Foundation (Grant number 320.6750.2021-22-34).
Acknowledgments
Thanks to all the patients included in this study. Without your clinical data, this study will not be completed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XD declared a shared parent affiliation with the authors to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1330644/full#supplementary-material
Supplementary Figure 1 | Comparison of serum HBsAg levels before and after PD-1 inhibitor administration in cancer patients under different clinical conditions. (A) Comparison of serum HBsAg levels in patients received different antiviral agents. (B-D) Comparison of serum HBsAg levels in patients with different baseline HBV-DNA levels. (E) Comparison of serum HBsAg levels in liver cirrhosis group and non-liver cirrhosis group. (F) Comparison of serum HBsAg levels in patients with and without prior use of antiviral therapy. (G) Comparison of serum HBsAg levels in patients received PD-1 inhibitors ≤ 5 cycles and > 5 cycles. (H) Comparison of serum HBsAg levels in patients under different types of PD-1 inhibitor therapy. ns, not statistically significant; ETV, Entecavir; TDF, Tenofovir disoprox fumarate; TAF, Tenofovir alafenamide fumarate. Due to limited cases, serum HBsAg levels were not compared in patients under Toripalimab therapy and NAs switched therapy.
References
1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
2. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z
3. Xia L, Wang H, Sun M, Yang Y, Yao C, He S, et al. Peripheral CD4+ T cell signatures in predicting the responses to anti-PD-1/PD-L1 monotherapy for Chinese advanced non-small cell lung cancer. Sci China Life Sci. (2021) 64:1590–601. doi: 10.1007/s11427-020-1861-5
4. Boni C, Fisicaro P, Valdatta C, Amadei B, Vincenzo PD, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. (2007) 81:4215–25. doi: 10.1128/JVI.02844-06
5. Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. (2008) 134:1938–1949, 1949.e1-3. doi: 10.1053/j.gastro.2008.03.037
6. Salimzadeh L, Nina LB, Dutertre CA, Gill US, Newell EW, Frey C, et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J Clin Invest. (2018) 128:4573–87. doi: 10.1172/JCI121957
7. Zhang E, Kosinska A, Lu M, Yan H, Roggendorf M. Current status of immunomodulatory therapy in chronic hepatitis b, fifty years after discovery of the virus search for the “magic bullet” to kill cccDNA. Antiviral Res. (2015) 123:193–203. doi: 10.1016/j.antiviral.2015.10.009
8. Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, et al. Antiviral intrahepatic T cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. (2010) 138:682–93. doi: 10.1053/j.gastro.2009.09.052
9. Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1: PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. (2007) 178:2714–20. doi: 10.4049/jimmunol.178.5.2714
10. Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. (2014) 61:1212–9. doi: 10.1016/j.jhep.2014.07.005
11. Gane E, Verdon DJ, Brooks AE, Gaggar A, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. (2019) 71:900–7. doi: 10.1016/j.jhep.2019.06.028
12. Wang G, Cui Y, Xie Y, Mao Q, Xie Q, Gu Y, et al. ALT flares were linked to HBsAg reduction, seroclearance and seroconversion: Interim results from a phase IIb study in chronic hepatitis B patients with 24-week treatment of subcutaneous PDL1 Ab ASC22 (Envafolimab) plus nucleos (t)ide analogs. J Hepatol. (2022) 77(S1):p.S70. doi: 10.1016/S0168-8278(22)00538-4
13. Yoo S, Lee D, Shim JH, Kim KM, Lim YS, Lee HC, et al. Risk of hepatitis B virus reactivation in patients treated with immunotherapy for anti-cancer treatment. Clin Gastroenterol Hepatol. (2022) 20:898–907. doi: 10.1016/j.cgh.2021.06.019
14. Wong LH, Wong WS, Hui WK, Yip CF, Chan SL. Hepatitis flare during immunotherapy in patients with current or past hepatitis B virus infection. Am J Gastroenterol. (2021) 116:1274–1283. doi: 10.14309/ajg.0000000000001142
