- 1The Unit of Proteomics and Flow Cytometry, Allergy and Clinical Immunology, Bnai-Zion Medical Center, Faculty of Medicine, Technion, Haifa, Israel
- 2Department of Pathology, Bnai-Zion Medical Center, Faculty of Medicine, Technion, Haifa, Israel
- 3Department of Dermatology, Hospital del Mar & Research Institute, Universitat Pompeu Fabra, Barcelona, Spain
- 4Institute of Allergology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 5Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Immunology and Allergology, Berlin, Germany
Background: The proximity of activated T cells and mast cells in the lesional skin of patients with chronic spontaneous urticaria (CSU) is held to contribute to the development of wheals and angioedema. In a previous study, we demonstrated that increased IL-17 expression in T cells and mast cells in skin lesions of patients with CSU is associated with T/mast cell proximity, but the mechanisms that drive T cell/mast cell co-localization remain unknown.
Objectives: To assess if chemokines expressed in lesional CSU skin contribute to T cell/mast cell proximity.
Patients and methods: Biopsies from lesional CSU skin were compared to biopsies from healthy skin for expression of CCR5 and its ligand CCL3 by CD4+ T cells and mast cells, respectively.
Results: Numbers of CCR5-positive CD4+ T cells in lesional CSU skin were significantly increased as compared to healthy normal skin (p < 0.0001). The number of mast cells expressing CCL3 (ligand for CCR5) in CSU skin was also increased (p < 0.0002) and significant association with T-cell close proximity (p < 0.0001) is noticed.
Conclusions: The close proximity of T cells and mast cells in the skin of severe CSU may be driven, at least in part by increased CCR5 and CCL3 expression. Therapies that target CCL3 interaction with CCR5 should be assessed for their effects in CSU.
1 Introduction
Type I and type II autoimmunity are both involved in the pathogenesis of chronic spontaneous urticaria (CSU) (1, 2). As part of this, activated CD4+ T cells, namely, autoreactive T cells, were reported in many studies to be key players in CSU, both in the peripheral blood and in the lesional skin of CSU patients (3). Lesional CSU skin is characterized by perivascular non-necrotizing cellular infiltrate around small venules that consist mainly of CD4+ T cells, eosinophils, basophils, and neutrophils (4). The crosstalk between activated CD4+ T cells and mast cells was reported more than two decades ago, resulting into mast cell degranulation and the release of histamine and other proinflammatory mediators (5). During the process of this crosstalk, mast cells have been found to be activated in T-cell-mediated inflammation by remaining in close physical proximity to activated CD4+ T cells (6). In a recent study, we described for the first time the intense infiltration of IL-17A expressing CD4+ T cells in the skin of CSU patients in close proximity with IL-17A expressing mast cells, suggesting this to be one of the important mechanisms in the induction of mast cell degranulation in CSU (7). However, the mechanisms that drive T-cell infiltration and their co-localization with mast cells in the skin of CSU patients are yet to be better identified and characterized. Chemokines (both chemokine receptors and chemokine ligands) are small bound and secreted proteins, promoting the recruitment of activated immune cells into the cellular infiltrate of many inflammatory skin diseases including into lesional CSU skin, contributing to their differentiation and release of inflammatory cytokines (8, 9). Based on their molecular structure, chemokines are divided into ligands such as CCL and CXCL, which bind to their specific receptors, namely, to CCR or CXCR. In this respect, chemokine axis such as SDF-1/CXCR4 or CCL5/RANTES are reported to be potent chemo-attractants of proinflammatory immune cells and increased angiogenesis in inflamed skin tissues, such as chronic spontaneous urticaria and psoriasis (10, 11). Chemokines such as CXCL1/2, CXCL8, CCR5, and others are recently reported to be released from a wide spectrum of cells, including endothelial cells, activated T cells, and basophils in both peripheral blood and lesional skin of CSU patients resulting in the increased crosstalk between all these cells, thus contributing to the pathogenesis of CSU (12, 13). We suggest that many chemokine receptors on activated T cells and many chemokine ligands on mast cells are involved in this crosstalk between immune cells in the skin of CSU patients. In this study, we chose to analyze the expression of CCR5 (expressed on T cells) and CCL3 (expressed on mast cells) in order to establish for the first time their role in promoting the proximity of these cells in lesional skin of CSU patients.