15. Zhang XY, Zhou YX, Chen C, Fang WF, Cai XY, Zhang XS, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. (2019) 7:322. doi: 10.1186/s40425-019-0808-5
16. Shen J, Wang X, Wang N, Wang N, Wen S, Yang G, et al. HBV reactivation and its effect on survival in HBV-related hepatocarcinoma patients undergoing transarterial chemoembolization combined with tyrosine kinase inhibitors plus immune checkpoint inhibitors. Front Cell Infect Microbiol. (2023) 13:1179689. doi: 10.3389/fcimb.2023.1179689
17. He MK, Peng C, Zhao Y, Liang RB, Lai ZC, Kan A, et al. Comparison of HBV reactivation between patients with high HBV-DNA and low HBV-DNA loads undergoing PD-1 inhibitor and concurrent antiviral prophylaxis. Cancer Immunol Immunother. (2021) 70:3207–16. doi: 10.1007/s00262-021-02911-w
18. Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. (2018) 320:1702–3. doi: 10.1001/jama.2018.13995
19. Maughan BL, Bailey E, Gill DM, Agarwal N. Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front Oncol. (2017) 7:56. doi: 10.3389/fonc.2017.00056
20. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
21. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. LBA38_PRCheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. (2019) 30:v874–5. doi: 10.1093/annonc/mdz394.029
22. Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
23. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. (2020) 17:725–741. doi: 10.1038/s41571-020-0413-z
24. Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The significance of transarterial chemo (Embolization) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: A systematic review. Front Immunol. (2022) 13:913464. doi: 10.3389/fimmu.2022.913464
25. Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. (2021) 15:1031–48. doi: 10.1007/s12072-021-10239-x
26. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (Ctcae-version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (EnglEd). (2021) 112:90–2. doi: 10.1016/j.ad.2019.05.009
27. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. (2018) 67:1560–99. doi: 10.1002/hep.29800
28. Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology. (2011) 53:2121–9. doi: 10.1002/hep.24364
29. Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. (2017) 67:370–98. doi: 10.1016/j.jhep.2017.03.021
30. Zhen S, Qiang R, Lu J, Tuo X, Yang X, Li X. Enhanced antiviral benefit of combination therapy with anti-HBV and anti-PD1 gRNA/cas9 produces a synergistic antiviral effect in HBV infection. Mol Immunol. (2021) 130:7–13. doi: 10.1016/j.molimm.2020.12.004
31. Scott B, Volodymyr G, Mason PJ, Susan C, Levine SM, Wichroski MJ, et al. Safety and efficacy of anti-PD-L1 therapy in the woodchuck model of HBV infection. PloS One. (2018) 13:e0190058. doi: 10.1371/journal.pone.0190058
32. Kim JH, Ghosh A, Ayithan N, Romani S, Khanam A, Park JJ, et al. Circulating serum HBsAg level is a biomarker for HBV-specific T and B cell responses in chronic hepatitis B patients. Sci Rep. (2020) 10:1835–46. doi: 10.1038/s41598-020-58870-2
33. Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. (2015) 60:1457–64. doi: 10.1007/s10620-014-3486-7
34. Xia Z, Zhang J, Chen W, Zhou H, Du D, Zhu K, et al. Hepatitis B reactivation in cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Infect Dis Poverty. (2023) 12:87. doi: 10.1186/s40249-023-01128-6
35. Chang Y, Jeong SW, Jang JY. Hepatitis B virus reactivation associated with therapeutic interventions. Front Med (Lausanne). (2022) 8:770124. doi: 10.3389/fmed.2021.770124
36. Jun BG, Kim YD, Kim SG, Kim YS, Jeong SW, Jang JY, et al. Hepatitis B virus reactivation after radiotherapy for hepatocellular carcinoma and efficacy of antiviral treatment: A multicenter study. PloS One. (2018) . 13:e0201316. doi: 10.1371/journal.pone.0201316
37. Smalls DJ, Kiger RE, Norris LB, Bennett CL, Love BL. Hepatitis B virus reactivation: risk factors and current management strategies. Pharmacotherapy. (2019) 39:1190–203. doi: 10.1002/phar.2340
38. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. (2017) 152:1297–309. doi: 10.1053/j.gastro.2017.02.009
39. Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. (2000) 62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0
40. Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology. (2014) 146:1193–207. doi: 10.1053/j.gastro.2013.12.036
41. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1 + regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. (2019) 116:9999–10008. doi: 10.1073/pnas.1822001116
42. Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. (2020) 5:eaay1863. doi: 10.1126/sciimmunol.aay1863
43. Strauss L, Guarneri V, Gennari A, Sica A. Implications of metabolism-driven myeloid dysfunctions in cancer therapy. Cell Mol Immunol. (2021) 18:829–41. doi: 10.1038/s41423-020-00556-w
44. Grigoriou M, Banos A, Hatzioannou A, Kloetgen A, Kouzis P, Aggouraki D, et al. Regulatory T-cell transcriptomic reprogramming characterizes adverse events by checkpoint inhibitors in solid tumors. Cancer Immunol Res. (2021) 9:726–34. doi: 10.1016/j.jhep.2020.04.013
45. Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis B surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J Hepatol. (2020) 73:409–22. doi: 10.1016/j.jhep.2020.04.013
46. Croagh CM, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol. (2014) 20:10395–404. doi: 10.3748/wjg.v20.i30.10395
47. Alpsoy A, Adanir H, Bayramoglu Z, Elpek GO. Correlation of hepatitis B surface antigen expression with clinicopathological and biochemical parameters in liver biopsies: A comprehensive study. World J Hepatol. (2022) 14:260–73. doi: 10.4254/wjh.v14.i1.260
48. Kumar P, Saini S, Prabhakar BS. Cancer immunotherapy with checkpoint inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin Cancer Biol. (2020) 64:29–35. doi: 10.1016/j.semcancer.2019.01.006
49. Pan S, Yu Y, Wang S, Tu B, Shen Y, Qiu Q, et al. Correlation of HBV DNA and hepatitis B surface antigen levels with tumor response, liver function and immunological indicators in liver cancer patients with HBV infection undergoing PD-1 inhibition combinational therapy. Front Immunol. (2022) 13:892618. doi: 10.3389/fimmu.2022.892618
50. Sheng J, Srivastava S, Sanghavi K, Lu Z, Schmidt BJ, Bello A, et al. Clinical pharmacology considerations for the development of immune checkpoint inhibitors. J Clin Pharmacol. (2017) 57:S26–42. doi: 10.1002/jcph.990
Keywords: cancer, PD-1 inhibitor, HBsAg loss, HBsAg increase, HBV reactivation, immune-related adverse events, risk factor identification
Citation: Zeng Y, Huang J, Pang J, Pan S, Wu Y, Jie Y, Li X and Chong Y (2024) The occurrence of immune-related adverse events is an independent risk factor both for serum HBsAg increase and HBV reactivation in HBsAg-positive cancer patients receiving PD-1 inhibitor combinational therapy. Front. Immunol. 15:1330644. doi: 10.3389/fimmu.2024.1330644
Received: 31 October 2023; Accepted: 20 February 2024;
Published: 15 March 2024.
Edited by:
Hakim Echchannaoui, Johannes Gutenberg University Mainz, GermanyReviewed by:
Xinpei Deng, Sun Yat-sen University Cancer Center (SYSUCC), ChinaSuresh Kalathil, University at Buffalo, United States
Jin Hou, Second Military Medical University, China
Copyright © 2024 Zeng, Huang, Pang, Pan, Wu, Jie, Li and Chong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinhua Li, bGl4aW5oOEBtYWlsLnN5c3UuZWR1LmNu; Yutian Chong, Y2hvbmd5dEBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
 Yingfu Zeng
Yingfu Zeng Jiwei Huang3†
Jiwei Huang3† Yusheng Jie
Yusheng Jie Xinhua Li
Xinhua Li Yutian Chong
Yutian Chong