2 Patients and methods
2.1 Patients
We analyzed 12 skin biopsies from lesional skin of severe CSU patients and compared them with eight biopsies from age- and sex-matched healthy individuals. Biopsies were taken following signing an informed consent and approved by the local Helsinki committee of Bnai-Zion Medical Center and the Charité—Universitätsmedizin Berlin. All CSU patients were unresponsive to high doses of anti-histamines, and short courses of steroids could not achieve sufficient remission. Biopsies were taken when patients did not use corticosteroids or cyclosporine A. Urticarial activity score (UAS7) values were available in all patients, ranging between 29 and 39 (mean ± 32) (see characteristics in Table 1). Biopsies from healthy individuals were taken from plastic surgeries, e.g., breast and abdomen. All biopsies were assessed for (1) the expression of CD4+ T cells and mast cells in lesional skin, in comparison with that in normal skin; (2) CCR5 expression on CD4+ T cells and the expression of CCL3 on mast cells; and (3) the proximity of CCL3-expressing mast cells with lymphocytes in the skin of CSU when compared to that in normal skin.
2.2 Skin immunofluorescence
Lesional skin biopsies from severe CSU patients (n=12) and control skin biopsies (n=8) from healthy subjects were studied by double staining immunofluorescence. Briefly, formalin-fixed paraffin-embedded skin tissue sections were sliced serially into 5-µm slices by a microtome. The slides were deparaffinized by xylene and then rehydrated by absolute ethanol, 96% ethanol, 70% ethanol, and two short-distilled water washes. The antigen retrieval was done by cooker pressure in an EDTA solution (2 mM, and pH 8.0), blocked with 3% glycine for 1 h and then with 1% BSA for an additional hour (14). Primary antibodies, mouse monoclonal anti-human CCR5 (1:25, Abcam), rabbit polyclonal anti-human cKit (CD117) (1:100, Abcam), and rabbit polyclonal anti-human CD4 (1:300, Abcam) were incubated with the slides overnight. On the second day, slides were washed three times with 1× PBS and then incubated with secondary antibodies for 45 min. Slides were rewashed and stained with DAPI. The whole slide was scanned and quantified for the amount of CD4+ and cKit+ (CD117) and double stained for CD4+ T cells/CCR5 cells by using Image J software.
2.3 Skin immunohistochemistry
Tissues were fixed in 4% paraformaldehyde, processed routinely, and embedded in paraffin. Sections (3 μm) were mounted on positively charged slides. Hematoxylin/eosin staining was used for histological evaluation under a light microscope. Sequential sections were used for immunohistochemistry staining.
The immunostains were conducted on an automated stainer (Benchmark Ultra; Ventana Systems, Phoenix, AZ) (14). Following antigen retrieval in Tris-based buffer, double staining of CCL3 and CD117 (c-Kit) was performed. Antibodies used were rabbit anti-human CD117 polyclonal antibody (1:200, DAKO), rabbit anti-human CCL3 (1:500, Abcam). OptiVIEW DAB detection kit and UltraVIEW Alkaline phosphatase red detection kit (760-700 and 760-501, accordingly; Roche Diagnostics GmbH, Mannheim, Germany) were used according to manufacturer-recommended protocol, followed by hematoxylin counterstain for color development. Pictures of sections mounted for CCL3+CD117+ (c-Kit) were taken using a DP70 Olympus camera.
2.4 Statistical analysis
Results were presented as mean standard deviation (SD). The intensity of CD4+ T cells, mast cells, and chemokine expression was compared between CSU biopsies and healthy skin. Statistical significance was performed by Student’s t-test. Statistical significance was considered with a p-value ≤ 0.05).
2.5 Bioethical standards
Bioethical approval of the Charité—Universitätsmedizin Berlin (No. EA4/052/19) and that of the Bnai-Zion Medical Center, Israel (0133-18-BNZ) was obtained.
3 Results
3.1 CD4+ T cells and mast cells are increased in the CSU skin
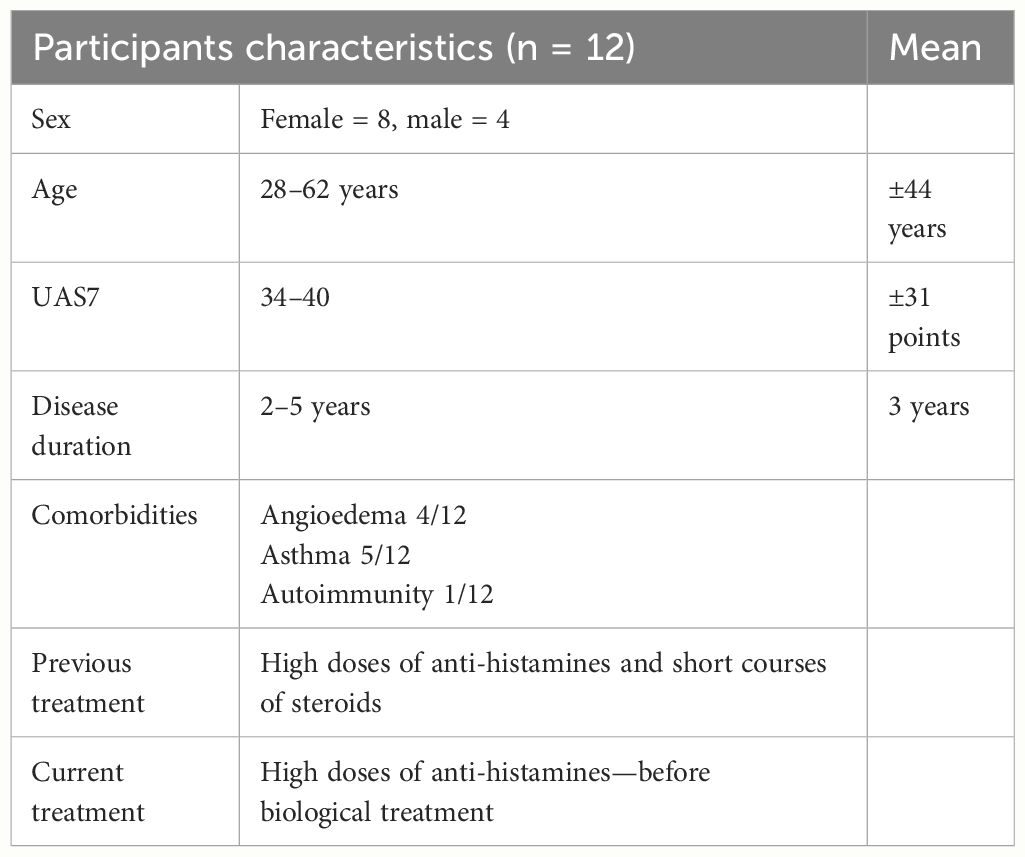
CD4+ T cells were markedly increased in the lesional skin of CSU patients, as assessed by quantitative histomorphometry. Specifically, CSU skin lesions had more than 2.8 fold higher numbers of CD4+ T cells as compared to the skin of healthy controls, i.e., 42.3 ± 2.1 vs. 14.7 ± 4.7 CD4+ T cells/µm2 (p < 0.0001, Figure 1A, I, II). Mast cell numbers, assessed by staining for CD117, were also substantially increased in the lesional skin of patients with CSU. As compared to healthy control skin, lesional CSU skin had >600% the number of mast cells, i.e., 50.5 ± 3.7 vs. 7.7 ± 2.4 mast cells/µm2 (p < 0.0001, Figure 1B, I, II).
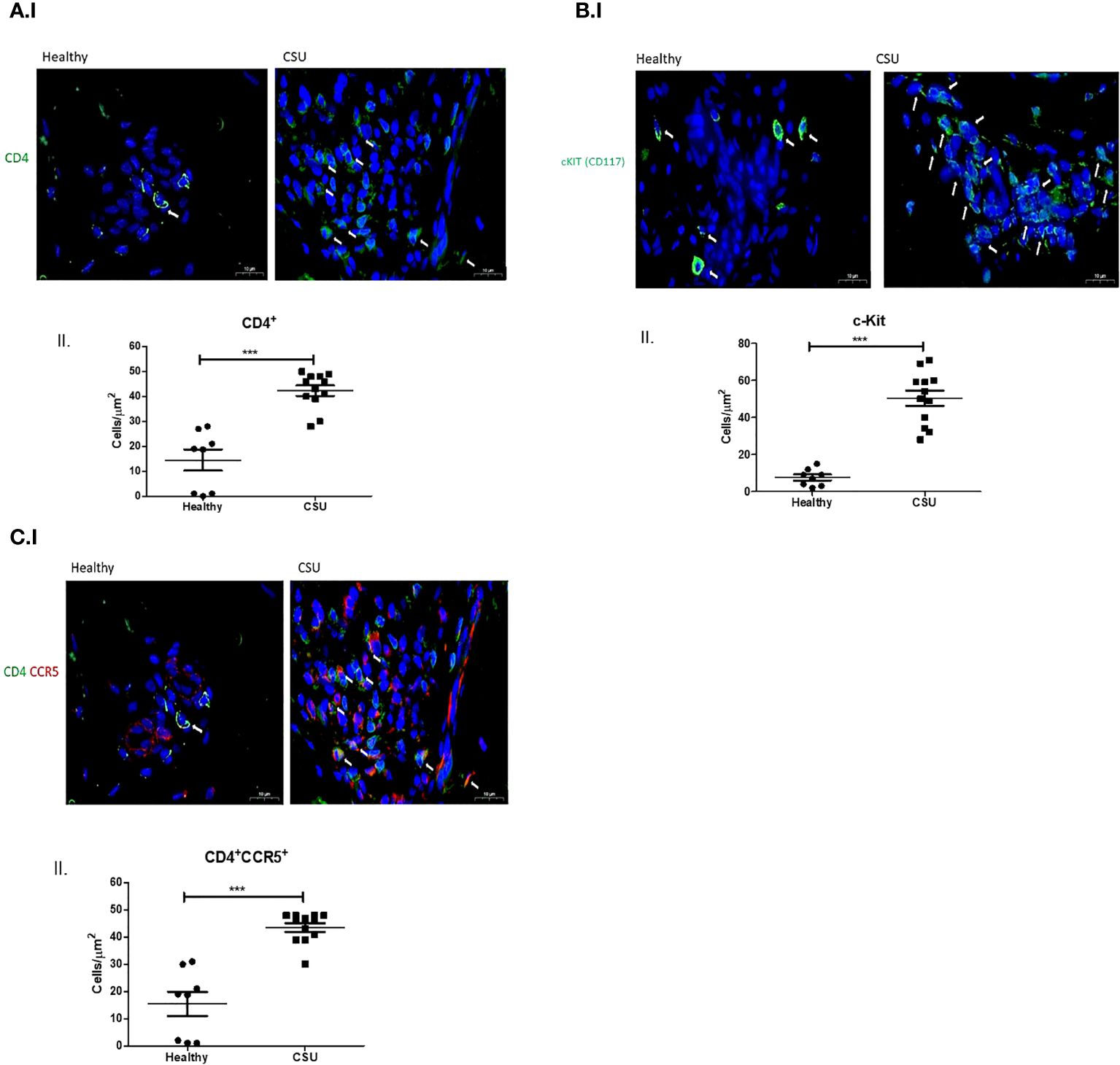
Figure 1 CD4+ T cells, mast cells, and CD4+CCR5+ in skin biopsies. (A) I. Normal skin shows small amount of CD4+ T cells while CSU skin shows numerous CD4+ T cells (in green, white arrows indicate CD4+ T cells). II. Quantitative analysis of number of CD4+ T cells in normal vs. CSU skin biopsies (p < 0.0001). (B) I. Normal skin shows small amount of mast cells, while CSU skin shows increased numbers of mast cells (in green, white arrows indicate mast cells). II. Quantitative analysis of number of mast cells in normal vs. CSU skin biopsies (p < 0.0001). (C) I. Double-staining immunofluorescence CD4+CCR5+ cells in CSU skin biopsies. Normal skin shows a small number of double-stained cells in comparison with CSU, in green and red, see white arrows. II. Quantitative analysis of number of double stained T cells in normal vs. CSU skin biopsies (p < 0.0001). Scale bar= 10 µm. *** statistically significant.
3.2 CCR5-positive CD4+ T cells are increased in CSU skin
In lesional CSU skin, numbers of CCR5-positive CD4+ T cells were almost threefold higher as compared to healthy skin, i.e., 43.6 ± 1.6 vs. 15.4 ± 4.9 cells/µm2 (p< 0. 0001, Figure 1C, I, II).
3.3 The chemokine ligand CCL3 on mast cells is increased in CSU skin
The numbers of mast cells that express CCL3, the ligand of CCR5, were markedly increased in lesional skin biopsies of CSU patient in comparison with normal skin biopsies, i.e., 27.1 ± 2.9 vs. 9.4 ± 1.4 cells/µm2 (p<0.0002) (Figure 2A, I, II). Most CCL3-positive mast cells, 23.4 ± 2.9 cells/µm2 (spindle-shaped nuclei with cytoplasmic granules) positioned in close proximity to T cells (round-shaped nuclei with small non-granular cytoplasm) (Figure 2B, I, II). The distance between T cells and mast cells CCL3+ was between 0.4 μm and 0.9μm in CSU skin biopsies comparing to 2.3–10.5 μm in healthy controls. Thus, a distance lower than 1 μm was considered to be in close proximity (see Supplementary Figures).
Figure 2 CCL3 expression on mast cells. (A) I. and II. Positive CCL3-stained mast cells in CSU skin biopsy. Black arrows denote mast cell that are positive for CCL3 staining; an increase in the CCL3-positive mast cell numbers is noted. ×200 magnification (p < 0.0002). Scale bar= 20 µm. (B) I. and II. CCL3-positive mast cells in CSU skin biopsy (black arrows), in proximity to CD4+ T cells (white arrows). ×400 magnification (p < 0.0001). Scale bar= 40 µm. *** statistically significant.
4 Discussion
IgE- and IgG-dependent autoimmunity is considered a major mechanism in the development of mast cell activation and degranulation in CSU skin. IgE-independent pathways are recently reported to play pivotal roles in the pathogenesis of mast cell activation (though of limited relevance). In this respect, human mast cells express several regulatory receptors such as toll-like receptors, Mas-related G-protein coupled receptor-X2 (MRGPRX2), and others. MRGPRX2 is expressed at high levels on skin mast cells and is activated by small molecule compounds during immune-mediated inflammation, leading to mast cell hypersensitivity reactions such as in CSU. This suggests that MRGPRX2 inhibitors may become a therapeutic strategy in allergic diseases (15). In addition, the crosstalk between infiltrating immune cells around small venules in deep dermis of CSU patients, namely, between T cells, mast cells, eosinophils, basophils, and neutrophils was previously shown to involve the presence of chemokines in the skin of atopic patients, thus becoming a highly relevant mechanism in mast cell degranulation in the pathogenesis of CSU (12). With this in mind, the proximity between activated CD4+ T cells and mast cells is highly appreciated to be a fundamental mechanism for the induction of mast cell degranulation. Y. Mekori was the first to characterize a novel mast cell activation pathway initiated by close physical contact with activated T cells. It was shown that mast cells were activated by the release of microparticles from activated CD4+ T cells involving MARK signaling pathway (16). In another study, microRNA-4443 derived by released microvesicles was shown to act as intercellular carriers, contributing to the downregulation of tyrosine phosphatase protein expression in mast cells, leading to their activation and degranulation (17). Later, we published our results where we demonstrated that increased IL-17 expression in both CD4+ T cells and mast cells were found to be in close proximity in lesional skin of CSU patients (7). In our current study, we clearly show for the first time that the expression of chemokine receptors and ligands on both CD4+ T cells and mast cells is significantly increased (Figure 1C, I, II, Figure 2A, I, II). In this respect, it is our additional finding that T cells are increasingly infiltrated in close proximity with CCL3 expressing mast cells (Figure 2B, I, II). In an early study, it was shown that following the binding of CCL3 to its CCR5 receptor on T cells, the phosphorylation and activation of Janus kinase 2 (JAK2) was observed, but this was not prevented by inhibition of JAK, indicating that this is only one of many other dependent signals (18). In another study, although not related to CSU, it was shown that the CCL3–CCR5 axis promotes cell migration and invasion of colon adenocarcinoma via Akt signaling pathway (19). Finally, increased expression of CCR5 and its ligand CCL3 was demonstrated in the tear film and ocular surface of patients with dry eye syndrome, especially in those with Sjogren’s syndrome (20). Our preliminary results in this study indicate clearly that CCR5–CCL3 axis is important in the crosstalk between mast cells and T cells in CSU lesional skin. This suggests that increased chemokine expression in CSU skin is of high relevance in attracting CD4+ T cells into the CSU skin and, by a crosstalk mechanism, may lead to mast cell activation and degranulation.
5 Study limitations
The limitations of our study are the relatively small number of available biopsies. This is because biopsies are not performed routinely in CSU patients, and they are rarely available for research purposes. In addition, functional tests are required in order to better establish the above-demonstrated observation. Additional studies may establish the idea that targeting this chemokine axis may become one of the optional therapeutic strategies in CSU (21).
6 Conclusions
Increased expression of CCR5 and its ligand CCL3 in T cells and mast cells in the skin of CSU patients may increase the proximity between these cells, which leads to disease worsening. Therefore, therapies that target CCL3 interaction with CCR5 should be assessed for their effects in CSU.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Bnai-Zion Local Helsinky Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it is a retrospective study on skin biopsies.
Author contributions
RM: Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. RS: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. AG: Conceptualization, Data curation, Writing – review & editing. MM: Conceptualization, Data curation, Writing – review & editing. JB: Investigation, Methodology, Visualization, Writing – review & editing. ET: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. ZV: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1327040/full#supplementary-material
References
1. Kolkhir P, Munoz M, Asero R, Ferrer M, Kocaturk E, Metz M, et al. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol. (2022) 149:1819–31. doi: 10.1016/j.jaci.2022.04.010
2. Kolkhir P, Kovalkova E, Chernov A, Danilycheva I, Krause K, Sauer M, et al. Autoimmune chronic spontaneous urticarial detection with IgG anti-TPO and total IgE. J Allergy Clin Immunol Pract. (2021) 9:4138–46. doi: 10.1016/j.jaip.2021.07.043
3. Auyeung P, Mittag D, Hodgkin PD, Harrison LC. Autoreactive T cells in chronic spontaneous urticarial target the IgE Fc receptor Iα subunit. J Allergy Clin Immunol. (2016) 138:761–68. doi: 10.1016/j.jaci.2016.04.036
4. Gimenez-Arnau AM, DeMontojoye L, Asero R, Cugno M, Kulthanan K, Yanase Y, et al. The pathogenesis of chronic spontaneous urticaria: The role of infiltrating cells. J Allergy Clin Immunol Pract. (2021) 9:2195–208. doi: 10.1016/j.jaip.2021.03.033
5. Bhattacharyya SP, Drucker I, Reshef T, Kirshenbaum AS, Metcalfe DD, Mekori YA. Activated T lymphocytes induce degranulation and cytokine production by human mast cells following cell-to-cell contact. J Leukoc Biol. (1998) 63:337–41. doi: 10.1002/jlb.63.3.337
6. Mekori YA, Metcalfe DD. Mast cell- T cell interactions. J Allergy Clin Immunol. (1999) 104:517–23. doi: 10.1016/S0091-6749(99)70316-7
7. Sabag DA, Matanes L, Bejar J, Sheffler H, Brazilai A, Church MK, et al. Interleukin-17 is a potential player and treatment target in severe chronic spontaneous urticaria. Clin Exp Allergy. (2020) 50:799–804. doi: 10.1111/cea.13616
8. Stone MJ, Hayward JA, Huang C, Huma ZE, Sanchez J. Mechanisms of regulation of the chemokine-receptor network. Int J Mol Sci. (2017) 18:342. doi: 10.3390/ijms18020342
9. Sun Z, Vattepu R, Zhang S. Chemolkines and innate lymphoid cells in skin inflammation. Cells. (2021) 10:3074. doi: 10.3390/cells10113074
10. Zgraggen S, Huggenberger R, Keri K, Detnar M. An important role of SDF-1/CSCR4 axis in chronic skin inflammation. PLoS. (2014) 9:e93665. doi: 10.1371/journal.pone.0093665
11. Puxeddu I, Panza F, Pratesi F, Bartaloni D, Casigliani Rabl S, Rocchi V, et al. CCL5/RANTES, sVCAM-1, and sICAM-1 in chronic spontaneous urticaria. Int Arch Allergy Immunol. (2013) 162:330–4. doi: 10.1159/000354922
12. Zhou B, Li J, Liu R, Zhu L, Peng C. The role of crosstalk of immune cells in pathogenesis of chronic spontaneous urticarial. Front Immunol. (2022) 13:879754. doi: 10.3389/fimmu.2022.879754
13. Kaplan A, Lebwohl, Gimenez-Arnau AM, Hide M, Armstrong AW, Maurer M. Chronic spontaneous urticarial: Focus on pathophysiology to unlock treatment advances. Allergy. (2023) 78:389–401. doi: 10.1111/all.15603
14. Sabag DA, Matanes L, Bejar J, Sheffer H, Barzilai A, Church MK, et al. Interleukin-17 is a potential player and treatment target in severe chronic spontaneous urticarial. Clin Exp Allergy. (2020) 50:799–804. doi: 10.1111/cea.13616
15. Ogasawara H, Noguchi M. Therapeutic potential of MRGPRX2 inhibitors on mast cells. Cells. (2021) 10:2906. doi: 10.3390/cells10112906
16. Shefler I, Salamon P, Reshef T, Mor A, Mekori YA. T cell-induced mast cell activation: a role for microparticles released from activated T cells. J Immunol. (2010) 185:4206–12. doi: 10.4049/jimmunol.1000409
17. Shefler I, Salamon P, Levi-Schaffer F, Mor A, Hershko AY, Mekori YA. MicroRNA-4443 regulates mast cell activation by T cell-derived microvesicles. J Allergy Clin Immunol. (2018) 141:2132–41. doi: 10.1016/j.jaci.2017.06.045
18. Mueller A, Strange PG. CCL3, acting via the chemokine receptor CCR5, leads to independent activation of Janus kinase 2 (JAK2) and Gi protein. FEBS Lett. (2004) 570:126–32. doi: 10.1016/j.febslet.2004.04.100
19. Guan B, Li H, Yao J, Guo J, Yu F, Li G, et al. CCL3-CCR5 axis promotes cell migration and invasion of colon adenocarcinoma via Akt signaling pathway. Environ Toxicol. (2023) 38:172–84. doi: 10.1002/tox.23675
20. Choi, Li Z, Oh H-J, Im S-K, Lee S-H, Park S-H, et al. Expression of CCR5 and its ligands CCL3, -4, -5 in the tear film and ocular surface of patients with dry eye disease. Curr Eye Res. (2012) 37:12–7. doi: 10.3109
Keywords: CSU, T cells, mast cells, chemokines, wheals, angioedema
Citation: Mubariki R, Samara R, Gimenez-Arnua AM, Maurer M, Bejar J, Toubi E and Vadasz Z (2024) CD4+CCR5+ T cells and CCL3+ mast cells are increased in the skin of patients with chronic spontaneous urticaria. Front. Immunol. 15:1327040. doi: 10.3389/fimmu.2024.1327040
Received: 14 November 2023; Accepted: 09 July 2024;
Published: 22 July 2024.
Edited by:
Maria Maslinska, National Institute of Geriatrics, Rheumatology and Rehabilitation, PolandReviewed by:
Eli Magen, Ben-Gurion University of the Negev, IsraelJie Li, Central South University, China
Rohit Saluja, All India Institute of Medical Sciences, Bibinagar, India
Copyright © 2024 Mubariki, Samara, Gimenez-Arnua, Maurer, Bejar, Toubi and Vadasz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zahava Vadasz, emFoYXZhLnZhZGFzQGItemlvbi5vcmcuaWw=
 Raeda Mubariki
Raeda Mubariki Reem Samara2
Reem Samara2 Marcus Maurer
Marcus Maurer Elias Toubi
Elias Toubi Zahava Vadasz
Zahava Vadasz